Abstract
Background
Allergies have long been observed in Inborn Errors of Immunity (IEI) and might even be the first presentation resulting in delayed diagnosis or misdiagnosis in some cases. However, data on the prevalence of allergic diseases among IEI patients are limited and contradictory.
Objective
To provide a worldwide view of allergic diseases, across a broad spectrum of IEI, and their impact on the timely diagnosis of IEI.
Methods
This is a worldwide study, conceived by the World Allergy Organization (WAO) Inborn Errors of Immunity Committee. A questionnaire was developed and pilot-tested and was sent via email to collect data from 61 immunology centers known to treat pediatric and/or adult IEI patients in 41 countries. In addition, a query was submitted to The United States Immunodeficiency Network (USIDNET) at its website.
Results
Thirty centers in 23 countries caring for a total 8450 IEI patients responded. The USIDNET dataset included 2332 patients. Data from responders showed that a median (IQR) of 16.3% (10–28.8%) of patients experienced allergic diseases during the course of their IEI as follows: 3.6% (1.3–11.3%) had bronchial asthma, 3.6% (1.9–9.1%) atopic dermatitis, 3.0% (1.0–7.8%) allergic rhinitis, and 1.3% (0.5–3.3%) food allergy. As per the USIDNET data, the frequency of allergy among IEI patients was 68.8% (bronchial asthma in 46.9%). The percentage of IEI patients who presented initially with allergic disorders was 8% (5–25%) and diagnosis delay was reported in 7.5% (0.9–20.6%). Predominantly antibody deficiencies had the highest frequency of allergic disease followed by combined immunodeficiency with a frequency of 40.3% (19.2–62.5%) and 20.0% (10–32%) respectively. As per the data of centers, anaphylaxis occurred in 25/8450 patients (0.3%) whereas per USIDNET dataset, it occurred in 249/2332 (10.6%); drugs and food allergy were the main causes in both datasets.
Conclusions
This multinational study brings to focus the relation between allergic diseases and IEI. Major allergies do occur in IEI patients but were less frequent than the general population. Initial presentation with allergy could adversely affect the timely diagnosis of IEI. There is a need for policies to raise awareness and educate primary care and other referring specialties on the association of allergic diseases with IEI. This study provides a network among centers for future prospective studies in the field.
Keywords: Primary immunodeficiency, Asthma, Atopic dermatitis, IVIG, Omalizumab, Anaphylaxis, Allergic rhinitis
Introduction
Primary immunodeficiency diseases (PIDs), recently termed human inborn errors of immunity (IEI), typically present with an unusual tendency to recurrent and/or severe infections.1 Allergies have long been observed in immune deficiency,2 and might even be the first clinical presentation resulting in delayed diagnosis or misdiagnosis in some cases. However, data on the prevalence of allergic diseases among patients with IEI are limited and contradictory; some reports pointed to an overall lower prevalence of food allergy (FA) (1.8%) and atopic dermatitis (AD) (6%) compared to the general population3 contrasting reports from other countries such as Tunisia, in which eczematous dermatitis was described in 21.38% of IEI patients.4 Thus, there is a need to better define atopic features in a carefully phenotyped cohort of patients with IEI.
AD has been reported with several of the IEIs such as hyper Ig-E syndrome (HIES), DOCK 8 deficiency, Omenn syndrome (OS), and Wiskott-Aldrich syndrome (WAS), among others.5 The course might be severe and recalcitrant leading to profound skin infections and sepsis.6 Selective IgA deficiency (SIgAD) is associated with multiple allergies. Some prospective studies have shown an increased risk of parentally/self-reported FA in SIgAD.7,8 This holds true for other IEIs such as CD40 ligand deficiency, primary hypogammaglobulinemia and combined immunodeficiency (CID).2 FA is a known cause of persistent diarrhea and failure to thrive and could potentially be life threatening through inducing anaphylaxis. Recurrent wheezy chest and asthma are part of everyday pediatric practice, and in the context of severe atopy, chest infections can sometimes be perceived as simply secondary to the allergic inflammation and not the result of an IEI.2 In a recent study, asthma was reported in 37.5% of patients with common variable immunodeficiency (CVID).9 Although both conditions have immune basis as the main background, both can co-exist, mimic or worsen each other.
Awareness of IEI by physicians of different specialties is suboptimal,10 and failure to recognize that some IEIs masquerade as allergic disease may interfere with early diagnosis and treatment and have significant bearing on the outcome. Furthermore, failure of recognition of the allergic nature of some of the clinical manifestations in patients with IEI may compound their disease course and deprive them of receiving the optimal allergy treatment including biologics. Indeed, several case series3,4 reported this association, yet the overall picture is still unclear. Hence, we conducted this study to outline the global extent and nature of allergic diseases across a broad spectrum of IEI, their impact on the timely diagnosis of IEI, and the modalities used for diagnosis and treatment of allergic diseases in these patients.
Methods
Study design and population
This study, conceived and planned by the World Allergy Organization (WAO) Inborn Errors of Immunity Committee, sought to obtain center-level retrospective data via a questionnaire sent by email to immunology centers in countries all over the world, recorded in the WAO database and/or registered with the European Society for Immunodeficiencies, and defined as treating pediatric and/or adult IEI patients. We obtained the contacts of 61 IEI experts working at 61 centers in 41 countries. In addition, a query was submitted to The United States Immunodeficiency Network (USIDNET) at its website (https://usidnet.org), to have a broad view of allergic diseases associating IEI. The database was searched to determine the responses to the questionnaire's fields in the core registry relevant to allergic diseases (also retrospective data). [The U.S. Immunodeficiency Network (USIDNET), a program of the Immune Deficiency Foundation (IDF), is supported by a cooperative agreement, U24AI86837, from the National Institute of Allergy and Infectious Diseases (NIAID)].
The questionnaire was constructed as a form with fields to be filled in with exact data extracted from the centers' registries. It was developed by 3 professors of allergy and immunology and was pilot tested in 5 centers. Some questions were re-phrased and a further 3 were added. The final form was distributed to expert physicians/professors in specialized immunology centers and the responses were compiled by the main author. The questionnaire first covered data about the participating center whether serving pediatric age patients, adults, or both. The surveyed core data were structured into 4 main domains that comprised in total 27 questions: The domains were: 1) the number of IEI patients in the center and the frequency of the different IEI groups according to the 2019 IUIS classification;1 2) the frequency of IEI patients presenting initially with allergic diseases and its impact on diagnosis delay of IEI; 3) the frequency of bronchial asthma (BA), atopic dermatitis (AD), allergic rhinitis (AR), food allergy (FA), anaphylaxis, urticaria, and other allergies encountered in each group of IEI (focusing on predominantly antibody deficiency [PAD] IUIS group III, combined immunodeficiency [CID] IUIS groups I & II, defective phagocytic number/function IUIS group V, immune dysregulation IUIS group IV, and autoinflammatory diseases IUIS group VII); and 4) the most common IEI associated with longstanding allergic disorder requiring continued allergy treatment, the allergy diagnostic tests and treatment used for allergic IEI patients. A free space was provided at the end to encourage reporting of an interesting relevant case.
Questionnaire reliability
The assessment was done using Chronbach's alpha test to assess internal consistency.
Statistical methods
Data were collected, revised, coded and entered to the statistical package for social science (SPSS) version 23. Prevalence data were collected from participating centers as frequencies and reported as median with interquartile range (IQR). Frequencies of allergic diseases among IEI groups were compared using Kruskall-Wallis test followed by post hoc Dunn test. Chi-square test was used to compare 2 groups of categorical data. All p-values were two-sided. P-values < 0.05 were considered significant. The data of the USIDNET were analyzed separately and were presented as frequencies.
Ethical consideration
It was determined by the WAO Inborn Errors of Immunity Committee that the collected data reported in aggregate did not require consent.
Results
Questionnaire reliability
The assessment was as follows: The internal consistency for the 4 domains was good, with Cronbach's alpha coefficients (α = 0.88, 0.72, 0.87, 0.73 respectively) meeting the minimum recommended threshold of α > 0.7 with the total questionnaire achieving Cronbach's alpha coefficient of 0.89.
Characteristics of the participating centers
Responses were received from 30 centers in 23 countries spanning the 6 continents (Fig. 1). Non-response (n = 22) despite sending 3 reminders over 3 months was perceived as refusal to participate or failure to reach. Nine centers apologized (reasons were: not having patients' records in 3, and not having the time in the rest). There were no apparent differences between responding and non-responding centers. Fourteen centers (46.7%) were pediatric IEI centers, 12 (40%) managed both pediatric and adult IEI patients and 3 (10%) managed only adults. One center (3.3%) did not specify the age group. The number of IEI patients per center upon which the responders based their responses ranged from 20 to 1395 with a median (IQR) number of 203.5 (42.25–490.25) and with a total of 8450 patients. The USIDNET provided the data of 2332 adult and pediatric patients with IEI.
Fig. 1.
Geographical distribution of the participating centers (including USIDNET)
Prevalence of allergic diseases among IEI patients and their impact on timely diagnosis of IEI:Fig. 2, Fig. 3
Fig. 2.
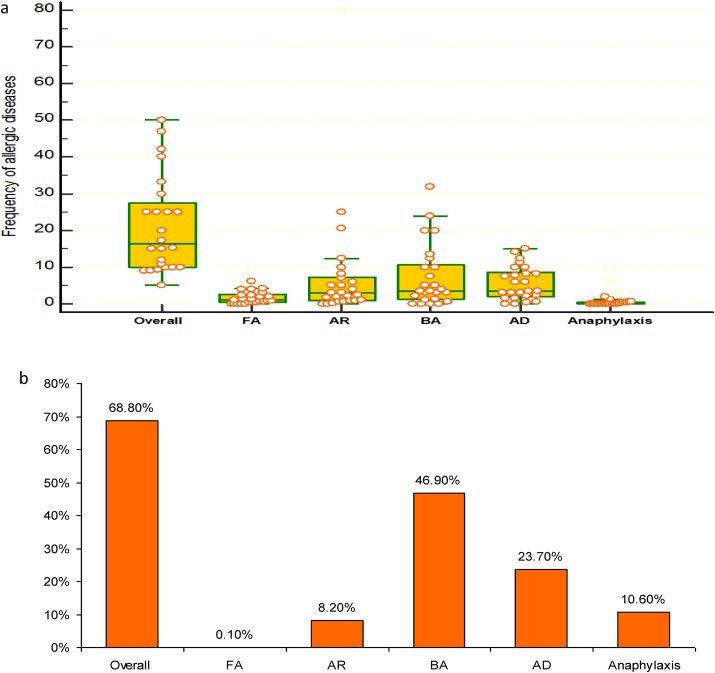
The frequencies of allergies among all IEI patients; a, results as per participating centers; b, results as per the USIDNET dataset. FA: food allergy; AR: allergic rhinitis; BA: bronchial asthma; AD: atopic dermatitis
Fig. 3.
The median (interquartile range) percentages of initial presentation with allergic diseases and diagnosis delay because of this presentation. IEI: inborn errors of immunity
Data from centers showed that a median frequency (IQR) of 16.3% (10–28.8%) of all patients suffered allergic diseases. The frequency of bronchial asthma (BA) among IEI patients was 3.6% (1.3–11.3%), that of atopic dermatitis (AD) was 3.6% (1.9–9.1%), that of allergic rhinitis (AR) was 3.0% (1.0–7.8%), and that of food allergy (FA) was 1.3% (0.5–3.3%). Fig. 2a. Their frequencies among allergic IEI patients were 35%, 30%, 20%, and 5% respectively. Eosinophilic esophagitis was reported only by 1 center in 12 patients, constituting 0.1% of all IEI patients; all had PAD. Other allergies, reported by few centers, were drug allergy, reported by 5 centers with a frequency of 3% (1.8–7.3), urticaria, reported by 3 at a frequency of 3% and allergic conjunctivitis (AC), reported also by 3 at a median frequency of 1%. According to the USIDNET data, 68.8% of IEI patients had associated allergic disorders, primarily BA in 46.9% followed by AD in 23.7%. AR was observed in 8.2%. Fig. 2b. AC and FA were infrequent (0.4% and 0.1% respectively).
Forty-three percent of the centers reported incidents of anaphylaxis in a total of 25/8450 IEI patients (0.3%), ranging from 0 to 4 patients per center, with a frequency of 0% (0–0.6%). Fig. 2a. The causes were drugs including antibiotics in 10/25 patients (40%), IVIG in 7/25 (28%), FA in 6/25 (24%), and fresh frozen plasma and bee sting 1 patient (4%) each. This represented 0.11%, 0.08%, 0.07%, 0.01%, and 0.01% of all IEI patients respectively.
In the USIDNET dataset, there were 249/2332 (10.6%) IEI patients who experienced 348 incidents of anaphylactic/anaphylactoid reactions in total. Fig. 2b. The most common trigger was drugs, seen in 6.9%. Food was the cause in 2.4%, environmental exposures in 1.0%, contrast media in 0.3%, and IVIG in 0.3%, whereas the cause was undetermined in the rest. It is to be noted that 16 patients (0.7%) developed anaphylaxis from multiple causes. In the USIDNET cohort, there were 1705 (73.1%) patients receiving IVIG, of these, 0.8% developed IVIG-induced anaphylaxis and 2.8% urticaria/generalized rash.
The median (IQR) percentage of IEI patients who presented initially with allergic disorders was 8% (5–25%). The delay in the establishment of a definitive diagnosis of IEI caused by the initial presentation with an allergic complaint (diagnosis delay) was reported by 14 centers, and showed a frequency of 7.5% (0.9–20.6%), ranging from 0 to 50% (Fig. 3). The USIDNET data showed that 732/2332 (31.4%) of the sample had 1 or more allergic disorders before or at diagnosis, but there were no data on the impact of this on the timely diagnosis of IEI.
Type of allergic disease in relation to type of IEI
PAD ranked as the most common IEI in this study with a frequency of 40.3% (19.2–62.5%) and ranged between 5 and 93%, followed by CID (IUIS groups I & II) which constituted 20.0% (10–32%) and ranged between 0 and 52%. The frequency of different IEI groups reported by the participating centers and those of the USIDNET were displayed in Fig. 4.
Fig. 4.
Percentages of the different groups of inborn errors of immunity
The study showed that allergic diseases were most commonly seen in PAD and CID with a frequency of 25% (15–70) and 20%,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 respectively, and this was especially obvious in SIgAD (53.6%) and CID with syndromic features (HIES: 43%; WAS: 38%). Meanwhile, allergy was less frequent in patients with immune dysregulation and congenital phagocytic defects with a frequency of 14% (0–33.8%) and 9% (0–20%) respectively (Fig. 5). The former group were mostly patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked disease (IPEX) and the latter were those with chronic granulomatous disease (CGD).
Fig. 5.
Frequency of allergy among different inborn errors of immunity
The frequency of PAD (60.4%) in the USIDNET dataset was 3 times higher than combined immunodeficiencies (19.1%). CVID was the most common IEI associated with BA affecting 59.4% of them, whereas ataxia telangiectasia and leukocyte adhesion deficiency had the least frequency of asthma (0.1%). AD was most commonly reported among WAS, HIES and CVID affecting 85%, 71% and 17.2% of these patients respectively. FA was reported in 3 patients: 2 with CVID and 1 with HIES.
Comparisons among IEI groups as regards the frequency of different allergic disorders
There were significant differences in the frequencies of allergic diseases among different IEI. BA was the main allergic disease reported in PAD (36% (10.7–50), whilst in CID, atopic dermatitis prevailed; (30% (10–60)). Post hoc analysis showed highly significant increase in the frequency of BA and AR among PAD patients compared to other types of IEI. AD was significantly more prevalent among CID patients as compared to PAD patients and those with congenital defects in phagocytic function and/or number. FA was significantly more frequent in patients with CID, PAD and immune dysregulation when compared to patients with autoinflammatory disease (Table 1). Drug allergy was recorded in 14.8% of IEI patients and was especially pronounced among PAD patients seen in 11%. Multi-drug allergy manifesting as severe cutaneous reaction was described in an ADA 2 deficiency patient (from the Tunisian cohort).
Table 1.
The frequencies of common allergic diseases among the studied IEI groups.
| Predominately antibody deficiency IUIS # III Median% (IQR). 40.3 (19.2–62.5) |
Combined immunodeficiencies IUIS #I&II Median% (IQR). 20 (10–32) |
Congenital phagocytic defects IUIS #V Median% (IQR). 10 (5–15.5) |
Immune dysregulation IUIS # IV Median% (IQR). 6 (1–10) |
Auto inflammatory IUIS # VII Median% (IQR). 2 (0–5) |
Test value• | P-value | |
|---|---|---|---|---|---|---|---|
| A. Food allergy Median (IQR) |
0.0 (0.0–5.0) | 0.8 (0.0–8.0) | 0.0 (0.0–0.0) | 0.0 (0.0–10.0) | 0.0 (0.0–0.0) | 10.598 | 0.031 |
| B. Allergic rhinitis Median (IQR) |
17.95 (5.0–40.0) | 5.0 (0.0–20,0) | 0.0 (0.0–11.3) | 0.0 (0.0–15.0) | 0.0 (0.0–0.0) | 27.854 | <0.001 |
| C. Bronchial asthma Median (IQR) |
36.0 (10.7–50.0) | 13.5 (0.0–20.0) | 0.0 (0.0–10.0) | 0.0 (0.0–17.5) | 0.0 (0.0–0.0) | 34.229 | <0.001 |
| D. Atopic dermatitis Median (IQR) |
8.0 (1.0–20.0) | 30.0 (10.0–60.0) | 0.0 (.00–11.1) | 9.5 (0.0–50.0) | – | 16.517 | 0.001 |
|
Post hoc analysis∗ |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 |
| A. Food allergy | 0.683 | 0.077 | 0.577 | 0.027 | 0.042 | 0.869 | 0.014 | 0.055 | 0.597 | 0.019 |
| B. Allergic rhinitis | 0.014 | 0.001 | 0.003 | <0.001 | 0.230 | 0.555 | 0.002 | 0.637 | 0.061 | 0.014 |
| C. Bronchial asthma | 0.015 | <0.001 | <0.001 | <0.001 | 0.019 | 0.126 | <0.001 | 0.368 | 0.212 | 0.033 |
| D. Atopic dermatitis | 0.007 | 0.032 | 0.799 | – | <0.001 | 0.108 | – | 0.038 | – | – |
∗ Kruskall-Wallis test followed by post hoc analysis. P values are significant if < 0.05.
P1: Predominantly antibody deficiency vs combined immunodeficienicies.
P2: Predominantly antibody deficiency vs congenital defects of phagocytosis.
P3: Predominantly antibody deficiency vs immune dysregulation.
P4: Predominantly antibody deficiency vs autoinflammatory disorders.
P5: Combined immunodeficienicies vs congenital defects of phagocytosis.
P6: Combined immunodeficienicies vs immune dysregulation disorders.
P7: Combined immunodeficienicies vs autoinflammatory disorders.
P8: Congenital defects of phagocytosis vs immune dysregulation disorders.
P9: Congenital defects of phagocytosis vs auto inflammatory disorders.
P10: Iimmune dysregulation vs auto inflammatory disorders
Allergy tests used
For the diagnosis of atopy in IEI patients, 76% of the centers used serum specific IgE, 62% used percutaneous skin testing, and 44% reported using oral food challenge for the diagnosis of FA. It is worth mentioning that 7% reported not using any allergy diagnostic tests, whereas 66% reported using more than one allergy test.
Longstanding allergic disease and allergy treatment
Almost half of the participants (42.9%) stated that HIES was the most common IEI associated with longstanding allergic disease among their patients followed by SIgAD (28.6%) and WAS in 25% of the responses. Fig. 6.
Fig. 6.
Inborn errors of immunity patients with chronic allergic diseases and on long-term treatment for allergy
The frequency of IEI patients whose allergic diseases necessitated long term treatment was 22.5%.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Corticosteroids were used in 12.5% (5–31.7%). Anti-IgE monoclonal antibody (omalizumab) was rarely used in IEI patients with a frequency of 0% (0–0.4%) and ranged between 0 and 20%. USIDNET dataset showed that systemic corticosteroids were used in 84 patients (5.2%) and omalizumab was used in 6 patients (0.4%).
Pediatric-only versus adult-and-pediatric centers
In centers serving children only (n = 14), allergic diseases occurred at a frequency of 13.5% (10–25%), whereas in centers serving adults only (n = 3) the frequency was 15.0% (9.0–33.3%). We compared pediatric-only centers to those managing both pediatric and adult patients (n = 12) and did not find significant differences regarding the frequency of different IEI groups or the frequencies of different allergic disorders except that of FA among patients with immune dysregulation disorders which was significantly higher among centers serving both pediatric and adult IEI patients (p = 0.03). The frequency of measuring serum specific IgE for diagnosis of atopy was significantly higher among centers caring for both adult and pediatric IEI patients (p = 0.03). Both types of centers (pediatric-only versus those managing both pediatric and adult patients) were comparable with respect to the use of long-term medications for allergic disorders as well as the frequency of anaphylaxis.
Of the IEI patients registered in USIDNET, 1254 patients (53.8%) were ≤18 years and 330 patients (14.2%) were >18 years while the ages of 748 patients (32.1%) were unavailable. The frequency of allergic diseases among pediatric IEI patients was significantly higher than their adult counterparts, especially AD and AR. The frequency of BA was comparable in both groups; (Table 2).
Table 2.
Variation of allergic diseases among pediatric and adult IEI patients in the USIDNET dataset
| ≤18 years N = 1254 |
>18 years N = 330 |
Chi Square (χ2) |
P-value |
|
|---|---|---|---|---|
| N (%) | N (%) | |||
| Allergic diseases | 892 (71.1) | 187 (56.7) | 25.173 | <0.001a |
| Asthma | 547 (43.6) | 153 (46.4) | 1.572 | 0.472 |
| Atopic dermatitis | 367 (29.3) | 35 (10.6) | 48.035 | <0.001a |
| Allergic rhinitis | 119 (9.5) | 11 (3.3) | 13.143 | <0.001a |
Significant
The impact of the level of development of the participating country
Based on the economic status, the participating countries were categorized as to having developing or developed economies according to the annex prepared by the Economic Analysis and Policy Division of the Department of Economic and Social Affairs of the United Nations.11 The diagnosis lag caused by initial presentation with allergic disease was similar in the 2 groups of countries. Moreover, no significant differences were noted in the percentages of centers using allergy diagnostic tests (p > 0.05). However, the use of anti-IgE monoclonal antibodies in management of severe allergic diseases associated with IEI was significantly noted in developed countries (p = 0.01). It was noticed that CID was more frequent among IEIs in developing countries compared to developed countries (28.5% (15–35%) vs 10% (5.5–20%); p = 0.032).
Interesting cases reported by participants
An interesting case reported from Sudan reflected the negative impact of initial presentation with eczema with delayed diagnosis of CID and poor outcome; clues to diagnosis were unresponsiveness to eczema treatment, sepsis, as well as lymphopenia. Participants reported a Mexican case of ataxia telangiectasia and bronchiectasis who was initially misdiagnosed as BA. An IPEX patient from Peru presented with severe early-onset diarrhea. At first, cow's milk allergy was suspected with high positive serum specific IgE; however, a switch to amino acid-based formula did not help, so further testing was done and unveiled the underlying IEI. Skin rash, high serum total IgE and eosinophilia were misinterpreted as AD and resulted in delayed diagnosis in an infant with Omenn syndrome in Brazil.
Discussion
Allergic diseases have long been observed among IEI patients. However, studies on the prevalence of allergic diseases in patients with IEI are limited.2 In this first world-wide project examining this association, we were able to provide a broad view of the overall prevalence of allergic diseases in IEI and to highlight the possible diagnostic lag of IEI caused by such allergic phenotype. The study calculated the frequency of allergic diseases among pediatric and adult IEI patients to be 16.3% (10–28.75%). The WAO estimate of allergy prevalence of the whole population by country ranged between 10 and 40%.12
BA, AD, and AR were the most commonly observed allergies at median frequencies of 3.6%, 3.6% and 3% of all IEI patients respectively. Based on the findings of this multinational study, it can be stated that major allergies were less frequent among IEI patients compared to those in the general population which is 5–10% for BA;13 whereas for AD, it is 15–20% among children and 1–3% among adults.14,15 Furthermore, the frequency of AR among IEI patients (3.0% (1.0–7.8%)) is lower than the rates reported in the general population: 10–30% in adults and up to 40% in children.12 The overlap of clinical manifestations of infections in IEI (bronchiectasis and non-allergic rhinosinusitis) with allergies (BA and allergic rhinosinusitis) could account for the under recognition of the allergic nature of the clinical condition. The association between allergy and IEI may represent a disruption of the complex balance within the immune system of effector and regulatory cells, perhaps also contributed to by differences in microbial colonization and infection patterns, most likely due to tolerance failure, T-cell receptor signaling defects, failure of production of counter regulating interferon-gamma and excess cytokines production like IL-13 which interfere with skin antimicrobial peptides as well as skin barrier disruption.2 The frequency of allergic disorders among IEI patients in the USIDNET cohort was much higher (68.8%), reflecting the higher prevalence rates in USA possibly due to geographical or environmental factors. This frequency was even higher than those reported by the 2 participating centers from USA (25% and 40%), perhaps related to variations in assessment or documentation. Once again, BA and AD were the most frequent allergic diseases; BA being the highest (46.9%). The frequencies of BA, AD and AR in the USIDNET data were far above the corresponding median frequencies as per the surveyed centers. An exception to that was FA, which was lower in the USIDNET cohort.
Allergies do have different prevalence rates among diverse countries and the overall lower frequency of allergies we observed in IEI compared to the general population may not hold true for all countries. Hence, we compared the data provided by contributing centers, country by country, versus the reported frequency of allergy of that country, taking into consideration the age group of IEI patients served at each center. In more than 50% of participating countries, the IEI patients had lower frequencies of allergic diseases, whereas in Algeria, Egypt, Iran, Portugal, and United States, they had frequencies comparable to the respective general population. Data from Lebanon, Peru, and the Czech Republic showed higher frequencies of allergic diseases in IEI patients.12,16, 17, 18, 19, 20 However, it is important to state here that some of the reports on the prevalence of allergic diseases are symptom-based and estimated from self-reports, whereas those we are documenting here for IEI patients are based on physician-diagnosis.
IEI could first present with non-infectious manifestations including autoimmunity, lymphoproliferation, malignancy and allergy. In this study, the initial presentation of IEI patients with allergic diseases has been reported by the participants at a frequency of 8% (5–25%). Consequent upon such a presentation is the possible delayed or missed diagnosis of IEI with negative impacts on the morbidity and mortality of the original disease. Delayed diagnosis of IEI because of allergic presentation was reported by 18 centers at a frequency that varied widely and with a median of 7.5%. By default, the diagnosis of IEI is delayed, and this is especially seen in CVID with a lag of as long as 6 years in an American cohort.21 In previous reports from European and Italian cohorts, the mean diagnostic delay of CVID was 7.522 and 8.9 years23 respectively. In another European study on CVID, the delay was considerably shorter in patients presenting after the age of 10 years, compared to those who became ill at younger ages (delay 3.1 vs 7.2 years, respectively).24 An Iranian cohort of PAD patients had a median delay of diagnosis of 34.5 months. In Iran, assessment of diagnosis delay of PAD showed significant improvement in recognition over the years.25 Australian researchers reported a significantly longer delay in diagnosis of CVID (median: 9 years), compared to X-linked agammaglobulinemia (XLA) (median: 1 year),26 probably related to the insidious complex presentation of CVID.27 Comberiati et al,28 reported a girl who developed refractory chronic urticaria at the age of 10 amidst recurrent pneumonias since the age of 5 years; it was only until late adolescence when she was immunologically evaluated and found to have CVID. In a case series, 7 patients (3 months–6 years) were misdiagnosed as cow milk allergy because of initial presentation with eczema and/or gastrointestinal symptoms. They were kept on replacement formula for months to years before the diagnosis of IEI was established.29
Our study is the first to record the frequency of diagnosis delay of IEI caused by initial presentation with allergic disease. An interesting case reported by participants from Sudan reflected the negative impact of initial presentation with recalcitrant eczema with delayed diagnosis of CID and poor outcome. A Mexican case of ataxia telangiectasia and bronchiectasis was initially misdiagnosed as BA. An IPEX patient from Peru with severe early-onset diarrhea was misdiagnosed as having cow milk's allergy but a switch to amino acid-based formula did not help, and further testing unveiled the underlying IEI. The misinterpretation of skin rash in the presence of high serum total IgE and eosinophilia was the cause of delayed diagnosis in an infant with Omenn syndrome in Brazil. A history of recurrent, severe or unusual infections, a family history of IEI together with other warning signs should be sought, especially in patients with severe and/or recalcitrant AD, poorly controlled BA, or gastrointestinal manifestations of FA. This would decrease the chances of missing the diagnosis of IEI.
IEI comprise 406 disorders, with PAD being the most prevalent.30,31 In line with this, the study showed that PAD was the most common IEI registered in the participating centers with a median frequency of 40.3%, and a frequency of 60.4% in the USIDNET cohort. More than a quarter of allergic diseases were reported in patients with PAD, particularly in SIgAD followed by CVID, whilst data of the USIDNET showed CVID to be the one most commonly associated with allergic disease. The reported incidence of allergies among patients with SIgAD range from 13% to 84%,8,32 considerably contributing to the clinical manifestation of the disease.33
BA ranked as the most frequent allergy, seen in more than a third of PAD patients followed in the participating centers, and in almost two-thirds of CVID patients registered in the USIDNET. In a study from Turkey, BA was more frequent in SIgAD compared to other PADs.6 CVID is commonly associated with BA as presented here; and, in a previous study, 83% of pediatric patients with CVID were diagnosed with asthma.34 A recent study reported that 37.5% of CVID patients had BA for which most were prescribed a controller medication; however, only 10% had proven IgE-mediated allergy.9,35 In contrast, a study of pediatric IEI demonstrated that BA was less frequent among CVID patients than those with SIgAD and IgG subclass deficiency.6 IgG3 subclass deficiency was the most common PAD associated with atopic asthma and a higher risk of asthma exacerbation in adults.36 It seems that patients with PAD have a bias towards Th2-mediated immune response as has been demonstrated in CVID patients where increased production of IL-4 and IL-10 were documented.37
Data from the participating centers revealed that 20% of all CID patients experienced allergic diseases. AD was the most frequent particularly among those with syndromic features (mainly HIES and WAS). In a recent study, severe combined immunodeficiency (SCID) came as the second IEI to present allergic manifestations next to CID with syndromic features.38 The USIDNET data accorded with these findings. All patients with HIES mainly DOCK-8 deficiency and WAS had AD in the study by Özcan and co-workers.6 A single-center study showed that atopic disease was present in 10/18 (55.6%) of patients with early onset ADA-SCID.39
AD can be among the early symptoms in patients with signal transducer and activator of transcription 3 loss of function mutations (STAT3-LOF), and although a timely differentiation of HIES from AD is difficult, it is extremely important for the early institution of the proper treatment.40 Despite marked IgE elevation, these patients were relatively protected from severe allergic reactions because of defects in mast cell degranulation and vascular responses to histamine caused by the STAT3 mutation itself41,42 DOCK8 deficiency exhibit severe atopy and high IgE but with significant viral skin infections and neoplastic phenotypes that overlap with WAS due to direct interactions of DOCK8 with WASP.43 Weak T cell receptor signaling during T-cell activation cause a skew towards default differentiation into T helper type 2 cells (Th2 cells), partly explaining the atopic features.44 The eczema in these patients resembles classic AD but is widespread, primarily appearing on the face, scalp, flexures, and diaper area with progressive lichenification. It gets better with age.45 HSCT do not only correct the immunodeficient state, but also result in amelioration of atopic symptoms, decrease in IgE and eosinophils and correction of Th1/Th2 imbalance.46
Significantly elevated total IgE (>1000 IU/ml) has been seen in several IEI disorders other than STAT 3-LOF HIES, DOCK8 deficiency HIES, including autosomal recessive mutations in the serine protease inhibitor gene Kazal-type 5 (SPINK5) resulting in Comèl-Netherton syndrome, phosphoglucomutase deficiency-PGM3 HIES as well as in WAS ALPS, IPEX, the innate immunity signaling pathways defects of IRA4 and MYD88 deficiency and some SCID disorders such as Omenn syndrome, ADA-SCID and ZAP 70 -SCID. Discordance between the elevated total IgE, and allergic symptoms as well as the specific IgE has been described not only in STAT3-HIES, but also in ALPS and IRAK4 and MYD88 deficiency.47,48
The association of FA with IEI was perceived as uncommon by most participants (save those from Sudan, Algeria and Tunis), with a frequency among all IEI patients of 1.2% (0.5–2.8%). FA was reported mainly in patients with HIES, IPEX and CVID. In line with these findings, the USIDNET data revealed that 0.1% only had FA. In a previous analysis of USIDNET data, FA was less frequent among IEI patients compared to the general population (1.8% versus 2.5%).3 In another study, FA was reported in 2.8% of 318 pediatric IEI patients mostly with HIES and WAS.6
In view of the scanty reports, we were keen on presenting data on anaphylaxis and its causes in IEI. Anaphylaxis was reported by 43% of the participating centers affecting 0.3% of the total number of IEI patients, closer to the lower limit of the range in normal individuals. The most common cause was drugs including antibiotics (40.0%). Twenty-eight percent of the reactions were induced by IVIG. Severe reactions to IVIG do occur, however, the rarity (1.0%)49 of these events (0.8% of IEI patients receiving IVIG as per USIDNET dataset) must be emphasized. Anaphylaxis to IVIG may be associated with IgG-anti-IgA antibodies and often associated with prior exposure to immunoglobulin therapy.50,51 However, a similar reaction was reported in an IVIG naïve CVID patient with absent IgA and in the absence of detectable IgG anti-IgA antibodies suggesting an IgE anti-IgA antibody mechanism.51 FA was the cause in 24% of our IEI patients with anaphylaxis. Anaphylaxis due to nut allergy was reported in a CID patient with ARPC1B mutation.52 Prolonged cold contact can cause anaphylaxis in patients with PLCγ2-associated antibody deficiency and immune dysregulation (PLAID).53 Anaphylaxis was a more common finding in the USIDNET dataset; 10.6% experienced at least 1 incident. Foods, drugs, IVIG, contrast media, environmental allergen and venoms were all recorded as causes of anaphylaxis in these patients. In a previous USIDNET study, anaphylaxis was the most frequent reaction (20%) reported in IEI patients with FA.3
In this study, patients with congenital phagocytic defects, immune dysregulation and autoinflammatory disorders had lower frequency of allergic diseases (9% (0–20%), 14% (0–33.8%) and 0% (0–10%) respectively), with the least frequencies, among other IEIs, of asthma, AR and AD. Ozcan et al.,6 found significantly lower AR and AD in phagocyte defects compared to PAD. IPEX syndrome which we found constituting 44.4% of the reported immune dysregulation disorders had frequencies of FA and AD comparable to those of CID and PAD. Autoimmune enteropathy and AD were reported among the main features of disease after 1 month of age.54
Allergy testing was commonly used by the participants (82.1%) to evaluate IEI patients with allergies, where measurement of serum specific IgE was the most common test used (76%) to distinguish IgE from non-IgE mediated allergies. Limited allergy testing among CVID patients with history suggestive of allergic diseases was found in a retrospective study by Bjelac and co-workers.9 It remained unclear whether the relatively low rate of skin testing in patients with respiratory concerns resulted from lack of recognition of potential for IgE-mediated disease in CVID patients.
More than one-fifth of IEI patients with allergic diseases required long term treatment to control the allergic manifestations with corticosteroids being used in 12.5% (5.0–31.7%) of all IEI patients. Considering their immunosuppressive action, it is challenging having to use them in patients with such vulnerability to infections. Omalizumab was rarely needed by the participants except in Japan, where it was used in 20% of allergic patients but no data is available about the indications of its use. The use of omalizumab in the setting of immunodeficiency is limited to a few case reports mainly among HIES55 and CVID28 with acceptable safety profile.
Allergic diseases are known to be more common among children than adults. In this study, allergic diseases in centers serving children only (n = 14) occurred at a frequency of 13.5% (10–25%). Centers serving adults only were 3 not permitting statistical comparison. They reported FA at a frequency much less than that reported by pediatric centers. Analysis of the USIDNET data revealed that FA and AD were more common among IEI patients younger than 18 years supporting a consistency in the age trends of allergy and pointing to the possibility that the mechanisms underlying allergic diseases are the same in IEI as they are in allergy in general.
We speculated that the level of development of countries could impact the timely diagnosis and the choices of treatment of IEI presenting allergic manifestations. This speculation proved true to some extent. The median frequency of delayed diagnosis of IEI brought about by the initial presentation with allergy was lower, yet statistically insignificant, in centers in developed (3%) versus developing countries (11%), probably due to the overall under and delayed diagnosis of IEI, as reflected by the number of patients registered in developing countries on the one hand, and the lack of neonatal screening for IEI in most of the countries worldwide on the other. This calls for more efforts to raise awareness of IEI among GPs and physicians of different specialties especially pediatricians, allergists and dermatologists. The use of omalizumab for allergic IEI patients was significantly higher in developed (1%) versus developing countries (0%), p = 0.007, obviously related to the cost of treatment. In spite that developed countries experience an allergy epidemic and outrun developing countries in this regard, their higher frequency of allergic disease was not statistically significant among IEI patients (20% vs 15% respectively).
This study did not inquire about the morbidities imposed by the allergic diseases and their long term treatment on IEI patients, or which groups of IEI initially presented with allergies, which we considered as limitations of the study. We were not able to capture the natural history of allergic diseases. The lack of validation is another limitation to our work. This study was aimed to include more countries and include data from other registries but failed to get responses from some of the centers contacted. However, this project provided a baseline network among centers to implement prospective studies in the field.
In summary, this study, provided a multinational view of the association of allergic diseases with IEI. The compiling of data of such a big number of IEI patients is the result of cooperation and contribution of centers from all over the world. Allergic diseases affect 16.3% of patients with IEI especially those with PAD and CID, and their existence at initial presentation, impacts the timely diagnosis of IEI in a median of 7.5% of cases. BA was the main allergic disease reported in patients with PAD, whilst in CID, atopic dermatitis prevailed. The study also highlighted the gaps in management of allergic diseases in IEI between developed and developing countries specifically the use of biologic therapies. Further studies are needed to address differences in the prevalence of allergic diseases in IEI among different countries, relevant to geographical locations, and among different age groups.
Conclusions
This worldwide study, incorporating data of an outstanding number of IEI patients, brings to focus the relation of allergic diseases with IEI. Major allergies do occur in IEI patients, yet were less frequent compared to the general population. The initial presentation with allergy could adversely affect the timely diagnosis of IEI with a possibility of jeopardizing the prognosis. Furthermore, the frequency of allergies are relatively high in some types of IEI in particular, imposing not only difficulties in diagnosis but also necessitating the use of long term therapies, some of which are immunosuppressant and might constitute a challenge to the prevention and treatment of infections in these patients. The use of newer allergy diagnostic testing and taking advantage of the latest treatment modalities would help decrease morbidities from allergic diseases in these immunodeficient patients. There is a strong need for policies to raise awareness and educate primary care and other referring specialties on the association of allergic diseases with IEI. This WAO IEI Committee project provided a network among IEI centers to implement prospective studies in the field.
Abbreviations
AC, Allergic Conjunctivitis; AD, Atopic Dermatitis; ADA, Adenosine Deaminase; AR, Allergic Rhinitis; BA, Bronchial asthma; CGD, Chronic Granulomatous Disease; CID, Combined Immunodeficiency; CVID, Common Variable Immunodeficiency; FA, Food Allergy; HIES, Hyperimmunoglobulin E Syndrome; IEI, Inborn Errors of Immunity; IPEX, Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked disease; IQR, Interquartile Range; IVIG, Intravenous Immunoglobulins; OS, Omenn Syndrome; PAD, Predominantly Antibody Deficiency; SCID, Severe Combined Immunodeficiency; SIgAD, Selective IgA Deficiency; WAS, Wiskott Aldrich Syndrome; XLA, X-linked Agammaglobulinemia;
Funding
The study received no funding.
Availability of data and materials
The dataset is available from Zeinab A El-Sayed.
Author contributions
ZAE-S, DHE, and KES conceived and designed the study. ZAE-S and DHE data analysis and drafted the manuscript. NR contributed data and shared in drafting. JAOM, NR, JCA,WA-H, MAA-N, AC-N, TC, BE, NHHE, SE, EF, NARF, RF, NG, EG, MH, KI, CI, NK, AK, ML, TM, MO, FNQ, SQ, IQ, RP, CP, NRa, NRe,JR; SSing, SSini, IT, LKT, BVD, AV, KS contributed data and revised the manuscript. All authors approved the manuscript.
Ethical considerations
The WAO IEI Committee and the Ethical Committee of Children's Hospital Ain Shams University determined that data reported in aggregate did not require consent.
Consent for publication
All authors revised the manuscript and approve its publication.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The authors acknowledge Dr Eman D El-Desouky, Epidemiology and Biostatistics, NCI, Cairo University, Cairo, Egypt, for statistical consultation.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Bousfiha A., Jeddane L., Picard C., et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40:66–81. doi: 10.1007/s10875-020-00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokol K., Milner J.D. The overlap between allergy and immunodeficiency. Curr Opin Pediatr. 2018 Dec;30(6):848–854. doi: 10.1097/MOP.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 3.Tuano K.S., Orange J.S., Sullivan K., Cunningham-Rundles C., Bonilla F.A., Davis C.M. Food allergy in patients with primary immunodeficiency diseases: prevalence within the US Immunodeficiency Network (USIDNET) J Allergy Clin Immunol. 2015 Jan;135(1):273–275. doi: 10.1016/j.jaci.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhouib N.G., Ben Khaled M., Ouederni M., et al. Cutaneous manifestations of primary immunodeficiency diseases in Tunisian children. Mediterr J Hematol Infect Dis. 2018 Nov 1;10(1) doi: 10.4084/MJHID.2018.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Herz W., Nanda A. Skin manifestations in primary immunodeficient children. Pediatr Dermatol. 2011;28:494–501. doi: 10.1111/j.1525-1470.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 6.Özcan C., Metin A., Erkoçoğlu M., Kocabaş C.N. Allergic diseases in children with primary immunodeficiencies. Turk J Pediatr. 2014 Jan-Feb;56(1):41–47. [PubMed] [Google Scholar]
- 7.Janzi M., Kull I., Sjoberg R., et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clin Immunol. 2009;133:78–85. doi: 10.1016/j.clim.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Aghamohammadi A., Cheraghi T., Gharagozlou M., et al. IgA deficiency: correlation between clinical and immuno-logical phenotypes. J Clin Immunol. 2009;29:130–136. doi: 10.1007/s10875-008-9229-9. [DOI] [PubMed] [Google Scholar]
- 9.Bjelac J.A., Blanch M.B., Fernandez J. Allergic disease in patients with common variable immunodeficiency at a tertiary care referral center. Ann Allergy Asthma Immunol. 2018 Jan;120(1):90–92. doi: 10.1016/j.anai.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 10.Boyarchuk O., Lewandowicz-Uszynska A., Kinash M., Haliyash N., Sahal I., Kovalchuk T. Physicians’ awareness concerning primary immunodeficiencies in Ternopil region, Ukraine. Pediatr Pol. 2018;93:221–228. [Google Scholar]
- 11.United Nations, editor. World Economic Situation and Prospects. 2020. Economic analysis and policy division of the department of economic and social Affairs annex.https://www.un.org/development/desa/dpad/publication/world-economic-situation-and-prospects-2020/ P165-6 published January 16, 2020. [Google Scholar]
- 12.Pawankar R., Canonica G.W., Holgate S.T., Lockey R.F., Blaiss M.S. World Allergy Organization; Milwaukee, WI: 2013. WAO White Book on Allergy: Update 2013.https://www.worldallergy.org/wao-white-book-on-allergy [Google Scholar]
- 13.Tarlo S.M., Balmes J., Balkissoon R., et al. Diagnosis and management of work-related asthma: American college of chest physicians consensus statement. Chest. 2008 Sep;134(3 Suppl):1S–41S. doi: 10.1378/chest.08-0201. [DOI] [PubMed] [Google Scholar]
- 14.Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- 15.Eichenfield L.F., Tom W.L., Chamlin S.L., et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher M.I., Montefort S., Björkstén B., et al. ISAAC Phase Three Study Group Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006 Aug 26;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 17.To T., Stanojevic S., Moores G., et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Publ Health. 2012 Mar 19;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgy V., Fahim H.I., El-Gaafary M., Walters S. Prevalence and socioeconomic associations of asthma and allergic rhinitis in northern [corrected] Africa. Eur Respir J. 2006 Oct;28(4):756–762. doi: 10.1183/09031936.06.00089005. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho D., Aguiar P., Ferrinho P., Mendes-Bastos P., Palma-Carlos A. Eczema and urticaria in the adult population in Portugal: a prevalence study. Actas Dermosifiliogr (Engl Ed). 2019 Nov;110(9):744–751. doi: 10.1016/j.ad.2019.03.005. English, Spanish. [DOI] [PubMed] [Google Scholar]
- 20.Khaldi F., Fakhfakh R., Mattoussi N., Ben Ali B., Zouari S., Khémiri M. Prevalence and severity of asthma, allergic rhinoconjunctivitis and atopic eczema in "Grand Tunis" schoolchildren: ISAAC. Tunis Med. 2005 May;83(5):269–273. [PubMed] [Google Scholar]
- 21.Cunningham-Rundles C., Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 22.Chapel H., Lucas M., Lee M., et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 23.Quinti I., Soresina A., Spadaro G., et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 24.Gathmann B., Mahlaoui N., Ceredih, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 25.Aghamohammadi A., Bahrami A., Mamishi S., et al. Impact of delayed diagnosis in children with primary antibody deficiencies. J Microbiol Immunol Infect. 2011;44:229–234. doi: 10.1016/j.jmii.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Slade C.A., Bosco J.J., Giang T.B., et al. Delayed diagnosis and complications of predominantly antibody deficiencies in a cohort of Australian adults. Front Immunol. 2018;9(694) doi: 10.3389/fimmu.2018.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urschel S., Kayikcl L., Wintergerst U., Notheis G., Jansson A., Belohradsky B. Common variable immunodeficiency disorders in children: delayed diagnosis despite typical clinical presentation. J Pediatr. 2009;154:888–894. doi: 10.1016/j.jpeds.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Comberiati P., Costagliola G., Carli N., et al. Refractory chronic spontaneous urticaria treated with omalizumab in an adolescent with common variable immunodeficiency. Front Immunol. 2019;10:1700. doi: 10.3389/fimmu.2019.01700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo K.M., Dantas E., DeMoraes-Pinto M.I., et al. Primary immunodeficiency may be misdiagnosed as cow's milk allergy: seven cases referred to a Tertiary Pediatric Hospital. ISRN Pediatr. 2013;2013 doi: 10.1155/2013/470286. Published online 2013 Sep. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durandy A., Kracker S., Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13(7):519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 31.Abolhassani H., Parvaneh N., Rezaei N., Hammarstrom L., Aghamohammadi A. Genetic defects in B-cell development and their clinical consequences. J Investig Allergol Clin Immunol. 2014;24:6–22. [PubMed] [Google Scholar]
- 32.Edwards E., Razvi S., Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111:93–97. doi: 10.1016/j.clim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Szczawinska-Poplonyk A. An overlapping syndrome of allergy and immune deficiency in children. J Allergy. 2012 doi: 10.1155/2012/658279. Article ID 658279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogershok P.R., Hogan M.B., Welch J.E., Corder W.T., Wilson N.W. Spectrum of illness in pediatric common variable immunodeficiency. Ann Allergy Asthma Immunol. 2006;97(5):653–656. doi: 10.1016/S1081-1206(10)61096-4. [DOI] [PubMed] [Google Scholar]
- 35.Agondi R.C., Barros M.T., Rizzo L.V., Kalil J., Giavina-Bianchi P. Allergic asthma in patients with common variable immunodeficiency. Allergy. 2010;65:510–515. doi: 10.1111/j.1398-9995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee S., Ban G., Kim S., et al. Association between primary immunodeficiency and asthma exacerbation in adult asthmatics. Korean J Intern Med. 2020;35:449–456. doi: 10.3904/kjim.2018.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezaei N., Aghamohammadi A., Kardar G.A., Nourizadeh M., Pourpak Z. T- helper 1 and 2 cytokine assay in patients with common variable immunodeficiency. J Investigational Allergology Clin ImmunoL. 2008;18(6):449–453. [PubMed] [Google Scholar]
- 38.Ghaini M., Jamee M., Mahdaviani S.A., et al. The prevalence of atopic manifestations in 313 Iranian patients with inborn errors of immunity. Int Arch Allergy Immunol. 2021;182(11):1122–1126. doi: 10.1159/000516596. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence M.G., Barber J.S., Sokolic R.A., et al. Elevated IgE and atopy in patients treated for early-onset ADA-SCID. J Allergy Clin Immunol. 2013;132:1444–1446. doi: 10.1016/j.jaci.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagl B., Heinz V., Schlesinger A., et al. Key findings to expedite the diagnosis of hyper-IgE syndromes in infants and young children. Pediatr Allergy Immunol. 2016;27:177–184. doi: 10.1111/pai.12512. [DOI] [PubMed] [Google Scholar]
- 41.Siegel A.M., Stone K.D., Cruse G., et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–1396. doi: 10.1016/j.jaci.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hox V., O'Connell M.P., Lyons J.J., et al. Diminution of signal transducer and activator of transcription 3 signaling inhibits vascular permeability and anaphylaxis. J Allergy Clin Immunol. 2016;138:187–199. doi: 10.1016/j.jaci.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssen E., Tohme M., Hedayat M., et al. A DOCK8-WIP-WASp complex links T-cell receptor to the actin cytoskeleton. J Clin Invest. 2016;126:3837–3851. doi: 10.1172/JCI85774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milner J.D., Fazilleau N., McHeyzer-Williams M., Paul W. Cutting Edge: lack of high affinity competition for peptide in polyclonal CD4 + responses unmasks IL-4 production. J Immunol. 2010;184:6569–6573. doi: 10.4049/jimmunol.1000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochs H.D., Thrasher A.J. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Al-Herz W., Chu J.I., van der Spek J., et al. Hematopoietic stem cell transplantation outcomes for 11 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2016 Sep;138(3):852–859. doi: 10.1016/j.jaci.2016.02.022. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponsford M.J., Klocperk A., Pulvirenti F., et al. Hyper-IgE in the allergy clinic––when is it primary immunodeficiency? Allergy. 2018;73:2122–2136. doi: 10.1111/all.13578. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence M.G. Patterns of allergic sensitization in high IgE syndromes. Curr Allergy Asthma Rep. 2015;15:8. doi: 10.1007/s11882-015-0574-5. [DOI] [PubMed] [Google Scholar]
- 49.Bonilla F.A., Khan D.A., Ballas Z.K., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015 Nov;136(5):1186–1205. doi: 10.1016/j.jaci.2015.04.049. e1-78. [DOI] [PubMed] [Google Scholar]
- 50.Burks A.W., Sampson H.A., Buckley R.H. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N Engl J Med. 1986;314:560–564. doi: 10.1056/NEJM198602273140907. 1986. [DOI] [PubMed] [Google Scholar]
- 51.Gharib A., Caperton C., Gupta S. Anaphylaxis to IGIV in immunoglobulin-naïve common variable immunodeficiency patient in the absence of IgG anti-IgA antibodies: successful administration of low IgA-containing immunoglobulin. Allergy Asthma Clin Immunol. 2016 May 17;12:23. doi: 10.1186/s13223-016-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuijpers T.W., Tool A.T.J., van der Bijl I., et al. Combined immunodeficiency with severe inflammation and allergy caused by ARPC1B deficiency. J Allergy Clin Immunol. 2017 Jul;140(1):273–277. doi: 10.1016/j.jaci.2016.09.061. e10. [DOI] [PubMed] [Google Scholar]
- 53.Milner J.D. PLAID: a Syndrome of complex patterns of disease and unique phenotypes. J Clin Immunol. 2015;35:527–530. doi: 10.1007/s10875-015-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barzaghi F., Hernandez L.C., Neven B., et al. Long-term follow-up of IPEX syndrome patients after different therapeutic strategies: an international multicenter retrospective study. J Allergy Clin Immunol. 2018;141:1036–1049. doi: 10.1016/j.jaci.2017.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bard S., Paravisini A., Aviles-Izquierdo J.A., Fernandez-Cruz E., Sanchez Ramon S. Eczematous dermatitis in the setting of hyper-IgE syndrome successfully treated with omalizumab. Arch Dermatol. 2008;144:1662–1663. doi: 10.1001/archdermatol.2008.510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available from Zeinab A El-Sayed.