Abstract
OBJECTIVE:
To evaluate whether 17α-hydroxyprogesterone caproate use in preventing preterm birth increases the risk of gestational diabetes mellitus (GDM).
DATA SOURCES:
Electronic databases (MEDLINE, Scopus, ClinicalTrials.gov PROSPERO, EMBASE, Scielo and the Cochrane Central Register of Controlled Trials) were searched for studies published before October 2018. Keywords included “gestational diabetes,” “preterm birth,” “pregnancy,” and “17-hydroxyprogesterone caproate.”
METHODS OF STUDY SELECTION:
Studies comparing 17α-hydroxyprogesterone caproate with unexposed control groups in women with singleton gestation and a history of a prior spontaneous preterm birth were included. The primary outcome was the development of GDM. Secondary outcomes included abnormal 1-hour, 50-g glucose screen results and mean venous blood glucose levels. Summary estimates were reported as mean differences and 95% CI for continuous variables or relative risk (RR) with 95% CI for dichotomous outcomes. Meta-analysis was performed using the random effects model of DerSimonian and Laird.
TABULATION, INTEGRATION AND RESULTS:
Six studies, four of which were cohort studies, met inclusion criteria and were included in the final meta-analysis. Of the 5,053 women, 1,538 (30.4%) received 17α-hydroxyprogesterone caproate and 3,515 (69.6%) were in unexposed control groups. The overall rate of GDM in women exposed to 17α-hydroxyprogesterone caproate was 10.9% vs 6.1% in women who were not exposed (RR 1.77, 95% CI 1.22–2.55). After exclusion of the cohort studies, the summary estimate of effect was nonsignificant among women who had been randomly allocated to 17α-hydroxyprogesterone caproate (RR 1.21, 95% CI 0.63–2.36).
CONCLUSION:
Women with singleton gestations receiving weekly 17α-hydroxyprogesterone caproate for recurrent preterm birth prevention had a significantly higher incidence of abnormal glucose test results and GDM compared with those in unexposed control groups, a finding that did not hold among women who had been randomly allocated to 17α-hydroxyprogesterone caproate.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42016041694.
If current trends persist, about 1 in 10 U.S. women who become pregnant will deliver preterm, according to the Centers for Disease Control and Prevention.1 From 2014 to 2016, the U.S. preterm birth rate rose 3%, from 9.57% to 9.85%2 despite increased understanding and use of preventive strategies such as supplementation with 17α-hydroxyprogesterone caproate.
Approved by the U.S. Food and Drug Administration in 2011,3 17α-hydroxyprogesterone caproate is a once-weekly 250-mg (1-mL) injection that reduces the risk of recurrent preterm birth by more than 30%.4 As longer-term data are becoming available from phase IV studies and postmarketing surveillance, maternal and neonatal outcomes related to 17α-hydroxyprogesterone caproate use has generated a lot of interest.4-7 Progestins are thought to exhibit diabetogenic properties through a reduction in glucose transporter 4 expressions by impairing the normal cell adaptive response of enhanced insulin secretion.8 Preclinical pharmacokinetic studies have demonstrated that progestins play a key role in pancreatic function and in signaling insulin release.9 These observations strengthen the biological plausibility of progestin-mediated gestational hyperglycemia, and have raised concerns about a possible association of 17α-hydroxyprogesterone caproate with an increased risk of developing gestational diabetes.
Available data on the association between 17α-hydroxyprogesterone caproate use in prevention of preterm birth and the risk of gestational diabetes are conflicting. Although some studies suggest an increased risk,5,10,11 others suggest little to no risk.12-14 Therefore, the aim of this systematic review and meta-analysis was to comprehensively synthesize the literature on the risk of glucose intolerance in women with singleton pregnancies treated with 17α-hydroxyprogesterone caproate compared with those in unexposed control groups.
SOURCES
The methodology conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. We searched ClinicalTrials.gov, MEDLINE, EMBASE, Scopus, Web of Science, and the Cochrane Library for studies that met our inclusion and exclusion criteria using a search strategy designed in collaboration with an experienced librarian at the Johns Hopkins University School of Public Health. Our PubMed search incorporated both controlled MEDLINE vocabulary (ie, MeSH terms) and free text keywords. Our search included controlled vocabulary terms (MeSH and free text keywords) for 17-hydroxyprogesterone (study intervention), gestational diabetes mellitus (GDM), impaired glucose tolerance, mean venous plasma glucose (study outcome), pregnancy, history of prior preterm birth (study population), cohort studies and randomized controlled trials (study design). The search strategy included Boolean operators “OR” (for related term) and “AND” (combination of different concepts). Our ClinicalTrials.gov search strategy incorporated similar search strategy, without incorporating the “cohort” and “randomized controlled trial” terms. Relevant synonyms and alternative spellings were identified using EMBASE’s controlled vocabulary, Emtree.
STUDY SELECTION
The protocol for this review was registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42016041694) before data extraction following the PRISMA guidelines for protocols (PRISMA-P). We searched for retrospective cohort studies, prospective cohort studies, and randomized controlled trials regarding women with a history of prior preterm birth treated with 17α-hydroxyprogesterone caproate compared with those in unexposed control groups. We included studies of pregnant women of any age, in any country, of any gestation or parity, in any trimester of pregnancy, with one or more history of prior spontaneous preterm birth. The primary outcome of interest of this systematic review was the proportion of women who developed GDM, defined as a 1-hour, 50-g glucose screen result of at least 200 mg/dL or two or more abnormal results identified on a confirmatory 3-hour, 100-g oral glucose tolerance test according to the Carpenter-Coustan criteria.11,14 We assessed the following secondary outcomes: abnormal 1-hour, 50-g glucose screen result and mean venous plasma glucose concentration. An abnormal 1-hour glucose screen result was defined as a nonfasting venous plasma glucose concentration of at least 135 mg/dL but less than 200 mg/dL. All primary and secondary outcomes were assessed as risk ratios, except mean venous plasma concentration, which was compared as a difference of means.
Using Covidence software, two reviewers (A.C.E. and E.M.G.) independently screened titles and abstracts for full-text review. Duplicate entries were removed by Covidence software based on matching titles, authors, and journals, and then manually confirmed. A third adjudicator (J.S.), whose decision was final, resolved discrepancies between reviewers. Studies were categorized by titles and abstracts as “include” or “exclude” based on relevance to the study question and adherence to the systematic review’s eligibility criteria. Titles and abstracts marked “include” were reviewed in full text. Two reviewers (A.C.E. and E.M.G.) independently read each full-text article to assess for eligibility based on the predefined eligibility criteria described previously in this protocol. A third adjudicator (J.S.) whose decision was final resolved discrepancies between reviewers. Full-text articles were categorized as “include” or “exclude.” Studies marked as “include” based on full-text review were included in the systematic review.
Quality assessment of the included studies was performed using the Risk of Bias In Non-randomized Studies of Interventions assessment tool for cohort studies.15 This tool includes three domains: preintervention, at-intervention, and postintervention. In the preintervention domain, bias due to confounding and participant selection were evaluated. Possible confounding factors for this review include maternal age, parity, race, body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), tobacco use, use of antenatal corticosteroids, number of prior preterm births, and gestational age during 17α-hydroxyprogesterone caproate injections. Bias due to misclassification of the intervention status (17α-hydroxyprogesterone caproate compared with unexposed control group) was assessed in the at-intervention domain. The postintervention domain included bias due to departures from the intended interventions, missing data, methods of outcome measurements, and selective reporting outcomes. Each nonrandomized study was rated as having either a low, moderate, serious, critical, or an unclear risk of bias.15
We obtained missing data and other relevant information through email requests to the authors of the studies and the Johns Hopkins University Welch Medical Library. For the purpose of this review, we allowed a period of one week for the author’s response. We determined whether to perform a meta-analysis based on qualitative assessment of whether study populations and interventions were reasonably comparable. We presumed that, if studies met our inclusion criteria, the outcome of GDM would be comparable across studies. We also assessed for clinical, methodologic, and statistical evidence of heterogeneity in the studies and considered this heterogeneity in our assessment to do a meta-analysis. We then performed a pairwise meta-analysis comparing 17α-hydroxyprogesterone caproate with unexposed control groups. We used a random effects model because we expected a great deal of heterogeneity in these studies given that the studies were likely to incorporate different patient characteristics and use varying methodologies.
In deciding whether to present summary relative risk (RR) estimates, we considered clinical and methodologic sources of heterogeneity across studies. We investigated statistical heterogeneity by calculating the Q-statistic, the I2, and the T2. For the Q-statistic, which tests the hypothesis of a common effect, we used a critical value of less than 0.1 as the cutoff for statistical significance. The I2 statistic, which showed the inter-study heterogeneity as a proportion of the total heterogeneity, we considered I2 values of 25–50%, 51–75% and 76–100% to be of low, moderate and high heterogeneity, respectively.16 We also examined the CIs of the risk estimates in the forest plots for overlap. We reported the T2, which reflects the amount of true heterogeneity. For T2, we used a critical value of less than 1.0 as the cutoff for statistical significance. We performed sensitivity analyses to assess the effect of excluding studies with higher risk of bias. In case of statistically significant heterogeneity (P<.1 for the Cochrane Q-statistic), the random-effects model of DerSimonian and Laird was used to obtain the pooled RR estimate. For studies that reported both unadjusted and adjusted risk for confounders statistically proven, we performed an aggregate data meta-analysis using generic inverse variance method to obtain the adjusted RR for the primary outcome and for the secondary outcomes in the main analysis.17,18 For the sensitivity analysis, we excluded studies that we had determined to be at high risk of bias in the domains of selection bias or attrition bias, because a high risk of bias in either of these domains is likely to have the greatest effect on the results of the studies included in this systematic review. Statistical analyses were conducted with Review Manager (RevMan) 5.3.
RESULTS
The search identified 675 bibliographic references, 578 through the PubMed database and 97 through ClinicalTrials.gov (Fig. 1). After 74 duplicate papers were removed, there were 601 records in title and abstract form available for further screening. We excluded 588 irrelevant references through reading of the abstracts. Thus, we assessed 13 references for eligibility into the systematic review and meta-analysis. After careful scrutiny, we further excluded seven of these references because they did not fulfill the inclusion criteria (Suhag A, Dutta R, Monga M, Sangi-Haghpeykar H, Ramin S, Hollier L. Early initiation of 17OHPC does not increase risk for gestational diabetes in women with prior preterm birth [abstract]. Obstet Gynecol 2017;129:S125.)19-24 (Fig. 1). Subsequently, six references describing six studies met the inclusion criteria for this systematic review (Fig. 1).
Fig. 1.
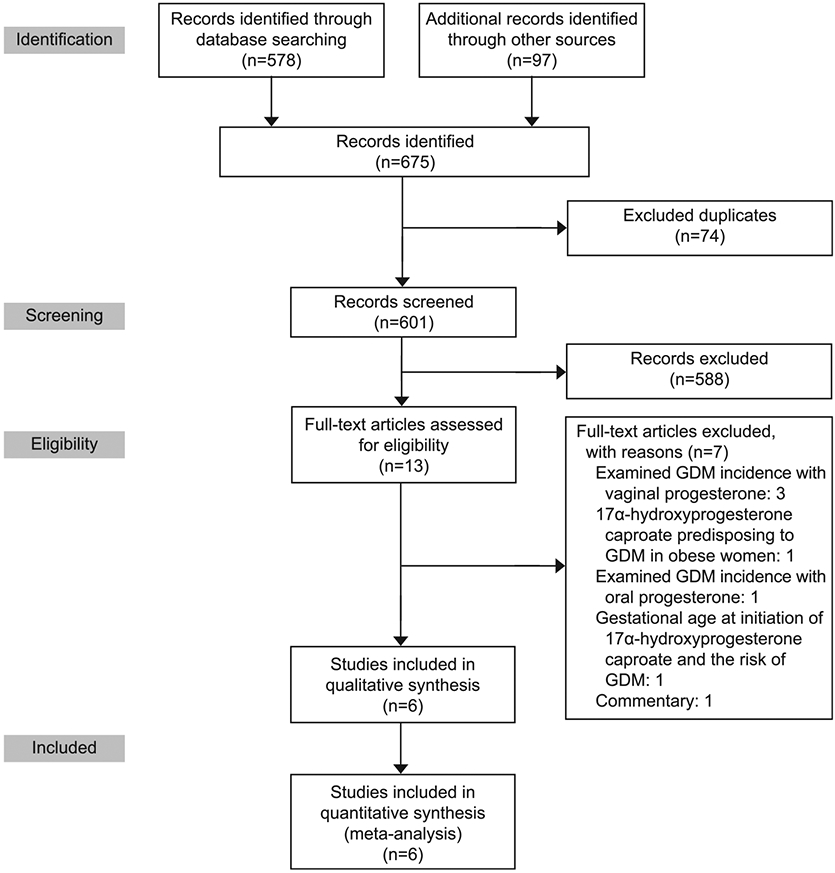
PRISMA study flow diagram showing study selection process. GDM, gestational diabetes mellitus.
The studies included 5,053 women, with 1,538 women receiving 17α-hydroxyprogesterone caproate and 3,515 in unexposed control groups (Table 1). One study was a randomized controlled trial of 17α-hydroxyprogesterone caproate in women with prior preterm birth compared with those in an unexposed control group.13 One study was a prospective cohort study,5 one study was a secondary analysis of two randomized controlled trials,12 and three studies were retrospective cohort studies.10,11,14 Five of the studies were carried out in the United States,5,10-12,14 and one was done in Iran.13 Two of the studies were published in 2009,11,12 one in 2007,10 one in 2011,14 one in 2015,13 and one in 2017.5 All the studies included women with singleton pregnancies and a history of prior preterm birth.
Table 1.
Characteristics of Included Studies Examining the Effect of 17α-Hydroxyprogesterone Caproate on Glucose Intolerance and Rate of Gestational Diabetes Mellitus
| Gyamfi et al12 | Nelson et al5 | Rebarber et al10 |
Rouholamin et al13 |
Waters et al11 | Wolfe et al14 | |
|---|---|---|---|---|---|---|
| Study type | Secondary analysis of 2 RCTs | Prospective cohort | Retrospective cohort | RCT | Retrospective cohort | Prospective cohort study |
| Study country | United States | United States | United States | Iran | United States | United States |
| Sample size | 441 | 1,720 | 2,081 | 164 | 440 | 207 |
| 17α-OHPC dose (mg/wk) | 250 | 250 | 250 | 250 | 250 | 250 |
| Control group | No 17 α -OHPC | No 17 α -OHPC | No 17 α -OHPC | No 17 α -OHPC | No 17 α -OHPC | No 17 α -OHPC |
| Gestational age at 17 α -OHPC initiation (wk) | 16–20 | 16–20 | 16–20 | 16–20 | 16–20 | 16–20 |
| OGTT testing algorithm | Criteria for diagnosis of GDM not predefined* | 1-h, 50-g test; GDM diagnosis with 100-mg, 3-h test | 1-h, 50-g test; GDM diagnosis with 100-mg, 3-h test | 1-h, 50-g test; GDM diagnosis with 100-mg, 3-h test | 1-h, 50-g test; GDM diagnosis with 100-mg, 3-h test | 1-h, 50-g test; GDM diagnosis with 100-mg, 3-h test |
| Inclusion criteria | Singleton, history of prior PTB at less than 36 wk | Singleton, history of prior PTB at less than 35 wk | Singleton, history of prior PTB at less than 37 wk | Singleton, history of prior PTB at less than 37 wk | Singleton, history of prior PTB at less than 37 wk | Singleton, history of prior PTB at less than 37 wk |
| Exclusion criteria | Multiple gestation, pregestational DM, unknown GDM diagnosis, lost to follow-up | Multiple gestation, pregnancy induced hypertension, placental abruption | Multiple gestation, pregestational DM, unknown GDM | Multiple gestation, BMI greater than 30, abnormal OGTT, family history of DM, unknown GDM | Multiple gestation, pregestational DM, unknown GDM | Multiple gestation, progestational diabetes, unknown GDM, younger than 18 or older than 50 y. |
RCT, randomized controlled trial; 17 α -OHPC, 17 α-hydroxyprogesterone caproate; OGTT, oral glucose tolerance test; GDM, gestational diabetes mellitus; PTB, preterm birth; BMI, body mass index; DM, diabetes mellitus.
Because this is a secondary analysis, criteria for diagnosis of GDM were not predefined. However, the centers that participated in the original study used similar guidelines to diagnose GDM.
Exclusion criteria were quite uniform across all the studies at baseline (Table 1). These include history of multiple gestations, family history of pregestational diabetes mellitus, history of unknown diabetes mellitus and loss to follow up. Exclusion criteria related to specific studies include history of preeclampsia, aspirin use, chronic hypertension, severe fetal growth restriction, history of intrauterine fetal demise before 28 weeks of gestation, and a history of liver or renal disease.13 Other exclusion criteria include women experiencing recurrent preterm delivery before 28 weeks,10 women younger than 18 years or older than 50 years, women who did not complete a glucose tolerance test, or had a major fetal anomaly, placental anomalies, or a medically indicated preterm delivery.5,14 All the studies compared 17α-hydroxyprogesterone caproate with unexposed control groups in women with a prior preterm birth with singleton gestations. 17α-hydroxyprogesterone caproate was started in the second trimester and continued until the 36th week of gestation, but the timing varied slightly between the studies. The mean timing of 17α-hydroxyprogesterone caproate commencement in these studies was 18±0.6 weeks.
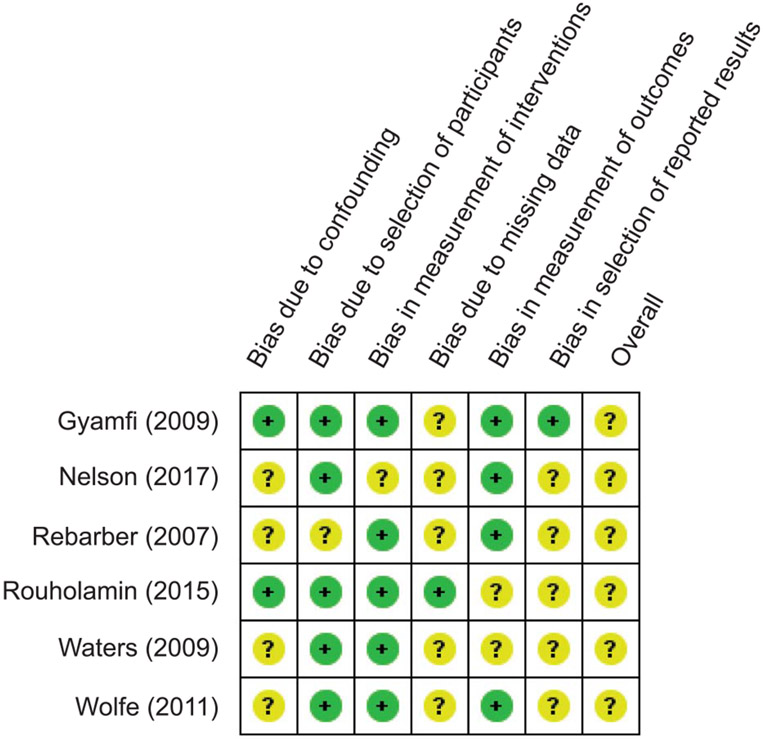
We assessed the quality of the included studies using the Risk of Bias In Non-randomized Studies of Interventions tool (Fig. 2). A majority of the included studies demonstrated an overall robust quality in the domains of selection and comparability between the study groups. Overall, the participants in these studies were clinically heterogeneous, with women having a history of one or more prior preterm births. Furthermore, most of the included studies excluded women with risk factors for preterm birth at baseline, and risk factors for GDM such as history of pregestational diabetes mellitus, multiple gestations, and history of unknown diabetes mellitus. There were also some methodologic heterogeneity issues in the included studies, including differences in the number of participants in the 17α-hydroxyprogesterone caproate and unexposed control groups and lack of information about study follow-up.
Fig. 2.
Risk of Bias In Non-randomized Studies of Interventions tool for assessing risk of bias. Review authors’ judgments about each risk of bias item for each included study. Each study was rated as having either a low (green circles) or unclear risk of bias (yellow circles).
Statistical heterogeneity was assessed using the Q-statistic, I2, and τ2. A priori, we defined a Q-statistic of less than 0.1 as statistically significant, and an I2 statistic of 25–50% as low heterogeneity, 51–75% as moderate heterogeneity, and 76–100% as high heterogeneity. We visually evaluated the CIs of the summary estimates of effect in the forest plots for evidence of overlap. Regarding our primary and secondary outcomes, the combination of an I2 statistic of 57%, a Q-statistic of P=.04, and a T2 of 0.1 in the primary outcome (GDM) suggest nonsubstantial heterogeneity. In making a decision whether or not to continue with a meta-analysis in the face of reasonable methodologic and clinical heterogeneity, moderate I2 statistic, overlapping CIs and a nonsubstantial T2, we deemed it reasonable to continue with a meta-analysis to examine the effect of 17α-hydroxyprogesterone caproate on abnormal glucose test results and GDM.
The meta-analysis showed a 77% increase in the risk of GDM in women treated with 17α-hydroxyprogesterone caproate compared with those in unexposed control groups. This risk increase was statistically significant at the 95% level (RR 1.77, 95% CI 1.22–2.55, Fig. 3). The secondary outcome–abnormal 1-hour, 50-g glucose screen results (1-hour nonfasting venous plasma glucose concentration of at least 135 mg/dL but less than 200 mg/dL)25–was assessed in three studies.11,13,14 Meta-analysis of these studies demonstrated a 55% increase in the risk of abnormal 1-hour, 50-g glucose screen results in women treated with 17α-hydroxyprogesterone caproate compared with those in unexposed control groups. This risk increase was statistically significant at the 95% level (RR 1.55, 95% CI 1.07–2.24);Figure 4. Three studies.11,13,14 evaluated the secondary outcome of mean venous plasma glucose concentration. Meta-analysis of these studies demonstrated an increase in mean venous plasma glucose concentration in women treated with 17α-hydroxyprogesterone caproate compared with those in unexposed control groups. The summary estimate of effect was not statistically significant when comparing women who received 17α-hydroxyprogesterone caproate to those who did not (RD 4.69, 95% CI −2.44 to 11.81); Figure 5. The number needed to treat (NNT) was 20 and 11 for the development of GDM and abnormal 1-hour glucose screen result, respectively. One additional woman is expected to develop gestational diabetes for every 20 women receiving 17α-hydroxyprogesterone caproate compared with those in unexposed control groups (NNT=20). One additional woman is expected to develop abnormal glucose tolerance for every 11 women receiving 17α-hydroxyprogesterone caproate compared with those in unexposed control groups (NNT=11).
Fig. 3.
Forest plot for the risk of gestational diabetes mellitus. 17-α-OHPC, 17α-hydroxyprogesterone caproate; M-H, Mantel–Haenszel; df, degrees of freedom.
Fig. 4.
Forest plot for the risk of abnormal 1-h glucose screen result. 17-α-OHPC, 17α-hydroxyprogesterone caproate; M-H, Mantel–Haenszel; df, degrees of freedom.
Fig. 5.
Forest plot for the risk of mean venous plasma glucose concentration. 17-α-OHPC, 17α-hydroxyprogesterone caproate; IV, independent variable; df, degrees of freedom.
Sensitivity analysis was evaluated on the primary outcome (GDM) excluding all cohort studies. After exclusion of these studies, the summary estimate of effect was nonsignificant (RR 1.21, 95% CI 0.63–2.36). Publication bias was assessed using funnel plots (Figs. 6 and 7). The funnel plots are scatter plots of the estimated effect size (RR estimates) of GDM, abnormal 1-hour, 50-g glucose screen results, and mean plasma glucose concentration plotted on the horizontal axis against the reciprocal of standard error of the estimated effect on the vertical axis for the studies identified. The asymmetric nature of the funnel plots (for the primary and secondary outcomes) would suggest possible publication bias. However, assessment of publication bias in this review is particularly difficult given that the number of studies were fewer than 10 (publication bias is difficult to assess, and funnel plots are thought to be unreliable methods of investigating potential bias if the number of studies is less than 10).
Fig. 6.
Funnel plot for the risk of gestational diabetes.
Fig. 7.
Funnel plot for the risk of abnormal 1-h glucose screen result and mean plasma glucose concentration.
DISCUSSION
We found that women with singleton gestations receiving weekly 17α-hydroxyprogesterone caproate for recurrent preterm birth prevention had a significantly higher incidence of abnormal glucose test results and GDM compared with women in unexposed control groups, but this finding did not hold among women who had been randomly allocated to 17α-hydroxyprogesterone caproate.
Our study has several strengths. First, the most important strength of our work rests on the fact that we were adequately powered to evaluate the development of gestational diabetes in the included studies. For our power analysis, we determined that with 5,053 women (in six studies) who met inclusion criteria for this meta-analysis, using a two-tailed alpha level of 0.05 (type-1 error rate of 5%), we had greater than 80% power to detect a difference in effect size of 30%. Second, we adjusted for potential confounding factors in our analysis. Third, there were many variables that were similar and uniformly reported across the included studies, such as BMI, maternal age, and gestational age at initiation of 17α-hydroxyprogesterone caproate.
Our study also had limitations. First, the retrospective nonrandomized design of some of the included studies, lack of information on other ethnicities in the included studies (American Indian, Alaska Native, Asian American, Native Hawaiian, and Other Pacific Islander), and lack of information on all risk factors for GDM were some of the limitations of this meta-analysis. Second, most of the studies did not stratify outcomes by parity, so it wasn’t possible to assess the effect of parity on the association between 17α-hydroxyprogesterone caproate and glucose intolerance, as high parity has been associated with increased incidence of gestational diabetes. Third, there was some heterogeneity in the included studies. However, we proceeded with a meta-analysis because the heterogeneity in the included studies was low to moderate.
To prove causality, temporal and dose-response relationships would be necessary through adequately powered, well designed prospective cohort studies or randomized controlled trials. The PROLONG (Progestin’s Role in Optimizing Neonatal Gestation) trial (NCT01004029),26 a confirmatory study of 7α-hydroxyprogesterone caproate compared with vehicle for the prevention of preterm birth in women carrying a singleton gestation who have a history of a prior singleton preterm birth, may answer this question.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. Maternal and reproductive health reports: Preterm birth. Atlanta (GA): Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 2.Martin JA, Osterman MJK. Describing the increase in preterm births in the United States, 2014–2016. NCHS Data Brief, no 312. Hyattsville (MD): National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Hydroxyprogesterone caproate (17P). Silver-Spring (MD): FDA; 2011. [Google Scholar]
- 4.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DB, McIntire DD, McDonald J, Gard J, Turrichi P, Leveno KJ. 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study. Am J Obstet Gynecol 2017;216:600.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med 2005;159:957–62. [DOI] [PubMed] [Google Scholar]
- 7.Vidaeff AC, Belfort MA. Critical appraisal of the efficacy, safety, and patient acceptability of hydroxyprogesterone caproate injection to reduce the risk of preterm birth. Patient Prefer Adherence 2013;7:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 9.Picard F, Wanatabe M, Schoonjans K, Lydon J, O’Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation. Proc Natl Acad Sci U S A 2002;99:15644–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebarber A, Istwan NB, Russo-Stieglitz K, Cleary-Goldman J, Rhea DJ, Stanziano GJ, et al. Increased incidence of gestational diabetes in women receiving prophylactic 17alpha-hydroxyprogesterone caproate for prevention of recurrent preterm delivery. Diabetes Care 2007;30:2277–80. [DOI] [PubMed] [Google Scholar]
- 11.Waters TP, Schultz BA, Mercer BM, Catalano PM. Effect of 17alpha-hydroxyprogesterone caproate on glucose intolerance in pregnancy. Obstet Gynecol 2009;114:45–9. [DOI] [PubMed] [Google Scholar]
- 12.Gyamfi C, Horton AL, Momirova V, Rouse DJ, Caritis SN, Peaceman AM, et al. The effect of 17-alpha hydroxyprogesterone caproate on the risk of gestational diabetes in singleton or twin pregnancies. Am J Obstet Gynecol 2009;201:392.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouholamin S, Zarean E, Sadeghi L. Evaluation the effect of 17-alpha hydroxyprogesterone caproate on gestational diabetes mellitus in pregnant women at risk for preterm birth. Adv Biomed Res 2015;4:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe K, Dearmond C, How H, Henderson ZT, Sibai B. The rates of abnormal glucose challenge tests and gestational diabetes in women receiving 17alpha-hydroxyprogesterone caproate. Am J Perinatol 2011;28:741–6. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JA, Hernan MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias. Available at: https://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm. Retrieved October 18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters J, Mengersen K. Selective reporting of adjusted estimates in observational epidemiology studies: reasons and implications for meta-analyses. Eval Health Prof 2008;31:370–89. [DOI] [PubMed] [Google Scholar]
- 19.Alimohammadi S, Parsapoor H, Esna-Ashari F, Zamani M. Impaired glucose tolerance test in pregnant women receiving vaginal progesterone: a 1 year cohort study. Int J Curr Res Acad Rev 2015;3:317–24. [Google Scholar]
- 20.Rosta K, Ott J, Kelemen F, Temsch W, Lahner T, Reischer T, et al. Is vaginal progesterone treatment associated with the development of gestational diabetes? A retrospective case-control study. Arch Gynecol Obstet 2018;298:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zipori Y, Lauterbach R, Matanes E, Beloosesky R, Weiner Z, Weissman A. Vaginal progesterone for the prevention of preterm birth and the risk of gestational diabetes. Eur J Obstet Gynecol Reprod Biol 2018;230:6–9. [DOI] [PubMed] [Google Scholar]
- 22.Egerman R, Ramsey R, Istwan N, Rhea D, Stanziano G. Maternal characteristics influencing the development of gestational diabetes in obese women receiving 17-alpha-hydroxyprogesterone caproate. J Obes 2014;2014:563243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosus A, Kosus N, Haktankacmaz SA, Ak D, Turhan NO. Effect of dose and duration of micronized progesterone treatment during the first trimester on incidence of glucose intolerance and on birth weight. Fetal Diagn Ther 2012;31:49–54. [DOI] [PubMed] [Google Scholar]
- 24.Tuffnell DJ, McClean S. Does treatment with 17 alpha-hydroxyprogesterone caproate increase the risk of gestational diabetes mellitus? Nat Clin Pract Endocrinol Metab 2008;4:130–1. [DOI] [PubMed] [Google Scholar]
- 25.Harper LM, Mele L, Landon MB, Carpenter MW, Ramin SM, Reddy UM, et al. Carpenter-Coustan compared with National Diabetes Data Group criteria for diagnosing gestational diabetes. Obstet Gynecol 2016;127:893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, Chauhan SP, Hughes BL, Louis JM, et al. PROLONG clinical study protocol: hydroxyprogesterone caproate to reduce recurrent preterm birth. Am J Perinatol 2018;35:1228–34. [DOI] [PubMed] [Google Scholar]