Abstract
Growth of the chitin-degrading marine bacterium S91 on solid surfaces under oligotrophic conditions was accompanied by the displacement of a large fraction of the surface-derived bacterial production into the flowing bulk aqueous phase, irrespective of the value of the surface as a nutrient source. Over a 200-h period of surface colonization, 97 and 75% of the bacterial biomass generated on biodegradable chitin and a nonnutritional silicon surface, respectively, detached to become part of the free-living population in the bulk aqueous phase. Specific surface-associated growth rates that included the cells that subsequently detached from the substrata varied depending on the nutritional value of the substratum and during the period of surface colonization. Specific growth rates of 3.79 and 2.83 day−1 were obtained when cells first began to proliferate on a pure chitin film and a silicon surface, respectively. Later, when cell densities on the surface and detached cells as CFU in the bulk aqueous phase achieved a quasi-steady state, specific growth rates decreased to 1.08 and 0.79 day−1 on the chitin and silicon surfaces, respectively. Virtually all of the cells that detached from either the chitin or the silicon surfaces and the majority of cells associated with the chitin surface over the 200-h period of surface colonization displayed no detectable expression of the chitin-degrading genes chiA and chiB. Cells displaying high levels of chiA-chiB expression were detected only on the chitin surface and then only clustered in discrete areas of the surface. Surface-associated, differential gene expression and displacement of bacterial production from surfaces represent adaptations at the population level that promote efficient utilization of limited resources and dispersal of progeny to maximize access to new sources of energy and maintenance of the population.
In the marine environment, hydrolysis of particulate organic matter (POM) to low-molecular-weight dissolved organic matter (DOM) is mediated primarily by ectohydrolytic enzymes produced by particle-associated bacteria (25). While some of the DOM derived from POM hydrolysis is respired as CO2, a portion is used for new bacterial production (BP) (53). It has also been hypothesized that a significant portion of the DOM derived from enzymatic attack of POM by POM-associated bacteria supports maintenance and reproduction of free-living bacteria in the pelagic marine environment (3, 9). In fact, POM-associated bacteria are thought to provide more DOM for production of the free-living bacterial populations than for production of POM-associated populations (4, 20, 45, 51). However, detachment of POM-derived cells could lead to overestimation of dissolved organic carbon-derived, free-living BP. Jacobsen and Azam (23) reported that bacteria associated with copepod fecal pellets were displaced into the surrounding water during fecal pellet degradation. While these researchers recognized the potential importance of detachment as a process that contributes to free-living BP, the magnitude of this process is currently unknown for the pelagic marine environment.
In the pelagic marine environment, bacterial degradation of detrital POM is carried out under conditions of constant flow as the detrital POM sinks through the water column (1). The DOM generated by ectoenzymatic attack of POM that is displaced into the surrounding seawater is quickly diluted as a result of particle sinking. Thus, free-living bacteria in the bulk seawater are likely to encounter this DOM at much lower concentrations than are cells that remain associated with the POM surface. The extent to which this influences bacterial growth and production in these different compartments of the water column is not clearly understood.
In order to gain a better understanding of the relative importance of free-living and particle-associated populations to total BP and how particle-associated BP changes during POM degradation, it is necessary to determine the extent of bacterial detachment from POM during the degradation process. Bacterial cells produced on POM that subsequently detach and become part of the free-living population must be distinguished from BP contributed by the free-living bacterial population growing on the POM-derived DOM displaced into the bulk aqueous phase.
In this investigation, solid chitin, an important form of POM in the pelagic marine environment (18), was used as the primary source of carbon, nitrogen, and energy in a system designed to study particle-associated BP. Chitin is a high-molecular-weight biopolymer of β-1,4-linked N-acetyl-d-glucosamine and is utilized by organisms that produce extracellular chitinase enzymes. Bacteria are the principal mediators of chitin degradation (18), and since chitin is an insoluble biopolymer, the bacterial populations that contribute to the dissolution of this material are primarily surface associated. The chitin-degrading marine bacterium Pseudoalteromonas strain S91 was employed in this study to determine surface-associated BP from chitin degradation. In order to determine which cells were involved in chitin degradation, a strain of S91 that produces green fluorescent protein (GFP) when chitin-degrading genes are expressed was used (6). This approach permitted the determination of POM-associated BP and the fate of the surface-associated bacterial population during the degradation of chitin. It also permitted the establishment of the relationship between BP and chitinase gene expression within the surface-associated cell population.
MATERIALS AND METHODS
Bacterial strains and media.
The study employed Pseudoalteromonas sp. strain S91, which was constructed to report the expression of chitinase genes through fluorescence from GFP during the degradation of solid chitin. Strain S91 was derived from the wild-type strain S9, described previously (22). Strain S91 contained the pDSK519 plasmid carrying a complete chitinase gene (chiA) and a truncated chitin binding gene (chiB) interrupted by the insertion of a functional copy of the GFP gene (gfp) (26). In this configuration, the gfp gene is under the control of both the chiA and chiB promoters. A set of fully functional chiA-chiB genes resides on the chromosome (47, 48, 49). The plasmid confers kanamycin resistance on strain S91, which is otherwise resistant to streptomycin.
Bacterial cultivation.
Cultures of S91 were prepared as described previously (6). Cells were cultured in defined seawater solution containing N-acetyl-β-d-glucosamine (GlcNAc) as the sole carbon, nitrogen, and energy source when up-expression of genes involved in chitin degradation was desired (6). Starved-cell suspensions were prepared from glutamate-grown cultures (6). CFU were monitored daily during the 400-h starvation period by plate counts on MB2216 agar (Difco, Detroit, Mich.) after incubation of plates for 24 h at 20°C.
Substratum preparation.
Thin films of pure chitin served as the only added sources of carbon, nitrogen, and energy during chitin degradation. Silicon coupons, 1 by 1 cm square and 1.5 mm thick (Harrick Scientific, Ossining, N.Y.), were used as nonnutritional controls. The methods used in the preparation of pure chitin thin films were adapted from previously published methods for spin-casting chitin films from chitosan solutions (5, 6, 38, 40). Details of preparation and characteristics of the chitin thin films used in this study are described elsewhere (6). The pen from a Loligo species of squid was used as a substratum in studies to compare the behavior of bacterial cells on chitin thin films with the behavior of cells on a natural form of chitin. Squid pen chitin consists of approximately 40% chitin and 60% protein (19). Squid pens were dissected and stored at −40°C. Prior to use, the squid pens were sterilized by autoclaving in defined seawater solution, cut into 1- by 1-cm squares, and rinsed in 70% ethanol before use.
Laminar flow cell (LFC) preparation.
An LFC was used to observe bacterial attachment, reproduction, and detachment during chitin degradation during exposure to constantly flowing defined seawater solution as described previously (6). Three separate LFCs were used to monitor surface-associated bacterial activity. The first LFC contained two silicon coupons with spun-cast chitin thin films prepared as described previously (6). This LFC was used to evaluate bacterial activity during the degradation of pure chitin. The second LFC contained two clean silicon coupons, which served as nonnutritional control surfaces. The third LFC contained two 1-cm2 strips of natural squid pen chitin in place of the silicon coupons. The LFCs were set up as previously described (6). The strict cleaning procedure described previously (6) was used for all containers and surfaces to which the bulk aqueous phase was exposed in order to minimize the introduction of chemical contaminants that might support bacterial growth.
LFC operation.
The LFCs containing the bare silicon and chitin-coated silicon coupons were inoculated with the 400-h, starved-cell suspension (3.2 × 106 CFU ml−1) from the continuously stirred tank reactor (CSTR) to promote bacterial colonization of the coupon surfaces as described previously (6). A second 400-h starved-cell suspension (2.3 × 106 CFU ml−1) in another CSTR was used to inoculate the LFC containing squid pen chitin. All three LFCs were inoculated for a period of 1 h at a flow rate of 0.5 ml min−1. After inoculation, the LFCs were fed sterile defined seawater solution containing kanamycin and streptomycin but no added carbon, nitrogen, or energy sources equilibrated to 18°C at a continuous flow rate of 0.5 ml min−1 for an additional 199 h as described previously (6). This flow rate mimics the conditions at the surface of a particle that is sinking through the water column of the ocean (1). At this flow rate, the residence time of the aqueous phase in the LFC was 55 s. Since this residence time is much less than the generation time of S91, growth of free-living cells in the bulk aqueous phase during the time that it passed through the LFC was insignificant.
Flow cell hydrodynamics.
The average fluid velocity and Reynolds number (Re) experienced by the attached bacteria were calculated from the fluid flow rate and LFC geometry. Re was calculated based on the relationship described in equation 1,
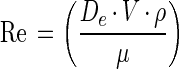 |
1 |
where De is a characteristic length that depends on the system geometry, V is the fluid velocity, ρ is the density of the fluid, and μ is the absolute viscosity (31). De was calculated based on the equation for a full-flowing rectangular conduit in cross section. The density and kinematic viscosity of the defined seawater solution were used to calculate the absolute viscosity (μ) using equation 2,
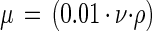 |
2 |
where ν is the kinematic viscosity in centipoise and ρ is the density in grams per cubic centimeter (55).
The shear stress at the point of cell attachment was calculated using the equation for parabolic flow velocity between two plates as described by equation 3,
 |
3 |
where x is the distance from the centerline of the flow cell in centimeters and y is 1/2 the flow channel height in centimeters (43). The shear stress (τ) was calculated by substituting equation 3 into equation 4 and solving to yield equation 5.
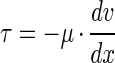 |
4 |
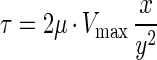 |
5 |
The units were converted to piconewtons per square micrometer and represent the shear stress at the surface of the silicon and chitin coupons in the LFCs.
Microscopy and image analysis.
Images of total S91 cells associated with the pure chitin and silicon surfaces were obtained every 10 min during the 1-h inoculation of the LFCs; 1, 2, 3, 5, 7, and 11 h after switching to sterile defined seawater solution; and every 12 h thereafter for the remainder of the 200-h experiment using reflected differential interference contrast (DIC) microscopy as described previously (6). Images of GFP-fluorescing, surface-associated cells up-expressed for chiA-chiB genes were obtained by epifluorescence microscopy for the same field of view used to image total cells by DIC as described previously (6). To compare relative fluorescence intensities (RFIs) of cells in different images, all images obtained by epifluorescence microscopy were captured at an exposure of 6 s using a gain of 4 and analyzed with Image-Pro Plus software (Media Cybernetics, Silver Spring, Md.). The only image manipulation performed on the DIC images was a background correction and in a few cases minor adjustment of the gray-scale contrast. No image manipulations were performed on the epifluorescence images. The software was used to count individual cells in all images using manually adjusted threshold values for each individual image. The intensity of GFP fluorescence was measured using Image-Pro Plus software by measuring the relative luminosity of chiA-chiB-gfp-expressing cells in each image. A minimum of six random fields (n = 6) on both coupons in each flow cell were imaged using both DIC and epifluorescence microscopy for every time point. Total surface-associated cell density, percentages of total cells up-expressed for chiA-chiB-gfp, and luminosity of cells up-expressed for chiA-chiB-gfp are presented as means ± standard deviations.
Detachment rate.
The rate of detachment of surface-derived bacterial cells was determined from the number of CFU recovered from 2 to 5 ml of effluent collected from the LFCs over a 4- to 10-min period at times over the 200-h period of surface colonization when microscopic images were obtained for the surface population. CFU were determined as described above for the starved-cell suspension. The rate of cell detachment at each sampling time was calculated as described by equation 6,
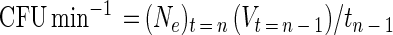 |
6 |
where (Ne)t = n is the CFU per milliliter of effluent at sampling time n, Vt = n − 1 is the volume in milliliters passing through the LFC since the previous sampling time n − 1, and tn − 1 is the time in minutes elapsed since the previous sampling time n − 1. The percentage of the total surface-derived bacteria that detached following LFC inoculation was determined at various times postinoculation by equation 7,
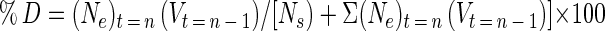 |
7 |
where Ns is the number of cells on the surface and all other terms are defined as in equation 6 above. Since detached cells were enumerated by CFU instead of total direct counts, moribund and viable but nonculturable cells in the detached population were not taken into account in the detachment rate calculation. Therefore, the detachment rates calculated from the data represent a minimum surface-associated bacterial detachment rate and are referred to as such.
Growth of bacterial cells in the bulk aqueous phase.
The extent to which DOM, released by the surface-associated bacterial population, supported growth of cells in the bulk aqueous phase after detachment from the chitin surface was determined during chitin degradation. Effluent (5 to 10 ml) from the chitin LFC was sampled at 50, 65, 78, 90, 102, 114, 126, 138, and 150 h postinoculation. Net growth of the free-living population on surface-derived DOM present in each sample was determined from plate counts obtained after further sample incubation in batch for 0, 24, 48, and 72 h at 20°C. CFU were determined as described above for the starved-cell suspension. The effluent of LFCs containing bare silicon coupons in place of the chitin-coated coupons was monitored for CFU to account for any growth derived from sources of DOM other than chitin degradation products.
Flow cytometric analysis.
The level of chiA-chiB expression by cells that detached from the surfaces over time was quantified by flow cytometric analysis using a Becton Dickinson FACSCalibur as previously described (6). Detached cells were partitioned on the basis of their RFI into one of three levels of chiA-chiB expression; no expression (1 to 10 RFI units), low-level expression (10 to 100 RFI units), and high-level expression (30 to 300 RFI units). There was some overlap in the fluorescence intensity histograms between the low and high levels of expression. These ranges were chosen to capture all of the cells associated with these subpopulations.
Surface-associated bacterial growth rate.
The growth rate of bacteria on the surfaces was calculated for two time intervals during chitin degradation. One interval (20 and 70 h postinoculation) coincided with the time of initial proliferation of bacteria on the surface. The other interval (100 and 200 h postinoculation) coincided with the time over which surface-associated bacterial cell densities and cell detachment rates remained fairly constant. The specific growth rate, k, was calculated over each of these time intervals from the increase in total surface-associated bacteria over that time interval plus the sum of the CFU obtained from all samples of LFC effluent collected during the same time interval. Specific growth rates associated with the squid pen chitin surface could not be determined, as surface-associated cells could not be accurately enumerated due to excessive surface roughness and autofluorescence of the natural chitin substratum. Since detached cells were enumerated by CFU instead of total direct counts, the growth rates calculated from the data, like the detachment rates described above, represent a minimum surface-associated bacterial specific growth rate and are referred to as such.
Bacterial biomass calculation.
Cell biomass (milligrams of C cell−1) was estimated from surface-associated cells in images of chitin and silicon surfaces and from detached cells collected on polycarbonate membranes (0.2-μm pore size; Nuclepore) following filtration of the LFC effluent. Images were captured with a Photometrics Imagepoint cooled charge-coupled device camera (Photometrics, Tucson, Ariz.), acquired at a magnification of ×1,000, and resolved with 32 bits of memory per pixel. The length and width of 40 randomly chosen bacteria in each of five captured images (n = 200) of the surface-associated bacterial population were obtained for this population of cells using Image-Pro Plus software (Media Cybernetics). The length and width of detached bacteria recovered on the polycarbonate membranes were obtained in a similar manner. The volume of cells of the surface-associated and detached populations was calculated from cell dimensions obtained from each population using equation 8,
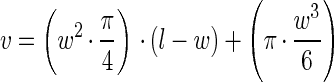 |
8 |
where v is cell volume and l and w are cell length and width, respectively (13). A mean cell volume ± 1 standard deviation was then calculated for the surface-associated and detached cell populations. Mean cell volume was then converted to mean cell carbon using equation 9,
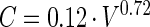 |
9 |
where V is the volume in cubic micrometers and C is in picograms of carbon per cell (36). Total surface-associated bacterial biomass was calculated by multiplying the mean carbon content of a surface-associated cell by the number of total surface-associated cells present at the end of the 200-h experiment. Total biomass of the detached bacterial population was calculated by multiplying the mean carbon content of the detached cell population by the total CFU present in the entire volume of LFC effluent discharged from the LFC over the 200-h experiment. Total surface-derived bacterial biomass was calculated as the sum of the total surface-associated and total detached bacterial biomass. Since detached cells were enumerated by CFU instead of total direct counts, the total detached bacterial biomass and total surface-derived bacterial biomass calculated from the data represent minimum values and are referred to as such.
BP.
BP by the surface-associated population on the 2-cm2 coupon surface area in the LFCs was calculated from the total surface-derived cell biomass produced over the 200-h experiment. This area-based production value (milligrams of C per square centimeter per hour) was converted to the more commonly encountered volume-based production value (milligrams of C per cubic meter per day) by replacing the area over which surface-derived cell biomass was generated with the volume of the bulk aqueous phase that passed through the LFCs over the 200-h experiment. Since detached cells were enumerated by CFU instead of total direct counts, the total detached BP and total surface-derived BP calculated from the data represent minimum values and are referred to as such.
RESULTS
Characteristics of starved population of S91.
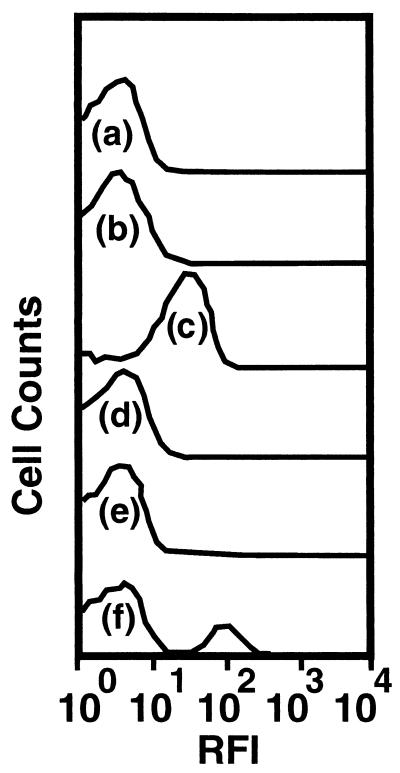
A culture of S91 suspended in defined seawater solution containing no added organic carbon, nitrogen, or energy sources in a CSTR achieved a stable cell density of 4 × 106 CFU ml−1 between 170 and 400 h of starvation (data not shown). During this time, the cells decreased in size and transformed from rods to coccoid forms. Cells of S91 displayed no detectable GFP fluorescence and hence no detectable chiA-chiB expression at any time during the 400-h period of starvation, based on epifluorescence microscopic examination of cells recovered from the starvation medium. Flow cytometric analysis of the starved-cell suspension revealed a distribution of RFIs among cells in the population that was similar to that displayed by a glutamate-grown culture of S91 (Fig. 1). In contrast, cells cultured in GlcNAc-containing medium displayed a range of RFIs that were approximately an order of magnitude higher than those of starved-cell suspension or glutamate-grown cultures (Fig. 1).
FIG. 1.
Histograms of RFI from GFP reporting expression of chiA-chiB genes in a population of S91 cells starved for 400 h in defined seawater solution (a), growing as a batch culture in defined seawater solution supplemented with glutamate (b), growing as a batch culture in defined seawater solution supplemented with GlcNAc (c), recovered in the effluent from the LFC containing silicon surfaces 96.5 h postinoculation (d), recovered in the effluent from the LFC containing pure chitin surfaces 96.5 h postinoculation (e), and recovered in the effluent from the LFC containing pure chitin surfaces 200 h postinoculation (f). Each histogram is based on evaluation of 5 × 105 cells.
Surface colonization.
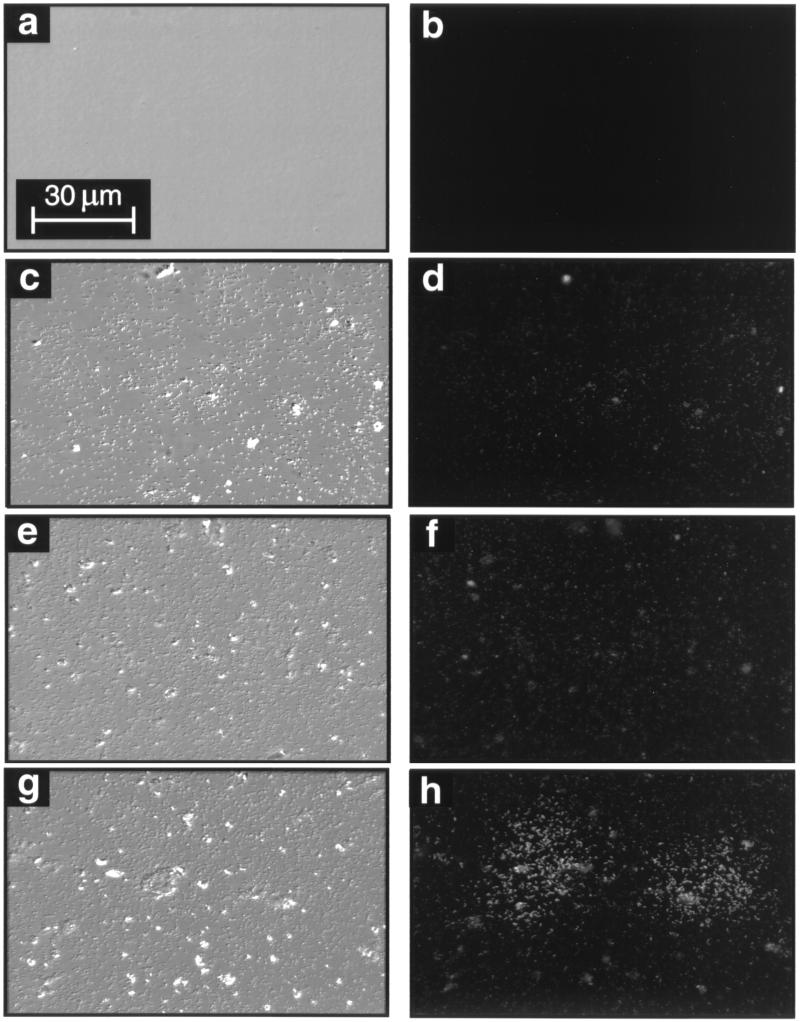
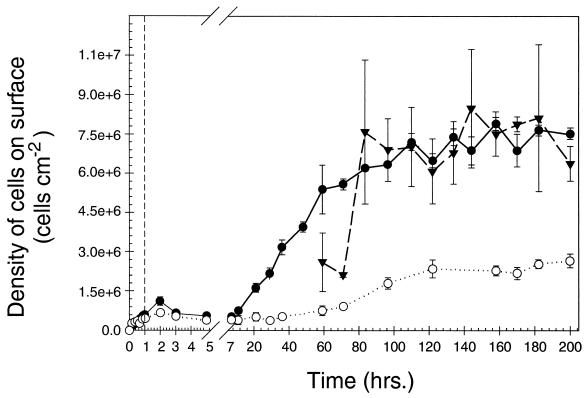
Prior to exposure to the starved-cell suspension, the surface of the chitin thin film was optically smooth and nonfluorescent (Fig. 2a and b). Both the chitin and silicon surfaces were rapidly colonized upon exposure to a flowing suspension of starved cells, accumulating 7.4 × 105 and 5.6 × 105 cells cm−2, respectively, within a 1-h period (Fig. 3). On the chitin surface, cell densities achieved a plateau at 7.1 × 106 cells cm−2 after 80 to 100 h, while on the silicon surface, they achieved a plateau at 2.2 × 106 cells cm−2 after 120 h. These results demonstrate that the chitin surface supplied essential nutrients for attached cell growth and replication that were not available on the silicon surface. However, cells that attached to the nonnutritional silicon surface were still able to grow and replicate, presumably utilizing the trace amounts of nutrients present as contaminants in the sterile defined seawater solution that flowed continuously across the coupon surface.
FIG. 2.
DIC (left panels) and epifluorescence (right panels) photomicrographs of clean, sterile chitin thin film surface (a and b), silicon surface 96.5 h postinoculation (c and d), pure chitin surface 96.5 h postinoculation (e and f), and pure chitin surface 200 h postinoculation (g and h).
FIG. 3.
Total cell densities on silicon surface (○), in areas of a pure chitin surface lacking clusters of cells displaying high-level chiA-chiB expression (●), and in areas of a pure chitin surface within clusters of cells displaying high-level chiA-chiB expression (▾) following inoculation of LFCs with starved-cell suspension (vertical dashed line). Vertical solid bars represent 1 standard deviation around the mean (n = 6 for each data point).
Chitinase gene expression of surface-associated bacteria.
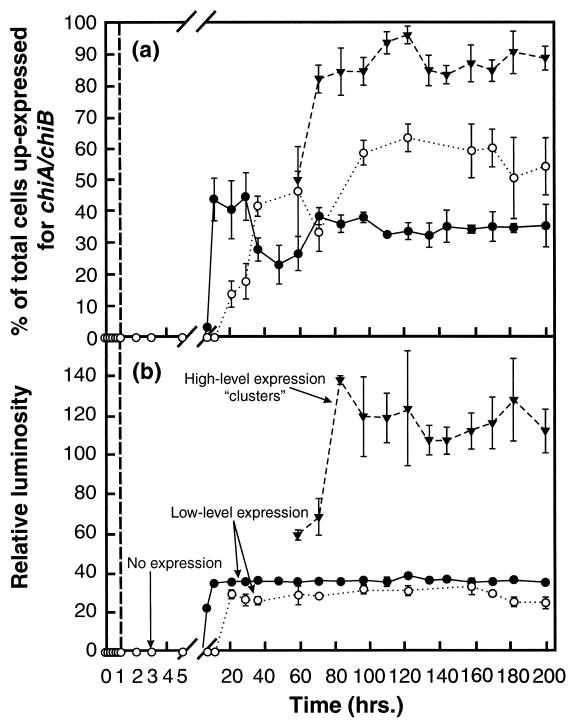
Upon initial attachment to the chitin or silicon surfaces, cells displayed no GFP fluorescence, indicating that detectable up-expression of the chiA-chiB genes was not required for initial adhesion of cells to the substratum. However, some cells on the chitin and silicon surfaces displayed GFP fluorescence 7 and 21 h postinoculation, respectively (Fig. 4a). The population of cells up-expressed for chiA-chiB on the silicon surface displayed a mean relative luminosity from GFP fluorescence of 28.6 ± 2.6 for the duration of the 200-h study (Fig. 4b). Over most areas of the chitin surface, cells that were up-expressed for chiA-chiB displayed a mean relative luminosity from GFP fluorescence of 35.9 ± 0.8 (Fig. 4b). The level of chiA-chiB expression among cells associated with most areas of the chitin surface is difficult to distinguish from that of cells expressing these genes on the silicon surface (Fig. 2c through f). This range of relative luminosity will be equated with low-level chiA-chiB expression.
FIG. 4.
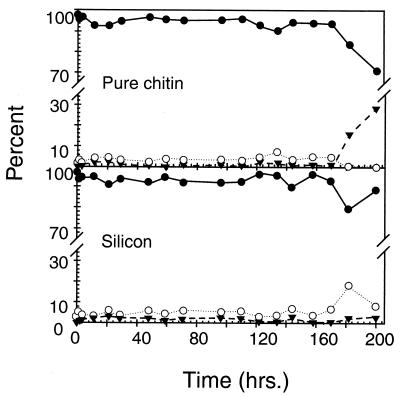
(a) Percentage of total surface-associated cells up-expressed for chiA-chiB on a silicon surface (○) and on the pure chitin surface in areas outside clusters of cells displaying high-level expression (●) and within clusters of cells displaying high-level expression (▾). (b) Relative luminosity of cells displaying different levels of chiA-chiB gene expression. The vertical dashed line identifies the time at which the feed to the LFCs was switched from the starved-cell suspension to the sterile defined seawater solution. Vertical solid bars represent 1 standard deviation around the mean (n = 6 for each data point).
Beginning at 60 h postinoculation, some areas of the chitin surface display clusters of cells with higher relative luminosity than those described above (Fig. 2g and h), averaging 117.5 ± 9.5 between 80 and 200 h postinoculation (Fig. 4b). Ninety-three percent of the cells in these clusters displayed this high relative luminosity (Fig. 4a). Thus, expression of chiA-chiB was significantly higher in cells in these clusters than in cells associated with the silicon surface or other areas of the chitin surface. This higher relative luminosity will be equated with high-level chiA-chiB expression. The density of cells in areas of the chitin surface where high-level expression occurred was not significantly different from that in surrounding areas of the surface where low-level expression was observed (Fig. 2g and h). At 96.5 h postinoculation, 37% of the total cells on the chitin surface expressed chiA-chiB at one of these levels, while 60% of the total cells on the silicon surface displayed low-level chiA-chiB expression (Fig. 4a). At no time during the 200-h study did any cells associated with the silicon surface display the high-level expression seen in clusters on the pure chitin surface. Thus, three subpopulations of cells were resolved on the chitin surface, based on levels of expression of chiA-chiB genes: one subpopulation that displayed no detectable expression, a second displaying low-level expression, and a third displaying a high level of expression. In contrast, only two subpopulations were resolved on the silicon surface based on levels of chiA-chiB expression; one subpopulation displaying no detectable expression and a second displaying low-level expression.
Clusters of cells displaying high-level expression varied in size from 308 to 49,125 μm2; the mean area of these clusters was 4,845 μm2. At the end of the 200-h experiment, clusters of cells displaying high-level expression covered approximately 20% of the total surface area of the chitin coupons. In a replicate experiment, the clusters of cells displaying high-level expression covered approximately 16% of the total surface area of the chitin coupons.
Detachment.
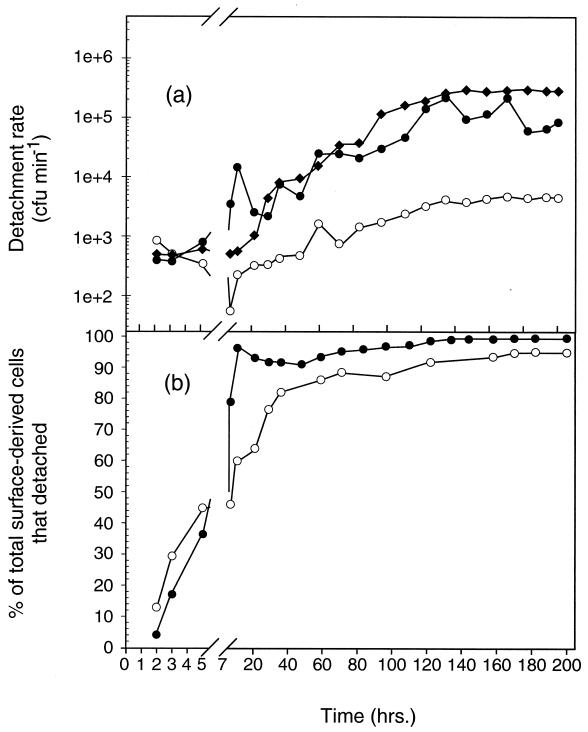
Rates of cell detachment from the pure chitin film, natural squid pen, and silicon surfaces, based on CFU recovered in LFC effluent, varied over the period of surface colonization. Cells of S91 detached from the pure chitin films and natural squid pen chitin surfaces at similar rates over the duration of the 200-h experiment (Fig. 5a). Detachment rates were lower on the silicon surface than on the chitin surfaces, however. Detachment rates on the pure chitin surface displayed the greatest increase during the time at which the cells accumulated most rapidly on that surface. The highest cell detachment rate was observed at 130 h postinoculation on all three surfaces (Fig. 5a). The detachment rate from the natural squid pen chitin and silicon surfaces remained at the maximum value for the remainder of the 200-h experiment, whereas the rate of cell detachment from the pure chitin film fluctuated over the 130- to 200-h interval (Fig. 5a). The detachment rates reported here likely underestimate the actual detachment rates since bacterial density determinations based on CFU did not take into account those cells that became moribund or nonculturable under the conditions employed in this study following detachment.
FIG. 5.
(a) Contribution of CFU in the LFC effluents by surface-derived cells that detached from the chitin (●), silicon (○), and natural squid pen chitin (⧫) surfaces. (b) Percentage of the total surface-derived population at different times of surface colonization that detached from the chitin (●) and silicon (○) surfaces.
Within 7 h of LFC inoculation, the percentage of surface-generated cells that detached from either the chitin or the silicon surfaces and were recovered as CFU in the bulk aqueous phase exceeded that which remained on the surface (Fig. 5b). No aggregation of cells was observed in effluent samples. The percentage of the total surface-generated cells that detached from the natural squid pen chitin surface could not be calculated, since the cells that remained associated with the surface could not be resolved. At 130 h postinoculation, 97.3 and 87.1% of the total cells generated on the pure chitin and silicon surfaces, respectively, had detached into the bulk aqueous phase (Fig. 5b). These percentages represent a minimum since not all the cells that detached were likely to have produced a CFU. Thus, the vast majority of surface-derived cells, regardless of the nutritional value of the surface, were displaced from the surface into the bulk aqueous phase over the 200-h experiment.
chiA-chiB gene expression in detached cells.
Flow cytometric analysis of effluent samples from both the silicon and pure chitin surfaces revealed that the population of detached cells displayed a range of RFIs from GFP fluorescence that was approximately the same as that observed for starved cells and cells cultured on glutamate, indicating that they were not expressing chiA-chiB (Fig. 1). The vast majority of cells that detached from either the pure chitin or the silicon surface over the entire 200-h period of surface colonization were down-expressed for chiA-chiB (Fig. 6). Some cells displaced from the pure chitin surface after 170 h of surface colonization displayed a higher level of fluorescence than those cells displaced from the surface at earlier times, suggesting a higher level of chiA-chiB gene expression (Fig. 1f). The appearance of cells displaying the higher level of fluorescence in the effluent of the chitin LFC after 170 h, coincided with an apparent deterioration and sloughing of portions of the pure chitin thin film from the coupon surface, presumably related to the biodegradation process. Thus, cells displaying the higher level of chiA-chiB expression that were detected in the LFC effluent after 170 h may have been displaced into the bulk aqueous phase with portions of the chitin thin film that sloughed from the silicon substratum, and not part of the population that detached from the intact chitin thin film.
FIG. 6.
Percentage of total cells that detached from the pure chitin (top) and silicon (bottom) surfaces that displayed no expression (●), 1 to 10 RFI units), low-level expression (○), 10 to 100 RFI units), and high-level expression (▾, 30 to 300 RFI units) of chiA-chiB genes.
Growth rates of surface-derived cells.
Taking into account the cells that detached from the surface based on CFU recovered in the effluent of the LFCs, as well as those that remained on the surface, a specific growth rate was calculated for the cells of the surface-derived populations over two different time intervals. Specific growth rates of 3.79 and 2.83 day−1 were obtained for cells generated on the pure chitin film and silicon surfaces, respectively, over the interval of 21 to 70 h postinoculation when cells first began to proliferate on these surfaces. Over the interval of 100 to 200 h postinoculation, when the density of the cells on the surfaces and CFU in the effluents had reached a steady state, specific growth rates decreased to 1.08 and 0.79 day−1 for cells generated on the pure chitin and silicon surfaces, respectively. These specific growth rates represent minimum values since CFU did not likely account for all the cells that detached from the surfaces. Specific growth rates could not be determined for the cells generated on natural squid pen chitin since surface roughness and autofluorescence precluded resolution and enumeration of single cells on this surface.
Biomass of surface-associated and detached cells.
At 200 h postinoculation, the mean volume and carbon content of cells that had accumulated on the pure chitin and silicon surfaces were 2.07 ± 0.15 μm3 (n = 40) and 2.03 × 10−10 mg of C cell−1, respectively. By comparison, the mean cell volume (1.01 ± 0.09 μm3, n = 50) and carbon content (1.21 × 10−10 mg of C cell−1) of bacteria that had detached from these surfaces were significantly less than those of the population of cells that remained associated with the surfaces. Comparing the biomass contributed by the cells that remained associated with the chitin (3.0 μg of C) and silicon (1.1 μg of C) surfaces at the end of the 200-h experiment to that contributed by the cells as CFU that had detached from the chitin (92.2 μg of C) and silicon (3.2 μg of C) surfaces over the 200-h experiment indicates that the detached cell population contributed the bulk of the surface-generated bacterial biomass in the system. This conclusion holds in spite of the smaller cell size and underestimation of total detached cells by the CFU enumeration method.
BP.
BP was determined on the basis of (i) cell biomass that remained associated with the chitin thin film and silicon surfaces at the end of the 200-h experiment, (ii) cell biomass that had detached from the surfaces over the duration of the 200-h experiment, and (iii) the combined biomass of cells that remained associated with the surfaces after 200 h and the cells that detached from the surfaces over the 200-h experiment (Table 1). The 55.3-s residence time of the bulk aqueous phase flowing through the LFCs was much less than the generation time of S91. Therefore, BP contributed by the detached cells, during the short time that they resided in the LFCs as a free-living population, was insignificant compared to the BP coming off the surface during that time.
TABLE 1.
Surface-derived BP
| Surface | BP that remained associated with surfacea (mg of C m−3 day−1) | BP displaced from the surfaceb (mg of C m−3 day−1) | Total surface-derived BP (mg of C m−3 day−1) | % Total surface-derived BP that detached |
|---|---|---|---|---|
| Silicon | 0.024 | 0.072 | 0.096 | 75 |
| Chitin | 0.068 | 2.060 | 2.128 | 96.8 |
| Squid pen chitin | NDc | 4.17 | ND | ND |
Based on total cells attached to 2.0 cm2 of surface at 200 h postinoculation and the total volume of seawater that passed through the LFCs over the 200-h period.
Based on CFU recovered from effluent samples collected over entire 200-h period.
ND, not determined.
While surface-retained BP on the chitin surface was nearly three times that on the silicon surface, surface-derived BP that detached from the chitin surface was nearly 30 times the BP that detached from the silicon surface (Table 1). Interestingly, BP that detached from the natural squid pen chitin was over twice that which had detached from the pure chitin film. Total chitin thin film-derived BP was 22 times the total silicon surface-derived BP after the 200-h incubation (Table 1). The total surface-derived and detached BP values for both chitin and silicon surfaces represent conservative estimates since they do not include that portion of the BP that had detached but failed to form a CFU. A minimum of 96.8 and 75% of the total surface-derived BP from the chitin and silicon surfaces, respectively, was displaced into the bulk seawater solution. Thus, irrespective of the nutritional value of the surface, and despite the conservative estimate of the number of cells that detached from the surfaces, the majority of the BP produced on the surfaces was displaced into the surrounding bulk aqueous phase.
BP by free-living cell population.
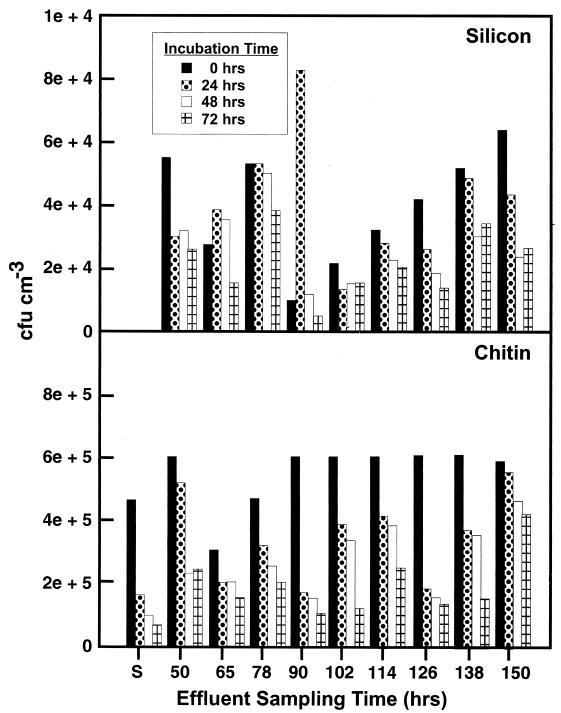
Once the cells detached from the chitin or silicon surface and became part of the free-living population, they did not contribute detectable BP in the effluent based on CFU recovered over a 72-h period following detachment. In fact, incubation of this population of cells in the seawater solution in which they were displaced resulted in a reduction in CFU over time (Fig. 7). A reduction in CFU was observed in effluents collected at every sampling period from 0 to 150 h postinoculation (Fig. 7). The decrease in CFU in the LFC effluents over the 72-h period following surface detachment resembled the decrease in CFU during the early stages of starvation of the LFC inoculum following resuspension of a glutamate-grown culture of S91 in defined seawater solution (Fig. 7).
FIG. 7.
CFU recovered from effluents of chitin- and silicon-containing LFCs at various times postinoculation after incubation as a batch culture at 20°C for 0, 24, 48, and 72 h. CFU recovered following exposure of a glutamate-grown culture of S91 to unsupplemented defined seawater solution for 0, 24, 48, and 72 h are presented for comparison (S).
Hydrodynamic conditions.
The 0.5-ml min−1 flow rate of the defined seawater solution through each LFC during the surface-associated cell growth and detachment corresponded to a fluid flow velocity of 75 m day−1. When crystal violet was injected into the LFCs at this flow rate, it moved in a plug flow manner with a Re of 1.30, suggesting laminar flow of the aqueous phase across the coupons. The shear stress experienced by the bacteria at the surface under these conditions was equivalent to 0.004 pN of force μm−2.
DISCUSSION
The cell density and cell morphology of a 400-h starved population of S91 resembled the density and cell morphology of natural bacterial populations in the pelagic marine environment (14, 15, 34, 50, 54). Thus, the physiological state of the cells used to inoculate the surfaces of the model system may have resembled that of natural bacterial populations colonizing POM in the pelagic marine environment. The rapid attachment of starved cells of S91 to the chitin and silicon surfaces and subsequent growth and replication on these surfaces are consistent with behavior displayed by free-living, starved cells of other marine bacteria that have been exposed to surfaces in oligotrophic environments (33). Growth of cells on the nonnutritional silicon surface was most likely the result of surface-associated cell scavenging and utilization of trace contaminants of DOM in the defined seawater solution that flowed continuously across the coupon surface.
Chitinases possess chitin-binding domains that aid in binding the enzyme to its substrate (39). These chitin-binding domains may also serve to anchor the cell to the chitin surface (38). That cells, upon initial attachment to either chitin or silicon surfaces, displayed no detectable GFP fluorescence and, hence, were not expressing chiA-chiB suggests that the products of these genes are not required for establishment of initial interactions between the cell and substratum, even when the substratum is pure chitin. However, the observed low-level expression of chiA-chiB genes following cell attachment to both the silicon and chitin surfaces is consistent with previous studies suggesting that expression of these genes was a surface-controlled response, independent of the presence of chitin.
The properties of a solid surface that affect gene expression have not been identified (10, 37, 44, 52, 57). Since chitin is one of the primary organic carbon and nitrogen sources in the pelagic marine environment, cells possessing chitinase genes may express these genes at a low level following contact with any solid surface to synthesize “sensing levels” of enzymes (6, 56). When a chitin surface is encountered, the small quantity of enzyme produced could generate sufficient amounts of GlcNAc or chitin oligomers, which, upon uptake by the cells, may promote higher levels of chiA-chiB expression, as was observed in cells on some areas of the chitin surface but not in cells on the silicon surface.
An unanticipated result of the present study was the displacement of the majority of the surface-derived BP as free-living cells to the bulk aqueous phase, regardless of the nutritional value of the surface. Even using the conservative measure of CFU to assess the number of surface-derived cells displaced into the bulk aqueous phase, the fraction of the total surface-derived BP displaced from the chitin and silicon surfaces over a 200-h period was 97 and 75%, respectively. Jacobsen and Azam (23) found that as much as 90% of bacteria produced by cells attached to copepod fecal pellets were recovered in the surrounding water. They hypothesized that the progeny of dividing cells leave the fecal pellet during the division cycle.
While hydrodynamic forces exerted over the surface of natural detrital POM during settling through the water column will vary as a consequence of complex geometry, those forces exerted upon the bacteria associated with the pure chitin and silicon surfaces by the flowing bulk aqueous phase were relatively constant in LFCs. The 0.004-pN μm−2 shear stress calculated for cells associated with surfaces exposed to a bulk aqueous phase flow rate of 0.5 ml min−1 approximates the shear stress at a point perpendicular to the flow of seawater across a particle sinking at a rate of 75 m day−1. This sinking velocity is very similar to the mean sinking velocity (74 ± 39 m day−1) of particles ranging from 2.4 to 75 mm in diameter off the coast of southern California (1). Thus, the shear stress experienced by bacteria attached to natural POM in the water column of the ocean approximates that experienced by the surface-associated cells in LFCs in the present study.
It is difficult to assess the significance of shear stress for the process of bacterial cell detachment from a surface. However, a study evaluating the effect of shear stress on the adhesion of Staphylococcus epidermidis to a variety of materials including glass, siliconized glass, plasma-conditioned glass, titanium, stainless steel, and Teflon suggested that shear stresses ranging from 0.2 to 1.0 pN μm−2 had little effect on the detachment rate of the surface-colonizing bacteria (32). Thus, the shear stress of 0.004 pN μm−2 calculated for cells of S91 attached to surfaces exposed to flowing seawater in the present study likely represents a negligible force for these bacteria.
In the absence of a significant shear stress, the cells themselves may have controlled their displacement from the surfaces by a yet-unknown mechanism of detachment. The higher detachment rates that were consistently observed over the study period for the chitin-associated population than for the silicon-associated population may involve a chitin-specific deadhesion process similar to that described for the marine chitin degrader Vibrio furnissii (56). The deadhesion displayed by progeny of cells of this bacterium during chitin utilization was proposed to promote colonization of new surfaces (56). Cells of another marine vibrio, MH3, associate with a surface only long enough to scavenge and metabolize surface-bound fatty acids before detachment (21). Detachment of S91 cells from either the chitin or silicon surfaces was not likely the result of space limitation, since detachment occurred even at early periods of cell accumulation on these surfaces when large areas of the surface remained unoccupied.
Jacobsen and Azam (23) recognized that detachment of POM-associated bacteria contributes to an underestimation of POM-derived BP and a corresponding overestimation of BP by the free-living bacterial population when conventional methods are used to determine BP in water samples collected from the environment. Methods of assessing BP in natural waters, such as those involving [3H]thymidine or [3H]leucine incorporation, assume that detachment of cells from POM is insignificant over the period of sample incubation (11, 12, 16, 27, 41). Results based on these approaches typically indicate that POM-associated BP is a small or insignificant fraction of the total BP in the system, with the free-living bacterial population always contributing the bulk of the BP (2, 20, 25, 51). Unfortunately, a simple means of assessing the displacement of particle-associated bacteria during determination of BP in water samples obtained from the environment does not exist at the present time.
By utilizing a model system that accounted for the surface-associated BP that was subsequently displaced into the bulk aqueous phase, it was possible in the present study to demonstrate quantitatively the contribution of the displaced BP to the surface-derived BP. That the displaced BP represented the bulk of the surface-derived BP supports the suggestion that detachment of POM-associated bacteria during determination of BP can result in errors in assignment of the bacterial populations responsible for the BP in the system when approaches that do not account for the displacement of cells from one phase to another are employed (23). Thus, existing methods of BP determination should be modified or new methods should be developed to account for not only detachment of bacteria from POM but also attachment of free-living bacteria to POM during BP determinations. The results also suggest that, in order to gain a better understanding of the dynamics of bacterial processes associated with POM degradation, it may be necessary to evaluate both detachment and BP nondestructively in real time, or at least at intervals which capture the significant changes in the rates of these processes during POM degradation.
On the basis of biomass calculations derived from measurements of cell dimensions, total biomass produced on the pure chitin surface was 40% greater than the estimated available carbon in the chitin thin films. The factors likely responsible for this discrepancy are the empirically derived factors for converting cell volume to biomass used in this study and the uncertainty of the thickness of the chitin thin films. The carbon content per volume can vary widely in marine bacteria (7, 17, 28, 29, 30). Since this was not determined in our study, the conversion factors may be inaccurate. Film thickness may vary as much as 30 to 40% when the casting temperature varies by 5°C, due to temperature-induced changes in the viscosity of the chitosan solution. Since temperature was not controlled during the casting of the films, estimated carbon content may be off by as much as 30 to 40%. Theoretically, these uncertainties can be minimized in future experiments, permitting an accurate determination of the efficiency of bacterial conversion of chitin to biomass in this model system.
Calculations based on the rates of POM-associated BP obtained by methods that do not account for bacterial detachment predict that it would take months to years for the bacteria retained on the particle to consume the carbon load of the particle (12, 24). This low rate of conversion of detrital particulate organic carbon to particle-associated BP cannot account for the observed rapid disappearance of these particles with depth, a phenomenon referred to as the “particle decomposition paradox” (24). One explanation that has been proposed is that POM-associated bacteria generate more DOM from the hydrolysis of POM than they can utilize, resulting in the release of DOM into the bulk aqueous phase for utilization by free-living bacteria (3, 9, 46). An alternative explanation may be that some POM-associated bacteria generate more DOM from the hydrolysis of POM than they themselves can utilize, and the excess is utilized by other POM-associated bacteria for BP, a significant portion of which becomes detached to become part of the free-living bacterial population.
Two surface-associated subpopulations were described with respect to chitinase gene expression: one comprised of cells up-expressed for chiA-chiB and one composed of cells down-expressed for these genes. These results are consistent with those of a previous study that monitored only chiA gene expression (6). Like the detached cells that displayed no detectable chiA gene expression in a previous study (6), the cells that detached from the surfaces in this study displayed no detectable chiA or chiB gene expression. Thus, in spite of the fact that chiA and chiB are each under the control of their own promoters (48), they appear to be coregulated in the surface-associated population. The evidence supporting the existence of chiA-up- and -down-expressed subpopulations, which also applies to chiA-chiB-up- and -down-expressed subpopulations, is presented elsewhere (6).
As was shown to be the case for a significant portion of cells up-expressed for chiA (6), a comparable portion of the cells up-expressed for chiA-chiB in the present study are also likely to be synthesizing and excreting active chitinase enzymes. On the chitin surface, the amount of BP supported by chitin degradation products created by the chitinase-producing subpopulation corresponds roughly to that represented by the difference in total surface-derived BP on the chitin and silicon surfaces (2.03 mg of C m−3 day−1). The surface-associated BP supported by chitin degradation products was 0.044 mg of C m−3 day−1 (Table 1), and the chiA-chiB-up-expressed subpopulation represented 37% of the total chitin surface-associated population during the time that BP achieved a quasi-steady state. Thus, the chiA-chiB-up-expressed subpopulation accounted for only 0.02 mg of C m−3 day−1, or approximately 1% of the total surface-derived BP attributable to that derived from the chitin degradation products they generated. These calculations suggest that the chiA-chiB-up-expressed subpopulation must have produced a large excess of chitin degradation products to support production of their chiA-chiB-down-expressed neighbors on the surface.
The means by which the chiA-chiB-down-expressed cells gain access to the soluble chitin degradation products remains to be determined. The matrix of extracellular polymeric substances excreted by surface-associated bacterial populations may play a role in this regard by impeding displacement of soluble chitin degradation products as well as chitinase enzyme molecules from the substratum surface to the flowing bulk aqueous phase. Channels and pores in the extracellular polymeric substance matrix near the substratum could then serve as conduits for transfer of soluble chitin degradation products to nearby cells that are not producing chitinase.
The present study also suggests that the DOM generated by the chitinase-active, surface-associated population supported no significant BP by the free-living population relative to that generated by the surface-associated population. No new BP, based on CFU, was detected in the LFC effluent containing the detached cells over a 72-h period of batch incubation, regardless of when detachment occurred during the period of surface colonization. In fact, the decrease in CFU over the 72-h incubation of all effluent samples and the smaller size of cells in the detached population than in the population that remained surface associated suggest that, once the cells became part of the free-living population, any DOM that they utilized was not sufficient to prevent many from entering a moribund, nonculturable or starvation state. Thus, the bulk of the surface-generated DOM that was utilized for BP supported surface population growth rather than growth of the free-living population in the model system employed in this study.
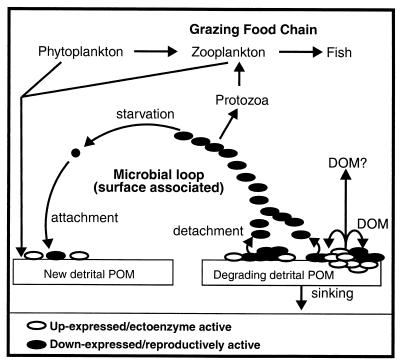
In summary, differential expression of chitinase genes among cells of this surface-associated bacterial population supports the idea that the chitin surface-associated subpopulation of cells, whose chiA-chiB genes are up-expressed, produces more soluble chitin degradation product than they can utilize and that the excess supports production of the surface-associated subpopulation of cells down-expressed for chiA-chiB. This then permits two energy-demanding activities (ectoenzyme production and BP) to proceed simultaneously but partitioned among different cells within a population of surface-associated bacteria (Fig. 8). Such altruistic behavior of cells in a bacterial population may be an adaptive response to conserve energy in oligotrophic environments such as the ocean which, when coordinated with a process like detachment for dispersal of the surface-generated BP, optimizes exposure to the widely dispersed, newly formed detrital POM in the pelagic water column for colonization and, ultimately, maintenance of the population. Other types of bacteria also appear to distribute different physiological activities among different members of the population as a means to enhance survival of the population as a whole (8, 42).
FIG. 8.
Schematic representation of the pathway involved in the degradation of detrital POM and resulting production of surface-derived microbial biomass based on results obtained using a model system. Starved cells of Pseudoalteromonas sp. strain S91 attach to a surface and form two subpopulations: one whose chitin-degrading genes become up-expressed and another whose chitin-degrading genes remain down-expressed. The former, through chitinase production and excretion, supplies the latter with soluble chitin degradation products for bacterial production at the surface. Progeny detach from the surface and disseminate into the bulk aqueous phase to seek out new detrital POM to repeat the cycle.
ACKNOWLEDGMENTS
We gratefully acknowledge Sandra Kurk at the Department of Veterinary Molecular Biology of Montana State University for her help in flow cytometric analysis. We also recognize Samuel Hudson at North Carolina State University for sharing his expertise in the preparation of chitin films.
Part of this work was supported by The Flinders University of South Australia and the Australian Research Council. Somkiet Techkarnjanaruk was supported by a Royal Thai Government Scholarship. This work was sponsored by the National Science Foundation under grant OCE 9720151 and the National Institutes of Health under grant S10RR11877 and under the National Science Foundation cooperative agreement EEC 8907039.
REFERENCES
- 1.Alldredge A L, Gotschalk C. In situ settling behavior of marine snow. Limnol Oceanogr. 1988;33:339–351. [Google Scholar]
- 2.Alldredge A L, Cole J J, Caron D A. Production of heterotrophic bacteria inhabiting macroscopic organic aggregates (marine snow) from surface waters. Limnol Oceanogr. 1986;31:68–78. [Google Scholar]
- 3.Azam F. Microbial control of oceanic carbon flux: the plot thickens. Science. 1998;280:694–696. [Google Scholar]
- 4.Azam F, Hodson R. Size distribution and activity of marine microheterotrophs. Limnol Oceanogr. 1977;22:492–501. [Google Scholar]
- 5.Bae H, Hudson S M. The cooperative binding behavior of sodium dodecyl sulfate to crosslinked chitosan films. J Appl Polymer Sci: Part A Polymer Chem. 1997;35:3755–3765. [Google Scholar]
- 6.Baty A M, III, Eastburn C C, Diwu Z, Techkarnjanaruk S, Goodman A E, Geesey G G. Differentiation of chitinase-active and non-chitinase-active subpopulations of a marine bacterium during chitin degradation. Appl Environ Microbiol. 2000;66:3566–3573. doi: 10.1128/aem.66.8.3566-3573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornsen P K, Kuparinen J. Determination of bacterioplankton biomass, net production and growth efficiency in the Southern Ocean. Mar Ecol Prog Ser. 1991;71:185–194. [Google Scholar]
- 8.Caldwell D E, Wolfaardt G M, Korber D R, Lawrence J R. Do bacterial communities transcend darwinism? Adv Microb Ecol. 1997;15:105–191. [Google Scholar]
- 9.Cho B C, Azam F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature. 1988;332:441–443. [Google Scholar]
- 10.Davies D G, Geesey G G. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl Environ Microbiol. 1995;61:860–867. doi: 10.1128/aem.61.3.860-867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducklow H W, Kirchman D L. Production and fate of bacteria in the oceans. Bioscience. 1983;33:494–499. [Google Scholar]
- 12.Ducklow H W, Kirchman D L, Rowe G T. Production and vertical flux of attached bacteria in the Hudson River Plume of the New York Bight as studied with floating sediment traps. Appl Environ Microbiol. 1982;43:769–776. doi: 10.1128/aem.43.4.769-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felip M, Sattler B, Psenner R, Catalan J. Highly active microbial communities in the ice and snow cover of high mountain lakes. Appl Environ Microbiol. 1995;61:2394–2401. doi: 10.1128/aem.61.6.2394-2401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuhrman J A. Influence of method on the apparent size distribution of bacterioplankton cells: epifluorescence microscopy compared to scanning electron microscopy. Mar Ecol Prog Ser. 1981;5:103–106. [Google Scholar]
- 15.Fuhrman J A, Azam F. Bacterioplankton secondary production estimates for coastal waters of British Columbia, Antarctica, and California. Appl Environ Microbiol. 1980;39:1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuhrman J A, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Biol. 1982;66:109–120. [Google Scholar]
- 17.Fukuda R, Ogawa H, Nagata T, Koike I. Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. Appl Environ Microbiol. 1998;64:3352–3358. doi: 10.1128/aem.64.9.3352-3358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gooday G W. The ecology of chitin degradation. Adv Microb Ecol. 1990;11:387–430. [Google Scholar]
- 19.Gooday G W. Physiology of microbial degradation of chitin and chitosan. Biodegradation. 1990;1:177–190. [Google Scholar]
- 20.Griffith P, Shiah F, Gloersen K, Ducklow H W, Fletcher M. Activity and distribution of attached bacteria in Chesapeake Bay. Mar Ecol Prog Ser. 1994;108:1–10. [Google Scholar]
- 21.Hermansson M, Marshall K C. Utilization of surface localized substrate by non-adhesive marine bacteria. Microb Ecol. 1985;11:91–105. doi: 10.1007/BF02010482. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey B, Kjelleberg S, Marshall K C. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol. 1983;45:43–47. doi: 10.1128/aem.45.1.43-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen J T, Azam F. Role of bacteria in copepod fecal pellet decomposition: colonization, growth rates and remineralization. Bull Mar Sci. 1984;35:495–502. [Google Scholar]
- 24.Karl D M, Knaur G A, Martin J H. Downward flux of particulate organic matter in the ocean: a particle decomposition paradox. Nature. 1988;332:438–441. [Google Scholar]
- 25.Karner M, Herndl G J. Extracellular enzymatic activity and secondary production in free-living and marine snow associated bacteria. Mar Biol. 1992;113:341–347. [Google Scholar]
- 26.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad host range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 27.Kirchman D L, Ducklow H W, Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982;44:1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogure K, Koike I. Particle counter determination of bacterial biomass in seawater. Appl Environ Microbiol. 1987;53:274–277. doi: 10.1128/aem.53.2.274-277.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroer N. Relationships between biovolume and carbon and nitrogen content of bacterioplankton. FEMS Microbiol Ecol. 1994;13:217–224. [Google Scholar]
- 30.Lee S, Fuhrman J A. Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol. 1987;53:1298–1303. doi: 10.1128/aem.53.6.1298-1303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeburg M R. Fluid properties. In: Lindeburg M R, editor. Engineer in training reference manual. 8th ed. Belmont, Calif: Professional Publications; 1992. pp. 14.1–14.2. [Google Scholar]
- 32.Linton C J, Sherriff A, Millar M R. Use of a modified Robbins device to directly compare the adhesion of Staphylococcus epidermidis RP62A to surfaces. J Appl Microbiol. 1999;86:194–202. doi: 10.1046/j.1365-2672.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- 33.Marshall K C. Adhesion and growth of bacteria at surfaces in oligotrophic habitats. Can J Microbiol. 1988;34:503–506. [Google Scholar]
- 34.Marshall K C, Stout R, Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971;17:1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery M T, Kirchman D L. Role of chitin-binding proteins in the specific attachment of the marine bacterium Vibrio harveyi to chitin. Appl Environ Microbiol. 1993;59:373–379. doi: 10.1128/aem.59.2.373-379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norland S. The relationship between biomass and volume of bacteria. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 303–307. [Google Scholar]
- 37.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 38.Qin Y. The chelating properties of chitosan fibers. J Appl Polymer Sci. 1993;49:727–731. [Google Scholar]
- 39.Raikhel N V, Lee H I, Broekaert W F. Structure and function of chitin binding proteins. Annu Rev Plant Mol Biol. 1993;44:591–615. [Google Scholar]
- 40.Rathke T D, Hudson S M. Review of chitin and chitosan as fiber and film formers. J. Mater. Sci.: Rev. Macromol Chem Phys. 1994;C34:375–437. [Google Scholar]
- 41.Reimann B, Bell R T. Advances in estimating bacterial biomass and growth in aquatic systems. Arch Hydrobiol. 1990;25:385–402. [Google Scholar]
- 42.Shapiro J A. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- 43.Siedlecki K J. M.S. thesis. Bozeman: Montana State University; 1995. Interactions between the pathogenic yeast Candida albicans and poly(vinyl chloride) pp. 33–37. [Google Scholar]
- 44.Silverman M, Belas R, Simon M. Genetic control of bacterial adhesion. In: Marshall K C, editor. Microbial adhesion and aggregation. New York, N.Y: Dahlein Conference, Springer-Verlag; 1984. pp. 95–107. [Google Scholar]
- 45.Simon M, Alldredge A L, Azam F. Bacterial carbon dynamics on marine snow. Mar Ecol Prog Ser. 1990;51:201–213. [Google Scholar]
- 46.Smith D C, Simon M, Alldredge A L, Azam F. Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature. 1992;359:139–141. [Google Scholar]
- 47.Stretton S, Techkarnjanaruk S, McLennan A M, Goodman A E. Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Appl Environ Microbiol. 1998;64:2554–2559. doi: 10.1128/aem.64.7.2554-2559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Techkarnjanaruk S, Goodman A E. Multiple genes involved in chitin degradation from the marine bacteria Pseudoalteromonas sp. strain S91. Microbiology. 1999;145:925–934. doi: 10.1099/13500872-145-4-925. [DOI] [PubMed] [Google Scholar]
- 49.Techkarnjanaruk S, Pongpattanakitshote S, Goodman A E. Use of a promoterless lacZ gene insertion to investigate chitinase gene expression in the marine bacterium Pseudoalteromonas sp. strain S9. Appl Environ Microbiol. 1997;63:2989–2996. doi: 10.1128/aem.63.8.2989-2996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torrella F, Morita R Y. Microcultural study of the bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981;41:518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turley C M, Mackie P J. Biogeochemical significance of attached and free living bacteria and the flux of particles in the NE Atlantic Ocean. Mar Ecol Prog Ser. 1994;115:191–203. [Google Scholar]
- 52.Van Loosdrecht M C M, Lyklema J, Norde W, Zehnder A J B. Influence of interfaces on microbial activity. Microbiol Rev. 1990;54:75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vetter Y A, Deming J W. Growth rates of marine bacterial isolates on particulate organic substrates solubilized by freely released extracellular enzyme. Microb Ecol. 1999;37:86–94. doi: 10.1007/s002489900133. [DOI] [PubMed] [Google Scholar]
- 54.Watson S W, Novitsky T J, Quinby H L, Valois F W. Determination of bacteria number and biomass in the marine environment. Appl Environ Microbiol. 1977;33:940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weast R C. CRC handbook of chemistry and physics. 68th ed. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. D–250. [Google Scholar]
- 56.Yu C, Lee A M, Bassler B L, Roseman S. Chitin utilization by marine bacteria. A physiological function for bacterial adhesion to immobilized carbohydrates. J Biol Chem. 1991;266:24260–24267. [PubMed] [Google Scholar]
- 57.Zhang J P, Normark S. Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]