SUMMARY
Hypusine is a post-translational modification on eukaryotic translation initiation factor 5A (eIF5A). The last step of hypusine biosynthesis, deoxyhypusine hydroxylation, is an oxygen-dependent reaction. Here we show that deletion of the deoxyhypusine hydroxylase Lia1 compromises yeast respiration through translation downregulation of selected proteins in the respiration pathway. The translation suppression, because of the lack of deoxyhypusine hydroxylation, mainly affects translation of the N termini of the proteins, independent of the presence of proline residues but likely dependent on the interaction between the N-terminal nascent peptide and the ribosomal peptide exit tunnel. Proteomics and biochemical studies reveal that Lia1 deletion decreases N-terminal translation of proteins involved in mitochondrial respiration, oxidative stress response, and protein folding. Our work uncovers functions of the hypusine modification by considering the substrate requirement of the post-translational modification, highlights the unique challenges of translating the N termini of proteins, and reveals an oxygen-sensing mechanism in eukaryotic cells.
Graphical Abstract
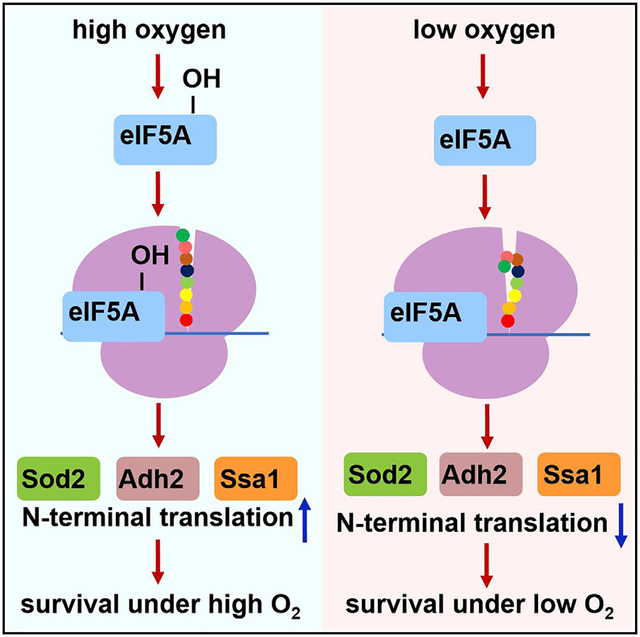
In brief
Cells have to regulate their metabolism in response to oxygen levels. Zhang et al. find that oxygen regulates hydroxylation of translation factor eIF5A in yeast. Hydroxylation promotes translation of the N termini of many proteins essential for cell survival in the presence of oxygen in a proline-independent manner.
INTRODUCTION
Organisms that live in aerobic environments must sense and respond to oxygen levels to survive. Oxygen is used in oxidative phosphorylation for energy production but can also cause oxidative stress, which leads to cellular damage and death. Cells develop multiple strategies to regulate protein expression in response to changing oxygen concentration at transcriptional, post-transcriptional, and translational levels (Nakayama and Kataoka, 2019; Uniacke et al., 2012). Under normoxia conditions, oxygen is utilized by prolyl-hydroxylase 2 (PHD2) to hydroxylate hypoxia-inducible factor 1α (HIF1α) (Semenza, 2014). Hydroxylation of HIF1α leads to its degradation (Semenza, 2014). HIF1α is stabilized under hypoxia conditions because of the lack of hydroxylation, turning on transcription of a series of genes, leading to hypoxia response (Nakayama and Kataoka, 2019; Semenza, 2014). Mammalian target of rapamycin (mTOR) activity is also regulated by oxygen through hypoxia-inducible transcription of REDD1 (Arsham et al., 2003; Braunstein et al., 2007; Brugarolas et al., 2004). Eukaryotic initiation factor 2α (eIF2α) and eEF2 are known to be inhibited by low oxygen levels (Koritzinsky et al., 2006; Liu et al., 2006). Through these mechanisms, a lower oxygen concentration suppresses translation globally.
Hypusine is a post-translationally modified lysine residue found solely on eIF5A (Cooper et al., 1983; Park et al., 1981; Shiba et al., 1971). It is synthesized in two steps. In the first step, spermidine is added to the lysine side chain through nicotinamide adenine dinucleotide (NAD+)-dependent deoxyhypusine synthase to form deoxyhypusine (Wolff et al., 1995). Deoxyhypusine is then hydroxylated by an iron-dependent deoxyhypusine hydroxylase (Kim et al., 2006; Park et al., 2006; Vu et al., 2009). It has been shown that eIF5A is essential for cell survival and that hypusine modification is essential for alleviating translation stalling in poly-proline sequences and other proline-containing motifs (Doerfel et al., 2013; Gutierrez et al., 2013; Saini et al., 2009; Schuller et al., 2017; Ude et al., 2013). The function of hypusine observed so far can be mostly attributed to deoxyhypusine because deletion of deoxyhypusine hydroxylase has no obvious phenotype in yeast (Park et al., 2006). However, deletion of the deoxyhypusine hydroxylase is lethal in higher eukaryotic model organisms (Park et al., 2010; Sasaki et al., 1996; Schnier et al., 1991). Thus, the hydroxylation step of hypusine may have important physiological functions.
Here we show that oxygen levels can regulate deoxyhypusine hydroxylation. In response to oxygen, deoxyhypusine hydroxylation increases, which, in turn, promotes translation of many selected proteins that are important for cell growth under aerobic conditions. We show that deletion of hydroxylation in most cases affects translation of the very N termini (~10 amino acids) of proteins, independent of proline but dependent on its interaction with the peptide exit tunnel of the ribosome. Thus, deoxyhypusine hydroxylation is another oxygen-sensing mechanism of eukaryotic cells.
RESULTS
Deoxyhypusine hydroxylation is regulated by the oxygen level
Hydroxylation of deoxyhypusine by oxygen resembles proline hydroxylation on HIF1α. Therefore, we hypothesized that deoxyhypusine hydroxylation is another mechanism for the cell to sense oxygen. To test whether low oxygen levels lead to low levels of hypusine in cells, we purified eIF5A from yeast cultured with different oxygen concentrations. eIF5A was fully hypusinated in yeast cultured with a normal oxygen concentration (Figure 1A). When oxygen was limited by sealing the culture tubes, the hypusine level dropped and the deoxyhypusine level increased (Figure 1A). When oxygen concentration was further limited by culturing yeast under anaerobic conditions, the hypusine level decreased further (Figure 1A). These results demonstrate that the oxygen level regulates deoxyhypusine hydroxylation.
Figure 1. Deoxyhypusine hydroxylation is dependent on oxygen levels and promotes oxidative phosphorylation.

(A) Yeast eIF5A hypusine levels are regulated by oxygen levels. eIF5A purified from WT yeast with different oxygen concentrations was analyzed using mass spectrometry (MS). Relative levels of hypusine and deoxyhypusine were quantified against the total amount of the peptide. eIF5A from ΔLia1 was used as a negative control. +O2, cultured under regular conditions; +limiting O2, cultured in a sealed flask filled with medium; −O2, cultured in an anaerobic chamber. The 26.7% of hypusine in yeast cultured in an anaerobic chamber may come from the starting yeast cells, which were cultured with oxygen.
(B) The ΔLia1 strain without deoxyhypusine hydroxylation shows a lower growth rate than the WT strain on nonfermentable carbon sources. The genotype of each strain is indicated on the left. Each row represents a serial dilution from left to right.
(C) The ΔLia1 strain without deoxyhypusine hydroxylation has a lower oxygen consumption rate than the WT strain. Error bars represent the standard deviation.
Deoxyhypusine hydroxylation promotes expression of proteins essential for oxidative metabolism
Given that deoxyhypusine hydroxylation is responsive to oxygen concentration, we next wanted to determine whether deoxyhypusine hydroxylation affects the expression of proteins essential for oxygen use. Thus, we set out to test whether mitochondrial respiration, the pathway consuming the most oxygen, is downregulated by deletion of Lia1, the gene responsible for deoxyhypusine hydroxylation in yeast. We cultured wild-type (WT) and ΔLia1 yeast on non-fermentable carbon sources, including ethanol and acetate, to force the yeast to utilize oxidative phosphorylation instead of glycolysis. Indeed, the ΔLia1 strain showed growth defects on these non-fermentable carbon sources (Figure 1B). The ΔLia1 strain also showed a lower oxygen consumption rate (Figures 1C and S1). These results indicate that deoxyhypusine hydroxylation senses the oxygen level and promotes respiration.
Because the ΔLia1 strain showed lower levels of oxygen consumption, we rationalized that certain proteins in the ethanol respiration pathway in the ΔLia1 strain should have lower expression. We found that alcohol dehydrogenase 2 (Adh2) in yeast was downregulated in the ΔLia1 strain (Figures 2A and S2). The lower expression was not attributed to its transcription because the mRNA level of Adh2 did not change in the ΔLia1 strain (Figure 2B). Under cycloheximide treatment, Adh2 protein stability was not decreased by Lia1 deletion (Figure 2C). Thus, we concluded that Adh2 downregulation in the ΔLia1 strain was due to a decrease in translation. To further confirm that the observed downregulation is through translation, we performed polysome profiling, isolated monosome and polysome fractions, and then measured the relative levels of Adh2 mRNA in the two fractions. Adh2 mRNA had a significantly higher distribution in the monosome fraction in ΔLia1 compared with the WT, demonstrating that translation was downregulated upon Lia1 deletion (Figures 2D and S3).
Figure 2. Downregulation of Adh2 in the ΔLia1 strain is due to inefficient translation of the N-terminal protein sequence.

(A) Adh2 is downregulated in the ΔLia1 strain.
(B) The mRNA level of Adh2 does not change between the WT and ΔLia1. Statistical analysis: n = 3, Student’s t test (not significant [ns], p = 0.36).
(C) Adh2 protein is stable in the WT and ΔLia1. Yeast was treated with 35 μg/mL cycloheximide for the indicated time. Protein level was monitored using western blotting. Error bars represent the standard deviation.
(D) Polysome profiling was carried out for WT and ΔLia1 yeast cells. The monosomes and polysomes were isolated, and the amount of Adh2 mRNA in them was quantified by qRT-PCR. Actin mRNA was used as a control. The ΔLia1 strain had more Adh2 mRNA in the monosome fraction than the WT, suggesting that Adh2 mRNA was translated less efficiently in the ΔLia1 strain. Statistical analysis: n = 3, Student’s t test (***p = 0.0002). Error bars represent the standard deviation.
(E) The sequence of the first 31 amino acids in Adh2 and the construct with different lengths of the Adh2 N-terminal coding sequence.
(F) The protein level of each construct was checked by western blot. The Adh2 original coding sequence was replaced with Renilla luciferase, which contained a 3×FLAG tag at the C terminus and the Adh2 N-terminal peptide at the N terminus. The length of the Adh2 N-terminal peptide in the construct is indicated above. For example, N4 indicates that Adh2(1–4) is followed by Renilla luciferase with a C-terminal 3×FLAG tag in the construct.
(G) The only proline in the first 9 amino acids of Adh2 is not required for the effect of hypusine on translation. The protein level of the fusion protein, Adh2(1–9) fused with Renilla luciferase with a C-terminal 3×FLAG tag, was monitored using western blotting. P4, without mutation; ΔP4, deletion of the proline; P4A, proline 4 mutated to alanine.
Hydroxylation of deoxyhypusine is important for translation of the N termini of selected proteins
Hypusine is known to promote translation of proline-rich motifs (Schuller et al., 2017) because proline is a poor substrate for peptide bond formation (Ude et al., 2013). However, whether hydroxylation plays an essential role in the translation of proline-containing peptide has not been tested. Adh2 contains a proline rich “25-PVPKPKP-31” sequence (Figure 2E). We hypothesized that this motif might be responsible for the translation suppression in ΔLia1. To test this hypothesis, we replaced the coding sequence of Adh2 after I24 or P31 with Renilla luciferase containing a C-terminal 3×FLAG tag in its original genome locus (Figure 2E). We expected that the P31 fusion would show lower expression in ΔLia1 compared with the WT and that the I24 fusion would show similar expression. Surprisingly, both fusions showed much lower expression levels in the ΔLia1 strain than in the WT strain (Figure 2F), suggesting that the sequence before I24 is important for enhanced translation by deoxyhypusine hydroxylation. We thus generated a series of constructs with different truncations of the N-terminal coding sequence of Adh2 fused to Renilla luciferase, followed by the C-terminal 3×FLAG tag in the original genome locus of Adh2 (Figure 2E). We found that the first 9 amino acids of Adh2 were enough to cause lower expression in ΔLia1 (Figure 2F). Deletion or mutation of the only proline in the first 9 amino acids did not abolish downregulation in ΔLia1 (Figure 2G). All of these results indicated that the lack of deoxyhypusine hydroxylation affected the translation of the very N-terminus of Adh2 instead of affecting proline-containing sequences.
It is well known that certain mRNA sequences can also affect translation efficiency because of codon use (Chu et al., 2011; Goodman et al., 2013; Han et al., 2014; Kudla et al., 2009; Pechmann and Frydman, 2013; Tuller et al., 2010; Verma et al., 2019; Weinberg et al., 2016). To test whether the mRNA sequence is important for the translation suppression caused by Lia1 deletion, we generated two constructs of the Adh2 N-terminal 10-codon sequence by altering the mRNA sequence but not the amino acid sequence. The codon decoding time in yeast was obtained from Chu et al. (2014). Using the decoding time, we were able to construct an Adh2 N-terminal sequence with the fastest codons (Adh2_Fast) and slowest codons (Adh2_Slow). These two constructs showed an expression difference similar to the original Ahd2 construct between the WT and ΔLia1 strains (Figure S4). This result indicated that codon use is not important for translation regulation by Lia1 deletion.
To gain a comprehensive understanding of proteins regulated by deoxyhypusine hydroxylation, we performed SILAC (stable isotope labeling in cell culture) proteomics analysis to compare the protein levels in WT and ΔLia1 yeast strains. WT cells were cultured in medium with heavy isotope-labeled lysine and arginine, and ΔLia1 cells were cultured in medium with light lysine and arginine. We took proteins with heavy-to-light (H/L) ratios larger than 1.2 as potential candidates for proteins that are upregulated with deoxyhypusine hydroxylation (false discovery rate < 1%) in three biological replicates with a p value of less than 0.05 (Figure S5; Table S1). With this cutoff, we identified 510 proteins.
Among the identified proteins, we chose proteins related to an observable phenotype for validation. We tagged these potential targets with a C-terminal 3×FLAG tag by PCR-mediated epitope tagging (Mathur and Kaiser, 2014). All eight targets tested (Lsc2, Sod2, Ssa1, Tom70, Tdh2, Atp2, Trr2, and Rki1) showed the expected changes in expression between the WT and ΔLia1, indicating that the SILAC results were reliable (Figure 3).
Figure 3. Verification of proteins identified in SILAC by western blot.

Western blotting was used to detect the protein levels of Lsc2, Sod2, Ssa1, Tom70, Tdh2, Atp2, Trr2, and Rki1 in the WT and ΔLia1 strains. The quantification of proteins of interest, obtained by dividing the anti-FLAG immunoblotting signal with that of anti-Act1, is normalized to the WT results. Statistical analysis: n = 3, Student’s t test. The p values are included in Figure S7.
To find out whether hypusine regulates translation of the N termini of these proteins, similar to the case of Adh2, we constructed a translation reporter system based on a centromeric plasmid, pTH644, with a bi-directional promoter (Chu et al., 2011). We put mCherry on one side of the bidirectional promoter and EGFP on the other side. We then inserted the N-terminal sequences of the proteins of interest at the N terminus of EGFP (Figure 4A). To test the very N-terminal sequence of the open reading frame (ORF), we inserted only the first 10 amino acids. The translation efficiency of the N-terminal sequence was represented by the EGFP-to-mCherry fluorescence intensity ratio. Then the fluorescence ratio in the ΔLia1 strain was normalized to that in the WT strain. A ratio of less than 1.0 indicates that the lack of deoxyhypusine hydroxylation suppresses translation of the N-terminal sequence fused to EGFP. The reporter system enabled us to easily test multiple N-terminal coding sequences. Among the nine verified protein targets downregulated in ΔLia1, six of them (Tom70, Atp2, Sod2, Tdh2, Adh2, and Ssa1) showed decreased translation of their N-terminal sequences in ΔLia1 (Figure 4B). These results indicated that deoxyhypusine hydroxylation affected translation of the majority of the identified proteins by promoting translation of the first 10 amino acids at the N termini of these proteins.
Figure 4. Interaction between the N-terminal nascent peptide and the ribosome peptide exit tunnel determines the effects of deoxyhypusine hydroxylation on translation.
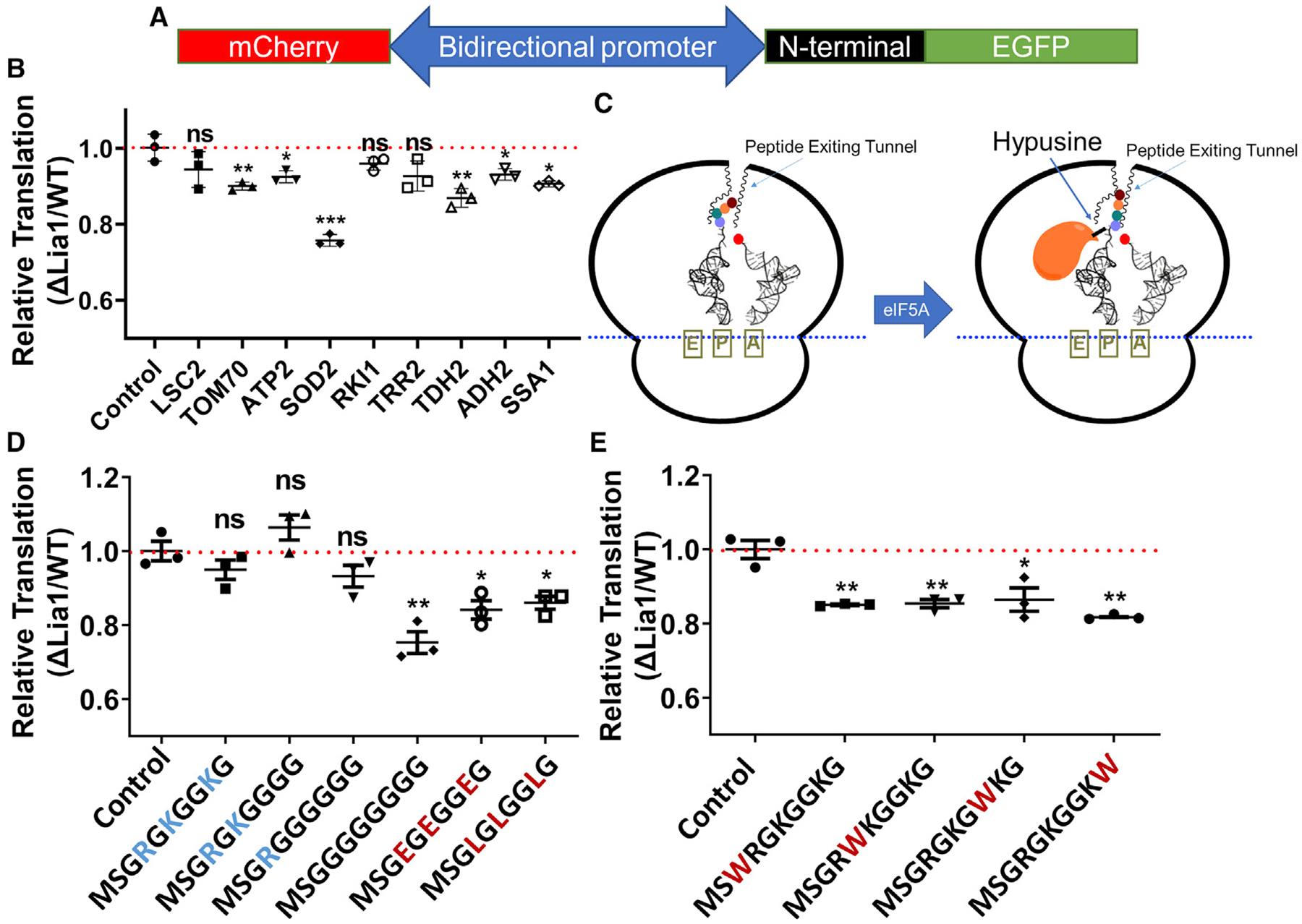
(A) Diagram showing the reporter construct. EGFP and mCherry were inserted at different sides of the bi-directional promoter. The N-terminal sequences from different proteins were inserted at the N terminus of EGFP.
(B) Testing the translation efficiency of the first 10 codons from potential targets in the ΔLia1 and WT strains using the reporter shown in (A). The construct without any insertion at the N terminus of EGFP was used as a control. The N-terminal sequences of six of nine targets showed a significant decrease in translation efficiency in the ΔLia1 strain.
(C) Proposed model of the function of deoxyhypusine hydroxylation. In the absence of hydroxylation, certain N-terminal nascent peptides have unfavorable interactions with the peptide exit tunnel, leading to a conformation in the peptide transferase center (PTC) detrimental to formation of new peptide bonds. In the presence of hydroxylation, the interaction between hypusine and the peptidyl-tRNA promotes a proper conformation in the PTC, favoring formation of new peptide bonds.
(D) Testing the translation efficiency of the N terminus of Hhf1 with mutations of positively charged residues using the reporter construct. Removal of positively charged residues led to decreases in translation efficiency in the ΔLia1 strain.
(E) Testing the translation efficiency of the N terminus of Hhf1 with Gly-to-Trp mutations. Mutation of Gly to Trp led to decreases in translation efficiency in the ΔLia1 strain.
Statistical significance in (B), (D), and (E) was determined using Student’s t test (n = 3). *p < 0.05, **p < 0.01. The exact p values are included in Table S3. Error bars represent the standard deviation.
Deoxyhypusine hydroxylation helps overcome unfavorable interactions between the N termini of proteins and the peptid exit tunnel of the ribosome
It has been proposed that ribosomes stall at the peptide sequences containing three consecutive proline residues because of the improper conformation between proline residues (Doerfel et al., 2013; Huter et al., 2017; Ude et al., 2013). Hypusine has been proposed to promote formation of peptide bonds between prolines through interaction with the peptidyl-tRNA (Doerfel et al., 2013; Huter et al., 2017; Ude et al., 2013). To promote translation of the N termini of proteins, we hypothesized that some N-terminal sequences with weak interaction with the peptide exit tunnel might not extend properly through the exit tunnel, rendering an improper conformation in the peptide transferase center (PTC), inhibiting peptide bond formation. With the help of fully hydroxylated hypusine, eIF5A forces the nascent peptide to extend into the peptide exit tunnel and promotes a proper conformation in the PTC, promoting peptide bond formation (Figure 4C). In line with this hypothesis, an N-terminal sequence with a favorable interaction with the peptide exit tunnel should translate equally well with or without deoxyhypusine hydroxylation, whereas an N-terminal sequence with an unfavorable interaction with the peptide exit tunnel may translate less efficiently without deoxyhypusine hydroxylation. The peptide exit tunnel is lined up by residues from ribosomal proteins and rRNA with a constriction point (Petrone et al., 2008; Riba et al., 2019). The peptide exit tunnel is also known to be narrow and negatively charged (Petrone et al., 2008; Riba et al., 2019). Thus, a peptide sequence with small and positively charged residues is favored by the peptide exit tunnel (Petrone et al., 2008; Riba et al., 2019).
We chose the first 10 codons of yeast histone H1 (Hhf1), MSGRGKGGKG, which contains mainly positively charged residues and the smallest residue, glycine, to test our model. If our model is correct, then translation of this sequence should not be affected by the lack of deoxyhypusine hydroxylation. As expected, the translation efficiency of the N-terminal of Hhf1 was not decreased by the lack of deoxyhypusine hydroxylation (Figure 4D). In line with our hypothesis, after removal of positively charged residues or adding a bulky hydrophobic residue, tryptophan, to perturb the interaction of the nascent peptide with the peptide exit tunnel, we observed significant translation suppression by the lack of deoxyhypusine hydroxylation (Figures 4D and 4E). The results are consistent with our hypothesis that the interaction between the very N-terminal sequence of a protein and the peptide exit tunnel determines whether the translation is facilitated by deoxyhypusine hydroxylation.
Deoxyhypusine hydroxylation promotes translation of proteins important for mitochondrial metabolism, protein folding, and oxidative stress removal
The SILAC proteomics results and the biochemical validation showed that ΔLia1 affects expression of many proteins. Through these proteins, we can gain a rather comprehensive understanding of biological processes affected by ΔLia1. The ATP synthase subunit Atp2 was downregulated in ΔLia1 (Figure 3). A subunit of the translocase of inner mitochondrial membrane (TIM)/translocase of outer mitochondrial membrane (TOM) complex, Tom70, was downregulated in ΔLia1 (Figure 3). Lsc2, the succinyl-coenzyme A (CoA) ligase that participates in the Krebs cycle, was downregulated in ΔLia1 (Figure 3). These SILAC targets further explain how respiration or mitochondrial function is compromised in the ΔLia1 strain.
We also noticed that the Hsp70 family proteins Ssa1, Ssb1, Ssb2, and Ssc1 were downregulated in ΔLia1. Expression of Ssa1 was confirmed by western blot (Figure 3). Hsp70 is a major chaperone responsible for protein folding (Mayer and Bukau, 2005). Downregulation of Hsp70 will likely render yeast heat sensitive because of protein folding stress under elevated temperatures. To test this, we cultured yeast at 37°C instead of 30°C. As expected, compared with the WT, ΔLia1 yeast showed a growth defect at 37°C (Figure 5A).
Figure 5. The ΔLia1 strain shows growth defects at elevated temperature or under oxidative stress.

(A) Yeast was cultured at 3°C (left) or 37°C (right). Each row represents a serial dilution from left to right.
(B) Yeast was cultured in synthetic medium with 2% glucose without H2O2 (left) or with H2O2 (right). Each row represents a serial dilution from left to right.
Based on the proteomics results, proteins related to oxidative stress response, including Sod2, Trr2, and Trx1, were downregulated in ΔLia1. The downregulation of Sod2 and Trr2 was confirmed by western blot (Figure 3). The downregulation of Sod2 translation was also confirmed by polysome profiling (Figure S6). These proteins are related to removal of reactive oxygen species, which could be produced during oxidative phosphorylation. To test whether ΔLia1 has defects in oxidative stress response, we treated yeast cells with 4 mM H2O2. Indeed, ΔLia1 showed growth defects under H2O2 treatment (Figure 5B).
We also performed STRING (search tool for the retrieval of interacting genes/proteins) analysis, which identified additional pathways affected by hypusine (Table S2). Many amino acid biosynthesis pathways are affected, which would be interesting to test in future studies. On the other hand, Lia1 deletion showed minor changes in protein expression for the majority of the proteins in the SILAC results under normal conditions. SILAC results obtained under stress conditions may identify more protein targets and pathways affected by Lia1 deletion.
DISCUSSION
Hypusine, a modification that is only present in eIF5A, is known to promote translation of various proteins (Gutierrez et al., 2013; Schuller et al., 2017). However, these effects may be due to the combined functions of deoxyhypusine and hypusine. Here, by examining the protein level changes in the WT and ΔLia1 strains, we were able to specifically examine the effect of hypusine or the effect of the hydroxyl group on the hypusine modification. We noticed the requirement of oxygen for addition of the hydroxyl group in hypusine and thus suspected that hypusine may sense oxygen levels to regulate translation of proteins important for respiration. Under low-oxygen conditions, the hydroxylation level is low. Low hydroxylation triggers downregulation of oxidative phosphorylation, oxidative stress response, and heat shock proteins. Under low-oxygen conditions, oxidative phosphorylation is not a good way to produce energy. A low oxygen level also lowers the concentration of reactive oxygen species produced during oxidative metabolism, rendering a low requirement for oxidative damage repair. There is also a lower requirement for folding of proteins with oxidative damage when oxidative stress is lower. Thus, it seems that nature uses deoxyhypusine hydroxylation to sense oxygen levels to fine-tune translation of proteins for maximal fitness (Figure 6). Our results suggest that hydroxylation of deoxyhypusine is an evolutionally conserved oxygen-sensing mechanism in eukaryotes. Compared with the well-known HIF-mediated oxygen sensing pathway, which senses oxygen levels to control transcription of specific genes, the hypusine oxygen-sensing pathway controls translation of selected proteins. Although oxygen levels have been reported to regulate translation, these previous mechanisms regulate translation globally (Arsham et al., 2003; Braunstein et al., 2007; Brugarolas et al., 2004; Koritzinsky et al., 2006; Liu et al., 2006). In contrast, the hypusine oxygen-sensing pathway is a way to selectively control translation of proteins important for oxidative metabolism.
Figure 6. Hypusine promotes cell survival in the presence of oxygen.

When the oxygen level is high, hydroxylation of deoxyhypusine in eIF5A increases. Hydroxylation promotes translation of N termini of many selected proteins in oxidative phosphorylation, oxidative stress response, and protein folding that promote cell survival in the presence of oxygen.
It is very interesting that the effect of deoxyhypusine hydroxylation on translation in many cases is on the very first 10 amino acids of the proteins and independent of prolines. This seems to be different from what has been suggested previously for the function of hypusine (Doerfel et al., 2013; Gutierrez et al., 2013; Saini et al., 2009; Schuller et al., 2017; Ude et al., 2013). However, we think this is not necessarily in conflict with previous reports about the function of hypusine. Deoxyhypusine synthase was deleted in the previous studies and, thus, even deoxyhypusine was removed (Doerfel et al., 2013; Gutierrez et al., 2013; Saini et al., 2009; Schuller et al., 2017; Ude et al., 2013). Therefore, the effect on translation of proline-rich sequences may be due to deoxyhypusine. In contrast, our study, by using the Lia1 deletion strain, specifically examines the effect of deoxyhypusine hydroxylation.
Translation initiation, elongation, and termination collectively control translation of proteins (Choi et al., 2018; Gingold and Pilpel, 2011; Subramaniam et al., 2014). The N termini of proteins have slower translation elongation rates because of the low abundance of tRNA coding (Pechmann and Frydman, 2013; Tuller et al., 2010). Slower translation of N termini ensures movement of the ribosome across the whole mRNA without collision (Subramaniam et al., 2014; Tuller et al., 2010). Such a 5’ ramp was observed with multiple methods and can affect translation initiation (Chu et al., 2014; Han et al., 2014; Weinberg et al., 2016). Codon use, secondary structure, and some amino acid sequences have been proposed to affect translation efficiency in this region (Chu et al., 2014; Goodman et al., 2013; Kudla et al., 2009; Verma et al., 2019). We demonstrated here that, through deoxyhypusine hydroxylation, the N-terminal sequences can be used as regulatory elements to control translation of selected proteins in response to oxygen levels to maximize fitness.
It is interesting that a modification exists to specifically promote translation of the N terminus of proteins. What is unique about the translation of the N terminus of proteins? The N terminus of proteins is special in translation because it is the first part of the protein that interacts with the peptide exit tunnel of the ribosome. In some cases, the N terminus of a protein does not have a favorable interaction with the peptide exit tunnel, which could result in the translating peptide adopting a conformation not suitable for new peptide bond formation in the PTC of the ribosome. The hypusine modification extends into the PTC and interacts with the translating peptide, forcing the peptide into a favorable conformation for forming the next peptide bond (Huter et al., 2017). The process is less efficient without hydroxylation. As translation continues, the translating peptide fully occupies the exit tunnel, providing more interaction to promote a favorable conformation for peptide bond formation in the PTC. The co-translational folding could further stabilize the conformation to promote peptide bond formation in the PTC. Because of this, the effect of deoxyhypusine hydroxylation would be less obvious in translation of sequences far from the N terminus.
The effect of N-terminal sequences on translation is not fully understood because many factors, including the amino acid sequence, mRNA sequence, mRNA structure, and co-translational folding, are involved. So far, we are not able to identify a common feature for what sequence will be affected by Lia1 deletion. In the case of Adh2, the first 9 codons from the Adh2 N-terminal sequence are enough to show the translation suppression caused by Lia1 deletion. However, this effect is abolished when only 4 codons are included, demonstrating that the minimum length requirement of the N-terminal sequence is longer than 4 amino acids. By using the N-terminal sequence of HHF1, we obtained insights into the translation of some N-terminal sequences affected by Lia1 deletion.
Here we discovered the function of deoxyhypusine hydroxylation by considering its substrate, oxygen. Because oxygen is a substrate for deoxyhypusine hydroxylation, we speculated that oxygen could regulate the level of deoxyhypusine hydroxylation, which, in turn, regulates translation of proteins important for oxidative metabolism. This line of thinking could potentially be useful for understanding other post-translational modifications with unknown biological functions.
Limitation of the study
We used SILAC proteomics to identify the N-terminal sequences regulated by deoxyhypusine hydroxylation. The ΔLia1 strain showed a phenotype difference under stress conditions, whereas SILAC was performed under optimal culture conditions with 2% glucose. This may omit some of the proteins causing the growth defect of ΔLia1 strain under stress conditions. Comprehensive SILAC under stress conditions may help unravel more common features of N-terminal sequence regulation. Our model of how hydroxylation affects N-terminal translation, although plausible, will need to be confirmed by future structural studies.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Hening Lin (hl379@cornell.edu).
Materials availability
All unique/stable reagents generated in this study are available from the lead contact with a completed materials transfer agreement.
Data and code availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange and are publicly available as of the date of publication. Dataset identifier number is listed in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| HRP-conjugated anti-Flag antibody | Sigma | Cat# A8592; RRID:AB_439702 |
| Mouse anti Actin antibody | Santa Cruz | Cat# SC-47778; RRID:AB_626632 |
| Anti-mouse IgG, HRP linked antibody | Cell Signaling | Cat#7076S; RRID:AB_330924 |
| Bacterial and virus strains | ||
| NEB 5-alpha | NEB | Cat#C2987H |
| Chemicals, peptides, and recombinant proteins | ||
| Antimycin A | Abcam | Cat#141904 |
| Yeast nitrogen base without amino acid and ammonium sulfate | BD | Cat#233520 |
| Ammonium Sulfate | VWR | Cat# BDH9216-2.5KG |
| Glucose | Sigma | Cat# G8270-10KG |
| Ethanol | VWR | Cat# 71001-866 |
| Potassium Acetate | VWR | Cat# BHD9254-500G |
| Adenine Sulfate | Amersco | Cat# 0607-50G |
| Alanine | VWR | Cat# AAJ60279-18 |
| Arginine | Alfa Aesar | Cat# A14730 |
| Asparagine | VWR | Cat# AAJ62869-22 |
| Aspartic acid | Chem Impex | Cat# 00027 |
| Cysteine | VWR | Cat# AAA10389-22 |
| Glutamine | VWR | Cat# AAJ60573-22 |
| Glutamic acid | Sigma | Cat# G8415 |
| Glycine | VWR | Cat# 0167-5KG |
| Inositol | VWR | Cat# A13586-22 |
| Isoleucine | VWR | Cat# AAA13699-18 |
| Leucine | VWR | Cat# AAJ62824-22 |
| Lysine | VWR | Cat# AAJ62225-14 |
| Methionine | Chem Impex | Cat# 00221-100G |
| para-Aminobenzoic acid | Sigma | Cat# A9878 |
| Phenylalanine | Santa Cruz | Cat# sc-394058 |
| Proline | VWR | Cat# AAA10199-22 |
| Serine | VWR | Cat# AAA11179-14 |
| Threonine | Alfa Aesar | Cat# A16851 |
| Tryptophan | VWR | Cat# AAA10230-14 |
| Tyrosine | Alfa Aesar | Cat# A11141 |
| Valine | Sigma | Cat# 94619 |
| Histidine | Sigma | Cat# H6034 |
| Uracil | VWR | Cat# AAA15570-18 |
| Agar | Millipore | Cat# 12177 |
| Trisbase | Santa Cruz | Cat# sc-3715C |
| Sodium Chloride | VWR | Cat# BDH9286-2.5KG |
| NP40 | VWR | Cat# 97064-730 |
| 3XFlag peptide | Sigma | Cat# F4799-25MG |
| Triethylammonium bicarbonate | Sigma | Cat# T7408 |
| Urea | Fisher Scientific | Cat# BP169-212 |
| Thiourea | Fisher Scientific | Cat# AC424540010 |
| Sodium lauryl sulfate | Fisher Scientific | Cat# S529-500 |
| Dithiothreitol | Fisher Scientific | Cat# BP172-5 |
| Iodoacetamide | Fisher Scientific | Cat# 122271000 |
| Phosphoric acid | Fisher Scientific | Cat# A260-500 |
| AspN | Promega | Cat# VA1160 |
| Formic acid | Fisher Scientific | Cat# A117-50 |
| Acetonitrile | Fisher Scientific | Cat# A955-4 |
| Lysine-13C6,15N2 hydrochloride | Sigma | Cat# 608041 |
| Arginie-13C6,15N4 hydrochloride | Sigma | Cat# 608033 |
| Sodium hydroxide | VWR | Cat# 97064-486 |
| 2-Mercaptoethanol | VWR | Cat# 97064-878 |
| Trichloroacetic acid | Sigma | Cat# T6399-100G |
| Sodium phosphate dibasic | Sigma | Cat# S9763 |
| Hydrochloric acid | VWR | Cat# 470301-260 |
| Potassium Chloride | Sigma | Cat# P9541 |
| Magnesium Chloride | VWR | Cat# BDH9244-500G |
| Critical commercial assays | ||
| Extracellular O2 Consumption Assay | Abcam | Cat#197243 |
| Deposited data | ||
| Proteomics Dataset | ProteomeXchange | PXD032321 |
| Experimental models: Organisms/strains | ||
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Horizon Discovery | YSC1048 |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX | Horizon Discovery | YSC6273-201937826 |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Adh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–4) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–9) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–14) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–19) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷ Adh2(1–24) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷ Adh2(1–31) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Adh2(1–4) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–9) Rluc This paper 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–14) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–19) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷ Adh2(1–24) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷ Adh2(1–31) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 atp2Δ0∷Atp2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX atp2Δ0∷Atp2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ssa1Δ0∷Ssa1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXssa1Δ0∷Ssa1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Lsc2Δ0∷Lsc2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Lsc2Δ0∷Lsc2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Sod2Δ0∷Sod2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Sod2Δ0∷Sod2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Trr2Δ0∷Trr2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Trr2Δ0∷Trr2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Rki1Δ0∷Rki1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX RkiΔ0∷Rki1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Tdh2Δ0∷Tdh21 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Tdh2Δ0∷Tdh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Tom70Δ0∷Tom70 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Tom70Δ0∷Tom70 3XFlag∷His3 | This paper | N/A |
| Oligonucleotides | ||
| ADH2-RT-F: GCCGCTCACATTCCTCAAGGTAC |
IDT | N/A |
| ADH2-RT-R: GCCCAGTGGCCTGCTCTC |
IDT | N/A |
| SOD2-RT-F: TCAAGTTCCATGGCGGTGG |
IDT | N/A |
| SOD2-RT-R: GCCAAAGCGCCGGTGG |
IDT | N/A |
| ACT1-RT-F: TGGCCGGTAGAGATTTGACTGAC |
IDT | N/A |
| ACT1-RT-R: GTTCGAAGTCCAAGGCGACG |
IDT | N/A |
| Other | ||
| Anti-Flag® M2 affinity gel | Sigma | Cat# A2220-25ML |
| Micro Bio-Spin® column | Bio-rad | Cat# 732-6221 |
| S-Trap micro spin column | PROTIFI | Cat# C02-micro-80 |
| Acclaim PepMap C18 RP trapping column | Thermo Scientific | Cat# 164564-CMD |
| Acclaim PepMap C18 RSLC Nano column | Thermo Scientific | Cat# 164,941 |
| PVDF membrane | Biorad | Cat# 1620177 |
| 400 micron Acid Washed Zirconium Beads | Ops Diagnostics | Cat# BAWZ 400-250-35 |
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Yeast strains
The strains were generated using PCR-based tagging method as previously described (Funakoshi and Hochstrasser, 2009). Please refer to Tagging of endogenous proteins with 3xFlag tag and Generation of fusion protein for Adh2 in the method details section.
Yeast culturing method
Yeast was cultured in synthetic complete media. The media was made with 1.70 g/L Yeast nitrogen base without amino acids and ammonium sulfate (BD), 5.0 g/L ammonium sulfate, 2.0 g/L amino acid mixture and 2% glucose. The amino acid mixture was made from the following table. Yeast culture media with ethanol or acetate was made by replacing 2% glucose with 2% ethanol or 2% potassium acetate. Yeast plate was made by adding 2% agar to the corresponding liquid media.
| Reagent | Amount to add/g |
|---|---|
| Adenine | 0.5 |
| Alanine | 2.0 |
| Arginine | 2.0 |
| Asparagine | 2.0 |
| Aspartic acid | 2.0 |
| Cysteine | 2.0 |
| Glutamine | 2.0 |
| Glutamic acid | 2.0 |
| Glycine | 2.0 |
| Inositol | 2.0 |
| Isoleucine | 2.0 |
| Leucine | 4.0 |
| Lysine | 2.0 |
| Methionine | 2.0 |
| para-Aminobenzoic acid | 0.2 |
| Phenylalanine | 2.0 |
| Proline | 2.0 |
| Serine | 2.0 |
| Threonine | 2.0 |
| Tryptophan | 2.0 |
| Tyrosine | 2.0 |
| Valine | 2.0 |
| Histidine | 2.0 |
| Uracil | 2.0 |
METHOD DETAILS
Yeast spot growth assay
WT and ΔLia1 yeast cells were cultured in synthetic complete medium at 30°C overnight, adjusted to OD600 of 0.2 with autoclaved water, and then diluted serially in four-fold increments. The serially diluted cells were spotted on SC media with 2% glucose or 2% potassium acetate or 2% ethanol agar plates using a replica plater. Plates were incubated at 30°C. Cell growth was recorded 2–3 days after plating.
Purification of eIF5A from yeast
WT or ΔLia1 yeast overexpressing P432 Met25 Hyp2 with C-terminal Flag tag was cultured overnight in H– SC media with 2% glucose overnight. Overnight culture was inoculated into 250 mL H− SC media. Yeast pellet was harvested when OD600 reaches 1.5. Yeast cultured in glove box anaerobically is considered no oxygen conditions. Yeast cultured in 250 mL round flask with rubber stopper is considered limiting oxygen conditions. Yeast pellet was resuspended in 1 mL buffer containing 250 mM Tris (pH = 8.0), 150 mM NaCl and 1× protease inhibitor. 600 μg acid-washed zirconium beads were added to the tube. The mixture was subject to Tissue lyser LT (Qiagen) for 50 Hz 1 min. The mixture was cooled on ice for 3 min. The lysing and cooling cycle was repeated six times. The mixture was centrifuged at 5000g for 5 min. The supernatant was collected. The zirconium beads were washed with 1 mL buffer containing 250 mM Tris (pH = 8.0) and 150 mM NaCl. The wash was collected with the supernatant and centrifuged at 17000g for 15 min. The supernatant from this centrifuge was incubated with 200 μL anti-Flaga® M2 affinity gel (Sigma) at 4°C for 2 h in the presence of 0.2% NP40. The anti-Flaga® M2 affinity gel was washed five times with buffer containing 25 mM Tris (pH = 8.0), 150 mM NaCl and 0.2% NP40. The anti-Flaga® M2 affinity gel was then incubated with 30 μM 3XFlag peptide (sigma) for 2 h at 4°C for elution. The elution was performed twice. The elution was combined and concentrated to 50 μL. The concentrated sample was desalted against buffer containing 25 mM Tris (pH = 8.0) and 150 mM NaCl using Micro Bio-Spina® column (BioRad). The sample was then used for mass spectroscopic detection of hypusine or deoxyhypusine.
In-solution AspN digestion
In solution digestion was performed using an S-Trap micro spin column (ProtiFi, Huntington, NY, USA) following a Strap protocol on as described previously (Yang et al., 2018) with slight modification. The protein sample (~20 μg) in 25 μL of 50mM tetraethylammonium bromide (TEAB), 6 M urea, 2 M thiourea, 1% SDS, 10 mM dithiothreitol (DTT) were reduced for 1 h at 34°C, followed by alkylation with 50 mM iodoacetamide for 45 min at room temperature in the dark and then quenched with a final concentration of 25 mM DTT. After quenching, 12% phosphoric acid was added to a final concentration of 1.2%. Followed by 1:7 dilution (v/v) with 90% methanol, 100 mM TEAB. The samples were then placed into the S-Trap spin column and centrifuged at 4000g for 30 s. Then washed three times with 150 μL 90% methanol, 100 mM TEAB. AspN (2μg) from Promega was reconstituted with double-distilled water. Digestion was performed with 25 μL AspN at 80 ng/μL (1:10 w/w) added to the top of the spin column. AspN solution absorbed into the highly hydrophilic matrix, the spin columns were incubated at 37°C overnight (16 h). Following incubation, the digested peptides were eluted off the S-trap column sequentially with 40 μL each of 50 mM TEAB followed by 0.2% formic acid (FA) and finally, 50% acetonitrile (ACN)-0.2% FA. Three eluted peptides washes were pooled together and evaporated to dryness by a Speedvac SC110 (Thermo Savant, Milford, MA).
NanoLC-tandem mass spectrometry (nanoLC-MS/MS) for hypusine analysis
The in-solution AspN digests were reconstituted in 50 μL of 0.5% FA for nanoLC- ESIMS/MS analysis, which was carried out using an Orbitrap Fusion™ Tribrid™ (Thermo-Fisher Scientific, San Jose, CA) mass spectrometer equipped with a nanospray Flex Ion Source, and coupled with a Dionex UltiMate 3000 RSLCnano system (Thermo, Sunnyvale, CA) (Yang et al., 2018). The peptides (4 μL) were injected onto a PepMap C-18 RP nano trapping column (5 μm, 100 μm i.d × 20 mm) at 20 μL/min flow rate for rapid sample loading and then separated on a PepMap C-18 RP nano column (2 μm, 75 μm × 25 cm) at 35°C. The AspN digested peptides were eluted in a 90 min gradient of 5%–35% ACN in 0.1% FA at 300 nL/min, followed by an 8 min ramping to 90% ACN-0.1% FA and an 8 min hold at 90% ACN-0.1% FA. The column was re-equilibrated with 0.1% FA for 25 min prior to the next run. The Orbitrap Fusion is operated in positive ion mode with spray voltage set at 2.0 kV and source temperature at 275°C. External calibration for FT, IT and quadrupole mass analyzers was performed. In data-dependent acquisition (DDA) analysis, the instrument was operated using FT mass analyzer in MS scan to select precursor ions followed by 3-s “Top Speed” data-dependent CID ion trap MS/MS scans for 3–4 charged ions that was toggled with an additional EThcD MS2 scan in ion trap for 3–8 charged ions (Lynch et al., 2019; Thomas et al., 2017). Precursor peptides were selected at 1.2 m/z quadrupole isolation with multiple charged ions above a threshold ion count of 10,000 and normalized collision energy of 30%. MS survey scans at a resolving power of 120,000 (fwhm at m/z 200), for the mass range of m/z 350–1550. The reaction time for 150 ms and maximal reagent injection time at 200 ms were applied for ETD fragmentation supplemented with an additional 20% HCD activation energy to yield EThcD MS2 spectra. Dynamic exclusion parameters were set at 50 s of exclusion duration with ±10 ppm exclusion mass width. All data were acquired by Xcalibur 4.4 operation software under Tune Application v. 3.4 (Thermo-Fisher Scientific).
Data processing for hypusine MS analysis
The raw files for EThcD-CID toggle MS/MS were subjected to database searches using Proteome Discoverer (PD) 2.4 software (Thermo Fisher Scientific, Bremen, Germany) with the Sequest HT algorithm (Lynch et al., 2019; Yang et al., 2018) against Saccharomyces cerevisiae database along with a common contaminants database. Two-missed cleavage sites by AspN were allowed for database search of raw files. The peptide precursor tolerance was set to 10 ppm and fragment ion tolerance was set to 0.6 Da. Variable modification of methionine oxidation, deamidation of asparagine/glutamine, hypusine/deoxyhypusine on lysine residue and fixed modification of cysteine carbamidomethylation, were set for the database search. Only high confidence peptides defined by Sequest HT with a 1% FDR by Percolator were considered for the peptide identification. All MS/MS spectra for identified relevant peptides from initial database searching were manually inspected and validated using Xcalibur 4.4. Confident identification of hypusine and deoxyhypusine modified peptides was achieved by manual confirmation of MS precursor ions and their associated EThcD MS/MS and CID MS/MS spectra. Relative quantitation of identified hypusine and deoxyhypusine peptides and their counterpart native peptides within single samples and between samples was determined by PD-assistant or manual extraction ion chromatograms (XICs). The XICs of each peptide (and its modified forms) with different charge states for each sample were obtained based on precursor ion m/z with mass tolerance of 5 ppm in Xcalibur software.
Oxygen consumption rate measurement
WT and ΔLia1 yeast cells were cultured in SC liquid medium with 2% ethanol at 30°C overnight. The next day overnight culture was diluted in fresh SC medium with 2% ethanol and then cultured until OD600 reached 0.5. The yeast culture was washed 3 times and then resuspended with fresh SC medium with 2% ethanol to adjust OD600 = 0.1. The oxygen consumption rate was measured using Extracellular O2 Consumption Assay (ab197243) following the protocol for the kit. Each measurement was performed in a well of a 96 well plate with 150 μL of yeast culture, 10 μL oxygen consumption rate reagent and 100 μL mineral oil (2 drops). The fluorescence measurement chamber was pre-heated to 30°C. The fluorescence was measured with 340 nm excitation and 645 nm emission and the emission integration was collected. The fluorescence lifetime was generated using the following equation.
F30 and F70 stands for fluorescence intensity integration of 30 μs after delay of 30 μs or 70 μs. The data points of 2 repeats were collected every 90s for 2 h. The background of measurement was collected with WT yeast with 2 ug/mL antimycin A (abcam 141904). The background was subtracted, and the oxygen consumption rate was generated by the slop of lifetime plot against time (Figure S1).
Tagging of endogenous proteins with 3xFlag tag
The strain expressing endogenous 3XFLAG tag were generated using PCR-based tagging method as previously described (Funakoshi and Hochstrasser, 2009) with the following modifications. The 500 bp fragment from the 3’ of the ORF before the stop codon was cloned into the plasmid pFA6a-6xGLY-3xFLAG-HIS3MX6 right before the 3xFlag tag with double digestion. The 500 bp fragment right after the stop codon of the ORF was cloned into the same vector after the His3MX6 selection marker with double digestion. The fragment from the first 500 bp to the second 500 bp was PCR amplified and transformed into yeast and plated on synthetic complete agar plates with histidine dropout for selection. Two positive colonies are used for Western Blots. pFA6a-6xGLY-3xFLAG-HIS3MX6 was a gift from Mark Hochstrasser (Addgene plasmid # 20753; http://n2t.net/addgene:20753; RRID:Addgene_20753)
Generation of fusion protein for Adh2
The fusion protein was generated following a reported method with modifications (Funakoshi and Hochstrasser, 2009). Renilla luciferase was inserted into pFA6a-6xGLY-3xFLAG-HIS3MX6 (Addgene plasmid 20753) with SalI and BamHI double digestion. An XhoI cut site was inserted before the SalI cut site during the process to generated plasmid pFA6a-6xGLY-Rluc-3xFLAG-HIS3MX6. 500 bp of the 3’ UTR of Adh2 was PCR amplified and inserted into the plasmid with EcoRI and SpeI double digestion to generate the plasmid pFA6a-6xGLY-Rluc-3xFLAG-HIS3MX6-3’UTR Adh2. 500 bp of the 5’ untranslated region (UTR) of Adh2 along with the desired Adh2 N-terminal fragment was PCR amplified and inserted into the plasmid with HindIII and XhoI double digestion. The PCR fragment was generated by amplifying from the 5’ of the 5’ UTR to the 3’ of 3’ UTR. The PCR product was transformed to yeast strain and selected with synthetic complete agar plates with histidine dropout.
Sample preparation for SILAC study
Saccharomyces cerevisiae BY4741 strain (HL813Y) was cultured in synthetic complete media (20 mL) with heavy lysine (Sigma 608041) (0.086 g L–1) and arginine (Sigma 608033) (0.086 g L–1) till OD600 reached approximately 0.5. The ΔLia1 (HL1410Y) strain was cultured in synthetic complete media with light lysine and arginine till OD600 reached approximately 0.5. Three biological replicates of WT and ΔLia1 cultures were made. Cells were harvested by centrifugation at 5000 rpm for 5 min. Equal number of WT and ΔLia1 cells were mixed. Yeast was resuspended in 400 μL of H2O. 68 μL of buffer containing 1.85 M NaOH and 7.4% 2-Mercaptoethanol was added. The mixture was incubated on ice for 10 min before adding of 32 μL of 100% trichloroacetic acid. The mixture was incubated on ice for 10 min and then centrifuged for 15 min at 17000 g. The supernatant was discarded and the pellet was washed with 500 μL ice cold acetone. The protein pellet was obtained after centrifuge for 10 min at 17000 g. The wash was repeated for three times. The protein pellet was dried with a SpeedVac and then 400 μL buffer containing 4% SDS and 50 mM Na2HPO4, pH 7.4 was added. To fully resuspend the protein pellet, the mixture was sonicated for 20 min at room temperature. The fully resuspended protein pellet was boiled at 95°C for 5 min. The supernatant was obtained after centrifugation at 17000g for 5 min and quantified with BCA assay.
Nano LC/MS/MS and data analysis of SILAC sample
The SILAC tryptic digests were reconstituted in 50 μL of 2% ACN-0.5% FA estimated at 0.1 μg/μL for nanoLC-ESI-MS/MS analysis, which was carried out on an Orbitrap Fusion mass spectrometer interfaced with a Dionex UltiMate 3000 RSLCnano system (Thermo-Fisher Scientific, San Jose, CA) as described above. Each SILAC peptide sample (5 μL) was injected and eluted with a 120-min gradient of 5%–38%. The Orbitrap Fusion was operated in 3 s “top speed” DDA under FT-IT mode All MS and MS/MS raw spectra were processed and searched using Sequest HT software within the Proteome Discoverer 2.4 (PD 2.4, Thermo Scientific) against the Saccharomyces cerevisiae database. The database search was performed under a search workflow with the “Precursor Ions Quantifier” node for SILAC 2plex (Arg10, Lys8) quantitation. The default setting for protein identification in Sequest node were: two miscleavages for full trypsin with fixed carbamidomethyl modification of cysteine, variable modifications of 10.008 Da on Arginine and 8.014 Da on Lysine, N-terminal acetylation, methionine oxidation and deamidation of asparagine and glutamine residues. The peptide mass tolerance and fragment mass tolerance values were 10 ppm and 0.6 Da, respectively. Only high confidence peptides defined by Sequest HT with a 1% FDR by Percolator and peptide mass accuracy ≤5 ppm.were considered for the peptide identification. The mass precision for expected standard deviation of the detected mass used to create extracted ion chromatograms was set to 5 ppm. The SILAC 2-plex quantification method within PD 2.4 was used with unique plus razor peptides only to calculate the heavy/light ratios of all identified proteins without normalization. The final protein group list was further filtered with two peptides per protein in which only No.1-rank peptides within top scored proteins were used.
Western blot
Yeast grown in 20 mL synthetic complete media with or without rapamycin treatment were collected when OD600 reached 0.5. Yeast pellet was lysed with 200 μL of glass beads in 200 μL buffer containing 25 mM Tris at pH 8.0 and 150 mM NaCl. 20 μg of total yeast lysate was used for western blot against HRP-conjugated anti-Flag antibody (Sigma A8592).
Polysome profiling
Polysome extracts were prepared as previously described with minor revisions (Arava et al., 2003; Kuhn et al., 2001). Polysome buffer was prepared in DEPC water with 20mM Tris-HCl, 140 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 1% Triton X-100 and 0.1 mg/mL cycloheximide, pH 8.0. Polysome buffer and all the following samples were kept on ice. 60 mL culture of wild-type and ΔLia1 yeast cells in YPD medium were harvested when OD600 reached 0.6 for each sucrose gradient. Cycloheximide was introduced to a final concentration of 0.1 mg/mL at the time of harvest. Cells were collected by centrifugation at 4,500 × g, 4 °C for 5 min. The pellets were washed twice with 3 mL polysome buffer and centrifugation. The final pellets were resuspended in 750 μL polysome buffer and transferred to 15 mL conical centrifuge tubes with 500 μL prechilled zirconium beads. The mixtures were vortexed at full speed for 20 s and incubated on ice for 100 s. This step was repeated three times. The cell lysate was then centrifuged at 3,500 g, 4 °C for 5 min and the supernatant (~500 μL) was transferred to a microcentrifuge tube containing 200 μL polysome buffer. The samples were further centrifuged at 8,000 g, 4 °C for 5 min and 650 μL supernatant of each sample was layered onto 14 mL of 15%–45% sucrose gradient prepared in DEPC water with 20 mM Tris-HCl, 140 mM KCl, 5 mM MgCl2, 0.5 mM DTT, and 0.1 mg/mL cycloheximide, pH 8.0. The samples are centrifuged for 150 min at 32,000 rpm, 4°C in a SW40 rotor. Gradients were fractioned with 700 μL per aliquot using Brandel Density Gradient Faction System.
The sucrose gradient fractions corresponding to monosome and polysome were pooled together and the mRNA was reverse-transcribed using the SuperScript III First-Strand Synthesis kit (Invitrogen) following the manufacturer’s instructions. For quantitative real-time PCR analysis, iTaq Universal SYBR Green Supermix (Bio-Rad) was used following the manufacturer’s instructions. The reaction was performed with QuantStudio™ 7 Flex Real-Time PCR System (Applied Biosystems™). The relative expression of each gene, normalized to actin, was calculated using the 2-ΔΔCt method. The relative Adh2 mRNA level of polysome versus monosome was calculated with ΔLia1 strain referenced to be 1. Primers used for RT-qPCR are listed in Key resources table.
Construction of the reporter system
EGFP was amplified with YZ849_EGFP_SalI_5’ agtcagGTCGACatggtgagcaagggcgaggag YZ850_EGFP_HindIII_Flag_3’ agtcagAAGCTTttaCTTATCGTCGTCATCCTTGTAATCcttgtacagctcgtccatgcc and inserted into pTH644-CENBEVY with SalI and HindIII double digestion. mCherry was amplified with YZ847_mCherry_Xmal_5’ agtcagCCCGGGatggtgagcaagggcga YZ848_mCherry_EcoRI_HA_3’ agtcagGAATTCctaAGCGTAATCTGGAACATCGTATGGGTActtgtacagctcgtccat and inserted into pTH644-CENBEVYEGFP with XmaI and EcoRI double digestion. The protein of interested was inserted right at N-terminal of EGFP. pTH644-CENBEVY was a gift from Tobias von der Haar (Addgene plasmid # 29695; http://n2t.net/addgene:29695; RRID:Addgene_29695)
Fluorescent quantification of N-terminal translation efficiency
Yeast was cultured to OD600 around 0.5 and harvested by centrifugation at 500 g for 4 min. Yeast was washed three times with water. After the final wash, the yeast pellet was resuspended in 700 μL of water. Fluorescent reading of yeast suspension was obtained using a BioTek Cytation 5 plate reader. The EGFP signal was excited at 479 nm and read at 520 nm. The mCherry signal was excited at 579 nm and read at 616 nm. The ratio of EGFP/mcherry was calculated and normalized to EGFP/mCherry signal without the N-terminal insertion in corresponding strains.
QUANTIFICATION AND STATISTICAL ANALYSIS
Two-tailed Student’s t-test was used for all statistical analyses without. All statistical analysis were conducted using GraphPad Prism 8.0. The definition of n, center, dispersion, and precision all follow the definition used in student’s t-test. The detail of statistical analysis can be found in the figure legend. For Figure 3, the statistical analysis was included in Figures S7–S9. For Figure 4, the statistical analysis was included in Table S3 p values of 0.05 or less were the threshold for statistical significance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. All analysis was under the standard assumption of Two-tailed Student’s t-test.
Supplementary Material
Highlights.
eIF5A deoxyhypusine hydroxylation is an oxygen-sensing mechanism
Hydroxylation facilitates N-terminal translation of selected proteins
Hydroxylation promotes oxidative metabolism and survival
ACKNOWLEDGMENTS
We thank Prof. Shu-Bing Qian and Dr. Leiming Dong for helping with the ribosomal profiling experiment. This work is supported in part by NIH grant R35GM131808. NIH SIG grant 1S10 OD017992-01 was used to fund the Orbitrap Fusion mass spectrometer.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.110855.
DECLARATION OF INTERESTS
H.L. is a founder of and consultant for Sedec Therapeutics.
REFERENCES
- Arava Y, Wang Y, Storey JD, Liu CL, Brown PO, and Herschlag D (2003). Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U S A 100, 3889–3894. 10.1073/pnas.0635171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsham AM, Howell JJ, and Simon MC (2003). A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J. Biol. Chem 278, 29655–29660. 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, and Schneider RJ (2007). A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell 28, 501–512. 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, and Kaelin WG Jr. (2004). Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904. 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Grosely R, Prabhakar A, Lapointe CP, Wang J, and Puglisi JD (2018). How messenger RNA and nascent chain sequences regulate translation elongation. Annu. Rev. Biochem 87, 421–449. 10.1146/annurev-biochem-060815-014818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Barnes DJ, and von der Haar T (2011). The role of tRNA and ribosome competition in coupling the expression of different mRNAs in Saccharomyces cerevisiae. Nucleic Acids Res. 39, 6705–6714. 10.1093/nar/gkr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Kazana E, Bellanger N, Singh T, Tuite MF, and von der Haar T (2014). Translation elongation can control translation initiation on eukaryotic mRNAs. EMBO J. 33, 21–34. 10.1002/embj.201385651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HL, Park MH, Folk JE, Safer B, and Braverman R (1983). Identification of the hypusine-containing protein hy+ as translation initiation factor eIF-4D. Proc. Natl. Acad. Sci 80, 1854–1857. 10.1073/pnas.80.7.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, and Rodnina MV (2013). EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 339, 85–88. 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- Funakoshi M, and Hochstrasser M (2009). Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast 26, 185–192. 10.1002/yea.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, and Pilpel Y (2011). Determinants of translation efficiency and accuracy. Mol. Syst. Biol 7, 481. 10.1038/msb.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman DB, Church GM, and Kosuri S (2013). Causes and effects of N-terminal codon bias in bacterial genes. Science 342, 475–479. 10.1126/science.1241934. [DOI] [PubMed] [Google Scholar]
- Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, and Dever TE (2013). eIF5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45. 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Gao XW, Liu BT, Wan J, Zhang XQ, and Qian SB (2014). Ribosome profiling reveals sequence-independent post-initiation pausing as a signature of translation. Cell Res. 24, 842–851. 10.1038/cr.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huter P, Arenz S, Bock LV, Graf M, Frister JO, Heuer A, Peil L, Starosta AL, Wohlgemuth I, Peske F, et al. (2017). Structural basis for polyproline-mediated ribosome stalling and rescue by the translation elongation factor EF-P. Mol. Cell 68, 515–527.e6. 10.1016/j.molcel.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kang KR, Wolff EC, Bell JK, McPhie P, and Park MH (2006). Deoxyhypusine hydroxylase is an Fe(II)-dependent, heat-repeat enzyme. J. Biol. Chem 281, 13217–13225. 10.1074/jbc.m601081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, et al. (2006). Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 25, 1114–1125. 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla G, Murray AW, Tollervey D, and Plotkin JB (2009). Coding-sequence determinants of gene expression in Escherichia coli. Science 324, 255–258. 10.1126/science.1170160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KM, DeRisi JL, Brown PO, and Sarnow P (2001). Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol. Cell Biol 21, 916–927. 10.1128/MCB.21.3.916-927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, and Simon MC (2006). Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol. Cell 21, 521–531. 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MJ, Miller M, James M, Zhang S, Zhang K, Li C, Charon NW, and Crane BR (2019). Structure and chemistry of lysinoalanine crosslinking in the spirochaete flagella hook. Nat. Chem. Biol 15, 959–965. 10.1038/s41589-019-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R, and Kaiser P (2014). PCR-mediated epitope tagging of genes in yeast. Methods Mol. Biol 1205, 37–44. 10.1007/978-1-4939-1363-3_4. [DOI] [PubMed] [Google Scholar]
- Mayer MP, and Bukau B (2005). Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci 62, 670–684. 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, and Kataoka N (2019). Regulation of gene expression under hypoxic conditions. Int. J. Mol. Sci 20, 3278. 10.3390/ijms20133278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Aravind L, Wolff EC, Kaevel J, Kim YS, and Park MH (2006). Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc. Natl. Acad. Sci. U S A 103, 51–56. 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Cooper HL, and Folk JE (1981). Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. U S A 78, 2869–2873. 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, and Valentini SR (2010). Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids 38, 491–500. 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann S, and Frydman J (2013). Evolutionary conservation of codon optimality reveals hidden signatures of cotranslational folding. Nat. Struct. Mol. Biol 20, 237–243. 10.1038/nsmb.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone PM, Snow CD, Lucent D, and Pande VS (2008). Side-chain recognition and gating in the ribosome exit tunnel. Proc. Natl. Acad. Sci. U S A 105, 16549–16554. 10.1073/pnas.0801795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba A, Di Nanni N, Mittal N, Arhne E, Schmidt A, and Zavolan M (2019). Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates. Proc. Natl. Acad. Sci. U S A 116, 15023–15032. 10.1073/pnas.1817299116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini P, Eyler DE, Green R, and Dever TE (2009). Hypusine-containing protein eIF5A promotes translation elongation. Nature 459, 118–121. 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Abid R, and Miyazaki M (1996). Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett. 384, 151–154. 10.1016/0014-5793(96)00310-9. [DOI] [PubMed] [Google Scholar]
- Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, and Hershey JW (1991). Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol. Cell Biol 11, 3105–3114. 10.1128/mcb.11.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller AP, Wu CCC, Dever TE, Buskirk AR, and Green R (2017). eIF5A functions globally in translation elongation and termination. Mol. Cell 66, 194–205.e5. 10.1016/j.molcel.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2014). Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol 9, 47–71. 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Shiba T, Mizote H, Kaneko T, Nakajima T, Yasuo K, and sano I (1971). Hypusine, a new amino acid occurring in bovine brain. Biochim. Biophys. Acta 244, 523–531. 10.1016/0304-4165(71)90069-9. [DOI] [PubMed] [Google Scholar]
- Subramaniam AR, Zid BM, O’Shea E, and O’Shea EK (2014). An integrated approach reveals regulatory controls on bacterial translation elongation. Cell 159, 1200–1211. 10.1016/j.cell.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CJ, Cleland TP, Zhang S, Gundberg CM, and Vashishth D (2017). Identification and characterization of glycation adducts on osteocalcin. Anal. Biochem 525, 46–53. 10.1016/j.ab.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, and Pilpel Y (2010). An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141, 344–354. 10.1016/j.cell.2010.03.031. [DOI] [PubMed] [Google Scholar]
- Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, and Jung K (2013). Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 339, 82–85. 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, Payette J, Holcik M, Pause A, and Lee S (2012). An oxygen-regulated switch in the protein synthesis machinery. Nature 486, 126–129. 10.1038/nature11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M, Choi J, Cottrell KA, Lavagnino Z, Thomas EN, Pavlovic-Djuranovic S, Szczesny P, Piston DW, Zaher HS, Puglisi JD, and Djuranovic S (2019). A short translational ramp determines the efficiency of protein synthesis. Nat. Commun 10, 5774. 10.1038/s41467-019-13810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu VV, Emerson JP, Martinho M, Kim YS, Munck E, Park MH, and Que L Jr. (2009). Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center. Proc. Natl. Acad. Sci 106, 14814–14819. 10.1073/pnas.0904553106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DE, Shah P, Eichhorn SW, Hussmann JA, Plotkin JB, and Bartel DP (2016). Improved ribosome-footprint and mRNA measurements provide insights into dynamics and regulation of yeast translation. Cell Rep. 14, 1787–1799. 10.1016/j.celrep.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EC, Lee YB, Chung SI, Folk JE, and Park MH (1995). Deoxyhypusine synthase from rat testis: purification and characterization. J. Biol. Chem 270, 8660–8666. 10.1074/jbc.270.15.8660. [DOI] [PubMed] [Google Scholar]
- Yang Y, Anderson E, and Zhang S (2018). Evaluation of six sample preparation procedures for qualitative and quantitative proteomics analysis of milk fat globule membrane. Electrophoresis 39, 2332–2339. 10.1002/elps.201800042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange and are publicly available as of the date of publication. Dataset identifier number is listed in the key resources table. All other data reported in this paper will be shared by the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| HRP-conjugated anti-Flag antibody | Sigma | Cat# A8592; RRID:AB_439702 |
| Mouse anti Actin antibody | Santa Cruz | Cat# SC-47778; RRID:AB_626632 |
| Anti-mouse IgG, HRP linked antibody | Cell Signaling | Cat#7076S; RRID:AB_330924 |
| Bacterial and virus strains | ||
| NEB 5-alpha | NEB | Cat#C2987H |
| Chemicals, peptides, and recombinant proteins | ||
| Antimycin A | Abcam | Cat#141904 |
| Yeast nitrogen base without amino acid and ammonium sulfate | BD | Cat#233520 |
| Ammonium Sulfate | VWR | Cat# BDH9216-2.5KG |
| Glucose | Sigma | Cat# G8270-10KG |
| Ethanol | VWR | Cat# 71001-866 |
| Potassium Acetate | VWR | Cat# BHD9254-500G |
| Adenine Sulfate | Amersco | Cat# 0607-50G |
| Alanine | VWR | Cat# AAJ60279-18 |
| Arginine | Alfa Aesar | Cat# A14730 |
| Asparagine | VWR | Cat# AAJ62869-22 |
| Aspartic acid | Chem Impex | Cat# 00027 |
| Cysteine | VWR | Cat# AAA10389-22 |
| Glutamine | VWR | Cat# AAJ60573-22 |
| Glutamic acid | Sigma | Cat# G8415 |
| Glycine | VWR | Cat# 0167-5KG |
| Inositol | VWR | Cat# A13586-22 |
| Isoleucine | VWR | Cat# AAA13699-18 |
| Leucine | VWR | Cat# AAJ62824-22 |
| Lysine | VWR | Cat# AAJ62225-14 |
| Methionine | Chem Impex | Cat# 00221-100G |
| para-Aminobenzoic acid | Sigma | Cat# A9878 |
| Phenylalanine | Santa Cruz | Cat# sc-394058 |
| Proline | VWR | Cat# AAA10199-22 |
| Serine | VWR | Cat# AAA11179-14 |
| Threonine | Alfa Aesar | Cat# A16851 |
| Tryptophan | VWR | Cat# AAA10230-14 |
| Tyrosine | Alfa Aesar | Cat# A11141 |
| Valine | Sigma | Cat# 94619 |
| Histidine | Sigma | Cat# H6034 |
| Uracil | VWR | Cat# AAA15570-18 |
| Agar | Millipore | Cat# 12177 |
| Trisbase | Santa Cruz | Cat# sc-3715C |
| Sodium Chloride | VWR | Cat# BDH9286-2.5KG |
| NP40 | VWR | Cat# 97064-730 |
| 3XFlag peptide | Sigma | Cat# F4799-25MG |
| Triethylammonium bicarbonate | Sigma | Cat# T7408 |
| Urea | Fisher Scientific | Cat# BP169-212 |
| Thiourea | Fisher Scientific | Cat# AC424540010 |
| Sodium lauryl sulfate | Fisher Scientific | Cat# S529-500 |
| Dithiothreitol | Fisher Scientific | Cat# BP172-5 |
| Iodoacetamide | Fisher Scientific | Cat# 122271000 |
| Phosphoric acid | Fisher Scientific | Cat# A260-500 |
| AspN | Promega | Cat# VA1160 |
| Formic acid | Fisher Scientific | Cat# A117-50 |
| Acetonitrile | Fisher Scientific | Cat# A955-4 |
| Lysine-13C6,15N2 hydrochloride | Sigma | Cat# 608041 |
| Arginie-13C6,15N4 hydrochloride | Sigma | Cat# 608033 |
| Sodium hydroxide | VWR | Cat# 97064-486 |
| 2-Mercaptoethanol | VWR | Cat# 97064-878 |
| Trichloroacetic acid | Sigma | Cat# T6399-100G |
| Sodium phosphate dibasic | Sigma | Cat# S9763 |
| Hydrochloric acid | VWR | Cat# 470301-260 |
| Potassium Chloride | Sigma | Cat# P9541 |
| Magnesium Chloride | VWR | Cat# BDH9244-500G |
| Critical commercial assays | ||
| Extracellular O2 Consumption Assay | Abcam | Cat#197243 |
| Deposited data | ||
| Proteomics Dataset | ProteomeXchange | PXD032321 |
| Experimental models: Organisms/strains | ||
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Horizon Discovery | YSC1048 |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX | Horizon Discovery | YSC6273-201937826 |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Adh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–4) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–9) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–14) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷Adh2(1–19) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷ Adh2(1–24) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 adh2Δ0∷ Adh2(1–31) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷Adh2(1–4) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–9) Rluc This paper 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–14) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXadh2Δ0∷Adh2(1–19) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷ Adh2(1–24) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX adh2Δ0∷ Adh2(1–31) Rluc 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 atp2Δ0∷Atp2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX atp2Δ0∷Atp2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ssa1Δ0∷Ssa1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMXssa1Δ0∷Ssa1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Lsc2Δ0∷Lsc2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Lsc2Δ0∷Lsc2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Sod2Δ0∷Sod2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Sod2Δ0∷Sod2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Trr2Δ0∷Trr2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Trr2Δ0∷Trr2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Rki1Δ0∷Rki1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX RkiΔ0∷Rki1 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Tdh2Δ0∷Tdh21 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Tdh2Δ0∷Tdh2 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 Tom70Δ0∷Tom70 3XFlag∷His3 | This paper | N/A |
| MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 lia1Δ0∷KANMX Tom70Δ0∷Tom70 3XFlag∷His3 | This paper | N/A |
| Oligonucleotides | ||
| ADH2-RT-F: GCCGCTCACATTCCTCAAGGTAC |
IDT | N/A |
| ADH2-RT-R: GCCCAGTGGCCTGCTCTC |
IDT | N/A |
| SOD2-RT-F: TCAAGTTCCATGGCGGTGG |
IDT | N/A |
| SOD2-RT-R: GCCAAAGCGCCGGTGG |
IDT | N/A |
| ACT1-RT-F: TGGCCGGTAGAGATTTGACTGAC |
IDT | N/A |
| ACT1-RT-R: GTTCGAAGTCCAAGGCGACG |
IDT | N/A |
| Other | ||
| Anti-Flag® M2 affinity gel | Sigma | Cat# A2220-25ML |
| Micro Bio-Spin® column | Bio-rad | Cat# 732-6221 |
| S-Trap micro spin column | PROTIFI | Cat# C02-micro-80 |
| Acclaim PepMap C18 RP trapping column | Thermo Scientific | Cat# 164564-CMD |
| Acclaim PepMap C18 RSLC Nano column | Thermo Scientific | Cat# 164,941 |
| PVDF membrane | Biorad | Cat# 1620177 |
| 400 micron Acid Washed Zirconium Beads | Ops Diagnostics | Cat# BAWZ 400-250-35 |
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


