Key Points
Question
What are the risks of venous thromboembolism (VTE) after lower extremity revascularization (LER) for peripheral artery disease (PAD), and is use of low-dose rivaroxaban plus aspirin associated with risk of VTE after LER?
Findings
In this cohort study of 6564 patients undergoing LER for PAD, the 3-year rate of VTE in patients receiving placebo was 1.66%, and low-dose rivaroxaban plus aspirin was associated with reduced risk for VTE.
Meaning
These findings suggest that patients who underwent LER for PAD were at risk for VTE in addition to arterial thrombotic events, and low-dose rivaroxaban plus aspirin should be considered for reduction of the full spectrum of thrombotic risk.
This cohort study assesses the risk factors and outcomes associated with venous thromboembolism after lower extremity revascularization among patients with peripheral artery disease.
Abstract
Importance
Prior studies have observed an association between the burden of atherosclerotic vascular disease and the risk of venous thromboembolism (VTE). The association is not well described in peripheral artery disease (PAD) after lower extremity revascularization (LER).
Objective
To describe the risk of, factors associated with, and outcomes after VTE, as well as the association of low-dose rivaroxaban plus antiplatelet therapy with VTE after LER.
Design, Setting, and Participants
This global, multicenter cohort study used data from the Vascular Outcomes Study of ASA (acetylsalicylic acid) Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization for PAD (VOYAGER PAD) randomized clinical trial, which enrolled patients from 2015 to 2018 with median follow-up of 28 months. Participants included patients with PAD undergoing LER. Patients with an indication for therapeutic anticoagulation were excluded. Data were analyzed from September 2020 to September 2021.
Exposure
Randomization to rivaroxaban 2.5 mg twice daily or placebo on a background of aspirin 100 mg daily; short-term clopidogrel was used at the discretion of the treating physician.
Main Outcomes and Measures
Symptomatic VTE was a prespecified secondary outcome and prospectively collected.
Results
Among 6564 patients (median [IQR] age, 67 [61-73] years; 4860 [74.0%] men), 66 patients had at least 1 VTE. The 3-year rate of VTE in patients receiving placebo was 1.7%, and the pattern of risk was linear (year 1: 0.5%; year 2: 1.1%). After multivariable modeling, weight (hazard ratio [HR], 3.04; 95% CI, 1.09-8.43), hypertension (HR, 2.11; 95% CI, 0.91-4.89), prior amputation (HR, 2.07; 95% CI, 0.95-4.53), and older age (HR, 1.81; 95% CI, 1.06-3.11) were associated with increased risk of VTE. VTE was associated with risk of subsequent mortality (HR, 7.22; 95% CI, 4.66-11.19). Compared with aspirin alone, rivaroxaban plus aspirin was associated with lower VTE risk (HR, 0.61; 95% CI, 0.37-0.998; P = .047), with benefit apparent early and sustained over time. This association was not modified by use of clopidogrel at randomization (without clopidogrel: HR, 0.55; 95% CI, 0.29-1.07; with clopidogrel: HR, 0.69; 95% CI, 0.32-1.48; P for interaction = .67).
Conclusions and Relevance
In this cohort study, there was continuous risk for VTE after LER in patients with PAD, with greater risk in patients who were older and had obesity and those with more severe PAD, as reflected by prior amputation. Low-dose rivaroxaban plus aspirin was associated with lower VTE risk compared with aspirin alone, with benefits apparent early and continued over time. The spectrum of venous and arterial thrombotic events and overall benefits of more potent antithrombotic strategies for prevention should be considered after LER for PAD.
Introduction
Peripheral artery disease (PAD) is associated with risk of arterial thrombotic complications and a broad range of manifestations of atherosclerosis-associated arterial thrombosis.1 Studies have demonstrated elevated risk of severe cardiovascular and limb ischemic events, including myocardial infarction (MI), stroke, acute limb ischemia, and major amputation, among patients with chronic PAD.2,3,4,5 After lower extremity revascularization (LER) for symptomatic PAD, the risk of arterial thrombotic complications is even greater, both early and in the long term after the procedure.1,5,6,7 The contribution of arterial thrombosis to ischemic risk in PAD has thus formed the basis for antithrombotic treatment.
Beyond risk for arterial thrombosis, data support an association between atherosclerosis and venous thromboembolism (VTE).8 Patients with VTE have been observed to have increased risk of concomitant atherosclerosis, as well as increased risk of subsequent atherosclerotic ischemic events.8,9,10 More recently, in a combined analysis11 of 2 antiplatelet therapy trials, Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–Thrombolysis in Myocardial Infarction 50 (TRA°2P-TIMI 50)12 and Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–TIMI 54 (PEGASUS-TIMI 54),13 patients with stable atherosclerotic cardiovascular disease, including PAD, were at risk for not only arterial thrombosis but also VTE, with higher VTE risk associated with greater burden of atherosclerosis. In the combined study by Cavallari et al,11 more intensive antiplatelet therapy was associated with lower risk of VTE. Similarly, the addition of a low-dose anticoagulant, rivaroxaban 2.5 mg twice daily, to aspirin vs aspirin alone was also associated with a 39% lower rate of VTE (hazard ratio [HR], 0.61; 95% CI, 0.37-1.00) in patients with stable atherosclerotic cardiovascular disease in the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial.14
These observations raise the question as to the pattern and magnitude of VTE risk in patients with symptomatic PAD in the acute postrevascularization setting and whether the potential benefits of more potent antithrombotic therapy with low-dose rivaroxaban observed in stable, chronic PAD might extend to the postprocedural setting, where use of dual antiplatelet therapy is common. Therefore, VTE was a prespecified outcome of the Vascular Outcomes Study of ASA (acetylsalicylic acid) Along With Rivaroxaban in Endovascular or Surgical Limb Revascularization for PAD (VOYAGER PAD) trial.15 This study describes the risk of, factors associated with, and outcomes after VTE, as well as the association between rivaroxaban plus antiplatelet therapy and VTE in the VOYAGER PAD trial.
Methods
This cohort study, a prespecified secondary analysis of the VOYAGER PAD study, was approved by institutional review boards at VOYAGER PAD participating institutions. All patients provided written, informed consent. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Source
Data for this cohort were from VOYAGER PAD (NCT02504216), the design and results of which have been previously published.15,16 VOYAGER PAD was a double-blind randomized clinical trial conducted at 542 sites in 34 countries, randomizing patients undergoing LER for symptomatic PAD to rivaroxaban 2.5 mg twice daily or placebo with a background of aspirin 100 mg daily. Concomitant clopidogrel use for no more than 6 months was allowed. The trial was designed and overseen by Colorado Prevention Center (CPC) Clinical Research (an academic research organization affiliated with the University of Colorado), the academic executive committee, and the sponsors, Bayer and Janssen Pharmaceuticals. CPC Clinical Research holds the clinical database and independently performed all analyses for this study.
Study Population
From 2015 to 2018, VOYAGER PAD enrolled patients with symptomatic PAD aged 50 years and older who underwent successful LER for claudication or critical limb ischemia via an endovascular (including hybrid) or surgical approach within the previous 10 days. Key exclusion criteria included planned dual antiplatelet therapy for more than 6 months, need for systemic anticoagulation (including acute treatment or long-term secondary prevention of VTE), recent acute limb ischemia or acute coronary syndrome, increased risk of bleeding, significantly impaired baseline kidney function, and prior intracranial hemorrhage, stroke, or transient ischemic attack. In VOYAGER PAD, race was self-reported as American Indian or Alaska Native, Asian, Black, Native Hawaiian or other Pacific Islander, multiple races, and White. These data were collected as part of an international clinical trial, as they can provide information about the study population.
Outcomes
In VOYAGER PAD, the primary efficacy end point was a composite of acute limb ischemia, major amputation of vascular etiology, MI, ischemic stroke, or cardiovascular death; symptomatic VTE, defined as pulmonary embolism or deep vein thrombosis, was prospectively ascertained by investigator report and was a prespecified secondary end point. For this analysis, symptomatic VTE was the primary end point. VTE severity was classified according to hospitalization or death within 30 days of the VTE event. An independent clinical events committee blinded to treatment assignment adjudicated all deaths and potential ischemic cardiovascular and limb events.
Statistical Analysis
Examining the association between rivaroxaban and risk of VTE was a prespecified secondary analysis of VOYAGER PAD. Categorical variables are reported as count (percentage), and continuous variables as median (IQR). Baseline characteristics grouped by incident VTE status during follow-up were compared using Wilcoxon rank-sum tests for continuous variables and χ2 or Fisher exact tests (where possible) for categorical variables.
A multivariable Cox regression model with stepwise selection was used to identify baseline demographic and clinical variables associated with VTE. Given the small number of VTE events, P = .10 was used for model entry or exit. Candidate variables are listed in the eTable in the Supplement. A model to estimate the association between all-cause death and incident VTE as a time-varying covariate was determined, with adjustment for treatment assignment and other baseline factors significantly related to VTE during follow-up, and stratification according to the type of procedure and according to whether clopidogrel was intended to be used. The risk of these events before vs after VTE was also summarized by the number of events per 100 patient-years of follow-up and associated 95% CIs. The association between rivaroxaban and time to first VTE was assessed by a Cox proportional hazards models, stratified by type of revascularization procedure and intended use of baseline clopidogrel. The treatment effect was summarized by an HR with associated Wald 95% CI and log-rank P value. A sensitivity analysis for the outcome accounted for death from any cause as a competing terminal event. Event probabilities were determined by Kaplan-Meier estimates of cumulative incidence. Potential heterogeneity of the association of rivaroxaban with VTE for subgroups defined by baseline demographic and clinical characteristics was assessed by the significance of interaction terms in proportional hazards models. Analyses of clinical outcomes were performed according to the intention-to-treat principle, including all patients and events from randomization to the study efficacy cutoff date. Two-tailed P < .05 was considered statistically significant, with no adjustment for multiple testing. Analyses were performed in SAS version 9.4 (SAS Institute) and S-Plus version 8.2 (MS Miami International Software). Data were analyzed from September 2020 to September 2021.
Results
Baseline Characteristics
Among 6564 patients randomized in VOYAGER PAD and followed for a median (IQR) of 28 (22-34) months, 66 patients (1.0%) had at least 1 VTE event, and the incidence of VTE was 0.42 per 100 patient-years. Baseline characteristics according to incident VTE status are shown in the Table. Patients with VTE, compared with patients without VTE, were older (median [IQR] age, 68 [64-75] years vs 67 [61-73] years), had greater body weight (7.6% vs 16.6% with weight ≤60 kg), and more frequently had hypertension (90.9% vs 81.3%). Patients with VTE vs those without also had more severe PAD, as indicated by more frequent history of LER (43.9% vs 35.5%) and amputation (10.6% vs 5.9%).
Table. Baseline Characteristics.
| Characteristic | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| Overall (N = 6564) | With VTE (n = 66) | Without VTE (n = 6498) | ||
| Age, y | ||||
| Median (IQR) | 67 (61-73) | 68 (64-75) | 67 (61-73) | .14 |
| ≥75 | 1330 (20.3) | 19 (28.8) | 1311 (20.2) | .09 |
| Sex | ||||
| Women | 1704 (26.0) | 16 (24.2) | 1688 (26.0) | .89 |
| Men | 4860 (74.0) | 50 (75.8) | 4810 (74.0) | |
| Weight ≤60 kg | 1082 (16.5) | 5 (7.6) | 1077 (16.6) | .046 |
| Race | ||||
| American Indian or Alaska Native | 5 (0.1) | 0 | 5 (0.1) | .25 |
| Asian | 966 (14.7) | 4 (6.0) | 962 (14.8) | |
| Black | 155 (2.4) | 0 | 155 (2.4) | |
| Native Hawaiian or Other Pacific Islander | 1 (<0.1) | 0 | 1 (<0.1) | |
| Multiple | 3 (0.1) | 0 | 3 (0.1) | |
| White | 5303 (80.8) | 59 (89.4) | 5244 (80.7) | |
| Not Reported | 131 (2.0) | 3 (4.6) | 128 (2.0) | |
| Geographic region | ||||
| North America | 694 (10.6) | 13 (19.7) | 681 (10.5) | .02 |
| Western Europe | 1826 (27.8) | 24 (36.4) | 1802 (27.7) | |
| Eastern Europe | 2599 (39.6) | 22 (33.3) | 2577 (39.7) | |
| Asia Pacific | 961 (14.6) | 4 (6.1) | 957 (14.7) | |
| South America | 484 (7.4) | 3 (4.6) | 481 (7.4) | |
| Risk factors and comorbidities | ||||
| Current smoker | 2279 (34.7) | 19 (28.8) | 2260 (34.8) | .41 |
| Hypertension | 5342 (81.4) | 60 (90.9) | 5282 (81.3) | .05 |
| Hyperlipidemia | 3939 (60.0) | 36 (54.6) | 3903 (60.1) | .38 |
| Coronary artery disease | 2067 (31.5) | 21 (31.8) | 2046 (31.5) | >.99 |
| Heart failure | 539 (8.2) | 5 (7.6) | 534 (8.2) | >.99 |
| Carotid artery disease | 575 (8.8) | 9 (13.6) | 566 (8.7) | .18 |
| Diabetes | 2629 (40.1) | 26 (39.4) | 2603 (40.1) | >.99 |
| eGFR <60 mL/min/1.73 m2 | 1327 (20.2) | 17 (25.8) | 1310 (20.2) | .28 |
| SBP, median (IQR), mm Hg | 135 (125-145) | 135 (125-150) | 125 (125-145) | .71 |
| Polyvascular disease | 2361 (36.0) | 25 (37.8) | 2336 (35.9) | .80 |
| History of cancer | 137 (2.1) | 2 (3.0) | 135 (2.1) | .40 |
| PAD history | ||||
| Index ABI, median (IQR) | 0.56 (0.42-0.67) | 0.54 (0.42-0.67) | 0.56 (0.42-0.67) | .69 |
| Prior revascularization | 2336 (35.6) | 29 (43.9) | 2307 (35.5) | .16 |
| Prior amputation | 390 (5.9) | 7 (10.6) | 383 (5.9) | .11 |
| Qualifying revascularization | ||||
| Revascularization approach | ||||
| Endovascular | 4379 (66.7) | 41 (62.1) | 4338 (66.8) | .87 |
| Surgical | 2185 (33.3) | 25 (37.9) | 2160 (33.2) | |
| Site of revascularization | ||||
| Popliteal or above | 5919 (90.2) | 58 (87.9) | 5861 (90.2) | .53 |
| Infrapopliteal or other | 645 (9.8) | 8 (12.2) | 637 (9.8) | |
| Randomization strata | ||||
| Endovascular with clopidogrel | 3080 (46.9) | 25 (37.9) | 3055 (47.0) | .31 |
| Endovascular without clopidogrel | 1213 (18.5) | 15 (22.7) | 1198 (18.4) | |
| Surgical intervention | 2271 (34.6) | 26 (39.4) | 2245 (34.6) | |
| Indication for revascularization | ||||
| Claudication | 5031 (76.6) | 49 (74.4) | 4982 (76.7) | .66 |
| Critical limb ischemia | 1533 (23.4) | 17 (25.8) | 1516 (23.3) | |
| Long (≥15 cm) target lesion length | 2252 (34.3) | 27 (40.9) | 2225 (34.2) | .049 |
| Medications | ||||
| Statin | 5249 (80.0) | 51 (77.3) | 5198 (80.0) | .54 |
| Clopidogrel | 3313 (50.5) | 27 (40.9) | 3286 (50.6) | .14 |
| Randomized to rivaroxaban | 3286 (50.1) | 25 (37.9) | 3261 (50.2) | .048 |
Abbreviations: ABI, ankle-brachial index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Factors Associated With VTE
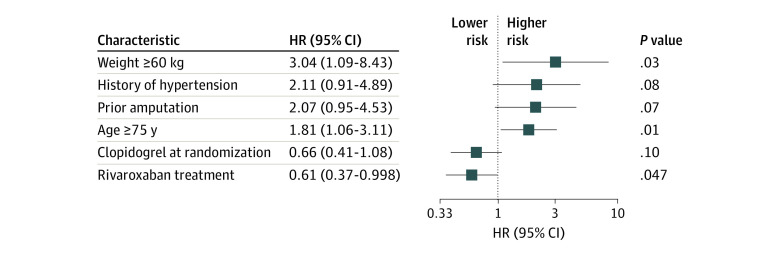
After multivariable modeling (Figure 1), baseline factors independently associated with VTE risk included older age (HR, 1.81; 95% CI, 1.06-3.11), weight (HR, 3.04; 95% CI, 1.09-8.43), and hypertension (HR, 2.11; 95% CI, 0.91-4.89). Prior amputation (HR, 2.07; 95% CI, 0.95-4.53), an indicator of PAD severity, was also associated with VTE risk. Clopidogrel use at randomization was not associated with VTE risk (HR, 0.66; 95% CI, 0.41-1.08), but rivaroxaban use was (HR, 0.60; 95% CI, 0.37-0.998; P = .047). Clopidogrel was used for a median (IQR) of 29 (26-50) days in the overall study population.
Figure 1. Baseline Characteristics Associated With Venous Thromboembolism .
Associated variables (candidate variables are listed in the eTable in the Supplement) were identified by stepwise selection with a P = .10 for model entry or exit. HR indicates hazard ratio.
Outcomes After VTE
Of the first VTE events occurring during follow-up, 27 (40.9%) were nonfatal and did not lead to hospitalization, 29 (43.9%) led to hospitalization, and 10 (15.2%) were associated with death within 30 days of the VTE event (Figure 2). Overall, of 66 patients who experienced a VTE event, 23 (34.8%) subsequently died over a median (IQR) of 1.2 (0.2-1.8) years. Across treatment groups, incident VTE was associated with a greater risk of subsequent death compared with no VTE, with a marked increase in events per 100 patient-years after a VTE event (all-cause mortality: 3.7 [95% CI, 3.4-4.0] deaths per 100 patient-years before VTE vs 28.8 [95% CI, 18.2-43.1] deaths per 100 patient-years after VTE). After adjusting for the factors significantly associated with VTE, VTE was associated with significantly increased risk of all-cause death during follow-up (HR, 7.22; 95% CI, 4.66-11.19).
Figure 2. Distribution of Venous Thromboembolism According to Event Severity.
Shown are the distributions of venous thromboembolism event severity in patients randomized to placebo or rivaroxaban.
Rivaroxaban vs Placebo and VTE
In patients randomized in VOYAGER PAD, rivaroxaban was associated with a 39% lower risk of VTE (HR, 0.61; 95% CI, 0.37-0.998; P = .047) (Figure 3), with event rates at 3 years of 1.7% in those receiving antiplatelet therapy alone, with a linear pattern of risk (year 1: 0.5%; year 2: 1.1%), and 0.8% in those receiving rivaroxaban in addition to antiplatelet therapy. Among 41 patients in the placebo group with VTE, 25 events were deep vein thrombosis and 16 events were pulmonary embolism; among 25 patients in the rivaroxaban group with VTE, 19 events were deep vein thrombosis and 6 events were pulmonary embolism. Furthermore, the association between rivaroxaban and decreased risk of VTE was unchanged when death from any cause was treated as a competing terminal event (HR, 0.61; 95% CI, 0.37-0.995; P = .048). The association between rivaroxaban and VTE was consistent among multiple subgroups and irrespective of use of clopidogrel and statin at randomization (Figure 4). There were no statistically significant differences in the number of events in each category of VTE severity (Figure 2).
Figure 3. Association Between Rivaroxaban and Venous Thromboembolism .
Shown are the cumulative incidence curves for venous thromboembolism in patients assigned to rivaroxaban vs placebo and the hazard ratio (HR) and associated 95% CI.
Figure 4. Association Between Rivaroxaban and Venous Thromboembolism in Selected Subgroups.
CAD indicates coronary artery disease; eGFR, estimated glomerular filtration rate; and HR, hazard ratio.
Discussion
This cohort study reports 3 novel findings on the risk of VTE and benefit associated with rivaroxaban in patients with PAD after LER. First, the rate of VTE after LER was approximately 0.6% per year, which is approximately 2-fold that seen in stable atherosclerosis populations,11,14 and both patient factors (eg, age, obesity) and severity of PAD (eg, prior amputation) were associated with VTE risk. Second, the pattern of risk for VTE was not front-loaded, as might be anticipated in the postprocedural setting, but rather was linear and continuous, suggesting that VTE was a consequence of the disease state rather than the procedure. Third, in addition to the arterial benefits described in the VOYAGER PAD trial, rivaroxaban was also associated with a lower rate of VTE, underscoring the broad thrombotic benefits of this strategy in patients at heightened thrombotic risk.
Although atherothrombosis, or atherosclerosis-associated arterial thrombosis, and VTE are often considered separate entities, an evolving body of literature suggests an association between atherosclerotic vascular disease and VTE. In a large population-based case-control study, risk for subsequent MI and stroke was 20% to 40% higher among patients with VTE compared with controls, and VTE was associated with 1.9-fold increased risk of MI and 2.7-fold increased risk of stroke.9 Conversely, patients with a history of symptomatic atherosclerotic cardiovascular disease have also been found to be at increased risk of VTE.11,17 In addition, arterial and venous thrombosis share common risk factors, such as age and obesity,18 and data suggest that thrombophilia and inflammation may play a role in both conditions.19,20,21 Statins, which have anti-inflammatory properties,22 have been associated with 20% to 30% decreased risk of VTE,23,24,25 and there was an observed trend for reduction in VTE associated with PCSK9-inhibitor use in acute coronary syndromes, with the risk of VTE related to reduction in the proinflammatory lipoprotein(a),26 but not low-density lipoprotein cholesterol.27 Further evidence for shared pathobiology in atherothrombosis and VTE comes from the finding of increased platelet activation in patients with VTE,28,29 suggesting cross-talk between platelet function and coagulation. Consistent with this finding is the additional 29% reduction in VTE associated with more intensive antiplatelet therapy demonstrated in TRA°2P-TIMI 50 and PEGASUS-TIMI 54.11,12,13
Given this association between atherosclerosis and VTE, we examined VTE as a potential complication after LER for symptomatic PAD. In patients with stable atherosclerotic vascular disease with mixed CAD and PAD assigned to placebo in a pooled analysis of the TRA°2P-TIMI 50 and PEGASUS-TIMI 54 trials and in the COMPASS trial, the rate of VTE was approximately 0.3% per year.11,12,13,14 In contrast, the risk of VTE among patients in the placebo group in VOYAGER PAD was almost 2-fold higher (approximately 0.6% per year). In this population, patient factors, such as older age and greater weight, as well as greater PAD severity, as evidenced by history of lower extremity amputation, were associated with VTE risk. These findings provide a more complete understanding of the true spectrum of thrombotic risk facing patients with PAD undergoing revascularization and may help to identify patients at higher risk for VTE after LER. Although attention has been focused on postprocedure arterial complications, VTE events are not benign and can be associated with significant morbidity, mortality, and cost.30,31 In our analysis, 44% of VTE complications required inpatient treatment, and 15% of patients died within 30 days of their VTE event. Increased risk of death after incident VTE within the first year, as well as long-term up to 30 years after, has also been observed.32
Beyond raising awareness of the heightened risk for VTE after LER, our results also provide insight into the underlying pathophysiology of VTE in this population. We hypothesized that VTE risk might be higher immediately after LER, perhaps related to procedure-associated hypercoagulability and reduced mobility. However, we observed a linear and continuous pattern of risk for VTE after LER. This pattern suggests that VTE risk is related to PAD itself, rather than only to procedure- or hospitalization-related factors. Along with the observed post-LER VTE risk that was almost 2-fold that in patients with stable vascular disease, these results suggest that more severe atherosclerosis (eg, symptomatic PAD treated with LER) is associated with VTE risk and further support an association between atherosclerosis and venous thrombotic events.
Interestingly, concomitant CAD was not identified as a factor associated with VTE in this analysis. Prior studies have demonstrated that among patients with stable atherosclerotic disease, polyvascular disease (ie, disease in more than 1 vascular territory) is associated with greater risk of major adverse cardiovascular events compared with disease in a single vascular territory.33,34 However, most patients in those studies had CAD; thus, the term polyvascular has often referred to the addition of PAD to CAD. In this study, CAD was not informative for risk: having baseline CAD in addition to PAD was not associated with greater risk of VTE. While this may be related to the presence of CAD in only approximately one-third of the population and low numbers of events, the data also suggest that perhaps in this population, the burden of atherosclerosis overall is not as important as the burden of PAD specifically. This is consistent with the identification of prior major lower extremity amputation reflecting severe PAD as a baseline characteristic associated with VTE. Functional limitations and decreased mobility associated with PAD may also contribute to increased risk of VTE in this setting. Furthermore, prior data have demonstrated that, in contrast to CAD, pathological manifestations of PAD are related to thrombosis irrespective of the extent of atherosclerosis,35 providing additional support for greater thrombotic risk associated with PAD than other atherosclerotic diseases.
Although antiplatelet agents remain first-line therapy for atherosclerotic vascular disease, most recently, greater intensity of antithrombotic therapy with dual pathway inhibition combining a factor Xa inhibitor with aspirin has been studied for the prevention of thrombotic complications in patients with vascular disease. In COMPASS,14 rivaroxaban 2.5 mg twice daily plus low-dose aspirin was associated with lower VTE risk in patients with stable atherosclerotic disease who were only treated with single antiplatelet therapy. Our study from VOYAGER PAD demonstrates that even in the higher risk, acute setting of LER for symptomatic PAD, among patients often treated with dual antiplatelet therapy, rivaroxaban plus aspirin was associated with a 39% reduction in VTE, with an effect apparent early after the procedure and irrespective of clopidogrel or statin use at randomization. These findings should be considered hypothesis-generating, since VTE was a low-event secondary outcome. Taken together with the primary results of VOYAGER PAD,15 these results suggest that this strategy of dual pathway inhibition may be associated with long-term protection against the spectrum of venous and arterial post-LER thrombotic complications. Importantly, both potential benefit and risk should be considered when selecting patients for more intensive antithrombotic therapy after LER. For example, older age, a factor associated with VTE risk in this study, is also a risk factor for bleeding procedural complications. However, the events prevented with rivaroxaban after LER extend beyond VTE, and there is an overall net benefit for use of rivaroxaban in this setting among older patients, with an estimated 38 ischemic events prevented at a cost of 8 TIMI major bleeds without intracranial hemorrhage or fatal bleeding among 1000 patients aged 75 years and older treated for 3 years with rivaroxaban plus aspirin after LER.36
Limitations
This study has several limitations. First, VTE was a secondary end point in VOYAGER PAD and was investigator reported; however, VTE was prospectively ascertained, and this analysis was prespecified. Second, adjusted models accounted for known baseline characteristics, but postrandomization variables were not included, and residual confounding may exist. Third, although associated factors were modeled, formal predictive modeling, including cross-validation, was not performed owing to the number of events. Fourth, the number of VTE events was small, which may have resulted in overfitting of models, and fifth, the median time to death after VTE likely reflects a combination of fatal VTE events and nonfatal VTE events. Sixth, although the P value associated with rivaroxaban effect on VTE was P < .05, it should be considered nominal, given its position in the hierarchy of testing of secondary outcomes in VOYAGER PAD.
Conclusions
This cohort study found that patients with symptomatic PAD undergoing LER were at heightened risk for VTE, with almost 2-fold the risk observed in patients with stable vascular disease. Postprocedure VTE risk accrued in a linear, continuous fashion, suggesting that risk was related more to PAD rather than the procedure. VTE was associated with poor prognosis, and patient and PAD characteristics may help clinicians identify patients at risk for VTE events. Rivaroxaban plus aspirin was associated with lower risk of VTE after LER. Combined with the arterial benefits reported in VOYAGER PAD, these findings highlight the broad thrombotic benefits of this approach in patients with PAD.
eTable. Candidate Variables for Factors Associated With VTE
References
- 1.Hess CN, Rogers RK, Wang TY, et al. Major adverse limb events and 1-year outcomes after peripheral artery revascularization. J Am Coll Cardiol. 2018;72(9):999-1011. doi: 10.1016/j.jacc.2018.06.041 [DOI] [PubMed] [Google Scholar]
- 2.Kumbhani DJ, Steg PG, Cannon CP, et al. ; REACH Registry Investigators . Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35(41):2864-2872. doi: 10.1093/eurheartj/ehu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2°P-TIMI 50. Circulation. 2013;127(14):1522-1529, 1529e1-6. doi: 10.1161/CIRCULATIONAHA.112.000679 [DOI] [PubMed] [Google Scholar]
- 4.Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719-2728. doi: 10.1016/j.jacc.2016.03.524 [DOI] [PubMed] [Google Scholar]
- 5.Hess CN, Huang Z, Patel MR, et al. Acute limb ischemia in peripheral artery disease: insights from EUCLID. Circulation. 2019;140(7):556-565. doi: 10.1161/CIRCULATIONAHA.119.039773 [DOI] [PubMed] [Google Scholar]
- 6.Bonaca MP, Gutierrez JA, Creager MA, et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50). Circulation. 2016;133(10):997-1005. doi: 10.1161/CIRCULATIONAHA.115.019355 [DOI] [PubMed] [Google Scholar]
- 7.Hess CN, Wang TY, Weleski Fu J, et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 2020;75(5):498-508. doi: 10.1016/j.jacc.2019.11.050 [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P, Bilora F, Marchiori A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348(15):1435-1441. doi: 10.1056/NEJMoa022157 [DOI] [PubMed] [Google Scholar]
- 9.Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370(9601):1773-1779. doi: 10.1016/S0140-6736(07)61745-0 [DOI] [PubMed] [Google Scholar]
- 10.Piazza G, Goldhaber SZ, Lessard DM, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with symptomatic atherosclerosis. Thromb Haemost. 2011;106(6):1095-1102. [DOI] [PubMed] [Google Scholar]
- 11.Cavallari I, Morrow DA, Creager MA, et al. Frequency, predictors, and impact of combined antiplatelet therapy on venous thromboembolism in patients with symptomatic atherosclerosis. Circulation. 2018;137(7):684-692. doi: 10.1161/CIRCULATIONAHA.117.031062 [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, Braunwald E, Bonaca MP, et al. ; TRA 2P–TIMI 50 Steering Committee and Investigators . Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366(15):1404-1413. doi: 10.1056/NEJMoa1200933 [DOI] [PubMed] [Google Scholar]
- 13.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators . Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. doi: 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 14.Eikelboom JW, Connolly SJ, Bosch J, et al. ; COMPASS Investigators . Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319-1330. doi: 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 15.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994-2004. doi: 10.1056/NEJMoa2000052 [DOI] [PubMed] [Google Scholar]
- 16.Capell WH, Bonaca MP, Nehler MR, et al. Rationale and design for the vascular outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am Heart J. 2018;199:83-91. doi: 10.1016/j.ahj.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Sørensen HT, Horvath-Puho E, Søgaard KK, et al. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2009;7(4):521-528. doi: 10.1111/j.1538-7836.2009.03279.x [DOI] [PubMed] [Google Scholar]
- 18.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93-102. doi: 10.1161/CIRCULATIONAHA.107.709204 [DOI] [PubMed] [Google Scholar]
- 19.Luxembourg B, Schmitt J, Humpich M, et al. Cardiovascular risk factors in idiopathic compared to risk-associated venous thromboembolism: a focus on fibrinogen, factor VIII, and high-sensitivity C-reactive protein (hs-CRP). Thromb Haemost. 2009;102(4):668-675. [DOI] [PubMed] [Google Scholar]
- 20.Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism: a prospective investigation in the ARIC cohort. Thromb Haemost. 2009;102(4):615-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchini M, Mannucci PM. Association between venous and arterial thrombosis: clinical implications. Eur J Intern Med. 2012;23(4):333-337. doi: 10.1016/j.ejim.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Liberale L, Carbone F, Montecucco F, Sahebkar A. Statins reduce vascular inflammation in atherogenesis: a review of underlying molecular mechanisms. Int J Biochem Cell Biol. 2020;122:105735. doi: 10.1016/j.biocel.2020.105735 [DOI] [PubMed] [Google Scholar]
- 23.Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31(10):1248-1256. doi: 10.1093/eurheartj/ehp556 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal V, Phung OJ, Tongbram V, Bhardwaj A, Coleman CI. Statin use and the prevention of venous thromboembolism: a meta-analysis. Int J Clin Pract. 2010;64(10):1375-1383. doi: 10.1111/j.1742-1241.2010.02439.x [DOI] [PubMed] [Google Scholar]
- 25.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851-1861. doi: 10.1056/NEJMoa0900241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orsó E, Schmitz G. Lipoprotein(a) and its role in inflammation, atherosclerosis and malignancies. Clin Res Cardiol Suppl. 2017;12(suppl 1):31-37. doi: 10.1007/s11789-017-0084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators . Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2020;141(20):1608-1617. doi: 10.1161/CIRCULATIONAHA.120.046524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vesterqvist O, Gréen K, Johnsson H. Thromboxane and prostacyclin formation in patients with deep vein thrombosis. Thromb Res. 1987;45(4):393-402. doi: 10.1016/0049-3848(87)90228-3 [DOI] [PubMed] [Google Scholar]
- 29.Chirinos JA, Heresi GA, Velasquez H, et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45(9):1467-1471. doi: 10.1016/j.jacc.2004.12.075 [DOI] [PubMed] [Google Scholar]
- 30.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4)(suppl):S495-S501. doi: 10.1016/j.amepre.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 31.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi: 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130(10):829-836. doi: 10.1161/CIRCULATIONAHA.114.009107 [DOI] [PubMed] [Google Scholar]
- 33.Bonaca MP. Polyvascular disease and risk: When two is not better than one. Vasc Med. 2018;23(6):531-533. doi: 10.1177/1358863X18796936 [DOI] [PubMed] [Google Scholar]
- 34.Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137(4):338-350. doi: 10.1161/CIRCULATIONAHA.117.032235 [DOI] [PubMed] [Google Scholar]
- 35.Narula N, Olin JW, Narula N. Pathologic disparities between peripheral artery disease and coronary artery disease. Arterioscler Thromb Vasc Biol. 2020;40(9):1982-1989. doi: 10.1161/ATVBAHA.119.312864 [DOI] [PubMed] [Google Scholar]
- 36.Krantz MJ, Debus SE, Hsia J, et al. Low-dose rivaroxaban plus aspirin in older patients with peripheral artery disease undergoing acute limb revascularization: insights from the VOYAGER PAD trial. Eur Heart J. 2021;42(39):4040-4048. doi: 10.1093/eurheartj/ehab408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Candidate Variables for Factors Associated With VTE