Abstract
The Knoevenagel–Michael cyclocondensation of malononitrile, aryl aldehydes, and resorcinol was used as a multicomponent green tandem strategy for the metal-free synthesis of 2-amino-4H-chromene scaffolds. Through a visible-light-induced process, the photo-excited state functions derived from Na2 eosin Y were used as direct hydrogen atom transfer catalysts in aqueous ethanol at ambient temperature. The purpose of this study was to examine the further use of an organic dye that does not contain metal and is inexpensive and commercially available. Na2 eosin Y is synthesized by photochemical means using the least amount of catalyst, which results in excellent yields, energy efficiency, and environmental friendliness, high atom economy, time-saving features, and ease of operation. As a result, some properties of green and sustainable chemistry are met. This kind of cyclization can be performed on a gram scale, indicating the potential utility of this reaction in industry.
Keywords: photoexcited Na2 eosin Y, photochemical synthesis, visible light mediated, 2-amino-4H-chromene scaffolds, green chemistry
Introduction
Eosin Y is a metal-free organic dye that has gained widespread use in recent years as a cost-effective and environment friendly alternative to transition-metal-based photocatalysts (Zhu et al., 2018; Wang et al., 2019; Yan et al., 2019; Chen et al., 2020). Successfully oxidized/reduced target substrates by their incited mode in eosin Y-catalyzed photoredox reactions are typically dependent on whether the substrates’ prospective oxidability or reducibility falls within the scope of eosin Y (Yan et al., 2019). The electrochemical requirements that have been discussed have limited the range of eosin Y-catalyzed photochemical processes. Eosin Y is distinguished from other organic dyes by its distinct xanthene and phenol moieties, as well as notable acid–base properties, which can result in four distinct constructions. In most earlier reports on photoreactions, there is ample evidence that the anionic kinds of eosin Y exhibit photocatalytic properties, but the neutral types are thought to have typical inactivity and are ignorable in potentially applied synthesis procedures (Hari and König, 2014; Majek et al., 2014). The structural properties of eosin Y have inspired a team of Wang (Zhao and Wang, 2018) and Wu (Fan et al., 2018) to innovate in the discovery of novel activating states of photoexcited eosin Y in recent years. The researchers observed that incited modes derived from neutral eosin Y may operate as photoacids and direct hydrogen atom transfer (HAT) catalysts for activating glycals and native C–H bonds in the order they were identified (Yan et al., 2019).
HAT (hydrogen atom transfer) is a fundamental stage that may be responsible for a variety of chemical, environmental, and biological processes. In recent years, benzophenone- and quinone-mediated direct HAT catalysis has been promoted as a tool for activating the C–H bond under light radiation (Ravelli et al., 2016; Romero and Nicewicz, 2016; Capaldo and Ravelli, 2017). Direct HAT catalysis mediated by benzophenone and quinone has recently been established as a viable method for irradiating the C–H bond (Ravelli et al., 2016; Romero and Nicewicz, 2016; Capaldo and Ravelli, 2017). Due to the similarities between eosin Y and quinones (Ravelli et al., 2016; Romero and Nicewicz, 2016), Wu and others suggested that when exposed to visible light, eosin Y may work as a direct HAT catalyst, activating a C–H bond and creating radical species for further functionalities (Fan et al., 2018). The radical species formed from eosin Y is unlikely to suffer the kinds of side reactions seen in HAT catalysis with diaryl ketones, allowing for a reverse transfer of hydrogen atom, due to its captodative and steric features. Wu and others demonstrated that when eosin Y in the neutral state is exposed to the visible spectrum, it may successfully initiate numerous C (sp3)-H and C (sp2)-H bonds to start generating the matching carbon radicals, allowing radical introduction to multiple alkenes with electron deficit. This method covers a wide range of substrates and has a high group tolerance. The needed C–H alkylation compounds were synthesized with good yields and site selectivity. A number of C (sp3)-H and C (sp2)-H bonds of ethers, thioethers, alcohols, aldehydes, and cyclohexanes were radically alkylated with acceptable site selection (e.g., 10 c). This approach can be used to a wide range of tri- and tetrasubstituted olefins with various properties. The substrate restrictions of traditional SET-based redox reactions are circumvented by this HAT catalysis technique (Yan et al., 2019).
In the eco-friendly synthesis of organic molecules, visible light irradiation has also proven to be a trustworthy strategy for green chemists because of its abundant energy reserves, low prices, and renewable source of energy (Mohamadpour, 2020; Mohamadpour, 2021a). In general, visible light sources such as light emitting diodes and tiny fluorescent lamps are used for various conversions.
Because of their biological actions, chromenes and their equivalents have gotten a lot of attention such as antimicrobial (Kathrotiya and Patel, 2012), antifungal (Alvey et al., 2009), anti-inflammatory (Moon et al., 2007), antibacterial (Kumar et al., 2009), antioxidant (Symeonidis et al., 2009), antileishmanial (Narender et al., 2004), anti-HIV (Flavin et al., 1996; Rueping et al., 2008), anticancer (Abdelrazek et al., 2004; Paliwal et al., 2013), and hypotensive (Cai et al., 2009). Also, they are used as inhibitors (Wang et al., 2000; Huynh et al., 2012).
Several multicomponent reactions for manufacturing 2-amino-4H-chromene scaffolds have been described against various catalysts such as glycine (Datta and Pasha, 2012), mesolite (Pawar et al., 2018), potassium phthalimide (Dekamin and Eslami, 2014), MgFe2O4NPs (Eshtehardian et al., 2020), POM@Dy-PDA (Hosseinzadeh-Baghan et al., 2020), P4VPy-CuI (Albadi and Mansournezhad, 2016), nanozeolite clinoptilolite (Baghbanian et al., 2013), water extract of lemon fruit shell ash (WELFSA) (Kantharaju and Khatavi, 2018), tungstic acid functionalized SBA-15 (Kundu et al., 2013), MIL-101(Cr)-SO3H (Saikia and Saikia, 2016) [Et2NH(CH2)2CO2H][AcO] (Shaikh et al., 2019), {[4,4′-BPyH][C(CN)3]2} (Zolfigol et al., 2016), DBU (Raghuvanshi and Singh, 2010), and hydrotalcite (Kale et al., 2013). Several cases arose from these surgeries. Some synthetic policies, however, include restrictions on the use of metal catalysts, harsh reaction conditions, expensive reagents, monotonous workup processes, unacceptable yields, long reaction times, environmental hazards, and the use of homogeneous catalysts that are problematically detached from the reaction mixture.
Given the foregoing factors and our interest in producing 2-amino-4H-chromenes, the key goal was to investigate the photocatalyst (Mohamadpour, 2021b; Mohamadpour, 2021c) under green conditions for the appropriate synthesis of these heterocyclic compounds. This study paves the new role for further usage of a metal-free organic dye with commercial availability and inexpensiveness, Na2 eosin Y in aforementioned photochemical synthesis. Evidence suggests that the photoexcited states of Na2 eosin Y act as a direct hydrogen atom transfer (HAT) catalyst in the photochemical synthesis of 2-amino-4H-chromenes via the Knoevenagel–Michael cyclocondensation reaction of aryl aldehydes, malononitrile, and resorcinol in aqueous ethanol at ambient temperature under air atmosphere. This is a successful one-pot reaction that uses extremely effective, moderate, and simple reaction conditions.
Experimental
Producing 4a-w
Under white light (LED) irradiation (18 W), Na2 eosin Y (0.5 mol%) was added to a mixture of aryl aldehydes, malononitrile, and resorcinol in an H2O/EtOH (2:1) (3 ml) (Supplementary Figure S2). At rt, the mixture was agitated, and TLC was used to track the reaction’s progress. The resulting solid was filtered and rinsed with H2O before the reaction was completed. The pure substance was then recrystallized crude solid from ethanol with no further purification. After that, the goods were classified by comparing the spectroscopic data (1HNMR). The spectroscopic data listed below can be found in the Supplementary Material file.
Results and Discussion
To prepare 4a in H2O/EtOH (2:1) (3 ml) at ambient temperature under LED irradiation, the reaction between malononitrile, benzaldehyde, and resorcinol was studied first. In 3 ml of H2O/EtOH (2:1) for 15 min with no photocatalysts, there was a 57% of 4a at rt. Various organic photocatalysts, such as Na2 eosin Y, erythrosin B, phenanthrenequinone, rhodamine B, acenaphthenequinone, riboflavin,9H-xanthen-9-one, fluorescein, and rose bengal, were tested in similar settings to stimulate the process. While achieving the matching product 4a, the progression of this reaction was seen in 51–93% yields. The results showed that in such a response, Na2 eosin Y performed better. The yield was enhanced to 93% by using 0.5 mol% Na2 eosin Y. (Table 1, entry 3). In addition, CHCl3, toluene, THF, DMSO, CH2Cl2, CH3CN, and DMF, all had reduced product yields. The reaction rate and yield were enhanced by performing the reaction in EtOAc, MeOH, EtOH, H2O/EtOH, H2O, and solvent-free. The reaction went well in a 2:1 mixture of H2O and EtOH. Table 1 shows that in identical conditions, a yield of 93% was attained (entry 3). Different light sources were employed to screen the yield, demonstrating the effect of white light. There was a minuscule of 4a without using the light source, according to the test control. To create product 4a successfully, visible light and Na2 eosin Y are required, according to the findings. Furthermore, the increased settings were specified by irradiating white LEDs of varied intensities (10, 12, 18, and 20 W). The best results were obtained under the irradiation of white LEDs (18 W), according to Table 1 (entry 3). It was discovered that the process may be used on a variety of substrates (Table 2; Scheme 1). (More data are provided in Supplementary Table S1 in the Supplementary Material file).
TABLE 1.
Optimization table of photocatalyst for the synthesis of 4a a .

| |||||
| Entry | Photocatalyst | Light source | Solvent (3 ml) | Time (min) | Isolated yields (%) |
| 1 | — | White light (18 W) | H2O/EtOH (2:1) | 15 | 57 |
| 2 | Na2 eosin Y (0.2 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 78 |
| 3 | Na 2 eosin Y (0.5 mol%) | White light (18 W) | H 2 O/EtOH (2:1) | 5 | 93 |
| 4 | Na2 eosin Y (1 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 93 |
| 5 | Erythrosin B (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 51 |
| 6 | Phenanthrenequinone (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 53 |
| 7 | Rhodamine B (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 72 |
| 8 | Acenaphthenequinone (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 57 |
| 9 | Riboflavin (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 64 |
| 10 | 9H-xanthen-9-one (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 60 |
| 11 | Fluorescein (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 77 |
| 12 | Rose bengal (0.5 mol%) | White light (18 W) | H2O/EtOH (2:1) | 5 | 68 |
Reaction conditions: benzaldehyde (1 mmol), malononitrile (1 mmol), resorcinol (1 mmol) in H2O/EtOH (2:1) (3 ml), white LED (18 W), and various photocatalysts at rt. Bold values provides optimal conditions for reaction.
TABLE 2.
Photoexcited Na2 eosin Y as a photocatalyst for the synthesis of 2-amino-4H-chromene scaffolds.

| ||
 4a (5min, 93%)MP. 234‐236°CLit. 232‐234°C [27]
4a (5min, 93%)MP. 234‐236°CLit. 232‐234°C [27] |
 4b (5min, 94%)Mp. 166‐168°CLit. 168‐170°C [32]
4b (5min, 94%)Mp. 166‐168°CLit. 168‐170°C [32] |
 4c (3min, 91%)Mp. 185‐187°CLit. 186‐188°C [33]
4c (3min, 91%)Mp. 185‐187°CLit. 186‐188°C [33] |
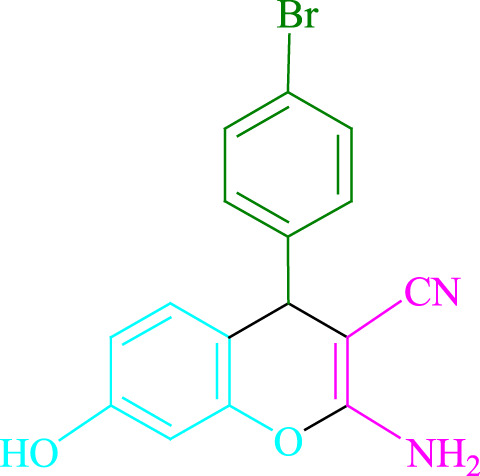 4d (10min, 88%)Mp. 223‐225°CLit. 222‐224°C [24]
4d (10min, 88%)Mp. 223‐225°CLit. 222‐224°C [24] |
 4e (7min, 91%)Mp. 179‐181°CLit. 180‐182°C [33]
4e (7min, 91%)Mp. 179‐181°CLit. 180‐182°C [33] |
 4f (5min, 95%)Mp. 192‐194°CLit. 190‐192°C [32]
4f (5min, 95%)Mp. 192‐194°CLit. 190‐192°C [32] |
 4g (10min, 84%)Mp. 249‐251°CLit. 250‐252°C [23]
4g (10min, 84%)Mp. 249‐251°CLit. 250‐252°C [23] |
 4h (5min, 86%)Mp. 187‐189°CLit. 189‐191°C [27]
4h (5min, 86%)Mp. 187‐189°CLit. 189‐191°C [27] |
 4i (5min, 92%)Mp. 194‐196°CLit. 194‐196°C [24]
4i (5min, 92%)Mp. 194‐196°CLit. 194‐196°C [24] |
 4j (9min, 87%)Mp. 200‐202°CLit. 198‐200°C [35]
4j (9min, 87%)Mp. 200‐202°CLit. 198‐200°C [35] |
 4k (5min, 92%)Mp. 211‐213°CLit. 210‐212°C [24]
4k (5min, 92%)Mp. 211‐213°CLit. 210‐212°C [24] |
 4l (3min, 93%)Mp. 146‐148°CLit. 148‐150°C [29]
4l (3min, 93%)Mp. 146‐148°CLit. 148‐150°C [29] |
 4m (7min, 89%)Mp. 175‐177°CLit. 176‐178°C [35]
4m (7min, 89%)Mp. 175‐177°CLit. 176‐178°C [35] |
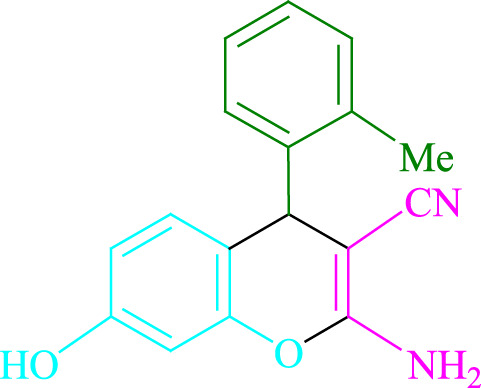 4n (3min, 94%)Mp. 229‐229°CLit. 223‐231°C [25]
4n (3min, 94%)Mp. 229‐229°CLit. 223‐231°C [25] |
 4o (3min, 96%)Mp. 189‐191°CLit. 188‐190°C [27]
4o (3min, 96%)Mp. 189‐191°CLit. 188‐190°C [27] |
 4p (7min, 88%)Mp. 208‐210°CLit. 210‐212°C [29]
4p (7min, 88%)Mp. 208‐210°CLit. 210‐212°C [29] |
 4q (7min, 88%)Mp. 259‐261°CLit. 257‐259°C [27]
4q (7min, 88%)Mp. 259‐261°CLit. 257‐259°C [27] |
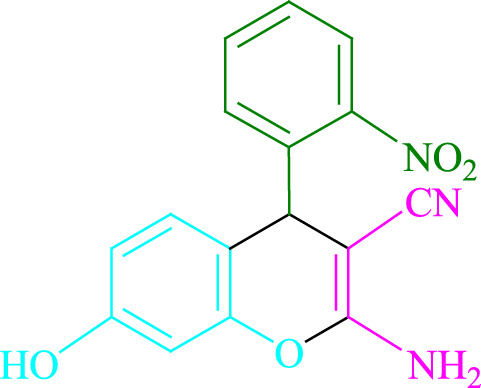 4r (3min, 96%)Mp. 160‐162°CLit. 162‐163°C [34]
4r (3min, 96%)Mp. 160‐162°CLit. 162‐163°C [34] |
 4s (8min, 87%)Mp. 218‐220°CLit. 219‐221°C [33]
4s (8min, 87%)Mp. 218‐220°CLit. 219‐221°C [33] |
 4t (9min, 85%)Mp. 229‐231°CLit. 227‐229°C [33]
4t (9min, 85%)Mp. 229‐231°CLit. 227‐229°C [33] |
 4u (3min, 94%)Mp. 201‐203°CLit. 200‐202°C [34]
4u (3min, 94%)Mp. 201‐203°CLit. 200‐202°C [34] |
 4v (7min, 87%)Mp. 163‐165°CLit. 162‐163°C [27]
4v (7min, 87%)Mp. 163‐165°CLit. 162‐163°C [27] |

|
|
SCHEME 1.
Synthesis of 2-amino-4H-chromene scaffolds.
The proposed technique is depicted in Scheme 2. Malononitrile (2) is exposed to tautomerization through the visible light to provide (A). Then, aldehydes (1) and (A) combine to make arylidenemalononitrile (B), which undergoes photochemical activation to yield a radical intermediate (C). The visible light can be changed in part by the application of more energy to speed up this reaction. Eosin Y-made photoexcited modes can operate as direct HAT catalysts to activate C–H bonds eosin, according to earlier findings (Fan et al., 2018; Yan et al., 2019; Chen et al., 2020). Through a HAT method, the malononitrile radical is produced to boost the visible light triggered Na2 eosin Y*. The reverse hydrogen atom transfer (RHAT) method between radical adduct C and eosin Na2 Y-H produces intermediate D and ground-state Na2 eosin Y. A hydrogen atom is then removed from (E) by the malononitrile radical, resulting in intermediate (F). Then, as a Michael acceptor, intermediates (F) and (D) coalesce to produce (G), which undergoes intramolecular cyclization and tautomerization to yield the product (4).
SCHEME 2.
The proposed mechanistic pathway to synthesize the 2-amino-4H-chromene scaffolds.
Table 3 compares the capability of some of the catalysts used in this investigation to generate 2-amino-4H-chromenes. It could be used for a variety of purposes, such as the use of a short-time reaction with the least amount of photocatalyst and no by-products when exposed to visible light. The multigram-scale atom-economic amazing protocol is operative because it contains the main industrial applications that achieve both good purity and excellent performance. The atomic economy was likewise carefully managed in this sense.
TABLE 3.
Comparison between the catalytic capacity of some catalysts in this work a .
| Entry | Catalyst | Conditions | Time/yield (%) | References |
|---|---|---|---|---|
| 1 | Glycine | H2O, sonication | 9 min/94 | Datta and Pasha, (2012) |
| 2 | Mesolite | EtOH, reflux | 30 min/93 | Pawar et al. (2018) |
| 3 | Potassium phthalimide | H2O, reflux | 12 min/94 | Dekamin and Eslami, (2014) |
| 4 | MgFe2O4NPs | EtOH, 65°C | 12 min/74 | Eshtehardian et al. (2020) |
| 5 | POM@Dy-PDA | EtOH/H2O, reflux | 15 min/95 | Hosseinzadeh-Baghan et al. (2020) |
| 6 | P4VPy-CuI | H2O, reflux | 15 min/94 | Albadi and Mansournezhad, (2016) |
| 7 | Nanozeolite clinoptilolite | H2O, reflux | 15 min/92 | Baghbanian et al. (2013) |
| 8 | WELFSA | H2O, rt | 1.5h/88 | Kantharaju and Khatavi, (2018) |
| 9 | Tungstic acid functionalized SBA-15 | H2O, 100°C | 12 min/86 | Kundu et al. (2013) |
| 10 | MIL-101(Cr)-SO3H | H2O, 100°C | 180 min/82 | Saikia and Saikia, (2016) |
| 11 | [Et2NH(CH2)2CO2H][AcO] | Solvent free, 60°C | 12 min/92 | Shaikh et al. (2019) |
| 12 | {[4,4′-BPyH][C(CN)3]2} | Solvent free, 80°C | 15 min/90 | Zolfigol et al. (2016) |
| 13 | DBU | EtOH,MW, 50°C | 3 min/94 | Raghuvanshi and Singh, (2010) |
| 14 | Hydrotalcite | H2O, 60°C | 4 h/95 | Kale et al. (2013) |
| 15 | Na 2 eosin Y | Visible light irradiation, H 2 O/EtOH (2:1), rt | 5 min/93 | This work |
Based on the three-component reaction of benzaldehyde, malononitrile, and resorcinol. Bold values provides optimal conditions for reaction.
Conclusion
The photo-excited state functions produced from Na2 eosin Y can be used to metal-free synthesis the 2-amino-4H-chromene scaffolds, according to the findings. This procedure is carried out in an aqueous ethanol air environment at room temperature using visible light. The most obvious benefits of this green protocol include the use of the least amount of catalyst, high yields, efficient sides of the reaction, secure reaction conditions, and a quick operation without the use of toxic solvents or catalysts. As a result, this procedure has more advantages when it comes to meeting industrial needs and environmental issues.
Acknowledgments
The author gratefully acknowledges the financial support from the Research Council of the Apadana Institute of Higher Education.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.880257/full#supplementary-material
References
- Abdelrazek F. M., Metz P., Farrag E. K. (2004). Synthesis and Molluscicidal Activity of 5-Oxo-5,6,7,8-Tetrahydro-4h-Chromene Derivatives. Arch. Pharm. Pharm. Med. Chem. 337, 482–485. 10.1002/ardp.200400881 [DOI] [PubMed] [Google Scholar]
- Albadi J., Mansournezhad A. (2016). Aqua-mediated Multicomponent Synthesis of Various 4H-Pyran Derivatives Catalyzed by Poly(4-Vinylpyridine)-Supported Copper Iodide Nanoparticle Catalyst. Res. Chem. Intermed 42, 5739–5752. 10.1007/s11164-015-2400-z [DOI] [Google Scholar]
- Alvey L., Prado S., Saint-Joanis B., Michel S., Koch M., Cole S. T., et al. (2009). Diversity-oriented Synthesis of Furo[3,2-F]chromanes with Antimycobacterial Activity. Eur. J. Med. Chem. 44, 2497–2505. 10.1016/j.ejmech.2009.01.017 [DOI] [PubMed] [Google Scholar]
- Baghbanian S. M., Rezaei N., Tashakkorian H. (2013). Nanozeolite Clinoptilolite as a Highly Efficient Heterogeneous Catalyst for the Synthesis of Various 2-Amino-4h-Chromene Derivatives in Aqueous media. Green. Chem. 15, 3446–3458. 10.1039/C3GC41302K [DOI] [Google Scholar]
- Cai S. X., Drewe J., Kemnitzer W. (2009). Discovery of 4-Aryl-4h-Chromenes as Potent Apoptosis Inducers Using a Cell- and Caspase-Based Anti-cancer Screening Apoptosis Program (ASAP): SAR Studies and the Identification of Novel Vascular Disrupting Agents. Acamc 9, 437–456. 10.2174/1871520610909040437 [DOI] [PubMed] [Google Scholar]
- Capaldo L., Ravelli D. (2017). Hydrogen Atom Transfer (HAT): a Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2056–2071. 10.1002/ejoc.201601485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-N., Di J.-Q., Li J.-M., Mo L.-P., Zhang Z.-H. (2020). Eosin Y-Catalyzed One-Pot Synthesis of spiro[4H-pyran-oxindole] under Visible Light Irradiation. Tetrahedron 76, 131059. 10.1016/j.tet.2020.131059 [DOI] [Google Scholar]
- Datta B., Pasha M. A. (2012). Glycine Catalyzed Convenient Synthesis of 2-Amino-4h-Chromenes in Aqueous Medium under Sonic Condition. Ultrason. Sonochem. 19, 725–728. 10.1016/j.ultsonch.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Dekamin M. G., Eslami M. (2014). Highly Efficient Organocatalytic Synthesis of Diverse and Densely Functionalized 2-Amino-3-Cyano-4h-Pyrans under Mechanochemical ball Milling. Green. Chem. 16, 4914–4921. 10.1039/C4GC00411F [DOI] [Google Scholar]
- Eshtehardian B., Rouhani M., Mirjafary Z. (2020). Green Protocol for Synthesis of MgFe2O4 Nanoparticles and Study of Their Activity as an Efficient Catalyst for the Synthesis of Chromene and Pyran Derivatives under Ultrasound Irradiation. J. Iran. Chem. Soc. 17, 469–481. 10.1007/s13738-019-01783-3 [DOI] [Google Scholar]
- Fan X. Z., Rong J. W., Wu H. L., Zhou Q., Deng H. P., Tan J. D., et al. (2018). Eosin Y as a Direct Hydrogen‐Atom Transfer Photocatalyst for the Functionalization of C−H Bonds. Angew. Chem. Int. Ed. 57, 8514–8518. 10.1002/anie.201803220 [DOI] [PubMed] [Google Scholar]
- Flavin M. T., Rizzo J. D., Khilevich A., Kucherenko A., Sheinkman A. K., Vilaychack V., et al. (1996). Synthesis, Chromatographic Resolution, and Anti-human Immunodeficiency Virus Activity of (±)-Calanolide A and its Enantiomers. J. Med. Chem. 39, 1303–1313. 10.1021/jm950797i [DOI] [PubMed] [Google Scholar]
- Hari D. P., König B. (2014). Synthetic Applications of Eosin Y in Photoredox Catalysis. Chem. Commun. 50, 6688–6699. 10.1039/C4CC00751D [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh-Baghan S., Mirzaei M., Eshtiagh-Hosseini H., Zadsirjan V., Heravi M. M., Mague J. T. (2020). An Inorganic–Organic Hybrid Material Based on a Keggin Type polyoxometalate@Dysprosium as an Effective and green Catalyst in the Synthesis of 2-Amino-4h-Chromenes via Multicomponent Reactions. Appl. Organomet. Chem. 34, e5793–5816. 10.1002/aoc.5793 [DOI] [Google Scholar]
- Huynh T. H. V., Abrahamsen B., Madsen K. K., Gonzalez-Franquesa A., Jensen A. A., Bunch L. (2012). Design, Synthesis and Pharmacological Characterization of Coumarin-Based Fluorescent Analogs of Excitatory Amino Acid Transporter Subtype 1 Selective Inhibitors, UCPH-101 and UCPH-102. Bioorg. Med. Chem. 20, 6831–6839. 10.1016/j.bmc.2012.09.049 [DOI] [PubMed] [Google Scholar]
- Kale S. R., Kahandal S. S., Burange A. S., Gawande M. B., Jayaram R. V. (2013). A Benign Synthesis of 2-Amino-4h-Chromene in Aqueous Medium Using Hydrotalcite (HT) as a Heterogeneous Base Catalyst. Catal. Sci. Technol. 3, 2050–2056. 10.1039/C3CY20856G [DOI] [Google Scholar]
- Kantharaju K., Khatavi S. Y. (2018). Microwave Accelerated Synthesis of 2‐Amino‐4H‐Chromenes Catalyzed by WELFSA: A Green Protocol. ChemistrySelect 3, 5016–5024. 10.1002/slct.201800096 [DOI] [Google Scholar]
- Kathrotiya H. G., Patel M. P. (2012). Microwave-assisted Synthesis of 3′-indolyl Substituted 4H-Chromenes Catalyzed by DMAP and Their Antimicrobial Activity. Med. Chem. Res. 21, 3406–3416. 10.1007/s00044-011-9861-4 [DOI] [Google Scholar]
- Kumar D., Reddy V. B., Sharad S., Dube U., Kapur S. (2009). A Facile One-Pot green Synthesis and Antibacterial Activity of 2-Amino-4h-Pyrans and 2-Amino-5-Oxo-5,6,7,8-Tetrahydro-4h-Chromenes. Eur. J. Med. Chem. 44, 3805–3809. 10.1016/j.ejmech.2009.04.017 [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Mondal J., Bhaumik A. (2013). Tungstic Acid Functionalized Mesoporous SBA-15: A Novel Heterogeneous Catalyst for Facile One-Pot Synthesis of 2-Amino-4h-Chromenes in Aqueous Medium. Dalton Trans. 42, 10515–10524. 10.1039/C3DT50947H [DOI] [PubMed] [Google Scholar]
- Majek M., Filace F., Wangelin A. J. v. (2014). On the Mechanism of Photocatalytic Reactions with Eosin Y. Beilstein J. Org. Chem. 10, 981–989. 10.3762/bjoc.10.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamadpour F. (2021b). A New Role for Photoexcited Na2 Eosin Y as Direct Hydrogen Atom Transfer (HAT) Photocatalyst in Photochemical Synthesis of Dihydropyrano[2,3-C]pyrazole Scaffolds Promoted by Visible Light Irradiation under Air Atmosphere. J. Photochem. Photobiol. A: Chem. 418, 113428. 10.1016/j.jphotochem.2021.113428 [DOI] [Google Scholar]
- Mohamadpour F. (2021a). Catalyst-free, Visible Light Irradiation Promoted Synthesis of Spiroacenaphthylenes and 1H-Pyrazolo[1,2-B]phthalazine-5,10-Diones in Aqueous Ethyl Lactate. J. Photochem. Photobiol. A: Chem. 407, 113041. 10.1016/j.jphotochem.2020.113041 [DOI] [Google Scholar]
- Mohamadpour F., New role for photoexcited organic dye, Na2 eosin Y via the direct hydrogen atom transfer (HAT) process in photochemical visible-light-induced synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b]phthalazine-5,10-diones under air atmosphere, Dyes and Pigments. 194 (2021c) 109628. 10.1016/j.dyepig.2021.109628 [DOI] [Google Scholar]
- Mohamadpour F. (2020). Visible Light Irradiation Promoted Catalyst-free and Solvent-free Synthesis of Pyrano[2,3-D]pyrimidine Scaffolds at Room Temperature. J. Saudi Chem. Soc. 24, 636–641. 10.1016/j.jscs.2020.06.006 [DOI] [Google Scholar]
- Moon D.-O., Kim K.-C., Jin C.-Y., Han M.-H., Park C., Lee K.-J., et al. (2007). Inhibitory Effects of Eicosapentaenoic Acid on Lipopolysaccharide-Induced Activation in BV2 Microglia. Int. Immunopharmacology 7, 222–229. 10.1016/j.intimp.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Narender T., Shweta S., Gupta S. (2004). A Convenient and Biogenetic Type Synthesis of Few Naturally Occurring Chromeno Dihydrochalcones and Their In Vitro Antileishmanial Activity. Bioorg. Med. Chem. Lett. 14, 3913–3916. 10.1016/j.bmcl.2004.05.071 [DOI] [PubMed] [Google Scholar]
- Paliwal P. K., Jetti S. R., Jain S. (2013). Green Approach towards the Facile Synthesis of Dihydropyrano(c)chromene and Pyrano[2,3-D]pyrimidine Derivatives and Their Biological Evaluation. Med. Chem. Res. 22, 2984–2990. 10.1007/s00044-012-0288-3 [DOI] [Google Scholar]
- Pawar G. T., Magar R. R., Lande M. K. (2018). Mesolite: An Efficient Heterogeneous Catalyst for One-Pot Synthesis of 2-Amino-4h-Chromenes. Polycyclic Aromatic Compd. 38, 75–84. 10.1080/10406638.2016.1159584 [DOI] [Google Scholar]
- Raghuvanshi D. S., Singh K. N. (2010). An Expeditious Synthesis of Novel Pyranopyridine Derivatives Involving Chromenes under Controlled Microwave Irradiation. Arkivok 2010, 305–317. 10.3998/ark.5550190.0011.a25 [DOI] [Google Scholar]
- Ravelli D., Protti S., Fagnoni M. (2016). Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 116, 9850–9913. 10.1021/acs.chemrev.5b00662 [DOI] [PubMed] [Google Scholar]
- Romero N. A., Nicewicz D. A. (2016). Organic Photoredox Catalysis. Chem. Rev. 116, 10075–10166. 10.1021/acs.chemrev.6b00057 [DOI] [PubMed] [Google Scholar]
- Rueping M., Sugiono E., Merino E. (2008). Asymmetric Organocatalysis: An Efficient Enantioselective Access to Benzopyranes and Chromenes. Chem. Eur. J. 14, 6329–6332. 10.1002/chem.200800836 [DOI] [PubMed] [Google Scholar]
- Saikia M., Saikia L. (2016). Sulfonic Acid-Functionalized MIL-101(Cr) as a Highly Efficient Heterogeneous Catalyst for One-Pot Synthesis of 2-Amino-4h-Chromenes in Aqueous Medium. RSC Adv. 6, 15846–15853. 10.1039/C5RA28135K [DOI] [Google Scholar]
- Shaikh M. A., Farooqui M., Abed S. (2019). Novel Task-specific Ionic Liquid [Et2NH(CH2)2CO2H][AcO] as a Robust Catalyst for the Efficient Synthesis of Some Pyran-Annulated Scaffolds under Solvent-free Conditions. Res. Chem. Intermed. 45, 1595–1617. 10.1007/s11164-018-3696-2 [DOI] [Google Scholar]
- Symeonidis T., Chamilos M., Hadjipavlou-Litina D. J., Kallitsakis M., Litinas K. E. (2009). Synthesis of Hydroxycoumarins and Hydroxybenzo[f]- or [h]coumarins as Lipid Peroxidation Inhibitors. Bioorg. Med. Chem. Lett. 19, 1139–1142. 10.1016/j.bmcl.2008.12.098 [DOI] [PubMed] [Google Scholar]
- Wang J.-L., Liu D., Zhang Z.-J., Shan S., Han X., Srinivasula S. M., et al. (2000). Structure-based Discovery of an Organic Compound that Binds Bcl-2 Protein and Induces Apoptosis of Tumor Cells. Proc. Natl. Acad. Sci. U.S.A. 97, 7124–7129. 10.1073/pnas.97.13.7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang X., Xia C., Wu L. (2019). Visible-light-promoted Oxidative Dehydrogenation of Hydrazobenzenes and Transfer Hydrogenation of Azobenzenes. Green. Chem. 21, 4189–4193. 10.1039/C9GC01618J [DOI] [Google Scholar]
- Yan D.-M., Chen J.-R., Xiao W.-J. (2019). New Roles for Photoexcited Eosin Y in Photochemical Reactions. Angew. Chem. Int. Ed. 58, 378–380. 10.1002/anie.201811102 [DOI] [PubMed] [Google Scholar]
- Zhao G., Wang T. (2018). Stereoselective Synthesis of 2‐Deoxyglycosides from Glycals by Visible‐Light‐Induced Photoacid Catalysis. Angew. Chem. Int. Ed. 57, 6120–6124. 10.1002/anie.201800909 [DOI] [PubMed] [Google Scholar]
- Zhu J., Cui W.-C., Wang S., Yao Z.-J. (2018). Radical Hydrosilylation of Alkynes Catalyzed by Eosin Y and Thiol under Visible Light Irradiation. Org. Lett. 20, 3174–3178. 10.1021/acs.orglett.8b00909 [DOI] [PubMed] [Google Scholar]
- Zolfigol M. A., Yarie M., Baghery S. (2016). Application of {[4,4′-BPyH][C(CN)3]2} as a Bifunctional Nanostructured Molten Salt Catalyst for the Preparation of 2-Amino-4Hchromene Derivatives under Solvent-free and Benign Conditions. Synlett 27, 1418–1422. 10.1055/s-0035-1561345 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.




