Abstract
Background
Optical coherence tomography angiography (OCTA) is a valuable imaging tool for the diagnosis of several retinal and choroidal diseases. Its role in ocular oncology is clinically promising but still controversial. In this review, we report the main applications and limits of the use of OCTA for the study of intraocular tumors.
Summary
OCTA allows a rapid, safe, low-cost, and high-resolution visualization of the retinal and choroidal vasculature. Attempts have been made to use this technology in ocular oncology to differentiate benign and malignant lesions and to assist physicians in the evaluation and monitoring of post-treatment complications. Main limitations include failure in correct segmentation due to the tumor inner profile or thickness, poor penetration of the laser into the lesion, masking effect from overlying fluid, media opacities and poor fixation.
Key Messages
The main applications of OCTA in ocular oncology consist of the documentation of tumor-associated choroidal neovascularizations and the study of vascular changes following tumor treatments. In particular, the diffusion of wide-field protocols makes OCTA suitable for the diagnosis and follow-up of radiation chorio-retinopathy, allowing a detailed visualization of both macular and peripheral ischemic changes. Optimistically, future innovations in OCTA technology may offer new perspectives in the diagnosis and follow-up of intraocular tumors.
Keywords: Angiography, Intraocular tumors, Optical coherence tomography angiography, Uveal melanoma
Introduction
In recent years, advances in optical coherence tomography (OCT) and, particularly, the introduction of enhanced-depth imaging technique and swept-source (SS) technology has revolutionized the imaging of choroidal tumors [1]. OCT is currently performed on a routine basis in the main ocular oncology facilities, providing valuable information at diagnosis and allowing for a precise, reliable follow-up of both retinal and choroidal tumors.
OCT angiography (OCTA) is a relatively new imaging technology which provides a fast, high-resolution, and depth-resolved visualization of the vascular networks of the eye without the need for dye injection [2]. The availability of SS devices has further improved the visualization of choroidal structures due to longer wavelength (1,050 nm), increased penetration in pigmented structures, and increased scan window depth up to 6 mm in thickness. Although numerous reports have widely documented the feasibility of OCTA in several ocular conditions including vascular disorders [3, 4], age-related, hereditary, and inflammatory retinal diseases, the application of OCTA in ocular oncology is clinically promising but still controversial [5]. In this article, the authors will discuss advantages and disadvantages of OCTA in assessing the most frequent intraocular neoplasms.
Melanocytic Tumors
Since its introduction in the clinical setting, one of the main topics of OCTA in the field of ocular oncology has been the study of melanocytic tumors. In particular, OCTA research has been primary directed to the study of tumor intrinsic microvasculature and secondary retinal alterations.
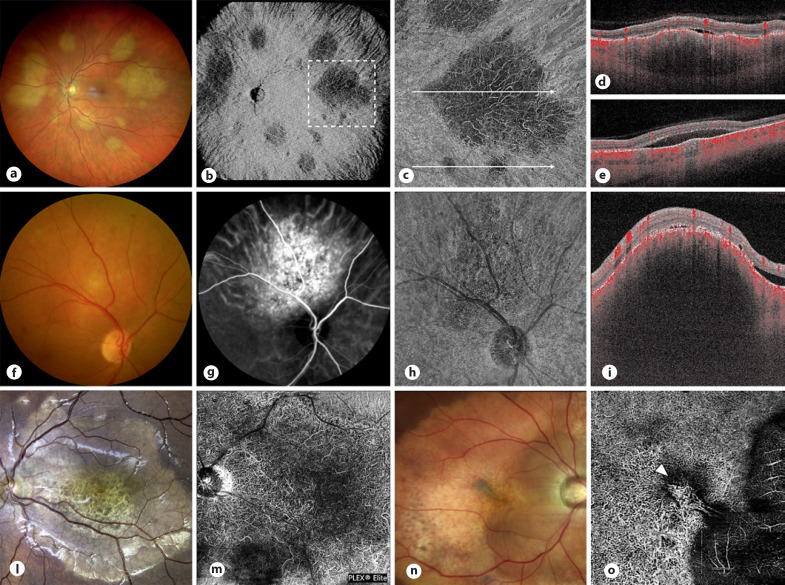
Intrinsic microcirculation in choroidal melanoma has been the focus of several studies and indicated as a possible diagnostic criterion. Folberg et al. [6] in 1992 analyzed the microcirculation of the ciliary body and choroidal melanomas and identified 9 vascular patterns possibly influencing the biological behavior of the tumor. In 1998, Mueller et al. [7] documented the same microvascular patterns using indocyanine green angiography (ICGA). Despite these reports, the role of conventional dye-based imaging techniques has been significantly downsized over the years, and the documentation of intrinsic vascularity in pigmented tumors has never been considered a reliable diagnostic element. Similarly, the first OCTA analyses were limited by several technical issues typical of the spectral domain technology, including a limited penetration into pigmented tissues; limited scan window depth; poor quality of the imaging, secondary to vitreous opacities or subretinal fluid; and segmentation failures. This allowed to study only tumors <2 mm in thickness, with suboptimal visualization of intratumoral vasculature [8]. As a consequence, most of the studies have been focused on changes occurring at the retinal plexi on the apex of the tumor or in the foveal/parafoveal area. In 2017, Shields et al. [9]studied 30 consecutive patients with unilateral melanoma by OCTA and documented a significant reduction in superficial and deep capillary vascular density and enlargement of the deep foveal avascular zone in affected eyes compared with fellow eyes [9, 10]. Other studies focused on the appearance of the choriocapillaris: the main findings were a decreased flow rate in choroidal melanomas compared to choroidal nevi, with nevi also presenting an intense vascular rim [11, 12, 13]. According to Garcia-Arumi Fuste et al. [14], choroidal nevi are hyper-reflective with few avascular areas but distinct margins compared to melanomas, which display ill-defined margins, multiple intrinsic avascular areas, and flow voids at the level of the choriocapillaris (Fig. 1).
Fig. 1.
Fundus photography (a, e, i, o, s) and the corresponding OCTA image of the superficial retinal plexus (b, f, l, p, t), choriocapillaris (c, g, m, q), and avascular zone (u) in 5 eyes with choroidal nevi. Superficial retinal plexus typically looks preserved apart from a possible reduction in vascular density for the thickest tumors or in case of chronic changes occurring at the level of the outer retina or RPE (l). Study of the choriocapillaris may reveal different patterns including a predominant hyporeflective core (flow voids) with a surrounding hyperreflective ring (c, g) or a diffuse hyperreflectivity (q). The increased reflectivity occurring at this level should be considered an artifact rather than a real increase in vascular flow; this phenomenon is mainly secondary to the well-known high reflectivity of choroidal nevi in OCT and is confirmed by the analysis of B-scans with flow overlay (d, h, n, r, v). Differently, in case of RPE atrophic changes (case 2; e–h) and amelanotic/partially pigmented tumors (case 3; i–n), increased light transmission allows for the detection of flow signal within the tumor likely corresponding to normal choroidal vasculature. o–v Two young patients presenting with a choroidal nevus complicated by subretinal fluid: in these cases, OCTA should be used to evidence a type II CNVm (u, v) or to confirm the absence of the CNV (q, r). RPE, retinal pigment epithelium; CNVm, choroidal neovascular membrane.
The introduction of SS-OCTA has later improved the imaging of ocular tumors, offering enhanced penetration into deep tissues and better visualization of the choroid. Also, newest SS-OCTA have allowed for an increased scan window depth and wide-field protocols up to 23 × 20 mm, facilitating the imaging of these lesions. Pellegrini et al. [15] in 2018 studied SS-OCTA feasibility in 22 consecutive choroidal melanomas, ranging from 1.5 to 8.9 mm in thickness, evidencing tumor intrinsic microvasculature in all cases (Fig. 2) [16]. Gunduz et al. [16] showed that choroidal melanoma, in contrast to nevi, was characterized by ill-defined borders, mixed hyperreflective-hyporeflective or hyperreflective internal structure, and demonstrated outer retinal involvement.
Fig. 2.
Fundus photography (a, d, g, l), OCTA (b, e, h, m), and OCT B-scan with an angio overlay (c, f, i, n) in 4 patients presenting with choroidal melanoma. SS-OCTA imaging in en-face visualization successfully reveals tumor-intrinsic microcirculation in a pigmented melanoma with an epicenter on the superotemporal vascular arcade and measuring 4.0 mm in thickness (b), in an 8.9-mm choroidal melanoma with massive exudative retinal detachment (e) and in a 3.3-mm amelanotic melanoma (h). Interestingly, no intrinsic vascularization can be reliably disclosed in the last case (m) despite a tumor measuring only 1.9 mm in thickness, minor subretinal exudation, and good quality of the imaging.
Despite these interesting reports, OCTA assessment of melanocytic tumors and in particular, the analysis of pigmented lesions remains challenging. To date, the diagnosis of uveal melanoma remains mainly clinical and is supported by ocular ultrasound (US); additional imaging including fundus autofluorescence and OCT is useful in documenting intrinsic and surface features and should be performed on a routine basis. In contrast, although SS-OCTA has allowed for a better imaging of melanocytic tumors, so far, it has not improved our diagnostic ability. The documentation of changes in the outer retina and choriocapillaris or the evidence of intrinsic microcirculation patterns by OCTA cannot be considered a reliable sign of malignancy (Fig. 1g, m). For these reasons, the main application of OCTA when studying melanocytic tumors mainly consists of the documentation of secondary choroidal neovascularizations in choroidal nevi, complicated by intra- or subretinal fluid [17] (Fig. 1u), and the study of retinal and choroidal vascular changes following ocular irradiation. The development of CNV secondary to the choroidal nevus is reported to occur in <1% of all choroidal nevi. In a recent analysis, despite the presence of intraretinal or subretinal fluid and hemorrhage, OCTA displayed the CNV network in all cases, whereas fluorescein angiography (FA) and ICGA in 90% and 83%, respectively [17].
Choroidal Metastases
Choroidal metastases represent the most common intraocular malignancy in adults. When presenting as a single lesion, especially in the absence of history of systemic cancer, the diagnosis may be challenging and is typically achieved by a detailed clinical and imaging study including US and OCT. On OCT, choroidal metastases present a typical, almost pathognomonic, undulated, lumpy-bumpy, inner contour, and an intrinsic low reflectivity with diffuse infiltration of the choriocapillaris and choroid [1].
The role of OCTA in visualizing choroidal metastases is controversial with no conclusive, diagnostic criteria published so far. It should be considered that compared to melanocytic tumors, the typical undulated tumor profile makes OCTA analysis even more complex with several segmentation failures. Most of the reports describe the presence of mixed areas of hypo- and hyperreflectivity within the choroid, with variable amount of intrinsic flow voids [11]. The apparent lack of intrinsic blood flow in OCTA has been correlated to either a shadowing artifact from the overlying intra/subretinal fluid or to a high blood flow velocity characterizing the tumor's vessels (fringe effect) [18]. Within the lesions, disorganized intratumoral vasculature showing both thick and fine vessels, anastomoses, and vascular loops have also been reported on SS-OCTA (Fig. 3a-e) [11].
Fig. 3.
Fundus photography (a) and wide-field OCTA (23 × 20 mm with flow analysis at the level of the inner choroid) (b) of the left eye of a 47-year-old female suffering from breast adenocarcinoma complicated by choroidal metastases. c 6 × 6 mm OCTA volume performed temporally to the macula showing a well-defined choroidal mass with diffuse decrease in flow signal secondary to diffuse choroidal infiltration visible also on the B-scan with a flow overlay (d); few vascular structures can be noticed within the lesion. Inferiorly to the main tumor, 2 sub-millimetric metastases can be recognized and corresponding with 2 areas of focal flow void on the B-scan (e). f–i Fundus photography (f) and ICGA (g) in a patient presenting with a CCH. Differently from ICGA where tumor margins can be clearly defined, OCTA typically does not offer a clear visualization of the hemangioma especially on en-face visualization (h); within a region of global hyporeflectivity, few large, dense, vascular trunks can be observed. On the contrary, a B-scan with a flow overlay clearly highlights the presence of preserved flow at the level of the choriocapillaris and inner choroid without the typical compression occurring in melanocytic tumors and metastases. l–o Fundus images (l, n) and OCTA analysis (m, o) of 2 patients suffering of choroidal osteoma; OCTA (m, o) displays tumor intrinsic vasculature in both cases and reveals the presence of a secondary CNVm (o). CNVm, choroidal neovascular membrane.
Circumscribed Choroidal Hemangioma
Secondary to their typical posterior location, their amelanotic appearance, and the notable role of dye-based imaging techniques for confirming their diagnosis (ICGA, in particular, shows a pathognomonic early filling and late washout corresponding to the tumor), circumscribed choroidal hemangiomas (CCHs) have been the focus of several studies since the very first introduction of SD-OCTA devices into clinical practice. According to the literature, on OCTA, CCHs are characterized by large, dense, intertwined vascular trunks, originating from a circular vascular arcade surrounding the lesion and well-defined margins (Fig. 3h) [11]. CCH vascularity may appear hyporeflective, isoreflective, or hyperreflective; in particular, intrinsic hyporeflectivity may be secondary to either a turbulent blood flow within the tumor or to a masking effect from the overlying intra-subretinal fluid [19]. Different from melanocytic tumors and choroidal metastases, on B-scan, with an angio overlay, the flow at the level of the choriocapillaris is usually preserved, and this vascular layer rather appears expanded. The overlying retinal flow is usually normal, but flow voids from retinal edema or schisis may be visible in the retinal deep capillary plexus [20].
So far, the diagnosis of CCH still requires a comprehensive clinical examination including indirect ophthalmoscopy, US and conventional angiography. In particular, the kinetic of tumor filling at ICGA remains essential for both the diagnosis and the planning of the proper treatment. OCTA may be superior to the abovementioned techniques only to document and follow noninvasively retinal, choriocapillaris and choroidal changes after treatment has been performed [21].
Miscellaneous
In the literature, several reports dealing with OCTA imaging of less frequent intraocular tumors can be found [22, 23, 24, 25]. In choroidal osteoma, OCTA has been able to document fine, dense vascular networks within the tumor (Fig. 3l-m); additionally, OCTA may be used to detect complications that may contribute to vision loss such as the development of choroidal neovascular membranes (Fig. 3n-o) [22]. In combined hamartoma of the retina and the retinal pigment epithelium, en-face OCT and OCTA have been used to document that both vascular traction and dragging occur at all retinal levels, leading to a global reduction in vascular density indexes [23]. In retinal capillary hemangioma, OCTA allows for a precise localization of the tumor and finely documents the hypervascularity of the lesion, whereas FA typically suffers from early and pronounced leakage due to the breakdown of the inner blood retinal barrier [24]. In retinal astrocytic hamartoma, OCTA can show ill-defined arborizing tumor vessels in both superficial and deep retinal plexi [25].
Radiation Retinopathy
Radiation retinopathy (RR) is the delayed and most common side effect of ocular radiotherapy. The primary involvement of the vascular structures makes this condition suitable to be studied with OCTA, and the use of OCTA in RR includes the study of both the macular region and the retinal periphery [10, 26]. Interestingly, studies report that in RR, the vascular damage involves both the retina and choroid. For these reasons, OCTA represents the ideal imaging tool for visualizing and studying independently these different vascular structures [26].
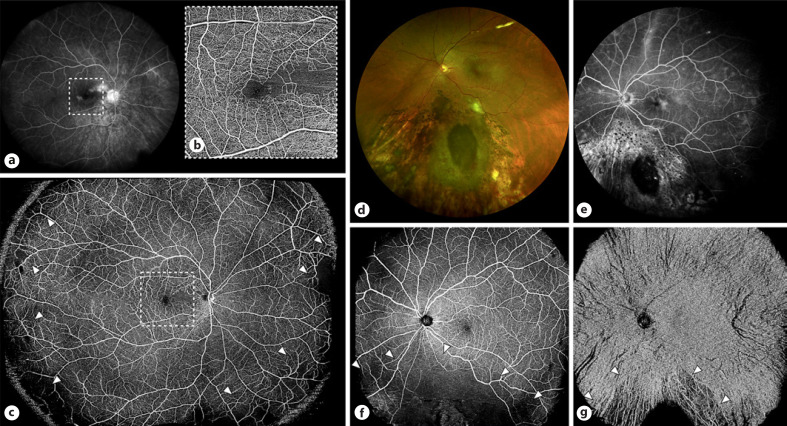
Macular changes occurring in RR are the result of both increased vascular hyperpermeability and capillary nonperfusion. These include retinal hemorrhages, vascular telangiectasias, microaneurysms, and retinal ischemia, finally leading to possible neovascularization. OCTA represents the ideal imaging technique for assessing nonperfusion and vascular remodeling, whereas OCT and FA remain the gold standard to document retinal edema and the breakdown of inner blood retinal barrier. Studies reported an enlargement of the foveal avascular zone and remodeling of both superficial and deep capillary plexi in patients with radiation maculopathy (Fig. 4) [27, 28]. Moreover, a rarefaction of the choriocapillaris can be detected after macular irradiation [29]. Finally, different reports indicate that OCTA can highlight subclinical ischemic changes before the appearance of ophthalmoscopically visible retinopathy [27]. The main limits of OCTA investigation emerge in the presence of severe macular edema, where the fluid may mask the underlying retinal structures and lead to segmentation failures or in patients suffering from poor central fixation [28].
Fig. 4.
a–c FA acquired using the Heidelberg 102° wide-field noncontact lens (a), 6 × 6 OCTA volume centered on the macula (b) and montage of two 23 × 20 mm volumes performed using an SS-OCTA device (c) in a case of a circumpapillary melanoma successfully treated using proton beam radiotherapy. Wide-field FA (a) displays parafoveal leakage, optic nerve staining, and peripheral areas of hyperfluorescence; OCTA (b, c) allows a detailed examination of the foveal and parafoveal region showing diffuse dropout of the superficial retinal capillary plexus nasally to the fovea but also focal areas of retinal-nonperfusion and vascular remodeling (arrowheads) in the extreme nasal, inferior, and temporal periphery (c). d–g Fundus photography (d), wide-field FA (e), and OCTA showing retinal (f) and choriocapillaris/choroidal flow (g), respectively, in a patient previously treated with brachytherapy and sectoral photocoagulation for an inferior choroidal melanoma. OCTA allows separate monitoring of radiation-related changes occurring at the level of the retina and choroid; OCTA scans were in this case acquired using a single 23 × 20 mm volume centered on the fovea.
Along with posterior pole changes, alterations of the peripheral retina are commonly observed in patients with RR [26]. If first devices were indeed characterized by small fields of view (from 2 mm to 9 mm), the introduction of wider protocols and the possibility to increase the field of examination by placing a convex lens of +20.00 diopter between the eye and an OCT probe with the extended field imaging technique have later allowed a better study of the retinal periphery. The great advantage of extended field imaging OCTA is the possibility to study both peripheral retinal and choroidal ischemia, obtaining high quality images with a single fast acquisition [26]. Currently, new wide-field OCTA algorithms available on SS devices can reach 23 mm in field of view and can be successfully performed in most patients regardless of their BCVA.
Conclusion
OCTA represents a valuable imaging tool for diagnosing and monitoring several chorioretinal pathologies. The main advantages consist of the possibility of obtaining a rapid, safe, low-cost, and high-resolution visualization of the retinal and choroidal vasculature with no need of dye injections. If SS technology has provided a better assessment of deep structures, the study of choroidal tumors still finds several technical limitations in the daily clinical setting. Poor penetration of the laser within the tumor, masking effect from overlying fluid and media opacities, failure of correct segmentation due to irregular inner profile, tumor thickness, poor fixation, or peripheral location of the lesion often characterize these exams. For these reasons, in contrast to other subspecialties, OCTA has not yet played a noticeable role in ocular oncology at least from a diagnostic standpoint. Even if the increasing use of SS devices has improved the quality of choroidal imaging, OCTA should be considered as a first-line imaging technique mainly to determine the presence of secondary CNV in small melanocytic tumors with subretinal fluid and to follow up vascular complications of treatments, such as in radiation retinopathy. Hopefully, the continuous improvement of OCTA devices as well as the introduction of new segmentation protocols and algorithms will add new perspectives in a field where conventional angiography has always had minor applications.
Statement of Ethics
The author has no ethical conflicts to disclose. The patients have given their written permission for publication their images.
Conflict of Interest Statement
Giovanni Staurenghi received grants and personal fees from Optovue Inc, Heidelberg Engineering, Zeiss Meditec, Nidek, and CenterVue.
Funding Sources
This study did not receive any funding.
Author Contributions
All the authors conceived the presented idea. M.P. and C.P. wrote the manuscript with the input from all the authors. M.P., G.S., and C.P. contributed substantively to the final paper.
Data Availability Statement
This is a review on imaging techniques. No real data are to be shared except for bibliographic references. Further inquiries can be directed to the corresponding author.
References
- 1.Shields CL, Pellegrini M, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of intraocular tumors: from placid to seasick to rock and rolling topography: the 2013 Francesco Orzalesi Lecture. Retina. 2014;34:1495–512. doi: 10.1097/IAE.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 2.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T, Kakihara S, Toriyama Y, Nittala MG, Murata T, Sadda S. Wide-field en face swept-source optical coherence tomography angiography using extended field imaging in diabetic retinopathy. Br J Ophthalmol. 2017;9:1–5. doi: 10.1136/bjophthalmol-2017-311358. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini M, Cozzi M, Staurenghi G, Corvi F. Comparison of wide field optical coherence tomography angiography with extended field imaging and fluorescein angiography in retinal vascular disorders. PLoS One. 2019;14((4)):e0214892. doi: 10.1371/journal.pone.0214892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naseripour M, Ghasemi Falavarjani K, Mirshahi R, Sedaghat A. Optical coherence tomography angiography (OCTA) applications in ocular oncology. Eye. 2020;34((9)):1535–45. doi: 10.1038/s41433-020-0819-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Peʼer J, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100((9)):1389–98. doi: 10.1016/s0161-6420(93)31470-3. [DOI] [PubMed] [Google Scholar]
- 7.Mueller AJ, Freeman WR, Folberg R, Bartsch DU, Scheider A, Schaller U, et al. Evaluation of microvascularization pattern visibility in human choroidal melanomas: comparison of confocal fluorescein with indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol. 1999 Jun;237((6)):448–56. doi: 10.1007/s004170050260. [DOI] [PubMed] [Google Scholar]
- 8.Maloca P, Gyger C, Hasler PW. A pilot study to image the vascular network of small melanocytic choroidal tumors with speckle noise-free 1050-nm swept source optical coherence tomography (OCT choroidal angiography) Graefes Arch Clin Exp Ophthalmol. 2016;254((6)):1201–10. doi: 10.1007/s00417-015-3259-9. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Say EAT, Ferenczy S, Agni M, Shields CL. Altered parafoveal microvasculature in treatment-naïve choroidal melanoma eyes detected by optical coherence tomography angiography. Retina. 2017;37((1)):32–40. doi: 10.1097/IAE.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Say EAT, Samara WA, Khoo CTL, Mashayekhi A, Shields JA, et al. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma. Comparison of irradiated versus nonirradiated eyes in 65 patients. Retina. 2016;36((8)):1493–505. doi: 10.1097/IAE.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 11.Toledo JJ, Asencio M, García JR, Morales LA, Tomkinson C, Cajigal C. OCT angiography: imaging of choroidal and retinal tumors. Ophthalmol Retina. 2018;2((6)):613–22. doi: 10.1016/j.oret.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Toledo JJ, Asencio-Duran M, García-Martinez JR, López-Gaona A. Use of OCT angiography in choroidal melanocytic tumors. J Ophthalmol. 2017;2017:1573154. doi: 10.1155/2017/1573154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghassemi F, Mirshahi R, Fadakar K, Sabour S. Optical coherence tomography angiography in choroidal melanoma and nevus. Clin Ophthalmol. 2018;12:207–14. doi: 10.2147/OPTH.S148897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Arumi Fuste C, Peralta Iturburu F, Garcia-Arumi J. Is optical coherence tomography angiography helpful in the differential diagnosis of choroidal nevus versus melanoma? Eur J Ophthalmol. 2020;30((4)):723–9. doi: 10.1177/1120672119851768. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrini M, Corvi F, Invernizzi A, Ravera V, Cereda MG, Staurenghi G. Swept-source optical coherence tomography angiography in choroidal melanoma: an analysis of 22 consecutive cases. Retina. 2019;39((8)):1510–9. doi: 10.1097/IAE.0000000000002205. [DOI] [PubMed] [Google Scholar]
- 16.Gunduz AK, Mirzayez I, Kasimoglu R, Ates FSO. Swept-source optical coherence tomography angiography findings in choroidal and retinal tumors. Eye. 2021;35((1)):4–16. doi: 10.1038/s41433-020-01151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrini M, Corvi F, Say EAT, Shields CL, Staurenghi G. Optical coherence tomography angiography features of choroidal neovascularization associated with choroidal nevus. Retina. 2018;38((7)):1338–46. doi: 10.1097/IAE.0000000000001730. [DOI] [PubMed] [Google Scholar]
- 18.Mathis T, Jardel P, Loria O, Delaunay B, Nguyen AM, Lanza F, et al. New concepts in the diagnosis and management of choroidal metastases. Prog Retina Eye Res. 2018;68:144–76. doi: 10.1016/j.preteyeres.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Konana VK, Shanmugam PM, Ramanjulu R, Mishra KCD, Sagar P. Optical coherence tomography angiography features of choroidal hemangioma. Indian J Ophthalmol. 2018;66((4)):581–3. doi: 10.4103/ijo.IJO_955_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takkar B, Azad S, Shakrawal J, Gaur N, Venkatesh P. Blood flow pattern in a choroidal hemangioma imaged on swept-source-optical coherence tomography angiography. Indian J Ophthalmol. 2017;65((11)):1240–2. doi: 10.4103/ijo.IJO_504_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giudice GL, Galan A. Optical coherence tomography angiography of circumscribed choroidal hemangioma treated with photodynamic therapy. Indian J Ophthalmol. 2017;65((10)):1049–51. doi: 10.4103/ijo.IJO_237_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagar P, Shanmugam M, Ramanjulu R, Konana VK. OCT angiography characteristics of choroidal osteoma. Ophthalmol Retina. 2018;2((1)):77–9. doi: 10.1016/j.oret.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Arrigo A, Corbelli E, Aragona E, Manitto MP, Martina E, Bandello F, et al. Optical coherence tomography and optical coherence tomography angiography evaluation of combined hamartoma of the retina and retinal pigment epithelium. Retina. 2019;39:1009–15. doi: 10.1097/IAE.0000000000002053. [DOI] [PubMed] [Google Scholar]
- 24.Thirumalesh MB, Jain A, Agrawal S, Bhujang Shetty K. In vivo microvascular pattern of solitary juxtapapillary capillary hemangioma on OCT angiography. Ophthalmic Surg Lasers Imaging Retina. 2017;48:592–5. doi: 10.3928/23258160-20170630-12. [DOI] [PubMed] [Google Scholar]
- 25.Yung M, Iafe N, Sarraf D. Optical coherence tomography angiography of a retinal astrocytic hamartoma. Can J Ophthalmol. 2016;51:e62–4. doi: 10.1016/j.jcjo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Preziosa C, Corvi F, Staurenghi G, Pellegrini M. Extended field imaging optical coherence tomography angiography for the study of retinal and choroidal changes after radiation therapy for choroidal melanoma: comparison with wide-field angiography. Retina. 2021;41((2)):373–80. doi: 10.1097/IAE.0000000000002848. [DOI] [PubMed] [Google Scholar]
- 27.Say EA, Samara WA, Khoo CT, Magrath GN, Sharma P, Ferenczy S, et al. Parafoveal capillary density after plaque radiotherapy for choroidal melanoma: analysis of eyes without radiation maculopathy. Retina. 2016;36:1670–8. doi: 10.1097/IAE.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 28.Matet A, Daruich A, Zografos L. Radiation maculopathy after proton beam therapy for uveal melanoma: optical coherence tomography angiography alterations influencing visual acuity. Invest Ophthalmol Vis Sci. 2017;58((10)):3851–61. doi: 10.1167/iovs.17-22324. [DOI] [PubMed] [Google Scholar]
- 29.Sellam A, Coscas F, Lumbroso-Le Rouic L, Dendale R, Lupidi M, Coscas G, et al. Optical coherence tomography angiography of macular features after proton beam radiotherapy for small choroidal melanoma. Am J Ophthalmol. 2017;181:12–9. doi: 10.1016/j.ajo.2017.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review on imaging techniques. No real data are to be shared except for bibliographic references. Further inquiries can be directed to the corresponding author.