This meta-analysis compares the efficacy and acceptability of different noninvasive brain stimulation interventions for treating negative symptoms.
Key Points
Question
Which noninvasive brain stimulation (NIBS) is associated with the best efficacy and acceptability in reducing negative symptoms in schizophrenia?
Findings
In this systematic review and network meta-analysis, the excitatory NIBS strategies (ie, high-definition transcranial random noise stimulation, intermittent theta-burst stimulation, anodal transcranial direct current stimulation, high-frequency repetitive transcranial magnetic stimulation, and extreme high-frequency repetitive transcranial magnetic stimulation) over the left dorsolateral prefrontal cortex with/without other inhibitory stimulation in the contralateral brain regions were associated with significantly larger reductions in negative symptoms than sham control. Acceptability did not significantly differ between groups.
Meaning
Excitatory NIBS over the left dorsolateral prefrontal cortex was associated with significantly large improvements in negative symptoms.
Abstract
Importance
Negative symptoms have a detrimental impact on functional outcomes and quality of life in people with schizophrenia, and few therapeutic options are considered effective for this symptomatic dimension. Studies have suggested that noninvasive brain stimulation (NIBS) interventions may be effective in treating negative symptoms. However, the comparative efficacy of different NIBS protocols for relieving negative symptoms remains unclear.
Objective
To compare the efficacy and acceptability of different NIBS interventions for treating negative symptoms.
Data Sources
The ClinicalKey, Cochrane CENTRAL, Embase, ProQuest, PubMed, ScienceDirect, ClinicalTrials.gov, and Web of Science electronic databases were systematically searched from inception through December 7, 2021.
Study Selection
A frequentist model network meta-analysis was conducted to assess the pooled findings of trials that evaluated the efficacy of repetitive transcranial magnetic stimulation (rTMS), theta-burst stimulation, transcranial random noise stimulation, transcutaneous vagus nerve stimulation, and transcranial direct current stimulation on negative symptoms in schizophrenia. Randomized clinical trials (RCTs) examining NIBS interventions for participants with schizophrenia were included.
Data Extraction and Synthesis
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was followed. Data were independently extracted by multiple observers. The pair-wise meta-analytic procedures were conducted using a random-effects model.
Main Outcomes and Measures
The coprimary outcomes were changes in the severity of negative symptoms and acceptability (ie, dropout rates owing to any reason). Secondary outcomes were changes in positive and depressive symptoms.
Results
Forty-eight RCTs involving 2211 participants (mean [range] age, 38.7 [24.0-57.0] years; mean [range] proportion of female patients, 30.6% [0%-70.0%]) were included. Compared with sham control interventions, excitatory NIBS strategies (standardized mean difference [SMD]: high-definition transcranial random noise stimulation, −2.19 [95% CI, −3.36 to −1.02]; intermittent theta-burst stimulation, −1.32 [95% CI, −1.88 to −0.76]; anodal transcranial direct current stimulation, −1.28 [95% CI, −2.55 to −0.02]; high-frequency rTMS, −0.43 [95% CI, −0.68 to −0.18]; extreme high-frequency rTMS, −0.45 [95% CI, −0.79 to −0.12]) over the left dorsolateral prefrontal cortex with or without other inhibitory stimulation protocols in the contralateral regions of the brain were associated with significantly larger reductions in negative symptoms. Acceptability did not significantly differ between the groups.
Conclusions and Relevance
In this network meta-analysis, excitatory NIBS protocols over the left dorsolateral prefrontal cortex were associated with significantly large improvements in the severity of negative symptoms. Because relatively few studies were available for inclusion, additional well-designed, large-scale RCTs are warranted.
Introduction
In the 2019 Global Burden of Disease Study, the number of schizophrenia cases was reported to have increased from 14.2 million in 1990 to 23.6 million in 2019. Furthermore, schizophrenia has contributed to 12.2% disability-adjusted life-years globally.1 The main symptomatic dimensions of schizophrenia included positive, cognitive, and negative symptoms.2 Although antipsychotic drugs are relatively effective in mitigating positive symptoms of schizophrenia, their efficacy is limited for the treatment of the negative symptoms of the illness.2 Evidence indicates that the negative symptoms play a key detrimental role in overall disability and quality of life of people with schizophrenia.2
Negative symptoms are believed to have a greater negative impact on patients’ quality of life and social function than positive symptoms.3 Regardless of the potentially beneficial effect on negative symptoms by oral antipsychotics, those regimens were frequently associated with poor acceptability and safety profile (ie, high all-cause discontinuation and overall adverse events).4 Noninvasive brain stimulation (NIBS), such as transcranial direct current stimulation (tDCS) or repetitive transcranial magnetic stimulation (rTMS), exerts various effects on the brain. Different stimulation protocols appear to have distinct effects on cortical activity. For example, high-frequency rTMS (hf-rTMS), extreme hf-rTMS, and intermittent theta-burst stimulation (iTBS) increase cortical excitability, whereas low-frequency rTMS and continuous theta-burst stimulation decrease it.5,6 Studies have suggested that NIBS helps manage neuropsychiatric diseases7,8,9,10,11 or modulate cognitive function.12 Although several meta-analyses have addressed the benefits of NIBS on positive symptoms of schizophrenia,13,14 no conclusive evidence of their efficacy on negative symptoms has been obtained.
According to previous functional magnetic resonance imaging studies, patients with schizophrenia present abnormal functional connectivity between dorsolateral prefrontal cortex (DLPFC) and midbrain region,15 which is referred to as the mesocortical pathway. A dysfunctional mesocortical pathway is known to be related to the presence of negative symptoms.16 Therefore, treatment-induced changes in connectivity in these regions could theoretically improve negative symptoms in schizophrenia. Randomized clinical trials (RCTs) examining the efficacy of NIBS for treatment of negative symptoms have provided inconsistent findings. A 2020 RCT demonstrated that application of anodal tDCS (a-tDCS) over left DLPFC (F3 region) resulted in greater improvements in negative symptoms than sham control.17 Similar findings were observed in an RCT focusing on hf-rTMS (10 Hz) and demonstrated a reduction in negative symptoms in schizophrenia.18 However, another RCT of low-frequency (1 Hz) deep rTMS failed to demonstrate any beneficial effects.19 Several pairwise meta-analyses of different NIBS for relieving negative symptoms in schizophrenia have revealed that only tDCS20,21 elicited a substantial decrease in these symptoms. However, other meta-analyses have challenged these findings.22,23,24 Although some studies have produced valuable findings, several factors must still be considered. First, the RCTs have used a wide variety of study designs. Second, conclusive evidence for the efficacy of various NIBS modalities, such as low-frequency rTMS, hf-rTMS, tDCS, high-definition tDCS (hd-tDCS), transcranial random noise stimulation (tRNS), transcutaneous vagus nerve stimulation, and iTBS, are lacking. Finally, direct comparisons between interventions are required to evaluate their relative efficacy. Because of these major gaps in the literature, further research is necessary.
A network meta-analysis of RCTs may help to evaluate the comparative efficacy and acceptability of different NIBS to manage negative symptoms of schizophrenia. Furthermore, it may clarify the relative merits of multiple interventions that the aforementioned pairwise meta-analyses were unable to address.25,26 To our knowledge, no network meta-analysis has been conducted on this topic. Therefore, we conducted this systematic review and network meta-analysis to compare the efficacy and acceptability of various NIBS protocols in negative symptoms management of schizophrenia.
Methods
This detailed description of this systematic review and network meta-analysis is available in the eMethods in the Supplement. We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (eTable 1 in the Supplement)27 and the flowcharts in accordance with the procedures of other network meta-analyses (eTable 2 in the Supplement).7,8,10,12,28,29,30,31 After registration in PROSPERO (CRD42022296839), 2 independent authors (P.-T.T. and B.-S.Z.) searched and screened ClinicalKey, Cochrane CENTRAL, Embase, ProQuest, PubMed, ScienceDirect, Web of Science, and ClinicalTrials.gov to find RCTs comparing the effects of different NIBS methods on the severity of negative symptoms among participants with schizophrenia. Two independent authors (P.-T.T. and C.-M.-H.) evaluated risk of bias according to Cochrane risk of bias tool32 and extracted data of coprimary outcomes: (1) changes of negative symptoms after NIBS and (2) acceptability (ie, dropout). The dropout rate was defined when patients left the study for any reason before the study’s completion. We summarized the measurement with the standardized mean differences (SMDs) with 95% CIs for continuous outcome and odds ratios and 95% CIs for categorical data. The inclusion criteria included (1) RCTs, (2) application of NIBS interventions, (3) participants with schizophrenia, and (4) studies comparing the efficacy of different NIBS strategies to manage participants’ negative symptoms. The diagnosis of schizophrenia could be based on a well-established criterion or by physicians’ clinical diagnosis. To include more comprehensive studies, we did not set limitations to patients with predominant negative symptoms. The nomenclature (ie, node definition) of the NIBS was based on our previous 6 network meta-analyses on NIBS approaches for other conditions based on 10 to 20 electroencephalography mapping.7,8,9,10,12,33 The network meta-analysis used mvmeta command in Stata version 16.0 (StataCorp).34 All pairwise meta-analyses and network meta-analysis procedures were conducted using random-effects and frequentist models, respectively. This study used a mixed comparison with generalized linear mixed models to analyze direct and indirect comparisons in the network meta-analysis.35 This study calculated the surface under the cumulative ranking curve (SUCRA) to rank the probabilities of the effects of all treatments on the target outcomes.36 Heterogeneity among the included studies was evaluated using the tau value, whereas inconsistencies were evaluated by using the loop-specific approach, node-splitting, and design-by-treatment model.37 We followed Cochrane Handbook for GRADE (Grading of Recommendations, Assessment, Development and Evaluations) ratings in the BMJ38 and 1 important network meta-analysis in the Lancet39 for quality assessment. We used funnel plots and Egger regression to evaluate potential publication bias. Finally, we assessed the reported efficacy of the different sham interventions (ie, changes in negative symptom severity) or waiting list controls to justify our assumption of transitivity. Furthermore, we conducted subgroup analyses focusing on RCTs with definite diagnostic criteria for schizophrenia.
Results
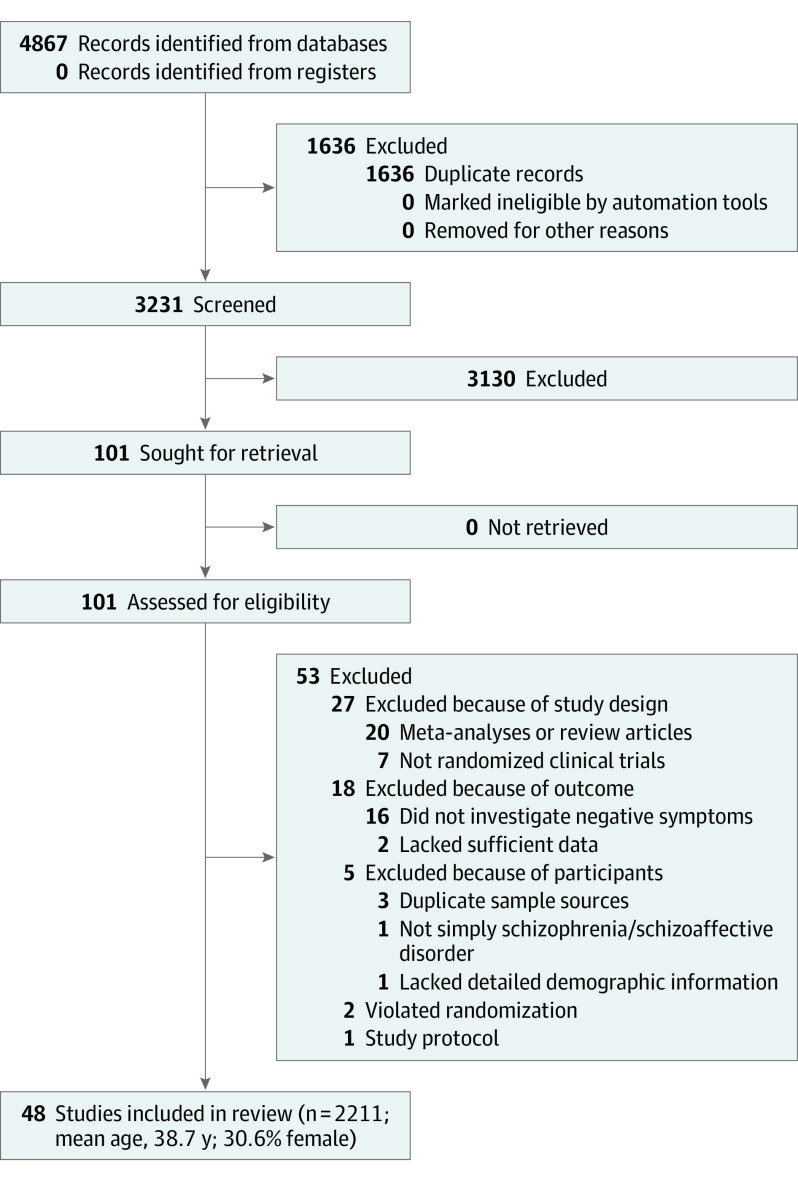
A total of 101 articles were considered for a full-text review (Figure 1). Fifty-three articles were excluded for various reasons (Figure 1 and eTable 3 in the Supplement). Finally, 48 RCTs were included (eTable 4 in the Supplement).15,17,19,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83
Figure 1. Network Meta-analysis Flowchart.
Characteristics of the Included Studies
A total of 2211 participants were included. The mean (range) age was 38.7 (24.0-57.0) years, and the mean (range) proportion of female participants was 30.6% (0%-70.0%). The mean (range) treatment duration was 2.8 (1-12) weeks. The mean (range) overall study duration (ie, treatment + follow-up duration) was 9 (1-32) weeks. The baseline characteristics of the included participants are listed in eTable 4 in the Supplement. All the included RCTs allowed concurrent treatment with antipsychotics during the study period.
Among the 48 RCTs, 37 recruited patients with schizophrenia and 11 recruited patients with schizophrenia or schizoaffective disorder were analyzed. Only 1 RCT84 did not clearly mention the diagnostic criteria, whereas the others applied codes from the DSM-IV, DSM-IV-TR, and DSM-5, Chinese Classification of Mental Disorders, International Classification of Diseases, 9th Revision, and Clinical Modification, International Classification of Diseases, 10th Revision, Clinical Modification as the criteria.
Coprimary Outcomes
Changes in Negative Symptom Severity
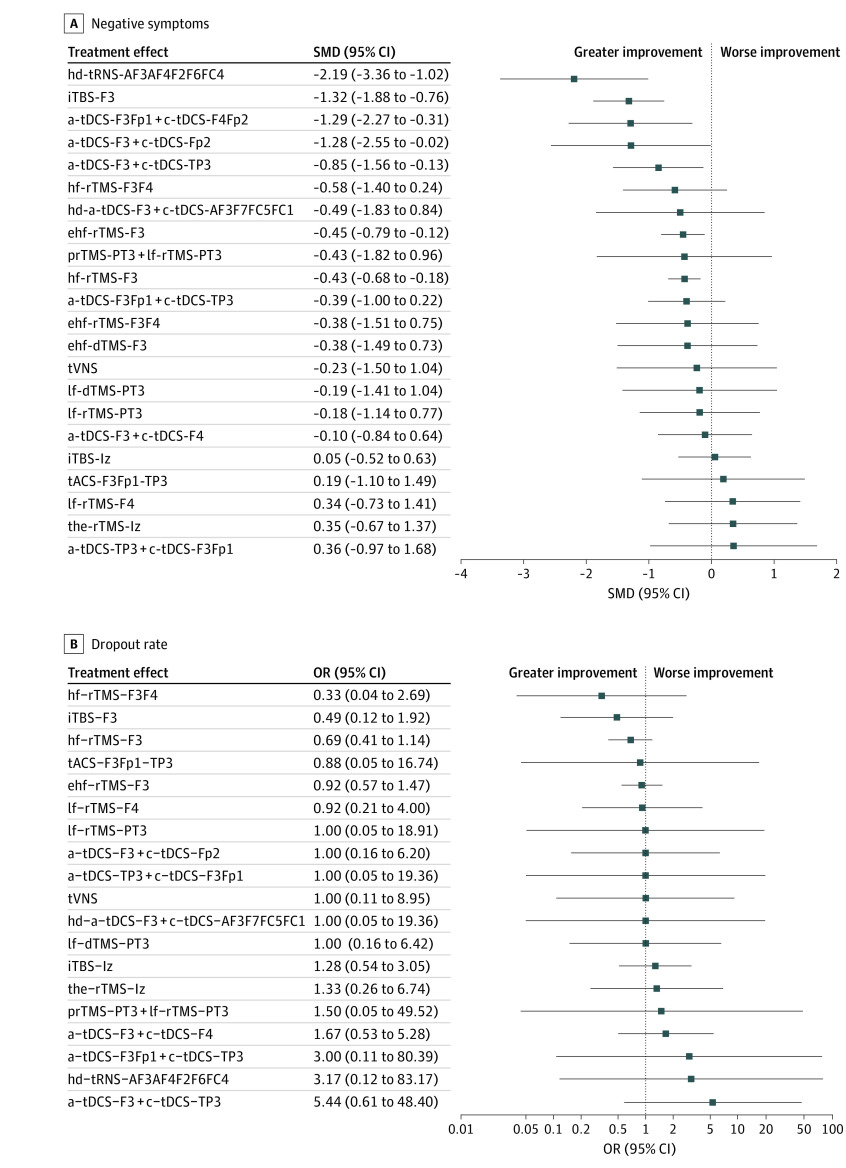
The following NIBS were associated with significantly greater improvements in negative symptom severity than sham control: hd 2-mA anode tRNS at AF3 plus a cathode at AF4, F2, F6, and FC4 (hd-tRNS-AF3AF4F2F6FC4; SMD = −2.19 [95% CI, −3.36 to −1.02]; iTBS at left DLPFC: iTBS-F3: SMD = −1.32 [95% CI, −1.88 to −0.76]), 2-mA anode tDCS at F3Fp1 plus a cathode at F4Fp2 (a-tDCS-F3Fp1 + cathodal [c]-tDCS-F4Fp2; SMD = −1.29 [95% CI, −2.27 to −0.31]), 2-mA anode tDCS at F3 plus a cathode at Fp2 (a-tDCS-F3 + c-tDCS-Fp2; SMD = −1.28 [95% CI, −2.55 to −0.02]), 2-mA anode tDCS at F3 plus a cathode at left TPJ (a-tDCS-F3 + c-tDCS-TP3; SMD = −0.85 [95% CI, −1.56 to −0.13]), 20-Hz rTMS at left DLPFC (extreme hf-rTMS-F3; SMD = −0.45 [95% CI, −0.79 to −0.12]), and 10-Hz rTMS at left DLPFC (hf-rTMS-F3; SMD = −0.43 [95% CI, −0.68 to −0.18]; eTable 10A in the Supplement and Figure 2A). According to SUCRA, hd-tRNS-AF3AF4F2F6FC4 was ranked the highest probability of being the best, followed by iTBS-F3 and a-tDCS-F3Fp1 + c-tDCS-F4Fp2 (SUCRA value: 2.4, 12.4, and 15.7, respectively) (eTable 5A in the Supplement). eFigure 6A in the Supplement shows the heat map diagram representation of the improvement of negative symptoms.
Figure 2. Forest Plot of Changes in Negative Symptom Severity.
When the effect size is less than 0, the specified intervention is associated with a greater improvement in negative symptom severity compared with sham controls. a Indicates anodal; c, cathodal; dTMS, deep repetitive transcranial magnetic stimulation; ehf, extreme high-definition; hd, high-definition; hf, high-frequency; iTBS, intermittent theta-burst stimulation; lf, low-frequency; OR, odds ratio; prTMS, priming repetitive transcranial magnetic stimulation; rTMS, repetitive transcranial magnetic stimulation; SMD, standardized mean difference; tACS, transcranial alternating current stimulation; tDCS; transcranial direct current stimulation; the, theta; tRNS, transcranial random noise stimulation; tVNS, transcutaneous vagus nerve stimulation.
The test of transitivity revealed that there was significant alleviation of negative symptom severity in both the rTMS–theta-burst stimulation and tDCS sham therapy groups (SMD = 0.468 [95% CI = 0.313-0.622]; P < .001 and SMD = 0.251 [95% CI, 0.022-0.480]; P = .03, respectively). Furthermore, no significant between-group differences were detected between the rTMS–theta-burst stimulation and tDCS sham therapy groups (rTMS sham control: SMD = 0.47 [95% CI, 0.31-0.62] vs tDCS sham control: SMD = 0.25 [95% CI, 0.02-0.48]; P = .12; eFigure 1 in the Supplement).
The subgroup analysis focusing on RCTs with definite diagnostic criteria revealed similar findings: hd-tRNS-AF3AF4F2F6FC4 (SMD = −2.19 [95% CI, −3.36 to −1.02), iTBS-F3 (SMD = −1.32 [95% CI, −1.88 to −0.76]), a-tDCS-F3Fp1 + c-tDCS-F4Fp2 (SMD = −1.29 [95% CI, −2.27 to −0.31]), a-tDCS-F3 + c-tDCS-Fp2 (SMD = −1.28 [95% CI, −2.55 to −0.02]), a-tDCS-F3 + c-tDCS-TP3 (SMD = −0.85 [95% CI, −1.56 to −0.13]), extreme hf-rTMS-F3 (SMD = −0.45 [95% CI, −0.79 to −0.12]), and hf-rTMS-F3 (SMD = −0.43 [95% CI, −0.68 to −0.18]) were associated with significantly greater alleviation of negative symptom severity than sham control (eTable 6A, eFigure 2A, and eFigure 3A in the Supplement). According to the SUCRA, hd-tRNS-AF3AF4F2F6FC4 was ranked the highest probability of being the best, followed by iTBS-F3 and a-tDCS-F3Fp1 + c-tDCS-F4Fp2 (SUCRA = 3.0, 12.4, and 16.2, respectively) (eTable 5B in the Supplement).
Acceptability
None of the investigated NIBS modalities were associated with significantly different acceptability rates relative to sham control groups (eTable 5C and eTable 10B in the Supplement, Figure 3B, and Figure 2B). eFigure 6B in the Supplement represent the heat map diagram representation of the tolerability in aspect of dropout rate.
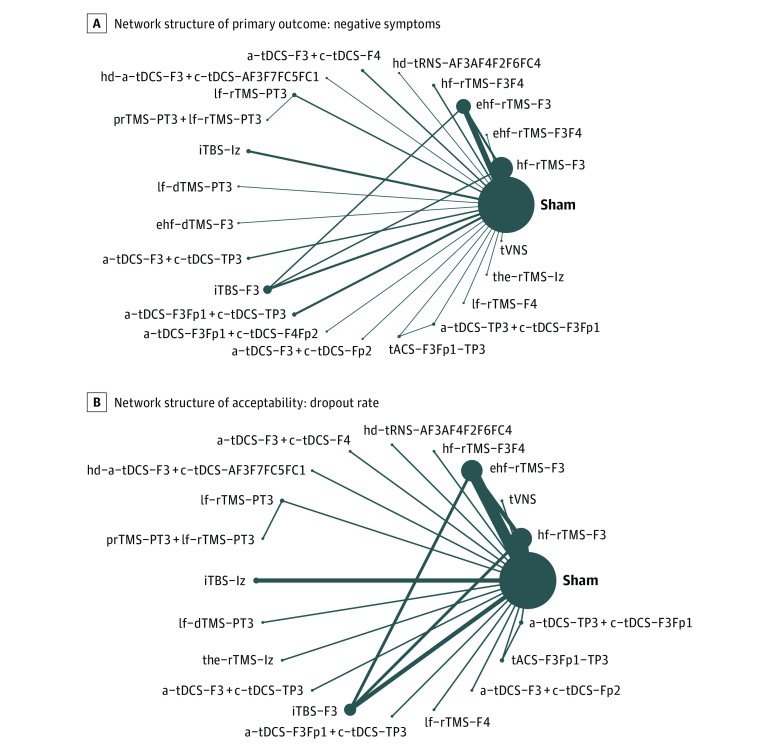
Figure 3. Network Structure of Changes in Negative Symptom Severity.
Lines between nodes represent direct comparisons between trials, and circle size is proportional to the size of the population that received each treatment. Line thickness is proportional to the number of studies providing data to the comparison. a Indicates anodal; c, cathodal; dTMS, deep repetitive transcranial magnetic stimulation; ehf, extreme high-definition; hd, high-definition; hf, high-frequency; iTBS, intermittent theta-burst stimulation; lf, low-frequency; prTMS, priming repetitive transcranial magnetic stimulation; rTMS, repetitive transcranial magnetic stimulation; tACS, transcranial alternating current stimulation; tDCS; transcranial direct current stimulation; the, theta; tRNS, transcranial random noise stimulation; tVNS, transcutaneous vagus nerve stimulation.
Secondary Outcome
Changes in Positive Symptom Severity
None of the investigated NIBS approaches was associated with significantly different changes in positive symptom severity compared with sham control groups, except for iTBS-F3. iTBS-F3 (SMD = 1.06 [95% CI, 0.32-1.80]) was associated with significantly worse positive symptom severity than sham control (eTable 6B, eFigure 2B, and eFigure 3B in the Supplement). According to the SUCRA, 6-Hz priming rTMS at PT3 plus 1-Hz rTMS at PT3 (priming [pr] TMS-PT3 + low-frequency [lf]–rTMS-PT3) (SUCRA value, 22.8) was associated with the most improvement but did not achieve statistical significance (SMD = −0.75 [95% CI, −2.38 to 0.88]; eTable 5D in the Supplement).
Changes in Depressive Symptom Severity
Only a-tDCS-F3Fp1 + c-tDCS-F4Fp2 (SMD = −0.79 [95% CI, −1.43 to −0.15]) was associated with significant alleviation of depressive symptom severity compared with sham control. By contrast, the use of 10-Hz rTMS at the left prefrontal cortex and right prefrontal cortex (hf-rTMS-F3F4; SMD = 0.73 [95% CI, 0.10-1.36]) was associated with significantly worse depressive symptoms (eTable 6C, eFigure 2C, and eFigure 3C in the Supplement). According to the SUCRA, a-tDCS-F3 + c-tDCS-Fp2 was associated with the most alleviation but did not achieve statistical significance (SMD = −0.93 [95% CI, −1.94 to 0.07]), followed by a-tDCS-F3Fp1 + c-tDCS-F4Fp2 (SUCRA value: 10.1 and 10.1, respectively) (eTable 5E in the Supplement).
Risk of Bias, Publication Bias, Inconsistency, and Heterogeneity
Of the included studies, 74.4% (250 of 336), 25.0% (84 of 336), and 0.6% (2 of 336 items) had overall low, unclear, and high risks of bias, respectively. The unclear reporting of allocation concealment contributed to bias risk (eFigure 4A and B in the Supplement). Funnel plots of publication bias across the included studies (eFigure 5A-H in the Supplement) revealed a general symmetry. No significant publication bias was detected among the articles included in network meta-analysis by using the Egger test. The network meta-analysis did not demonstrate inconsistency in terms of either local inconsistency (assessed using the loop-specific approach and node splitting) or global inconsistency (determined using the design-by-treatment method; eTable 7 in the Supplement). The heterogeneity test revealed some significant heterogeneity noted in some treatment comparison (eTable 8A and B in the Supplement). The GRADE ratings revealed that the quality of evidence in the network meta-analysis ranged from low to medium (eTable 9A and B in the Supplement).
Discussion
To our knowledge, this is the first network meta-analysis to directly investigate the potential benefits of NIBS for treating negative symptom severity in patients with schizophrenia. This study demonstrated that, compared with sham controls, excitatory stimulations (ie, tRNS, iTBS, a-tDCS, hf-rTMS, and extreme hf-rTMS) over left DLPFC (F3 region) with/without other inhibitory stimulation protocols in the contralateral brain regions were associated with the highest probability of being the best reductions in negative symptom severity. Of these, a-tDCS-F3Fp1 + c-tDCS-F4Fp2 was also associated with significant improvements in depressive symptom severity compared with sham controls. None of the investigated NIBS were associated with significantly different dropout rates compared with sham controls.
The key finding of this network meta-analysis was that the excitatory NIBS applied over left DLPFC (F3 region) were associated with significant alleviation of negative symptoms compared with sham controls. A functional magnetic resonance imaging study demonstrated abnormal functional connectivity of the DLPFC to the ventral tegmental area,15 which is the origin of mesocorticolimbic dopamine projections. Previous studies have indicated significant association between this abnormal functional connectivity and abnormal working memory performance85 and anticipated reward (wanting) function.86 Among these deficits, anticipated reward is considered to be linked to the negative symptoms of schizophrenia.87 Therefore, the restoration of this abnormal connectivity has become a rationale for negative symptom management. A recent seed-based functional magnetic resonance imaging in treatment-resistant schizophrenia demonstrated that iTBS over left DLPFC could both decrease patients’ negative symptom severity and increase functional connectivity between left DLPFC and brain regions with dopamine neuron cell bodies.40 Therefore, excitatory stimulation over left DLPFC would be a hypothetically reasonable strategy. This hypothesis is supported by the main finding of the present study, in which the most excitatory NIBS (tRNS, iTBS, a-tDCS, hf-rTMS, and extreme hf-rTMS) over F3 region significantly alleviated negative symptom severity in schizophrenia.
Of the excitatory NIBS, tRNS (hd-tRNS-AF3AF4F2F6FC4) was associated with the greatest alleviation of negative symptoms. This is a newly developed NIBS technique in which the current is delivered at randomly alternating intensities and frequencies and is believed to induce random neural activity, resulting in neural noise effects.41 The theory of neural noise effects88 explains the contradictory mechanism of transcranial electrical stimulations.89,90 The hypothesis of neural noise proposes that transcranial electrical stimulations, either through depolarization or hyperpolarization in different cortices, would improve cognitive performance.12 The application of tRNS has been revealed to be effective in different cortical dysfunction diseases, such as tinnitus with unknown origin.10 In a head-to-head trial, tRNS was revealed to be superior to tDCS at modulating neural activity after a single stimulation session.91 This superiority was also identified in the present network meta-analysis, in which tRNS stimulation was ranked superior to tDCS strategies in relieving negative symptom severity. However, only 1 RCT in this study, with a relatively short duration (a total of 5 weeks with a study duration of 1 week), reported the effect of tRNS on schizophrenia and schizoaffective disorder.41 Therefore, clinicians should pay particular attention when applying these findings in their clinical practice.
In addition to the aforementioned neural noise hypothesis, bimodal tDCS (a-tDCS-F3Fp1 + c-tDCS-F4Fp2) demonstrated favorable results. Specifically, a-tDCS-F3Fp1 + c-tDCS-F4Fp2 was beneficial to both negative and depressive symptoms in schizophrenia. This result was derived from 1 pioneer RCT,42 in which the authors applied a bianodal-bicathodal tDCS mode to successfully reduce patients’ negative and depressive symptoms. This finding might be explained by the 2-fold accumulated electrical dosage, synergistic effect, and deeper brain stimulation.42 However, because this result is derived from a pioneer RCT, the underlying mechanism beyond the bimodal tDCS remains unclear.
Limitations
This study has some limitations. First, this network meta-analysis may have been underpowered because of the heterogeneity of participants (eg, comorbidities, concomitant antipsychotics, baseline negative symptom severity, instruments used in each study, timing of NIBS intervention, and follow-up duration). All included studies were relatively small trials, presenting small samples and heterogeneous technical procedures. Second, although most RCTs included a sham control in their study design, the blindness of the RCTs may not have been complete because of the limitations of the commercial instruments used. Third, significant alleviation of negative symptom severity was demonstrated in both the rTMS-TBS and tDCS sham therapy groups (P < .001 and P = .03, respectively). Furthermore, no significant between-group difference was detected between these 2 groups (P = .12), which might indicate fair transitivity in this network meta-analysis. The significant placebo effect in the rTMS-TBS and tDCS sham therapy groups might be caused by (1) direct placebo effect of sham therapy and (2) potential therapeutic effect of concurrent antipsychotics on negative symptoms. However, we cannot completely exclude the potential therapeutic effect of the concurrent antipsychotics because no RCTs excluded antipsychotics from their study design. Fourth, this network meta-analysis only investigated dropout rate as an indicator of safety. Fifth, the study was designed to assess changes in negative symptom severity as a primary outcome. Therefore, we might have missed RCTs that mainly reported changes in positive or depressive symptoms and other outcomes in schizophrenia. Sixth, a new hypothesis has been proposed that potential beneficial effects of different NIBS would result from their neuroprotection, antiapoptosis, neurogenesis, angiogenesis, or neuroplasticity effects.92 However, we could not further investigate whether the negative symptoms were alleviated because of the neurogenesis effect of NIBS because of limited information. Seventh, our study was limited by the number of RCTs available for inclusion and the absence of long-term studies. In addition, because of the limited numbers of RCTs available, the overall network structures were relatively weak (ie, star-shaped network). Therefore, the interpretation of the SUCRA results should be more conservative. This weak network structure would also limit the application of inconsistency evaluation. To be specific, the inconsistency model would be less suitable for those network with star-shaped network structure. Besides, the heterogeneity test revealed some significant heterogeneity noted in some treatment comparisons. Therefore, clinicians should pay special attention when applying the results of our study in clinical practice.
Conclusions
This network meta-analysis revealed that excitatory stimulation over left DLPFC with/without other inhibitory stimulation protocols in contralateral brain regions is associated with alleviation of negative symptom severity. None of the investigated NIBS were associated with significantly different dropout rates compared with sham controls. In addition, this study noted significant alleviation of negative symptom severity in both the rTMS-TBS and tDCS sham therapy groups. However, because the NIBS had not been approved to have indication of negative symptoms management in schizophrenia, the results of the current network meta-analysis should be interpreted and applied in a more preserved way. Our findings might serve as a starting point for future large-scale RCTs with longer follow-up periods and sham control to investigate the association between NIBS and negative symptoms in schizophrenia.
eMethods.
eTable 1. PRISMA 2020 checklist of current network meta-analysis
eTable 2. Keywords in each database and search result
eTable 3. Excluded studies and reason
eTable 4. Characteristics of the included studies
eTable 5. SUCRA of the improvement of symptoms and tolerability in aspect of drop-out rate
eTable 6. League table of the improvement of symptoms
eTable 7. Inconsistency of different intervention
eTable 8. Estimated between-studies standard deviation of different outcome and between-treatment heterogeneity
eTable 9. Quality of evidence for primary outcomes
eTable 10. League table of the improvement of negative symptoms and tolerability in aspect of drop-out rate
eFigure 1. Test for transivity assumption: changes in negative symptoms
eFigure 2. Network structure of primary and secondary outcomes
eFigure 3. Forest plot of primary and secondary outcomes
eFigure 4. Overview and detailed risk of bias
eFigure 5. Funnel plot and Egger regression of primary and secondary outcomes and funnel plot of acceptability
eFigure 6. Heatmap diagram representation of primary outcomes and tolerability in aspect of drop-out rate
eReferences
References
- 1.GBD 2019 Mental Disorders Collaborators . Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9(2):137-150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia: an overview. JAMA Psychiatry. 2020;77(2):201-210. doi: 10.1001/jamapsychiatry.2019.3360 [DOI] [PubMed] [Google Scholar]
- 3.Medalia A, Lim R. Treatment of cognitive dysfunction in psychiatric disorders. J Psychiatr Pract. 2004;10(1):17-25. doi: 10.1097/00131746-200401000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951. doi: 10.1016/S0140-6736(19)31135-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milev RV, Giacobbe P, Kennedy SH, et al. ; CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4: neurostimulation treatments. Can J Psychiatry. 2016;61(9):561-575. doi: 10.1177/0706743716660033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mix A, Benali A, Eysel UT, Funke K. Continuous and intermittent transcranial magnetic theta burst stimulation modify tactile learning performance and cortical protein expression in the rat differently. Eur J Neurosci. 2010;32(9):1575-1586. doi: 10.1111/j.1460-9568.2010.07425.x [DOI] [PubMed] [Google Scholar]
- 7.Tseng PT, Jeng JS, Zeng BS, et al. Efficacy of non-invasive brain stimulation interventions in reducing smoking frequency in patients with nicotine dependence: a systematic review and network meta-analysis of randomized controlled trials. Addiction. Published online August 4, 2021. doi: 10.1111/add.15624 [DOI] [PubMed] [Google Scholar]
- 8.Zeng BY, Zeng BS, Chen YW, et al. Efficacy and acceptability of noninvasive brain stimulation interventions for weight reduction in obesity: a pilot network meta-analysis. Int J Obes (Lond). 2021;45(8):1705-1716. doi: 10.1038/s41366-021-00833-2 [DOI] [PubMed] [Google Scholar]
- 9.Chu CS, Li CT, Brunoni AR, et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: a component network meta-analysis. J Neurol Neurosurg Psychiatry. 2021;92(2):195-203. doi: 10.1136/jnnp-2020-323870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JJ, Zeng BS, Wu CN, et al. Association of central noninvasive brain stimulation interventions with efficacy and safety in tinnitus management: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2020;146(9):801-809. doi: 10.1001/jamaoto.2020.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hung YY, Yang LH, Stubbs B, et al. Efficacy and tolerability of deep transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109850. doi: 10.1016/j.pnpbp.2019.109850 [DOI] [PubMed] [Google Scholar]
- 12.Hung CM, Zeng BY, Zeng BS, et al. The efficacy of transcranial direct current stimulation in enhancing surgical skill acquisition: a preliminary meta-analysis of randomized controlled trials. Brain Sci. 2021;11(6):707. doi: 10.3390/brainsci11060707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Fang X, Tang W, et al. Effects and potential mechanisms of transcranial direct current stimulation (tDCS) on auditory hallucinations: a meta-analysis. Psychiatry Res. 2019;273:343-349. doi: 10.1016/j.psychres.2019.01.059 [DOI] [PubMed] [Google Scholar]
- 14.Tranulis C, Sepehry AA, Galinowski A, Stip E. Should we treat auditory hallucinations with repetitive transcranial magnetic stimulation? a metaanalysis. Can J Psychiatry. 2008;53(9):577-586. doi: 10.1177/070674370805300904 [DOI] [PubMed] [Google Scholar]
- 15.Giordano GM, Stanziano M, Papa M, et al. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: a resting state functional MRI study. Eur Neuropsychopharmacol. 2018;28(5):589-602. doi: 10.1016/j.euroneuro.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 16.Brisch R, Saniotis A, Wolf R, et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;5:47. doi: 10.3389/fpsyt.2014.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valiengo LDCL, Goerigk S, Gordon PC, et al. Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):121-129. doi: 10.1001/jamapsychiatry.2019.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Yin M, Lyu XL, Zhang LL, Du XD, Hung GC. Delayed effect of repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia: Findings from a randomized controlled trial. Psychiatry Res. 2016;240:333-335. doi: 10.1016/j.psychres.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg O, Gersner R, Klein LD, Kotler M, Zangen A, Dannon P. Deep transcranial magnetic stimulation add-on for the treatment of auditory hallucinations: a double-blind study. Ann Gen Psychiatry. 2012;11:13. doi: 10.1186/1744-859X-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: A meta-analysis of randomized controlled trials. Eur Psychiatry. 2018;49:69-77. doi: 10.1016/j.eurpsy.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 21.Cheng PWC, Louie LLC, Wong YL, et al. The effects of transcranial direct current stimulation (tDCS) on clinical symptoms in schizophrenia: a systematic review and meta-analysis. Asian J Psychiatr. 2020;53:102392. doi: 10.1016/j.ajp.2020.102392 [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Iwata Y, Plitman E, et al. A meta-analysis of transcranial direct current stimulation for schizophrenia: “is more better?”. J Psychiatr Res. 2019;110:117-126. doi: 10.1016/j.jpsychires.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Fang X, Chen Y, Wang Y, Wang D, Zhang C. Efficacy of transcranial direct current stimulation in ameliorating negative symptoms and cognitive impairments in schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2020;224:2-10. doi: 10.1016/j.schres.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: meta-analysis of controlled trials. Neurosci Biobehav Rev. 2018;89:111-118. doi: 10.1016/j.neubiorev.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386(9994):628-630. doi: 10.1016/S0140-6736(15)61478-7 [DOI] [PubMed] [Google Scholar]
- 26.Naci H, Salcher-Konrad M, Kesselheim AS, et al. Generating comparative evidence on new drugs and devices before approval. Lancet. 2020;395(10228):986-997. doi: 10.1016/S0140-6736(19)33178-2 [DOI] [PubMed] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895-905. doi: 10.1111/obr.12835 [DOI] [PubMed] [Google Scholar]
- 29.Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535. doi: 10.1001/jamapsychiatry.2018.4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng PT, Yang CP, Su KP, et al. The association between melatonin and episodic migraine: a pilot network meta-analysis of randomized controlled trials to compare the prophylactic effects with exogenous melatonin supplementation and pharmacotherapy. J Pineal Res. 2020;69(2):e12663. doi: 10.1111/jpi.12663 [DOI] [PubMed] [Google Scholar]
- 31.Yang CP, Tseng PT, Pei-Chen Chang J, Su H, Satyanarayanan SK, Su KP. Melatonergic agents in the prevention of delirium: a network meta-analysis of randomized controlled trials. Sleep Med Rev. 2020;50:101235. doi: 10.1016/j.smrv.2019.101235 [DOI] [PubMed] [Google Scholar]
- 32.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. [Google Scholar]
- 33.Cheng YC, Zeng BY, Hung CM, et al. Effectiveness and acceptability of noninvasive brain and nerve stimulation techniques for migraine prophylaxis: a network meta-analysis of randomized controlled trials. J Headache Pain. 2022;23(1):28. doi: 10.1186/s10194-022-01401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: a network meta-analysis. Laryngoscope. 2016;126(4):951-955. doi: 10.1002/lary.25688 [DOI] [PubMed] [Google Scholar]
- 35.Tu YK. Use of generalized linear mixed models for network meta-analysis. Med Decis Making. 2014;34(7):911-918. doi: 10.1177/0272989X14545789 [DOI] [PubMed] [Google Scholar]
- 36.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. doi: 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Del Giovane C, Chaimani A, Caldwell DM, Salanti G. Evaluating the quality of evidence from a network meta-analysis. Value Health. 2014;17(7):A324. doi: 10.1016/j.jval.2014.08.572 [DOI] [PubMed] [Google Scholar]
- 38.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 39.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bation R, Magnin C, Poulet E, Mondino M, Brunelin J. Intermittent theta burst stimulation for negative symptoms of schizophrenia: a double-blind, sham-controlled pilot study. NPJ Schizophr. 2021;7(1):10. doi: 10.1038/s41537-021-00138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang CC, Lin YY, Tzeng NS, Kao YC, Chang HA. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia: a randomized, double-blind, sham-controlled pilot study. J Psychiatr Res. 2021;132:151-160. doi: 10.1016/j.jpsychires.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 42.Chang CC, Kao YC, Chao CY, Tzeng NS, Chang HA. Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: a randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109715. doi: 10.1016/j.pnpbp.2019.109715 [DOI] [PubMed] [Google Scholar]
- 43.Basavaraju R, Ithal D, Thanki MV, et al. Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: a randomized controlled trial. Schizophr Res. 2021;238:108-120. doi: 10.1016/j.schres.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chauhan P, Garg S, Tikka SK, Khattri S. Efficacy of intensive cerebellar intermittent theta burst stimulation (iCiTBS) in treatment-resistant schizophrenia: a randomized placebo-controlled study. Cerebellum. 2021;20(1):116-123. doi: 10.1007/s12311-020-01193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dharani R, Goyal N, Mukherjee A, Umesh S. Adjuvant high-definition transcranial direct current stimulation for negative symptoms in schizophrenia: a pilot study. J ECT. 2021;37(3):195-201. doi: 10.1097/YCT.0000000000000756 [DOI] [PubMed] [Google Scholar]
- 46.Pan Z, Xiong D, Xiao H, et al. The effects of repetitive transcranial magnetic stimulation in patients with chronic schizophrenia: insights from EEG microstates. Psychiatry Res. 2021;299:113866. doi: 10.1016/j.psychres.2021.113866 [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Zhang W, Zhu Y, et al. Cerebellar theta burst stimulation for the treatment of negative symptoms of schizophrenia: a multicenter, double-blind, randomized controlled trial. Psychiatry Res. 2021;305:114204. doi: 10.1016/j.psychres.2021.114204 [DOI] [PubMed] [Google Scholar]
- 48.Guan HY, Zhao JM, Wang KQ, et al. High-frequency neuronavigated rTMS effect on clinical symptoms and cognitive dysfunction: a pilot double-blind, randomized controlled study in Veterans with schizophrenia. Transl Psychiatry. 2020;10(1):79. doi: 10.1038/s41398-020-0745-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar N, Vishnubhatla S, Wadhawan AN, Minhas S, Gupta P. A randomized, double blind, sham-controlled trial of repetitive transcranial magnetic stimulation (rTMS) in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2020;13(3):840-849. doi: 10.1016/j.brs.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 50.Li X, Yuan X, Kang Y, et al. A synergistic effect between family intervention and rTMS improves cognitive and negative symptoms in schizophrenia: A randomized controlled trial. J Psychiatr Res. 2020;126:81-91. doi: 10.1016/j.jpsychires.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 51.Singh S, Kumar N, Verma R, Nehra A. The safety and efficacy of adjunctive 20-Hz repetitive transcranial magnetic stimulation for treatment of negative symptoms in patients with schizophrenia: a double-blinded, randomized, sham-controlled study. Indian J Psychiatry. 2020;62(1):21-29. doi: 10.4103/psychiatry.IndianJPsychiatry_361_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiu MH, Guan HY, Zhao JM, et al. Cognitive enhancing effect of high-frequency neuronavigated rTMS in chronic schizophrenia patients with predominant negative symptoms: a double-blind controlled 32-week follow-up study. Schizophr Bull. 2020;sbaa035. doi: 10.1093/schbul/sbaa035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CC, Kao YC, Chao CY, Chang HA. Enhancement of cognitive insight and higher-order neurocognitive function by fronto-temporal transcranial direct current stimulation (tDCS) in patients with schizophrenia. Schizophr Res. 2019;208:430-438. doi: 10.1016/j.schres.2018.12.052 [DOI] [PubMed] [Google Scholar]
- 54.Zhuo K, Tang Y, Song Z, et al. Repetitive transcranial magnetic stimulation as an adjunctive treatment for negative symptoms and cognitive impairment in patients with schizophrenia: a randomized, double-blind, sham-controlled trial. Neuropsychiatr Dis Treat. 2019;15:1141-1150. doi: 10.2147/NDT.S196086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomes JS, Trevizol AP, Ducos DV, et al. Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: a phase II randomized sham-controlled trial. Schizophr Res Cogn. 2018;12:20-28. doi: 10.1016/j.scog.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeon DW, Jung DU, Kim SJ, et al. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr Res. 2018;197:378-385. doi: 10.1016/j.schres.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 57.Mellin JM, Alagapan S, Lustenberger C, et al. Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur Psychiatry. 2018;51:25-33. doi: 10.1016/j.eurpsy.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasan A, Wobrock T, Guse B, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22(6):857-864. doi: 10.1038/mp.2016.161 [DOI] [PubMed] [Google Scholar]
- 59.Fröhlich F, Burrello TN, Mellin JM, et al. Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry. 2016;33:54-60. doi: 10.1016/j.eurpsy.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 60.Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res. 2016;243:413-420. doi: 10.1016/j.psychres.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 61.Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporo-parietal junction in patients with schizophrenia. Schizophr Bull. 2016;42(2):318-326. doi: 10.1093/schbul/sbv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palm U, Keeser D, Hasan A, et al. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr Bull. 2016;42(5):1253-1261. doi: 10.1093/schbul/sbw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dlabac-de Lange JJ, Bais L, van Es FD, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. 2015;45(6):1263-1275. doi: 10.1017/S0033291714002360 [DOI] [PubMed] [Google Scholar]
- 64.Gan J, Duan H, Chen Z, et al. [Effectiveness and safety of high dose transcranial magnetic stimulation in schizophrenia with refractory negative symptoms: a randomized controlled study]. Zhonghua Yi Xue Za Zhi. 2015;95(47):3808-3812. [PubMed] [Google Scholar]
- 65.Hasan A, Wolff-Menzler C, Pfeiffer S, et al. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2015;265(7):589-600. doi: 10.1007/s00406-015-0618-9 [DOI] [PubMed] [Google Scholar]
- 66.Quan WX, Zhu XL, Qiao H, et al. The effects of high-frequency repetitive transcranial magnetic stimulation (rTMS) on negative symptoms of schizophrenia and the follow-up study. Neurosci Lett. 2015;584:197-201. doi: 10.1016/j.neulet.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 67.Ray P, Sinha VK, Tikka SK. Adjuvant low-frequency rTMS in treating auditory hallucinations in recent-onset schizophrenia: a randomized controlled study investigating the effect of high-frequency priming stimulation. Ann Gen Psychiatry. 2015;14:8. doi: 10.1186/s12991-015-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77(11):979-988. doi: 10.1016/j.biopsych.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 69.Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacol. 2014;28(7):686-690. doi: 10.1177/0269881114533600 [DOI] [PubMed] [Google Scholar]
- 70.Zhao S, Kong J, Li S, Tong Z, Yang C, Zhong H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. 2014;26(1):15-21. doi: 10.3969/j.issn.1002-0829.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prikryl R, Ustohal L, Prikrylova Kucerova H, et al. A detailed analysis of the effect of repetitive transcranial magnetic stimulation on negative symptoms of schizophrenia: a double-blind trial. Schizophr Res. 2013;149(1-3):167-173. doi: 10.1016/j.schres.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 72.Barr MS, Farzan F, Tran LC, Fitzgerald PB, Daskalakis ZJ. A randomized controlled trial of sequentially bilateral prefrontal cortex repetitive transcranial magnetic stimulation in the treatment of negative symptoms in schizophrenia. Brain Stimul. 2012;5(3):337-346. doi: 10.1016/j.brs.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 73.Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724. doi: 10.1176/appi.ajp.2012.11071091 [DOI] [PubMed] [Google Scholar]
- 74.Prikryl R, Mikl M, Prikrylova Kucerová H, et al. Does repetitive transcranial magnetic stimulation have a positive effect on working memory and neuronal activation in treatment of negative symptoms of schizophrenia? Neuro Endocrinol Lett. 2012;33(1):90-97. [PubMed] [Google Scholar]
- 75.Zheng LN, Guo Q, Li H, Li CB, Wang JJ. [Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients]. Beijing Da Xue Xue Bao Yi Xue Ban. 2012;44(5):732-736. [PubMed] [Google Scholar]
- 76.Fitzgerald PB, Herring S, Hoy K, et al. A study of the effectiveness of bilateral transcranial magnetic stimulation in the treatment of the negative symptoms of schizophrenia. Brain Stimul. 2008;1(1):27-32. doi: 10.1016/j.brs.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 77.Mogg A, Purvis R, Eranti S, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93(1-3):221-228. doi: 10.1016/j.schres.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 78.Prikryl R, Kasparek T, Skotakova S, Ustohal L, Kucerova H, Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res. 2007;95(1-3):151-157. doi: 10.1016/j.schres.2007.06.019 [DOI] [PubMed] [Google Scholar]
- 79.Rosa MO, Gattaz WF, Rosa MA, et al. Effects of repetitive transcranial magnetic stimulation on auditory hallucinations refractory to clozapine. J Clin Psychiatry. 2007;68(10):1528-1532. doi: 10.4088/jcp.v68n1009 [DOI] [PubMed] [Google Scholar]
- 80.Novák T, Horácek J, Mohr P, et al. The double-blind sham-controlled study of high-frequency rTMS (20 Hz) for negative symptoms in schizophrenia: negative results. Neuro Endocrinol Lett. 2006;27(1-2):209-213. [PubMed] [Google Scholar]
- 81.Saba G, Verdon CM, Kalalou K, et al. Transcranial magnetic stimulation in the treatment of schizophrenic symptoms: a double blind sham controlled study. J Psychiatr Res. 2006;40(2):147-152. doi: 10.1016/j.jpsychires.2005.02.008 [DOI] [PubMed] [Google Scholar]
- 82.Hajak G, Marienhagen J, Langguth B, Werner S, Binder H, Eichhammer P. High-frequency repetitive transcranial magnetic stimulation in schizophrenia: a combined treatment and neuroimaging study. Psychol Med. 2004;34(7):1157-1163. doi: 10.1017/s0033291704002338 [DOI] [PubMed] [Google Scholar]
- 83.Holi MM, Eronen M, Toivonen K, Toivonen P, Marttunen M, Naukkarinen H. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr Bull. 2004;30(2):429-434. doi: 10.1093/oxfordjournals.schbul.a007089 [DOI] [PubMed] [Google Scholar]
- 84.Klein E, Kolsky Y, Puyerovsky M, Koren D, Chistyakov A, Feinsod M. Right prefrontal slow repetitive transcranial magnetic stimulation in schizophrenia: a double-blind sham-controlled pilot study. Biol Psychiatry. 1999;46(10):1451-1454. doi: 10.1016/s0006-3223(99)00182-1 [DOI] [PubMed] [Google Scholar]
- 85.Dauvermann MR, Moorhead TW, Watson AR, et al. Verbal working memory and functional large-scale networks in schizophrenia. Psychiatry Res Neuroimaging. 2017;270:86-96. doi: 10.1016/j.pscychresns.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 86.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36(5):919-934. doi: 10.1093/schbul/sbq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Waltz JA, Gold JM. Motivational deficits in schizophrenia and the representation of expected value. Curr Top Behav Neurosci. 2016;27:375-410. doi: 10.1007/7854_2015_385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McDonnell MD, Ward LM. The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci. 2011;12(7):415-426. doi: 10.1038/nrn3061 [DOI] [PubMed] [Google Scholar]
- 89.Forogh B, Yazdi-Bahri SM, Ahadi T, Fereshtehnejad SM, Raissi GR. Comparison of two protocols of transcranial magnetic stimulation for treatment of chronic tinnitus: a randomized controlled clinical trial of burst repetitive versus high-frequency repetitive Transcranial Magnetic Stimulation. Neurol Sci. 2014;35(2):227-232. doi: 10.1007/s10072-013-1487-5 [DOI] [PubMed] [Google Scholar]
- 90.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Front Syst Neurosci. 2014;8:2. doi: 10.3389/fnsys.2014.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry. 2013;4:158. doi: 10.3389/fpsyt.2013.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boonzaier J, van Tilborg GAF, Neggers SFW, Dijkhuizen RM. Noninvasive brain stimulation to enhance functional recovery after stroke: studies in animal models. Neurorehabil Neural Repair. 2018;32(11):927-940. doi: 10.1177/1545968318804425 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. PRISMA 2020 checklist of current network meta-analysis
eTable 2. Keywords in each database and search result
eTable 3. Excluded studies and reason
eTable 4. Characteristics of the included studies
eTable 5. SUCRA of the improvement of symptoms and tolerability in aspect of drop-out rate
eTable 6. League table of the improvement of symptoms
eTable 7. Inconsistency of different intervention
eTable 8. Estimated between-studies standard deviation of different outcome and between-treatment heterogeneity
eTable 9. Quality of evidence for primary outcomes
eTable 10. League table of the improvement of negative symptoms and tolerability in aspect of drop-out rate
eFigure 1. Test for transivity assumption: changes in negative symptoms
eFigure 2. Network structure of primary and secondary outcomes
eFigure 3. Forest plot of primary and secondary outcomes
eFigure 4. Overview and detailed risk of bias
eFigure 5. Funnel plot and Egger regression of primary and secondary outcomes and funnel plot of acceptability
eFigure 6. Heatmap diagram representation of primary outcomes and tolerability in aspect of drop-out rate
eReferences