Abstract
Politically authorized reports on personalized and precision medicine stress an urgent need for finer-grained disease categories and faster taxonomic revision, through integration of genomic and phenotypic data. Developing a data-driven taxonomy is, however, not as simple as it sounds. It is often assumed that an integrated data infrastructure is relatively easy to implement in countries that already have highly centralized and digitalized health care systems. Our analysis of initiatives associated with the Danish National Genome Center, recently launched to bring Denmark to the forefront of personalized medicine, tells a different story. Through a “meta-taxonomy” of taxonomic revisions, we discuss what a genomics-based disease taxonomy entails, epistemically as well as organizationally. Whereas policy reports promote a vision of seamless data integration and standardization, we highlight how the envisioned strategy imposes significant changes on the organization of health care systems. Our analysis shows how persistent tensions in medicine between variation and standardization, and between change and continuity, remain obstacles for the production as well as the evaluation of genomics-based taxonomies of difference. We identify inherent conflicts between the ideal of dynamic revision and existing regulatory functions of disease categories in, for example, the organization and management of health care systems. Moreover, we raise concerns about shifts in the regulatory regime of evidence standards, where clinical care increasingly becomes a vehicle for biomedical research.
Keywords: Big data, Data infrastructure, Denmark: Disease categories, Disease taxonomies, Genomics, Health care regulation, Personalized medicine, Precision medicine
Highlights
-
•
Personalized medicine has led to calls for speedy revisions of disease taxonomies.
-
•
A “meta-taxonomy” of disease taxonomic revisions is presented and discussed.
-
•
Fine-graining disease categories has regulatory implications for healthcare systems.
-
•
A Danish case illustrates difficulties in aligning multiple functions of disease codes.
-
•
We argue that the political call for speed should be treated with caution.
1. Introduction
This paper discusses important limitations and trade-offs inherent in contemporary political visions of personalized medicine as a route to finer-grained and more dynamic disease taxonomies. What is in Europe mostly called personalized medicine and, in the US, mostly precision medicine broadly refer to data-intensive strategies that describe and stratify diseases and patient groups according to an increasing number of biomolecular factors. The concepts remain “future-oriented” and sufficiently flexible to embrace various hopes and expectations for the future (Brown and Michael, 2003; De Grandis and Halgunset, 2016). As technologies still in the making, their clinical implications for disease diagnostics are inherently difficult to predict. Still, the visions and political plans are worth analysing because the projection of future goals, whether realistic or not, drive organizational developments with regulatory implications for science, medicine, and health care (Hogle, 2018).
The concept of regulation carries different meanings in different disciplines (Drahos, 2017). Rather than reserving the term for factors external to the clinical practice, such as legal statutes, we view regulatory structures as enacted in practice and as constitutive of knowledge production (Cambrosio et al., 2017). We approach regulation in health care through an analysis of practices that simultaneously enable and constrain the co-production of biomedical knowledge and disease categorizations. We emphasize co-production in this context, because the identification of genetic biomarkers is dependent on the establishment of new data infrastructures. While policy reports acknowledge the need for legislative changes to set up such infrastructures, less attention has been paid to the challenges of using and updating diagnostic codes that serve other clinical and regulatory functions. Taxonomic revision depends not only on the availability of genetic data, but also on the possibility of aligning the new aims with data's existing regulatory functions.
Our aim is to show how epistemic and regulatory challenges are intertwined in attempts to realize a more dynamic and finer-grained disease taxonomy. We are interested not only in what disease categories are as epistemic categories, but also in what disease categories do. Disease categories define what count as disease and relate symptoms and test results to treatment plans, but they also increasing serve as regulatory devices in the management of health care systems and allocation of social services (Jutel and Nettleton, 2011; Rose, 2013). Diagnostic codes are thus performative units that bring together the domains of health, science, administration, and policy (Bossen et al., 2016). We therefore not only consider disease taxonomies as epistemic tools for classification of disease, but also regulatory devices. The regulatory uses of disease categories may not be as visible as their classificatory role, but the infrastructures installed through these are central to the operation of health systems (Bowker and Star, 2000; Hoeyer, 2016). Because infrastructures are relational, changes in one domain impact other parts of the regulatory network. As a result, taxonomic revision is simultaneously constituted and constrained by the possibilities offered within a “regulatory web” of mutually dependent relations (Cambrosio et al., 2017).
By viewing disease categories as performative and regulatory units, we highlight how they are shaped through negotiations between scientific, clinical, and political aims. Correspondingly, we not only view personalized medicine as a scientific development but also as a political priority embraced and propagated in strategy reports by political leaders around the world (NAS, 2011; European Science Foundation, 2012; Ministry of Social Affairs and Health, 2015; Cyranoski, 2016; Ministry of Health and Danish Regions, 2016; Genomics England, 2017; National Institutes of Health, 2018; Australian Genomics Health Alliance, 2019). The current politics of personalized medicine are characterized by a strong sense of urgency for acquiring ever more data and for the fast implementation of new diagnostic categories (Tarkkala et al., 2018). Although personalized medicine is still a vision for the future, the ambitions for personalized medicine are already shaping the development of new data infrastructures.
The political visions are articulated in large-scale projects such as the US-based All of Us study (National Institutes of Health, 2018), and the UK-based 100.000 Genomes Project (Genomics England, 2017). Making sense of the collected genomic data, however, depends on access to infrastructures containing information about current diagnostic categories. Specifically, it depends on high quality health data on clinical phenotypes that are sufficiently standardized to allow for analysis via computational tools. The facilitation of this access has resulted in both American and British e-health initiatives aimed at creating digitally available health data through which each unique case can be compared to other cases and new patterns can be established (Minari et al., 2018). Similarly, the European Commission granted another 21 million Euros in 2019 to facilitate digital exchange of health data within the EU to promote personalized health (European Commission, 2019).
The vision of an integrated health data infrastructure is to a large extent already established in Denmark, a small European welfare state with a well-entrenched and centralized system for tracking each individual patient via unique personal identification numbers and digital diagnostic coding systems. To take advantage of this infrastructure, Denmark has recently embarked on a strategy for personalized medicine to ensure political and legislative facilitation of a national genome database (Ministry of Social Affairs and Health, 2015). To establish and operate the infrastructure of the National Genome Center, the Danish Ministry of Health has recently received approximately 137 million US$ by the Novo Nordisk Foundation (2018), the primary owner of Denmark's biggest pharmaceutical company. The aim of the Genome Center is to store copies of patient's genomes, initially 60,000 people and in time the whole population, in a manner that facilitates integration and combination with other types of health data (Danske Regioner, 2015a, 2015b: 11; 34). This is highlighted as important for ensuring that the data can serve multiple purposes for diagnosis, treatment, and as a unique data resource for commercial as well as academic research. In the following, we demonstrate the difficulties of aligning these purposes, by illustrating tensions between the epistemic and regulatory functions of disease codes.
A central aim of recent investments in data infrastructures, as emphasized by the Danish Minister for Health, is to “accelerate the process by which our healthcare system will increasingly treat people based on knowledge about their genes” (Novo Nordisk Foundation, 2018). To clarify what a genomics-based diagnostic system entails in practice, we propose what we call a “meta-taxonomy” of taxonomic revisions. By opening the “regulatory black box” (Cambrosio et al., 2017) of diagnostic infrastructures, we outline a set of important challenges related to these revisions. We argue that to address these, policymakers need to understand the epistemic, organizational, and regulatory landscapes in which knowledge and evidence is produced. This involves acknowledging the challenges of genomic data analysis, and how diagnostic information, on which this analysis relies, already serves other regulatory functions. A call for speedy revision works against existing regulatory uses and risks undermining the quest for safe and trustworthy information about likely treatment effects.
2. Methods
This paper began as a theoretical reflection on the need for a speedy and finer-grained revision of disease taxonomies, often articulated in reports and at conferences on personalized medicine. Rather than an ethnographic account, we aim to provide an interdisciplinary and practice-oriented reflection on hopes and challenges articulated by actors in the field. For this purpose, we combine methods from STS-research and philosophy of science. Together and separately we have participated in more than 70 conferences, meetings, and workshops in Denmark and the UK during the period from 2014 to 2018. These meetings have been documented in extensive field notes. In addition, we have analysed reports and other forms of written material describing precision medicine initiatives and their associated restructuring of data infrastructures. Furthermore, Hoeyer has conducted interviews with administrators, data analysts, and clinicians in the Danish health services working with health data and data-intensive medicine in the course of a larger project on data-intensification in health care. Submitting qualitative research for ethics committee approval is not possible in Denmark, but the project complies with the EU GDPR-requirements. We draw on insights from the exploration of the Danish data infrastructure to tease out how epistemic and regulatory challenges are intertwined in the pursuit of personalized medicine.
3. The vision of data integration and speedy taxonomic revisions
Personalized medicine is often promoted as a way to overcome important limitations within evidence-based medicine (EBM). With Randomized Clinical Trails seen as the gold standard of evidence, EBM gives priority to statistical evidence and standardization (Cartwright, 2011; Timmermans and Berg, 2003). Accordingly, diagnostic manuals such as the International Classification of Disease (ICD), the Diagnostic and Statistical Manual of Mental Disorders (DSM), and the International Classification of Primary Care (ICPC) prioritize requirements for large-scale studies of the diagnosis of morbidity, treatment effects, and causes of mortality (Bowker and Star, 2000). This emphasis places constraints on the speed of taxonomic revision and the ability to account for special cases (Solomon, 2011). Although clinicians in practice seek to overcome the problem of de-individualization (Timmermans and Berg, 2003; Timmermans, 2015), concerns have been raised about the increasing bureaucratic management of EBM and the lack of relevance of many statistical measures for clinical care (Greenhalgh et al., 2014; Tonelli and Shirts, 2017).
In response to such limitations, personalized medicine is increasingly promoted as a route to a more dynamic disease taxonomy that can better account for patient variation. Already in 2011, an influential report commissioned by the National Academy of Science (NAS), entitled “Precision Medicine. A Framework for Developing a New Taxonomy of Disease”, stressed an “obvious need to categorize diseases with finer granularity” (NAS, 2011: 29). The report highlights how the use of multiple molecular-based parameters will lead to a “more accurate and finer-grained classification of disease” (46). The aim is to take advantage of a transformative opportunity provided by the “explosion of disease-related data with the potential to dramatically alter disease classification” (4). Similar visions have been promoted in many other countries. For instance, the Danish Ministry of Health and the Danish Regions (which are responsible for the management of the public health system) maintain that by using genetic knowledge it is possible to “diagnose diseases more precisely and target treatment more accurately” (Ministry of Health and Danish Regions, 2016: 3).
Increasing precision in the context of personalized medicine is a vision of reduced uncertainty of disease diagnostics through molecular biomarkers that can be “measured accurately and precisely” (NAS, 2011: 42). This is contrasted with the current system, which is described as being grounded on an outdated one-size-fits-all-approach that is primarily symptom-based (Ministry of Health and Danish Regions, 2016: 11; NAS, 2011: 10, 42). It is debatable how well this description captures the current state of the field. But we shall here focus on how the ability to update diagnostic categories and tests through genomic data depends on integration with clinical data on disease development, treatments, and health outcomes.
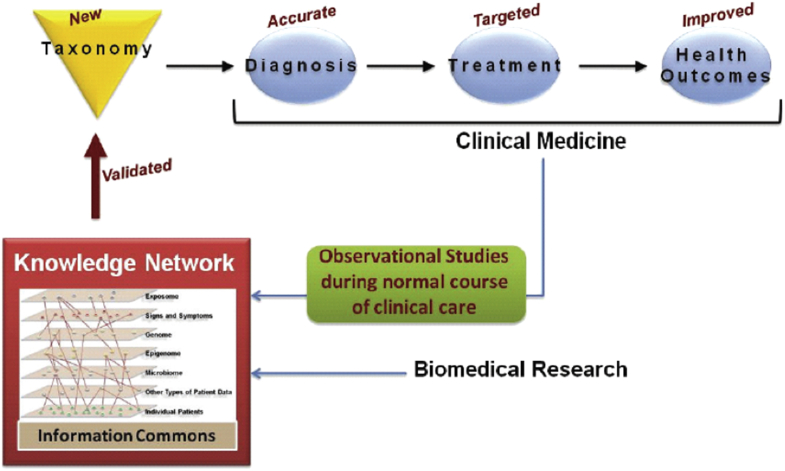
The US NRC report illustrates this task through the development of a shared infrastructure for clinical practice and biomedical research in a central figure (Fig. 1), from which the Danish Regions also draw inspiration (Jylling, 2017). A centralized database entitled Information Commons (or Knowledge Commons in the equivalent Danish figure) is anticipated to give rise to a Knowledge Network that continuously revises and validates new disease categories.
Fig. 1.
Illustration of the organization of the new taxonomy (NAS, 2011: 2). Reprinted with permission by courtesy of the National Academies Press, Washington, D.C.
The figure from the NRC report gives the impression that the integration of genomic data and health data is a fairly straightforward process of merging existing databases with new types of data, akin to the development of GIS-type structure underlying Google Maps (NAS, 2011: 17; see also Prainsack, 2015). Yet, data-driven taxonomic revision via computer analysis presupposes an ability to re-use data across different contexts and for a variety of purposes (Leonelli, 2016). As we shall see, integrating data – even of the same type and level – often turns out to be very challenging due to the existence of diverse practices for diagnosing and coding. We highlight that attempts to overcome such challenges through regulatory amendments, such as data standardization, entail significant disruptions in health care practices as well as in standards of evidence. It is not as easy as it may sound.
4. A meta-taxonomy of taxonomic revision
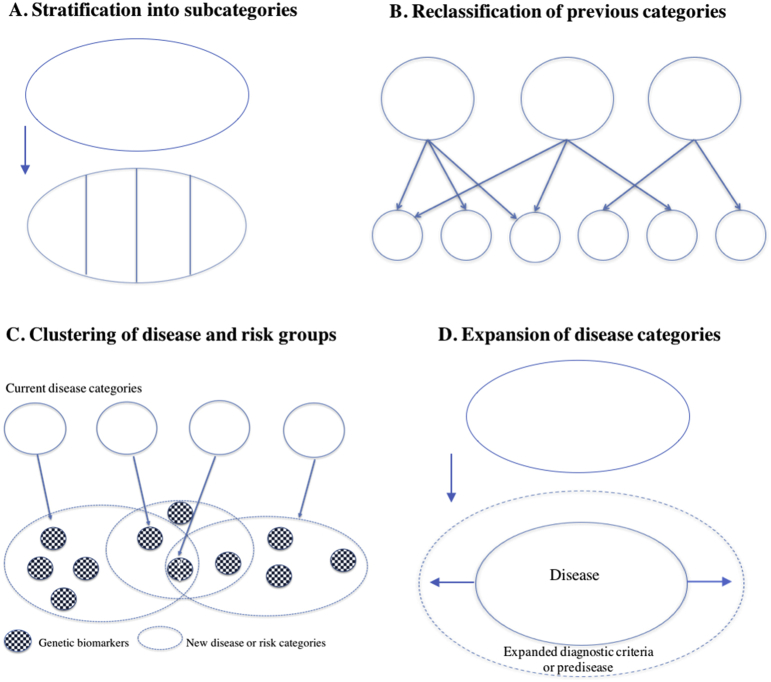
Policy reports on personalized or precision medicine highlight the urgency of implementation (see also Section 7), but they consistently lack a specification of how the new taxonomy is supposed to be developed. To explore what a finer-grained taxonomy entails in practice, we outline what we call a “meta-taxonomy” of taxonomy revision. We illustrate each type of revision with examples from the reports or recent publications on personalized medicine and discuss the associated implications below. Our aim is to show that the development of new nosological categories are intertwined with changes in regulatory practices (cf. Cambrosio et al., 2017).
The most straightforward interpretation of what more fine-grained disease categories might entail is what we call stratification into subgroups of diseases (Fig. 2A). This type of revision is exemplified through recently proposed sub-categories of diabetes or cancer through genomic profiling (Danske Regioner, 2015b: 5, 16). Breast cancer has since the 1970s has been differentiated into multiple subtypes based on genetic markers, such as HER2-positive or HER2-negative breast cancers, and recently further into an increasing number of rare disease variants (Hedgecoe, 2004; Keating et al., 2016). More generally, research within cancer genomics, such as The Cancer Genome Atlas, has expanded the list of cancer subtypes (Tomczak et al., 2015). This type of reclassification keeps the overall taxonomic label but distinguishes the category into more fine-grained disease variants.
Fig. 2.
A meta-taxonomy illustrating different ways of fine-graining disease categories through personalized medicine.
The second type, which we call reclassification of previous categories (Fig. 2B), illustrates how diseases may be redefined in relation to traits that differ from the previous categorization. This type can result in merging previously distinct categories according to shared molecular characteristics. For example, colon and rectal cancers previously were considered distinct groups but then became grouped together because of similar patterns of genomic and epigenetic changes (Tomczak et al., 2015). Alternatively, new disease categories may emerge, as seen in the recategorization of some organ specific cancers into biomarker specific cancers. In some phase 1 clinical trials, genetic analysis of tumour biopsies gives rise to the allocation of patients diagnosed with one cancer type to another, (e.g., HER2-related subtypes of breast and colon cancer, or BRAF and RAS mutations for lung cancer and melanoma). Recategorizations of this type can thus result in boundary changes involving both lumping and splitting of medical conditions (Featherstone and Atkinson, 2012: 107)
Type 3 in our meta-taxonomy, clustering of disease and risk groups (Fig. 2C), represents how different diseases may be linked via genetic markers and lead to clustering of categories based on a network of risk factors and observed co-morbidities. For example, network analyses of omics data (genomics, proteomics and metabolomics) have established links between obesity, diabetes mellitus, and asthma (Barabási et al., 2011). Similarly, genome-wide association studies have recently identified overlaps in genetic markers associated with psychiatric disorders such as autism, depression, bipolar disorder, and schizophrenia (Gandal et al., 2018). A central idea is that patients currently belonging to one disease or risk group are likely also to have or develop other genetically linked diseases, and that treatment and prevention therefore need to target several related diseases.
Type 4, expansion of disease categories (Fig. 2D), illustrates how technological possibilities for detection of finer-grained abnormalities and pathologies may result in expansion of current disease concepts. When the definitions of disease and diagnostic tests are extended to include phenomena that were previously not part of the diagnosis (e.g., genetic markers), the result is often a conceptual expansion of diagnostic criteria (Hedgecoe, 2003), as well as an expansion of diagnosed disease incidence at the population level (Hofmann, 2017). Similarly, increased focus on early detection and disease prevention has led to the emergence of risk or predisease categories, which represent a spectrum between health and disease (Dumit, 2012). Examples are risk categories of heritable forms of heart disease and pre-diabetes, which have recently been highlighted as actionable targets for disease prevention in pilot projects of personalized medicine (Price et al., 2017; Perkins et al., 2018).
Even though it may sound simple and good with a taxonomic revision, our meta-taxonomy shows how a revision can imply many different things and be anything but simple. While our meta-taxonomy allows an initial appraisal of the different types of diagnostic revision, it is now time to discuss in more depth some of the challenges involved. We begin with the emphasis on genetic variation and the enduring uncertainties of genetic information, and then in the subsequent section explore uncertainties related to phenotypic information that reflect the multiple regulatory functions of disease categories.
5. The focus on genetic variation and the quest of persistent uncertainty
Our meta-taxonomy illustrates some aspects of what the epidemiologist Abby Lippman (1992) described as the effects of genetic technology, namely differentiation, expansion, and geneticization of disease concepts. Lippman viewed these developments as driven by a reduction of biological variation to molecular factors that entails essentialist tendencies. One should be cautious in forecasting the effects of the application of genetic knowledge in clinical contexts (Arribas-Ayllon, 2016), especially since implementation often happens in a piecemeal way where genetic and non-genetic diagnostic practices are integrated (Hedgecoe, 2002, 2003; Featherstone and Atkinson, 2012). But since the vision of a finer-grained disease taxonomy foregrounds genetic variation as a basis for improved disease diagnostics, it is worth analysing the assumptions underlying the envisioned “genomic enlightenment”, as well as the organizational consequences of the proposed strategy (cf. Hedgecoe, 2001).
The borders around the circles in types A and B on Fig. 2 not only demarcate disease categories but also patient groups that decrease dramatically in size. Through finer-grained stratification of diseases and patient groups, personalized medicine promises to overcome the limitations of classical phase III trials that agencies like the American Food and Drug Administration (FDA) and the European Medicines Agencies (EMA) traditionally have seen as the gold standard of evidence. Whereas classical trials are criticized for being based on population-based averages, accounting for genetic variation is expected to offer higher diagnostic precision. It should not, however, be assumed that more genetic data will solve the current problems.
We should keep in mind that biomarkers cannot be “read” in the genome as there is typically no direct or linear causal link to diseases. Rather, genetic biomarkers are established on the basis of statistical comparisons of patient groups, or case/control studies, which make the result prone to potential biases and uncertainties concerning sampling procedures, size, and variation of the compared data and population groups. In this context, the interpretation of genetic data thus critically depends on the definition of population cohorts with specific diagnostic histories and treatment outcomes. But these previous diagnostics are exactly what personalized medicine is supposed to move beyond or revise. Taxonomic revision exemplifies an attempt to promote regulatory objectivity (Cambrosio et al., 2017), through systematic co-production of new nosological entities and new regulatory norms of action. It is therefore important not only to identify existing epistemic uncertainties but also to analyse whether and how policymakers respond to such challenges.
Despite the grand hopes and expectations expressed in the reports on personalized medicine, the clinical utility of genetic markers remains a controversial topic and many genetic tests continue to be marked by great uncertainty (Timmermans et al., 2016; Senn, 2016). The long-term clinical benefits of targeted cancer therapies are ongoing issues of debate (Hutchinson and Romero, 2016), and so is the assumption that privileging genetic markers will give more accurate disease diagnostics (Senn, 2016). The implications of such uncertainties have been most clearly documented in preventive strategies (C and D on Fig. 2). At a recent conference in Copenhagen on personalized medicine, one of the speakers admitted that “we haven't yet developed good practices for analysing genomic data”. Genomic data are supposed to give a more precise image of individual patients. However, the speaker had had his genome sequenced 5 times, and each time the “genetic mirror” showed a different picture of disease risks. The example illustrates the common problem that different sequencing platforms often give different results, and the status of implemented biomarkers are consistently revised (Timmermans, 2015).
One study found that almost 200 known gene variants previously seen as predicting risk of disease were found not to be associated with increased risk anyway (Lek et al., 2016). Similarly, a recently developed test for inherited thrombophilia does not change treatment recommendations, but nevertheless the US Medicare system received 280,000 claims for the test just in 2014. Despite questionable medical benefits, this test costs taxpayers approximately half a billion USD a year (Ross, 2016). Reliance on uncertain genetic tests can result not only in wasted resources but also in harm to patients. Recently, 21 women underwent prophylactic mastectomies and removal of ovaries at a Norwegian hospital after testing positive for a variant of the BRCA2-gene that is no longer considered predictive of disease risk (Dagens Medicin, 2017). Similarly, a recent case report from the US documents how two dozen members of the same family were misdiagnosed with LQT1 (a hereditary heart disease), because a genetic test result of uncertain relevance was prioritized over phenotypic evidence indicating normal ECG test results (Ackerman et al., 2016). The misdiagnosis led to unnecessary implementation of preventive pacemakers in more than 20 individuals, in one case leading to two shock incidents in an otherwise healthy individual.
Prioritizing genetic variation is a common feature of many policy strategies across countries, despite a remarkable lack of convincing clinical examples to justify this focus (Tarkkala et al., 2018). For instance, a Danish report admits that recent genomics-based studies of diabetes and ischemic cardiac disease have not resulted in improved prediction or explanation of these diseases (Danske Regioner, 2015b: 17), but this does not lower the expectations expressed in the same report of genomics-based disease risk and disease stratification. Following a more general trend, disappointed expectations do not lead to reflection on whether these are realistic (Brown and Michael, 2003). Rather, expectations are redefined to be realized at an indeterminate point in the future – whenever enough data have been collected (Hoeyer, forthcoming). Thus, the ‘blame’ is shifted onto the quantity of data currently available, and uncertainties become incentives to collect ever more data (see also Timmermans et al., 2016). Much the same happens in scientific publications when proponents of personalized medicine are confronted with problems relating to false positive tests and overdiagnosis (Hood et al., 2015). This should give further reason for concern about shifts in evidence standards (discussed in Section 6 below).
Why, then, do proponents move so quickly from a description of the problem of inefficient treatments and poor preventive strategies to claims that integration of (gen)omics data will improve the situation? In our view, we cannot see this as an epistemic question alone; it is as much an economic and political issue. Scientists are under pressure to align their aspirations with political interests to raise funding, and governmental agencies need to present themselves as accountable to public health, which implies opting for immediate health benefits. In an interview, a person working at the state-level with development of personalized medicine explained the focus on genetics as a matter of public accountability: genetics is identified by the appropriate experts, she said, as the only area where it is possible to use the data gathered from the public right away. The state cannot provide funding into pure research databases documenting metabolism, physical activity, diet, compliance, or social and psychological factors when such data types have not been standardized enough to be clinically useful, she argued. This is the case even though these data might be relevant from a biomedical perspective (James, 2014; Smolen and Aletaha, 2013). The current investments in genetics might not (yet) deliver epistemologically speaking, but they do respond to a political need.
In consequence, the proposed solution seems to amount to what Senn (2016) calls a phenotypic squeeze. By this, Senn refers to the trend to account for complex sources of variation by a reductive foregrounding of genetic factors. At the same time, most scientists and even proponents of personalized medicine acknowledge the difficulty of accounting for biomedical complexity through genomics. Speakers at conferences on personalized medicine often articulate endless socio-biological variation and continuous interaction between multiple factors that in effect make each individual unique. Nevertheless, when moving to concrete suggestions to be implemented, the same people typically narrowed their focus to disease prediction through analysis of genomic variation (Ackerman et al., 2016). Personalized medicine initiatives thus opt for the data that are assumed to be most conveniently collected and standardized. However, in reality, the convenience of genomic data is hampered by organizational requirements, and by the complexity of interpretation. An interesting insight from conferences we have attended is that people often intervene in arguments by saying: “But we don't know the phenotype!” As argued above, the phenotype is needed to identify the significance of the genotype. For instance, Genome-Wide Association Studies identify genetic variants on the basis of a statistical comparison of genomic data from a patient population, compared to a control group. But, in the absence of reliable phenotypes, i.e., diagnostic categories to classify population data, the existing diagnostic uncertainty is inherited by the genetic algorithm (Lemoine, 2017). This underscores how the interpretation of genomic data is inherently dependent on existing systems of classification, and thus how genomic data does not offer an Archimedian point for disease diagnostics.
In the following, we highlight how practical problems of integrating and standardizing phenotypic data reveal inherent structural trade-offs between different functions of disease codes. The call for speedy revisions of taxonomic systems conflicts with existing regulatory functions of disease codes and may work against the very ability to make valid interpretations of phenotypic information.
6. Organizational challenges for data integration
To clarify the organizational challenges associated with speedy taxonomic revision, we examine the Danish context where minimal challenges should be expected. Denmark has a long tradition of registering health data and other information on all citizens in connection to a personal identifier, the so-called CPR-number derived from the Central Person Register (Bauer, 2014; Hoeyer, 2018). Denmark should therefore be an ideal context to realize data integration, and achieving personalized medicine is considered primarily as a matter of “realizing the potential by linking what we already know about the population's diseases with genetic knowledge” (Ministry of Health and Danish Regions, 2016: 3). Again, this may sound easy, but it is not.
Five biorepositories including the Danish Blood Donor Biobank have undergone integration in 2016 and are administered by the newly established Bio- and Genome Bank Denmark. Moreover, new and more standardized software platforms are being implemented in Danish hospitals and general practice. Currently, the National Board of Health Data works to implement a Health Data Programme aimed at enhancing national integration of phenotypic data while the new National Genome Centre works to collect, store, and establish standards for genotypic data. The digital infrastructures through which the data are to be gathered are not always easy to integrate. For example, two of the five regions have recently implemented a new system bought from the US firm EPIC, and this system faces severe integration problems with the national databases and registers (Wadman and Hoeyer, 2018).
At various conferences, we have observed how the Danish health data infrastructure is framed as a unique and available raw material: a form of ‘oil’ that just needs digital tools to be released. However, even in this relatively homogenous and highly digitized health care system, there is considerable variation in local clinical practices for collection, use, and coding of data. Data might be there, but they are not easy to find and require specific skills to analyse. To illustrate, we provide a telling example from one of Hoeyer's interviews. Even something as simple as lab results for a cholesterol measurement have been reported with different digits, or as a yes/no to being above a specific clinical guideline level. Just as the reporting format has differences within and between laboratories, guideline levels have changed over time. Also, the facilities performing the measurements change over time when municipalities, general practitioners or other healthcare providers take on or give up on particular functions. As a result, a patient may have had the same cholesterol level measured continuously for 15 years, but be reported first as healthy, later as ill, and then with measurements of different digits. Moreover, data records may be discontinuous or broken if measurements or information from particular periods are missing. To know what the data mean over time takes not just a lot of data cleaning but also quite fine-grained organizational and historical knowledge.
The former president of the International Epidemiological Association, Rodolfo Saracci (2018) recently commented on the effectiveness of so-called “real world evidence” for realizing precision medicine. He emphasizes that population-based health registers are the best sources for this purpose and highlights Danish registers as an exemplary case. But he then stresses that even in this context, the records do not always provide the information necessary for realizing precision medicine. He mentions a discussion on a recent Danish cohort study that claims to document treatment advantages of beta-blockers against atrial fibrillation in patients with and without heart failure. The study is impressive in terms of a high population recruitment (200,000 patients) as well as standardized procedures for reporting of morbidity, co-morbidity, treatments, etc. However, because the data registers lack standardized information on ejection fraction (heart pumping capacity), the patients could not be stratified according to the severity of hemodynamic dysfunction. As a result, concerns were raised about insufficient control for confounding effects resulting from within-group differences. Saracci uses the examples to highlight that the “real world studies have ‘local value’ and are indispensable to measure treatment effectiveness within specific local (in time and place) circumstances”, but such differences also make “inherently uncertain any generalization of results” (Saracci, 2018: 5).
As has been experienced in multiple research contexts, strategies to strengthen data-reuse can compromise local uses through a loss of system-specific knowledge (Leonelli, 2016). Complex and non-standardized information is difficult to mine via algorithms, but is often central to delivering primary health care services to individual patients. Standardization procedures also raise questions about who is accountable for decisions concerning which classificatory systems or codes to adopt when there are discrepancies in existing datasets. The Danish health care system currently relies on both ICPC coding and ICD-10, and Electronic Medical Records may contain as many as 140,000 codes that can be taken from drop-down menus. Although such systems provide shared languages for data analysis, they easily become intractable in practice.
Through interviews we have learned that one important challenge for the creation of large-scale searchable databases with structured patient data is that time pressure and clinical variation tends to make clinicians opt for broader diagnostics codes, higher in the taxonomy, and then write the specifics in free-text. It makes the treatment of the particular patient easier, but the subsequent (re)use of the data more difficult. Often doctors record breast cancer patients as “DC509, unspecified breast cancer”, partly because it is quicker and partly because DC500-DC508 provide specifications that are not fully adequate for the patient at hand. Even when specific codes are chosen, the codes can be difficult to interpret in isolation from the historical continuity and social embedding of local practices of data use and data production.
Another key challenge is that the revision of disease taxonomies is constrained by the embedding of disease categories in various additional infrastructures. Taxonomic systems play increasing regulatory roles for record keeping, institutional remuneration schemes, financial administration, performance management, quality assurance, as well as legal functions related to liability issues (Hoeyer, 2016, 2018). New standardized and finer-grained diagnostic codes cannot be assumed to be practically applicable in all these systems. In Denmark and many other countries, disease categories are for instance related to regulatory categories called Diagnose-Related Groups (DRG) that are central to the administration of the finances of the hospitals (Bossen et al., 2016). There are currently 740 DRG-groups with associated tens of thousands of codes for diagnoses and treatment to categorize patient care. When a patient is diagnosed and treated, such codes are used to calculate payments to the hospitals for health and treatment services. For reasons unrelated to personalized medicine these remuneration systems are also subject to reforms. Rapid changes in diagnostic codes will therefore interact with other regulatory changes and cause disruptions in coding infrastructures that are central to the operation of health systems.
The practical challenges highlight that disease categories are performative and multi-relational units that are embedded in complex infrastructures, or “regulatory webs”. An important concern is therefore which effects altered disease codes will have on the various practices in health care systems. A tension between clinical practice and research seems to be acknowledged in the US NAS report in a passage stressing that the taxonomy should “be highly dynamic, at least when used as a research tool, continuously incorporating newly emerging disease information” (NAS, 2011: 4, our emphasis). However, this statement seems at odds with the main aim of developing a joint infrastructure that simultaneously will be used by research and clinical practice, and no suggestions are offered for how to deal with this tension.
7. The need for speed and the remaking of evidence
So far, we have documented how reclassification of disease categories based on biomarkers is epistemically and organizationally dependent on the possibility of aligning multiple uses of disease categories. This can be considered as a “challenge of creating equivalences between different domains of activity, such as laboratory and clinical activities” (Cambrosio et al., 2017: 165). Cambrosio and colleagues highlight that regulatory challenges also extend to evidence practices such as clinical trials. Below, we add to this analysis by highlighting the regulatory challenges associated with using data from clinical practice to facilitate research and taxonomic revisions.
The data infrastructure depicted in Fig. 1 reflects an attempt to address the problem that the timeframe for clinical intervention studies is much longer than genomic innovation (Danske Regioner, 2015b: 23). The iterative loop of taxonomic revision is framed as a process of “co-production of health and evidence” (Jylling, 2017). While data integration and “real time” production of evidence may sound like a convenient regulatory fix to the slowness of current clinical trials, it involves a quite significant change in the organization of health care. Progress is here seen as dependent upon developing a “rapid-learning health care system” that is characterised by a more dynamic relationship between evidence and treatment (Friedman et al., 2010). This dynamism does, however, come with significant practical and ethical risks (Minari et al., 2018). At the Danish conferences we have attended, one of the most frequently recurring questions revolves around how to discover new categories of disease in the course of applying them in treatment. This amounts to a shift in the regulatory regime of evidence standards, where evidence is supposed to result from new treatments rather than being the basis for them.
The vision of faster translation and implementation runs counter to the evidence hierarchy of EBM that prioritizes statistical evidence as a precondition for treatment recommendations. While EBM has its critics (Cartwright, 2011; Solomon, 2011; Greenhalgh et al., 2014; Stegenga, 2018), not least because it fails to take proper account of clinical judgement and experience (Tonelli and Shirts, 2017; Rees, 2000), we cannot see how personalized medicine presents a feasible alternative at this stage (see also Lambert, 2006). Admittedly, the use of treatment outcomes as evidence is not new to medicine, being well documented in the history of medicine: see for example, Le Fanu's, (1999/2011)account of the emergence of clinical medicine between the two world wars (1999/2011). On a more statistically significant scale, Danish (and other) registers that follow patients over time have been an important source of knowledge about side-effects and other unwarranted aspects of treatment. However, the evidence that emerges from treatment usually has a tentative status; for example, post-hoc subgroup analyses are usually considered as exploratory only as these are amenable to biases associated with p-hacking and data fishing (Germain and Baetu, 2017). It is not in itself surprising or even unreasonable that treatments give rise to evidence. But it is worrying that the reversal of the relationship between evidence and treatment is built into the taxonomic reconfiguration of disease and treatment guidelines, to be adopted on a regulatory level, without a clear rationale or justification.
Despite the uncertainties outlined, the political ambition is nevertheless to realize the potential of personalized medicine in Denmark by moving fast (Danske Regioner, 2015a, 2015b). The webpage on personalized medicine by the Danish Regions stresses that “we should not linger on the debate - we have to act for the benefit of the patients. Other countries are already well underway, and Denmark must participate in the race to take advantage of the opportunities” (webpage of Danske Regioner, our translation, see also Tupasela, 2017, for an analysis of national branding in Finland). Similarly, a central message at a recent conference on personalized medicine was that we do not have time for further professional or public debate, and that “the talking days are over!”
The ambition to dissolve existing regulatory thresholds of evidence without having a viable replacement is particularly disconcerting considering how Danish reports very explicitly highlight that the new data infrastructure will open new possibilities for data economies and public-private partnerships (DAMVAD Analytics, 2016). Similarly, in the context of initiatives for personalized medicine in Finland, Tarkkala, Helén, and Snell (2018) observe how expectations have shifted from a focus on health and targeted treatments to a focus on ‘wealth’, i.e., on potentials for economic growth (Vezyridis and Timmons, 2017). Meanwhile, evidence for mutual benefits for wealth and health is in both contexts projected into the future, while existing doubts are ignored (Hoeyer, forthcoming). This raises important questions about who will benefit from the new strategies, and about who will pay if the expectations are not realized.
We do not deny that iterative improvement of diagnostic categories must be done in an anti-foundational manner – nor do we see this challenge as specific to personalized medicine. The philosopher Otto Neurath used the analogy of boat repair at sea to describe a similar challenge in economics and science (Stanovich, 2004). What we question is thus not the idea of iterative corrections within existing systems of belief. We have raised concerns about the combination of the epistemic and organizational challenges outlined with an accelerated speed of revision and implementation. Neurath's bootstrapping analogy may be taken to extremes in personalized medicine. A speaker at one of the international conferences in Denmark even compared the current development of personalized medicine to the thrilling challenge of constructing airplanes while flying. The analogies illustrate how iterative corrections must be made without causing structural disruptions that would make the boat sink or the plane crash. Similarly, attention should be taken to revise disease taxonomies in a way that does not risk undermining health care.
8. Conclusion
The promotion of a need for a more dynamic and finer-grained disease taxonomy exemplifies how epistemic, organizational, regulatory, and political issues intersect when personalized medicine is to materialize in practice. We have focused specifically on how policy reports call for a dynamic taxonomy that more rapidly can accommodate insights from data-intensive research. Personalized medicine promotes the epistemic ideal of diagnostic accuracy through a more precise stratification of disease categories. In practice, it does so primarily through emphasis on genetic variation, typically without further justification of why genetic variation is considered causally privileged (see also Hedgecoe, 2001).
We have suggested that taxonomic revision is not just a straightforward matter of using genetic data to create “more fine-grained taxonomies”. Since taxonomic revisions can take several forms, each with different epistemic and organizational challenges, we have proposed a meta-taxonomy of taxonomic revisions as a basis for discussions of evidence thresholds and implications for health care systems. Here, we have suggested a first attempt with four types, but just like disease taxonomies, it will have to develop along with new experiences and data. A necessary requirement for all types of fine-graining via genomics is, however, that genetic data can be evaluated on the basis of existing information in terms of reliable, standardized, and continuous phenotypic data.
Developing an integrated database for research and healthcare is often pictured as relatively straightforward. However, disease categories are embedded within larger organizational and regulatory infrastructures that simultaneously constitute and constrain the possibilities for diagnostic innovation. By examining the variety of epistemic and regulatory roles of disease codes, we have pointed to inherent trade-offs in the strategies proposed. The ambition of personalized medicine to ensure a dynamic revision of disease taxonomies is at odds with requirements for continuity of data, to ensure the preservation and support of existing health system but also to generate the data sources personalized medicine needs. Personalization is dependent on the ability to identify similar patients, which presupposes continuity in the (historical) health records and across sites. Yet, the aim of a highly dynamic taxonomy risks undermining existing practices of data generation and validation, and thereby the very data that personalized diagnostics need.
Moreover, drawing on the Danish case, we have shown how the proposed strategy risks disrupting the current regulatory structure of health care in ways that have previously not received much attention. Information must be gathered from systems also used as regulatory devices for various purposes such as institutional remuneration, quality assurance, financial administration, data integration, as well as legal functions related to liability issues. To serve these and clinical purposes well, and to gather information about local coding practices needed to assess validity, stable rather than rapidly shifting taxonomies are needed. We view the competing demands as trade-offs that must be carefully balanced, as personalized medicine has not found a way to overcome persistent tensions between the quest for fast revision and requirements for continuity in codes and datasets, and between variation and standardization.
The regulatory challenge is threefold. How can knowledge and evidence be generated when the status of diagnostic markers, causal understandings of disease, and principles of categorization and treatment allocation are all under revision at the same time? How will a highly dynamic revision be possible when diagnostic codes are subject to multiple regulatory pressures - as tools for performance measurement, as well as epistemic tools for clinical research and practice? How does the call for taxonomic revision affect standards of evidence? Legitimate changes in health care regulation need to attend to institutionalized thresholds of evidence. In the case of personalized medicine, however, one of the key sources of evidence – phenotypic information – is envisioned to serve seemingly incompatible global and local, and epistemic and regulatory, roles. We have suggested that the current inexhaustible demand for data, together with a sense of time pressure to develop and implement these strategies, aims to change notions of evidence in tandem with reclassification of disease categories.
Political reports outlining the strategies for realizing personalized medicine do not acknowledge the existence of trade-offs or necessary compromises associated with the proposed strategies. Expectations also appear unaffected by disappointing results and remaining uncertainty concerning the causal status and clinical utility of genetic markers. We find it particularly disturbing that the emphasis on fast implementation means that policymakers side-step well-known uncertainties and challenges, and praise accelerated implementation of new treatment forms as a self-evident solution to the current problem in health care systems. In this fast-track environment, individual treatment plans do not need to rest on ‘best available evidence’ in the classical sense: instead, the individual case constitutes an experiment that might generate evidence. Accordingly, we have raised concerns about what may be considered a form of temporal disruption of evidence, where evidence does not precede, but follows treatment, and is used to construct the categories it claims to be based on. Despite the justified criticisms of EBM, it is not clear that personalised medicine has yet put in place an alternative system for knowledge and evidence production that could authoritatively supersede it.
Without dismissing the potential of personalized medicine to improve the future of medicine and health care, we wish to stress that it takes time and careful consideration to provide the desired certainty. Where such certainty is not available, the appropriate attitude is one of humility (Jasanoff, 2007). If developing personalized medicine is akin to constructing an airplane while flying, it may turn out to be as difficult, and as dangerous, as it sounds. Instead of this ‘daredevil’ attitude, that lends a kind of glamour to personalized medicine, slowing down and moderating claims for personalization may be a sounder regulatory route.
Declarations of interest
None.
Acknowledgement
We are grateful for our informants’ time and confidence and thank them sincerely though they must remain anonymous. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement number 682110). Green’s research is supported by The Carlsberg Foundation (Semper Ardens grant CF17-0016). A previous version of the paper was presented at the meeting for the Society for Philosophy of Science in Practice (2018) in Ghent. We thank participants as well as members of the research group Personalized Medicine in the Welfare State (MeInWe) for feedback. Moreover, we would like to express our gratitude to three anonymous reviewers and guest editor Fiona A. Miller for very helpful comments to an earlier version of the paper.
Contributor Information
Sara Green, Email: sara.green@ind.ku.dk.
Annamaria Carusi, Email: a.carusi@sheffield.ac.uk.
Klaus Hoeyer, Email: klho@sund.ku.dk.
References
- Ackerman J.P., Bartos D.C., Kapplinger J.D., Tester D.J., Delisle B.P., Ackerman M.J. The promise and peril of precision medicine: Phenotyping still matters most. Mayo Clin. Proc. 2016;91:1606–1616. doi: 10.1016/j.mayocp.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman S.L., Darling K.W., Lee S.S.-J., Hiatt R.A., Shim J.K. Accounting for complexity: gene–environment interaction research and the moral economy of quantification. Sci. Technol. Hum. Val. 2016;41:194–218. doi: 10.1177/0162243915595462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribas-Ayllon M. After genetization. Soc. Sci. Med. 2016;159:132–139. doi: 10.1016/j.socscimed.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Australian Genomics Health Alliance Integrating genomics into healthcare. 2019. https://www.australiangenomics.org.au/about-us/australian-genomics/ Availbable at:
- Barabási A., Gulbahce N., Loscalzo J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S. From administrative infrastructure to biomedical resource: Danish population registries, the "scandinavian laboratory," and the "epidemiologist's dream. Sci. Context. 2014;27:187–213. doi: 10.1017/s0269889714000040. [DOI] [PubMed] [Google Scholar]
- Bossen C., Danholt P., Klausen M.B. Diagnoser som styringshybrider. Diagnoserelaterede grupper i sundhedsvæsenet. Tidsskrift for Forskning i Sygdom og Samfund. 2016;13 doi: 10.7146/tfss.v13i25.24995. [DOI] [Google Scholar]
- Bowker G.C., Star S.L. MIT Press; Cambridge, MA: 2000. Sorting Things Out: Classification and its Consequences. [Google Scholar]
- Brown N., Michael M. A sociology of expectations: retrospecting prospects and prospecting retrospects. Technol. Anal. Strateg. 2003;15:3–18. [Google Scholar]
- Cambrosio A., Bourret P., Keating P., Nelson N. Opening the regulatory black box of clinical cancer research: transnational expertise networks and “disruptive” technologies. Minerva. 2017;55:161–185. [Google Scholar]
- Cartwright N. A philosopher's view of the long road from RCTs to effectiveness. The Lancet. 2011;377:1400–1401. doi: 10.1016/s0140-6736(11)60563-1. [DOI] [PubMed] [Google Scholar]
- Cyranoski D. China embraces precision therapy: strong genomics record bodes well for health-care revolution. Nature. 2016;529:9–11. doi: 10.1038/529009a. [DOI] [PubMed] [Google Scholar]
- Dagens Medicin 21 kvinner fikk fjernet bryst og eggstokker – skulle ikke vært operert. 02-17. 2017. https://www.dagensmedisin.no/artikler/2017/02/17/21-kvinner-fikk-fjernet-bryst-og-eggstokker--skulle-ikke-vart-operert/ Available online:
- DAMVAD Analytics Analyse af personlig medicin. 2016. https://www.regioner.dk/media/3128/damvad-rapport-analyse-af-personlig-medicin.pdf Available online.
- Danske Regioner https://www.regioner.dk/sundhed/medicin/personlig-medicin webpage:
- Danske Regioner Handlingsplan for personlig medicin. København: Danske Regioner, 2015 [Google Scholar]
- Danske Regioner . 2015. Personlig medicin og individualiseret behandling. Oplæg til en samlet dansk indsats. København: Danske Regioner. [Google Scholar]
- De Grandis G., Halgunset V. Conceptual and terminological confusion around personalised medicine: a coping strategy. BMC Med. Ethics. 2016;17:43. doi: 10.1186/s12910-016-0122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drahos P. ANU Press; Acton Act, Australia: 2017. Regulatory Theory: Foundations and Applications. [Google Scholar]
- Dumit J. Duke University Press; Durham and London, UK: 2012. Drugs for Life: How Pharmaceutical Companies Define Our Health. [Google Scholar]
- European Commission Citizen-centred EU-EHR exchange for personalised health. 2019. https://cordis.europa.eu/project/rcn/219932/factsheet/en, 14/2-2019 Grant agreement available at:
- European Science Foundation . European Science Foundation; Strasbourg: 2012. Personalised Medicine for the European Citizen. Towards More Precise Medicine for the Diagnosis, Treatment and Prevention of Disease (iPM) [Google Scholar]
- Featherstone K., Atkinson P. Routledge; New York: 2012. Creating Conditions: the Making and Remaking of a Genetic Syndrome. [Google Scholar]
- Friedman C.P., Wong A.K., Blumenthal D. Achieving a nationwide learning health system. Sci. Transl. Med. 2010;2:1–3. doi: 10.1126/scitranslmed.3001456. [DOI] [PubMed] [Google Scholar]
- Gandal M.J., Haney J.R., Parikshak N.N., Liu C. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–697. doi: 10.1126/science.aad6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomics England . 2017. The 100,000 Genomes Project Protocol V3. Available online. [DOI] [Google Scholar]
- Germain L., Baetu T. In: Philosophy of Molecular Medicine. Foundational Issues in Research and Practice. Boniolo G., Nathan M.J., editors. Routledge; New York: 2017. Forms of extrapolation in molecular medicine; pp. 217–235. [Google Scholar]
- Greenhalgh T., Howick J., Maskrey N. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725. doi: 10.1136/bmj.g3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecoe A. Schizophrenia and the narrative of enlightened geneticization. Soc. Stud. Sci. 2001;31:875–911. doi: 10.1177/030631201031006004. [DOI] [PubMed] [Google Scholar]
- Hedgecoe A.M. Reinventing diabetes: classification, division and the geneticization of disease. New Genet. Soc. 2002;21:7e27. [Google Scholar]
- Hedgecoe A.M. Expansion and uncertainty: cystic fibrosis, classification and genetics. Sociol. Health Illness. 2003;25:50–70. doi: 10.1111/1467-9566.t01-2-00324. [DOI] [PubMed] [Google Scholar]
- Hedgecoe A.M. Cambridge University Press; Cambridge UK: 2004. The Politics of Personalized Medicine. Pharmacogenetics in the Clinic. [Google Scholar]
- Hoeyer, K., forthcoming. Data as promise: reconfiguring Danish public health through personalised medicine, Soc. Stud. Sci. [DOI] [PubMed]
- Hoeyer K. In: The Ethics of Biomedical Big Data. Floridi L., Mittelstadt B., editors. Springer; Dordrecht: 2016. Denmark at a crossroad? Intensified data sourcing in a research radical country; pp. 73–94. [Google Scholar]
- Hoeyer K. In: Personalized Medicine, Individual Choice and the Common Good. Beers B., Sterckx S., Dickenson D., editors. Cambridge University Press; Cambridge: 2018. Lost and found: relocating the individual in the age of intensified data sourcing in European healthcare; pp. 144–166. [Google Scholar]
- Hofmann B. The overdiagnosis of what? On the relationship between the concepts of overdiagnosis, disease, and diagnosis. Med. Health Care Philos. 2017;20:453–464. doi: 10.1007/s11019-017-9776-z. [DOI] [PubMed] [Google Scholar]
- Hogle L.F. In: Global Perspectives on Stem Cell Technologies. Bharadwaj A., editor. Palgrave Macmillan; Cham: 2018. Intersections of technological and regulatory zones in regenerative medicine; pp. 51–84. [Google Scholar]
- Hood L., Lovejoy J.C., Price N.D. Integrating big data and actionable health coaching to optimize wellness. BMC Med. 2015;13:4. doi: 10.1186/s12916-014-0238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L., Romero D. Precision or imprecision medicine? Nat. Rev. Clin. Oncol. 2016;13:713. doi: 10.1038/nrclinonc.2016.190. [DOI] [PubMed] [Google Scholar]
- James J.E. Personalised medicine, disease prevention, and the inverse care law: more harm than benefit? Eur. J. Epidemiol. 2014;29:383–390. doi: 10.1007/s10654-014-9898-z. [DOI] [PubMed] [Google Scholar]
- Jasanoff S. Technologies of humility. Nature. 2007;450:33. doi: 10.1038/450033a. [DOI] [PubMed] [Google Scholar]
- Jutel A., Nettleton S. Towards a sociology of diagnosis: reflections and opportunities. Soc. Sci. Med. 2011;73:793–800. doi: 10.1016/j.socscimed.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Jylling E. Registreringskrav i sundhedsvæsenet: støj eller værdiskabende? OUH Talks September. 2017;6:2017. http://www.ouh.dk/wm502925 Slides available online. [Google Scholar]
- Keating P., Cambrosio A., Nelson N.C. “Triple negative breast cancer”: translational research and the (re) assembling of diseases in post-genomic medicine. Stud. Hist. Philos. Biol. Biomed. Sci. 2016;59:20–34. doi: 10.1016/j.shpsc.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Lambert H. Accounting for EBM: notions of evidence in medicine. Soc. Sci. Med. 2006;62:2633–2645. doi: 10.1016/j.socscimed.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Le Fanu J. Little Brown Book Group; London: 1999/2011. The Rise and Fall of Modern Medicine. [DOI] [PubMed] [Google Scholar]
- Lek M., Karczewski K.J., Minikel E.V., Tukiainen T. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine M. In: Philosophy of Molecular Medicine. Foundational Issues in Research and Practice. Boniolo G., Nathan M.J., editors. Routledge; New York: 2017. Molecular complexity. Why has psychiatry not been revolutionized by genomics (yet)? pp. 81–99. [Google Scholar]
- Leonelli S. University of Chicago Press; Chicago and London: 2016. Data-centric Biology: A Philosophical Study. [Google Scholar]
- Lippman A. Led (astray) by genetic maps: the cartography of the human genome and health care. Soc. Sci. Med. 1992;35:1469–1476. doi: 10.1016/0277-9536(92)90049-v. [DOI] [PubMed] [Google Scholar]
- Minari J., Brothers K.B., Morrison M. 2018. Tensions in Ethics and Policy Created by. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Social Affairs and Health . 2015. Improving Health through the Use of Genomic Data. Finland's Genome Strategy. Finland's Genome Strategy Working Group Proposal. Ministry of Social Affairs and Health Raportteje Ja Muistioita 34/2015: 34. [Google Scholar]
- Ministry of Health and Danish Regions Personalized medicine for the benefit of patients. Clear diagnosis, targeted treatment, strengthened research. National Strategy for personalized medicine 2017-2010. 2016. https://www.regioner.dk/sundhed/medicin/personlig-medicin Available online:
- National Academy of Sciences (NAS) Committee on A Framework for Developing a New Taxonomy of Disease. The National Academies Press; Wastington, DC: 2011. Toward precision medicine. Building a knowledge-network for biomedical resarch and a new taxonomy of disease. [PubMed] [Google Scholar]
- National Institutes of Health All of Us research program. Protocol Version v.1.7. 2018. https://allofus.nih.gov/about/all-us-research-program-protocol Available online:
- Novo Nordisk Foundation Novo Nordisk Foundation supports new national genome center. 2018. https://novonordiskfonden.dk/en/news/novo-nordisk-foundation-supports-new-national-genome-centre/
- Perkins B.A., Caskey C.T., Brar P., Hands G. Precision medicine screening using whole-genome sequencing and advanced imaging to identify disease risk in adults. Proc. Natl. Acad. Sci. U.S.A. 2018;115:3686–3691. doi: 10.1073/pnas.1706096114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prainsack B. Is personalized medicine different? (Reinscription: the sequel) A response to Troy Duster. Br. J. Sociol. 2015;66:28–35. doi: 10.1111/1468-4446.12117. [DOI] [PubMed] [Google Scholar]
- Price N.D., Magis A.T., Earls J.C., Qin S. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat. Biotechnol. 2017;35:747–756. doi: 10.1038/nbt.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees J. Evidence-based medicine: the epistemology that isn't. J. Am. Acad. Dermatol. 2000;43:727–729. doi: 10.1067/mjd.2000.108368. [DOI] [PubMed] [Google Scholar]
- Rose N. Institute of Psychiatry; London: 2013. What Is Diagnosis for? DSM 5 and the Future Of Diagnosis.https://nikolasrose.com/wp-content/uploads/2013/07/Rose-2013-What-is-diagnosis-for-IoP-revised-July-2013.pdf 4th June 2013. Available at: [Google Scholar]
- Ross C. Genetic test costs taxpayers $500 million a year, with little to show for it. Stat News Nov 2. 2016. https://www.statnews.com/2016/11/02/genetic-test-medical-costs/ Available online:
- Saracci R. Epidemiology in wonderland: big Data and precision medicine. Eur. J. Epidemiol. 2018;33:245. doi: 10.1007/s10654-018-0385-9. [DOI] [PubMed] [Google Scholar]
- Senn S. Mastering variation: variance components and personalised medicine. Stat. Med. 2016;35:966–977. doi: 10.1002/sim.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J.S., Aletaha D. Forget personalised medicine and focus on abating disease activity. Ann. Rheum. Dis. 2013;72:3–6. doi: 10.1136/annrheumdis-2012-202361. [DOI] [PubMed] [Google Scholar]
- Solomon M. Just a paradigm: evidence-based medicine in epistemological context. Euro. Jnl. Phil. Sci. 2011;1:451. [Google Scholar]
- Stanovich K.E. University of Chicago Press; Chicago: 2004. The Robot's Rebellion: Finding Meaning in the Age of Darwin. [Google Scholar]
- Stegenga J. Oxford University Press; Oxford: 2018. Medical Nihilism. [Google Scholar]
- Tarkkala H., Helén I., Snell K. From health to wealth: the future of personalized medicine in the making. Futures. 2018 doi: 10.1016/j.futures.2018.06.004. [DOI] [Google Scholar]
- Timmermans S. Trust in standards: transitioning clinical exome sequencing from bench to bedside. Soc. Stud. Sci. 2015;45:77–99. doi: 10.1177/0306312714559323. [DOI] [PubMed] [Google Scholar]
- Timmermans S., Berg M. Temple University Press; Philadelphia: 2003. The Gold Standard: the Challenge of Evidence-Based Medicine and Standardization in Health Care. [Google Scholar]
- Timmermans S., Tietbohl C., Skaperdas E. Narrating uncertainty: variants of uncertain significance (VUS) in clinical exome sequencing. BioSocieties. 2016;12:439–458. [Google Scholar]
- Tomczak K., Czerwińska P., Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp. Oncol. 2015;19:A68. doi: 10.5114/wo.2014.47136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli M.R., Shirts B.H. Knowledge for precision medicine: mechanistic reasoning and methodological pluralism. J. Am. Med. Assoc. 2017;318:1649–1650. doi: 10.1001/jama.2017.11914. [DOI] [PubMed] [Google Scholar]
- Tupasela A. Populations as brands in medical research: placing genes on the global genetic atlas. BioSocieties. 2017;12:47–65. [Google Scholar]
- Vezyridis P., Timmons S. Understanding the care. data conundrum: new information flows for economic growth. Big Data & Society. 2017;4 doi: 10.1177/2053951716688490. [DOI] [Google Scholar]
- Wadmann S., Hoeyer K. Big Data & Society; 2018. Dangers of the Digital Fit: Rethinking Seamlessness and Social Sustainability in Data-Intensive Healthcare. [DOI] [Google Scholar]