Key Points
Question
Are time-dependent changes in antithrombin activity associated with anti–factor Xa (anti-FXa) levels in response to enoxaparin prophylaxis and with risk of trauma-related venous thromboembolism (VTE) in patients with trauma, and is ex vivo antithrombin supplementation associated with improved enoxaparin sensitivity?
Findings
In this cohort study of 150 patients at a level 1 trauma center, antithrombin activity was lowest among patients with subprophylactic anti-FXa levels and was independently associated with VTE development. Ex vivo supplementation of antithrombin to 150% was associated with the highest prophylactic anti-FXa levels and reductions in thrombin generation.
Meaning
These findings suggest that antithrombin deficiency is common after trauma and may contribute to enoxaparin resistance and VTE.
Abstract
Importance
Venous thromboembolism (VTE) affects 2% to 20% of recovering trauma patients, despite aggressive prophylaxis with enoxaparin. Antithrombin is a primary circulating anticoagulant and crucial component of enoxaparin thromboprophylaxis. Approximately 20% of trauma patients present with antithrombin deficiency (antithrombin activity <80%).
Objective
To examine time-dependent changes in antithrombin activity, responsiveness to enoxaparin, as measured by anti–factor Xa (anti-FXa) levels, and incidence of VTE after severe trauma and to assess the association of ex vivo antithrombin supplementation with patients’ sensitivity to enoxaparin prophylaxis.
Design, Setting, and Participants
This single-center, prospective cohort study was performed at a level 1 trauma center between January 7, 2019, and February 28, 2020. Adult trauma patients admitted to the trauma service at high risk for VTE, based on injury pattern and severity, were screened and enrolled. Patients who were older than 70 years, were pregnant, had a known immunologic or coagulation disorder, or were receiving prehospital anticoagulants were excluded.
Exposures
Blood samples were collected on emergency department arrival and daily for the first 8 days of hospitalization.
Main Outcomes and Measures
Patients’ antithrombin activity and anti-FXa levels were measured by a coagulation analyzer, and thrombin generation was measured by calibrated automated thrombography. Responsiveness to enoxaparin was assessed by measuring anti-FXa levels 4 to 6 hours after the first daily enoxaparin dose and compared between patients who developed VTE and who did not. In addition, the associations of ex vivo supplementation of antithrombin with plasma anti-FXa levels were assessed.
Results
Among 150 patients enrolled (median [IQR] age, 35 [27-53] years; 37 [24.7%] female and 113 [75.3%] male; 5 [3.3%] Asian, 32 [21.3%] Black, and 113 [75.3%] White; and 51 [34.0%] of Hispanic ethnicity), 28 (18.7%) developed VTE. Patients with VTE had significantly lower antithrombin activity on admission compared with patients without VTE (median [IQR], 91% [79%-104%] vs 100% [88%-112%]; P = .04), as well as lower antithrombin activity on hospital days 5 (median (IQR), 90% [83%-99%] vs 114% [99%-130%]; P = .011), 6 (median [IQR], 97% [81%-109%] vs 123% [104%-134%]; P = .003), 7 (median [IQR], 82% [74%-89%] vs 123% [110%-140%]; P < .001), and 8 (median [IQR], 99% [85%-100%] vs 123% [109%-146%]; P = .011). Anti-FXa levels were significantly lower in patients with VTE vs those without VTE at hospital day 4 (median [IQR], 0.10 [0.05-0.14] IU/mL vs 0.18 [0.13-0.23] IU/mL; P = .006), day 6 (median [IQR], 0.12 [0.08-0.14] IU/mL vs 0.22 [0.13-0.28] IU/mL; P = .02), and day 7 (median [IQR], 0.11 [0.08-0.12] IU/mL vs 0.21 [0.13, 0.28] IU/mL; P = .002). Multivariable analyses found that for every 10% decrease in antithrombin activity during the first 3 days, the risk of VTE increased 1.5-fold.
Conclusions and Relevance
The results of this cohort study suggest that after severe trauma, antithrombin deficiency is common and contributes to enoxaparin resistance and VTE. Interventional studies are necessary to determine the efficacy of antithrombin supplementation in the reduction of VTE incidence.
This cohort study of patients from a level 1 trauma center examines the association of changes in antithrombin activity over time with anti–factor Xa levels in response to enoxaparin prophylaxis and with risk of trauma-related venous thromboembolism.
Introduction
Venous thromboembolism (VTE), defined as deep vein thrombosis (DVT) with or without pulmonary embolism (PE), affects 2% to 20% of hospitalized patients with traumatic injury and is a leading cause of morbidity and mortality.1,2,3 Venous thromboembolism rates remain at greater than 10% after hospital discharge, and 11% of potentially preventable trauma-related fatalities that occur after initial hospitalization result from PE.1,4 Understanding clinical and physiological factors contributing to the pathogenesis of trauma-related VTE is of high clinical relevance.
Ongoing hypercoagulability is a well-described independent risk factor for trauma-related VTE.5 Plasma thrombin generation is significantly increased on admission among trauma patients who develop VTE compared with those who do not,5,6 in addition to pronounced increases in whole-blood viscoelastic parameters, which continue through at least day 5.7,8,9 To mitigate VTE risk, institutional protocols that include early use (in the first 24 hours after injury) of prophylactic low-molecular-weight heparin are widespread.10 While these measures have significantly reduced the incidence of VTE, thromboembolic complications persist in this population.2
Past work has demonstrated low responsiveness to the low-molecular-weight heparin enoxaparin among trauma patients, with 50% to 70% of patients failing to achieve the recommended peak anti–factor Xa (anti-FXa) range of 0.2 to 0.4 IU/mL.11,12 Importantly, these patients have higher incidences of VTE, which is not attenuated on escalation of the enoxaparin dosage.13 It is known that enoxaparin’s mechanism of action is dependent on endogenous antithrombin because its inhibitory activity for FXa and thrombin is accelerated on binding heparin. Recent data show that 20% to 70% of trauma patients have acquired antithrombin deficiency on admission, defined as antithrombin activity below 80%, which is dependent on injury severity and associated with VTE.3,6 Previous data reported by this research team6 found that ex vivo antithrombin supplementation (addition of antithrombin to plasma) was associated with increases in anti-FXa levels and reductions in thrombin generation. However, these experiments were limited by the use of samples collected only on hospital admission.
The goals of this study were to assess (1) time-dependent changes in antithrombin activity in trauma patients at high risk for VTE, (2) the association between antithrombin activity and responsiveness to enoxaparin prophylaxis, and (3) the association of ex vivo antithrombin supplementation with improvements in enoxaparin sensitivity among patients who fail to achieve prophylactic anti-FXa levels.
Methods
Study Population
This analysis was a single-center, prospective cohort study conducted at The University of Texas Health Science Center at Houston and Memorial Hermann Hospital–Texas Medical Center in Houston. This study was performed under an existing protocol approved by The University of Texas Health Science Center Committee for the Protection of Human Subjects and Memorial Hermann. The protocol covers collection of blood samples for all trauma patients admitted to Memorial Hermann for creation of a deidentified blood bank and registry for exploratory observations and investigations. The analysis of these blood samples, including the tests described in this article, is also covered by this protocol. We used blood samples from patients who fit our eligibility criteria collected during the designated dates to perform our study. Patients or their legally authorized representatives provided written informed consent to participate in the study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.14
Patients were eligible if they were aged 18 to 70 years, were admitted to the trauma service of Memorial Hermann Hospital–Texas Medical Center, and started receiving therapeutic doses of anticoagulants between January 7, 2019, and February 28, 2020 (eTable 1 in the Supplement). Patients were excluded if they had an ineligible injury pattern, were pregnant, were a prisoner, were not admitted to the trauma service, died or were discharged from the emergency department (ED), immediately started receiving therapeutic doses of enoxaparin or heparin, were taking anticoagulants prior to hospitalization, or had a known hematologic or immunologic disorder (eTable 1 in the Supplement). In addition, individuals older than 70 years were excluded to reduce enrollment of patients receiving prehospital anticoagulants.
Standard-of-care VTE prophylaxis at our institution is 30 mg of enoxaparin every 12 hours for patients weighing 90 kg or less and 40 mg every 12 hours for patients weighing more than 90 kg. For those with reduced estimated glomerular filtration rate (<30 mL/min/per 1.73 m2), heparin 5000 IU every 8 hours is substituted for enoxaparin (7500 IU for those 90 kg or less). Anti-FXa levels are measured 4 hours after the third dose of enoxaparin. Dose escalations occur when a patient does not achieve an anti-FXa level of at least 0.2 IU/mL. In these cases, the twice-daily enoxaparin dose is increased by 10 mg, and anti-FXa measurements are repeated after 3 doses to determine whether another 10-mg adjustment is necessary.
Venous thromboembolism surveillance at our institution is limited to monitoring for PE symptoms without routine DVT screening. Consequently, only symptomatic VTEs are diagnosed unless otherwise identified intraoperatively. Computed tomographic angiography of the chest is performed to establish a diagnosis of PE in patients who present with cough, tachypnea, or hypoxia. DVT is identified by ultrasound on presentation of extremity edema, redness, or tenderness and confirmed by computed tomographic angiography.
Data Collection
Patient demographic characteristics, vital signs, injury mechanism, and prehospital fluid administration were collected by direct observation on admission to the ED. Patient race (Asian, Black, and White) and ethnicity (Hispanic or Latino) were reported by the patient or a legally authorized representative and included in analyses as a confounder because of known contributions of race and/or ethnicity to VTE risk. Race and ethnicity were classified based on standard classifications used at Memorial Hermann and abstracted from the patient records for this study. No patients were excluded based on race and ethnicity. Daily hospital laboratory results, as well as initiation times, duration, and doses of all anticoagulants, and outcomes (DVT; PE; hospital-, ventilator-, and intensive care unit [ICU]–free days; and death), and 24-hour blood product and fluid administration were collected from medical records. Injury Severity Scores (measured on a scale of 1 to 75, with 1 representing the least severe injury and 75 representing the most severe injury) were collected from the trauma registry.
Sample Collection
Whole blood was collected in vacutainer tubes containing sodium citrate, 3.2%, on ED arrival, in conjunction with clinical sample collection (day 0). Whole-blood samples were collected once daily during hospitalization, 4 to 6 hours after the first daily enoxaparin dose, to assess peak anti-FXa levels. Blood was centrifuged at 3200 rpm for 20 minutes to obtain platelet-poor plasma and frozen at −80 °C until analysis. Serial blood samples were obtained for 8 days after ED arrival or until a patient died or was discharged, was diagnosed with VTE, or advanced to therapeutic enoxaparin or unfractionated heparin.
Plasma Analysis
Plasma samples were thawed at 37 °C immediately prior to use. Antithrombin activity, international normalized ratio, and anti-FXa and fibrinogen levels were determined using an ACL TOP coagulation analyzer (Werfen). Neutrophil elastase (kit No. ab270204; Abcam) and thrombin-antithrombin complex levels (kit No. ab108907; Abcam) were measured by enzyme-linked immunosorbent assay . Thrombin generation was measured using calibrated automated thrombography (Thermo Fisher Scientific Inc) as previously described.15 Clot formation was assessed kinetically by measuring plasma turbidity in the presence of calcium chloride (10 mmol), tissue factor (1 pmol), and phospholipids (4 μmol) at 405 nm using a SpectraMax Plus plate reader (Molecular Devices LLC). Antithrombin deficiency was defined as antithrombin activity below 80%. An adequate anti-FXa level for patients receiving enoxaparin was defined as 0.2 IU/mL or greater. Antithrombin was supplemented ex vivo up to 120%, 150%, or 180% using antithrombin concentrate (Thrombate III; Grifols) based on baseline antithrombin values and confirmed by measuring antithrombin activity with an ACL TOP coagulation analyzer.
Statistical Analysis
Medians and IQRs were reported for all continuous variables. The Mann-Whitney U test and χ2 test were used to evaluate differences between continuous and dichotomous variables, respectively. The average antithrombin value over the first 3 days was used as a continuous variable in multivariable logistic regression models to evaluate the independent association between antithrombin activity and VTE, while controlling for age, sex, Injury Severity Score , and body mass index (calculated as weight in kilograms divided by height in meters squared). Associations between antithrombin activity and neutrophil elastase, thrombin-antithrombin complex, and liver enzyme levels were determined by Pearson correlation analysis. For ex vivo spiking experiments, differences in anti-FXa and thrombin generation were evaluated using repeated-measures one-way analysis of variance using the Tukey multiple comparisons test. Statistical significance was defined as 2-tailed P < .05. Statistical analyses were performed using Stata software, version 12.1 (StataCorp LLC).
Results
During the study period, 2150 patients were screened, and 237 met eligibility criteria (eFigure 1 in the Supplement). Eighty-seven patients declined participation or could not give consent, leaving 150 patients enrolled in the study (median [IQR] age, 35 [27-53] years; 37 [24.7%] female and 113 [75.3%] male; 5 [3.3%] Asian, 32 [21.3%] Black, and 113 [75.3%] White; and 51 [34.0%] of Hispanic ethnicity). Of these, 28 patients (18.7%) developed VTE, including 12 with DVT, 14 with PE, and 2 with DVT and PE. Three VTE events were identified intraoperatively, and the patients with the remaining 25 were symptomatic. Of the 12 DVTs, all were unilateral; 9 (75.0%) were proximal, and 3 (25.0%) were distal. The median (IQR) time to VTE was 4.0 (1.3-8.8) days.
Patient Characteristics
No differences in demographic characteristics, admission vital signs, or injury mechanism and severity were identified between patients with and without VTE. Although the differences were not significant, the mean (IQR) systolic blood pressure (109 [90-135] vs 119 [98-141] mm Hg; P = .09) and mean [IQR] arterial base deficit (−7 [−12 to −2] vs −5 [−7 to −2]; P = .06) were lower among patients with VTE, suggesting a more critically ill population. The VTE group also received significantly more blood products in the first 24 hours after ED arrival (given as median [IQR]): red blood cells, 4 (1-9) vs 0 (0-3) units (P < .001); plasma, 3 (1-9) vs 1 (0-3) units (P = .001); whole blood, 0 (0-1) vs 0 (0-0) units (P = .04); and cryoprecipitate, 0 (0) vs 0 (0) units (P = .007). Patients with VTE had significantly fewer median (IQR) ICU-free (19 [8-26] vs 25 [21-27]; P = .006) and hospital-free (6 [0 to 15] vs 17 [9 to 21]; P < .001) days, compared with patients without VTE. No significant differences in mortality were observed, and no in-hospital deaths were directly attributed to VTE (Table 1).
Table 1. Demographic and Clinical Characteristics of Patients Who Did and Did Not Develop VTE.
| Characteristic | Participants, No. (%) | P value | |
|---|---|---|---|
| No VTE (n = 122) | VTE (n = 28) | ||
| Age, median (IQR), y | 35 (28-50) | 40 (27 to 57) | .51 |
| Sex | |||
| Female | 27 (22.1) | 10 (35.7) | .13 |
| Male | 95 (77.9) | 18 (64.2) | |
| Race | |||
| Asian | 5 (4.1) | 0 | .51 |
| Black | 25 (20.5) | 7 (25.0) | |
| White | 92 (75.4) | 21 (75.0) | |
| Ethnicity, No. (% Hispanic/Latino) | 40 (32.8) | 11 (39.3) | .51 |
| BMI, median (IQR) | 28 (24-31) | 29 (26-32) | .24 |
| Admission vital statistics, median (IQR) | |||
| Systolic blood pressure, mm Hg | 119 (98-141) | 109 (90-135) | .09 |
| Heart rate, beats/min | 105 (85-124) | 108 (87-139) | .33 |
| Arterial base deficit, mmol/L | −5 (−7 to −2) | −7 (−12 to −2) | .06 |
| Injury | |||
| Blunt | 111 (91.0) | 23 (82.1) | .28 |
| Glasgow Coma Scale score, median (IQR)a | 14 (3-15) | 8 (3-15) | .25 |
| Injury Severity Score, median (IQR)b | 27 (17-34) | 29 (20-41) | .22 |
| AIS head >2c | 53 (43.4) | 13 (46.4) | .77 |
| Visceral injury | 56 (45.9) | 15 (53.6) | .46 |
| Fracture injury | 117 (95.9) | 25 (89.1) | .16 |
| Transfusion volumes, median (IQR) | |||
| Prehospital crystalloid, mL | 0 | 0 | .47 |
| 24-h Crystalloid, mL | 0 (0-1000) | 0 (0-1000) | .50 |
| 24-h Red blood cells, U | 0 (0-3) | 4 (1-9) | <.001 |
| 24-h Plasma, U | 1 (0-3) | 3 (1-9) | .001 |
| 24-h Platelets, U | 0 (0-0) | 0 (0-2) | .01 |
| 24-h Whole blood, U | 0 (0-0) | 0 (0-1) | .04 |
| 24-h Cryoprecipitate, U | 0 (0-0) | 0 (0-0) | .007 |
| Outcomes, median (IQR) | |||
| Ventilator-free days | 28 (26-30) | 27 (13-30) | .11 |
| ICU-free days | 25 (21-27) | 19 (8-26) | .006 |
| Hospital-free days | 17 (9-21) | 6 (0-15) | <.001 |
| In-hospital mortality | 4 (3.3) | 3 (10.7) | .09 |
Abbreviations: AIS, Abbreviated Injury Scale; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ICU, intensive care unit; VTE, venous thromboembolism.
Scored from 3 to 15, with 3 being the worst response and 15 being the best.
Measured on a scale of 1 to 75, with 1 representing the least severe and 75 representing the most severe injury.
Measured on a scale of 0 to 6, with 0 representing no involvement and 6 representing devastating injury.
Chemoprophylaxis Administration
Of enrolled patients, 131 (87.3%) received enoxaparin prophylaxis, while 19 (12.7%) received subcutaneous unfractionated heparin. Additionally, 52 patients (34.7%) received aspirin therapy owing to blunt cerebrovascular or arterial injuries. No differences in VTE incidence, anti-FXa levels, or antithrombin activity were identified among patients receiving aspirin.
We noted that 39 patients (26.0%) received enoxaparin dose escalations during their clinical course, of which 38 were a result of the patients’ weight. Of 28 patients with VTE, 10 (35.7%) received a dose escalation before VTE diagnosis. Patients with VTE experienced a slight but nonsignificant delay in time to first chemoprophylaxis dose (median [IQR], 35.3 [12.5-44.5] hours since arrival) compared with patients without VTE (median [IQR], 31.2 [15.5-45.4] hours since arrival; P = .56). Time to achieve an anti-FXa level of 0.2 IU/mL or greater was significantly delayed in patients with VTE compared with patients without VTE (median [IQR], 8 [5-8] days vs 4 [3-7] days; P = .04).
Coagulation Parameters on Admission and Over Time
We identified no significant differences in platelet counts, international normalized ratio, fibrinogen, rapid thrombelastographic parameters, or thrombin generation between and patients with and without VTE on admission (eTable 2 in the Supplement) or over time (eFigure 2A through 2C in the Supplement). Thrombin generation was evaluated over time among patients who had received 3 or more doses of enoxaparin. Although no significant differences were found, we observed that patients without VTE exhibited relatively stable peak thrombin, endogenous thrombin potential, and rate of thrombin generation over time, while patients with VTE demonstrated time-dependent increases in these parameters (eFigure 2D through 2F in the Supplement).
Association of Early Antithrombin Levels With Trauma-Related VTE
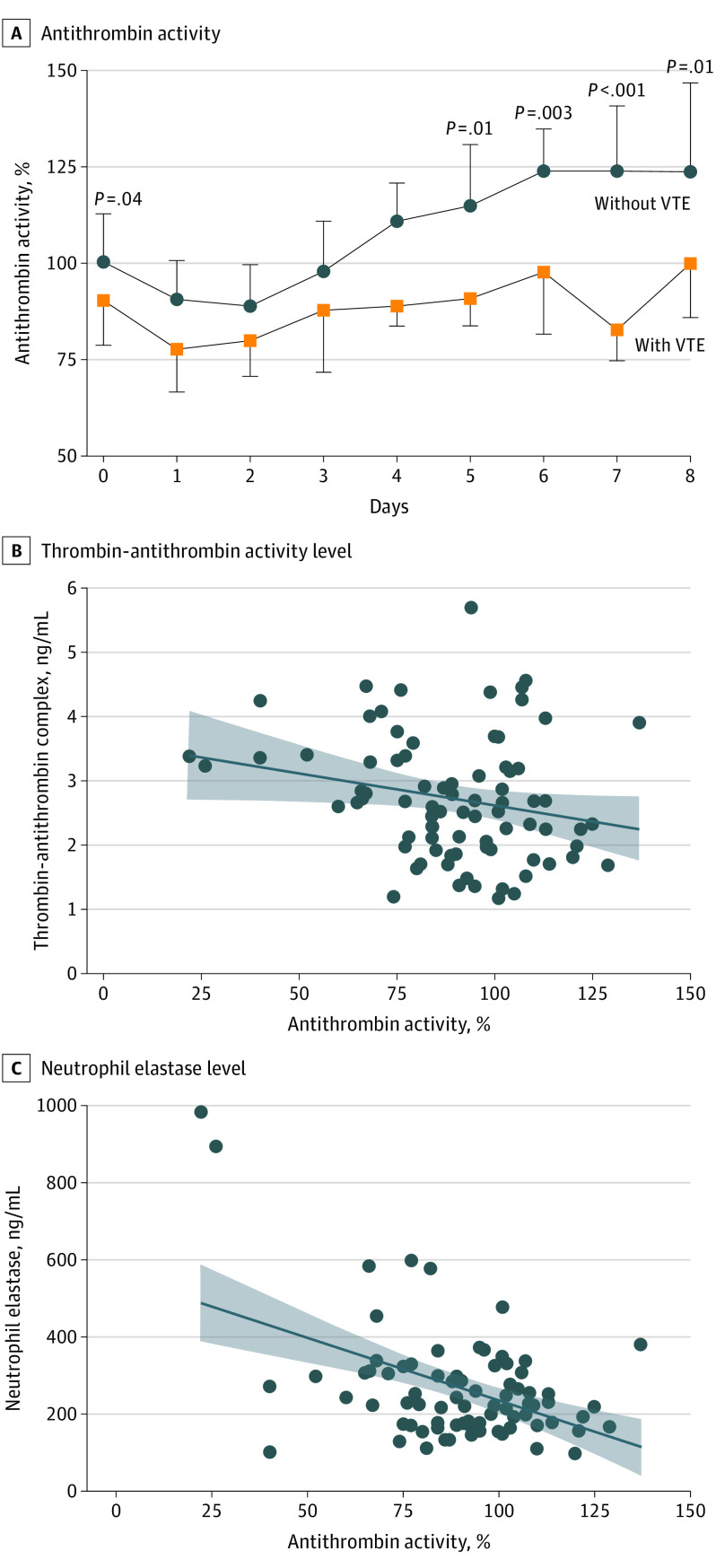
Admission antithrombin values were available for 126 patients, of whom 22 (17.5%) presented with antithrombin activity below 80%. Patients with VTE had significantly lower antithrombin activity on admission compared with patients without VTE (median [IQR], 91% [79%-104%]) vs 100% [88%-112%]; P = .04), as well as lower antithrombin activity on hospital days 5 (median [IQR], 90% [83%-99%] vs 114% [99%-130%]; P = .011), 6 (median [IQR], 97% [81%-109%] vs 123% [104%-134%]; P = .003), 7 (median [IQR], 82% [74%-89%] vs 123% [110%-140%]; P < .001), and 8 (median [IQR], 99% [85%-100%] vs 123% [109%-146%]; P = .011) (Figure 1A). Overall, antithrombin activity declined during the first 2 days; it rebounded in patients without VTE starting between days 3 and 4 but did not in patients with VTE.
Figure 1. Antithrombin Activity Levels Over Time After Severe Trauma.
A, Antithrombin activity was measured chromogenically by ACL TOP coagulation analyzer (Werfen) over time among those patients who did and did not develop venous thromboembolism (VTE). Data are presented as medians; whiskers represent IQRs (upper, 75th percentile [without VTE]; lower, 25th percentile [with VTE]). Plasma thrombin-antithrombin complex and neutrophil elastase levels were measured by enzyme-linked immunosorbent assay. B and C, Scatterplots show plasma thrombin-antithrombin or neutrophil elastase levels and antithrombin activity on hospital day 2. The best-fit line is presented with 95% confidence bands (shaded areas). R values are provided in the text.
Multivariable logistic regression modeling was performed to evaluate the association between antithrombin activity and VTE using average antithrombin activity of the first 3 days for each patient. This period was selected because of increased missing data at later time points and because the median (IQR) time to VTE was 4.0 (1.3-8.8) days. When controlling for age, sex, obesity (defined as a body mass index ≥25), and Injury Severity Score, we found that female sex (odds ratio [OR], 3.07 [95% CI, 1.12-8.46]), obesity (OR, 5.33 [95% CI, 1.12-25.31]), and average antithrombin activity (OR, 1.52 [95% CI, 1.12-2.06]) were independently associated with VTE (Table 2). For every 10% decrease in average antithrombin activity during the first 3 days, the risk of VTE increased 1.5-fold (OR, 1.52 [95% CI, 1.12-2.04]; P < .001).
Table 2. Multivariable Logistic Regression Analysis to Evaluate Association of Average Antithrombin Level Over the First 3 Days With Incidence of VTE.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Average antithrombin (per 10% activity reduction) | 1.52 (1.12-2.06) | .007 |
| Age (per 10-y increment) | 1.04 (0.73-1.46) | .85 |
| Sex | ||
| Male | 1 [Reference] | .03 |
| Female | 3.07 (1.12-8.46) | |
| Obesity (BMI ≥25) | 5.33 (1.12-25.31) | .04 |
| Injury Severity Score (per 10-point increment)a | 0.97 (0.64-1.47) | .88 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); VTE, venous thromboembolism.
aMeasured on a scale of 1 to 75, with 1 representing the least severe and 75 representing the most severe injury.
Association of Antithrombin Levels With Antithrombin Consumption and Inhibition
To assess possible mechanisms associated with antithrombin deficiency following trauma, we analyzed correlations between antithrombin activity and thrombin-antithrombin complex levels, neutrophil elastase levels, and markers of liver function on hospital day 2, the time at which overall antithrombin activity was lowest. No correlations were identified between antithrombin and alanine aminotransferase, aspartate aminotransferase, or albumin, indicating no association between altered liver function and antithrombin biosynthesis. Antithrombin activity was significantly inversely correlated with thrombin-antithrombin complex level (R = −0.22 [95% CI, −0.42 to −0.004]; P < .046) (Figure 1B) and neutrophil elastase (R = −0.47; [95% CI, −0.62 to −0.27]; P < .001) (Figure 1C), suggesting a combination of antithrombin consumption by thrombin and inhibition by neutrophil elastase.
Association of Antithrombin Levels and VTE Rates With Enoxaparin Responsiveness
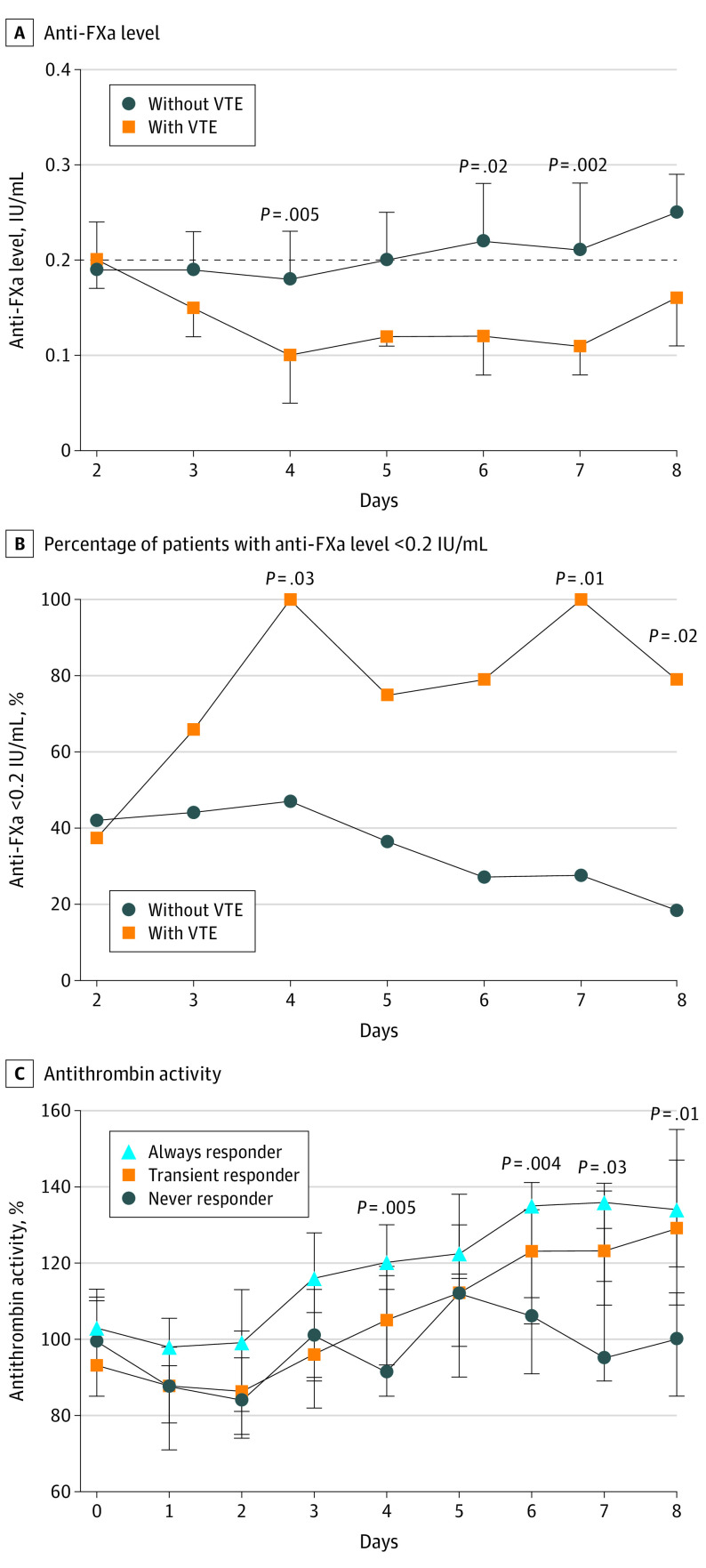
To evaluate temporal changes in enoxaparin responsiveness, anti-FXa levels were measured in patients who received 3 or more consecutive enoxaparin doses. Patients receiving unfractionated heparin were excluded. Of the 150 patients enrolled, 110 patients (73.3%) met these criteria. Patients received their third dose of enoxaparin on hospital day 2 at the earliest; therefore, anti-FXa was not assessed on admission and day 1. Anti-FXa levels over time are presented in Figure 2A, with a dashed line at the prophylactic target of 0.2 IU/mL. Patients without VTE exhibited relatively stable anti-FXa levels around 0.2 IU/mL between days 2 and 5 before surpassing this target. Patients with VTE exhibited a reduction in anti-FXa levels between days 2 and 4, which remained low. Significantly lower anti-FXa levels were observed in patients with VTE than in patients without VTE on hospital day 4 (median [IQR], 0.10 [0.05-0.14] IU/mL vs 0.18 [0.13-0.23] IU/mL; P = .006), day 6 (median [IQR], 0.12 [0.08-0.14] IU/mL vs 0.22 [0.16-0.28] IU/mL; P = .02), and day 7 (median [IQR], 0.11 [0.08-0.12] IU/mL vs 0.21 [0.13-0.28] IU/mL; P = .002). Anti-FXa levels greater than 0.4 IU/mL occurred in 4 patients (2.7%) during the study period; however, no bleeding complications were reported.
Figure 2. Associations Among Anti–Factor Xa (Anti-FXa), Venous Thromboembolism (VTE), and Antithrombin Activity.
A, Anti-FXa levels were measured chromogenically by ACL TOP coagulation analyzer (Werfen) over time, and values were compared between patients who did and did not develop VTE. B, The incidence of anti-FXa levels less than 0.2 IU/mL was calculated for each day and compared between patients who did and did not develop VTE. C, Patients were stratified by anti-FXa levels as always responders, transient responders, and never responders, and antithrombin activity was compared over time between groups. Data are presented as median (IQR).
The incidence of anti-FXa levels of 0.2 IU/mL or greater declined over time among patients without VTE; however, there was a time-dependent increase among patients with VTE in the incidence of subprophylactic anti-FXa levels, which was significant on hospital day 4 (7 of 7 patients [100.0%] vs 45 of 78 patients [57.7%]), day 7 (5 of 5 patients [100.0%] vs 24 of 57 patients [42.1%], and day 8 (5 of 6 patients [83.3%] vs 17 of 49 patients [34.7%]) (Figure 2B).
Patients were categorized into 3 groups: always responders, defined as having an anti-FXa level of 0.2 IU/mL or greater following each course of enoxaparin (measured after the third dose); transient responders, defined as having an anti-FXa level of 0.2 IU/mL or greater at least once after the third enoxaparin dose; and never responders, defined as never achieving an anti-FXa level of 0.2 IU/mL or greater at any time after the third enoxaparin dose. Of the 110 patients evaluated, 18 (16.4%) were always responders, 66 (60.0%) were transient responders, and 26 (23.6%) were never responders. While the incidence of VTE was similar between always responders and transient responders (1 [5.6%] and 4 [6.1%], respectively), never responders had a VTE incidence of 30.8% (8 patients) (P = .009). In addition, we identified significant differences in the median (IQR) antithrombin activity between groups on hospital day 4 (always responders, 120% [113%-130%]; transient responders, 105% [93%-119%]; never responders, 91.5% [85%-116.5%]; P = .005), day 6 (always responders, 135% [123%-141%]; transient responders, 123% [104%-134%]; never responders, 106% [91%-111%]; P = .004), day 7 (always responders, 136% [129%-139%]; transient responders, 123% [109%-141%]; never responders, 95% [89%-115%]; P = .03), and day 8 (always responders, 134% [119%-155%]; transient responders, 129% [109%-147%]; never responders, 100% [85%-112%]; P = .01).
Association of Ex Vivo Supplementation of Antithrombin With Enoxaparin Sensitivity Among Never Responders
We identified 14 never responders (77.8% of 18) with at least 3 plasma samples over time for antithrombin supplementation experiments. Baseline antithrombin values were used to supplement antithrombin ex vivo up to 120%, 150%, and 180%. Compared with baseline, supplementation of antithrombin to 120%, 150%, and 180% was significantly associated with increased anti-FXa levels at all time points; on day 2, for example, the mean (SD) anti-FXa level at supplementation to 120% was 0.24 (0.03) IU/mL compared with a mean (SD) level of 0.16 (0.01) IU/mL at baseline (P = .02); the exception to this finding was supplementation to 120% on day 5 (mean [SD], 0.15 [0.07] IU/mL vs. 0.11 [0.06] IU/mL; P = 0.5) (Figure 3). We observed an overall reduction in this increase at the later time points, with a greater amount of antithrombin (180%) necessary to achieve an anti-FXa level of 0.2 IU/mL and reduce thrombin generation on days 7 and 8. To assess the association of antithrombin supplementation with clot kinetics, we supplemented day 2 plasma samples with 120% antithrombin and measured changes in clot formation by turbidity. We found that 120% antithrombin significantly prolonged time to initiation of clot formation (lag time) and reduced clotting rates (slope) (eFigure 3A and 3B in the Supplement). Supplementing antithrombin into antithrombin-depleted plasma was associated with attenuated overall clot kinetics in a dose-dependent manner; however, no differences in fibrin formation (peak turbidity) were identified even when supplementing to 150% (eFigure 3C in the Supplement). In agreement with this, no anti-FXa level surpassed 0.37 IU/mL in antithrombin supplemented samples.
Figure 3. Association of Ex Vivo Antithrombin Supplementation With Anti–Factor Xa (Anti-FXa) Levels and Peak Thrombin Generation Among Never Responders.
Plasma from patients identified as never responders was supplemented with antithrombin to achieve a final activity of 120%, 150%, or 180%, and associations with anti-FXa levels and thrombin generation compared.
aP < .05 vs baseline.
bP < .01 vs baseline.
cP < .001 vs baseline.
Discussion
This cohort study found that only 16% of severely injured patients consistently achieved a prophylactic anti-FXa level. Additionally, the time to achieve a first anti-FXa level of 0.2 IU/mL or greater was significantly delayed in patients with VTE compared with patients without VTE, and 24% of patients never achieved an anti-FXa level of 0.2 U/mL or greater, despite dose escalations. Further, ongoing acquired antithrombin deficiency was associated with reduced responsiveness to enoxaparin prophylaxis and was independently associated with VTE. Finally, ex vivo studies found that supplementation of antithrombin was associated with improved responsiveness to enoxaparin, especially during the first 3 to 4 days of hospitalization, when most of the VTE occurred at our center. Findings of our study are consistent with past work3,6,16,17,18 demonstrating that severe injury is associated with acquired antithrombin deficiency and that these patients have lower responsiveness to enoxaparin, as measured by anti-FXa level.
Venous thromboembolism prevention in critically injured patients is a challenge because of poor early-risk stratification criteria, chemoprophylaxis contraindications, risks of bleeding, and lack of optimized thromboprophylaxis guidelines. While enoxaparin has been a mainstay for VTE prevention because of its safety and effectiveness, failure to achieve a prophylactic anti-FXa response is exceedingly common and can necessitate repeated dose escalations.11
Our data provide several novel and clinically relevant findings. Patients who did not achieve a prophylactic anti-FXa level were 5 times more likely to develop VTE than always and transient responders, which is consistent with a study by Malinoski et al,19 which found an increased risk of thrombotic complications among patients who failed to achieve the target anti-FXa level. Recent work12,13 similarly found that nearly half of trauma patients never achieved an anti-FXa level of 0.2 IU/mL or greater.12,13 However, in contrast to our findings, those studies12,13 did not find a difference in VTE incidence between groups. Our findings could be explained by our longitudinal approach, in which we measured anti-FXa levels daily as opposed to once after the third enoxaparin dose. This allowed us to isolate never responders from transient responders, which included patients who did not initially achieve a prophylactic anti-FXa level but subsequently did. Such patients had similarly low VTE rates to always responders.
We found that every 10% decrease in antithrombin activity increased VTE risk 1.5-fold. We found that antithrombin activity declined in all patients during the first 2 days after injury before rebounding in the majority of patients. Patients without VTE had a median antithrombin activity level of 123% on days 6 through 8. Conversely, patients with VTE did not exhibit this antithrombin rebound, with a median antithrombin activity from 97% to 99% on days 6 through 8. This suggests that perhaps the classic hematologic definition of antithrombin deficiency, antithrombin activity below 80%, is not appropriate for the critically injured patient population. Our group recently found that an antithrombin threshold of 90% yielded the greatest sensitivity for predicting VTE.3 In an effort to establish the mechanistic basis for reductions in antithrombin activity in the days after injury, we report herein that antithrombin activity was inversely correlated with circulating thrombin-antithrombin complex, in agreement with our past work,3 indicating that antithrombin is consumed secondary to thrombin generation. However, we found no significant differences in thrombin generation over time between patients with and without VTE, perhaps as a result of high variability in patients receiving enoxaparin. Interestingly, we found a stronger inverse correlation between antithrombin activity and plasma neutrophil elastase level. Neutrophil elastase is known to proteolytically degrade antithrombin and has been shown to contribute to antithrombin depletion in other disease conditions.20,21,22 Therefore, therapeutic agents that directly inhibit neutrophil elastase release or attenuate the innate immune response induced by major trauma could ultimately reduce VTE risk by preserving antithrombin activity.
Antithrombin activity was significantly lower among patients who never responded to enoxaparin. Our data agree with those of Droege et al,16 who found that patients with subprophylactic anti-FXa levels had lower antithrombin activity and higher incidence of antithrombin deficiency. Droege et al further randomized patients to receive either enoxaparin dose or frequency adjustments, demonstrating slightly greater efficacy in the dose-adjusted group.16 Although our institution adjusts enoxaparin dosing based on weight and anti-FXa levels, prophylaxis remained suboptimal, and VTEs persisted.
Our data suggest that changes in antithrombin and anti-FXa over the first 4 days are most important to VTE risk. The median (IQR) time to VTE was 4.0 (1.3-8.8) days, the same time point at which we observed antithrombin activity rebound in patients without VTE. Additionally, this time point identified significant differences in enoxaparin responsiveness, as demonstrated by anti-FXa levels, with obvious divergence leading up to day 4.
Our analyses found that ex vivo antithrombin supplementation was associated with the most improvement within the first 3 days of hospitalization. While we observed decreasing effectiveness of antithrombin supplementation on days 5 through 8, our data suggest that patients only need to achieve transient responsiveness to enoxaparin for effective VTE prevention (similar VTE rates in transient responders and always responders). Furthermore, supplementation was not associated with supratherapeutic anti-FXa levels (>0.4 IU/mL), even at 180%, suggesting that in vivo supplementation may be associated with a low risk of bleeding complications.
Limitations
This study had several limitations. This was a single-center study, and, therefore, applicability of our findings needs to be examined in a multicenter study. Relevance of our findings to asymptomatic patients with VTE would need to be examined in other cohorts or on implementing routine VTE screening. In addition, our study population comprised patients at highest risk for VTE and included critically ill patients, and our findings may not be relevant to lower acuity patients. Also, antithrombin supplementation experiments were performed ex vivo, and the relevance of these findings to in vivo use would require an interventional study design.
Conclusions
This cohort study found that time-dependent changes in antithrombin activity over the initial hospitalization period were associated with responsiveness to enoxaparin prophylaxis and subsequent risk of VTE. Ex vivo experiments found that early supplementation of antithrombin may substantially improve the rate of achieving a prophylactic anti-FXa level, thereby mitigating the frequency of VTE in trauma patients.
eTable 1. Enrollment Criteria
eTable 2. Admission Coagulation Parameters Between Patients Who Did and Did Not Develop VTE
eFigure 1. Flow Diagram Depicting Patient Screening, Enrollment, and Outcomes
eFigure 2. Standard Coagulation Tests and Thrombin Generation Over Time Among Trauma Patients Who Do and Do Not Develop VTE
eFigure 3. Effects of ex vivo Antithrombin Supplementation on Clot Formation
References
- 1.Godat LN, Kobayashi L, Chang DC, Coimbra R. Can we ever stop worrying about venous thromboembolism after trauma? J Trauma Acute Care Surg. 2015;78(3):475-480. doi: 10.1097/TA.0000000000000556 [DOI] [PubMed] [Google Scholar]
- 2.Hamada SR, Espina C, Guedj T, et al. High level of venous thromboembolism in critically ill trauma patients despite early and well-driven thromboprophylaxis protocol. Ann Intensive Care. 2017;7(1):97. doi: 10.1186/s13613-017-0315-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahbar E, Cotton BA, Wade CE, Cardenas JC. Acquired antithrombin deficiency is a risk factor for venous thromboembolism after major trauma. Thromb Res. 2021;204:9-12. doi: 10.1016/j.thromres.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake SA, Holcomb JB, Yang Y, et al. Establishing a regional trauma preventable/potentially preventable death rate. Ann Surg. 2020;271(2):375-382. doi: 10.1097/SLA.0000000000002999 [DOI] [PubMed] [Google Scholar]
- 5.Park MS, Spears GM, Bailey KR, et al. Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: A prospective cohort study. J Trauma Acute Care Surg. 2017;83(3):381-387. doi: 10.1097/TA.0000000000001466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas JC, Wang YW, Karri JV, et al. Supplementation with antithrombin III ex vivo optimizes enoxaparin responses in critically injured patients. Thromb Res. 2020;187:131-138. doi: 10.1016/j.thromres.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72(6):1470-1475. doi: 10.1097/TA.0b013e31824d56ad [DOI] [PubMed] [Google Scholar]
- 8.McCully BH, Connelly CR, Fair KA, et al. ; PROPPR Study Group . Onset of coagulation function recovery is delayed in severely injured trauma patients with venous thromboembolism. J Am Coll Surg. 2017;225(1):42-51. doi: 10.1016/j.jamcollsurg.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pommerening MJ, Schwartz DA, Cohen MJ, et al. Hypercoagulability after injury in premenopausal females: a prospective, multicenter study. Surgery. 2014;156(2):439-447. doi: 10.1016/j.surg.2014.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6)(suppl):381S-453S. doi: 10.1378/chest.08-0656 [DOI] [PubMed] [Google Scholar]
- 11.Costantini TW, Min E, Box K, et al. Dose adjusting enoxaparin is necessary to achieve adequate venous thromboembolism prophylaxis in trauma patients. J Trauma Acute Care Surg. 2013;74(1):128-133. doi: 10.1097/TA.0b013e3182788fa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karcutskie CA, Dharmaraja A, Patel J, et al. Relation of antifactor-Xa peak levels and venous thromboembolism after trauma. J Trauma Acute Care Surg. 2017;83(6):1102-1107. doi: 10.1097/TA.0000000000001663 [DOI] [PubMed] [Google Scholar]
- 13.Karcutskie CA, Dharmaraja A, Patel J, et al. Association of anti–factor Xa–guided dosing of enoxaparin with venous thromboembolism after trauma. JAMA Surg. 2018;153(2):144-149. doi: 10.1001/jamasurg.2017.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Equator Network . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. https://www.equator-network.org/reporting-guidelines/strobe/. Accessed February 10, 2022.
- 15.Cardenas JC, Rahbar E, Pommerening MJ, et al. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. J Trauma Acute Care Surg. 2014;77(6):839-845. doi: 10.1097/TA.0000000000000348 [DOI] [PubMed] [Google Scholar]
- 16.Droege ME, Droege CA, Philpott CD, et al. Impact of antithrombin III and enoxaparin dosage adjustment on prophylactic anti-Xa concentrations in trauma patients at high risk for venous thromboembolism: a randomized pilot trial. J Thromb Thrombolysis. 2021;52(4):1117-1128. doi: 10.1007/s11239-021-02478-4 [DOI] [PubMed] [Google Scholar]
- 17.Owings JT, Bagley M, Gosselin R, Romac D, Disbrow E. Effect of critical injury on plasma antithrombin activity: low antithrombin levels are associated with thromboembolic complications. J Trauma. 1996;41(3):396-405. doi: 10.1097/00005373-199609000-00004 [DOI] [PubMed] [Google Scholar]
- 18.Owings JT, Gosselin R. Acquired antithrombin deficiency following severe traumatic injury: rationale for study of antithrombin supplementation. Semin Thromb Hemost. 1997;23(suppl 1):17-24. [PubMed] [Google Scholar]
- 19.Malinoski D, Jafari F, Ewing T, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010;68(4):874-880. doi: 10.1097/TA.0b013e3181d32271 [DOI] [PubMed] [Google Scholar]
- 20.Chung MC, Jorgensen SC, Popova TG, Bailey CL, Popov SG. Neutrophil elastase and syndecan shedding contribute to antithrombin depletion in murine anthrax. FEMS Immunol Med Microbiol. 2008;54(3):309-318. doi: 10.1111/j.1574-695X.2008.00480.x [DOI] [PubMed] [Google Scholar]
- 21.Jordan RE, Kilpatrick J, Nelson RM. Heparin promotes the inactivation of antithrombin by neutrophil elastase. Science. 1987;237(4816):777-779. doi: 10.1126/science.3649921 [DOI] [PubMed] [Google Scholar]
- 22.Jordan RE, Nelson RM, Kilpatrick J, Newgren JO, Esmon PC, Fournel MA. Inactivation of human antithrombin by neutrophil elastase. Kinetics of the heparin-dependent reaction. J Biol Chem. 1989;264(18):10493-10500. doi: 10.1016/S0021-9258(18)81648-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Enrollment Criteria
eTable 2. Admission Coagulation Parameters Between Patients Who Did and Did Not Develop VTE
eFigure 1. Flow Diagram Depicting Patient Screening, Enrollment, and Outcomes
eFigure 2. Standard Coagulation Tests and Thrombin Generation Over Time Among Trauma Patients Who Do and Do Not Develop VTE
eFigure 3. Effects of ex vivo Antithrombin Supplementation on Clot Formation