Abstract
Long-term non-healing diabetic wounds are always a serious challenge and a global healthcare burden that needs to be resolved urgently in the clinic. Prolonged inflammation and impaired angiogenesis are the main direct causes of diabetic wounds. With the development of polymer biomaterials, various wound dressings have been created, but a few of them have been applied to the clinical management of diabetic wounds. Here, we developed a mussel-inspired bioactive scaffold consisting mainly of collagen and hyaluronic acid, which are natural biopolymer materials contained in human tissues. First, we fabricated different polydopamine modified lyophilized collagen hyaluronic acid scaffolds under different concentrations of dopamine alkaline solutions, 0.5, 1, 2 mg/mL, so named CHS-PDA-0.5, CHS-PDA-1, CHS-PDA-2. After testing their physical and chemical properties, antioxidant effect, inflammation regulation, as well as drug loading and release capabilities, we obtained a bioactive endothelial growth factor (EGF)-loaded wound dressing, CHS-PDA-2@EGF, which can resist reactive oxygen species (ROS) and promote the regeneration of chronic wounds in diabetic rats by reducing inflammation. In addition, the scaffold showed excellent swelling ability, a certain coagulation effect and reasonable degradation. Therefore, the scaffold has great potential to be used in clinical diabetic wound treatment as a low-cost and easily available wound dressing to accelerate chronic wound healing.
Keywords: Diabetic wound healing, Collagen, Hyaluronic acid, Polydopamine, Antioxidant, Anti-inflammatory
Graphical abstract
Highlights
-
•
The scaffold contains collagen and hyaluronic acid, both natural biopolymers found in human tissues.
-
•
By modifying polydopamine, the scaffold is capable of suppressing ROS, inflammation and macrophage M1 polarization.
-
•
The scaffold exhibited excellent swelling ability, a certain coagulation effect, and reasonable degradation.
-
•
The scaffoldis a low-cost, readily available wound dressing with great potential for use in clinical treatment.
1. Introduction
Diabetes complications include diabetic wounds, which are very common among diabetics [1]. In clinic, a minor trauma of diabetic patient can develop into nonhealing ulcers, which finally leads to suffering from amputation and permanent disability [2]. Skin wound healing is a complicated physiological process that involves four dynamic and connected phases, covering hemostasis, inflammatory response, tissue regeneration and remodeling [3]. Previous studies have shown that excessive production of Advanced glycation end products (AGE) due to elevated concentration of blood glucose is the main factor causing chronic trauma of diabetes, which can lead to increased oxidative stress [4]. In the inflammatory phase, reactive oxygen species (ROS) produced by macrophages increase continuously in the wound microenvironment, resulting in cellular dysfunctions as well as overproduction of ROS and inflammatory cytokines impairs wound healing [5]. Increasing studies discovered that the effective ROS scavenging and regulation of inflammation in the wound microenvironment are critical to treating unhealing diabetic wound [6,7].
Polydopamine coating is an emerging technology to alter the surface of materials and change the materials’ properties, which originally inspired by mussels [8]. Since it was first reported in 2007, polydopamine nanoparticles (pDA-NPs) have been widely implemented in biomedical engineering, including cell affinity, self-adhesiveness, and antioxidant and so on [[8], [9], [10]]. It has been proved by Liu et al. that pDA has good biocompatibility with low cytotoxicity and stability in vivo [11]. And the free radical scavenging ability of pDA-NPs has been convinced by Zhao et al. [12]. They deployed pDA-NPs to treat acute inflammation-induced lung injury successfully. Under alkaline ambient solutions, dopamine (DA) oxidatively self-polymerize into pDA-NPs and adhere to the surface of various material or substances, such as metals, hydrogels, and polymers through covalent bonding and hydrogen bonding [[13], [14], [15]]. Due to the amine and catechol functional groups, pDA actually has the surface immobilization of bioactive molecules. For example, Zhang et al. reported that a pDA-modified collagen sponge scaffold with sustained release of platelet-rich plasma (PRP) to promote skin repair [16]. Consequently, pDA coating has great potential to prevent initial burst in drug delivery systems.
In recent years, studies have indicated that natural polymer hydrogels have potential as wound dressings because they provide a moist environment that is favorable for wound healing [17,18]. For diabetic chronic wound, many kinds of wound dressing with free radical scavenging activity have been created to decrease oxidative damage to the wound [7,19]. Collagen and hyaluronic acid have been commonly used in research as the raw material of hydrogel for various diseases treatment [20]. Collagen, which is primarily circulated in the extracellular matrix (ECM), has excellent biocompatibility, biodegradability, and low immunogenicity. Numerous bi-layer dermal substitutes consisting of collagen have been deployed for the management of chronic wound [21,22]. The same is true for hyaluronic acid, which likewise is a component that can be found in many tissues [23]. Hyaluronic acid has been extensively utilized in wound healing to accelerate wound repair, because of its anti-inflammatory, strong moisturizing effect, good biocompatibility, and biodegradability [[24], [25], [26]].
In this research, we developed a pDA-modified and epidermal growth factor (EGF) loaded collagen hyaluronic acid composite scaffold, with a series of features for promoting cell proliferation, ROS scavenging and skin wound healing. EGF have been widely applied for tissue engineering, especially its efficacy in chronic wound healing has been widely proven [27]. Firstly, Type I atelocollagen and hyaluronic acid were homogenized in proportion, then lyophilized and cross-linked by EDC-NHS solution to produce collagen hyaluronic acid composite scaffolds, atelocollagen has lower immunogenicity [28]. We analyzed the degradation and humidity retention of collagen hyaluronic acid scaffolds modified with different concentrations of DA solutions, but also investigated their abilities of antioxidant and regulating inflammatory response ability. Ultimately, cytokine EGF was injected and immobilized in CHS-PDA, and the full-thick skin defect repair experiment in diabetic rats demonstrated that the EGF-loaded CHS-PDA stimulated diabetic wound healing. All of these results imply that the EGF-loaded CHS-PDA may be a suitable candidate for clinical tissue engineering applications to promote chronic wound repair (see Scheme 1).
Scheme 1.
Schematic illustration of the production processes of the CHS-PDA @EGF and the mechanism of CHS-PDA@EGF for promoting chronic wound healing on the full-thickness wound in the diabetic Male Sprague–Dawley Rat model.
2. Experimental section
2.1. Materials
Type I atelocollagen (Mw = 270,000–300,000) was purchased from Pashion (Beijing, China), hyaluronic acid sodium salt (Mw = 150,000–250,000) and dopamine hydrochloride were purchased from Yuanye (Shanghai, China). 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC-HCl), N-Hydroxysulfosuccinimide sodium salt (sulfo-NHS), Calcein AM and propidium iodide were purchased from Solarbio (Beijing, China). Human epidermal growth factor (EGF) was purchased from PrimeGene Bio-Tech (Shanghai, China). Streptozotocin (STZ, 99%), lipopolysaccharide (LPS), sodium citrate and citric acid monohydrate were supplied by Sigma-Aldrich. Dulbecco's modified eagle medium (DMEM), fetal bovine serum (FBS) and 4′,6-diamidino-2-phenylindole (DAPI) were supplied by Thermo Fisher Scientific. F4/80, CD206, CD31 and Ki67 antibodies were purchased from Proteintech (Wuhan, China) and Servicebio (Wuhan, China). All reagents were of analytical grade and used as received. Milli Q water was used throughout the experiment.
2.2. Preparation of composite scaffolds
The porous collagen-HA scaffold was fabricated as previously reported with some improvement [29,30]. Briefly, 0.4 M NaCl was firstly added into 1% HA solution to prevent the formation of poly-ion complex (PIC) when HA solution was blended with collagen solution. 1% HA solution was added to 1% Type I atelocollagen dispersion, forming a HA/collagen (v/v = 1:4) homogenized solution. The resulting solution was poured into mould (diameter 20 mm), frozen at −50 °C,and then lyophilized at −70 °C for 24 h. The fabricated CHSs were immersed in 95% ethanol solution of 50 mM of EDC-HCl and 5 mM sulfo-NHS for 24 h. The CHSs obtained were washed in distilled water using a sonicator, and then re-lyophilized at −70 °C for 24 h. Finally, CHSs immersed in four concentrations of dopamine with 0, 2.0, 1.0 and 0.5 mg/mL dissolved in 10 mM Tris-HCl and reacted in a horizontal rotation mode (50 rpm, 12 h) at room temperature. The composite scaffolds were designated as CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5, respectively. All scaffolds were sterilized by UV irradiation before cell and animal experiments.
2.3. Physicochemical characterizations
The cross sections and the surfaces of composite scaffolds were visually characterized by scanning electron microscope (SEM, SU-8010, Hitachi Japan) with an accelerating voltage of 3 kV. The chemical bonds of composite scaffolds after polydopamine modification were analyzed via ATR-FTIR spectra (Nicolet iS50, Thermo Fisher, America).
CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were individually weighted (Wd) and immersed in distilled water at room temperature for 20 s. After removal from the water, they were gently held with blunt tweezers for 1 min until no dripping water was observed and then weighted (Ws). The swelling rate was calculated as follows:
| Swelling rate (%) = [(Ws – Wd) / Ws] × 100% |
where Wd is the weight of the dry scaffold and Ws is the weight of the swollen scaffold.
The weight of dry scaffolds (Wd0) was precisely measured, and then placed in distilled water for 10 min to absorb enough water. The samples were gently held with blunt tweezers, drained for about 1 min, and immediately weight (Ws). Then we put them in a dish at 37 °C for three days, the weight of samples was accurately measured for each day (Wd1). The water retention of composite scaffolds was calculated as follows:
| Water retention (%) = (Wd1 - Wd0) / (Ws - Wd0) × 100% |
where Wd0 is the weight of the dry scaffold, Ws is the weight of the swollen scaffold and Wd1 is the weight of scaffold measured each day.
In vitro enzymatic degradation test of all scaffolds was performed using 2 mg/mL lysozyme dissolved in PBS (pH = 7.4) solution. Scaffolds were separately weighted (Wd0) and then immersed into lysozyme solution at 37 °C for 2, 4, 8, 16 days. At each point in time, Scaffolds were taken out and dipped into 10 mL distilled water three times and lyophilized. Finally, the weight of each scaffold was measured (Wd1). The degradation rate of composite scaffolds was calculated as follows:
| Degradation rate (%) = (Wd0 -Wd1) / Wd0 × 100% |
where Wd0 is the weight of the dry scaffold before degradation, Wd1 is the weight of scaffold after degradation at each point in time.
2.4. Cytotoxicity and hemolysis test
RAW264.7 and NIH3T3 cell line was employed to explore the cytotoxicity of the specimens by (3-(4,5-dimethyl-2-thiazoLyl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Each group of scaffolds were immersed into 1 mL DMEM complete medium for 24 h. The cells were respectively seeded on each group of scaffold's leaching solution in a 96-well plate at density of 105 cells/mL and cultured at 37 °C in an atmosphere containing 5% CO2. At the scheduled time intervals, the culture medium was replaced and supplemented with 10 μL MTT solution (5 mg/mL MTT/PBS) for further 3 h incubation. Then the medium was removed and 300 μL dimethylsulfoxide (DMSO) was added to dissolve the formazan crystals. 200 μL supernatant were pipetted and placed in another 96-well plate to test the absorbance at 490 nm and calculated by means of at least three wells and present as viability of cells compared to the control group (DMEM culture only).
According to previous reports [31], packed red blood cells (RBCs) were obtained by centrifugation of citrated whole blood (CWB) under 3000 rpm for 5 min and rinsed. Then 100 μL of RBC per 2 mL of PBS to prepare 5% hematocrit of RBC suspension. All scaffolds are ground into powder in liquid nitrogen, 10 mg the powder of sample in 0.8 mL of PBS was mixed with 0.2 mL of the RBC suspension. PBS and water (0.8 mL) were used as negative and positive controls, respectively. The blend was co-culture for an hour under 37 °C. Subsequently, the specimens were isolated by centrifugation, 3000 rpm for 5 min, and the supernatant absorbance at 541 nm was tested by spectrophotometer (Multiskan FC, Thermo Fisher, America). The hemolysis of RBCs was calculated as follows:
| Hemolysis rate (%) = (ODs - ODnc) / (ODpc -ODnc) × 100% |
where ODs is the absorbance of sample group, ODnc is the absorbance of negative control and ODpc is the absorbance of positive control.
2.5. Antioxidant efficiency of the hydrogel
The antioxidant efficiency of scaffolds was determined using DPPH-scavenging test and in vitro ROS-scavenging. First the CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were immersed in 3 mL, respectively. Then 100 μL 1,1-diphenyl-2-picrylhydrazyl (DPPH, 100 μM, 1 mL) solution were dispersed into different samples. After different reaction times (30, 60, 90, 120 min) in the dark, the absorption of the samples at 517 nm was measured using a spectrophotometer (Multiskan FC, Thermo Fisher, America). The DPPH-scavenging efficiency was calculated as follows:
| DPPH-scavenging efficiency (%) = (OD0 - ODS) / OD0 × 100% |
where OD0 is the absorption of the blank (DPPH + ethanol) and ODS is absorption of the scaffold group (DPPH + ethanol + scaffold) at 517 nm.
RAW264.7 cells (5 × 105 cells/sample) were cultured with the CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 in a 12-well plate with 1 mL of DMEM, respectively, which were sterilized with UV for 1 h. After co-culturing for 12 h, each group of cells were washed three times with serum-free culture medium. Then, 2′,7′-dichlorofluorescein diacetate (DCFH-DA, 10 Mm, 1 μL) probe molecules were dispersed into each group. After 20 min, each group of cells was washed three times with PBS. ROS levels were measured using a confocal laser scanning microscope (CLSM, TCSSP8, Leica, Germany). The cells were treated in the same way as in the quantitative analysis of flow cytometry. The cell suspension (1 × 105 viable cells) was collected in each tube and analyzed by flow cytometry (CytoFLEX S, Beckman, America). An unstained control sample was also prepared as a compensation control to determine the background levels. Each tube of cell suspension was sieved by a 40 μm cell strainer before flow cytometry testing. The percentage of positive events in the FL1 (FITC) channel was recorded. The Flow Jo Software was used to analyze the data.
2.6. Gene expression of M1, M2 phenotype macrophage
The RAW264.7 cells were seeded at a density of 2 × 105 cells/well to 6-well plate and cultured with or without scaffolds overnight, and which was treated with or not with 0.5 μg/mL LPS for 24 h. Following 24 h of stimulation, total RNA was extracted using Trizol reagent (TakaRa, Japan) according to the protocol, and the concentration was determined by a NanoDrop 2000c spectrophotometer (Thermo Fisher). The following instructions were followed for reverse transcription of 1 μg total RNA using a First Strand cDNA Synthesis SuperMix for qPCR Kit (Yeasen, Shanghai, China). The RT-PCR was subsequently performed on qPCR SYBR Green Master Mix Kit (Yeasen, Shanghai, China). The sequences of the primers were listed in Table S2.
2.7. Scratch wound healing and live/dead staining
For monitoring the effect of H2O2 on cells’ function with or without scaffold. HaCaT was applied for scratch wound assay. For the scratch wound healing assay, HaCaT cells were seeded into 12-well plates at 2.5 × 105 cells/well (3 replicates per group) and incubated for 12 h. Then the pipette tip was used to make a scratch on the cell layer at a constant speed on the bottom of the plate. After 3 washes with PBS, the medium was replaced by serum-free medium with or without 50 μM of H2O2 and scaffolds CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were immersed into medium of the corresponding group carefully. The cell migration of each group was photographed and recorded at 0 h, 12 h, 24 h and 36 h. Cell migration rate was calculated as follows:
| Migration rate (%) = (R0 – Rs) / R0 × 100% |
where R0 was the area of initial scratch area, Rs was remaining unhealing scratch area.
HUVECs were also applied for evaluating the antioxidation capacity of scaffolds by Live/Dead staining, HUVECs were seeded into 12-well plates at 1 × 105 cells/well (3 replicates per group) and incubated for 12 h, and then the medium was replaced by serum-free medium with or without 50 μM of H2O2 and scaffolds CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were immersed into medium of the corresponding group carefully. After 24 h co-culture with scaffolds, each group was stained with Live/Dead staining kit (Solarbio, Beijing, China) and observed by an inverted fluorescence microscope (AXIO Observer A1, Zeiss, German).
2.8. Animal experiments
Animal experiments were performed according to Guildlines for Animal Care and Use Committee of Zhejiang University. Sprague-Dawley (SD) rats (male, six weeks old) were obtained from Zhejiang Academy of Medical Sciences and housed under a 12-h light-dark cycle with free access to water and chow in a SPF environment. After fed high-fat-diet (25.4% fat, 42.1% carbohydrate, 24.2% protein). (Research diets, Biotech Co., Ltd, Beijing, China) for 4 weeks, SD rats were given a single injection of freshly dissolved streptozotocin (STZ), 40 mg/kg, in a 0.1 M citrate buffer (pH 4.5) into peritoneum. One week later, the rats with fasting random blood glucose levels greater than 16.7 mM were selected as the type II diabetic rats.
All diabetic rats were anesthetized by intraperitoneal injection of 4% (w/w) pentobarbital solution (0.2 mg/100 g). The hair on the back of the rat was shaved cleanly to expose the skin and sterilized with iodine. Then, a 20 mm full thickness skin excisional wound was created using a 20 mm punch biopsy.
The rats were randomly divided into four groups (parallel sample ≥4): blank group (tr), CHS, CHS-PDA-2, and CHS-PDA-2@EGF groups. After wounding, the rat was housed individually in a conventional vivarium with dietary accommodations. All wounds were photographed at 0, 3, 7, 14, 21 days post-wounding. Digital images were measured using ImageJ, and percentage of wound closure was calculated as follows:
| Wound closure rate = (A0 - At) / A0 × 100% |
where A0 is the initial wound area, and At is the wound at each time point post-wounding.
2.9. Immunofluorescence staining and immunohistochemistry
To investigate the cytological behaviors of macrophages and newly generated blood vessels, the macrophages recognizing markers F4/80, M2 macrophages phenotype marker CD206, endothelial cell marker CD31 and Ki67, Fission and proliferation related proteins in the nucleus, were detected by Immunofluorescence staining and Immunohistochemistry, respectively.
For Immunohistochemistry, the paraffin sections were deparaffinized and hydrated with a graded series of ethanol until to water. Antigen of tissue was repaired by microwave in 0.1 M Citric acid repair solution (pH = 6.0). The edge of tissue was drawn with immunity staining guard pen, the sections were blocked with 5% goat serum for 30 min and then covered by F4/80 rabbit polyclonal antibody (1:200, Proteintech, Wuhan, China), rabbit anti-CD31 polyclonal antibody (1:200, Servicebio, Wuhan, China) and mouse anti-Ki67 monoclonal antibody (1:200, Servicebio, Wuhan, China) at 4 °C overnight, respectively. After being washed three times with PBS, the slides were incubated with goat anti-rabbit HRP secondary antibodies (1:200, Servicebio, Wuhan, China), goat anti-mouse HRP secondary antibodies (1:200, Servicebio, Wuhan, China) and further developed with 3, 30-diaminobenzidine tetrahydrochloride (DAB) solution and finally counterstained with hematoxylin. Positive staining was observed and photographed by a brown color observed under an optical microscope (IX-81, Olympus, Japan).
The tissues for immunofluorescence were embedded in an optical cutting temperature compound (OCT) and sliced into 5 μm thick sections. Tissue sections were incubated at 37 °C for 1 h, and then washed with PBST (PBS with 0.1% Tween-20) three times for 5 min to remove the OCT. then blocked with 5% goat serum for 30 min and incubated overnight at 4 °C with F4/80 rabbit polyclonal antibody (1:200, Proteintech, Wuhan, China) and CD206 mouse polyclonal antibody (1:200, Proteintech, Wuhan, China). After rinsed with PBST, the slides were incubated with Cy3-conjugated goat anti mouse polyclonal antibody (1:200, Servicebio, Wuhan, China) and Alexa fluor@488-conjugated goat anti rabbit polyclonal antibody (1:200, Proteintech, Wuhan, China). After washing three times in PBS, DAPI was applied to stain cell nuclei for 10 min at room temperature. Immunofluorescence was observed and photographed by confocal laser microscope (SP8, Lecia, German).
2.10. Statistical analysis
All quantitative results are expressed as mean ± sd. Statistical significance was performed by one- and two-way ANOVA, and Student's t-tests using Graphpad Prism8 software as indicated. A p-value below 0.05 was considered significant and indicated with asterisk: ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05.
3. Results and discussion
3.1. Characterization of composite scaffolds
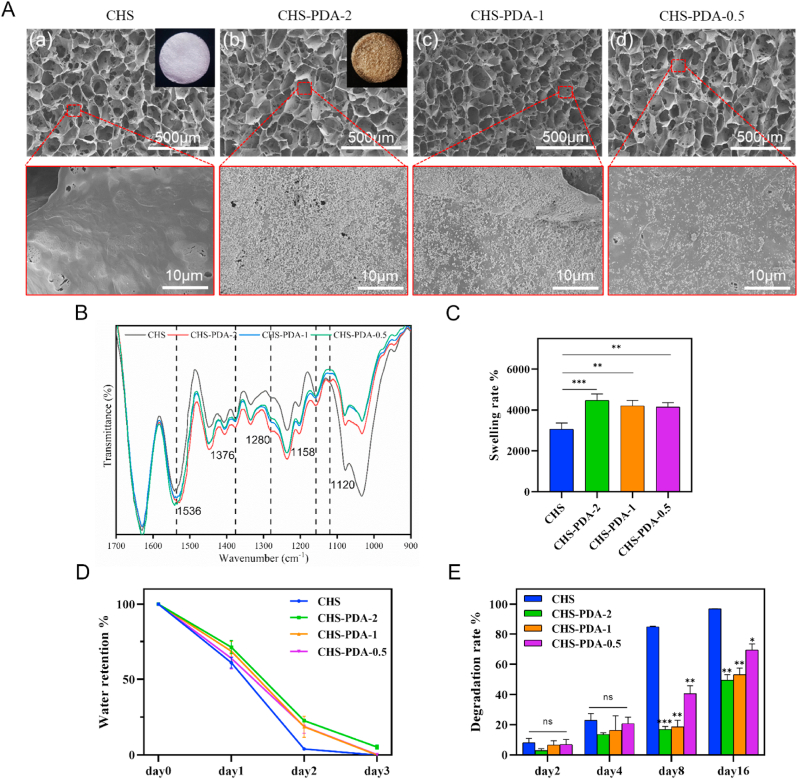
In this experiment, collagen hyaluronic acid composite scaffolds were synthesized and then immersed into four concentrations of dopamine alkaline solution with 0, 2.0, 1.0 and 0.5 mg/mL to get CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5. After the modification of polydopamine, the colors of composite scaffolds were deepened. As shown in Fig. 1A, the SEM images at low magnification proved that CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5 all retained porous network structures like CHS without change, meaning the original structure of CHS was seldom destroyed after the introduction of pDA. We know that the surface morphology and roughness of materials play an important role in changing the cell-scaffold interaction, the micro surfaces of CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were further observed by SEM with a higher magnification. We found that different amounts of pDA NPs were attached to the micro surface of CHS (Fig. 1A), after modification in different concentration of dopamine solution. The FTIR spectra of CHS-PDA composite scaffolds showed peaks assigned to the chemical bonds in Fig. 1B. The intensified peaks of chemical bonds related to PDA were observed such as polyphenols (1280 cm−1), C–O bonds (1158 and 1120 cm−1), C C bonds (1536 cm−1) and C–N–C bonds (1376 cm−1) for CHS, CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5, characterized by ATR-FTIR. These results revealed that the CHS-PDA composite scaffolds were successfully produced.
Fig. 1.
Morphology, characterization, and degradation profile of CHS, CHS-PDA scaffolds. (A) The SEM images of CHS (a), CHS-PDA-2 (b), CHS-PDA-1 (c), CHS-PDA-0.5 (d), the porous structure and different concentrations of polydopamine modified surfaces were observed. (B) FTIR spectrum of as-prepared CHS, CHS-PDA scaffolds. (C) Swelling rate and (D) water retention of CHS-PDA scaffolds. (E) The degradation rate of CHS, CHS-PDA scaffolds. Data represented mean ± SD (n = 3). ∗∗∗P < 0.001, ∗∗P < 0.01, ns, P > 0.05 compared with CHS group.
Wet healing environment is conducive to the growth of granulation, facilitates the proliferation of cells and promotes the wound healing [32]. Owing to the hydrophilic catechol groups of pDA, the swelling rate of CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5 were higher than CHS as shown in Fig. 1C, especially CHS-PDA-2 had the best swelling property (Movie S1), and it also had the best water retention effect compared with other scaffolds (Fig. 1D). The resistance to enzymatic degradation of CHS, CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5 illustrated in Fig. 1E, PDA modified CHS had a better ability against enzymatic hydrolysis, especially CHS-PDA-2, only about 50% had degraded at 16 days, than CHS that almost had degraded at 8 days. Therefore, synthetic CHS-PDA composite scaffolds have great potential for wound dressing.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.mtbio.2022.100320.
The following is the supplementary data related to this article:
3.2. Cytotoxicity and blood biocompatibility
The cell biocompatibility of CHS, CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5 was evaluated by MTT assay. Different mass of the pDA modified scaffold was incubated with cell culture medium for 24 h at 37 °C, then the leaching medium of pDA modified scaffold was used for cell culture of NIH3T3 and RAW264.7. MTT assay was conducted to identify the metabolic activity of cells treated with different scaffolds. As shown in Fig. 2A and B, there was no obvious difference between the experiment group and the control group, meaning that these scaffolds had no cytotoxicity to NIH3T3 and RAW264.7. Hemolysis is another essential index for assessing the biocompatibility of wound dressing. The blood compatibility of scaffolds was detected with rabbit red blood cells (RBCs) using water as positive control. The hemolysis rate of each experiment group was significantly lower than 5% (Fig. 2C), which demonstrated that there was negligible hemolytic capacity for erythrocytes for all experiment groups (Fig. 2D). Therefore, the CHS-PDA scaffold was nontoxic and proved good biocompatibility for RBC, which is what an ideal biomaterial should have.
Fig. 2.
Cytotoxicity and hemolysis of CHS, CHS-PDA scaffolds. The proliferation of (A) RAW264.7 cells and (B) NIH3T3 cells being respectively cultured in DMEM culture medium containing with soaking solution of CHS and different CHS-PDA scaffolds. (C) The percentage of the hemolysis rate of CHS, CHS-PDA scaffolds. (D) Image of RBCs incubate with different samples for 1 h under 37 °C. Data represented mean ± SD (n = 3). ∗∗P < 0.01 compared with control group.
3.3. Reactive oxygen species-scavenging and hemostasis properties
In diabetic wounds, persistent hyperglycemia and bacterial infections can lead to persistent inflammation and excessive ROS accumulation in wound sites, which also provoke a significant increase in oxidative stress in the wound microenvironment and eventually lead to delayed wound healing [33,34]. CHS-PDA-2 has excellent antioxidant properties, which are attributed to the dynamic redox behavior caused by catechol groups of pDA. The integration of antioxidants into scaffolds can effectively scavenge ROS, promote enzymatic repair and metabolic improvement. DPPH free radical scavenging test was conducted to investigate the in vitro antioxidant efficiency of the scaffolds. After reacting in the dark for 30, 60, 90 and 120 min, the DPPH-scavenging ratio was calculated based on the absorption of the DPPH solutions. The results showed that the DPPH-scavenging ratios of CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 increased over time. The CHS-PDA-2 exhibited the maximum DPPH-scavenging ratio of 61% at 90 min (Fig. 3A and B). Moreover, to identify the intracellular ROS scavenging capability of scaffolds, DCFH-DA probes were applied to label the intracellular ROS level in RAW264.7 cells cultured with different scaffolds (Fig. 3C). Comparing with positive control group (only H2O2), apparent fluorescence quenching increased with increase the mass of PDA in scaffold. The CHS-PDA-2 displayed the lowest fluorescence intensity, which was close to the negative control group. Quantitative flow cytometry analysis of flow cytometry also verified the efficiency of CHS-PDA-2 and other scaffolds on scavenging ROS in cells (Fig. 3D). For the negative group and the positive group, the ROS-positive expression rates were 0.86% and 78.2%, respectively. When the CHS was added, the ROS-positive expression rate was 77.1%. In sharp contrast, when CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5 were added, the positive expression of ROS was dropped to 3.05%, 9.4% and 26%, respectively.
Fig. 3.
Antioxidant efficiency of scaffolds and transformation of macrophage phenotype from M1 to M2. (A) DPPH scavenging capabilities by the CHS, CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5 and (B) the images of DPPH scavenging of all groups for 1 h and 12 h. (C) Intracellular ROS-scavenging performance of CHS, CHS-PDA-2, CHS-PDA-1 and CHS-PDA-0.5. Scale bars, 100 μm. (D) Flowcytometry of DCFH-DA labeled Raw264.7 cells in fluorescein isothiocyanate FITC-A channel on the different scaffolds. The percentage of both sides were also shown. (E–G) The expression of anti-inflammatory factors CD206, Arg-1 and pro-inflammatory factors IL-6 and iNOS in macrophage (Raw264.7) after treated with different scaffolds by RT-PCR. Data represented mean ± SD (n = 3). ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, ns, P > 0.05 compared with the LPS-only treated group.
The blood clotting capability of wound dressing is essential for the hemostatic period of wound healing [35,36]. Compared with severe trauma, diabetic chronic wound is associated with less bleeding, but most patients with diabetic foot need to undergo debridement surgery or even amputation surgery, so the hemostatic effect of wound dressings also plays an important role in the management of diabetic wounds after surgery. In vitro via blood clot index (BCI) was used to assess the blood clotting ability of CHS, CHS-PDA-2, CHS-PDA-1, CHS-PDA-0.5, in which a lower clotting rate represents a higher absorbance value of the hemoglobin solution. As shown in Fig. S2A (Supplementary data), the BCI value of CHS-PDA-2 (8.55% ± 0.79%) was significantly lower than that of CHS (13.32% ± 2.81%). After that, the hemostatic mechanism of scaffolds was further analyzed, hemagglutination is a dominant factor for the primary hemostasis. Hence, the surface attachment of RBCs was observed by SEM and the RBCs attachment rate was also tested. As shown in Fig. S2B, C (Supplementary data), CHS-PDA-2 exhibited the best cell absorption ability for RBCs. In conclusion, CHS-PDA-2 has a good antioxidant effect and procoagulant effect and can also be used as a hemostatic dressing for general acute wounds.
3.4. In vitro induction of M1-to-M2 phase transition in macrophages
In the wound healing process, macrophages can differentiate into pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes. M1 macrophages promote inflammatory response to clear the infection in the inflammatory phase of wound healing, on the contrary, M2 macrophages play a crucial role in immune regulation and tissue remodeling [37,38]. Strengthening the M1-to-M2 phase transition of macrophages can help block persistent inflammation and spur chronic wound healing. To support the in vitro anti-inflammatory properties of CHS-PDA scaffolds, macrophage polarization was assessed by real-time polymerase chain reaction (RT-PCR). RAW264.7 macrophages can be induced to M1 phenotype by lipopolysaccharide (LPS) stimulation, which was used as a positive control. Based on the RT-PCR results, it was found that in the CHS-PDA-2 treated group, inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) were significantly downregulated compared to the positive control group, and its effect was stronger than CHS-PDA-1 and CHS-PDA-0.5 treated groups, CHS-PDA-0.5 even had no significant effect for iNOS expression (Fig. 3E and F). As for the expression of M2-phenotype markers, macrophage mannose receptors (CD206) and arginase-1 (Arg-1) as well as transforming growth factor β (TGF-β), were upregulated in CHS-PDA-2 treated group, its effect was also stronger than CHS-PDA-1 and CHS-PDA-0.5 treated groups, and CHS-PDA-0.5 had no significant effect for CD206 expression (Fig. 3G and H; Fig. S3A, Supplementary data). As a result, we discovered that CHS-PDA-2 promoted the transformation from the M1 phenotype to the M2 phenotype nicely and could potentially promote wound healing with anti-inflammatory effects.
3.5. Protection of cells against harmful oxidative damage
We evaluated whether scaffolds could protect the vitality of cells from harmful peroxide. The scratch assay of HaCaT was performed to identify the cell migration rate under culture medium containing hydrogen peroxide and with or without different scaffolds and the results are displayed in Fig. 4A. HaCaT cells cultured in the control group and in the presence of CHS-PDA-2 showed a significantly enhanced migration rate at 12 h after scratching compared to other groups. At 36 h, the gap between control HaCaT cells and H2O2&CHS-PDA-2 treated groups were likewise closed. The migration rate of HaCaT in the H2O2&CHS, H2O2&CHS-PDA-2, H2O2&CHS-PDA-1, H2O2&CHS-PDA-0.5 was 21.03 ± 5.11%, 76.01 ± 5.89%, 44.73 ± 7.79%, 26.80 ± 2.91%, respectively, which was statistically significant (Fig. 4C). The result demonstrated that CHS-PDA-2 does indeed protect the activity of cells against the oxidative damage. And live/dead cell staining of HUVECs revealed that CHS-PDA-2 could defend cells against harmful oxidative damage as well (Fig. 4B). To simulate an oxidative microenvironment, HUVECs were cultured with scaffolds and then exposed to H2O2. The CHS-PDA-2 group showed the minimum reduction in cell viability compared to other experimental groups.
Fig. 4.
Scratch assay and live/dead cell staining to detect the damage of cell activity caused by peroxide. (A) The migration state of HaCaT under different treatment. Scale bars, 50 μm. (B) Treatment of HUVECs with different scaffolds abrogated H2O2-induced cell death as indicated by live/dead staining with Calcein-AM (green) and propidium iodide (red). (a) control, (b) H2O2, (c) H2O2 + CHS, (d) H2O2 + CHS-PDA-2, (e) H2O2 + CHS-PDA-1, (f) H2O2 + CHS-PDA-0.5. Scale bars, 50 μm. (C) Quantification of HaCaT migration rate. Data represented mean ± SD (n = 3). ∗∗P < 0.01, ∗P < 0.05, ns, P > 0.05 compared with only H2O2 group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Bioactive growth factor immobilization in scaffolds
We have confirmed in previous experiments that the CHS-PDA-2 scaffold has excellent water absorption characteristics, which is vital for wound dressings. Continuous delivery of bioactive drugs is an essential function of wound dressing for treatment of some chronic wound as well. Thanks to excellent water absorption ability and pDA modification, the CHS-PDA scaffold could effectively absorb and immobilize bioactive drugs or proteins that dissolved in aqueous solutions. In this research, we used the epidermal growth factor (EGF) as a bioactive drug, which has been commonly employed to promote wound healing [39,40]. We calculated the release of EGF from CHS-PDA scaffolds by immersing them in phosphate buffered saline (PBS) at 37 °C. As shown in Fig. S3B (Supplementary data), CHS-PDA-2 had the best effect of sustained release of EGF compared to CHS, and which was the weakest burst release at the beginning. These results furthermore proved that the high affinity to proteins of functional groups of pDA-NPs played an important role.
3.7. Evaluation of in vivo diabetic rat skin regeneration
We next evaluated the therapeutic effect of these scaffolds, CHS, CHS-PDA-2 and CHS-PDA-2@EGF, in full-thickness diabetic wound rat model that was created by high fat diet, streptozotocin (STZ) injection and creating a 20-mm diameter incisional wound on dorsum of diabetic rats. An acrylic ring was placed according to wound size and fixed by suturing with skin tissue around the wound to prevent wound contraction. The specific process of the in vivo experiment is shown in the Fig. 5A, we changed each group of wound dressing at day 14 and observed wounds and photographed at day 3, 7, 14, 21. As shown in Fig. 5B and C, CHS-PDA-2@EGF showed the best effect in promoting diabetic wound healing. Histological analysis of wound samples that were harvested at day 14 and 21 also indicated that CHS-PDA-2@EGF achieved the best effect of re-epithelization, granulation tissue formation and tissue remodeling (Fig. 5D). In contrast, the wounds in the control group represented delayed wound healing. As displayed in the quantitative results of Fig. 5E and F, the wound closure rate of and wound length rate of CHS-PDA-2 at day 14, 21 were better than that of CHS and control groups, indicating that PDA had a positive effect on diabetic wound healing, and those rates of CHS-PDA-2@EGF still were best among these groups. These results revealed that PDA and EGF synergistically accelerated wound healing in diabetic rats. However, we cannot ignore the effect of CHS in promoting diabetic wound repair in this experiment, which may be due to the anti-inflammatory effect of hyaluronic acid.
Fig. 5.
Treatment efficiency of the diabetic chronic wound by CHS-PDA-2@EGF in rat. (A) The schematic illustration of establishment and treatment of the diabetic chronic wound in SD rat. (B) Representative photographs of the diabetic chronic wound treated with different dressing. (C) Simulation plots of wound closure. (D) H&E staining of the wound indicated the healing condition at 14 and 21 days. (E) The quantitative analysis of wound closure and (F) H&E staining. Data represented mean ± SD (n ≥ 3). ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, ns, P > 0.05 compared with control group.
3.8. In vivo anti-inflammatory and antioxidant effects
It is now well known that persistent inflammation in chronic wounds critically hinders the process of wound healing, leading to the failure of the wounds to transit into the proliferation phase. Hence, the anti-inflammation effect of wound dressing is crucial to enhance chronic wound healing. As an excellent antioxidant agent, it was demonstrated that pDA NPs had an outstanding anti-inflammation effect [41,42]. And we detected the distribution of F4/80 positive macrophages on the sections of the wound tissue to verify the anti-inflammation effect of the pDA-modified scaffold. As shown in Fig. 6A and B, On Day 7, there was still a strong infiltration of F4/80 positive macrophages in the wound tissues treated with CHS, and in the control group, as well. Nevertheless, F4/80 positive macrophage infiltration was remarkably milder in the CHS-PDA-2 and CHS-PDA-2@EGF groups. At the same time, the transformation of the M1 and M2 morphology of macrophages also plays a key role in the regulation of wound inflammation. Then, the immunofluorescence staining of F4/80 and CD206 were conducted to identify macrophage phenotypes (Fig. 6C). The CHS-PDA-2 and CHS-PDA-2@EGF groups had higher ratios of CD206 positive macrophages, which represent M2 phenotype macrophages that can secrete anti-inflammation cytokines and reparative growth factors. By contrast, in the control group and CHS treated group, most macrophages remained in the M1 phenotype (F4/80+ and CD206-). Imbalanced oxidative stress is one of the main reasons for chronic wounds, especially caused by hyperglycemia microenvironment, and superoxide dismutase (SOD) is an antioxidative enzyme that exists in many kinds of tissues including skin tissue, which plays an important role in the antioxidative defense system. Thus, we detect the degree of SOD activity in each group to inspect the antioxidant effect of the CHS-PDA-2@EGF scaffold. The degree of SOD activity in the CHS-PDA-2 group (101.5 ± 7.8 U/mg) and CHS-PDA-2@EGF (106.6 ± 5.5 U/mg) group were definitely higher than the CHS group (50.1 ± 10.0 U/mg) and control group (41.5 ± 3.8 U/mg). These results disclose that pDA and EGF can synergistically promote anti-inflammatory effects to aid diabetic wound healing, and pDA can lower oxidative stress during the wound healing process.
Fig. 6.
Modulation of inflammation microenvironment and antioxidant effect in diabetic wound. (A) Immunohistochemistry staining of F4/80 on the wound sections at day 7. (B) Quantitative analysis of F4/80 positive area. (C) Immunofluorescence images of macrophages in wound tissue after treatment stained with F4/80 (Green) and CD206 (Red). Nuclei were stained with DAPI (blue). (D) Superoxide dismutase (SOD) activity (U/mg) in the wound tissue. ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, compared with control group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.9. Assessment of tissue regeneration and remodeling
In addition to wound epithelialization and inflammation response, cell proliferation and tissue remodeling are also the gold standards to judge wound healing. Herein, we detected the cell proliferation on the wound site, Ki67 is an antigen specifically expressed in the nucleus during cell division, which can show the cells in the proliferation cycle via immunohistochemical staining assay. And the result of immunohistochemical staining of Ki67 on the wounded skin showed that the presence of more Ki67 positive cells in the CHS-PDA-2@EGF, CHS-PDA-2 groups than in the CHS and control groups, and the difference between CHS-PDA-2@EGF group and control group was most significant (Fig. 7A). The quantification of Ki67-positive cells on day 7 demonstrated that the percentage of these cells in CHS-PDA-2@EGF was highest, 44.8% ± 4.5%, among all groups (Fig. 7C). Except for the significant effect of EGF to promote cell proliferation in the wound, the result also showed that pDA itself had the potential to accelerate cell proliferation by reducing inflammation and that was better than the non-modified CHS group.
Fig. 7.
Tissue section examination of related indicators about wound healing. (A) Immunohistochemistry staining of Ki67 on the wound sections at day 7. (B) Immunohistochemistry staining of CD31 on the wound sections at day 14 and day 21. (C–D) Quantitative analysis of Ki67, CD31 positive area. ∗∗∗P < 0.001, ∗∗P < 0.01 compared with control group.
The neovascularization effects of those scaffolds were investigated by immunohistochemistry staining of CD31 in Fig. 7B, comparing postoperative days 14 and 21, we can see that the CHS-PDA-2@EGF group had the highest vessel density. For further evaluation, on day 21, the quantitative result of CD31 immunohistochemistry staining demonstrated that CHS-PDA-2@EGF increased the vessel area most obviously (26.9% ± 0.5%), which was better than CHS-PDA-2 group (19.5% ± 0.6%), CHS group (11.6% ± 0.9%), and control group (8.0% ± 0.5%), and the results on day 12 showed the same trend (Fig. 7D).
During the remodeling stage of skin wound healing, collagen deposit is an important process and a criterion to assess the wound healing [43]. Several types of collagen fiber exist, including types I and III [44], the type I collagen is the main component of skin and thick, whereas type III collagen is thin and forms the structure of reticular fibers. During the remodeling of skin tissue, collagens I and III are expressed in coordinated ways [43]. Masson's trichrome stain was used to detect deposited collagen and keratin, which were stained in blue and red, respectively. Therefore, the results of Masson’ trichrome stain indicated that the group of CHS-PDA-2@EGF had less keratin-positive area at day 14 and had more thicker collagen fibers at day 21 than CHS-PDA-2 group, CHS group and control group (Fig. 8A). For CHS-PDA-2 group, collagen deposit of wound was better than CHS group at day 14 and day 21 (Fig. 8A), and it indicated that PDA itself improved ECM deposition and collagen arrangement in diabetic wound. As shown in Fig. 8B and C, the collagen I/III ratio in each group increased on day 21 compared to day 14, due to the increase in collagen I fibers, and the group of CHS-PDA-2@EGF had the most significant increase than other groups. The results of the CHS-PDA-2 group also showed that PDA had a certain ability to promote tissue remodeling of the diabetic wound. Finally, the long-term system toxicity of CHS, CHS-PDA-2 and CHS-PDA-2@EGF in vivo was detected. At 30 days after wound treatment, as shown in Fig. S4 of the Supplementary data, these scaffolds did not cause any damage to the heart, liver, spleen, lung, or kidney.
Fig. 8.
(A) Masson's staining and (B) Sirius red staining of the wound sections at day 14 and day 21. (C) Quantitative analysis of the ratio of collagen I (yellow) and collagen III (green). ∗∗∗P < 0.001, ∗∗P < 0.01, ns, P > 0.05 compared with control group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Conclusion
In general, we successfully created a polydopamine modified collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor to promote wound healing in a rat. In terms of composition, Type I collagen and hyaluronic acid are biomolecular materials that are commonly used in the medical field, specifically in micro plastic surgery, and they are readily available through the current mature extraction techniques. The scaffold CHS had a good swelling rate and after the modification of pDA, its swelling rate and the blood clotting ability were greatly improved. Therefore, it has great potential to as a wound dressing in clinical wound treatment. CHS-PDA-2 also exerted excellent antioxidant, anti-inflammatory, modulation of macrophage polarization, as well as immobilization and sustained release of EGF. All of these factors contributed to a promising wound healing efficiency in chronic diabetic wounds. We believe that the facile production process, adequate degradation time, excellent biocompatibility, convenient replacement operation and bioactivity of the CHS-PDA-2@EGF scaffold may provide a promising and rational combined treatment for effective diabetic chronic wound healing in clinic. Furthermore, other studies have shown that pDA has a bactericidal effect by photothermal effect under 808 nm near infrared light. To maximize the applicability fo this scaffold, it should be tested on other clinically complex wounds, including infected diabetic wounds. In addition to EGF, many other growth factors that play a role in wound healing, such as vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF1), platelet derived growth factor (PDGF) can be combined with this scaffold for chronic wound repair [45]. Meanwhile, gene therapy also can be combined as a new treatment method [46].
Credit author statement
Yong Wang, Li Chen provided the concept of this research, conducted experimental design, completed most of the experiments and wrote the original draft of the article. Dan-Yang Ren, Zi-Xuan Feng completed data sorting and analysis work. Li-Yun Zhang, Yu-Fan Zhong, Ming-Yuan Jin, Fa-Wei Xu conducted deep review and editing. Chun-Yan Feng, Yong-Zhong Du helped revise the paper and gave some advice. Wei-Qiang Tan supervised the whole study and obtained funding support for this research.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Wei-Qiang Tan reports administrative support was provided by National Natural Science Foundation of China. Yong-Zhong Du is a part-time professor of our department from School of Pharmacy, Zhejiang University.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No.82172206 and 81671918), Zhejiang Provincial Medical and Healthy Science Foundation of China (No. 2019KY757 and 2019KY754) and Zhejiang Provincial Science and Technology Project of China (LGF22H150002). Thanks for the technical support by the Core Facilities Plat Form of Zhejiang University School of Medicine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100320.
Appendix ASupplementary data
The following are the Supplementary data to this article:
References
- 1.Fui L.W., Lok M.P.W., Govindasamy V., et al. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process. J. Tissue Eng. Regen. Med. 2019;13(12):2218–2233. doi: 10.1002/term.2966. [DOI] [PubMed] [Google Scholar]
- 2.Sloan G., Selvarajah D., Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021;17(7):400–420. doi: 10.1038/s41574-021-00496-z. [DOI] [PubMed] [Google Scholar]
- 3.Broughton G., Janis J.E., Attinger C.E. Wound healing: an overview. Plast. Reconstr. Surg. 2006;117(7 Suppl) doi: 10.1097/01.prs.0000222562.60260.f9. 1e-S-32e-S, 2nd. [DOI] [PubMed] [Google Scholar]
- 4.Indyk D., Bronowicka-Szydełko A., Gamian A., et al. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-92630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakkala J.R., Li Z., Ahmad W., et al. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomater. 2021;123:1–30. doi: 10.1016/j.actbio.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Qi Y., Qian K., Chen J., et al. A thermoreversible antibacterial zeolite-based nanoparticles loaded hydrogel promotes diabetic wound healing via detrimental factor neutralization and ROS scavenging. J. Nanobiotechnol. 2021;19(1):414. doi: 10.1186/s12951-021-01151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H., Huang J., Li Y., et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials. 2020;258 doi: 10.1016/j.biomaterials.2020.120286. [DOI] [PubMed] [Google Scholar]
- 8.Lu J., Cai L., Dai Y., et al. Polydopamine-based nanoparticles for photothermal therapy/chemotherapy and their synergistic therapy with autophagy inhibitor to promote antitumor treatment. Chem. Rec. 2021;21(4):781–796. doi: 10.1002/tcr.202000170. [DOI] [PubMed] [Google Scholar]
- 9.Han L., Zhang Y., Lu X., et al. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl. Mater. Interfaces. 2016;8(42):29088–29100. doi: 10.1021/acsami.6b11043. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Ai K., Lu L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 11.Gong M., Liu C., Liu C., et al. Biomimetic hydroxyapate/polydopamine composites with good biocompatibility and efficiency for uncontrolled bleeding. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(11):1876–1892. doi: 10.1002/jbm.b.34849. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H., Zeng Z., Liu L., et al. Polydopamine nanoparticles for the treatment of acute inflammation-induced injury. Nanoscale. 2018;10(15):6981–6991. doi: 10.1039/c8nr00838h. [DOI] [PubMed] [Google Scholar]
- 13.Wei G., Jiang D., Hu S., et al. Polydopamine-decorated microcomposites promote functional recovery of an injured spinal cord by inhibiting neuroinflammation. ACS Appl. Mater. Interfaces. 2021;13(40):47341–47353. doi: 10.1021/acsami.1c11772. [DOI] [PubMed] [Google Scholar]
- 14.Jia Z., Gong J., Zeng Y., et al. Bioinspired conductive silk microfiber integrated bioelectronic for diagnosis and wound healing in diabetes. Adv. Funct. Mater. 2021;31(19) doi: 10.1002/adfm.202010461. [DOI] [Google Scholar]
- 15.Hong S., Yun S.N., Choi S., et al. Non-covalent self-assembly and covalent polymerization Co-contribute to polydopamine formation. Adv. Funct. Mater. 2012;22(22):4711–4717. doi: 10.1002/adfm.201201156. [DOI] [Google Scholar]
- 16.Zheng Z., Li M., Shi P., et al. Polydopamine-modified collagen sponge scaffold as a novel dermal regeneration template with sustained release of platelet-rich plasma to accelerate skin repair: a one-step strategy. Bioact. Mater. 2021;6(8):2613–2628. doi: 10.1016/j.bioactmat.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu J., Zhao X., Liang Y., et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 2018;183:185–199. doi: 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Francesko A., Petkova P., Tzanov T. Hydrogel dressings for advanced wound management. Curr. Med. Chem. 2018;25(41):5782–5797. doi: 10.2174/0929867324666170920161246. [DOI] [PubMed] [Google Scholar]
- 19.Augustine R., Zahid A.A., Hasan A., et al. Cerium oxide nanoparticle-loaded gelatin methacryloyl hydrogel wound-healing patch with free radical scavenging activity. ACS Biomater. Sci. Eng. 2021;7(1):279–290. doi: 10.1021/acsbiomaterials.0c01138. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q., Torres J.E., Hakim M., et al. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021;146 doi: 10.1016/j.mser.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C., Shi Z., Sun H., et al. Tissue factor-loaded collagen/alginate hydrogel beads as a hemostatic agent. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109(8):1116–1123. doi: 10.1002/jbm.b.34774. [DOI] [PubMed] [Google Scholar]
- 22.Pallaske F., Pallaske A., Herklotz K., et al. The significance of collagen dressings in wound management: a review. J. Wound Care. 2018;27(10):692–702. doi: 10.12968/jowc.2018.27.10.692. [DOI] [PubMed] [Google Scholar]
- 23.Pereira H., Sousa D.A., Cunha A., et al. Hyaluronic acid. Adv. Exp. Med. Biol. 2018;1059:137–153. doi: 10.1007/978-3-319-76735-2_6. [DOI] [PubMed] [Google Scholar]
- 24.Neuman M.G., Nanau R.M., Oruña-Sanchez L., et al. Hyaluronic acid and wound healing. J. Pharm. Pharmaceut. Sci. 2015;18(1):53–60. doi: 10.18433/j3k89d. [DOI] [PubMed] [Google Scholar]
- 25.Cortes H., Caballero-Florán I.H., Mendoza-Muñoz N., et al. Hyaluronic acid in wound dressings. Cell. Mol. Biol. 2020;66(4):191–198. doi: 10.14715/cmb/2020.66.4.23. [DOI] [PubMed] [Google Scholar]
- 26.Litwiniuk M., Krejner A., Speyrer M.S., et al. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88. [PubMed] [Google Scholar]
- 27.Augustine R., Hasan A., Dalvi Y.B., et al. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;118 doi: 10.1016/j.msec.2020.111519. [DOI] [PubMed] [Google Scholar]
- 28.Wyganowska-Swiatkowska M., Duda-Sobczak A., Corbo A., et al. Atelocollagen application in human periodontal tissue treatment-A pilot study. Life. 2020;10(7) doi: 10.3390/life10070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S.N., Lee H.J., Lee K.H., et al. Biological characterization of EDC-crosslinked collagen-hyaluronic acid matrix in dermal tissue restoration. Biomaterials. 2003;24(9):1631–1641. doi: 10.1016/s0142-9612(02)00550-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen S., Zhang Q., Nakamoto T., et al. Highly active porous scaffolds of collagen and hyaluronic acid prepared by suppression of polyion complex formation. J. Mater. Chem. B. 2014;2(34):5612–5619. doi: 10.1039/c4tb00780h. [DOI] [PubMed] [Google Scholar]
- 31.Wang C., Niu H., Ma X., et al. Bioinspired, injectable, quaternized hydroxyethyl cellulose composite hydrogel coordinated by mesocellular silica foam for rapid, noncompressible hemostasis and wound healing. ACS Appl. Mater. Interfaces. 2019;11(38):34595–34608. doi: 10.1021/acsami.9b08799. [DOI] [PubMed] [Google Scholar]
- 32.Ousey K., Cutting K.F., Rogers A.A., et al. The importance of hydration in wound healing: reinvigorating the clinical perspective. J. Wound Care. 2016;25(3):124–130. doi: 10.12968/jowc.2016.25.3.122. 122. [DOI] [PubMed] [Google Scholar]
- 33.Deng L., Du C., Song P., et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/8852759. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Y., Niu H., Liu Z., et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci. Adv. 2021;7(35) doi: 10.1126/sciadv.abj0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keast D.H., Janmohammad A. The hemostatic and wound healing effect of chitosan following debridement of chronic ulcers. Wounds. 2021;33(10):263–270. [PubMed] [Google Scholar]
- 36.Song F., Kong Y., Shao C., et al. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021;136:170–183. doi: 10.1016/j.actbio.2021.09.056. [DOI] [PubMed] [Google Scholar]
- 37.Hesketh M., Sahin K.B., West Z.E., et al. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aitcheson S.M., Frentiu F.D., Hurn S.E., et al. Skin wound healing: normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules. 2021;26(16) doi: 10.3390/molecules26164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang P., Han L., Li P., et al. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl. Mater. Interfaces. 2019;11(8):7703–7714. doi: 10.1021/acsami.8b18931. [DOI] [PubMed] [Google Scholar]
- 40.Han L., Li P., Tang P., et al. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale. 2019;11(34):15846–15861. doi: 10.1039/c9nr03095f. [DOI] [PubMed] [Google Scholar]
- 41.Barros N., Chen Y., Hosseini V., et al. Recent developments in mussel-inspired materials for biomedical applications. Biomater. Sci. 2021;9(20):6653–6672. doi: 10.1039/d1bm01126j. [DOI] [PubMed] [Google Scholar]
- 42.Bartzanas T. Recent advances in antioxidant polymers: from sustainable and natural monomers to synthesis and applications. Polymers. 2021;13 doi: 10.3390/polym13152465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues M., Kosaric N., Bonham C.A., et al. Wound healing: a cellular perspective. Physiol. Rev. 2019;99(1):665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davison-Kotler E., Marshall W.S., García-Gareta E. Sources of collagen for biomaterials in skin wound healing. Bioengineering (Basel) 2019;6(3) doi: 10.3390/bioengineering6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Augustine R., Ur Rehman S.R., K S.J., et al. Stromal cell-derived factor loaded co-electrospun hydrophilic/hydrophobic bicomponent membranes for wound protection and healing. RSC Adv. 2020;11(1):572–583. doi: 10.1039/d0ra04997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lou D., Luo Y., Pang Q., et al. Gene-activated dermal equivalents to accelerate healing of diabetic chronic wounds by regulating inflammation and promoting angiogenesis. Bioact. Mater. 2020;5(3):667–679. doi: 10.1016/j.bioactmat.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.