Abstract
Introduction
Proton beam therapy is an established primary treatment for patients with nonmetastasized uveal melanoma. Adjuvant local interventions, like intravitreal injections or surgery, were shown to improve long-term eye preservation; however, their impact on the patient's quality of life (QOL) remains unknown.
Methods
In a post-radiotherapeutic follow-up, we prospectively collected data on QOL, visual acuity, and interventional adjuvant procedures. QOL was measured with QOL-C30 questionnaire and quality of life questionnaire OPT30 at baseline, and at 3 and 12 months after proton therapy. Patients were grouped by the type of adjuvant treatment. The impact on QOL was analyzed by comparing changes in the mean score values and visual acuity for different interventional subgroups, with generalized linear mixed models and Wilcoxon signed-rank tests.
Results
We received 108 (100%) and 95 (88.0%) questionnaires at 3 and 12 months post-therapy, respectively. Adjuvant interventions included observation (n = 61, 56.5%), intravitreal injections (n = 17, 15.7%), and an intraocular surgical procedure (n = 30, 27.8%). In the latter group, several QOL items significantly declined after the 3-month adjuvant interval, but they partially recovered at the 12-month follow-up. In all adjuvant-intervention groups, global QOL scores returned to baseline levels at 12 months.
Conclusion
Posttreatment adjuvant interventions had no long-lasting effects on QOL in patients with uveal melanoma.
Keywords: Adult ocular oncology, Quality of life, Radiotherapy, Uveal melanoma
Introduction
Uveal melanoma is a rare malignancy that affects 5–8 individuals per million annually [1]. It is the most common primary intraocular malignant disease in adults in the Northern Hemisphere. The established eye-conserving treatment options include brachytherapy, with iodine or ruthenium, proton beam radiotherapy, and photon beam radiotherapy. Treatment selection is based on the tumor's location and prominence and on the availability of treatment modalities.
Several primary treatment methods are associated with low local recurrence. Therefore, treatments for patients with nonmetastasized uveal melanoma aim to provide long-term eye preservation, the best possible visual acuity, and an acceptable quality of life (QOL) [2]. However, eye preservation can often be challenging, particularly for large, highly prominent tumors. Proton therapy (PT) often causes complications, including extensive tumor exudation, retinal detachment, rubeosis iridis, and neovascular glaucoma, which may inevitably lead to secondary enucleation [3, 4, 5, 6, 7, 8]. Different adjuvant treatment strategies have been developed to prevent these complications, and at some institutions, adjuvants have become routine in a multimodal approach.
For most patients with small tumors, which were located centrally or in the mid-periphery, observation with regular follow-up visits is a reasonable approach [9]. In other patients, radiation-induced optic nerve and retinal changes can be reduced with repeated intravitreal injections with corticosteroids or an anti-vascular endothelial growth factor agent [10, 11]. Alternatively, for patients with highly prominent tumors, early adjuvant endoresection or endodrainage can limit gross inflammatory reactions after PT and improve eye preservation rates [6, 7, 12]. However, adjuvant interventions may substantially lower the QOL outcome of radiotherapy for uveal melanoma; thus, adjuvants may outweigh the potential benefits of eye preservation. To our knowledge, no previous study has investigated how different adjuvant interventions after PT might affect patient-reported QOL. The present study aimed to determine how adjuvant strategies applied after PT affected QOL in patients treated for uveal melanoma.
Methods
Patients and Study Design
This prospectively designed QOL study included 131 patients with a primary diagnosis of nonmetastasized uveal melanoma and an indication for proton beam therapy, between May 2019 and January 2020. All patients completed 2 questionnaires from the European Organization for Research and Treatment of Cancer (EORTC). These questionnaires surveyed QOL, in general (quality of life questionnaire (QLQ)-C30), and QOL specifically for patients with uveal melanoma (EORTC-OPT30). The study was approved by the Charité Ethics Committee, and it was conducted in accordance with the Declaration of Helsinki.
Questionnaires were completed immediately after PT (baseline, t1), after 3 months (t2), and at 12 months (t3) after completing PT. To determine the potential impact of different adjuvant interventional strategies on the QOL of our patients, we compared results from the EORTC QLQ-C30 and EORTC-OPT30 questionnaires and from visual acuity tests performed at t1 to the same results collected at t2 and t3. To compare the effects of different adjuvant interventions, we analyzed the following groups: observation group: patients who received no adjuvant treatment; injection group: patients who received an adjuvant intravitreal injection and laser treatment; and surgery group: patients who underwent an adjuvant vitrectomy with endoresection or endodrainage.
Measures
The EORTC QLQ-C30 (version 3.0) is a validated questionnaire and is frequently used in the context of cancer care [13]. It comprises 30 questions and 6 functional scales, covering a wide range of physical and mental symptoms. The EORTC QLQ-OPT30 is an ophthalmologic extension module for the QLQ-C30, designed to assess QOL in patients with ocular tumors. It focuses on visual and ocular symptoms [14]. Both EORTC questionnaires were evaluated with standardized procedures, according to the respective EORTC scoring manuals. Visual acuity was assessed as the best corrected visual acuity. Results are expressed in decimal notation.
Procedures
Proton Beam Therapy
For treatment planning, we performed fundoscopy, ultrasound, CT, and, in some cases, MRI. These data were integrated into a three-dimensional eye model.
In preparation for proton beam therapy, 4 tantalum clips were sutured onto the sclera to ensure an accurate eye position and gaze angle during the irradiation procedure. Immediately before each proton beam session, the correct eye position, according to the treatment plan, was verified with 2-plane X-ray imaging. During treatment, patients were immobilized with a thermoplastic mask and an integrated bite block.
PT was delivered on 4 consecutive days, at fractions of 15 cobalt gray equivalent per day, with a 40- to 60-s beam-on time. Detailed procedures and beam specifications were described previously [15, 16].
Intravitreal Injection and Laser Treatment
Intravitreal injections of corticosteroid (triamcinolone) or anti-vascular endothelial growth factor antibody (bevacizumab) were administered for treating minor subretinal fluid exudations from the tumor. Injections were performed with a standardized procedure, according to national guidelines. Briefly, either 0.1 mL triamcinolone (10 mg/mL) or 0.05 mL bevacizumab (5 mg/0.2 mL) was injected with a 30-g syringe into the vitreous body, at 3.5 mm from the limbus. When residual fluid persisted, the injection could be repeated after an interval of at least 4 weeks.
When intravitreal injections did not suffice, transpupillary thermotherapy was applied to the surface of the tumor to reduce tumor exudation. Briefly, after applying analgesia and local anesthesia, thermotherapy was performed at 810 nm with a diode laser mounted on a slit-lamp biomicroscope. Deeper penetration was achieved with a long exposure time and lower energy settings. The entire tumor surface was covered in an overlapping manner. To treat postradiation peripheral retinal ischemia, we applied retinal laser scatter treatment to the ischemic areas.
Surgical Intervention with Vitrectomy and Endoresection or Endodrainage
Patients with large, highly prominent tumors (i.e., height >6 mm) underwent preplanned endoresections as part of a multimodal treatment. Smaller tumors with excessive exudation received vitrectomy, followed by endodrainage of the subretinal fluid.
Prior to an endoresection, the crystalline lens was removed by phacoemulsification, and the posterior chamber lens was implanted to facilitate the intraocular procedure. After a pars plana vitrectomy, the irradiated tumor tissue was removed with a vitrectome. During resection, the systemic blood pressure was lowered to a mean arterial pressure of 60–65 mm Hg, and the intraocular pressure was elevated to reduce the risk of bleeding complications.
Prior to an endodrainage, a pars plana vitrectomy was performed. Then, a retinal tear was surgically performed in the periphery of the retina, within the 10–2 clock-hour section of the eye. Next, a perfluorocarbon liquid was injected into the vitreous cavity to drive out any fluid from the subretinal space. Finally, the perfluorocarbon liquid was replaced with silicon oil, which was removed after 6 months.
Statistics
Statistical evaluations were performed with R (version 4.1.0). To examine the effects of adjuvant treatments over time, we constructed generalized linear mixed models with a baseline adjustment. Exploratory interaction analyses included a post hoc comparison of mean questionnaire scores recorded at different time points in all subgroups; scores were compared with a paired Wilcoxon signed-rank test and the Bonferroni correction for multiple testing. Changes with p values ≤0.05 were considered significant. Differences between independent subgroups regarding patient and tumor characteristics were analyzed with Kruskal-Wallis and Mann-Whitney U tests, with a significance threshold of p ≤ 0.05.
Results
Study Sample
Of 131 prospectively included participants, 120 (91.6%) returned the questionnaires immediately after proton beam therapy (t1). Three months after proton beam therapy (t2), 108 (82.4%, 50 female) patients completed the follow-up assessment and therefore were included in the analyses. Of these, 95 (88.0%) patients completed the questionnaires at 12 months after PT (t3). During the 12-month follow-up, 1 patient was excluded for an unplanned enucleation; thus, the eye preservation rate was 99.1%. Three patients (2.8%) developed distant metastasis, and no patient experienced local tumor recurrence. The mean time intervals between the end of radiotherapy and questionnaire completion were 98.8 (SD 7.0) days (t2) and 383.4 (SD 12.3) days (t3).
During t2, 61 (56.5%) patients were observed, 17 (15.7%) patients received injections, and 30 (27.8%) patients received intraocular surgery. Overall, 87.0% of the actual adjuvant treatments adhered to the pretreatment tumor board protocol. Adherence rates were 95.1% in the observation group, 64.7% in the injection group, and 83.3% in the surgery group.
Five patients in the injection group received additional transpupillary thermotherapy due to persistent or recurring subretinal fluid. The average time from proton beam therapy to thermotherapy was 6 months. In 1 patient, retinal laser treatment was performed 11 months after proton beam therapy due to radiation-induced retinopathy.
In the surgery group, 23 patients received a pars plana vitrectomy with endoresection of the tumor. Additionally, 7 patients underwent a pars plana vitrectomy with endodrainage of the subretinal fluid.
Among the 108 included patients, the mean age was 59.2 (SD 13.7) years. The subgroups were equivalent with respect to age and sex. Significant differences among subgroups included tumor stage (p ≤ 0.001), tumor volume (p ≤ 0.001), maximal basal diameter (p ≤ 0.001), tumor thickness (p ≤ 0.001), and distance between the tumor and fovea (p ≤ 0.034; Table 1).
Table 1.
Patient and tumor characteristics for patients treated for uveal melanoma, stratified by adjuvant treatment strategy
| Characteristics | All patients | Adjuvant treatment strategy |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| no adjuvant treatment (OS) | intravitreal injection (IN) | endoresection (SU) | |||||||
| Patients, n | 108 | 61 | 56.5% | 17 | 15.7% | 30 | 27.8% | ||
| Male | 52 | 48.1% | 28 | 45.9% | 8 | 47.1% | 16 | 53.3% | 0.797* |
| Female | 56 | 51.9% | 33 | 54.1% | 9 | 52.9% | 14 | 46.7% | |
| Mean age, years | 59.8 | ±13.7 | 59.8 | ±14.1 | 58.5 | ±13.6 | 60.5 | ±13.3 | 0.919 |
| AJCC tumor stages | |||||||||
| T1a/c | 42 | 38.9% | 41 | 67.2% | 1 | 5.9% | 0 | 0.0% | |
| T2a/b | 37 | 34.3% | 18 | 29.5% | 9 | 52.9% | 10 | 33.3% | <0.001* |
| T3a/b | 23 | 21.3% | 2 | 3.3% | 6 | 35.3% | 15 | 50.0% | |
| T4a/b | 6 | 5.6% | 0 | 0.0% | 1 | 5.9% | 5 | 16.7% | |
| Tumor characteristics | |||||||||
| Tumor prominence, mm | 4.4 | ±3.0 | 2.6 | ±1.2 | 4.6 | ±2.2 | 8.1 | ±2.8 | <0.001 |
| Tumor base diameter, mm | 14.8 | ±3.8 | 12.9 | ±3 | 16.7 | ±4.1 | 17.7 | ±2.7 | <0.001 |
| Tumor volume, mm3 | 458 | ±445 | 196 | ±156 | 608 | ±491 | 904 | ±430 | <0.001 |
| Distance between tumor and fovea, mm | 1.9 | ±3.1 | 1.6 | ±3.1 | 1.5 | ±1.8 | 3 | ±3.4 | 0.034 |
| Distance between tumor and optic disc, mm | 2.6 | ±3.3 | 2.3 | ±2.9 | 1.3 | ±1.3 | 4.1 | ±4.4 | 0.090 |
Values are the mean and standard deviation (±SD). AJCC, American Joint Cancer Committee. * Significant differences between independent subgroups based on the Kruskal-Wallis test (p < 0.05). or χ2 test (p < 0.05), as appropriate.
Quality of Life
EORTC QLQ-C30
At t2 and t3, we observed a trend of gradual improvement in global QOL (Table 2) in the observation and injection groups, although the changes did not reach significance. In contrast, in the surgery group, the global QOL declined insignificantly during t1-t2, but then, it significantly improved at t3 (t1 QOL = 68.52 vs. t3 QOL = 72.57, p = 0.018).
Table 2.
Results from the EORTC QLQ evaluated over time in different adjuvant treatment groups
| EORTC QLQ-C30 | Type of adjuvant treatment |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no adjuvant treatment (OS) |
intravitreal injection (IN) |
endoresection/drainage (SU) |
||||||||||
| mean | ±SD | t1:t2 | t2:t3 | mean | ±SD | t1:t2 | t2:t3 | mean | ±SD | t1:t2 | t2:t3 | |
| Global health score | ||||||||||||
| t1 | 68.27 | 20.26 | p = 0.319 | 54.90 | 25.01 | p = 0.061 | 68.52 | 17.35 | p = 0.229 | |||
| t2 | 71.25 | 18.44 | p = 0.495 | 67.22 | 16.80 | p = 0.665 | 64.37 | 17.66 | p = 0.018 | |||
| t3 | 73.21 | 19.12 | 67.86 | 21.40 | 72.57 | 19.58 | ||||||
| Functional scales | ||||||||||||
| Physical functioning | ||||||||||||
| t1 | 88.05 | 18.20 | p = 0.049 | 84.71 | 22.33 | p = 0.526 | 91.67 | 10.28 | p = 0.020 | |||
| t2 | 85.39 | 21.45 | p = 0.041 | 89.17 | 18.68 | p = 0.312 | 86.89 | 13.62 | p = 0.879 | |||
| t3 | 88.81 | 17.76 | 86.67 | 26.41 | 85.56 | 19.23 | ||||||
| Role functioning | ||||||||||||
| t1 | 74.71 | 27.79 | p = 0.954 | 68.63 | 33.27 | p = 0.748 | 80.25 | 24.04 | p = 0.002 | |||
| t2 | 74.32 | 26.45 | p = 0.649 | 73.96 | 20.16 | p = 0.277 | 67.82 | 25.95 | p = 0.183 | |||
| t3 | 76.19 | 26.56 | 65.48 | 25.71 | 71.53 | 25.29 | ||||||
| Emotional functioning | ||||||||||||
| t1 | 71.05 | 22.55 | p = 0.050 | 63.73 | 25.51 | p = 0.365 | 70.99 | 24.61 | p = 0.314 | |||
| t2 | 75.91 | 22.85 | p = 0.730 | 70.56 | 24.57 | p = 0.477 | 69.35 | 18.29 | p = 0.689 | |||
| t3 | 78.17 | 21.41 | 70.63 | 25.73 | 67.59 | 22.91 | ||||||
| Cognitive functioning | ||||||||||||
| t1 | 86.84 | 19.60 | p = 0.403 | 77.45 | 29.43 | p = 0.490 | 83.95 | 17.59 | p = 0.210 | |||
| t2 | 83.06 | 20.30 | p = 0.214 | 77.78 | 22.42 | p = 0.645 | 80.00 | 22.49 | p = 0.202 | |||
| t3 | 82.14 | 20.31 | 80.95 | 15.82 | 77.78 | 17.49 | ||||||
| Social functioning | ||||||||||||
| t1 | 81.58 | 21.75 | p = 0.062 | 74.51 | 29.53 | p = 0.591 | 80.86 | 24.33 | p = 0.146 | |||
| t2 | 86.67 | 22.51 | p = 0.046 | 80.00 | 24.56 | p = 0.833 | 77.01 | 20.61 | p = 0.710 | |||
| t3 | 86.31 | 22.04 | 80.95 | 24.33 | 77.78 | 25.85 | ||||||
| Single item | ||||||||||||
| Pain | ||||||||||||
| t1 | 15.23 | 24.22 | p = 0.229 | 19.61 | 32.93 | p = 0.450 | 9.87 | 15.51 | p = 0.063 | |||
| t2 | 11.20 | 21.88 | p<0.001 | 9.38 | 22.75 | p = 0.045 | 16.11 | 18.82 | p = 0.289 | |||
| t3 | 18.97 | 25.59 | 16.96 | 24.51 | 17.86 | 24.31 | ||||||
Values are the mean scores and standard deviations (SD). t1, immediately after PT; t2,3 months after PT; t3,12 months after PT. Bold font: p value ≤0.05 indicates a significant change between the indicated assessment points, according to the paired Wilcoxon signed-rank test. QLQ, quality of life questionnaire; EORTC, European Organization for Research and Treatment of Cancer; PT, proton therapy.
In the surgery group, the mean scores for all the individual QLQ-C30 functional scales declined during the t1-t2 period. In this group, “physical functioning” (p = 0.020) and “role functioning” (p = 0.002) declined significantly. After these marked declines, neither score recovered fully, although “role functioning” partially recovered at the 12-month follow-up (t1 = 80.25, t2 = 67.82, t3 = 71.53). These declines reflected the substantial impact of intraocular surgery on everyday activities. In the observation and injection groups, “emotional functioning” improved at t2. This change was significant in the observation group (p = 0.05), and it continued to improve at t3. In contrast, the surgery group showed no improvement in “emotional functioning” at t2 or t3.
EORTC QLQ-OPT30
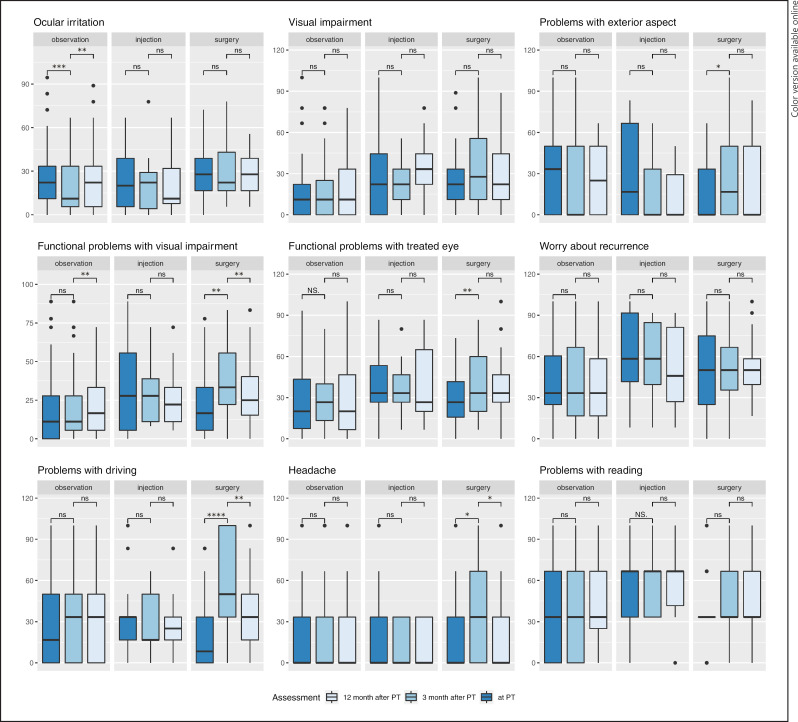
In the observation subgroup, we observed a significant improvement in “ocular irritation” at t2 (p ≤ 0.001, Table 3). In contrast, in the other 2 groups, patients reported only minor, nonsignificant changes in symptoms related to “ocular irritation.” However, at t2, the surgery group reported significant increases in symptoms for several QLQ-OPT30 items, including “problems with exterior aspect” (p = 0.041), “functional problems with visual impairment” (p = 0.002), “functional problems with the treated eye” (p = 0.005), and “problems with driving” (p ≤ 0.001). In contrast, at t2, the injection and observation groups showed no change or improvements in most QLQ-OPT30 items, and the only significant change was in “ocular irritation” in the observation group.
Table 3.
Results from the EORTC QLQ, ophthalmologic module (OPT), evaluated over time in different adjuvant treatment groups
| EORTC QLQ-C30 | Type of adjuvant treatment |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no adjuvant treatment (OS) |
intravitreal injection (IN) |
endoresection/drainage (SU) |
||||||||||
| mean | ±SD | t1:t2 | t2:t3 | mean | ±SD | t1:t2 | t2:t3 | mean | ±SD | t1:t2 | t2:t3 | |
| Functional scales time | ||||||||||||
| Ocular irritation* | ||||||||||||
| t1 | 26.59 | 20.81 | p < 0.001 | 24.05 | 20.43 | p = 0.807 | 28.72 | 16.89 | p = 0.389 | |||
| t2 | 19.34 | 17.75 | p < 0.003 | 21.18 | 20.31 | p = 0.432 | 30.37 | 19.17 | p = 0.201 | |||
| t3 | 24.11 | 21.25 | 20.32 | 19.38 | 26.62 | 13.90 | ||||||
| Visual impairment | ||||||||||||
| t1 | 16.27 | 22.32 | p = 0.741 | 28.76 | 29.15 | p = 0.788 | 25.10 | 25.71 | p = 0.053 | |||
| t2 | 16.67 | 20.71 | p = 0.701 | 24.31 | 16.34 | p = 0.075 | 34.44 | 26.96 | p = 0.079 | |||
| t3 | 17.58 | 23.20 | 35.04 | 23.94 | 29.71 | 22.45 | ||||||
| Worry about recurrence | ||||||||||||
| t1 | 40.77 | 29.72 | p = 0.885 | 61.76 | 30.05 | p = 0.629 | 50.93 | 30.69 | p = 0.585 | |||
| t2 | 40.35 | 28.01 | p = 0.843 | 55.56 | 28.60 | p = 0.506 | 53.06 | 27.81 | p = 0.867 | |||
| t3 | 38.39 | 27.19 | 51.79 | 30.52 | 50.35 | 22.98 | ||||||
| Problems with exterior aspect* | ||||||||||||
| t1 | 29.41 | 28.40 | p = 0.091 | 29.41 | 30.35 | p = 0.246 | 17.28 | 23.33 | p = 0.041 | |||
| t2 | 21.11 | 25.83 | p = 0.121 | 16.67 | 23.57 | p = 0.528 | 26.11 | 27.57 | p = 0.411 | |||
| t3 | 25.60 | 25.42 | 11.90 | 17.82 | 20.83 | 27.47 | ||||||
| Functional problems visual impairment* | ||||||||||||
| t1 | 19.39 | 22.51 | p = 0.481 | 31.05 | 28.94 | p = 0.932 | 24.31 | 22.66 | p = 0.002 | |||
| t2 | 19.76 | 20.60 | p = 0.006 | 29.90 | 19.13 | p = 0.272 | 36.48 | 24.49 | p = 0.009 | |||
| t3 | 22.54 | 21.58 | 26.19 | 20.26 | 29.40 | 22.07 | ||||||
| Functional problems with treated eye** | ||||||||||||
| t1 | 27.67 | 23.08 | p = 1.000 | 39.22 | 24.48 | p = 0.615 | 31.55 | 20.82 | p = 0.005 | |||
| t2 | 27.93 | 19.72 | p = 0.241 | 34.90 | 19.65 | p = 0.263 | 42.41 | 25.49 | p = 0.429 | |||
| t3 | 31.24 | 32.10 | 39.40 | 26.92 | 37.22 | 22.75 | ||||||
| Problems with driving* | ||||||||||||
| t1 | 27.33 | 30.45 | p = 0.473 | 34.44 | 27.07 | p = 0.454 | 21.79 | 27.39 | p < 0.001 | |||
| t2 | 28.95 | 27.19 | p = 0.063 | 28.13 | 21.70 | p = 0.345 | 53.45 | 34.90 | p = 0.009 | |||
| t3 | 32.08 | 29.02 | 30.95 | 26.03 | 35.51 | 29.43 | ||||||
| Single items | ||||||||||||
| Headache* | ||||||||||||
| t1 | 16.07 | 24.61 | p = 0.374 | 21.57 | 33.21 | p = 0.114 | 16.05 | 28.30 | p = 0.037 | |||
| t2 | 18.03 | 29.55 | p = 0.818 | 10.42 | 15.96 | p = 0.336 | 31.03 | 32.04 | p = 0.012 | |||
| t3 | 18.45 | 26.91 | 11.90 | 16.57 | 15.28 | 25.97 | ||||||
| Problems with reading | ||||||||||||
| t1 | 36.31 | 29.32 | p = 0.148 | 56.86 | 30.65 | p =1.000 | 35.71 | 23.88 | p = 0.148 | |||
| t2 | 39.89 | 30.32 | p = 0.897 | 56.86 | 25.72 | p = 0.213 | 40.23 | 27.28 | p = 0.891 | |||
| t3 | 41.67 | 34.38 | 61.90 | 22.81 | 43.06 | 25.02 | ||||||
Values are the mean scores and standard deviations (SD). t1, immediately after PT; t2,3 months after PT; t3,12 months after PT. Bold font indicates a significant change between the indicated assessment points (p value ≤ 0.05), according to the paired Wilcoxon signed-rank test. QLQ, quality of life questionnaire; EORTC, European Organization for Research and Treatment of Cancer; PT, proton therapy. * p ≤ 0.05 and ** p ≤ 0.1 indicate a significant interaction between the treatment type and time, according to a mixed generalized linear model.
At t3, the surgery group showed improvements in all QLQ-OPT30 items, with significant recoveries noted in “functional problems with visual impairment” (p = 0.009), “problems with driving” (p = 0.009), and “headache” (p = 0.012). At the same time, the observation and injection groups showed a trend of worsening ocular symptoms. Only the observation group showed significant deteriorations at t3 in “ocular irritation” (p = 0.003) and “functional problems with visual impairment” (p = 0.006).
Correlations between the QOL Outcome and Baseline (t1) Tumor Characteristics
Immediately after PT (t1), no tumor characteristics were significantly correlated to the QOL items on the QLQ-C30 except one: a short distance between the tumor and fovea was correlated to the global QOL (p = 0.013) and “emotional functioning” (p = 0.036). However, the baseline QLQ-OPT30 showed significant correlations between tumor prominence and “visual impairment” (p = 0.04), “functional problems with visual impairment” (p = 0.021), and “functional problems with the treated eye” (p = 0.013) (Fig. 1). In addition, tumor volume was significantly correlated with “functional problems with the treated eye” (p = 0.013). No tumor characteristics were significantly correlated with “ocular irritation,” “headache,” “problems with reading,” “problems with exterior aspect,” or “problems with driving.”
Fig. 1.
Mean score values for the multi-item scales and single items of the European Organization for Research and Treatment of Cancer Quality of Life ophthalmologic cancer module (EORTC QLQ-OPT30). Bars show values recorded directly after PT (dark blue), 3 months after PT (medium blue), and 12 months after PT (light blue), grouped by the adjuvant treatment type. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001 indicate significant changes between assessments, detected with the paired Wilcoxon signed-rank test. ns, not significant; PT, proton therapy; EORTC, European Organization for Research and Treatment of Cancer; QLQ, quality of life questionnaire.
Visual Acuity
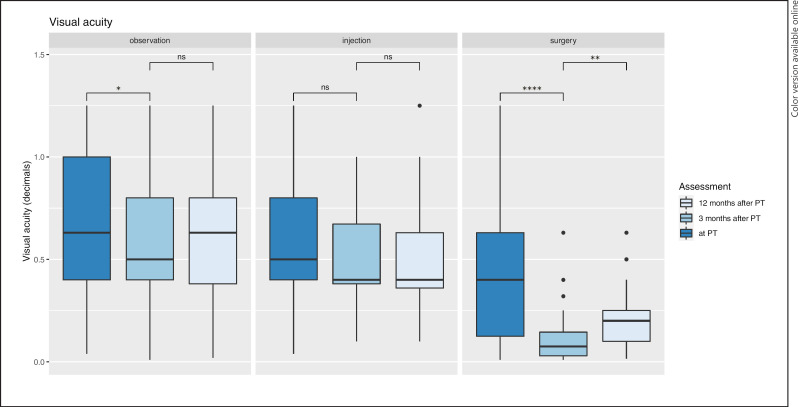
In the observation group, the median visual acuity declined from 0.63 (SD 0.38) at baseline to 0.50 (SD 0.34) at t2 (p = 0.020) and then recovered to 0.63 (SD 0.35) at t3. In the surgery group, visual acuity significantly deteriorated, from 0.4 at baseline (SD 0.34) to 0.08 (SD 0.15) at t2 (p ≤ 0.001), and then, it subsequently improved to 0.20 (SD 0.16) at t3 (p = 0.003). In the injection group, visual acuity did not significantly change over time (Fig. 2).
Fig. 2.
Visual acuity in patients with uveal melanoma treated with PT, with or without a subsequent adjuvant intervention. Bars show values recorded directly after PT (dark blue), 3 months after PT (medium blue), and 12 months after PT (light blue), grouped by the adjuvant treatment type. A mixed generalized linear model showed a significant interaction between the treatment type and time (p = 0.018). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001 indicate significant changes between assessments, detected with the paired Wilcoxon signed-rank test. ns, not significant; PT, proton therapy.
Visual acuity was significantly correlated with worsening QOL in several functional subscales. At t2, worse visual acuity was associated with declines in “role functioning” (p = 0.009), “visual impairment” (p ≤ 0.001), “functional problems with visual impairment” (p ≤ 0.001), “functional problems with the treated eye” (p = 0.005), and “problems with driving” (p = 0.004).
Discussion
The present study revealed that all patients had stable global health scores in the short-term after proton beam therapy, regardless of the adjuvant treatment strategy. A direct comparison of the QOL outcome between the subgroups was not feasible because the baseline QOL results clearly reflected a selection bias for adjuvant treatments. Indeed, the adjuvant treatment strategies proposed by the local tumor board were based on significantly different tumor characteristics and tumor stages among the 3 subgroups. Therefore, we analyzed longitudinal changes in QOL for each subgroup by comparing QOL questionnaire results collected immediately after the radiotherapy to those collected at 3 and 12 months.
We observed a nonsignificant trend in improved global QOL at t2 in the observation group, which was not observed in the surgery subgroup. This trend could be essentially explained by recoveries in the emotional (p = 0.05) and social functioning (p = 0.062) subscales after the initial treatment. At the same time, physical functioning declined in the observation group (p = 0.049), which could be explained by the deterioration in visual acuity. These results were consistent with observations in several previous studies on the general QOL of patients with uveal melanoma after various primary treatments [17, 18, 19, 20]. For example, Suchocka-Capuano et al. [19] and Brandberg et al. [17] found that plaque therapy or enucleation had an overall low impact on the general QOL at 1 year after treatment, and anxiety symptoms declined within 2 months after treatment. However, our results contrasted with those of Suchocka-Capuano et al. [19], who described a decline in “social functioning” during a 12-month follow-up.
Our findings on social functioning in the observation group may reflect a faster reintegration into their social life due to less hospitalization and better outcomes in “problems with exterior aspect” (p = 0.091) and “ocular irritation” (p ≤ 0.001). Interestingly, “worry about recurrence” did not significantly improve during the follow-up, regardless of the adjuvant treatment. In contrast, previous studies consistently reported improvements in anxiety issues for patients with uveal melanoma within the 1st 2 years after a primary treatment, compared to pretreatment assessments [19, 21, 22]. However, another study found that anxiety receded, to a large extent, immediately after PT and remained relatively stable thereafter [23].
We observed a similar trend of improved global QOL in the injection subgroup. However, we did not detect any significant changes in the EORTC QLQ-C30 subscales, in the QLQ-OPT30 subscales or in visual acuity.
As expected, the surgery group showed the worst QOL outcome, in terms of “physical functioning” (p < 0.001) and “role functioning” (p < 0.001) at t2 compared to t1. In previous studies without pretreatment assessments, these early changes were attributed to the more advanced tumor stages in this group, or they were considered early radiation side effects. Indeed, our surgery group showed a significant recovery of global QOL (p = 0.018) at t3, which supported the notion that major parts of the early transient QOL decline could be related to the type of adjuvant treatment.
In the surgery subgroup, the most significant deteriorations at t2 reflected the functional aspects of ocular impairment, like “functional problems with visual impairment” (p = 0.002), “functional problems with the treated eye” (p = 0.005), and “problems with driving” (p < 0.001). Importantly, all these ocular deteriorations recovered at t3 in the surgery subgroup. Significant recoveries were observed in “functional problems with visual impairment” (p = 0.002) and “problems with driving” (p = 0.009). These characteristic changes were clearly related to the adjuvant treatment period, and they were only observed in the surgery group. Therefore, these changes most likely reflected the impact of the surgical interventions on QOL issues and visual acuity.
Previous studies reported improved eye preservation rates in patients with large tumors after an adjuvant endoresection compared to an observational control group [7]. However, it remains unclear whether the QOL in those patients was related to successful eye preservation alone or whether the QOL might also have been influenced by additional morbidities associated with the adjuvant surgical treatment. Several authors reported that QOL was similar between patients who underwent enucleation and patients who underwent eye-preserving therapy [17, 18, 21, 24, 25, 26]. In a large study with 1,596 patients, Damato et al. found that QOL outcomes were worse after primary enucleation than after radiotherapy [26]. A more detailed look at those data revealed that patients who underwent enucleation were older and had more comorbidities than patients who received radiotherapy; thus, these differences might have influenced the results.
Study Strengths and Limitations
The present study strengths were the prospectively collected data and the longitudinal design, which allowed comparisons between subgroups with very different tumor characteristics. Another strength was the high adherence to adjuvant strategies planned by the pretreatment tumor board protocol. This adherence minimized confounding factors related to ad hoc interventions that are occasionally performed during the adjuvant period.
Our study also had some limitations. The overall follow-up time was relatively short; thus, it mainly reflected transient ocular symptoms associated with adjuvant interventions. However, long-term studies run the risk that these side effects might be confounded with evolving radiation-induced late toxicity. Consequently, our findings may be limited in portraying the long-term impact of adjuvant interventions on the QOL of patients treated for uveal melanoma. In addition, our adjuvant treatment subgroups varied considerably in size. In particular, the injection subgroup only included 17 patients; therefore, the results for that subgroup should be interpreted with caution.
Conclusion
The present study showed that, after 12 months, the global QOL may be unaffected, regardless of the adjuvant therapy applied. Nevertheless, patients and treating physicians should be aware that adjuvant surgical interventions may compromise the ophthalmological QOL of the patient. Our findings may inform patients and treating physicians in making individually tailored decisions, when selecting the appropriate primary treatment and consecutive interventional strategies.
Statement of Ethics
This study protocol was reviewed and approved by the Charité Ethics Committee, approval number EA4-031-19. Written informed consent was obtained from all participants to participate in the study.
Conflict of Interest Statement
Johannes Gollrad was involved in scientific presentations at symposia for Varian Medical Systems International AG in 2019 and 2020, with no conflict of interest to the actual manuscript. Christopher Rabsahl, Andrea Stroux, and Alexander Böker have no conflict of interest to declare. Antonia M. Joussen, Volker Budach, and Dirk Böhmer have no conflict of interest to declare related to the manuscript.
Funding Sources
There was no funding for this study.
Author Contributions
Johannes Gollrad made substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work; drafting the work; and revising it critically for important intellectual content. Christopher Rabsahl made substantial contributions to the acquisition and analysis of data for the work and drafting the work. Antonia M. Joussen made substantial contributions to the interpretation of data for the work and revising it critically for important intellectual content. Andrea Stroux made substantial contributions to analysis and interpretation of data for the work and revising it critically for important intellectual content. Volker Budach made substantial contributions to the conception of the work and revising it critically for important intellectual content. Dirk Böhmer made substantial contributions to the conception of the work and revising it critically for important intellectual content. Alexander Böker made substantial contributions to the design of the work, the analysis and interpretation of data for the work, drafting the work, and revising it critically for important intellectual content.
Data Availability Statement
The data that support the findings of this study are not publicly available due to concerns related that could compromise the privacy of research participants but are available from the corresponding author A.B. upon reasonable request.
References
- 1.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007 Dec;114((12)):2309–15. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Verma V, Mehta MP. Clinical outcomes of proton radiotherapy for uveal melanoma. Clin Oncol. 2016 Aug;28((8)):e17–27. doi: 10.1016/j.clon.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Damato B, Groenewald C, McGalliard J, Wong D. Endoresection of choroidal melanoma. Br J Ophthalmol. 1998 Mar;82((3)):213–8. doi: 10.1136/bjo.82.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechrakis NE, Petousis V, Krause L, Wachtlin J, Willerding G, Foerster MH. [Surgical treatment modalities in uveal melanomas] Klin Monbl Augenheilkd. 2009 Nov;226((11)):921–6. doi: 10.1055/s-0028-1109458. [DOI] [PubMed] [Google Scholar]
- 5.Ly LV, Bronkhorst IH, van Beelen E, Vrolijk J, Taylor AW, Versluis M, et al. Inflammatory cytokines in eyes with uveal melanoma and relation with macrophage infiltration. Invest Ophthalmol Vis Sci. 2010 Nov;51((11)):5445–51. doi: 10.1167/iovs.10-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seibel I, Cordini D, Willerding G, Riechardt AI, Joussen AM. Endodrainage, tumor photocoagulation, and silicone oil tamponade for primary exudative retinal detachment due to choroidal melanoma persisting after proton beam therapy. Ocul Oncol Pathol. 2014 Oct;1((1)):24–33. doi: 10.1159/000365333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibel I, Riechardt AI, Heufelder J, Cordini D, Joussen AM. Adjuvant ab interno tumor treatment after proton beam irradiation. Am J Ophthalmol. 2017 Jun;178:94–100. doi: 10.1016/j.ajo.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Boker A, Pilger D, Cordini D, Seibel I, Riechardt AI, Joussen AM, et al. Neoadjuvant proton beam irradiation vs. adjuvant ruthenium brachytherapy in transscleral resection of uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2018 Sep;256((9)):1767–75. doi: 10.1007/s00417-018-4032-7. [DOI] [PubMed] [Google Scholar]
- 9.Eckstein D, Riechardt AI, Heufelder J, Zeitz O, Boker A, Brockmann C, et al. Radiation-induced optic neuropathy: observation versus intravitreal treatment: can visual acuity be maintained by intravitreal treatment? Am J Ophthalmol. 2019 Dec;208:289–94. doi: 10.1016/j.ajo.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Chang M, Dalvin LA, Mazloumi M, Martin A, Yaghy A, Yang X, et al. Prophylactic intravitreal bevacizumab after plaque radiotherapy for uveal melanoma: analysis of visual acuity, tumor response, and radiation complications in 1131 eyes based on patient age. Asia Pac J Ophthalmol. 2020 Jan-Feb;9((1)):29–38. doi: 10.1097/APO.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seibel I, Vollhardt D, Riechardt AI, Rehak M, Schmied S, Schiller P, et al. Influence of ranibizumab versus laser photocoagulation on radiation retinopathy (RadiRet): a prospective randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2020 Apr;258((4)):869–78. doi: 10.1007/s00417-020-04618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bechrakis NE, Höcht S, Martus P, Kreusel KM, Heese J, Foerster MH. [Endoresection following proton beam irradiation of large uveal melanomas] Ophthalmologe. 2004 Apr;101((4)):370–6. doi: 10.1007/s00347-003-0911-2. [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993 Mar 3;85((5)):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 14.Brandberg Y, Damato B, Kivela T, Kock E, Seregard S, Force EOOT, et al. The EORTC ophthalmic oncology quality of life questionnaire module (EORTC QLQ-OPT30). Development and pre-testing (Phase I-III) Eye. 2004 Mar;18((3)):283–9. doi: 10.1038/sj.eye.6700639. [DOI] [PubMed] [Google Scholar]
- 15.Hocht S, Bechrakis NE, Nausner M, Kreusel KM, Kluge H, Heese J, et al. Proton therapy of uveal melanomas in Berlin. 5 years of experience at the Hahn-Meitner Institute. Strahlenther Onkol. 2004 Jul;180((7)):419–24. doi: 10.1007/s00066-004-1222-5. [DOI] [PubMed] [Google Scholar]
- 16.Marnitz S, Cordini D, Bendl R, Lemke AJ, Heufelder J, Simiantonakis I, et al. Proton therapy of uveal melanomas: intercomparison of MRI-based and conventional treatment planning. Strahlenther Onkol. 2006 Jul;182((7)):395–9. doi: 10.1007/s00066-006-1512-1. [DOI] [PubMed] [Google Scholar]
- 17.Brandberg Y, Kock E, Oskar K, af Trampe E, Seregard S. Psychological reactions and quality of life in patients with posterior uveal melanoma treated with ruthenium plaque therapy or enucleation: a one year follow-up study. Eye. 2000 Dec;14((Pt 6)):839–46. doi: 10.1038/eye.2000.233. [DOI] [PubMed] [Google Scholar]
- 18.Melia M, Moy CS, Reynolds SM, Hayman JA, Murray TG, Hovland KR, et al. Quality of life after iodine 125 brachytherapy vs enucleation for choroidal melanoma: 5-year results from the Collaborative Ocular Melanoma Study: COMS QOLS Report No. 3. Arch Ophthalmol. 2006 Feb;124((2)):226–38. doi: 10.1001/archopht.124.2.226. [DOI] [PubMed] [Google Scholar]
- 19.Suchocka-Capuano A, Bredart A, Dolbeault S, Rouic LL, Levy-Gabriel C, Desjardins L, et al. [Quality of life and psychological state in patients with choroidal melanoma: longitudinal study] Bull Cancer. 2011 Feb;98((2)):97–107. doi: 10.1684/bdc.2011.1300. [DOI] [PubMed] [Google Scholar]
- 20.van Beek JGM, Buitendijk GHS, Timman R, Muller K, Luyten GPM, Paridaens D, et al. Quality of life: fractionated stereotactic radiotherapy versus enucleation treatment in uveal melanoma patients. Acta Ophthalmol. 2018 Dec;96((8)):841–8. doi: 10.1111/aos.13823. [DOI] [PubMed] [Google Scholar]
- 21.Hope-Stone L, Brown SL, Heimann H, Damato B. Comparison between patient-reported outcomes after enucleation and proton beam radiotherapy for uveal melanomas: a 2-year cohort study. Eye. 2019 Sep;33((9)):1478–84. doi: 10.1038/s41433-019-0440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scannell O, OʼNeill V, Dunne M, Baily C, Salih A, Cunningham M, et al. Quality of life in uveal melanoma patients in Ireland: a single-centre survey. Ocul Oncol Pathol. 2020 Mar;6((2)):99–106. doi: 10.1159/000501692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollrad J, Rabsahl C, Riechardt AI, Heufelder J, Stroux A, Goerling U, et al. Quality of life and treatment-related burden during ocular proton therapy: a prospective trial of 131 patients with uveal melanoma. Radiat Oncol. 2021 Sep 8;16((1)):174. doi: 10.1186/s13014-021-01902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chabert S, Velikay-Parel M, Zehetmayer M. Influence of uveal melanoma therapy on patientsʼ quality of life: a psychological study. Acta Ophthalmol Scand. 2004 Feb;82((1)):25–31. doi: 10.1046/j.1600-0420.2003.0210.x. [DOI] [PubMed] [Google Scholar]
- 25.Amaro TA, Yazigi L, Erwenne C. Depression and quality of life during treatment of ocular bulb removal in individuals with uveal melanoma. Eur J Cancer Care. 2010 Jul;19((4)):476–81. doi: 10.1111/j.1365-2354.2009.01073.x. [DOI] [PubMed] [Google Scholar]
- 26.Damato B, Hope-Stone L, Cooper B, Brown SL, Salmon P, Heimann H, et al. Patient-reported outcomes and quality of life after treatment of choroidal melanoma: a comparison of enucleation versus radiotherapy in 1596 patients. Am J Ophthalmol. 2018 Sep;193:230–51. doi: 10.1016/j.ajo.2018.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to concerns related that could compromise the privacy of research participants but are available from the corresponding author A.B. upon reasonable request.