Abstract
Fatigue is a commonly reported but not well-documented symptom following burn injury. This study’s objective was to determine the frequency and severity of fatigue over time and to identify predictors of fatigue in the adult burn population. Data from the Burn Model System National Database (April 1997 to January 2006) were analyzed. Individuals over 18 years of age who were alive at discharge were included. The vitality subscale of the Short-Form 36 Item Health Survey was examined at preinjury and discharge and at 6, 12, and 24 months postinjury. Mean and number of low vitality scores were calculated at each time interval. Descriptive statistics were generated for demographic and medical data. Cross-sectional regression models analyzed predictors of vitality at 6, 12, and 24 months postinjury. The study included 945 subjects. The population was 72.5% male and had a mean age of 40.6 years and mean burn size of 17.4%. Fatigue symptoms were present in a majority of the population (74.6%) and were most commonly reported at discharge. Although fewer burn survivors reported fatigue symptoms at each subsequent follow-up (P < .001), approximately one-half (49%) of the population continued to report fatigue symptoms at 24 months postinjury. Larger burn size was the only variable that was significant or approaching significance at all follow-up time points (P < .0167). Fatigue symptoms are common after burns and many burn survivors continue to report symptoms at 2 years postinjury. Burn survivors did not return to preinjury fatigue levels, highlighting the importance of understanding and monitoring fatigue.
After burn injuries, many individuals experience a variety of long-term physical, psychological, and social challenges. Both patients and clinicians are increasingly identifying fatigue as a significant issue following burn injury; however, it remains loosely defined. Fatigue may be physical, psychological, or mental. In a general sense, it has been described as a subjective feeling of tiredness, weakness, or lack of energy.1 It is associated with a wide range of medical conditions, including fibromyalgia,2 multiple sclerosis,3,4 traumatic brain injury,5 and cancer,6 among others. In many cases, it may severely limit physical activities and increase risk of disability.7,8 Fatigue has been shown to have a significant impact on well-being and quality of life both during and after recovery; however, it remains “under-recognized, under-assessed, and under-treated.”1
There are several reasons why burn survivors may experience fatigue symptoms. Major burns result in a pathophysiological stress response, which in turn leads to a hypermetabolic state that reflects an increase in whole-body oxygen consumption above the normal range.9 Burn-induced hypermetabolism is associated with poor clinical outcomes and has been shown to persist long-term in large injuries,10 possibly contributing to fatigue for years postinjury. Additionally, muscle wasting can delay healing and greatly contribute to the long-term morbidity of burn survivors.9 This may result in postinjury weakness that can make activities of daily living challenging. In burns involving more than 30% total body surface area (TBSA), muscle wasting can persist for several years postinjury.9 Similarly, physical and functional impairments following burn injury may cause regular activities to be more tiresome and contribute to higher fatigue levels.11 Depression and anxiety following burn injury may also lead to reductions in physical functioning and higher levels of fatigue.12 There are many non–burn-specific causes of posthospitalization fatigue, including deconditioning and general weakness following prolonged hospitalization and bed rest, infection, hypotension, insomnia, viruses, immune system issues, inflammation, and arthritis.
A burn survivor must overcome multiple physical and psychological barriers in order to return to regular daily activities. In the authors’ experience, fatigue is a commonly reported symptom following burn injury; however, it is rarely included in outcomes studies and is not well documented in the literature. Fatigue is strongly associated with poorer health-related quality of life and greater work-related disability, and the risk of developing moderate to severe fatigue following burn injury has been found to increase with burn size.13 Women and people living in rural and remote areas have been found to be at greater risk of developing moderate-to-severe fatigue.13 Fatigue has been shown to persist as long as 17 years postinjury14; however, no previous studies have examined the progression of fatigue beyond a 1-year follow-up. As fatigue symptoms can contribute to depression, social isolation, inability to care for oneself and others, and inability to work, fatigue can have a serious impact on long-term quality of life and impair a burn survivor’s ability to achieve preinjury levels of satisfaction.
The extent to which fatigue is a barrier in burn recovery has not been extensively examined. Studies to date have been limited by small sample size, non-North American burn populations, and short follow-up windows. The objective of this study was to determine the frequency and severity of fatigue symptoms, as well as changes in the severity of fatigue over time, in the adult burn population. Additionally, predictors of fatigue at each follow-up time point will be determined.
METHODS
Data Source
Data from the prospectively collected Burn Model System (BMS) National Database between April 1997 and January 2006 were analyzed. Six burn centers in the United States have contributed to this database over the lifespan of the project (1993 to current).15 Individuals over 18 years of age who were alive at the time of acute care discharge were included. For this time period, the BMS National Database included data on survivors who met the American Burn Association criteria for a severely burned person, namely those with 1 or more of the following:
Deep second- and third-degree burns greater than 10% TBSA in patients over 50 years of age.
Deep second- and third-degree burns greater than 20% TBSA.
Deep second- and third-degree burns with serious threat of functional or cosmetic threat that involve face, hands, feet, genitalia, perineum, or major joints.
Third-degree burns greater than 5% TBSA.
Deep electrical burns including lightning injury.
Burn injury with inhalation injury.
Circumferential burns of the extremity or chest.
Modifications were made to the inclusion criteria over time. Details of the BMS National Database inclusion criteria, data collection process, and data collection sites can be found at http://burndata.washington.edu/.
Variables
Demographic (age, race, gender, marital status, and employment status) and medical (burn etiology, burn size, acute care length of stay, drug abuse, alcohol abuse, psychiatric treatment, and preexisting physical disability) variables were collected through self-report or medical records at discharge. Drug and alcohol abuse were evaluated using the CAGE questionnaire.16
Outcome
The vitality subscale of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), which was administered by BMS centers between April 1997 and January 2006, was used to determine fatigue symptoms. A lower vitality score indicates greater fatigue. The subscale has been used in cancer,17 head injury,18 and fibromyalgia19 populations to assess fatigue levels. It includes 4 questions that are scored on a 1–5 scale, where 1 is “All of the time” and 5 is “None of the time.” Two of the 4 questions were reverse coded. Vitality scores were examined at preinjury (collected at discharge, with a preinjury recall period) and discharge, as well as at 6, 12, and 24 months postinjury.
Data Analysis
Descriptive statistics were generated for demographic and medical data. Responses to the items of the vitality scale questions were transformed to a t-score with a mean of 50 and SD of 10 based on a U.S. population.20 Fatigue was defined as a score less than 50. Mean vitality scores and the percentage of the population reporting fatigue were calculated at each time interval. Wilcoxon signed rank-sum tests were conducted to compare vitality scores preinjury and at 24 months postinjury, as well as at discharge and at 24 months postinjury. Multivariate linear regression models were utilized to identify demographic and clinical predictors of fatigue at 6, 12, and 24 months postinjury. Due to concerns about multiple testing, a Bonferroni correction to the alpha level of 0.05 was used, resulting in a P value of < .0167 for statistical significance.
A secondary analysis was conducted to evaluate relationships between vitality scores and employment status and between vitality scores and mental health scores at 6, 12, and 24 months postinjury. The SF-36 mental health score has been used to measure depression in several other injury populations.21,22
RESULTS
Population Characteristics
The study included 945 subjects who reported vitality score data at discharge. The population was 72.5% male, had a mean age of 40.6 ± 14.6 years, and a mean burn size of 17.4 ± 15.7% TBSA (Table 1). Fire/flame was the most common burn etiology (58.1%). Of the 945 participants, 71% (n = 672) were working at the time of injury, 47% (n = 441) were single, and 68% (n = 2409) were white and non-Hispanic.
Table 1.
Demographic and medical characteristics of study population at discharge
| Variable | Total (N = 945) |
|---|---|
|
| |
| Age, mean (SD) | 40.6 (14.6) |
| Male gender, n (%) | 685 (72.5) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 632 (66.9) |
| Black, non-Hispanic | 178 (18.9) |
| Hispanic | 93 (9.8) |
| Asian | 11 (1.2) |
| Native American | 8 (0.9) |
| Multiracial | 4 (0.4) |
| Pacific Islander | 3 (0.3) |
| Other | 3 (0.3) |
| Employment status, n (%) | |
| Working | 672 (71.1) |
| Not working | 195 (20.6) |
| Homemaker | 7 (0.7) |
| Volunteer | 2 (0.2) |
| Retired | 66 (7.0) |
| Marital status at the time of burn, n (%) | |
| Single | 441 (46.7) |
| Married | 387 (41.0) |
| Partner/significant other | 45 (4.8) |
| Etiology of injury, n (%) | |
| Fire/flame | 549 (58.1) |
| Scald | 108 (11.4) |
| Contact with hot object | 39 (4.1) |
| Grease | 81 (8.6) |
| Electricity | 55 (5.8) |
| Other burn | 86 (9.1) |
| TBSA burned, mean (SD) | 17.4 (15.7) |
| Length of hospital stay, mean (SD) | 23.1 (20.1) |
| Inhalation injury, n (%) | 102 (10.8) |
| Drug abuse, n (%) | 87 (9.2) |
| Alcohol abuse, n (%) | 97 (10.3) |
| Psychiatric treatment, n (%) | 402 (11.2) |
| Preexisting physical disability, n (%) | 398 (11.1) |
Vitality Score
The mean vitality score before injury was 55.9 ± 10.9. The mean vitality score was lowest at discharge (43.1 ± 10.9) and improved at each subsequent time period (P < .001). The highest mean vitality score postinjury was exhibited at 24 months (49.6 ± 11.5); however, the vitality scale score at 24 months did not return to the preinjury level (P < .001). Fatigue symptoms were most commonly reported at discharge and seen in a majority of the population (vitality score < 50: 74.6%). Although fewer burn survivors reported fatigue symptoms at each subsequent follow-up (Figure 1), approximately one-half (49%) of the population continued to report fatigue symptoms at 24 months postinjury.
Figure 1.
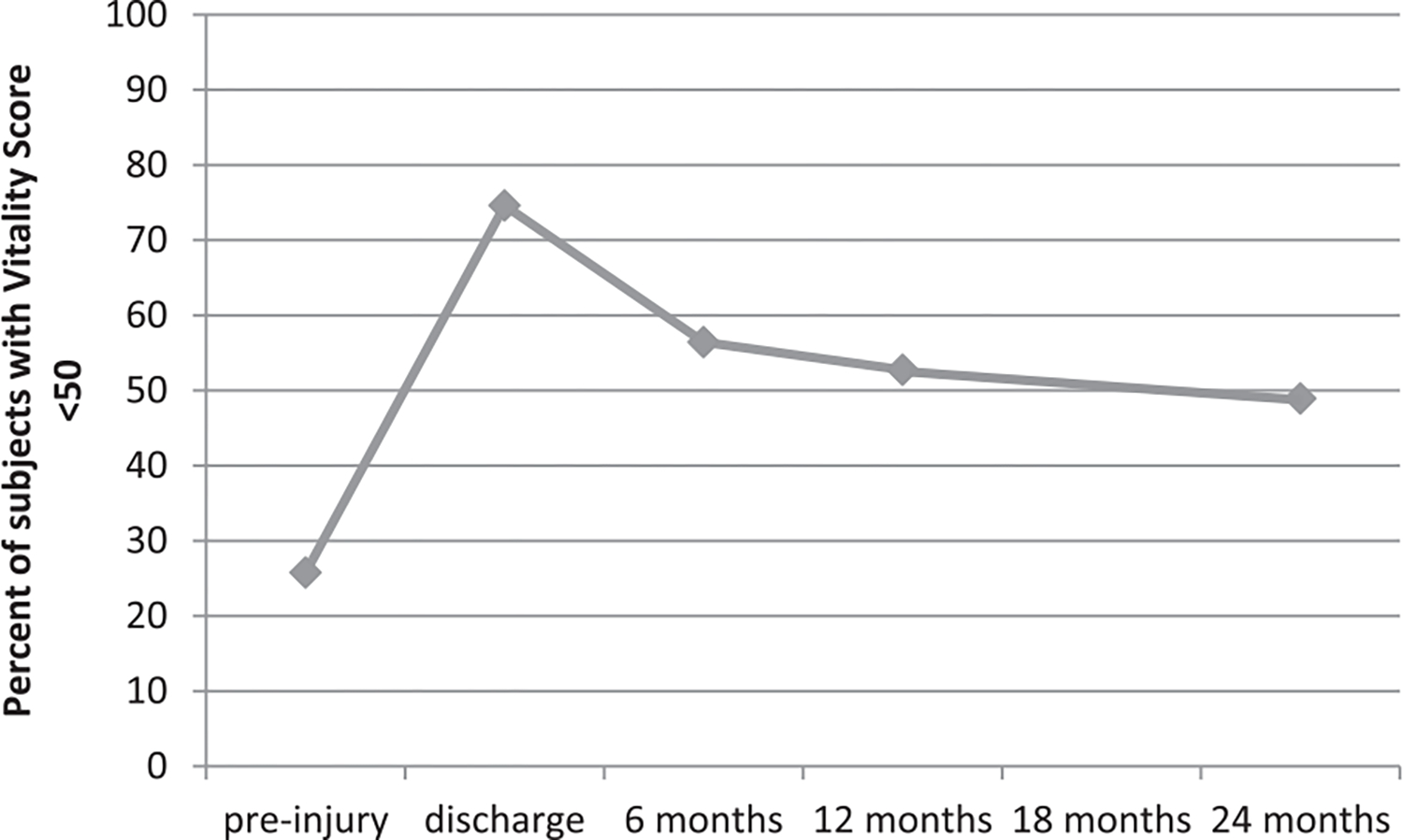
Frequency of fatigue over time. Responses to the items of the SF-36 vitality scale questions were transformed to a t-score with a mean of 50 and SD of 10 based on a U.S. population. Fatigue was defined as a score less than 50, and the percentage of subjects with low vitality scores was calculated at preinjury (collected at discharge) and discharge, as well as at 6, 12, and 24 months postinjury. SF, Short Form.
Regression Analysis
Larger burn size (6, 12 months), older age (6 months), female sex (12 months), electrical injury (24 months), and other burn etiology (6, 12 months) were strongly correlated with greater fatigue (P < .0167; Table 2). Larger burn size at 24 months was approaching significance (P = .018).
Table 2.
Linear regression analysis results examining predictors of the SF-36 vitality scores at 6, 12, and 24 months after injury
| 6 mo | 12 mo | 24 mo | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variables | β | P | β | P | β | P |
|
| ||||||
| Age | −0.138* | .008* | −0.122 | .020 | −0.074 | .232 |
| Female gender | −0.060 | .240 | −0.154* | .004* | −0.141 | .018 |
| Race/ethnicity, compared with white | ||||||
| Black, non-Hispanic | −0.110 | .026 | −0.058 | .253 | −0.089 | .122 |
| Other | 0.001 | .987 | −0.041 | .419 | 0.016 | .780 |
| Working at the time of injury | 0.061 | .277 | 0.032 | .566 | 0.075 | .263 |
| Married | 0.015 | .772 | 0.036 | .493 | 0.013 | .825 |
| TBSA burned | −0.179* | .005* | −0.174* | .011* | −0.181 | .018 |
| Etiology, compared with fire/flame | ||||||
| Scald | −0.098 | .058 | −0.111 | .033 | −0.042 | .482 |
| Grease | 0.002 | .974 | −0.019 | .720 | 0.013 | .833 |
| Electricity | −0.083 | .107 | −0.119 | .029 | −0.153* | .012* |
| Other | −0.154* | .003* | −0.158* | .003* | −0.060 | .325 |
| Length of hospital stay | 0.065 | .299 | 0.017 | .790 | 0.095 | .199 |
| Inhalation injury | 0.012 | .817 | −0.059 | .256 | 0.029 | .627 |
| Drug abuse | −0.063 | .206 | −0.046 | .378 | 0.034 | .600 |
| Alcohol abuse | −0.080 | .115 | −0.054 | .298 | −0.049 | .425 |
| Psychiatric treatment | −0.099 | .048 | −0.084 | .109 | −0.114 | .068 |
| Preexisting physical disability | −0.038 | .493 | −0.112 | .038 | −0.019 | .763 |
SF, Short Form.
Indicates significance (P < .0167).
Secondary Analysis
Unemployment was associated with greater fatigue at all 3 follow-up time points (P < .0167). Mental health scores were correlated with vitality scores at all 3 follow-up time points (r = 0.7479 at 6 months; r = 0.7140 at 12 months; r = 0.7647 at 24 months).
DISCUSSION
Little attention has been given to fatigue symptoms following burn injury, and fatigue is unlikely to be prioritized by burn care clinicians. This is the second study to examine fatigue after burn injury.13 In addition to confirming the prevalence of postinjury fatigue among adult burn survivors, this study evaluates fatigue at 2 years postinjury, examines a North American population, and identifies predictors of fatigue at 3 follow-up time points. Together, these 2 studies offer a greater understanding of an important but not well-documented symptom.
Fatigue is common following burn injury, and a majority of burn survivors continue to report symptoms at 24 months postinjury. Symptoms seem to gradually subside over time; however, burn survivors did not return to preinjury baseline levels. The causes of fatigue are not well understood; however, the high prevalence of fatigue long-term, as well as the detrimental impact these symptoms can have on work, family, and social activities, indicates that this is a persistent issue that must be addressed in order to improve quality of life for burn survivors.
In the present study, burn size was positively associated with the severity of fatigue symptoms at 6 and 12 months postinjury. This result is unsurprising, and existing literature offers several explanations, including hypermetabolism, deconditioning, muscle wasting, and other burn sequelae.9,14 Previous studies demonstrating persistent muscle weakness and lower levels of aerobic fitness among adult burn survivors further support these explanations.23,24 Electrical injury was also associated with greater fatigue at 24 months postinjury, indicating that the nature of electrical injuries has a greater impact on long-term fatigue than fire/flame injuries. This is consistent with previous research showing that individuals with electrical burns are more likely to experience complications such as amputations.25 Female gender was also associated with greater fatigue at 12 months postinjury. This reflects findings related to chronic fatigue in the general population,26 as well as a previous study using the BMS database that showed women experience greater depression following burn injury.27
Though treatment options for chronic fatigue symptoms are limited, addressing the potential underlying causes may help improve long-term outcomes. Psychological distress is common after a traumatic event, and it may be linked to greater postinjury fatigue.12 More specifically, depression is a well-known long-term problem following burn injury that may contribute to the development of fatigue symptoms.28,29 In the present study, burn survivors with greater fatigue had lower mental health scores at all follow-up time points, further suggesting a relationship between fatigue and depression following burn injury. Psychological issues, as well as physical sequelae such as pain and itch, may also interfere with sleep. Sleep dissatisfaction has been found to be associated, in a dose-dependent fashion, with increasing burn size, as well as with many quality of life measures, including pain, itch, physical function, social interactions, family concerns, and work reintegration.30 Moreover, up to 61% of burn survivors report sleep dissatisfaction 1 year postinjury.31 Thus, poor sleep quality following burn injury is common, and it is likely that problems with sleep decrease a survivor’s overall energy and increase levels of fatigue. Addressing sleep concerns, as well as psychological distress, at long-term burn care follow-up may help alleviate lasting fatigue.
Exercise may also improve persistent fatigue symptoms after burn injury. Burn survivors have been shown to have impaired muscle strength years following injury.32 Burn-induced muscle wasting is a hallmark of severe burn trauma, and reduced muscle mass has been shown to persist years following injury.9 Thus, exercise is a key component of rehabilitation. Exercise helps restore muscle mass and cardiovascular fitness following prolonged hospitalization,33 and better overall fitness can make daily tasks less challenging. However, one challenge to intensive rehabilitation following burn injury is thermoregulation. Compared with unburned skin, burned skin does not allow for the release of heat. It is expected that burn survivors’ internal temperature will increase, more than that of someone without a burn, during exercise. Several recent studies have shown that exercise following injury is safe, though burn survivors do experience a moderate increase in temperature and heart rate compared with the non-burned controls.34,35
Additionally, management of the pathophysiological stress response to burn injury may aid the treatment of fatigue. Oxandrolone, an anabolic steroid, has been shown to increase muscle protein synthesis and weight gain and is associated with decreased hospital stays in severe burns.36 It is a safe and effective treatment for hypermetabolism and thus may help attenuate fatigue symptoms. Skeletal muscle protein damage resulting from mitochondrial hypermetabolism might represent a new target for interventions aimed at treating and preventing muscle wasting and hypermetabolism.37 Proper nutrition also plays an important role in combating muscle wasting and hypermetabolism9 and should be taken into account when treating chronic fatigue. Despite past research efforts, further investigation is needed to better understand the pathophysiology of long-term fatigue symptoms after burn injury and to evaluate the impact of these and other potential interventions. It is possible that mechanistic information for the cause of postburn fatigue could be obtained by examining burn-induced metabolism–related transcriptome changes within energy production genes and mitochondria.38,39
Postburn injury fatigue can have many far-reaching implications on community reintegration, including employment. Energy is an important factor in returning to work after burn injury, and long-term fatigue symptoms pose a large barrier to work productivity in all populations. Workers with fatigue are significantly more likely to miss work than workers without fatigue.40 One study estimated that U.S. workers with fatigue cost employers over $100 billion annually in lost productive time when compared with workers without fatigue.41 Fatigue is a highly prevalent issue for burn survivors, and unemployment was associated with fatigue at all 3 follow-up time points examined in this study. Consistent with this finding, the authors have found that patients often cite fatigue as a primary reason for not returning to preinjury jobs. This is particularly important, given that burn survivors who are not working appear to have more pain and lower quality of life compared with those who are working.42 Additional research is needed to further explore the relationship between fatigue and employment and other community reintegration outcomes following burn injury.
Study Limitations
Several limitations to this study must be noted. The preinjury data are collected at the time of acute care discharge, allowing for the possibility of recall bias. Although the vitality score is used in other studies to assess fatigue, there are other, newer measures that are specifically designed to assess fatigue. Additionally, the data were collected between 1997 and 2006 and thus may not reflect the most current state of fatigue after burn injury. Nonetheless, given the paucity of literature on the topic and the size of the multicenter database, this is an important first step in establishing fatigue as a common symptom complex after burns.
Acknowledgments
The contents of this article were developed under grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) grant numbers 90DP0035, 90DP0053, and 90DP0055. NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this article do not necessarily represent the policy of NIDILRR, ACL, HHS, and one should not assume endorsement by the Federal Government.
REFERENCES
- 1.Radbruch L, Strasser F, Elsner F, et al. ; Research Steering Committee of the European Association for Palliative Care (EAPC). Fatigue in palliative care patients—an EAPC approach. Palliat Med 2008;22:13–32. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 3.Zajicek JP, Ingram WM, Vickery J, Creanor S, Wright DE, Hobart JC. Patient-orientated longitudinal study of multiple sclerosis in south west England (The South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort. BMC Neurol 2010;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynia K, Middel B, van Dijk JP, De Keyser JH, Reijneveld SA. The impact of disabilities on quality of life in people with multiple sclerosis. Mult Scler 2008;14:972–80. [DOI] [PubMed] [Google Scholar]
- 5.Cantor JB, Ashman T, Gordon W, et al. Fatigue after traumatic brain injury and its impact on participation and quality of life. J Head Trauma Rehabil 2008;23:41–51. [DOI] [PubMed] [Google Scholar]
- 6.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist 2007;12(Suppl 1):4–10. [DOI] [PubMed] [Google Scholar]
- 7.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord 2007;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones J, Rutledge DN, Jones KD, Matallana L, Rooks DS. Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues 2008;18:406–12. [DOI] [PubMed] [Google Scholar]
- 9.Porter C, Tompkins RG, Finnerty CC, Sidossis LS, Suman OE, Herndon DN. The metabolic stress response to burn trauma: current understanding and therapies. Lancet 2016;388:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr 1999;23:160–8. [DOI] [PubMed] [Google Scholar]
- 12.Edwards RR, Smith MT, Klick B, et al. Symptoms of depression and anxiety as unique predictors of pain-related outcomes following burn injury. Ann Behav Med 2007;34:313–22. [DOI] [PubMed] [Google Scholar]
- 13.Gabbe BJ, Cleland H, Watterson D, et al. ; BRANZ Adult Long Term Outcomes pilot project participating sites and working party. Predictors of moderate to severe fatigue 12 months following admission to hospital for burn: results from the Burns Registry of Australia and New Zealand (BRANZ) Long Term Outcomes project. Burns 2016;42:1652–61. [DOI] [PubMed] [Google Scholar]
- 14.Holavanahalli RK, Helm PA, Kowalske KJ. Long-term outcomes in patients surviving large burns: the musculoskeletal system. J Burn Care Res 2016;37:243–54. [DOI] [PubMed] [Google Scholar]
- 15.Goverman J, Mathews K, Holavanahalli RK, et al. The National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model System: twenty years of contributions to clinical service and research. J Burn Care Res 2016;38:e240–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien C The CAGE questionnaire for detection of alcoholism. JAMA 2008;300(17):2054–6. [DOI] [PubMed] [Google Scholar]
- 17.Brown LF, Kroenke K, Theobald DE, Wu J. Comparison of SF-36 vitality scale and Fatigue Symptom Inventory in assessing cancer-related fatigue. Support Care Cancer 2011;19:1255–9. [DOI] [PubMed] [Google Scholar]
- 18.de Leon MB, Kirsch NL, Maio RF, et al. Baseline predictors of fatigue 1 year after mild head injury. Arch Phys Med Rehabil 2009;90:956–65. [DOI] [PubMed] [Google Scholar]
- 19.Neumann L, Berzak A, Buskila D. Measuring health status in Israeli patients with fibromyalgia syndrome and widespread pain and healthy individuals: utility of the short form 36-item health survey (SF-36). Semin Arthritis Rheum 2000;29:400–8. [DOI] [PubMed] [Google Scholar]
- 20.Ware J, Kosinski M, Dewey J. How to score version 2 of the SF-36® health survey. Medical Outcomes Trust and QualityMetric Incorporated, Lincoln, RI; 2002. [Google Scholar]
- 21.Friedman B, Heisel M, Delavan R. Validity of the SF-36 fiveitem Mental Health Index for major depression in functionally impaired, community-dwelling elderly patients. J Am Geriatr Soc 2005;53:1978–85. [DOI] [PubMed] [Google Scholar]
- 22.Bell JA, daCosta DiBonaventura M, Witt EA, Ben-Joseph R, Reeve BB. Use of the SF-36v2 Health Survey as a screen for risk of major depressive disorder in a US population-based sample and subgroup with chronic pain. Med Care 2016;55(2):111–6. [DOI] [PubMed] [Google Scholar]
- 23.Ganio MS, Pearson J, Schlader ZJ, et al. Aerobic fitness is disproportionately low in adult burn survivors years after injury. J Burn Care Res 2013;201423:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helm P, Herndon DN, Delateur B. Restoration of function. J Burn Care Res 2007;28:611–4. [DOI] [PubMed] [Google Scholar]
- 25.Soto CA, Albornoz CR, Peña V, Arriagada C, Hurtado JP, Villegas J. Prognostic factors for amputation in severe burn patients. Burns 2013;39:126–9. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Wood DR, Mangelsdorff AD, Meier NJ, Powell JB. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA 1988;260(7):929–34. [PubMed] [Google Scholar]
- 27.Wiechman SA, Ptacek JT, Patterson DR, Gibran NS, Engrav LE, Heimbach DM. Rates, trends, and severity of depression after burn injuries. J Burn Care Rehabil 2001;22:417–24. [DOI] [PubMed] [Google Scholar]
- 28.Ward HW, Moss RL, Darko DF, et al. Prevalence of postburn depression following burn injury. J Burn Care Rehabil 1987;8:294–8. [DOI] [PubMed] [Google Scholar]
- 29.Corfield EC, Martin NG, Nyholt DR. Co-occurrence and symptomatology of fatigue and depression. Compr Psychiatry 2016;71:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Lee AF, Ryan CM, Schneider JC, et al. Quantifying risk factors for long-term sleep problems after burn injury in young adults. J Burn Care Res 2015:510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masoodi Z, Ahmad I, Khurram F, Haq A. Changes in sleep architecture after burn injury: ‘waking up’ to this unaddressed aspect of postburn rehabilitation in the developing world. Can J Plast Surg 2013;21:234–8. [PMC free article] [PubMed] [Google Scholar]
- 32.St-Pierre DM, Choinière M, Forget R, Garrel DR. Muscle strength in individuals with healed burns. Arch Phys Med Rehabil 1998;79:155–61. [DOI] [PubMed] [Google Scholar]
- 33.de Lateur BJ, Shore WS. Exercise following burn injury. Phys Med Rehabil Clin N Am 2011;22:347–50, vii. [DOI] [PubMed] [Google Scholar]
- 34.Austin KG, Hansbrough JF, Dore C, Noordenbos J, Buono MJ. Thermoregulation in burn patients during exercise. J Burn Care Rehabil 2003;24:9–14. [DOI] [PubMed] [Google Scholar]
- 35.McEntire SJ, Chinkes DL, Herndon DN, Suman OE. Temperature responses in severely burned children during exercise in a hot environment. J Burn Care Res 2010;31:624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Guo Y, Yang Z, Roy M, Guo Q. The efficacy and safety of oxandrolone treatment for patients with severe burns: a systematic review and meta-analysis. Burns 2016;42:717–27. [DOI] [PubMed] [Google Scholar]
- 37.Ogunbileje JO, Herndon DN, Murton AJ, Porter C. The role of mitochondrial stress in muscle wasting following severe burn trauma. J Burn Care Res 2017. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouquet J, Soloski MJ, Swei A, et al. Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. 2016;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gräns H Transcriptome analysis of patients with chronic fatigue syndrome [dissertation]. Stockholm: Karolinska Institutet; 2005. [Google Scholar]
- 40.Janssen N, Kant IJ, Swaen GMH, Janssen PPM, Schröer CAP. Fatigue as a predictor of sickness absence: results from the Maastricht cohort study on fatigue at work. Occup Environ Med 2003;60(Suppl 1):i71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricci JA, Chee E, Lorandeau AL, Berger J. Fatigue in the U.S. workforce: prevalence and implications for lost productive work time. J Occup Environ Med 2007;49:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Dyster-Aas J, Kildal M, Willebrand M, Gerdin B, Ekselius L. Work status and burn specific health after work-related burn injury. Burns 2004;30:839–42. [DOI] [PubMed] [Google Scholar]


