Abstract
Purpose of Review
While vascular endothelial growth factor receptor inhibitors (VEGFRis) have dramatically improved cancer survival, these drugs cause hypertension in a majority of patients. This side effect is often dose limiting and increases cardiovascular mortality in cancer survivors. This review summarizes recent advances in our understanding of the molecular mechanisms and clinical findings that impact management of VEGFRi-induced hypertension.
Recent Findings
Recent studies define new connections between endothelial dysfunction and VEGFRi-induced hypertension, including the balance between nitric oxide, oxidative stress, endothelin signaling, and prostaglandins and the potential role of microparticles, vascular smooth muscle cells, vascular stiffness, and microvessel rarefaction. Data implicating genetic polymorphisms that might identify patients at risk for VEGFRi-induced hypertension and the growing body of literature associating VEGFRi-induced hypertension with antitumor efficacy are reviewed.
Summary
These recent advances have implications for the future of cardio-oncology clinics and the management of VEGFRi-induced hypertension.
Keywords: Vascular endothelial growth factor receptor inhibitor, Hypertension, Cardio-oncology, Endothelial cell, Nitric oxide, Endothelin
Introduction and Background
Overview
Inhibition of vascular endothelial growth factor receptor (VEGFR)-mediated tumor blood vessel growth is a major advance in the management of patients with solid organ malignancies [1]. While it was hoped that therapies targeted to tumor-specific mechanisms would avoid side effects, many targeted agents induce cardiovascular (CV) toxicity that can limit cancer treatment and overall survival [2, 3, 4, 5, 6, 7, 8]. A recent meta-analysis demonstrated that VEGFR inhibitor (VEGFRi) treatment significantly increased the risk of cardiac dysfunction with an odds ratio (OR) of 1.35 and arterial thromboembolism (OR 1.52), but by far the most common side effect of VEGFRi is hypertension (OR 5.28), which is the focus of this review. Increased blood pressure (BP) is noted in almost all VEGFRi-treated patients, resulting in new or worsening hypertension in up to 70%, a side effect that often requires treatment dose adjustment or discontinuation with a negative impact on cancer outcomes [9•]. In addition to the negative impact of dose adjustment on cancer outcome, age adjusted mortality from hypertension has increased over the past 2 decades and CV mortality has overtaken cancer mortality for some long-term cancer survivors [10, 11]. While data is not available for long-term cardiovascular outcomes in VEGFRi-treated cancer patients, women exposed to preeclampsia during pregnancy, a disorder frequently associated with overexpression of a soluble VEGF trap, have a significant increase risk of early cardiovascular adverse events [12]. Finally, the rising use of combination therapies, each with distinct CV toxicities that are often synergistic, further enhances the CV risk of state-of-the-art cancer treatment regimens [13]. Thus, there has been a recent focus on understanding the mechanisms driving VEGFRi-induced hypertension to improve both cancer and cardiovascular outcomes by predicting those at greatest risk for side effects and identifying the best therapies to lower BP.
Role of VEGF in Normal Vascular Function and Cancer Progression
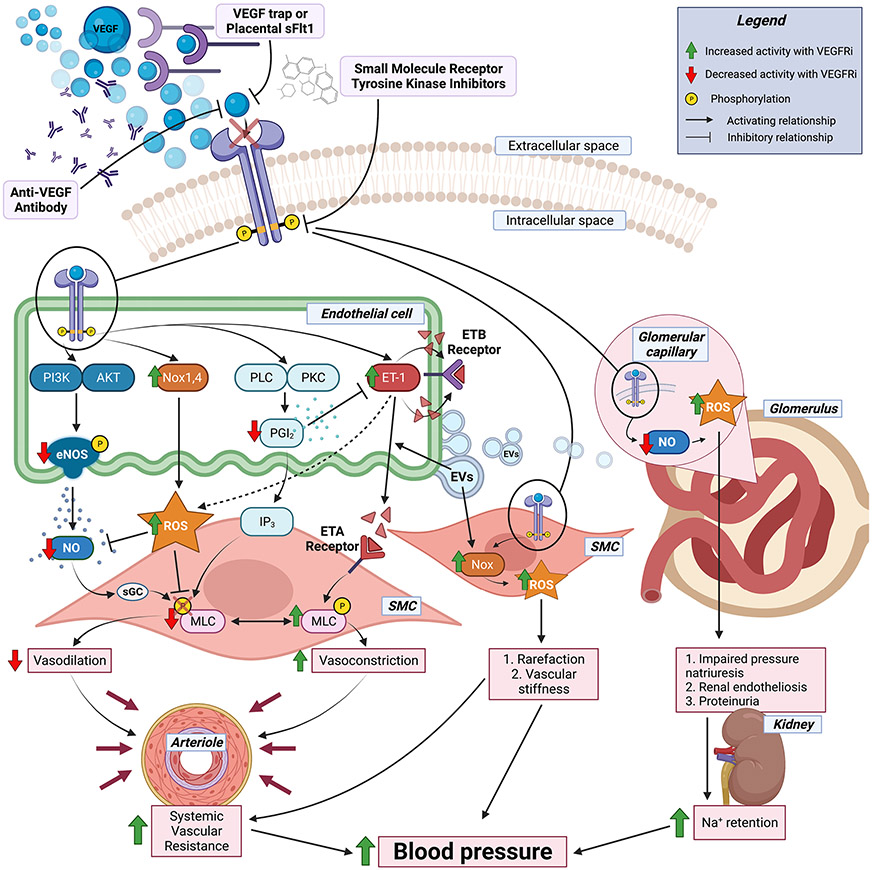
The VEGFs are a family of secreted proteins that are necessary for normal blood vessel growth and health. Blood vessels are composed of 3 layers. The innermost endothelium is a single layer of endothelial cells (ECs) that lines the lumen and hence is in contact with blood and all systemically circulating drugs. The medial layer is made up of vascular smooth muscle cells (SMCs) that constrict or relax to regulate lumen diameter, blood flow, and peripheral vascular resistance and hence contribute to BP control. The outer adventitia provides structure and support to the inner layers. VEGFs signal through VEGFRs, tyrosine kinase receptors which are predominantly expressed on the surface of ECs (although they are also be expressed on injured SMC) [14]. The healthy endothelium is anti-inflammatory, anti-thrombotic and releases vasodilatory factors that relax SMC to regulate vessel diameter. Factors released by ECs to control vessel tone include the vasodilators nitric oxide (NO) and prostacyclin (also known as prostaglandin I2 (PGI2)), as well as vasoconstrictors, including endothelin-1 (ET-1), which promotes vasoconstriction by interacting with SMC endothelin A receptors (ETAs) to phosphorylate myosin light chain leading to vasoconstriction (see Fig. 1). In addition to triggering ECs to release vasodilatory factors, activation of VEGFR signaling promotes proliferation and survival of ECs, enhances vascular permeability, and drives angiogenesis [15, 16]. Tumor growth is critically dependent on a sufficient blood supply [17]. As solid tumors grow, the core becomes hypoxic triggering the release of pro-angiogenic factors which induce neo-angiogenesis (the growth of new blood vessels from existing ones), a process that is dependent on VEGF signaling [15, 16]. VEGFR activation also enhances EC permeability which may contribute to cancer cell metastasis [18].
Fig. 1.
Mechanisms of VEGFRi-induced hypertension. Model summarizing the current understanding of molecular mechanisms driving the rise in blood pressure in patients treated with drugs blocking VEGF receptor signaling. The top indicates the classes of VEGF receptor inhibitors (VEGFRis) and their mechanisms of action. The middle summarizes the molecular impact of VEGFRi on endothelial cell, smooth muscle cell (SMC), or glomerular cell functions with the red arrows indicating decreases and green arrows indicating increases in response to VEGFRi treatment. The bottom indicates the physiologic impact of these molecular events on arteriolar and kidney function that contribute to VEGFRi-induced blood pressure elevation. Figure created with Biorender. VEGF vascular endothelial growth factor, ROS reactive oxygen species, PI3K phosphoinositide 3-kinases, Akt protein kinase B, Nox NADPH oxidase, PEC phospholipase C, PKC protein kinase C, PGI2 prostacyclin, IP3 inositol trisphosphate, ET-1 endothelin-1, ETA/B endothelin receptor A/B, NO nitric oxide, sGC soluble guanylate cyclase, MLC myosin light chain kinase, EVs extracellular vesicles
Impact of VEGFRi on Cancer Outcomes and Blood Pressure in Human and Canine Cancer Patients
Over the last 15 years, multiple classes of anti-angiogenic therapies have been developed that inhibit VEGF signaling to treat cancer. The earliest drugs were monoclonal antibodies (bevacizumab) and decoy receptors (aflibercept) which act by sequestering VEGFs to prevent binding to their receptors. Newer agents are small molecule tyrosine kinase inhibitors (TKIs) (sunitinib, sorafenib, axitinib, and many others) that directly inhibit VEGFR signaling as well as other non-VEGFR tyrosine kinases (Fig. 1, top). By targeting the cancer vasculature (rather than the tumor itself), VEGFR inhibitors block tumor angiogenesis to decrease tumor size and decrease vascular permeability which may prevent metastasis [19, 20]. By these mechanisms, VEGFR inhibition improves cancer outcomes, more than doubling progression free survival in patients with hepatocellular or renal cell carcinoma among other cancer types [21].
Consistent with the known function of VEGFR signaling in maintaining EC health, VEGFRis induce endothelial dysfunction and hypertension in a variety of settings [9•, 16, 20, 21]. In women with preeclampsia, a syndrome of hypertension and renal protein loss in pregnancy, release of soluble VEGFR1 (also known as sFlt1) from the ischemic placenta acts like the decoy receptors to sequester circulating VEGFs, leading to hypertension and endothelial dysfunction (reviewed in [22]). In human cancer patients, VEGFRi treatment increases BP in most patients, with a number needed to harm of 6 for hypertension and 17 for severe hypertension [9•]. This mechanism is highly conserved as hypertension is also a common complication of VEGFRi cancer therapy in veterinary oncology practice. The VEGFR TKI toceranib was FDA approved for canines in 2009, and several others are used off-label based on human experience [23]. VEGFRi has benefits in a variety of canine cancers including mast cell tumor, several carcinomas, neuroendocrine cancers, and multiple myeloma [24, 25, 26, 27]. Clinical toxicides in canines include both protein losing nephropathy and hypertension, similar to VEGFRi side effects seen in humans [23, 28]. In one study of dogs undergoing toceranib treatment for cancer, 67% developed an increase of 10 mmHg in systolic BP, and 37% had increases greater than 20 mmHg [28]. Additionally, 37% of these dogs had a systolic BP ≥ 160 mmHg. Interestingly, the hypertension was not readily managed by angiotensin-converting enzyme inhibitor (ACEi) monotherapy, and it was especially challenging for those already hypertensive prior to toceranib administration [28]. Thus, enhanced understanding of the etiology and evidence-based treatment of VEGFRi-induced hypertension has potential to benefit canine as well as human cancer patients, and veterinary practice may allow for more rapid translation of advances in this field. Moreover, understanding whether the mechanisms driving hypertension are independent or overlapping with the mechanisms for the cancer efficacy of VEGFRi is critical to safely treating hypertension and improving cancer and CV outcomes in these patients.
Thus, this review will summarize recent advances in the following: (1) molecular mechanisms driving VEGFRi-induced hypertension, (2) genetic predictors of the hypertensive response to VEGFRi treatment, (3) the relationship between cancer efficacy and BP response, and (4) available evidence supporting strategies to treat or prevent VEGFRi-induced hypertension.
Advances in Understanding Molecular Mechanisms of VEGFRi-Induced Hypertension
While the mechanisms underlying the hypertensive effects of VEGFRi remain to be fully elucidated, preclinical data support several molecular mechanisms. As this area has recently been reviewed (see [2]), this section summarizes our current understanding of mechanisms driving VEGFRi-induced hypertension with a focus on new findings in the past 3 years. This section reviews advances in the role of the endothelium summarizing the impact on the following: (1) nitric oxide (NO) and redox balance, (2) endothelin (ET-1) signaling, (3) prostacyclin production, and (4) the role of endothelial microparticles; the impact of blood vessel remodeling includes the following: (1) capillary rarefaction, (2) vascular stiffening, and (3) a potential direct impact on vascular smooth muscle cells. Finally, we describe the role of the kidney with a comparison to preeclampsia-like physiology (Fig. 1).
Advances in Understanding Endothelial Mechanisms for VEGFRi-Induced Hypertension
Nitric Oxide and Redox Balance: Nitric oxide (NO) is a potent vasodilator produced by ECs via the endothelial nitric oxide synthase (eNOS) enzyme. VEGF binding to type 1 (VEGFR1) or type 2 (VEGFR2) VEGF receptors activates the intracellular receptor tyrosine kinase leading to stimulation of the PI3K/Akt signaling pathway, which enhances eNOS phosphorylation and results in NO release [29]. As such, ample data in preclinical animal models and in cultured ECs confirms that VEGFRi decreases NO availability (reviewed in [2]). In a rat model of sunitinib-induced hypertension, sunitinib treatment decreased urinary NO metabolites and impaired endothelium-dependent vasorelaxation in mesenteric arteries [21, 28]. Decreased bioavailable NO may be due to decreased production of NO by eNOS or by increased oxidative stress, as reactive oxygen species (ROS) inactivate NO. A recent study showed that treatment of human aortic ECs with the VEGFRi vatalanib both decreased NO production, by decreasing phosphorylation of Ser1177 on eNOS, and increased superoxide production, by increasing NADPH oxidase (Nox)1 and Nox4 and decreasing anti-oxidant gene expression [30•] (Fig. 1). These findings are consistent with human data in which bevacizumab infusion impaired endothelial-dependent vasodilation to acetylcholine [31]. As such, polymorphisms in the eNOS gene that are associated with decreased eNOS activity and decreased plasma NO levels are also associated with the development of high-grade hypertension during sunitinib therapy [32].
Endothelin 1 Signaling: Endothelin 1 (ET-1) is a peptide secreted by ECs that regulates vasoconstriction by interacting with the G-protein-coupled membrane-bound ETA and ETB receptors on vascular SMC. The ETB receptor is also expressed on ECs where its activation induces eNOS-mediated NO and prostacyclin synthesis and mediates ET-1 clearance; hence, ETB receptor activation on ECs is vasodilatory. However, the predominant impact of ET-1 is that it is released from ECs and acts as a paracrine peptide via binding to SMC ETA receptors to induce vasoconstriction. VEGFRi treatment has been associated with an increase in circulating ET-1 levels in rats and humans which correlates with the degree of VEGFRi-induced hypertension [27, 29, 31, 32, 33•]. Furthermore, ET-1 enhances NADPH oxidases activity thereby increasing ROS production and thus may also contribute to hypertension by further decreasing available NO [34]. Therefore, it is difficult to completely disentangle the role of ET-1 from NO-ROS balance (Fig. 1). Kappers et al. explored this complex relationship in a swine model of sunitinib-induced hypertension. In swine treated with sunitinib for 1 week, BP and systemic vascular resistance increased without a change in coronary or pulmonary blood flow, suggesting that VEGFRi may have distinct impact on different vascular beds [35]. In the swine model, treatment with an eNOS inhibitor resulted in a greater increase in BP after sunitinib treatment, supporting a more nuanced role for eNOS in the BP response to sunitinib. However, treatment with the nonselective endothelin receptor antagonist tezosentan completely rescued the BP effect of sunitinib, more effectively than ROS scavenging with Tempol and N-acetylcysteine [35]. Similar findings in a rat model showed that endothelin receptor inhibition was more effective than ROS scavenging with Tempol at reducing sunitinib-induced hypertension [36]. More recently, using selective endothelin receptor antagonists, Colafella et al. further clarified that sunitinib-induced hypertension and albuminuria are mediated by ETA, not ETB, receptors, consistent with the known role of ETA in mediating SMC constriction [33•]. Mesenteric artery ROS generation was also attenuated by ETA receptor antagonism suggesting that ET-1/ETA receptor signaling may potentiate vascular ROS generation during VEGFRi treatment [29, 31]. Overall, substantial preclinical data support the concept that ETA inhibition improves both endothelin-induced vasoconstriction and ROS-NO balance, supporting the potential of endothelin antagonists as effective therapies to ameliorate VEGFRi-induced hypertension. However, whether ETA activation also contributes to the cancer efficacy of VEGFRi should be clarified before considering endothelin antagonism in cancer patients.
Prostacyclin: VEGF signaling in ECs also activates production of PGI2 via the PLC/PKC pathway [16]. Together with NO, PGI2 is a potent vasodilatory molecule that also inhibits ET-1 expression and secretion in cultured ECs [33•]. There is generally a dearth of experimental studies investigating the relationship between prostanoids like PGI2 and VEGFRi-induced hypertension. One recent study in rats found that co-treatment with high-dose aspirin (a nonselective COX inhibitor that prevents prostacyclin production) blunted the BP rise to sunitinib by 50% and inhibited the rise in albuminuria to a similar extent as high-dose sitaxentan (a selective ETA receptor inhibitor) [33•]. Interestingly, in contrast to preeclampsia which is characterized by a reduction in PGI2 levels, the same study observed an increase in circulating and urinary PGI2 levels following sunitinib treatment [33•]. Furthermore, in this same study, macitentan (a nonselective ETA/ETB receptor antagonist) did not block the reduction in PGI2 levels induced by sunitinib, pointing to possible negative counter-regulatory effects of ETB receptors on PGI2 release [33•]. In mice treated with sFlt1 (soluble VEGFR1 that acts as a VEGF trap and mimics preeclampsia), ex vivo responsiveness of isolated carotid (but not mesenteric) artery segments to ET-1 was enhanced and this effect was abrogated by indomethacin, a nonspecific COX inhibitor, implicating prostaglandins in the constrictive response [37•]. Once again, these data support careful attention to the vascular bed of interest. In vivo, sFlt1-induced hypertension was also attenuated by high-dose aspirin [37•]. Overall, the totality of these data suggests a possible role of PGI2 in the pathophysiology of VEGFRi-induced hypertension in cancer patients and in preeclampsia, and further studies are warranted to clarify the mechanisms (Fig. 1).
Endothelial Microparticles: Extracellular vesicles (EVs) are cell-derived microparticles released in response to cell perturbation or stress that act as a form of cell–cell communication. These microparticles transport information, in the form of membrane proteins and lipids and intra-vesicle cargo including microRNAs and proteins, between cells and tissues [38, 39]. EVs have been shown to play an important role in the cross-talk between cancer cells and other cells in their vicinity [40]. EVs from aggressive cancer cell lines enhanced vascular permeability, which may contribute to cancer progression and metastasis [38, 39]. EC-derived microparticles have also been found to communicate with SMC and other cells and thus may act as both drivers and potential biomarkers of vascular disease [38]. Neves et al. recently demonstrated that VEGFRi treatment promotes formation of pro-inflammatory EVs in cancer patients [41•]. Furthermore, when human ECs were treated with EVs from VEGFRi-treated ECs, this resulted in increased ET-1 gene expression, inhibition of eNOS activity, decreased NO, and increased ROS compared to treatment with EVs from untreated ECs [41•]. Thus, the impact of VEGFRi treatment on EC EVs may contribute to both the anticancer benefits and the vascular toxicity that drives hypertension. Further studies are needed to determine if EVs may be a therapeutic target or a biomarker to assist with diagnosis or monitoring of VEGFRi-induced hypertension.
Role of Blood Vessel Remodeling and SMCs and VEGFRi-Induced Hypertension
Microvessel Rarefaction: Microvascular rarefaction (reduction in microvessel density), leading to impaired microcirculation and increased vascular resistance, has been proposed to contribute to VEGFRi-induced hypertension [3, 40]. Capillary rarefaction may initially be functional, due to intense vasoconstriction, and later structural, due to apoptosis of ECs and SMCs, and this may be exacerbated by chronic vasoconstriction. Furthermore, EC dysfunction may precipitate thrombosis which leads to a further reduction in vascular perfusion, increased apoptosis, and microvascular obliteration [2, 42]. Thus, rarefaction may be a longer-term consequence, rather than a cause of VEGFRi-associated hypertension, which is further supported by the fact that VEGFRi-associated hypertension resolves rapidly after therapy discontinuation [29, 36].
Vascular Stiffness: The vasculature stiffens with aging and exposure to CV risk factors and the degree of stiffness predicts adverse CV event risk [43]. Stiffer vessels result in faster reflection of the cardiac pressure wave and hence can be measured noninvasively by quantifying pulse wave velocity. This faster and greater amplitude pressure wave contributes directly to higher central BP and to microvascular damage. In a recent study of 84 patients with metastatic renal cell carcinoma, increased vascular stiffness was reported within the first weeks of therapy with the VEGFRi sunitinib, as measured by carotid-femoral pulse wave velocity [44]. Vascular stiffness can be caused acutely by increased intrinsic EC stiffness due to alterations in cellular ion trafficking and chronically by structural remodeling and fibrosis of the vessels [45]. While the mechanism of VEGFRi-induced vascular stiffening has not been explored, the rapid onset and reversibility support an acute mechanism rather than structural remodeling, but further studies are needed in this area.
Direct Impact of VEGFRi on SMC Function: VEGF signaling impacts SMC function via the factors released from ECs including NO which is critical for vascular SMC homeostasis and vasomotor tone regulation (Fig. 1). Thus, VEGFRi impairs SMC function via ROS production and disruption of NO bioavailability [46]. While VEGFRs were originally thought to be expressed only on ECs, studies have shown that injured SMC can express VEGFR raising the possibility of direct effects of VEGFRi on SMC in patients with damage SMC due to underlying vascular disease or CV risk factors [14]. However, whether VEGFR inhibition directly impairs SMC function remains controversial. In humans treated with the VEGFRi telatinib, one study demonstrated a diminished response of SMC to a direct NO donor rather than a decrease in NO bioavailability [47]. However, another study showed that bevacizumab treatment impaired endothelium-dependent but not endothelial-independent dilation to sodium nitroprusside, a direct SMC dilator [31]. In rats exposed to sunitinib, another group found that ex vivo vascular responsiveness to sodium nitroprusside was decreased [48]. Interestingly, this same group found a diminished response to the vasoconstrictor angiotensin II, suggesting a generalized SMC contractile dysfunction during sunitinib administration in the coronary microcirculation [48]. These studies differ in the duration of VEGFRi treatment, the vascular bed tested, and the underlying CV risk profile when tested in human patients. In addition, most VEGFR TKIs inhibit other TKIs, including platelet-derived growth factor receptor (PDGFR), which is necessary for normal SMC function. Thus, concomitant inhibition of PDGFR and other pathways by VEGFRi can also lead to changes in SMC function [49], and further studies are needed to clarify the direct impact of VEGFRi treatment on SMCs.
Kidney Involvement in VEGFRi-Induced Hypertension and Similarities to Preeclampsia
Impact of VEGFRi on the Kidney: The glomeruli of the kidney are made up of ECs and podocytes that together are responsible for filtration and barrier functions of the kidney. When exposed to increased vascular tone due to renal vasoconstriction, the healthy kidney excretes additional sodium thereby normalizing BP in a process known as renal pressure-natriuresis. VEGF is secreted by podocytes, and VEGFRs are expressed on ECs and podocytes. VEGF-induced glomerular NO production plays a critical role in regulating renal perfusion and sodium reabsorption (recently reviewed in [50]). VEGF signaling inhibition therefore has deleterious effects on renal function and renovascular homeostasis that contributes to hypertension. VEGFRi treatment shifts the pressure natriuresis curve to attenuate sodium excretion, contributing to VEGFRi-induced hypertension, and increases podocyte permeability, leading to proteinuria (reviewed in [51]). In cancer patients, bevacizumab treatment decreased renal perfusion by functional MRI with an associated increase in proteinuria [52]. In vitro, VEGFRi decreases eNOS protein expression in glomerular ECs and long-term therapy with anti-VEGF antibodies in mice produced glomerular endotheliosis, proteinuria, and decreased nephrin expression, which is critical for podocyte foot process structural integrity [51]. Sunitinib combined with high-salt diet in rats resulted in hypertension associated with proteinuria with dose-dependent glomerular endotheliosis, evidence of glomerular EC damage, and the hallmark of kidney disease in preeclampsia [53]. Although renal damage is evident in humans and in preclinical models with VEGFRi, multiple studies suggest that BP rises prior to kidney damage and at a lower dose of VEGFRi [53, 54, 55]. The acuity of the BP response to VEGFRi suggests that functional changes in vascular tone may be the initial trigger of the hypertensive response with renal dysfunction following to maintain the BP elevation [42].
Parallels with Preeclampsia: The renal damage and dysfunction caused by VEGFRi treatment mimic the pathophysiology of preeclampsia. In preeclampsia, increased placental production of a splice variant of VEGFR1 (soluble fms-like tyrosine kinase 1 (sFlt1)) sequesters VEGFs resulting in functionally impaired VEGFR signaling [56]. Indeed, preeclamptic women and VEGFRi-treated cancer patients both develop hypertension, proteinuria, and renal endotheliosis [57]. Both syndromes are also associated with elevated ET-1 levels that correlate with serum sFlt1 in preeclampsia and dosage of VEGFRi in cancer patients [58]. In animal models of sFlt1-induced preeclampsia syndrome, interventions that increase NO, scavenge ROS, or block endothelin receptors appear to alleviate the preeclampsia phenotype [10, 21, 53, 54].
Advances in Predicting Susceptibility to VEGFRi Hypertension
Elevated serum VEGF is a poor prognostic marker in many cancers; however, serum biomarkers have not yet been identified to predict response to VEGFRi therapy [59]. Rather, genetic polypmorphisms have been shown to correlate with both cancer response and susceptibility to VEGFRi-induced hypertension. Early data in breast cancer patients showed that the VEGF-2578-AA and VEGF-1154-AA genotypes were associated with improved overall survival (OS) in bevacizumab-treated patients but not in those treated with placebo. Interestingly, two other genotypes, VEGF-634-CC and VEGF-1498-TT, were strongly associated with decreased incidence of grade 3 or 4 VEGFRi-induced hypertension but were not associated with improved OS [60]. The COMET trial was a prospective study to identify biomarkers in metastatic breast cancer patients receiving VEGFRi therapy. Recent data from that trial analyzing associations with single nucleotide polymorphisms (SNPs) showed that the VEGFA-(rs833061)-C/C, VEGFR1-(rs9582036)-C/A or -C/C, and VEGFR2-(rs1870377)-T/A or -A/A all were poor prognostics indicators, but their association with VEGFRi-induced hypertension was not reported [61]. In addition to genetic variations in VEGF itself, Frey et al. identified associations between haplotypes of WNK1, KLKB1, and GRK4 and increased incidence of grade 3 or 4 VEGFRi-induced hypertension in patients with solid tumors treated with bevacizumab [62]. A composite risk score using these genetic variants has an odds ratio of 6.45 for developing grade 3 or 4 VEGFRi-induced hypertension [62]. WNK1 encodes a sodium transporter in the distal convoluted tubule, KLKB1 encodes kallikrein which is a protease important in the bradykinin pathway involved in vasodilation, and GRK4 is a GPCR linked to dopamine regulation of blood flow in the kidney. As has been discussed above, vasomotor function, renal blood flow, and alterations in sodium balance have all been implicated in VEGRi-induced hypertension (Fig. 1), suggesting a potential causal relationship with these SNPs [51]. In addition to the above, Li et al. showed that polymorphisms in SLC29A1 and HSP90AB1 were associated with increased susceptibility to VEGFRi hypertension in colorectal cancer patients receiving bevacizumab. They then showed that overexpression of SLC29A1 in ECs was associated with greater decrease in NO levels in bevacizumab-treated ECs, supporting a potential mechanism for this genetic association [63]. While most literature regarding genetic polymorphisms and VEGFRi hypertension has been examined in the setting of bevacizumab treatment, Eechoute et al. also showed the polymorphisms in VEGFA and eNOS were associated with increased grade 3 hypertension in renal cell carcinoma patients treated with the VEGFR-TKI sunitinib [32]. Taken together, these studies show that variations among individuals in genes involved in the VEGF signaling pathway and in the pathways implicated in VEGFRi-induced vascular and renal dysfunction can lead to changes both in the cancer response to VEGFRi therapy and VEGFRi hypertension (Fig. 1).
The Relationship between VEGFRi-Induced Hypertension, Cancer Survival, and Treatment Efficacy
VEGFRi Hypertension as a Marker of Cancer Prognosis: outcome of efforts to predict cancer response to VEGFRi therapy is the finding that the development of VEGFRi hypertension itself may be a strong prognostic factor. Although initially debated, as clinical experience with VEGFRi therapies has grown, particularly with the expansion of the oral TKI therapies in recent years, it has become clear that the development of VEGFRi-induced hypertension is associated with increased survival with varying degrees of clinical significance (Table 1) [64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79]. This finding raises the possibility that the mechanism of cancer efficacy is the same as or at least overlapping with the mechanism of VEGFRi-induced hypertension. If so, the potential for mechanism-directed antihypertensive therapy to negatively impact cancer outcomes becomes a concern. As such, Langenberg et al. showed that antihypertensive prophylaxis with a calcium channel blocker decreased the incidence of severe hypertension without affecting cancer treatment efficacy; however, this was a relatively small study (N = 125) with a VEGFR-TKI not commonly used clinically [80]. More recently, McKay et al. performed a retrospective analysis of metastatic RCC patients treated with VEGFRi showing that ACEi co-treatment had better overall survival compared to other antihypertensive agents or patients not treated for hypertension [73]. They also showed that treatment with ACEi or angiotensin receptor blocker (ARB), but not calcium channel blocker or beta blocker, enhanced the antitumor efficacy of sunitinib in RCC cell lines in vitro [73]. These data support a possible direct link between the choice of antihypertensive agent and VEGFRi cancer treatment efficacy. Given that RCC cells express the angiotensin II receptor however, it is unclear if this is a vascular or a tumor specific effect. Also, due to the retrospective nature of the study, data about the reasoning behind the antihypertensive agent choice, dose, and efficacy were not available and confound the interpretation of the results. Indeed, a retrospective study by Hamnvik et al. showed that VEGFRi-treated patients who developed hypertension had better overall survival compared to those who did not but this analysis showed no significant difference in outcomes in patients treated with ACEi, ARB, or other antihypertensive agents [78]. Given the myriad mechanisms contributing to VEGFRi hypertension discussed above, as well as the growing repertoire of antihypertensive agents, the possibility of finding antihypertensive agents that effectively treat VEGFRi hypertension without compromising or better yet enhancing antitumor efficacy is an important area for future research. Conversely, as the development of hypertension correlates with cancer survival, it is critical that the antihypertensive therapy does not attenuate the benefits of VEGRi treatment on the tumor. As the benefits on the tumor are mediated by anti-angiogenic mechanisms, it will be important to test these antihypertensive treatments not only on isolated tumor cells but also in tumor angiogenesis models.
The Impact of Combination Cancer Therapy: An emerging treatment paradigm in oncology is the combination of VEGFRi therapy with immunotherapy for treatments of many solid tumors. This has led to a shift in frontline therapy for metastatic hepatocellular carcinoma and RCC in recent years [78, 79]. In these trials, patients treated with bevacizumab plus atezolizumab (a programmed cell death ligand checkpoint inhibitor) had similar rates of low and high-grade hypertension compared to patients receiving sorafenib [81]. Similarly, patients treated for RCC with axinitib and pembrolizumab (another programmed cell death ligand checkpoint inhibitor) had comparable rates of hypertension to patients treated with sunitinib [82]. These studies suggest that the addition of immunotherapy to VEGFRi does not increase the incidence of hypertension, although longer-term data about other cardiovascular side effects of VEGFRi when combined with immunotherapy is not yet available. Although we are still exploring the mechanisms behind the synergy between VEGFRi therapy and immunotherapy in the tumor, there is significant emerging data that “vascular normalization” through the effects of VEGFRi on the endothelium plays an important part in the process (reviewed by [59]). Given that many of possible mechanisms behind VEGFRi hypertension are also mediated by effects on the endothelium and that many of the antihypertensive agents used to treat VEGFRi hypertension act on the vasculature, potential interactions between antihypertensive agents and the efficacy of combination VEGFRi and immunotherapy deserve further investigation.
Table 1.
Evidence for development of hypertension as a prognostic indicator of cancer outcome during VEGF receptor inhibitor treatment
| Year | Agent studied | Type of study | N | Patients with VEGFi hypertension | Patients without VEGFi hypertension |
p-value | Ref |
|---|---|---|---|---|---|---|---|
| 2007 | Sunitinib | Retrospective | 40 | 66% disease response | 26% disease response | 0.009 | [66] |
| 2009 | Bevacizumab | Retrospective | 39 | Median PFS 14.5 months | Median PFS 3.1 Months | 0.04 | [67] |
| 2010 | Bevacizumab | RCT | 878 | Median OS 15.9 months | Median OS 11.5 Months | < 0.0001 | [68] |
| 2011 | Bevacizumab | Prospective | 101 | Median OS 25.8 months | Median OS 11.7 Months | < 0.0001 | [69] |
| 2012 | Sunitinib | Retrospective | 319 | Median OS 19.8 months | Median OS 14.5 months | 0.0003 | [70] |
| 2013 | Bevacizumab | Retrospective | 6486 | Early rise in BP > 10 or > 20 mmHG systolic only predicted benefit in 1/7 clinical trials analyzed | varied | [71] | |
| 2013 | Bevacizumab | Retrospective | 60 | Trend towards higher median OS for patients with grade 3 HTN compared to grades 0–2, but not significant | 0.058 | [72] | |
| 2014 | Bevacizumab | Retrospective | 82 | Median OS 11.7 months | Median OS 4.9 months | < 0.001 | [79] |
| 2015 | Multiple | Retrospective | 1120 | HR for OS, 0.76 (95% CI, 0.65–0.89) for HTN compared to no HTN | 0.0004 | [78] | |
| 2018 | Bevacizumab | Meta-analysis | 2292 | HR for OS 0.51 (95% CI: 0.39–0.65) for HTN compared to no HTN | < 0.001 | [74] | |
| 2019 | Apatinib | Retrospective | 110 | Median OS 9.9 months | Median OS 7.8 months | 0.005 | [65] |
| 2019 | Aflibercept | Retrospective | 78 | Median OS 10.6 months | Median OS 4.0 months | < 0.001 | [75] |
| 2019 | Multiple TKIs | Meta-analysis | 4661 | HR for PFS 0.56 (95% CI: 0.40–0.77) for studies 2011–2014, 0.61 (95% CI: 0.48–0.77) for 2015–2017 | < 0.001 for both | [77] | |
| 2021 | Alontinib | Post hoc | 109 | HR for OS 0.74 (95% CI: 0.31–1.77) in patients with preexisting HTN and 0.37 (95% CI: 0.21–0.67) in patients without preexisting HTN | Not given | [64] | |
| 2021 | Bevacizumab | Retrospective | 117 | Median OS 7.7 months | Median OS 4.4 months | 0.011 | [76]* |
| 2021 | Multiple TKIs | Retrospective | 38 | Median OS 8.6 months | Median OS 3.6 months | 0.03 | [76]* |
Studies in patients treated with VEGF inhibitors comparing outcomes in patients who developed hypertension versus those who did not. VEGFRi VEGF receptor inhibitor, PFS progression free survival, OS overall survival, HR hazard ratio, BP blood pressure, HTN hypertension, and 95% CI 95% confidence interval. All differences in PFS or OS were statistically significant unless mentioned in the table.
Subgroup analyses from a single study
Current Understanding of Management for VEGFRi-Induced Hypertension
Current Recommendations: Given the prevalence of VEGFRi-induced hypertension and the fact that preexisting hypertension is the most common comorbidity in patients with cancer, numerous agencies have issued recommendations for hypertension management in cancer patients in general and of VEGFRi hypertension specifically [83, 84, 85, 86]. Current recommendations are based on expert opinion as there are no published clinical trials to drive official evidence-based treatment guidelines. Current recommendations are to measure BP prior to starting VEGFRi therapy and consider delaying treatment at initial or subsequent treatment if BP is above 160/100. Initial agents of choice are either ACEi or ARB [81, 83]. Of note, non-dihydropyridine calcium channel blocker use is not recommended with VEGFRi-TKIs as they inhibit CYP3A and can increase levels of these drugs [85]. Most recommendations also suggest the addition of another antihypertensive agent prior to increasing the dose of the initial agent and referral to cardiology if BP is refractory to two agents. In recent years, the concept of the cardio-oncology clinic has arisen to help provide expert and evidence-based recommendations for monitoring and management of CV complications in oncology patients including those with VEGFRi-induced hypertension [87].
Emerging Treatments for VEGFRi Hypertension: Despite advances in our understanding of mechanisms driving the pathophysiology behind VEGFRi hypertension, current management algorithms are similar to current guidelines for essential hypertension. Studies are beginning to investigate potential treatments specific for VEGFRi hypertension using in vitro and preclinical animal models but human trials are lacking. Sharma et al. used human-induced pluripotent stem cells to quantify the effects of VEGFRi-TKIs on the phosphoprotein state of fibroblasts, cardiomyocytes, and ECs [88]. In that study, human pluripotent stem-derived ECs were found to develop an “EC toxicity profile” with drugs including sorafenib, regorafenib, and ponatinib [88]. The EC toxicity profile for these TKIs was different from the toxicity profile in cardiomyocytes and fibroblasts, and the use of this technique as a screening method for drug toxicity and interventions to reduce toxicity is an avenue for future research [88]. Other pre-clinical studies have begun to focus on antihypertensive agents that mitigate the specific molecular mechanisms recently implicated in VEGFRi hypertension. As mentioned above, Colafella et al. showed that selective ETA or dual ET A/B blockade was effective in preventing sunitinib-induced hypertension in rats, consistent with a role for ET-1 acting on SMC ETA receptors [33•]. Dabiré et al. showed that targeting the NO pathway with sildenafil, a phosphodiesterase inhibitor that prevents the breakdown of NO, can mitigate VEGFRi hypertension caused by sorafenib in rats [89]. However, there does not appear to be any pre-clinical or clinical studies to date investigating the impact of antihypertensive treatments on the antitumor efficacy of VEGFRi therapy. This is important as unlike other therapies targeted at the tumor cells, both the tumor efficacy and the pro-hypertensive effects of VEGFRi are mediated by impact on vascular cells.
Current Clinical Trials in VEGFRi-induced Hypertension: There are a number of ongoing and planned clinical trials to help expand the evidence base for predicting, monitoring, and treating VEGFRi hypertension. The UNICO (NCT03882580) and TITAN (NCT01621659) trials are a prospective and a randomized controlled trial respectively, which seek to provide more evidence base and guidance for the role of the cardio-oncology clinic in patients treated with VEGFRi and other cardiotoxic cancer treatments and to evaluate clinical outcomes of patients seen in a multidisciplinary clinic. The CHA-RISMA (NCT04467021) trial is a 60 patient randomized control trial of intensive (< 120/80) versus standard of care (systolic BP < 140) BP management in patients with metastatic renal cell and thyroid cancer initiating treatment with VEGFRi-TKIs. NCT03709771 is a prospective trial of 80 patients that seeks to investigate VEGFRi hypertension in patients undergoing combined VEGFRi therapy and immunotherapy. However, larger clinical studies are needed randomizing VEGFRi-treated cancer patients to different antihypertensive agents, with follow-up to determine the short-term impact on BP control and the longer-term impact on cancer outcomes and cardiovascular disease risk.
Conclusions
Inhibition of VEGF signaling has become a common part of treatment regimens for many malignancies. One of the most consistent and clinically significant side effects from VEGFRi treatment is the development of hypertension, which is of particular concern given the advanced age and high rate of cardiovascular comorbidities of patients who typically receive these therapies. In recent years, advances have been made with regard to defining the pathophysiology behind VEGFRi-induced hypertension, including the role of endothelial dysfunction. Genetic studies have also elucidated candidate polymorphisms in these pathways implicated in VEGFRi signaling that predict patients at risk for developing VEGFRi-induced hypertension. Pre-clinical investigations and the growing field of cardio-oncology researchers are working to further elucidate the most effective monitoring and management strategies for VEGFRi-induced hypertension. All of these changes are also occurring in the background of a firm body of literature showing that patients who develop hypertension on VEGFRi therapy have better oncologic outcomes, but worse cardiovascular outcomes, compared to those who do not develop hypertension on VEGFRi therapy. Further investigation into the interplay between antitumor and pro-hypertensive effects of VEGFRi is warranted to maximize the anticancer benefits from VEGFRi treatment while managing its side effects.
Funding
This work was supported by grants from the NIH (CA243542 to IZJ and CL and HL155078 to IZJ).
References
Papers of particular interest, published recently, have been highlighted as: ● Of importance
- 1.Dobbin SJH, Cameron AC, Petrie MC, Jones RJ, Touyz RM, Lang NN. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart. 2018;104:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neves KB, Montezano AC, Lang NN, Touyz RM. Vascular toxicity associated with anti-angiogenic drugs. Clin Sci (Lond). 2020;134:2503–20. [DOI] [PubMed] [Google Scholar]
- 3.Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precision One. 2018;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B, Papoian T. Preclinical approaches to assess potential kinase inhibitor-induced cardiac toxicity: past, present and future. J Appl Toxicol. 2018;38:790–800. [DOI] [PubMed] [Google Scholar]
- 5.Akam-Venkata J, Franco VI, Lipshultz SE. Late cardiotoxicity: issues for childhood cancer survivors. Curr Treat Options Cardio Med. 2016;18:47. [DOI] [PubMed] [Google Scholar]
- 6.Gopal S, Miller KB, Jaffe IZ. Molecular mechanisms for vascular complications of targeted cancer therapies. Clin Sci. 2016;130:1763–79. [DOI] [PubMed] [Google Scholar]
- 7.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–67. [DOI] [PubMed] [Google Scholar]
- 8.Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. [DOI] [PubMed] [Google Scholar]
- 9.•. Abdel-Qadir H, Ethier J-L, Lee DS, Thavendiranathan P, Amir E Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treatment Reviews. 2017;53:120–7 (Large meta-analysis quantifying the cardiovascular toxicity of VEGFRi treatment including increased risk of hypertension, cardiac ischemia, cardiac dysfunction, and arterial thromboembolism).
- 10.Shah NS, Lloyd-Jones DM, O’Flaherty M, Capewell S, Kershaw K, Carnethon M, Khan SS. Trends in Cardiometabolic Mortality in the United States, 1999–2017. JAMA. 2019;322:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology challenges and perspectives. Circ Res. 2019;124:1094–112. [DOI] [PubMed] [Google Scholar]
- 13.Jammal N, Pan E, Hurwitz M, Abramovitz RB. Outcomes of combination therapy with tyrosine kinase inhibitors and immune checkpoint inhibitors in metastatic renal cell carcinoma – a retrospective study. J Oncol Pharm Pract. 2020;26:556–63. [DOI] [PubMed] [Google Scholar]
- 14.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vase Biol. 2014;34:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller H Endothelial Function. Drugs. 1997;53:1–10. [DOI] [PubMed] [Google Scholar]
- 16.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–25. [DOI] [PubMed] [Google Scholar]
- 17.Ribatti D, Annese T, Ruggieri S, Tamma R, Crivellato E. Limitations of anti-angiogenic treatment of tumors. Translational Oncology. 2019;12:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saharinen P, Eklund L, Pulkki K, Bono P, Alitalo K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17:347–62. [DOI] [PubMed] [Google Scholar]
- 19.Wirth LJ, Tahara M, Robinson B, et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124:2365–72. [DOI] [PubMed] [Google Scholar]
- 20.Iacovelli RN, Sternberg C, Porta C, Verzoni E, de Braud F, Escudier B, Procopio G. Inhibition of the VEGF/VEGFR pathway improves survival in advanced kidney cancer: a systematic review and meta-analysis. Current Drug Targets. 2015;16:164–70. [DOI] [PubMed] [Google Scholar]
- 21.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic.Cold Spring Harb Perspect Med. 2012;2:a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol. 2017;37:386–97. [DOI] [PubMed] [Google Scholar]
- 23.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–65. [DOI] [PubMed] [Google Scholar]
- 24.Berger EP, Johannes CM, Jergens AE, Allenspach K, Powers BE, Du Y, Mochel JP, Fox LE, Musser ML. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of gastrointestinal stromal tumors of dogs. J Vet Intern Med. 2018;32:2045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lew FH, McQuown B, Borrego J, Cunningham S, Burgess KE. Retrospective evaluation of canine heart base tumours treated with toceranib phosphate (Palladia): 2011–2018. Vet Comp Oncol. 2019;17:465–71. [DOI] [PubMed] [Google Scholar]
- 26.London C, Mathie T, Stingle N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®)in solid tumours. Veterinary and Comparative Oncology. 2012;10:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rippy SB, Gardner HL, Nguyen SM, et al. A pilot study of toceranib/vinblastine therapy for canine transitional cell carcinoma.BMC Vet Res. 2016;12:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjostheim SS, Stepien RL, Markovic LE, Stein TJ. Effects of toceranib phosphate on systolic blood pressure and proteinuria in dogs. J Vet Intern Med. 2016;30:951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lankhorst S, Kappers MHW, van Esch JHM, Danser AHJ, vanden Meiracker AH. Hypertension during vascular endothelial growth factor inhibition: focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal. 2014;20:135–45. [DOI] [PubMed] [Google Scholar]
- 30.•. Neves KB, Rios FJ, van der Mey L, Alves-Lopes R, Cameron AC, Volpe M, Montezano AC, Savoia C, Touyz RM. VEGFR (vascular endothelial growth factor receptor) inhibition induces cardiovascular damage via redox-sensitive processes. Hypertension. 2018;71:638–47 This study shows that VEGFRi treatment of human endothelial cells in vitro decreased eNOS phosphorylation and inhibition of Nox1/4 reduced this effect.Mice treated with VEGFRi showed impaired vessel relaxation and downregulation of anti-oxidant genes. This implicates oxidative stress as an important mediator and potential target for VEGFRi toxicity..
- 31.Thijs AMJ, van Herpen CML, Sweep FCGJ, Geurts-Moespot A, Smits P, van der Graaf WTA, Rongen GA. Role of endogenous vascular endothelial growth factor in endothelium-dependent vasodilation in humans. Hypertension. 2013;61:1060–5. [DOI] [PubMed] [Google Scholar]
- 32.Eechoute K, van der Veldt AAM, Oosting S, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92:503–10. [DOI] [PubMed] [Google Scholar]
- 33.•. Mirabito Colafella KM, Neves KB, Montezano AC, et al. Selective ETA vs. dual ETA/B receptor blockade for the prevention of sunitinib-induced hypertension and albuminuria in WKY rats. Cardiovascular Research. 2020;116:1779–90 This study in rats demonstrated that VEGFRi treatment induced mesenteric vessel oxidative stress, proteinuria and hypertension all of which were reversed by co-treatment with an endothelin A receptor antagonist..
- 34.Kim Y-W, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123:625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kappers MHW, de Beer VJ, Zhou Z, Danser AHJ, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012:59:151–7. [DOI] [PubMed] [Google Scholar]
- 36.Kappers MHW, Smedts FMM, Horn T, van Esch JHM, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension. 2011;58:295–302. [DOI] [PubMed] [Google Scholar]
- 37.Amraoui F, Spijkers L, Lahsinoui HH, Vogt L, van der Post J, Peters S, Afink G, Ris-Stalpers C, van den Born B-J. SFlt-1 elevates blood pressure by augmenting endothelin-l-mediated vasoconstriction in mice. PLOS ONE. 2014;9:e91897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71. [DOI] [PubMed] [Google Scholar]
- 39.Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017:120:1658–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuchi S, Yoshioka Y, Prieto-Vila M, Ochiya T. Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. Int J Mol Sci. 2019:20:2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.•. Neves KB, Rios FJ, Jones R, Evans TRJ, Montezano AC, Touyz RM. Microparticles from vascular endothelial growth factor pathway inhibitor-treated cancer patients mediate endothelial cell injury. Cardiovasc Res. 2019:115:978–88 The authors demonstrate that treatment with VEGFRi induces EC microparticle release, which is a biomarker for EC injury. They also show that the microparticles isolated from patients on VEGFRi therapy induce expression of ET-1 and reduce eNOS expression in human primary aortic endothelial cells, suggesting that EC microparticles contribute to VEGFRi-induced hypertension..
- 42.van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ, Lang NN. Hypertension and prohypertensive antineoplastic therapies in cancer patients. Circ Res. 2021;128:1040–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–86. [DOI] [PubMed] [Google Scholar]
- 44.Catino AB, Hubbard RA, Chirinos JA, et al. (2018) Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circulation: Heart Failure 11:e004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumor K, Shoemaker-Moyle M, Nistala R, Whaley-Connell A. Arterial stiffness in hypertension: an update. Curr Hypertens Rep. 2018:20:72. [DOI] [PubMed] [Google Scholar]
- 46.Hsu P-Y, Mammadova A, Benkirane-Jessel N, Désaubry L, Nebigil CG. Updates on anticancer therapy-mediated vascular toxicity and new horizons in therapeutic strategies. Frontiers in Cardiovascular Medicine. 2021:8:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steeghs N, Gelderblom H, Roodt J op’t, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–6. [DOI] [PubMed] [Google Scholar]
- 48.Kappers MHW, van Esch JHM, Sluiter W, Sleijfer S, Danser AHJ, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–81. [DOI] [PubMed] [Google Scholar]
- 49.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. [DOI] [PubMed] [Google Scholar]
- 50.Van Wynsberghe M, Flejeo J, Sakhi H, Ollero M, Sahali D, Izzedine H, Henique C. Nephrotoxicity of anti-angiogenic therapies. Diagnostics. 2021:11:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG, Moslehi J. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor–associated hypertension and vascular disease. Hypertension. 2018. 10.1161/HYPERTENSIONAHA.117.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirashima Y, Yamada Y, Tateishi U, Kato K, Miyake M, Akiyoshi K, Horita Y, Nagashima K, Shirao K. New analysis of the hypertension mechanism in bevacizumab-treated patients using 3-tesla dynamic contrast-enhanced magnetic resonance imaging. JCO. 2011:29:450–450. [Google Scholar]
- 53.Lankhorst S, Baelde HJ, Clahsen-van Groningen MC, Smedts FMM, Danser AHJ, van den Meiracker AH. Effect of high salt diet on blood pressure and renal damage during vascular endothelial growth factor inhibition with sunitinib. Nephrol Dial Transplant. 2016;31:914–21. [DOI] [PubMed] [Google Scholar]
- 54.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol. 2010. 10.1016/j.semnephrol.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seki H Balance of antiangiogenic and angiogenic factors in the context of the etiology of preeclampsia. Acta Obstet Gynecol Scand. 2014;93:959–64. [DOI] [PubMed] [Google Scholar]
- 57.Gatford KL, Andraweera PH, Roberts CT, Care AS. Animal models of preeclampsia: causes, consequences, and interventions. Hypertension. 2020;75:1363–81. [DOI] [PubMed] [Google Scholar]
- 58.Verdonk K, Saleh L, Lankhorst S, et al. Association studies suggest a key role for endothelin-1 in the pathogenesis of preeclampsia and the accompanying renin–angiotensin–aldosterone system suppression. Hypertension. 2015;65:1316–23. [DOI] [PubMed] [Google Scholar]
- 59.Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–24. [DOI] [PubMed] [Google Scholar]
- 60.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gal J, Milano G, Brest P, et al. VEGF-related germinal polymorphisms may identify a subgroup of breast cancer patients with favorable outcome under bevacizumab-based therapy—a message from COMET, a French Unicancer Multicentric Study. Pharmaceuticals. 2020:13:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frey MK, Dao F, Olvera N, Konner JA, Dickler MN, Levine DA. Genetic predisposition to bevacizumab-induced hypertension. Gynecol Oncol. 2017;147:621–5. [DOI] [PubMed] [Google Scholar]
- 63.Li M, Mulkey F, Jiang C, et al. Identification of a genomic region between SLC29A1 and HSP90AB1 associated with risk of bevacizumab-induced hypertension: CALGB 80405 (Alliance). Clin Cancer Res. 2018;24:4734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Y, Xiao J, Fang W, et al. The relationship between treatment-induced hypertension and efficacy of anlotinib in recurrent or metastatic esophageal squamous cell carcinoma. Cancer Biol Med. 2021:18:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang S-C, Huang W, Zhang Y-M, Zhang H-T, Xie W-P. Hypertension as a predictive biomarker in patients with advanced non-small-cell lung cancer treated with apatinib. Onco Targets Ther. 2019;12:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;18:1117. [DOI] [PubMed] [Google Scholar]
- 67.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30. [DOI] [PubMed] [Google Scholar]
- 68.Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non–small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Österlund P, Soveri L-M, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer. 2011;104:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib ‡. Ann Oncol. 2012;23:3180–7. [DOI] [PubMed] [Google Scholar]
- 71.Hurwitz HI, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA, Chen D, Rosen O. Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist. 2013;18:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morita S, Uehara K, Nakayama G, Shibata T, Oguri T, Inada-Inoue M, Shimokata T, Sugishita M, Mitsuma A, Ando Y. Association between bevacizumab-related hypertension and vascular endothelial growth factor (VEGF) gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2013;71:405–11. [DOI] [PubMed] [Google Scholar]
- 73.McKay RR, Rodriguez GE, Lin X, Kaymakcalan MD, Hamnvik O-PR, Sabbisetti VS, Bhatt RS, Simantov R, Choueiri TK. Angiotensin system inhibitors and survival outcomes in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C-J, Zhang S-Y, Zhang C-D, Lin C-R, Li X-Y, Li Q-Y, Yu H-T. Usefulness of bevacizumab-induced hypertension in patients with metastatic colorectal cancer: an updated meta-analysis. Aging (Albany NY). 2018;10:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montes AF, Lago NM, Rúa MC, et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: Prognostic and predictive markers. Cancer Med. 2019;8:882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duco MR, Murdock JL, Reeves DJ (2021) Vascular endothelial growth factor inhibitor induced hypertension: retrospective analysis of the impact of blood pressure elevations on outcomes. J Oncol Pharm Pract 1078155220985915 [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Zhou L, Chen Y, Liao B, Ye D, Wang K, Li H. Hypertension as a prognostic factor in metastatic renal cell carcinoma treated with tyrosine kinase inhibitors: a systematic review and meta-analysis. BMC Urol. 2019;19:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamnvik O-PR, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, Kaymakcalan MD, Williams JS. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong J, Ali AN, Voloschin AD, Liu Y, Curran WJ Jr, Crocker IR, Shu H-KG. Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer. 2015;121:1456–62. [DOI] [PubMed] [Google Scholar]
- 80.Langenberg MHG, van Herpen CML, De Bono J, et al. Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: results from a phase II randomized, factorial, double-blind study of cediranib in patients with advanced solid tumors. JCO. 2009;27:6152–9. [DOI] [PubMed] [Google Scholar]
- 81.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]
- 82.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 83.Piccirillo JF. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441. [DOI] [PubMed] [Google Scholar]
- 84.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. JNCI. 2010;102:596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel S, Dushenkov A, Jungsuwadee P, Krishnaswami A, Barac A. Team-based approach to management of hypertension associated with angiogenesis inhibitors. J of Cardiovasc Trans Res. 2020;13:463–77. [DOI] [PubMed] [Google Scholar]
- 86.Plummer C, Michael A, Shaikh G, Stewart M, Buckley L, Miles T, Ograbek A, McCormack T. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer. 2019;121:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hassen LJ, Lenihan DJ, Baliga RR. Hypertension in the cardio-oncology clinic. Heart Fail Clin. 2019;15:487–95. [DOI] [PubMed] [Google Scholar]
- 88.Sharma A, Burridge PW, McKeithan WL, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017;9:eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dabiré H, Dramé F, Cita N, Ghaleh B. The hypertensive effect of sorafenib is abolished by sildenafil. Cardio-Oncology. 2020;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]