Key Points
Question
Are long-term outcomes similar after laparoscopic sleeve gastrectomy (LSG) and laparoscopic Roux-en-Y gastric bypass (LRYGB) in patients with severe obesity?
Findings
In this 10-year follow-up of a randomized clinical trial including 240 patients, both LSG and LRYGB resulted in good and sustainable weight loss. The estimated mean percentage excess weight loss (%EWL) was somewhat lower after LSG than LRYGB with no statistically significant difference in remission of type 2 diabetes, dyslipidemia, obstructive sleep apnea, or prevalence of Barrett esophagus; esophagitis was more prevalent after LSG, and hypertension remission was superior after LRYGB.
Meaning
Both procedures resulted in good and sustainable weight loss at 10 years; reflux was more prevalent after LSG, but the cumulative incidence of Barrett esophagus was similar after both procedures and markedly lower than in previous trials.
This follow-up of a randomized clinical trial evaluates long-term weight loss and remission of obesity-related conditions 10 years after laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass.
Abstract
Importance
Long-term results from randomized clinical trials comparing laparoscopic sleeve gastrectomy (LSG) with laparoscopic Roux-en-Y-gastric bypass (LRYGB) are limited.
Objective
To compare long-term outcomes of weight loss and remission of obesity-related comorbidities and the prevalence of gastroesophageal reflux symptoms (GERD), endoscopic esophagitis, and Barrett esophagus (BE) after LSG and LRYGB at 10 years.
Design, Setting, and Participants
This 10-year observational follow-up evaluated patients in the Sleeve vs Bypass (SLEEVEPASS) multicenter equivalence randomized clinical trial comparing LSG and LRYGB in the treatment of severe obesity in which 240 patients aged 18 to 60 years with median body mass index of 44.6 were randomized to LSG (n = 121) or LRYGB (n = 119). The initial trial was conducted from April 2008 to June 2010 in Finland, with last follow-up on January 27, 2021.
Interventions
LSG or LRYGB.
Main Outcomes and Measures
The primary end point was 5-year percentage excess weight loss (%EWL). This current analysis focused on 10-year outcomes with special reference to reflux and BE.
Results
At 10 years, of 240 randomized patients (121 randomized to LSG and 119 to LRYGB; 167 women [69.6%]; mean [SD] age, 48.4 [9.4] years; mean [SD] baseline BMI, 45.9 [6.0]), 2 never underwent surgery and there were 10 unrelated deaths; 193 of the remaining 228 patients (85%) completed follow-up on weight loss and comorbidities, and 176 of 228 (77%) underwent gastroscopy. Median (range) %EWL was 43.5% (2.1%-109.2%) after LSG and 50.7% (1.7%-111.7%) after LRYGB. Mean estimate %EWL was not equivalent between the procedures; %EWL was 8.4 (95% CI, 3.1-13.6) higher in LRYGB. After LSG and LRYGB, there was no statistically significant difference in type 2 diabetes remission (26% and 33%, respectively; P = .63), dyslipidemia (19% and 35%, respectively; P = .23), or obstructive sleep apnea (16% and 31%, respectively; P = .30). Hypertension remission was superior after LRYGB (8% vs 24%; P = .04). Esophagitis was more prevalent after LSG (31% vs 7%; P < .001) with no statistically significant difference in BE (4% vs 4%; P = .29). The overall reoperation rate was 15.7% for LSG and 18.5% for LRYGB (P = .57).
Conclusions and Relevance
At 10 years, %EWL was greater after LRYGB and the procedures were not equivalent for weight loss, but both LSG and LRYGB resulted in good and sustainable weight loss. Esophagitis was more prevalent after LSG, but the cumulative incidence of BE was markedly lower than in previous trials and similar after both procedures.
Trial Registration
ClinicalTrials.gov Identifier: NCT00793143
Introduction
Laparoscopic sleeve gastrectomy (LSG) is the most common bariatric and metabolic surgery procedure, accounting for up to 60% of all bariatric procedures both globally and in the US.1,2 The transition to relying on LSG took place before long-term randomized clinical trial (RCT) results comparing outcomes, safety, and technical ease of LSG with the criterion standard Roux-en-Y-gastric bypass (LRYGB) procedure were available.1,3,4,5 The long-term results of different bariatric surgery techniques are of vital importance regarding increasing obesity rates, with bariatric surgery being the only effective treatment for patients with severe obesity in terms of long-term and substantial weight loss, remission of obesity-related comorbidities, improvement of quality of life (QOL), and longer life expectancy.4,5,6,7,8,9,10,11 Recent studies have also shown a high incidence of worsening of de novo gastroesophageal reflux (GERD), esophagitis, and Barrett esophagus (BE) after LSG.12,13,14,15
In the Sleeve vs Bypass (SLEEVEPASS) trial,5,9 at 5 and 7 years after surgery, both LSG and LRYGB resulted in good weight loss outcomes, similar remission of type 2 diabetes and dyslipidemia, and no difference in quality of life (QOL) or morbidity; LRYGB was superior for hypertension remission. LRYGB resulted in somewhat greater weight loss, but based on prespecified equivalence margins, this difference was not clinically significant. The current article provides 10-year outcomes and, to our knowledge, this is the largest RCT with the longest follow-up comparing LSG and LRYGB. In addition to weight loss and remission of obesity-related comorbidities, this 10-year follow-up also focuses on the controversial issues of long-term GERD symptoms, endoscopic esophagitis, and BE after LSG compared with LRYGB.
Methods
Trial Design, Participants, and Interventions
The study design, rationale, and methods for the SLEEVEPASS trial5,9,16,17 have been previously reported (trial protocol in Supplement 1), with 1 amendment to the study protocol at 10-year follow-up: the addition of upper gastrointestinal endoscopy. The trial protocol was approved by the ethics committees of the 3 participating hospitals (Turku, Helsinki, and Vaasa), and the study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. All patients gave written informed consent.
Briefly, the trial was a multicenter, multisurgeon, open-label randomized clinical equivalence trial conducted from March 2008 to June 2010 in Finland involving 240 patients with severe obesity randomized to undergo either LSG or LRYGB in a 1:1 equal allocation ratio to the study groups. Eligibility criteria included age 18 to 60 years, body mass index (BMI) 40 and higher or 35 and higher with a significant obesity-related comorbidity, and previous failed adequate conservative treatment. Exclusion criteria were BMI higher than 60, serious psychiatric or eating disorder, active alcohol or substance misuse, active gastric ulcer disease, severe GERD with a large hiatal hernia, and previous bariatric surgery. The treating surgeons were all part of the study team and the surgical techniques have been previously reported in detail.5 The objective for the current 10-year follow-up study was to determine the long-term outcomes of weight loss and remission of obesity-related comorbidities after LSG and LRYGB with a special focus on the prevalence of GERD symptoms, esophagitis, and BE.
Long-term Follow-up
The previous predefined follow-up points were 30 days16; 6 months17; 1, 2, 3, and 5 (primary end point assessment) years5; and 7 years.9 The final follow-up date for this 10-year report was January 27, 2021, and the predefined follow-up plan extends up to 15 and 20 years. For patients who could not be reached for follow-up by clinical visit or telephone, a search of electronic hospital records was performed to retrieve information.
Trial Outcomes
The primary end point of the trial was weight loss defined by percentage excess weight loss (%EWL), calculated as (initial weight − follow-up weight) / (initial weight – ideal weight for BMI 25) × 10018 and predefined to be assessed at 5-year follow-up.5 Prespecified secondary outcomes included remission of obesity-related comorbidities, including type 2 diabetes, dyslipidemia, hypertension, obstructive sleep apnea, QOL, overall morbidity, and mortality. Additional post hoc outcomes added in the study protocol to be assessed at 10 years included GERD symptoms assessed by a health-related GERD-QOL questionnaire,19 use of proton pump inhibitors (PPIs), upper gastrointestinal endoscopic findings of esophagitis, and BE. In addition, the prevalence of percentage total weight loss (%TWL) less than 5% was assessed and weight regain was evaluated as percentage of maximum weight lost: (100 × (postnadir weight – nadir weight) / (presurgery weight – nadir weight), which may best associate with most clinical outcomes.20
Prior to randomization, all enrolled patients underwent upper gastrointestinal endoscopy. At the time of study initiation, the relative importance of GERD was not yet recognized and systematic GERD symptom assessment was not performed. At 10-year follow-up, patients provided a retrospective subjective assessment between current and preoperative GERD symptoms. Long-term follow-up endoscopy at 10 years with a separate written informed consent was offered to all patients, enabling comparison with preoperative endoscopic status. All endoscopies were performed by experienced surgeons, as in Finland all digestive surgeon specialists routinely perform all of their own endoscopies. Esophagitis was classified according to the Los Angeles classification21 (grade A: 1 or more mucosal breaks confined to the mucosal folds, each no longer than 5 mm; grade B: at least 1 mucosal break more than 5 mm long confined to the mucosal folds but not continuous between the tops of 2 mucosal folds; grade C: at least 1 mucosal break continuous between the tops of 2 or more mucosal folds but not circumferential; grade D: circumferential mucosal break) and the endoscopic diagnosis was confirmed by histopathology. Endoscopically BE was defined by columnar mucosa extending above the gastroesophageal junction (Z line).22 This finding was photographed and classified according to the Prague C & M criteria23 with endoscopic biopsies taken above the Z line according to the American Society for Gastrointestinal Endoscopy Seattle biopsy protocol: 4-quadrant biopsy sampling at 1- to 2-cm intervals starting from the top of the gastric folds up to the most proximal extent of the suspected BE, along with targeted biopsy sampling from any mucosal abnormality.24 Histopathological confirmation of BE required the presence of columnar intestinal metaplasia with goblet cells according to American Society for Gastrointestinal Endoscopy definition.24,25 Presence of only gastric metaplasia was not defined as BE in contrast to the British Society of Gastroenterology definition.26 The endoscopic findings were compared with preoperative endoscopic examinations and biopsies to confirm de novo BE. If possible, hiatal hernia was evaluated in inversion inspecting the gastroesophageal flap valve using the Hill classification.27
For type 2 diabetes, remission was defined according to the new recommendation and consensus of American Diabetes Association28 (a return of HbA1c to less than 6.5% or 48 mmol/mol that occurs spontaneously or following an intervention and persists for at least 3 months in the absence of usual glucose-lowering pharmacotherapy). Preoperative type 2 diabetes duration was classified and assessed in 3 categories: 0 to 2 years, 2 to 10 years, and more than 10 years. For dyslipidemia, remission was defined by no need for medication and normal lipid values based on European Society of Cardiology/European Atherosclerosis Society guidelines (low-density lipoprotein cholesterol less than 115.8 mg/dL [3.0 mmol/L] and no dyslipidemia medication).29 Hypertension was assessed as persisting (no change in medications compared with baseline), improved (reduction in medications), or remission (no medications). For obstructive sleep apnea, remission was defined by discontinuation of continuous positive airway pressure (CPAP) mask use and improvement (reduction in CPAP settings) or no change in CPAP settings. QOL was evaluated using the Moorehead-Ardelt QOL questionnaire total score.30
All complications between 7 and 10 years were reviewed and added to the previously reported complications at 7 years.9 Complications were classified as major or minor31 and according to Clavien-Dindo classification.32 Causes of death were extracted from the Official Statistics Finland registry on causes of death. Two patients died unexpectedly soon after completing the 10-year follow-up before the final data analyses, and both of these patients were included in the analyses.
Statistical Analysis
The sample size calculations have been reported previously.5 Equivalence of %EWL between LSG and LRYGB at different time points was estimated using a linear mixed model for repeated measures. Model-based estimates with 95% CIs were calculated for difference between operations to be able to evaluate the predefined margins for equivalence (−9 to 9).
Linear mixed models suitable for repeated measures were used to evaluate the differences between operations in BMI, percentage excess BMI loss, %TWL, weight, fasting plasma glucose, glycated hemoglobin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, and Moorehead-Ardelt QOL total score. Logarithmic transformation was used for skewed variables (fasting plasma glucose, glycated hemoglobin, high-density lipoprotein cholesterol, and triglycerides) and for those variables, estimates were transformed back to the original scale. Assumptions for models were checked with studentized residuals. For categorical variables, differences between study groups and other associations between categorical variables were tested using χ2 test or Fisher exact test in case of small frequencies. To compare the total score of GERD-HRQL questionnaire and weight regain between study groups, nonparametric Mann-Whitney U test was used.
The main analyses were performed using modified intention-to-treat population (patients who did not undergo surgery at all were excluded from the analyses). For the primary outcome of %EWL, a per-protocol analysis was also performed by excluding all the patients who had undergone conversion to another bariatric procedure. Missing data were excluded from the analyses. Multiple imputation was used for the primary end point sensitivity analyses. Multivariate imputation by fully conditional specification method was performed. The predictive mean matching method was used to construct 10 imputed data sets and a linear mixed model for repeated measures was fitted for each. Results were combined for the inference and compared with the original analyses. Two-tailed P values less than .05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute) and all figures were drawn with R version 4.0.3 (R Foundation for Statistical Computing) (protocol in Supplement 1; statistical analysis plan in in Supplement 2; eMethods in Supplement 3).
Results
Trial Patients
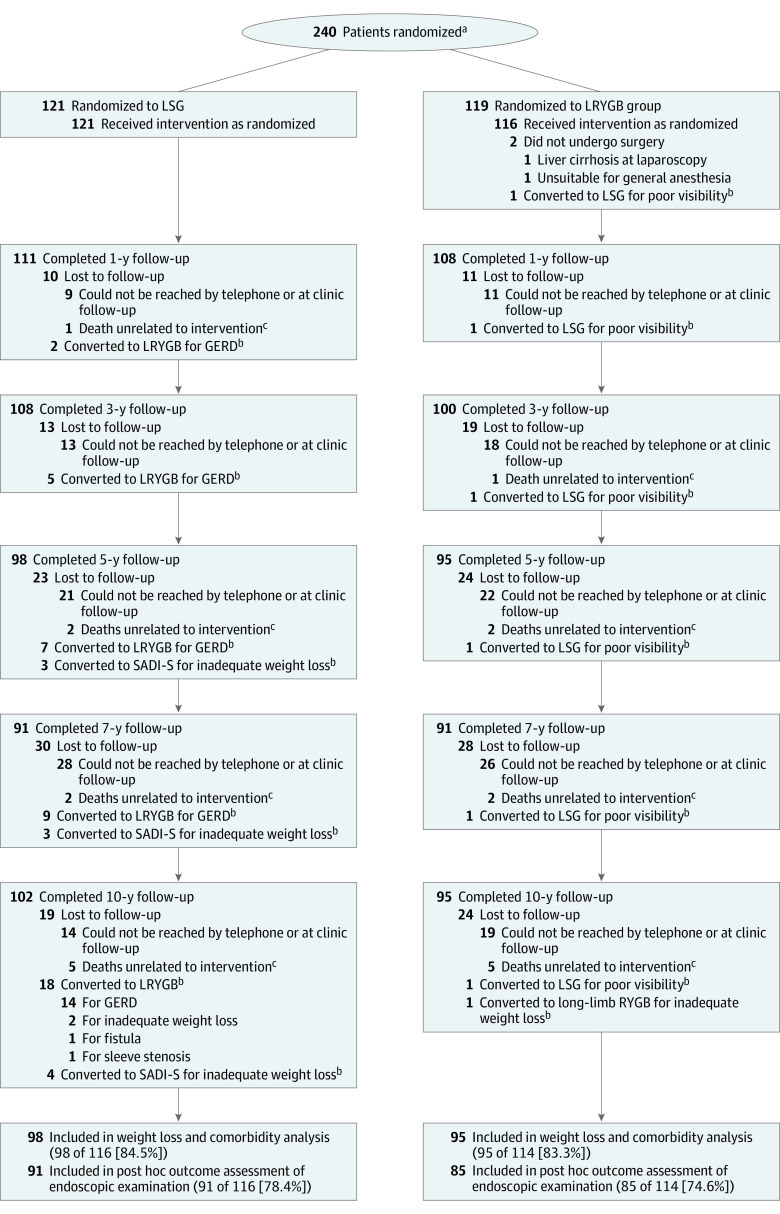
Figure 1 shows the trial profile. Baseline characteristics of the initial 240 trial patients (121 randomized to LSG and 119 to LRYGB; 167 women [69.6%]; mean [SD] age, 48.4 [9.4] years; mean [SD] baseline BMI, 45.9 [6.0]) have been previously reported5 and are presented in eTable 1 in Supplement 3. Two patients in the LRYGB group never underwent surgery, resulting in a total of 238 patients who underwent operations. Altogether, there were 10 deaths unrelated to intervention (5 in each group). Of the 228 available patients, 193 (84.6%) completed the 10-year follow-up on weight loss, remission of comorbidities, QOL, and GERD symptoms, and 176 (77.2%) underwent gastroscopy.
Figure 1. Flow Diagram for the Sleeve vs Bypass (SLEEVEPASS) Trial.
GERD indicates gastroesophageal reflux disease; LSG, laparoscopic sleeve gastrectomy; LRYGB, laparoscopic Roux-en-Y gastric bypass; SADI-S, single anastomosis duodenoileal bypass with sleeve gastrectomy.
aThe number of patients assessed for eligibility was not adequately recorded.
bAnalyzed according to intention-to-treat.
cThe specific causes of death were: 1 traffic incident, 1 drowning, 1 ketoacidosis, 1 pulmonary embolism, 1 uterine cancer, 1 cholangiocarcinoma, 1 lung cancer, 1 pancreatic cancer, and 2 alcohol overdose.
Weight Loss
The %EWL and %TWL trajectories at the predefined time points during the 10-year follow-up are shown in Figure 2. At 10 years, the estimated mean %EWL was 43.5% (95% CI, 39.8-47.2) after LSG and 51.9% (95% CI, 48.1-55.6) after LRYGB. The model-based estimate of mean %EWL was 8.4 percentage points (95% CI, 3.1-13.6) higher after LRYGB. Based on predefined margins of equivalence (−9 to 9), the 2 groups were not equivalent for weight loss as the whole confidence interval was not within the predefined margins. The per-protocol analyses of %EWL were similar; the difference was 10.4 percentage points (95% CI, 5.1-15.8). For patients with type 2 diabetes, the difference in %EWL between the groups was 8.4 percentage points (95% CI, 0.3-16.6). The %EWL results were similar after using multiple imputation for missing values. From baseline to 10 years, %EWL, BMI, percentage excess BMI loss, and %TWL data are reported in detail in Table 1. At 10 years, %TWL less than 5% was present in 5 of 98 patients (5.1%) after LSG and in 3 of 95 (3.2%) after LRYGB (P = .72). Median (range) weight regain, measured as the percentage of maximum weight lost, was 35.0% (0-95.5) after LSG and 24.7% (0-95.5) after LRYGB (P = .16). Weight in kilograms is reported in eTable 2 in Supplement 3.
Figure 2. Percentage Excess Weight Loss (%EWL) and Percentage Total Weight Loss (%TWL) for All Patients and Individual Patients After Laparoscopic Sleeve Gastrectomy (LSG) and Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) From Baseline to 10 years.
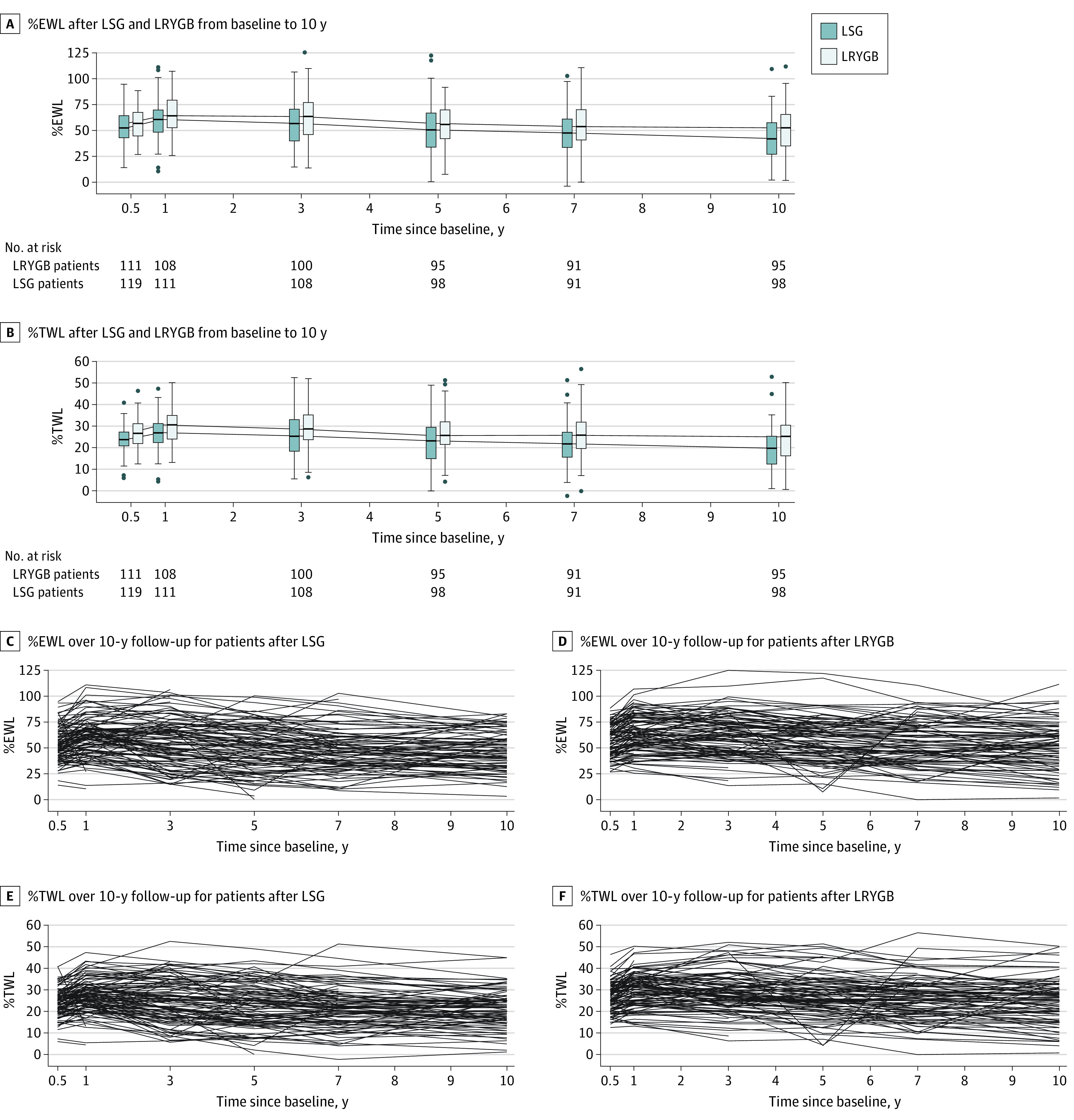
Table 1. Model-Based Estimates of Percentage Excess Weight Loss (EWL), Body Mass Index (BMI), Percentage Excess BMI Loss, and Percentage Total Weight Lossa.
| Time | LSG | LRYGB | LRYGB vs LSG difference (95% CI) | P value |
|---|---|---|---|---|
| %EWL, No.b,c,d | ||||
| Baseline | 121 | 119 | NA | NA |
| 0.5 y | 119 | 111 | 4.7 (−0.4 to 9.7) | NA |
| 1 y | 111 | 108 | 5.7 (0.6 to 10.8) | NA |
| 3 y | 108 | 100 | 8.6 (3.4 to 13.7) | NA |
| 5 y | 98 | 95 | 8.4 (3.1 to 13.7) | NA |
| 7 y | 91 | 91 | 9.0 (3.6 to 14.3) | NA |
| 10 y | 98 | 95 | 8.4 (3.1 to 13.6) | NA |
| BMI, mean estimate (95% CI)c,e,f | ||||
| Baseline | 47.3 (46.2 to 48.4) | 48.4 (47.2 to 49.5) | ||
| 0.5 y | 35.8 (34.7 to 37.0) | 35.3 (34.1 to 36.5) | −0.5 (−2.1 to 1.1) | .54 |
| 1 y | 34.4 (33.3 to 35.6) | 33.6 (32.4 to 34.8) | −0.9 (−2.5 to 0.8) | .30 |
| 3 y | 35.3 (34.2 to 36.5) | 34.0 (32.8 to 35.2) | −1.3 (−2.9 to 0.3) | .12 |
| 5 y | 36.5 (35.4 to 37.7) | 35.4 (34.2 to 36.6) | −1.1 (−2.8 to 0.6) | .19 |
| 7 y | 37.1 (36.0 to 38.3) | 35.8 (34.6 to 37.0) | −1.3 (−3.0 to 0.4) | .13 |
| 10 y | 37.8 (36.6 to 39.0) | 36.5 (35.3 to 37.7) | −1.3 (−3.0 to 0.4) | .13 |
| %EBL, mean estimate (95% CI)c,e,g | 50.8 (48.0 to 53.7) | 58.2 (55.3 to 61.2) | 7.4 (3.4 to 11.5) | <.001 |
| %TWL, mean estimate (95% CI)c,e,h | 23.4 (22.1 to 24.7) | 26.9 (25.6 to 28.2) | 3.5 (1.6 to 5.4) | <.001 |
Abbreviations: BMI, body mass index; %EBL, percentage excess BMI loss; %EWL, percentage excess weight loss; LSG, laparoscopic sleeve gastrectomy; LRYGB, laparoscopic Roux-en-Y gastric bypass; NA, not applicable; %TWL, percentage total weight loss.
All results adjusted for center and diabetes status.
Equivalence design was used in the analyses, and equivalence margins were set from −9 to 9.
Repeated-measurements analysis of variance.
Percentage EWL calculated as (initial weight − follow-up weight) / (initial weight − ideal weight for BMI 25).
Superiority design was used in the analysis.
P < .001 for operation × time interaction.
P = .36 for operation × time interaction; P < .001 for main effect of operation; P < .001 for main effect of time.
P = .49 for operation × time interaction; P < .001 for main effect of operation and P < .001 for main effect of time.
Upper Gastrointestinal Endoscopy and Reflux Symptoms
Esophagitis, BE, GERD symptoms with GERD-HRQL total scores, and PPI intake are reported in detail in Table 2. GERD-HRQL assessment for all dimensions is presented in eTable 3 in Supplement 3. The prevalence of esophagitis was significantly higher after LSG than LRYGB; 31% (28 of 91) vs 7% (6 of 85), respectively (P < .001). De novo BE was found in 4 of 91 patients (4%) after LSG and in 3 of 85 (4%) after LRYGB (P = .29). One patient in the LSG group at retrospective assessment had a very short-segment BE with mild dysplasia already at baseline, and this short-segment BE remained unchanged at 10 years (ie, the finding was not de novo BE), and this patient was excluded from the BE analysis. All de novo BE findings were short-segment with no dysplasia at histopathology.
Table 2. Proton Pump Inhibitor (PPI) Intake, Gastroesophageal Reflux Disease (GERD) Symptoms, GERD–Health-Related Quality of Life (HRQL), and Endoscopic Findings Between Laparoscopic Sleeve Gastrectomy (LSG) vs Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) at 10 Years.
| No./total No. (%) | P value | ||
|---|---|---|---|
| LSG (n = 91) | LRYGB (n = 85) | ||
| All patients who underwent endoscopy | 91/121 (75.2) | 85/119 (71.4) | |
| PPI intake preoperatively | 11/89 (12) | 5/81 (6) | .20a |
| PPI intake at 10 y | 58/90 (64) | 30/84 (36) | <.001a |
| GERD symptoms | |||
| No symptoms preoperatively or at any point | 18/90 (20) | 39/85 (46) | <.001a |
| Symptoms similar to preoperatively | 16/90 (18) | 6/85 (7) | |
| Symptoms alleviated postoperatively | 12/90 (13) | 32/85 (38) | |
| Symptoms worsened postoperatively | 44/90 (49) | 8/85 (9) | |
| GERD-HRQL total score, median (range) | 10.5 (0.0-47.0) | 0.0 (0.0-47.0) | <.001b |
| Hiatal herniac | 57/91 (63) | NA | NA |
| All patients with esophagitis | 28/91 (31) | 6/85 (7) | <.001d |
| Los Angeles classification | |||
| Gradus A | 14/28 (50) | 3/6 (50) | .66a |
| Gradus B | 12/28 (43) | 2/6 (33) | |
| Gradus C | 2/28 (7) | 1/6 (17) | |
| Gradus D | 0/28 (0) | 0/6 (0) | |
| PPI intake preoperatively | 3/28 (11) | 1/5 (20) | .50a |
| PPI intake at 10 y | 16/28 (57) | 2/5 (40) | .64a |
| GERD symptoms | |||
| No symptoms preoperatively or at any point | 6/28 (21) | 3/6 (50) | .02a |
| Symptoms similar to preoperatively | 6/28 (21) | 0/6 (0) | |
| Symptoms alleviated postoperatively | 4/28 (14) | 3/6 (50) | |
| Symptoms worsened postoperatively | 12/28 (43) | 0/0 (0) | |
| GERD-HRQL total score, median (range) | 15.0 (0.0-47.0) | 0.0 (0.0-18.0) | .03b |
| Hiatal herniac | 26/28 (93) | NA | NA |
| All patients with Barrett esophaguse | 4/91 (4) | 3/85 (4) | .29a |
| PPI intake preoperatively | 0/4 (0) | 1/2 (50)f | .33a |
| PPI intake at 10 y | 3/4 (75) | 2/3 (67) | .99a |
| GERD symptoms | |||
| No symptoms preoperatively or at any point | 0/4 (0) | 1/3 (33) | .49a |
| Symptoms similar to preoperatively | 1/4 (25) | 0/3 (0) | |
| Symptoms alleviated postoperatively | 0/4 (0) | 1/3 (33) | |
| Symptoms worsened postoperatively | 3/4 (75) | 1/3 (33) | |
| GERD-HRQL total score, median (range) | 11.0 (3.0-20.0) | 4.5 (0.0-9.0) | .25b |
| Hiatal herniac | 2/4 (50) | NA | NA |
Abbreviation: NA, not applicable.
Fisher exact test.
Nonparametric Mann-Whitney U test.
Descriptive data; hiatal hernias in LRYGB group could not be evaluated reliably owing to problems with inverse.
χ2 Test.
All short-segment de novo Barrett esophagus.
One patient had missing information.
Patients in the LSG group had significantly greater PPI intake (58 of 90 [64%] vs 30 of 84 [36%]; P < .001), higher GERD-HRQL total score (10.5 vs 0.0; P < .001), and more reflux symptoms (Table 2) compared with patients in the LRYGB group at 10 years. Patients with esophagitis after LSG had significantly more de novo GERD symptoms compared with the retrospective subjective assessment of the preoperative status (Table 2) and higher GERD-HRQL total scores (15.0 vs 0.0; P = .03) compared with patients in the LRYGB group presenting with esophagitis.
Remission of Obesity-Related Comorbidities
Type 2 Diabetes
At baseline, 101 patients (42%) had type 2 diabetes (LSG, 52 of 121 [43%]; LRYGB, 49 of 119 [41%]). At 10 years, remission of type 2 diabetes was seen in 11 of 42 patients (26%) after LSG and in 13 of 39 (33%) after LRYGB (P = .63). Type 2 diabetes preoperative duration was statistically significantly associated with remission of type 2 diabetes: 0 to 2 years, 12 of 23 (52%), more than 2 to 10 years, 12 of 48 (25%), and more than 10 years, 0 of 9 (0%) (P = .01). There was no statistically significant difference between the groups across the 10-year follow-up in estimated mean of fasting plasma glucose level value (6.9 mmol/L; 95% CI, 6.6-7.3 in LSG and 6.8 mmol/L; 95% CI, 6.4-7.1 in LRYGB; P = .42) or mean estimated values of glycated hemoglobin at 10 years (6.9%; 95% CI, 6.6-7.2 and 7.0%; 95% CI, 6.7-7.4, respectively; P = .64). The glycemic status, fasting glucose, and glycated hemoglobin of trial patients with type 2 diabetes at all follow-up points are shown in detail in eTable 4 in Supplement 3.
Dyslipidemia
At baseline, 84 patients (35%) had dyslipidemia (LSG, 39 of 121 [32%]; LRYGB, 45 of 119 [38%]). At 10 years, remission of dyslipidemia with normal lipid values and no medication was seen in 4 of 21 patients (19%) after LSG and in 11 of 31 (35%) after LRYGB, (P = .23). All lipid values at all time points are reported in detail in eTable 5 in Supplement 3.
Hypertension
At baseline, 170 patients (70.8%) had medication for hypertension (LSG, 83 of 121 [69%]; LRYGB, 87 of 119 [73%]). At 10 years, 6 of 72 patients (8%) after LSG vs 16 of 68 (24%) after LRYGB had discontinued medication, 23 of 72 (32%) vs 16 of 68 (24%) had reduced antihypertensive medications, and 43 of 72 (60%) vs 36 of 68 (53%) had no change in medication, respectively (P = .04).
Obstructive Sleep Apnea Syndrome
At baseline, 65 patients (27.1%) had obstructive sleep apnea (LSG, 30 of 121 [24.8%]; LRYGB, 35 of 119 [29.4%]). At 10 years, 5 of 31 patients (16%) in the LSG group vs 9 of 29 (31%) in the LRYGB group had discontinued using CPAP, 8 of 31 (26%) vs 4 of 29 (14%) had reduced CPAP settings, and 18 of 31 (58%) vs 16 of 29 (55%) had no change in CPAP settings, respectively (P = .30).
Quality of Life
At baseline, the mean (SD) Moorehead-Ardelt QOL total score was 0.10 (0.94) in the LSG group and 0.12 (1.12) in the LRYGB group. At 10 years, the mean (SD) QOL total score was 0.64 (1.24) after LSG and 0.41 (1.23) after LRYGB, (P = .91 for main effect of operation). The total QOL was significantly better at 10 years (mean estimate 0.49; 95% CI, 0.34-0.63) compared with baseline (mean estimate 0.11; 95% CI, 0.00-0.23) (P = .001).
Morbidity and Mortality
The detailed 30-day, 6-month, 5-year, and 7-year minor and major early (ie, 30 days or less) and late (more than 30 days) complications have been reported previously.5,9,16,17 For this long-term follow-up, all the minor and major complications after LSG and LRYGB from 30 days to 10 years were cumulatively evaluated. The overall minor complication rate (Clavien-Dindo I-IIIa) at 10 years was 34.7% (42 of 121) for LSG and 24.4% (29 of 119) for LRYGB (P = .08). The overall major complication rate (ie, reoperation rate, Clavien-Dindo IIIb) was 15.7% (19 of 121) for LSG and 18.5% (22 of 119) for LRYGB (P = .57). Most of the reoperations in the LSG group (14 of 19) were because of GERD, and most of the reoperations in the LRYGB group (18 of 22) were because of internal herniation. The minor and major complications are presented in detail in Table 3. There were 12 deaths altogether (7 in LSG and 5 in LRYGB), all unrelated to the intervention, and 10 deaths occurred before the 10-year follow-up.
Table 3. Minor and Major Late Complications After Laparoscopic Sleeve Gastrectomy (LSG) and Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) Reported Cumulatively After 30 Days to 10 Years of Follow-up.
| No. (%) | P value | ||
|---|---|---|---|
| LSG (n = 121) | LRYGB (n = 119) | ||
| Minor complications | |||
| Vomiting/dehydration | 0 | 3 (2.5) | NA |
| Gastroesophageal reflux | 38 (31.4) | 8 (6.7) | NA |
| Ulcer/stricture at gastrojejunal anastomosis | 2 (1.7) | 8 (6.7) | NA |
| Dumping | 1 (0.8)a | 3 (2.5) | NA |
| Fistula and abscess | 1 (0.8)b | 0 (0.0) | NA |
| Ureterolithiasis | 0 | 1 (0.8) | NA |
| Adhesion-related intestinal obstruction | 0 | 1 (0.8) | NA |
| Ventral hernia | 0 | 1 (0.8) | NA |
| Suspected internal herniation | 0 | 1 (0.8) | NA |
| Nonspecific abdominal pain | 0 | 1 (0.8) | NA |
| Anemia | 0 | 1 (0.8) | NA |
| Hypokalemia | 0 | 1 (0.8) | NA |
| Total | 42 (34.7) | 29 (24.4) | .08c |
| Major complications | |||
| Fistulectomia | 1 (0.8)b | 0 (0.0) | NA |
| Gastroesophageal reflux | 14 (11.6)a | 0 (0.0) | NA |
| Internal herniation | 0 | 18 (15.1)d | NA |
| Incisional hernia | 3 (2.5) | 3 (2.5)d | NA |
| Candy cane/blind loop resection | 0 | 1 (0.8) | NA |
| Abdominal pain and stricture | 0 | 1 (0.8) | NA |
| Sleeve stenosis | 1 (0.8) | 0 (0.0) | NA |
| Total | 19 (15.7) | 22 (18.5)d | .57c |
Abbreviation: GERD, gastroesophageal reflux disease.
One patient converted from sleeve to bypass for GERD at 6 years and later at 10 years experienced dumping as a complication from bypass.
Conversion from sleeve to bypass for fistula and abscess, and later fistulectomia.
P values calculated with χ2 test.
One patient underwent laparotomy 1 year after gastric bypass and later at 9 years incisional hernia, calculated only once in total count of major complications.
Discussion
The results of this 10-year follow-up analysis of the SLEEVEPASS RCT5,9,16,17 showed that both LSG and LRYGB resulted in significant and sustained long-term weight loss. The procedures did not meet the %EWL criteria for equivalence, and LRYGB was associated with greater weight loss at 10 years, similar to the 5-year5 and 7-year9 follow-up results. However, based on the study design and the prespecified equivalence margins, the superiority of LRYGB could not be shown. The prevalence of de novo BE was similar after LSG (4%) and LRYGB (4%) and significantly lower than in earlier studies reporting alarming rates of BE up to 17% after LSG.14,15 Esophagitis, reflux symptoms, and PPI use were significantly more prevalent after LSG compared with LRYGB. There were no statistically significant differences in either long-term complication rates or remission of type 2 diabetes, dyslipidemia, or obstructive sleep apnea between the procedures, but hypertension remission was superior after LRYGB.
The weight-loss trajectories between LSG and LRYGB in the current trial have been consistent throughout all time points. In the merged 5-year data11 of the 2 largest RCTs (SLEEVEPASS5 and the Swiss Multicenter Bypass or Sleeve Study [SM-BOSS] trial4), LRYGB induced greater weight loss evaluated by percentage excess BMI loss in contrast to both separate trials showing no difference in weight loss. This finding of superior weight loss is concurrent with the results of a recent large cohort study showing 6.2% to 8.1% greater %TWL after LRYGB than LSG.33 This difference in outcomes between the merged data and the initial trials4,5 was also true for late complication rate, which was higher after LRYGB in the merged data11 assessed by the comprehensive complication index taking into account the burden of all complications using the Clavien-Dindo classification.32 This variability of all bariatric surgery outcomes based on the used definitions highlights the importance of uniform definitions and standardized reporting of metabolic surgery outcomes enabling comparison between trials resulting in improved patient care.18,34
With increasing obesity rates and the popularity of LSG as the most common metabolic surgery procedure, the previously reported high cumulative incidence of BE ranging between 14%14 and 17%15 after LSG could have a major impact on both the associated risk of esophageal adenocarcinoma and the need for continuous endoscopic surveillance. To our knowledge, this report represents the first available long-term RCT data comparing LSG and LRYGB on the cumulative incidence of de novo BE and esophagitis. Cumulative BE incidence was significantly lower than previously reported, and a not statistically significant difference in the prevalence of BE was found between the 2 most common metabolic surgery procedures. This is in concurrence with the outcomes of a recent prospective cohort at 10.5-year follow-up with BE incidence of 4%.13 In addition, a large retrospective bariatric surgery registry trial with long-term follow-up data showed no difference in the incidence of postoperative BE or esophageal adenocarcinoma between LSG and LRYGB, but in concurrence with the results of the current study, the risk of GERD symptoms and esophagitis was higher after LSG compared with LRYGB.12 The discrepancies in BE rates between the studies may be largely attributed to the varying definitions of BE regarding intestinal and gastric metaplasia22 and also the potential variability of endoscopic assessment of BE. However, the significantly higher rate of endoscopic esophagitis, GERD symptoms, and PPI use after LSG compared with LRYGB underline the importance of systematic preoperative assessment of GERD and the associated endoscopic findings. For patients with clinical GERD, LSG may not be the optimal procedure of choice.
A recent large meta-analysis of matched cohort and prospective controlled studies concluded that metabolic surgery was associated with substantially lower all-cause mortality rates and longer life expectancy. These survival benefits were much more pronounced for patients with preoperative type 2 diabetes35 further underlining the need to detect differences between the 2 most common bariatric surgical procedures in type 2 diabetes remission and the related cardiovascular and end-organ complications. At short-term follow-up of the Oseberg trial,36 LRYGB was superior to LSG in type 2 diabetes remission and a large secondary analysis37 of a matched cohort concluded that LRYGB may be associated with better diabetes control. Most RCTs,4,5,10,38,39 even with merged data,11 are thus far underpowered to detect clinically significant differences. International scientific collaboration using individual patient data meta-analysis is needed. To detect a 10–percentage point difference in type 2 diabetes remission rate between the operations, approximately 700 patients with type 2 diabetes would need to be enrolled. As observed in multiple trials,10,11,40,41 longer preoperative type 2 diabetes duration was associated with lower remission rates, emphasizing the importance of early surgical treatment of patients with obesity and type 2 diabetes.
Limitations
The limitations of our study have been stated earlier. Briefly, there was a potential learning curve effect in both groups owing to the small number of bariatric procedures performed in Finland during trial enrollment. There was a lack of standardized preoperative GERD symptom assessment even though patients with severe GERD and large hiatal hernia were excluded. RCTs are always somewhat limited based on the original study design, especially in setting the minimal clinically important difference, as this sometimes has to be set arbitrarily owing to the lack of available clinical information at study planning and initiation. The multicenter and multisurgeon design of this RCT and the high follow-up rate at 10 years can be considered strengths of the study. Systematic baseline endoscopy and the high endoscopic follow-up rate and the availability of the preoperative endoscopic findings enabling the assessment of de novo esophagitis and BE are additional strong elements of the study.
Conclusions
At 10 years, %EWL was greater after LRYGB compared with LSG, and the procedures were not equivalent for weight loss. The cumulative incidence of BE was markedly lower than in previous trials and similar after both procedures, but endoscopic esophagitis, GERD symptoms, and PPI use were more prevalent after LSG, underlining the importance of preoperative GERD assessment and patient selection. There was no statistically significant difference in type 2 diabetes, dyslipidemia, and obstructive sleep apnea, but LRYGB resulted in superior remission of hypertension.
Trial protocol
Statistical analysis plan
eMethods. Additional detailed statistical methods outside article statistical methods and statistical analysis plan
eTable 1. Baseline characteristics
eTable 2. Weight after LSG an LRYGB from baseline to 10 years of follow-up
eTable 3. Detailed GERD-HRQL comparison between LSG and LRYGB at 10 years (No./total (%))
eTable 4. Improvement in glycemic control in patients with diabetes after LSG and LRYGB from baseline to 10 years of follow-up
eTable 5. Lipid profiles for the whole study group after LSG and LRYGB from baseline to 10 years of follow-up
Data sharing statement
References
- 1.Angrisani L, Santonicola A, Iovino P, et al. IFSO Worldwide Survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783-3794. doi: 10.1007/s11695-018-3450-2 [DOI] [PubMed] [Google Scholar]
- 2.Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg. 2020;271(2):201-209. doi: 10.1097/SLA.0000000000003554 [DOI] [PubMed] [Google Scholar]
- 3.Howard R, Chao GF, Yang J, et al. Comparative safety of sleeve gastrectomy and gastric bypass up to 5 years after surgery in patients with severe obesity. JAMA Surg. 2021;156(12):1160-1169. doi: 10.1001/jamasurg.2021.4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255-265. doi: 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen P, Helmiö M, Ovaska J, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241-254. doi: 10.1001/jama.2017.20313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams TD, Davidson LE, Hunt SC. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2018;378(1):93-96. doi: 10.1056/NEJMc1714001 [DOI] [PubMed] [Google Scholar]
- 7.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753-761. doi: 10.1056/NEJMoa066603 [DOI] [PubMed] [Google Scholar]
- 8.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313(1):62-70. doi: 10.1001/jama.2014.16968 [DOI] [PubMed] [Google Scholar]
- 9.Grönroos S, Helmiö M, Juuti A, et al. Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: the SLEEVEPASS randomized clinical trial. JAMA Surg. 2021;156(2):137-146. doi: 10.1001/jamasurg.2020.5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wölnerhanssen BK, Peterli R, Hurme S, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: 5-year outcomes of merged data from two randomized clinical trials (SLEEVEPASS and SM-BOSS). Br J Surg. 2021;108(1):49-57. doi: 10.1093/bjs/znaa011 [DOI] [PubMed] [Google Scholar]
- 12.Bevilacqua LA, Obeid NR, Yang J, et al. Incidence of GERD, esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma after bariatric surgery. Surg Obes Relat Dis. 2020;16(11):1828-1836. doi: 10.1016/j.soard.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 13.Csendes A, Orellana O, Martínez G, Burgos AM, Figueroa M, Lanzarini E. Clinical, endoscopic, and histologic findings at the distal esophagus and stomach before and late (10.5 years) after laparoscopic sleeve gastrectomy: results of a prospective study with 93% follow-up. Obes Surg. 2019;29(12):3809-3817. doi: 10.1007/s11695-019-04054-5 [DOI] [PubMed] [Google Scholar]
- 14.Felsenreich DM, Ladinig LM, Beckerhinn P, et al. Update: 10 years of sleeve gastrectomy-the first 103 patients. Obes Surg. 2018;28(11):3586-3594. doi: 10.1007/s11695-018-3399-1 [DOI] [PubMed] [Google Scholar]
- 15.Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568-574. doi: 10.1016/j.soard.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 16.Helmiö M, Victorzon M, Ovaska J, et al. SLEEVEPASS: a randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26(9):2521-2526. doi: 10.1007/s00464-012-2225-4 [DOI] [PubMed] [Google Scholar]
- 17.Helmiö M, Victorzon M, Ovaska J, et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: a prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg. 2014;103(3):175-181. doi: 10.1177/1457496913509984 [DOI] [PubMed] [Google Scholar]
- 18.Brethauer SA, Kim J, El Chaar M, et al. ; ASMBS Clinical Issues Committee . Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg. 2015;25(4):587-606. doi: 10.1007/s11695-015-1645-3 [DOI] [PubMed] [Google Scholar]
- 19.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130-134. doi: 10.1111/j.1442-2050.2007.00658.x [DOI] [PubMed] [Google Scholar]
- 20.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA. 2018;320(15):1560-1569. doi: 10.1001/jama.2018.14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111(1):85-92. doi: 10.1053/gast.1996.v111.pm8698230 [DOI] [PubMed] [Google Scholar]
- 22.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371(9):836-845. doi: 10.1056/NEJMra1314704 [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131(5):1392-1399. doi: 10.1053/j.gastro.2006.08.032 [DOI] [PubMed] [Google Scholar]
- 24.Qumseya B, Sultan S, Bain P, et al. ; ASGE STANDARDS OF PRACTICE COMMITTEE; ASGE Standards of Practice Committee Chair . ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2. doi: 10.1016/j.gie.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 25.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ; American Gastroenterological Association . American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140(3):e18-e52. doi: 10.1053/j.gastro.2011.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald RC, di Pietro M, Ragunath K, et al. ; British Society of Gastroenterology . British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63(1):7-42. doi: 10.1136/gutjnl-2013-305372 [DOI] [PubMed] [Google Scholar]
- 27.Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc. 1996;44(5):541-547. doi: 10.1016/S0016-5107(96)70006-8 [DOI] [PubMed] [Google Scholar]
- 28.Riddle MC, Cefalu WT, Evans PH, et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care. 2021;44(10):2438–2444. doi: 10.2337/dci21-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catapano AL, Reiner Z, De Backer G, et al. ; European Society of Cardiology (ESC); European Atherosclerosis Society (EAS) . ESC/EAS Guidelines for the management of dyslipidaemias the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011;217(1):3-46. doi: 10.1016/j.atherosclerosis.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 30.Oria HE, Moorehead MK. Bariatric analysis and reporting outcome system (BAROS). Obes Surg. 1998;8(5):487-499. doi: 10.1381/096089298765554043 [DOI] [PubMed] [Google Scholar]
- 31.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383-393. doi: 10.1016/S0016-5107(91)70740-2 [DOI] [PubMed] [Google Scholar]
- 32.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McTigue KM, Wellman R, Nauman E, et al. ; PCORnet Bariatric Study Collaborative . Comparing the 5-Year diabetes outcomes of sleeve gastrectomy and gastric bypass: the National Patient-Centered Clinical Research Network (PCORNet) bariatric study. JAMA Surg. 2020;155(5):e200087. doi: 10.1001/jamasurg.2020.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burger PM, Monpellier VM, Deden LN, et al. Standardized reporting of co-morbidity outcome after bariatric surgery: low compliance with the ASMBS outcome reporting standards despite ease of use. Surg Obes Relat Dis. 2020;16(11):1673-1682. doi: 10.1016/j.soard.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 35.Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397(10287):1830-1841. doi: 10.1016/S0140-6736(21)00591-2 [DOI] [PubMed] [Google Scholar]
- 36.Hofsø D, Fatima F, Borgeraas H, et al. Gastric bypass versus sleeve gastrectomy in patients with type 2 diabetes (Oseberg): a single-centre, triple-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(12):912-924. doi: 10.1016/S2213-8587(19)30344-4 [DOI] [PubMed] [Google Scholar]
- 37.Aminian A, Wilson R, Zajichek A, et al. Cardiovascular outcomes in patients with type 2 diabetes and obesity: comparison of gastric bypass, sleeve gastrectomy, and usual care. Diabetes Care. 2021;44(11):2552-2563. doi: 10.2337/dc20-3023 [DOI] [PubMed] [Google Scholar]
- 38.Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914-1918. doi: 10.1007/s00125-013-2965-2 [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Wang C, Cao G, et al. Long-term effects of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m(2). BMC Surg. 2015;15:88. doi: 10.1186/s12893-015-0074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aminian A, Vidal J, Salminen P, et al. Late relapse of diabetes after bariatric surgery: not rare, but not a failure. Diabetes Care. 2020;43(3):534-540. doi: 10.2337/dc19-1057 [DOI] [PubMed] [Google Scholar]
- 41.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297-2304. doi: 10.1001/jama.2014.5988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods. Additional detailed statistical methods outside article statistical methods and statistical analysis plan
eTable 1. Baseline characteristics
eTable 2. Weight after LSG an LRYGB from baseline to 10 years of follow-up
eTable 3. Detailed GERD-HRQL comparison between LSG and LRYGB at 10 years (No./total (%))
eTable 4. Improvement in glycemic control in patients with diabetes after LSG and LRYGB from baseline to 10 years of follow-up
eTable 5. Lipid profiles for the whole study group after LSG and LRYGB from baseline to 10 years of follow-up
Data sharing statement