Abstract
Alcohol dependence is a chronic mental disorder that leads to decreased quality of life for patients and their relatives and presents a considerable burden to society. Incretin hormones, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are endogenous gut-brain peptides, which can travel across the blood-brain barrier and access the nervous system. Their respective receptors, GIPR and GLP-1R, are expressed in the reward-related brain areas and are involved in memory formation and neurogenesis, which results in behavioral changes in rodent models. The current study investigated the potential association of genetic variability of incretin receptors with alcohol dependence and alcohol-related psychosymptomatology. Alcohol dependence and comorbid psychosymptomatology were assessed in a cohort of Slovenian male participants, comprised of 89 hospitalized alcohol-dependent patients, 98 abstinent alcohol-dependent patients, and 93 healthy blood donors. All participants were genotyped for GIPR rs1800437 and GLP1R rs10305420 and rs6923761 polymorphisms. For the statistical analysis Kruskal–Wall and Mann–Whitney tests were used in additive and dominant genetic models. Our findings indicated that GIPR rs1800437 genotypes were associated with an increased risk of alcohol dependence. Statistically significant association between GIPR rs1800437 GG genotype and Brief Social Phobia Scale scores were observed in the abstinent alcohol-dependent patients, while GLP1R rs6923761 GG genotype was associated with Zung anxiety scores in healthy controls. Our pilot study indicates that GIPR rs1800437 may play some role in susceptibility to alcohol dependence, as well as in alcohol-related psychosymptomatology symptoms. To our knowledge, this is the first study that indicates the involvement of GIPR in alcohol dependence. However, studies with larger cohorts are needed to confirm these preliminary findings.
Keywords: alcohol dependence, alcohol-related psychosymptomatology, incretin receptors, GIPR, GLP-1R, polymorphism
Introduction
Alcohol dependence is a chronic mental disorder characterized by an intense craving for alcohol and the inability to control or stop alcohol consumption, usually accompanied by a history of excessive drinking (Carvalho et al., 2019; Domi et al., 2021). Regarding its epidemiology, alcohol dependence is one of the most prevalent mental disorders worldwide, and it is five times more frequent in men than in women. In addition, alcohol dependence was found to be more frequent in high-income and upper -middle- income countries for both males and females (Carvalho et al., 2019). Alcohol dependence is also associated with high morbidity and mortality rates. Alcohol dependence is also related to other comorbid mental disorders, such as major depressive disorder, anxiety disorders, schizophrenia, bipolar disorder, and attention deficit hyperactivity disorder (Gandal et al., 2018; Kranzler and Soyka, 2018; Walters et al., 2018; Rudenstine et al., 2020; Zhou et al., 2020).
Alcohol dependence leads to decreased quality of life for patients and their relatives and presents a considerable burden to society (Carvalho et al., 2019; Klausen et al., 2022). Alcohol dependence and alcohol abuse used to be separate disorders in the Diagnostic and Statistical Manual of mental disorders (DSM-IV), whereas, in DSM-V, they are integrated into one broader category of alcohol use disorder (AUD) which includes sub-classifications, depending on the severity of the symptoms (Kathryn Mchugh and Weiss, 2019; Nutt et al., 2021). The stages of dependence can be divided into the acute and chronic state, followed by short-term and long-term abstinence. What sets them apart is the duration of each stage and the underlying molecular and cellular mechanisms involved (Nestler and Aghajanian, 1997; Koob and Volkow, 2016).
The emergence and perpetuation of AUD can be due to several factors, including genetic, environmental risk factors, and gene-environment interactions (Nestler and Aghajanian, 1997; Carvalho et al., 2019). Family, twin, and adoption studies (Cloninger et al., 1981; Heath et al., 1997; Verhulst et al., 2015) and a recent meta-analysis (Verhulst et al., 2015) indicated that heritability estimates are pretty high. Preclinical and clinical studies have shown that genetic variability is associated with susceptibility and development of AUD (Hiroi and Agatsuma, 2005; Jones et al., 2015; Bowen et al., 2022). A genome-wide meta-analysis on AUD and problematic alcohol use, which included 435,563 subjects of European ancestry, identified 29 independent risk variants, 19 of which were novel (Zhou et al., 2020).
Glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide, also known as gastric inhibitory polypeptide (GIP), are endogenous gut-brain peptides that function both as a hormone and neuropeptide and are released from intestinal L-cells and K-cells, respectively, in response to food intake in humans and mice (Adner and Nygren, 1992; Dalgaard et al., 2004; Alvarez et al., 2005; Pannacciulli et al., 2007; Seino et al., 2010; Seino and Yabe, 2013; Abraham et al., 2015; Jerlhag, 2020; Marty et al., 2020; Eren-Yazicioglu et al., 2021). They stimulate glucose-induced insulin secretion, inhibit glucagon secretion, and decrease appetite in humans and mice (Adner and Nygren, 1992; Dalgaard et al., 2004; Seino et al., 2010; Underwood et al., 2010; Seino and Yabe, 2013; Abraham et al., 2015; Klausen et al., 2022). They have the ability to travel across the blood-brain barrier and access the nervous system in humans and rats (Isbil-Buyukcoskun et al., 2009; Eren-Yazicioglu et al., 2021). GLP-1 and GIP act by binding with their receptors GLP-1R and GIPR, respectively, which are members of the G-protein coupled receptors and are expressed in the peripheral and central nervous system (Seino et al., 2010; Seino and Yabe, 2013). GLP-1 and GIP and their receptors are involved in memory formation and neurogenesis in rats (Seino et al., 2010). GIPR is expressed in neurons, and GLP-1R can be found in several tissues, including human, rat and mice brain regions related to reward and addiction (Merchenthaler et al., 1999; Alvarez et al., 2005; Pannacciulli et al., 2007; Rinaman, 2010; Seino et al., 2010; Suchankova et al., 2015; Jensen et al., 2018; Eser et al., 2020; Eren-Yazicioglu et al., 2021). It can also modulate dopamine levels and glutamatergic neurotransmission, which results in behavioral changes in rats, and mice (Alhadeff et al., 2012; Reddy et al., 2016; Eren-Yazicioglu et al., 2021). Also, it displayed neuroprotective effects in male rats, mice, non-human primates (Klausen et al., 2022) and humans (Erbil et al., 2019). Furthermore, both preclinical and clinical studies indicated the crucial role of GLP-1R in reward function and addictive disorders, including alcohol-seeking and AUD (Egecioglu et al., 2013; Suchankova et al., 2015; Jayaram-Lindström et al., 2016; Marty et al., 2020; Eren-Yazicioglu et al., 2021). Animal model studies have suggested that peptides like GLP-1 regulate behavioral responses to alcohol consumption (Seino et al., 2010; Jerlhag, 2020), however, the role of GIP and its receptor has not been studied so well and the exact mechanism of action is not yet fully known.
GLP-1R also seems to have a neuroprotective role and, thus, has been investigated as a target in the cerebral infarction treatment (Seino and Yabe, 2013). There is also evidence that GLP-1R stimulation regulates alcohol-seeking and wanting behaviors (Suchankova et al., 2015; Eren-Yazicioglu et al., 2021). Nevertheless, based on the mechanism of action of FDA-approved GLP-1 receptor agonists, reduction in alcohol consumption can be due to the discomfort felt during alcohol use and abstinence and reduction in rewarding effects. GLP-1R agonists reduce the rewarding effects of alcohol, which leads to decreased alcohol intake. Exentin-4 is a well know example of GLP-1R agonist that affects the signal transmission, and according to studies, GLP-1R and GIPR have similar molecular mechanisms (Seino et al., 2010).
Genetic variability of GLP-1R and GIPR has been investigated in human pathologies, such as metabolic and cardiovascular diseases, and bone mineral density. According to the literature, genetic variability influences response to incretin peptides and their antagonists (Jensterle et al., 2015; Klen and Dolžan, 2022). More specifically, GLP1R rs10305420 has been associated with response to exenatide in overweight patients with type 2 diabetes (Yu et al., 2019) and liraglutide in obese women with polycystic ovary syndrome (Jensterle et al., 2015). Regarding GLP1R rs6923761, it has been shown to relate with metabolic and obesity parameters, such as body mass index, weight, fat mass, waist circumference, triglycerides, insulin, HOMA-IR, and HDL cholesterol (de Luis et al., 2013, 2014a,b,c,e, 2015b,d, 2018), weight loss (de Luis et al., 2014a,d), cardiovascular risk in patients with obesity (de Luis et al., 2018), type 2 diabetes (de Luis et al., 2015a). It has also been associated with gliptin therapies, like the DPP-4 inhibitor sitagliptin and vildagliptin (Javorský et al., 2016; Urgeová et al., 2020; Mashayekhi et al., 2021), liraglutide and exenatide (de Luis et al., 2015c; Chedid et al., 2018). GIPR rs1800437 has been associated with glucose homeostasis (Sauber et al., 2010), obesity (Vogel et al., 2009), heart failure prognosis in obese patients (Agra et al., 2019), bone mineral density and fracture risk (Torekov et al., 2014).
Glucagon-like peptide 1 variability has been investigated in two preclinical studies with mice AUD models, one of which also included a cohort of AUD patients and controls (Koole et al., 2011; Suchankova et al., 2015). However, to our knowledge, there are no studies that focus on GIPR polymorphisms and alcohol.
The current study aimed to investigate the potential association of GLP1R rs10305420 and rs6923761 and GIPR rs1800437 with alcohol dependence, as well as alcohol-related comorbid psychosymptomatology.
Materials and Methods
Study Population
The study cohort included three groups of participants: hospitalized alcohol-dependent patients, abstinent alcohol-dependent patients, and healthy controls with no alcohol dependence history. All participants were male of Slovenian origin, aged 18 to 66. Experienced psychiatrists recruited patients hospitalized for treatment of alcohol dependence at the University Clinical Center Maribor and the University Psychiatric Clinic Ljubljana. The inclusion criteria for the hospitalized alcohol-dependent patients were a diagnosis of alcohol-dependence, according to the DSM-IV (American Psychiatric Association, 2000), with no significant symptoms of abstinence, after hospitalization for at least 2 weeks. The abstinent alcohol-dependent patients were recruited from support group meetings, and the inclusion criterion was abstinence for at least 2 years. Exclusion criteria for these two groups of patients were: a medical history of mental or neurological disorders or significant medical conditions and a previous diagnosis of dependence (nicotine not included) according to DSM-IV. The healthy controls were blood donors with no DSM-IV axis I mental disorders or alcohol consumption problems. The study was approved by the Slovenian National Medical Ethics committee (approval No. 117/06/10 and 148/02/1011).
Written informed consent was obtained from all the participants after they were informed about the scope of the study. At the baseline, all the demographic and clinical data of each patient were also recorded. Demographic variables included age, residence, marital status, academic years, and smoking status. In addition, questionnaires that evaluate comorbid psychosymptomatology were employed in all groups of participants. More specifically, depression and anxiety symptoms were accessed using the Zung Depression (Zung, 1965) and Anxiety (Zung, 1971) scale, social anxiety symptoms, using the Brief Social Phobia Scale (BSPS) (Davidson et al., 1997), drinking habits, and severity of alcohol use and dependence using the Alcohol Use Disorders Identification Test (AUDIT) (Rush et al., 2008), obsessive-compulsive traits, using the Yale-Brown Obsessive-Compulsive Scale (YBOCS) (Goodman and Price, 1992) and Obsessive-Compulsive Drinking Scale (OCDS) (Anton, 2000), and symptoms of aggression and hostility, were evaluated using the and the Buss-Durkee Hostility Inventory (BDHI) (Buss and Durkee, 1957). More information about the cohorts can be found in our previous articles (Plemenitas et al., 2015; Ilješ et al., 2021).
Molecular Genetic Analysis
DNA was extracted from whole blood for hospitalized alcohol-dependent patients and healthy controls, whereas DNA was extracted from buccal swabs for the abstinent alcohol-dependent group of patients. QIAamp Blood Mini kit was used for the DNA extraction from whole blood, collected using ethylenediaminetetraacetic acid (EDTA) and QIAamp Mini kit for the DNA extraction from buccal swabs, according to the manufacturer’s protocols (Qiagen GmbH, Hilden, Germany).
Genotyping was performed using fluorescence-based competitive allele-specific PCR (KASP) amplification combining KASP Master mix and custom validated KASP Genotyping Assays with a KASP reporting system, according to the manufacturer’s instructions (LGC Genomics, United Kingdom). Thermal cycling conditions are presented in Supplementary Table 1.
Statistical Analysis
The statistical analyses were performed with IBM SPSS Statistics, version 27.0 (IBM Corporation, Armonk, NY, United States). The cut-off for the statistical significance was set at 0.05. Pearson’s chi-square test was used to assess deviation from Hardy–Weinberg equilibrium (HWE) in healthy individuals for all studied polymorphisms. Additive and dominant genetic models were used in the analysis. To compare clinical characteristics between patient groups, we used Fisher’s exact test for categorical variables and the Kruskal–Wallis test with 2 degrees of freedom for continuous variables. Fisher’s exact test was also used to compare the frequencies of the rs10305420, rs6923761, and rs1800437 between the three studied groups. In logistic regression, odds ratios (ORs) and 95% confidence intervals (CIs) were determined. Age, residence place, marital status, academic years, and smoking status were considered as covariates and significant variables were used for adjustment in regression analysis. The association of genotypes with psychosymptomatology scores was evaluated using the Kruskal–Wallis and Mann–Whitney non-parametric tests for additive and dominant genetic models, respectively.
Results
Our cohort comprised of 89 hospitalized alcohol-dependent patients, 98 abstinent alcohol-dependent patients, and 93 healthy controls with no alcohol dependence history (Table 1). Regarding the demographic characteristics, the median age of the hospitalized alcohol-dependent and abstinent alcohol-dependent patients was significantly higher compared to healthy controls (p < 0.001). The distribution of the years of education also differed among groups (p < 0.001), but there were no differences in residence place (p = 0.265). However, the majority of healthy controls and abstinent alcohol-dependent patients were smokers (p < 0.001) and had a partner (p = 0.005) in comparison with hospitalized alcohol-dependent patients (Table 1).
TABLE 1.
Cohort’s characteristics.
| Characteristic | Healthy controls (N = 93) | Abstinent alcohol-dependent (N = 98) | Hospitalized alcohol-dependent (N = 89) | P * | |
| Age | Years, median (25–75%) | 36 (26–44.5) | 49 (44–54.3) | 47 (39–54) | <0.001 |
| Education | Years, median (25–75%) | 12 (12–12) | 12 (11–12) | 12 (11–12) | <0.001 |
| Partnership | Single, N (%) | 25 (26.9) | 21 (21.4) | 38 (42.7) | 0.005 |
| Partnership, N (%) | 68 (73.1) | 77 (78.6) | 51 (57.3) | ||
| Environment | Rural, N (%) | 37 (39.8) | 46 (46.9) | 46 (51.7) | 0.265 |
| Urban, N (%) | 56 (60.2) | 52 (53.1) | 43 (48.3) | ||
| Smoking | No, N (%) | 24 (25.8) | 48 (49.0) | 58 (65.2) | <0.001 |
| Yes, N (%) | 69 (74.2) | 50 (51.0) | 31 (34.8) |
*Calculated using Fisher’s exact test for categorical variables and Kruskal–Wallis test for continuous variables.
Regarding the questionnaires, differences were observed between the three groups in the scores of Zung Depression and Anxiety scale, YBOCS obsession and compulsion scale, AUDIT, OCDS, and BDHI questionnaires (all p < 0.05), but not for BSPS (p = 0.623) (Table 2 and Supplementary Table 2).
TABLE 2.
Questionnaire scores.
| Questionnaire | Healthy controls (N = 93) | Abstinent alcohol-dependent (N = 98) | Hospitalized alcohol-dependent (N = 89) | P * | |
| YBOCS obsession | Points, median (25–75%) | 1 (1–1) | 1 (1–1.3) | 2 (1–7) | <0.001 |
| YBOCS compulsion | Points, median (25–75%) | 1 (1–1) | 1 (1–1) | 1 (1–3) | <0.001 |
| BSPS | Points, median (25–75%) | 9 (5.5–14) | 10 (5–18.3) | 10 (4–18.5) | 0.623 |
| AUDIT | Points, median (25–75%) | 5 (4–7) | 3 (3–5) | 23 (19–28.5) | <0.001 |
| OCDS | Points, median (25–75%) | 3 (2–4) | 2 (2–3) | 18 (9–26.5) | <0.001 |
| Zung depression | Points, median (25–75%) | 22 (20–24) | 29 (25–35) | 34 (27–45) | <0.001 |
| Zung anxiety | Points, median (25–75%) | 22 (20–24) | 28 (25–35) | 34 (29–39) | <0.001 |
| BDHI | Points, median (25–75%) | 17 (10.5–23) | 24 (15.8–31) | 30 (22–40) | <0.001 |
*Calculated using Kruskal–Wallis test.
The genotype distributions for all the studied polymorphisms were in HWE for the healthy controls (GG: 65.6%, GC: 26.9%, CC: 7.5%; p = 0.068 for rs1800437, CC: 51.1%, CT: 37.8%, TT: 11.1%; p = 0.340 for rs10305420 and GG: 46.2%, GA: 45.2%, AA: 8.6%; p = 0.614 for rs6923761).
When comparing all three groups, no differences in the distribution of genotype frequencies were observed for any of the studied polymorphisms (p = 0.155 for rs1800437; p = 0.645 for rs10305420 and p = 0.632 for rs6923761) (Supplementary Table 3).
Given that both the groups of hospitalized and abstinent patients had the diagnosis of alcohol dependence, we merged these two groups into one and we compared the genotype frequencies with those of the healthy controls, an association was observed for GIPR rs1800437 GC (OR = 1.77, 95% CI = 1.02–3.09, p = 0.043) and GC + CC genotypes (OR = 1.69, 95% CI = 1.01–2.84, p = 0.045), but it did not remain statistically significant after adjustment for age, education, smoking, and partnership. No associations were observed for GLP1R polymorphisms (Table 3).
TABLE 3.
Comparison of genotype frequencies between all alcohol-dependent patients and healthy controls.
| Gene | SNP | Genotype | OR (95% CI) | P | OR (95% CI) adj | Padj |
| GIPR | rs1800437 | GG | Reference | Reference | ||
| GC | 1.77 (1.02–3.09) | 0.043 | 1.71 (0.85–3.44) | 0.135 | ||
| CC | 1.41 (0.55–3.62) | 0.477 | 1.79 (0.59–5.46) | 0.303 | ||
| GC + CC | 1.69 (1.01–2.84) | 0.045 | 1.73 (0.90–3.31) | 0.100 | ||
| GLP1R | rs10305420 | CC | Reference | Reference | ||
| CT | 1.07 (0.63–1.82) | 0.809 | 1.24 (0.62–2.47) | 0.548 | ||
| TT | 1.44 (0.64–3.23) | 0.374 | 1.26 (0.46–3.47) | 0.653 | ||
| CT + TT | 1.15 (0.70–1.89) | 0.583 | 1.24 (0.66–2.35) | 0.505 | ||
| GLP1R | rs6923761 | GG | Reference | Reference | ||
| GA | 0.96 (0.57–1.61) | 0.866 | 1.13 (0.58–2.21) | 0.721 | ||
| AA | 0.65 (0.24–1.73) | 0.389 | 0.38 (0.11–1.31) | 0.124 | ||
| GA + AA | 0.91 (0.55–1.49) | 0.702 | 0.96 (0.51–1.82) | 0.910 |
Adj: adjusted for age, education, smoking, and partnership. Statistically significant p values are printed in bold.
We also compared each group of alcohol-dependent patients with the controls separately. When comparing genotype frequencies between abstinent alcohol-dependent patients and healthy controls, no statistically significant difference was observed for any of the three studied polymorphisms, neither before nor after adjustments for age, education and smoking (Supplementary Table 4).
However, GIPR rs1800437 GC + CC and GC genotypes were significantly more frequent in hospitalized alcohol-dependent patients than in healthy controls (OR = 2.13, 95% CI = 1.17–3.87, p = 0.013 and OR = 2.21, 95% CI = 1.16–4.19, p = 0.015, respectively). The association remained statistically significant for GC + CC genotypes in the dominant model after adjustment for age, education, smoking, residence, and partnership (OR = 2.42, 95% CI = 1.07–5.48, p = 0.035). No significant differences in GLP1R genotype frequencies’ distribution were observed between these two groups (Table 4).
TABLE 4.
Comparison of genotype frequencies between hospitalized alcohol-dependent patients and healthy controls.
| Gene | SNP | Genotype | OR (95% CI) | P | OR (95% CI) adj | Padj |
| GIPR | rs1800437 | GG | Reference | Reference | ||
| GC | 2.21 (1.16–4.19) | 0.015 | 2.15 (0.90–5.15) | 0.087 | ||
| CC | 1.87 (0.65–5.41) | 0.250 | 3.69 (0.93–14.70) | 0.064 | ||
| GC + CC | 2.13 (1.17–3.87) | 0.013 | 2.42 (1.07–5.48) | 0.035 | ||
| GLP1R | rs10305420 | CC | Reference | Reference | ||
| CT | 1.08 (0.57–2.03) | 0.818 | 1.20 (0.50–2.88) | 0.680 | ||
| TT | 1.88 (0.77–4.60) | 0.167 | 1.50 (0.43–5.20) | 0.524 | ||
| CT + TT | 1.25 (0.70–2.24) | 0.450 | 1.27 (0.57–2.85) | 0.556 | ||
| GLP1R | rs6923761 | GG | Reference | Reference | ||
| GA | 0.93 (0.51–1.70) | 0.820 | 0.66 (0.29–1.55) | 0.343 | ||
| AA | 0.36 (0.09–1.44) | 0.148 | 0.23 (0.04–1.41) | 0.112 | ||
| GA + AA | 0.84 (0.47–1.51) | 0.560 | 0.58 (0.26–1.30) | 0.186 |
Adj: adjusted for age, education, smoking, residence, and partnership. Statistically significant p values are printed in bold.
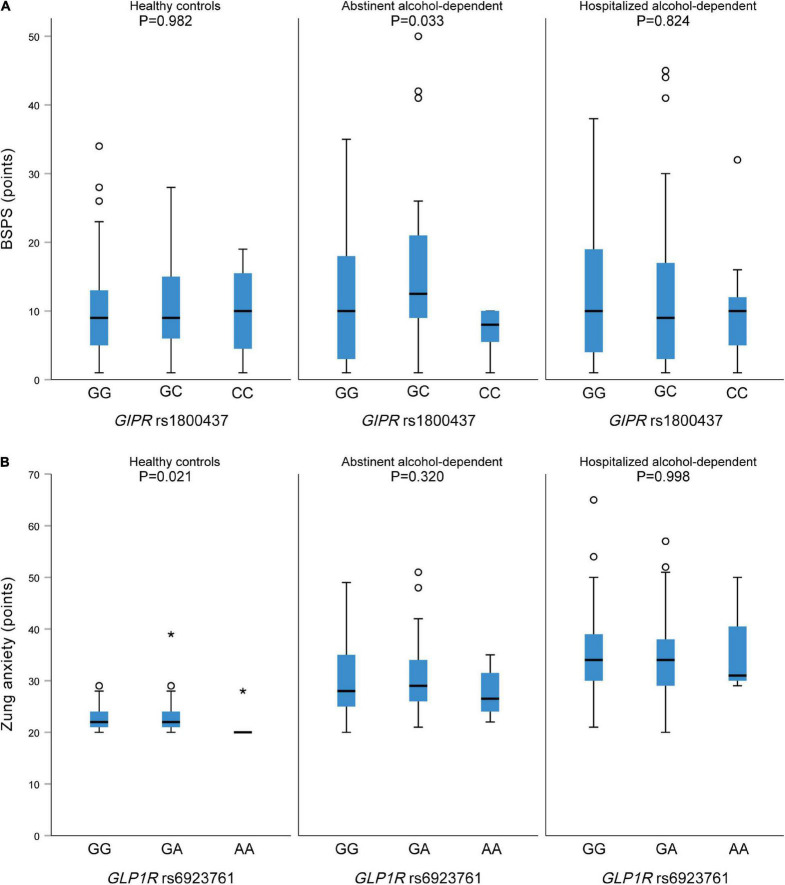
Regarding the potential relation between the studied polymorphisms and psychosymptomatology scores, we observed a statistically significant association between GIPR rs1800437 CC genotype and lower BSPS scores in the abstinent alcohol-dependent patients (p = 0.033) (Figure 1A). GIPR genotypes were not associated with any of the other psychosymptomatology scores (Table 5).
FIGURE 1.
(A) Association between GIPR rs1800437 and BSPS score in healthy controls (left), abstinent (middle) and hospitalized alcohol-dependent (right) patients. (B) Association between GLP1R rs6923761 and Zung anxiety score in healthy controls (left), abstinent (middle) and hospitalized alcohol-dependent (right) patients.
TABLE 5.
Associations between GIPR rs1800437 and the assessed psychosymptomatology scores.
| Scale | GIPR rs1800437 genotype | Healthy controls (N = 93) |
Abstinent alcohol-dependent (N = 98) | Hospitalized alcohol-dependent (N = 89) | |||
|
|
|
|
|||||
| Median (25–75%) | P * | Median (25–75%) | P * | Median (25–75%) | P * | ||
| YBOCS obsession | GG | 1 (1–1) | 0.880 | 1 (1–2) | 0.227 | 2 (1–7) | 0.129 |
| GC | 1 (1–1.5) | 1 (1–1) | 1 (1–8.3) | ||||
| CC | 1 (1–4) | 1 (1–2) | 1 (1–1.5) | ||||
| GC + CC | 1 (1–1.8) | 0.991 | 1 (1–1) | 0.118 | 1 (1–7) | 0.228 | |
| YBOCS compulsion | GG | 1 (1–1) | 0.653 | 1 (1–1) | 0.098 | 1 (1–6) | 0.213 |
| GC | 1 (1–1) | 1 (1–1) | 1 (1–2.3) | ||||
| CC | 1 (1–2) | 1 (1–2) | 1 (1–1) | ||||
| GC + CC | 1 (1–1) | 0.677 | 1 (1–1) | 0.315 | 1 (1–2) | 0.356 | |
| BSPS | GG | 9 (5–13.5) | 0.982 | 10 (3–18) | 0.033 | 10 (4–19) | 0.824 |
| GC | 9 (6–16) | 12.5 (8.8–21.5) | 9 (3–17.5) | ||||
| CC | 10 (3–18) | 8 (3–10) | 10 (3–14) | ||||
| GC + CC | 9 (6–16.5) | 0.894 | 11 (8–20) | 0.118 | 9 (3–17) | 0.714 | |
| AUDIT | GG | 5 (4–7) | 0.194 | 3 (3–4) | 0.999 | 23 (18.8–29.3) | 0.208 |
| GC | 4 (3–6) | 3 (3–5) | 23 (19.8–28) | ||||
| CC | 6 (5–8) | 3 (3–5) | 27 (22.5–32.5) | ||||
| GC + CC | 5 (3–6) | 0.297 | 3 (3–5) | 0.959 | 23 (20–28) | 0.792 | |
| OCDS | GG | 3 (2–4) | 0.444 | 2 (2–3) | 0.885 | 16 (9.8–26) | 0.889 |
| GC | 3 (2–4) | 2 (2–3.3) | 18 (8.8–29) | ||||
| CC | 4 (2–5) | 2 (2–4) | 19 (8–24.5) | ||||
| GC + CC | 3 (2–4) | 0.535 | 2 (2–4) | 0.792 | 18 (8–28) | 0.736 | |
| Zung depression | GG | 22 (20.5–24.5) | 0.493 | 28 (24–34) | 0.252 | 36 (25–49) | 0.550 |
| GC | 22 (20–22.5) | 29.5 (27–35.3) | 31.5 (27–40.5) | ||||
| CC | 21 (20–26) | 32 (29–35) | 35 (29–42) | ||||
| GC + CC | 22 (20–22.8) | 0.251 | 31 (27–35) | 0.106 | 32 (28–40) | 0.300 | |
| Zung anxiety | GG | 22 (20.5–25) | 0.484 | 28 (25–32.5) | 0.202 | 34.5 (29–42.5) | 0.491 |
| GC | 21 (20–23) | 30 (26–37.3) | 33.5 (29.8–37.3) | ||||
| CC | 21 (20–26) | 30 (23–35) | 32 (27–39.5) | ||||
| GC + CC | 21 (20–23) | 0.238 | 30 (26–36.5) | 0.078 | 33 (29–38) | 0.319 | |
| BDHI | GG | 17 (12–24.5) | 0.483 | 24 (17–29) | 0.420 | 33.5 (19.8–40) | 0.080 |
| GC | 13 (8–22.5) | 25.5 (14.8–33) | 33 (25.8–42.3) | ||||
| CC | 20 (11–22) | 21 (14–25) | 22 (15.5–29.5) | ||||
| GC + CC | 14.5 (8–22) | 0.369 | 24 (14–31) | 0.759 | 29 (24–42) | 0.793 | |
*Kruskal–Wall test for additive and Mann–Whitney test for the dominant model. Statistically significant p values are printed in bold.
No statistically significant associations were observed between GLP1R rs10305420 and the assessed psychosymptomatology scores in any of the study groups (data not shown). However, GLP1R rs6923761 AA genotype was associated with lower Zung anxiety scores among healthy controls (p = 0.021) (Figure 1B and Supplementary Table 5).
Discussion
We conducted a pilot study to investigate the role of GLP1R rs10305420 and rs6923761 and GIPR rs1800437 in alcohol dependence and related psychosymptomatology in a cohort of hospitalized alcohol-dependent patients, abstinent alcohol-dependent patients, and healthy individuals. To our knowledge, this is the first study that focuses on the role of GIPR on alcohol dependence and one of a few that investigated the relation of GLP1R with alcohol dependence in humans (Koole et al., 2011; Suchankova et al., 2015).
According to our results, GIPR rs1800437 genotypes were associated with an increased risk of alcohol dependence. No statistically significant associations were found for GLP1R rs10305420 and rs6923761 with alcohol dependence. We also observed statistically significant association between GIPR rs1800437 GG genotype and BSPS scores in the abstinent alcohol-dependent patients as well as the association between GLP1R rs6923761 GG genotype and Zung anxiety scores in healthy controls.
It is crucial to mention that this is the first study that indicates the involvement of GIPR in alcohol dependence and alcohol-related comorbid psychosymptomatology. The potential participation of GIP and its receptor in the etiology and pathophysiology of alcohol is limited. We know that GIPR is expressed in the adult rat hippocampus, a brain region related to memory (Nyberg et al., 2005). An animal model study has shown that mice with GIPR deficiency have synaptic plasticity deterioration, impaired neurogenesis, and learning disabilities (Faivre et al., 2011). Interestingly, GIP regulates progenitor cell proliferation (Nyberg et al., 2005) and neurotransmitter release and has a protective role on the synapses during synaptic plasticity (Gault and Hölscher, 2008). Alcohol use impacts the activity of the synapses, i.e., the points of contact between neurons, which affects the transmission of the information from one neuron to the next (Nestler and Aghajanian, 1997). Further studies are therefore needed to elucidate the role of GIPR genetic variability in AUD.
Regarding GLP-1, we observed an association between GLP1R rs6923761 GG genotype and Zung anxiety scores in healthy controls, but not between GLP1R rs10305420 and rs6923761 and alcohol dependence. Our results are in contrast with the findings of preclinical and clinical studies. An animal study indicated an interaction between alcohol use and the GLP-1 system, which might further elucidate the role of GLP-1R containing brain areas in reducing alcohol reinforcement through GLP-1R agonists and support the usage of GLP-1R as potential treatment targets for AUD. More specifically, the expression of the GLP-1 receptor in nucleus accumbens, which is the neural interface between motivation and action, was increased in high alcohol-consuming rodents compared to those under low alcohol consumption (Vallöf et al., 2019). In addition, Suchankova et al. (2015) investigated the impact of GLP-1R genetic variability on AUD in humans and a mouse model. Initially, they performed a case-control study that included 670 AUD patients and 238 controls with no current or past alcohol abuse. Then, the emerged significant associations were examined on a genome-wide association cohort of 1,917 patients with alcohol dependence and 1,886 healthy individuals. For functional validation of the findings, they included 84 participants who underwent intravenous self-administration of alcohol. To evaluate brain activity changes, they performed functional magnetic resonance imaging (fMRI) in 22 patients with alcohol dependence. Finally, they investigated the impact of GLP-1R agonism on alcohol dependence in a mouse model. Overall, their results indicated that the rs6923761 A (Ser) allele was nominally associated with increased AUD. Also, rs6923761 heterozygotes had higher alcohol self-administration and higher Blood-oxygen-level-dependent imaging (BOLD) signal in the globus pallidus when participants received rewarding outcomes during the Monetary Incentive Delay task. Lastly, from the preclinical model emerged a significant reduction of alcohol use after pharmacological GLP-1R agonist (Suchankova et al., 2015). An earlier in vitro study has also shown that rs6923761 has a functional role, given that the A (Ser) allele is associated with reduced GLP-1R expression levels in the cell’s surface (Koole et al., 2011). In our study, only potential association with psychosymptomatology was observed for rs6923761, while no differences in genotype frequencies were observed among different groups.
Regarding GLP-1 and its influences on reward processing through globus pallidus, ventral tegmental area and nucleus accumbens could explain detected association of GIPR rs1800437 and social anxiety scores, since neural activation in globus pallidus, among others, is associated with social phobia and anxiety disorders (Hattingh et al., 2013; Suchankova et al., 2015; Ashworth et al., 2021). Decreased connectivity between the nucleus accumbens and putamen was also reported in connection with social anxiety disorder. To the best of our knowledge, no human study explored the association of GLP-1 and GIP on the expression of anxiety symptoms. GLP-1 receptor gene polymorphism rs1042044 was associated with anhedonia, a symptom of major depressive disorder (Eser et al., 2020). Another human study reported about abnormal gene expression of GLP-1R in post-mortem brain of individuals with mood disorder (Mansur et al., 2019). The gut-brain axis with gastrointestinally derived neuropeptides like GLP-1, are emerging as potential key regulators of anxiety behavior. A study performed on rats reported chemogenetic activation of neurons and anxiolytic response. Another animal studies on rats reported anxiogenic and antidepressant effects of GLP-1 receptor stimulation and anti-anxiety effect of liraglutide which is GLP-1 agonist (Sharma et al., 2015; Anderberg et al., 2016). So further studies on this are warranted to elucidate this issue.
Nevertheless, our study has some limitations, such as the small sample size, and the lack of data on metabolic parameters of the participants. Given that age and the proportion of smokers differed between the three studied groups, differences in subjects’ characteristics were considered as an adjustment in logistic regression analysis. Another limitation is the inclusion of a cohort comprised only of male participants. However, it should be noted that all animal model studies focus on male animals, and the innovative research of Suchankova mentioned above also indicated that the association between AUD and GLP-1R was more significant in men (Suchankova et al., 2015). In a mice AUD model, it has been shown that males and females are different in terms of alcohol consumption and response during the potential forced abstinence, which is known to affect interconnected networks of neural circuits that are associated with depression and anxiety symptoms. Female mice consumed more alcohol, but they could transit to an abstinence-induced depressive state more quickly than male mice (Dao et al., 2020). Murano et al. (2017) found a similar pattern of gene activity in the hippocampus and the prefrontal cortex of men with alcoholism and infants’ developing brains, but not with women. These brain regions are associated with memory deficiency and cognitive problems, which are also symptoms of patients with alcoholism. They concluded that it is possible that these two brain region alterations are associated with the predisposition of patients to the alcohol abuse (Murano et al., 2017).
Concluding, our pilot study revealed a potential association between GIPR and alcohol dependence. Confirming this association in studies with bigger sample sizes and deciphering the role of genetic susceptibility may help with the identification of high-risk individuals and may also open the way to conceive novel treatment strategies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
This study involving human participants was reviewed and approved by the Slovenian National Medical Ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EET, API, and VD: conceptualization. EET, KG, BKP, API, and VD: methodology and writing – review and editing. EET, KG, and API: formal analysis and visualization. KG: statistical analysis. EET and API: writing – original draft preparation. API and VD: supervision. VD: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We kindly acknowledge Savica Soldat from the Pharmacogenetics Laboratory for her the expert technical assistance.
Funding
This study was financially supported by the Ministry of Education, Science and Sport of Slovenia (Grant No P1-0170).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.908948/full#supplementary-material
References
- Abraham K. A., Kearney M. L., Reynolds L. J., Thyfault J. P. (2015). Red wine enhances glucose-dependent insulinotropic peptide (GIP) and insulin responses in type 2 diabetes during an oral glucose tolerance test. Diabetol. Int. 7 173–180. 10.1007/s13340-015-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adner N., Nygren A. (1992). The influence of indomethacin, theophylline, and propranolol on ethanol augmentation of glucose-induced insulin secretion. Metabolism 41 1165–1170. 10.1016/0026-0495(92)90004-t [DOI] [PubMed] [Google Scholar]
- Agra R. M., Gago-Dominguez M., Paradela-Dobarro B., Torres-Español M., Alvarez L., Fernandez-Trasancos A., et al. (2019). Obesity-related genetic determinants of heart failure prognosis. Cardiovasc. Drugs Ther. 33 415–424. 10.1007/s10557-019-06888-8 [DOI] [PubMed] [Google Scholar]
- Alhadeff A. L., Rupprecht L. E., Hayes M. R. (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153 647–658. 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez E., Martínez M. D., Roncero I., Chowen J. A., García-Cuartero B., Gispert J. D., et al. (2005). The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J. Neurochem. 92 798–806. 10.1111/j.1471-4159.2004.02914.x [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: American Psychiatric Association. Text Revision (DSM-IV-TR). [Google Scholar]
- Anderberg R. H., Richard J. E., Hansson C., Nissbrandt H., Bergquist F., Skibicka K. P. (2016). GLP-1 is both anxiogenic and antidepressant; divergent effects of acute and chronic GLP-1 on emotionality. Psychoneuroendocrinology 65 54–66. 10.1016/j.psyneuen.2015.11.021 [DOI] [PubMed] [Google Scholar]
- Anton R. F. (2000). Obsessive-compulsive aspects of craving: development of the obsessive compulsive drinking scale. Addiction 95(Suppl. 2), S211–S217. 10.1080/09652140050111771 [DOI] [PubMed] [Google Scholar]
- Ashworth E., Brooks S. J., Schiöth H. B. (2021). Neural activation of anxiety and depression in children and young people: a systematic meta-analysis of fMRI studies. Psychiatry Res. Neuroimaging 311:111272. 10.1016/j.pscychresns.2021.111272 [DOI] [PubMed] [Google Scholar]
- Bowen M. T., George O., Muskiewicz D. E., Hall F. S. (2022). Factors Contributing to the Escalation of Alcohol Consumption. Neurosci. Biobehav. Rev. 132 730–756. 10.1016/j.neubiorev.2021.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A. H., Durkee A. (1957). An inventory for assessing different kinds of hostility. J. Consult. Psychol. 21 343–349. 10.1037/h0046900 [DOI] [PubMed] [Google Scholar]
- Carvalho A. F., Heilig M., Perez A., Probst C., Rehm J. (2019). Alcohol use disorders. Lancet 394 781–792. [DOI] [PubMed] [Google Scholar]
- Chedid V., Vijayvargiya P., Carlson P., Van Malderen K., Acosta A., Zinsmeister A., et al. (2018). Allelic variant in the glucagon-like peptide 1 receptor gene associated with greater effect of liraglutide and exenatide on gastric emptying: a pilot pharmacogenetics study. Neurogastroenterol. Motil. 30:e13313. 10.1111/nmo.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C. R., Bohman M., Sigvardsson S. (1981). Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch. Gen. Psychiatry 38 861–868. 10.1001/archpsyc.1981.01780330019001 [DOI] [PubMed] [Google Scholar]
- Dalgaard M., Thomsen C., Rasmussen B. M., Holst J. J., Hermansen K. (2004). Ethanol with a mixed meal decreases the incretin levels early postprandially and increases postprandial lipemia in type 2 diabetic patients. Metabolism 53 77–83. 10.1016/j.metabol.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Dao N. C., Suresh Nair M., Magee S. N., Moyer J. B., Sendao V., Brockway D. F., et al. (2020). Forced abstinence from alcohol induces sex-specific depression-like behavioral and neural adaptations in somatostatin neurons in cortical and amygdalar regions. Front. Behav. Neurosci. 14:86. 10.3389/fnbeh.2020.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. R. T., Miner C. M., De Veaugh-Geiss J., Tupler L. A., Colket J. T., Potts N. L. S. (1997). The brief social phobia scale: a psychometric evaluation. Psychol. Med. 27 161–166. 10.1017/s0033291796004217 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., de la Fuente B., Primo D., Conde R., Izaola O., et al. (2015d). Relation of the rs6923761 gene variant in glucagon-like peptide 1 receptor with weight, cardiovascular risk factor, and serum adipokine levels in obese female subjects. J. Clin. Lab. Anal. 29 100–105. 10.1002/jcla.21735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., Izaola O., Bachiller R. (2015a). Role of rs6923761 gene variant in glucagon-like peptide 1 receptor in basal GLP-1 levels, cardiovascular risk factor and serum adipokine levels in naïve type 2 diabetic patients. J. Endocrinol. Invest. 38 143–147. 10.1007/s40618-014-0161-y [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., Izaola O., Romero E. (2015b). Effects of a high-protein/low-carbohydrate versus a standard hypocaloric diet on adipocytokine levels and cardiovascular risk factors during 9 months, role of rs6923761 gene variant of glucagon-like peptide 1 receptor. J. Endocrinol. Invest. 38 1183–1189. 10.1007/s40618-015-0304-9 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Diaz Soto G., Izaola O., Romero E. (2015c). Evaluation of weight loss and metabolic changes in diabetic patients treated with liraglutide, effect of RS 6923761 gene variant of glucagon-like peptide 1 receptor. J. Diabetes Complications 29 595–598. 10.1016/j.jdiacomp.2015.02.010 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., Izaola O., Bachiller R., Pacheco D. (2014a). Cardiovascular risk factors and adipocytokines levels after two hypocaloric diets with different fat distribution in obese subjects and rs6923761 gene variant of glucagon-like peptide 1 receptor. J. Endocrinol. Invest. 37 853–859. 10.1007/s40618-014-0116-3 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., Izaola O., Lopez J. J., Gomez E., Torres B., et al. (2014b). Effect of rs6923761 gene variant of glucagon-like peptide 1 receptor on metabolic response and weight loss after a 3-month intervention with a hypocaloric diet. J. Endocrinol. Invest. 37 935–939. 10.1007/s40618-014-0117-2 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Bachiller R., Izaola O., De La Fuente B., Aller R. (2014c). Relation of the rs6923761 gene variant in glucagon-like peptide 1 receptor to metabolic syndrome in obese subjects. Ann. Nutr. Metab. 65 253–258. 10.1159/000365295 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Pacheco D., Aller R., Izaola O., Bachiller R. (2014e). Roles of rs 6923761 gene variant in glucagon-like peptide 1 receptor on weight, cardiovascular risk factor and serum adipokine levels in morbid obese patients. Nutr. Hosp. 29 889–893. 10.3305/nh.2014.29.4.7218 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Pacheco D., Aller R., Izaola O. (2014d). Role of the rs6923761 gene variant in glucagon-like peptide 1 receptor gene on cardiovascular risk factors and weight loss after biliopancreatic diversion surgery. Ann. Nutr. Metab. 65 259–263. 10.1159/000365975 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Aller R., Izaola O., De La Fuente B., Primo D., Conde R., et al. (2013). Evaluation of weight loss and adipocytokine levels after two hypocaloric diets with different macronutrient distribution in obese subjects with the rs6923761 gene variant of glucagon-like peptide 1 receptor. Ann. Nutr. Metab. 63 277–282. 10.1159/000356710 [DOI] [PubMed] [Google Scholar]
- de Luis D. A., Ballesteros M., Lopez Guzman A., Ruiz E., Muñoz C., Penacho M. A., et al. (2018). rs6923761 gene variant in glucagon-like peptide 1 receptor: allelic frequencies and influence on cardiovascular risk factors in a multicenter study of Castilla-Leon. Clin. Nutr. 37 2144–2148. 10.1016/j.clnu.2017.10.013 [DOI] [PubMed] [Google Scholar]
- Domi E., Domi A., Adermark L., Heilig M., Augier E. (2021). Neurobiology of alcohol seeking behavior. J. Neurochem. 157 1585–1614. 10.1111/jnc.15343 [DOI] [PubMed] [Google Scholar]
- Egecioglu E., Engel J. A., Jerlhag E. (2013). The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8:e69010. 10.1371/journal.pone.0069010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbil D., Eren C. Y., Demirel C., Küçüker M. U., Solaroǧlu I., Eser H. Y. (2019). GLP-1’s role in neuroprotection: a systematic review. Brain Inj. 33 734–819. 10.1080/02699052.2019.1587000 [DOI] [PubMed] [Google Scholar]
- Eren-Yazicioglu C. Y., Yigit A., Dogruoz R. E., Yapici-Eser H. (2021). Can GLP-1 be a target for reward system related disorders? A qualitative synthesis and systematic review analysis of studies on palatable food, drugs of abuse, and alcohol. Front. Behav. Neurosci. 14:614884. 10.3389/fnbeh.2020.614884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser H. Y., Appadurai V., Eren C. Y., Yazici D., Chen C. Y., Ongur D., et al. (2020). Association between GLP-1 receptor gene polymorphisms with reward learning, anhedonia and depression diagnosis. Acta Neuropsychiatr. 32 218–225. 10.1017/neu.2020.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre E., Gault V. A., Thorens B., Hölscher C. (2011). Glucose-dependent insulinotropic polypeptide receptor knockout mice are impaired in learning, synaptic plasticity, and neurogenesis. J. Neurophysiol. 105 1574–1580. 10.1152/jn.00866.2010 [DOI] [PubMed] [Google Scholar]
- Gandal M. J., Haney J. R., Parikshak N. N., Leppa V., Ramaswami G., Hartl C., et al. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault V. A., Hölscher C. (2008). Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. J. Neurophysiol. 99 1590–1595. 10.1152/jn.01161.2007 [DOI] [PubMed] [Google Scholar]
- Goodman W. K., Price L. H. (1992). Assessment of severity and change in obsessive compulsive disorder. Psychiatr. Clin. North Am. 15 861–869. [PubMed] [Google Scholar]
- Hattingh C. J., Ipser J., Tromp S. A., Syal S., Lochner C., Brooks S. J., et al. (2013). Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front. Hum. Neurosci. 6:347. 10.3389/fnhum.2012.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A. C., Bucholz K. K., Madden P. A. F., Dinwiddie S. H., Slutske W. S., Bierut L. J., et al. (1997). Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol. Med. 27 1381–1396. 10.1017/s0033291797005643 [DOI] [PubMed] [Google Scholar]
- Hiroi N., Agatsuma S. (2005). Genetic susceptibility to substance dependence. Mol. Psychiatry 10 336–344. [DOI] [PubMed] [Google Scholar]
- Ilješ A. P., Plesnièar B. K., Dolžan V. (2021). Associations of NLRP3 and CARD8 gene polymorphisms with alcohol dependence and commonly related psychiatric disorders: a preliminary study. Arh. Hig. Rada Toksikol. 72 191–197. 10.2478/aiht-2021-72-3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbil-Buyukcoskun N., Cam-Etoz B., Gulec G., Ozluk K. (2009). Effect of peripherally-injected glucagon-like peptide-1 on gastric mucosal blood flow. Regul. Pept. 157 72–75. 10.1016/j.regpep.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Javorský M., Gotthardová I., Klimčáková L., Kvapil M., Židzik J., Schroner Z., et al. (2016). A missense variant in GLP1R gene is associated with the glycaemic response to treatment with gliptins. Diabetes. Obes. Metab. 18 941–944. 10.1111/dom.12682 [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N., Ericson M., Steensland P., Jerlhag E. (2016).“Dopamine and alcohol dependence: from bench to clinic,” in Recent Advances in Drug Addiction Research and Clinical Applications, eds Meil W., Ruby C. (Norderstedt: Books on Demand; ). [Google Scholar]
- Jensen C. B., Pyke C., Rasch M. G., Dahl A. B., Knudsen L. B., Secher A. (2018). Characterization of the glucagonlike peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology 159 665–675. 10.1210/en.2017-00812 [DOI] [PubMed] [Google Scholar]
- Jensterle M., Pirš B., Goričar K., Dolžan V., Janež A. (2015). Genetic variability in GLP-1 receptor is associated with inter-individual differences in weight lowering potential of liraglutide in obese women with PCOS: a pilot study. Eur. J. Clin. Pharmacol. 71 817–824. 10.1007/s00228-015-1868-1 [DOI] [PubMed] [Google Scholar]
- Jerlhag E. (2020). Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res 1727:146562. 10.1016/j.brainres.2019.146562 [DOI] [PubMed] [Google Scholar]
- Jones J. D., Comer S. D., Kranzler H. R. (2015). The Pharmacogenetics of alcohol use disorder. Alcohol. Clin. Exp. Res. 39 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathryn Mchugh R., Weiss R. D. (2019). Alcohol use disorder and depressive disorders. Alcohol Res. 40 e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen M. K., Thomsen M., Wortwein G., Fink-Jensen A. (2022). The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br. J. Pharmacol 179 625–641. 10.1111/bph.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klen J., Dolžan V. (2022). Glucagon-like peptide-1 receptor agonists in the management of type 2 diabetes mellitus and obesity: the impact of pharmacological properties and genetic factors. Int. J. Mol. Sci. 23:3451. 10.3390/ijms23073451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. (2016). Neurobiology of addiction: a neurocircuitry analysis. The lancet. Psychiatry 3 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C., Wootten D., Simms J., Valant C., Miller L. J., Christopoulos A., et al. (2011). Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Mol. Pharmacol. 80 486–497. 10.1124/mol.111.072884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler H. R., Soyka M. (2018). Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 320 815–824. 10.1001/jama.2018.11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur R. B., Fries G. R., Trevizol A. P., Subramaniapillai M., Lovshin J., Lin K., et al. (2019). The effect of body mass index on glucagon-like peptide receptor gene expression in the post mortem brain from individuals with mood and psychotic disorders. Eur. Neuropsychopharmacol. 29 137–146. 10.1016/j.euroneuro.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty V. N., Farokhnia M., Munier J. J., Mulpuri Y., Leggio L., Spigelman I. (2020). Long-acting glucagon-like peptide-1 receptor agonists suppress voluntary alcohol intake in male wistar rats. Front. Neurosci. 14:599646. 10.3389/fnins.2020.599646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi M., Wilson J. R., Jafarian-Kerman S., Nian H., Yu C., Shuey M. M., et al. (2021). Association of a glucagon-like peptide-1 receptor gene variant with glucose response to a mixed meal. Diabetes. Obes. Metab. 23 281–286. 10.1111/dom.14216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I., Lane M., Shughrue P. (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 403 261–280. [DOI] [PubMed] [Google Scholar]
- Murano T., Koshimizu H., Hagihara H., Miyakawa T. (2017). Transcriptomic immaturity of the hippocampus and prefrontal cortex in patients with alcoholism. Sci. Reports 7:44531. 10.1038/srep44531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Aghajanian G. K. (1997). Molecular and cellular basis of addiction. Science 278 58–63. [DOI] [PubMed] [Google Scholar]
- Nutt D., Hayes A., Fonville L., Zafar R., Palmer E. O. C., Paterson L., et al. (2021). Alcohol and the Brain. Nutrients 13:3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg J., Anderson M. F., Meister B., Alborn A. M., Ström A. K., Brederlau A., et al. (2005). Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 25 1816–1825. 10.1523/JNEUROSCI.4920-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N., Le D. S. N. T., Salbe A. D., Chen K., Reiman E. M., Tataranni P. A., et al. (2007). Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage 35 511–517. 10.1016/j.neuroimage.2006.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemenitas A., Kastelic M., Porcelli S., Serretti A., Rus Makovec M., Kores Plesnicar B., et al. (2015). Genetic variability in CYP2E1 and catalase gene among currently and formerly alcohol-dependent male subjects. Alcohol Alcohol 50 140–145. 10.1093/alcalc/agu088 [DOI] [PubMed] [Google Scholar]
- Reddy I. A., Pino J. A., Weikop P., Osses N., Sørensen G., Bering T., et al. (2016). Glucagon-like peptide 1 receptor activation regulates cocaine actions and dopamine homeostasis in the lateral septum by decreasing arachidonic acid levels. Transl. Psychiatry 6:e809. 10.1038/tp.2016.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 1350 18–34. 10.1016/j.brainres.2010.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenstine S., Espinosa A., Kumar A. (2020). Depression and anxiety subgroups across alcohol use disorder and substance use in a national epidemiologic study. J. Dual Diagn. 16 299–311. 10.1080/15504263.2020.1784498 [DOI] [PubMed] [Google Scholar]
- Rush A. J., First M. B., Blacker D. (2008). “Task force for the handbook of psychiatric measures,” in Handbook of Psychiatric Measures, ed. American Psychiatric Association (Washington, DC: American Psychiatric Association; ), 828. 10.1002/14651858.CD012081.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauber J., Grothe J., Behm M., Scherag A., Grallert H., Illig T., et al. (2010). Association of variants in gastric inhibitory polypeptide receptor gene with impaired glucose homeostasis in obese children and adolescents from Berlin. Eur. J. Endocrinol. 163 259–264. 10.1530/EJE-10-0444 [DOI] [PubMed] [Google Scholar]
- Seino Y., Fukushima M., Yabe D. (2010). GIP and GLP-1, the two incretin hormones: similarities and differences. J. Diabetes Investig. 1 8–23. 10.1111/j.2040-1124.2010.00022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y., Yabe D. (2013). Glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1: incretin actions beyond the pancreas. J. Diabetes Investig. 4 108–130. 10.1111/jdi.12065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. N., Pise A., Sharma J. N., Shukla P. (2015). Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metab. Brain Dis. 30 719–730. 10.1007/s11011-014-9627-z [DOI] [PubMed] [Google Scholar]
- Suchankova P., Yan J., Schwandt M. L., Stangl B. L., Caparelli E. C., Momenan R., et al. (2015). The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl. Psychiatry 5:e583. 10.1038/tp.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torekov S. S., Harsløf T., Rejnmark L., Eiken P., Jensen J. B., Herman A. P., et al. (2014). A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J. Clin. Endocrinol. Metab. 99 E729–E733. 10.1210/jc.2013-3766 [DOI] [PubMed] [Google Scholar]
- Underwood C. R., Garibay P., Knudsen L. B., Hastrup S., Peters G. H., Rudolph R., et al. (2010). Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J. Biol. Chem. 285 723–730. 10.1074/jbc.M109.033829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgeová A., Javorský M., Klimčáková L., Zidzik J., Šalagovič J., Hubáček J. A., et al. (2020). Genetic variants associated with glycemic response to treatment with dipeptidylpeptidase 4 inhibitors. Pharmacogenomics 21 317–323. 10.2217/pgs-2019-0147 [DOI] [PubMed] [Google Scholar]
- Vallöf D., Vestlund J., Jerlhag E. (2019). Glucagon-like peptide-1 receptors within the nucleus of the solitary tract regulate alcohol-mediated behaviors in rodents. Neuropharmacology 149 124–132. [DOI] [PubMed] [Google Scholar]
- Verhulst B., Neale M. C., Kendler K. S. (2015). The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol. Med. 45 1061–1072. 10.1017/S0033291714002165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. I. G., Scherag A., Brönner G., Nguyen T. T., Wang H. J., Grallert H., et al. (2009). Gastric inhibitory polypeptide receptor: association analyses for obesity of several polymorphisms in large study groups. BMC Med. Genet. 10:19. 10.1186/1471-2350-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. K., Polimanti R., Johnson E. C., McClintick J. N., Adams M. J., Adkins A. E., et al. (2018). Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 21 1656–1669. 10.1038/s41593-018-0275-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Wang K., Liu H., Cao R. (2019). GLP1R variant is associated with response to exenatide in overweight Chinese Type 2 diabetes patients. Pharmacogenomics 20 273–277. 10.2217/pgs-2018-0159 [DOI] [PubMed] [Google Scholar]
- Zhou H., Sealock J. M., Sanchez-Roige S., Clarke T. K., Levey D. F., Cheng Z., et al. (2020). Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat. Neurosci. 23 809–818. 10.1038/s41593-020-0643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung W. W. K. (1965). A self-rating depression scale. Arch. Gen. Psychiatry 12 63–70. [DOI] [PubMed] [Google Scholar]
- Zung W. W. K. (1971). A rating instrument for anxiety disorders. Psychosomatics 12 371–379. 10.1016/S0033-3182(71)71479-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.