SUMMARY
The anabolic response to dietary protein can be defined as the difference between protein synthesis and breakdown, or the net protein balance, in response to ingestion of protein alone or a mixed meal containing protein. Others have concluded that a maximal anabolic response can be achieved with ingestion of 20–35 g of a high quality protein, leading to the formulation of a popular concept that the maximal anabolic response can be achieved by distributing the total protein intake evenly throughout the day, rather than eating a majority of dietary protein with dinner. However, this concept was based entirely on the measurement of muscle protein synthesis and thus ignored the potential contributions of suppression of protein breakdown to the anabolic response, as well as the possibility that tissues and organs other than muscle may also play a role in the anabolic response. In this review we discuss the factors comprising the total anabolic response, discuss relevant methodological issues, derive a theoretical maximal anabolic response based on current literature values, and interpret recent papers addressing the issue of maximal anabolic response as well as meal distribution of dietary protein. We conclude that it is not likely that there is a practical limit to the maximal anabolic response to a single meal, and the most efficient way in which to maximize the total anabolic response over a 24-h period is to increase dietary protein at breakfast and lunch without reducing protein intake with dinner.
Keywords: Optimal protein intake, Muscle protein synthesis, Essential amino acid, Protein intake pattern, Protein quality
1. Introduction
Dietary protein intake serves many physiological roles, but the most prominent is the maintenance or gain of body protein stores. This is accomplished by stimulation of protein synthesis, the inhibition of protein breakdown, or a combination thereof. A net gain in protein balance (i.e., synthesis minus breakdown) is called an anabolic response, as opposed to a catabolic response caused by the rate of protein breakdown exceeding the rate of protein synthesis. An anabolic response usually refers to gain of muscle protein, but can involve the entire body. Optimal protein nutrition in an individual meal could be defined as the minimal amount of protein intake that results in the maximal anabolic response, as that will be the most likely approach to maintaining or increasing lean body mass (LBM) over time. Consumption of dietary protein in excess of the amount needed to elicit the maximal anabolic response could be considered excessive, since no further stimulation of the net gain of body protein can occur. Consequently, determining the amount of dietary protein needed to elicit the maximal anabolic response is directly relevant to defining the “optimal” amount of dietary protein in a meal. Determining the optimal amount of dietary protein in a meal involves quantification of the rates of protein synthesis, breakdown, and the balance between synthesis and breakdown in response to dietary protein in the context of a complete mixed meal. There is presumably a limit to the extent to which protein synthesis can be stimulated by dietary protein intake, and protein breakdown cannot be suppressed to less than zero. Therefore, there must be some level of protein intake beyond which no further gains in net balance can occur, which we will define as the maximal anabolic response.
It has been postulated that the maximal anabolic response can be elicited with intake of 20–35 g of high quality protein solely based on the stimulation of muscle protein synthesis (MPS) [1]. If true, this would mean that the typical, uneven distribution of dietary protein intake in the American diet results in considerable excessive protein consumption in the dinner meal, as the average protein intake at dinner may be as much as 40 g or more [2]. As a consequence, it has been proposed that the anabolic response to protein intake would be improved if the traditional pattern of the American diet in which approximately half of dietary protein is consumed at dinner were altered so that daily intake of protein is more evenly distributed throughout breakfast, lunch and dinner. In this paper we will examine the validity of this perspective within the practical range of protein intake (i.e., the Acceptable Macronutrient Distribution Range or AMDR, 10–35% of daily calorie intake). In that context we will discuss if the maximal anabolic response to dietary protein is important physiologically, the physiological determinants of the net anabolic response, and the methodologies that have been used to evaluate the “even distribution” hypothesis. We will discuss relevant experimental data from studies performed in human subjects, including consideration of the difference between the responses to pure protein intake as opposed to intake of a protein-containing mixed meal. Finally, we will conclude that, based on currently-available data, there is no practical limit to the anabolic response to dietary protein intake at least within the range of AMDR in a single meal. Further, we conclude that the total amount of dietary protein over the day is more relevant to the total anabolic response to dietary protein than the distribution of intake over the course of the day. From a practical standpoint this conclusion can most readily be incorporated into daily nutritional pattern by increasing the amount of dietary protein as part of breakfast and lunch without diminishing the amount of dietary protein eaten at dinner.
2. Is a maximal anabolic response important? Implications for health and disease
The anabolic response in the fed state affects protein mass in many tissues and organs, but prominently involves repletion of skeletal muscle proteins lost in the post-absorptive or fasting state. The issue is thus important in maintaining or increasing skeletal muscle mass. In the fasted state the net breakdown of muscle protein (i.e., protein breakdown exceeding protein synthesis) provides amino acids into the circulation. The transient increase in muscle protein breakdown (MPB) in the post-absorptive or fasting state is very effective in this process, as fasting amino acid levels remain constant for days and even weeks if muscle mass is adequate [3]. Many of the processes maintained by the supply of plasma amino acids in the fasted state involve protein synthesis in tissues such as skin and brain that have little protein reserve and therefore must maintain a balance between protein synthesis and breakdown, even in the fasted state. In addition to tissue protein synthesis, plasma amino acids are involved in maintaining other processes during fasting, such as synthesis of enzymes, neurotransmitters, and nitric oxide (important for regulation of blood flow and immune function) and gluconeogenesis. The importance of skeletal mass (reflected in LBM) are evident in many (patho) physiological circumstances such as starvation, cancer, obesity, osteoporosis, and sarcopenia [4]. In this regard, the fast turning-over gut tissue plays an important role for reducing the burden placed on muscle tissue to provide amino acids to the circulation for maintaining the processes mentioned above. This will be discussed later in this paper. Further, the basal rates of protein synthesis and breakdown of skeletal muscle constitute a significant component of total resting energy expenditure, so maintenance of muscle (protein) mass plays an important role in energy balance [4]. Finally, and perhaps most obviously, maintenance of muscle mass is central to physical strength and function. Since it is undeniable that maintaining or increasing skeletal muscle mass is important, and the anabolic response to protein intake at the muscle level is the principle metabolic basis to achieve this goal, it follows that maximizing the anabolic response to dietary protein over the entire day is an important nutritional goal.
3. The balance between protein synthesis and breakdown: the metabolic determinants of the anabolic responses
Changes in muscle protein mass are a consequence of net changes in anabolic or catabolic responses to factors including nutritional intake, hormonal milieu, physical exercise, starvation, inflammation, and more serious physiological stress such as sepsis or trauma. Changes in muscle mass are most conventionally considered the primary target of anabolic and catabolic responses, although imbalances between protein synthesis and breakdown can occur in many body tissues and organs to some extent. Thus, to determine the extent of net anabolism or catabolism it is necessary to quantify both rates of protein synthesis and breakdown not only at the muscle level but at the whole-body level. There is a relationship between protein turnover (i.e., protein synthesis and protein breakdown) and the availability of amino acids that can be incorporated into proteins. Intracellular amino acids are derived from the inward transport from plasma and from protein breakdown. The possible fates of intracellular amino acids are new protein synthesis, efflux back to plasma, or, in some cases, oxidation. In the post-absorptive state, the net efflux of amino acids from the muscle into the blood predominates. However, ingestion of a protein rich meal increases inward transport of plasma amino acids into muscle, which in turn increases intracellular amino acid availability and thus protein synthesis. Quantitatively, protein breakdown is the most important source of the intracellular amino acid pool and thus precursors for protein synthesis [5]. There is thus a close link between protein synthesis and breakdown that is evident even in various catabolic circumstances including type I diabetes, cancer cachexia, and burn injury. In these catabolic circumstances, the rate of protein synthesis is typically normal or often elevated even though there is net loss of muscle tissue over time [6]. The reason for this counterintuitive phenomenon of an elevated rate of protein synthesis in a catabolic state is that availability of intracellular amino acids (regardless of the source – in this case protein breakdown) is the main determinant of the rate of protein synthesis. In other words, acceleration of protein breakdown drives protein synthesis as a consequence of increased availability of amino acids. However, because some portion of intracellular amino acids are either oxidized or released from the cell into the plasma, protein synthesis will be stimulated less than the elevation in breakdown, leading to a net loss of muscle protein over time.
The extent to which the interplay between protein synthesis and breakdown determines if the body gains or loses protein underscores the importance of simultaneous determination of both protein synthesis and protein breakdown when assessing the maximal anabolic response to dietary protein. If only the stimulated protein synthesis in a seriously burned patient is measured, for example, one could erroneously conclude that the patient was in anabolic state, when the exact opposite was actually the case. This point is relevant to the quantification of the response to dietary protein intake, in that if only the synthetic response is measured the total net response is unknown.
4. Methodological considerations in determination of the anabolic response
There are various methods that estimate anabolic responses to dietary protein at the muscle and the whole body levels in response to a single meal or over the course of an entire day [5,7]. We will discuss representative stable isotope tracer methods for exploring anabolic responses at the muscle and the whole-body levels. It has been shown that the tracer methods to quantify protein kinetics (protein synthesis, protein breakdown, and net protein balance) in response to dietary protein in acute metabolic studies predict changes in LBM over a longer time period [8], which can be translated to improvement in muscle strength [9].
Differences between MPS and MPB determine the magnitude of the muscle anabolic response. MPS is typically determined as the fractional synthetic rate (FSR) using the tracer incorporation method [10]. To measure muscle protein FSR, any amino acid tracer (phenylalanine is popular choice) is either infused or injected as a bolus, and protein FSR is calculated as changes in isotopic enrichment of the representative amino acid in the bound proteins at the beginning and the end of the measurement from the sampled muscle tissues, and then divided by mean enrichment of the precursor amino acid during the same time frame. For a complete understanding of muscle anabolic response, it is necessary to simultaneously determine FSR and FBR. For the measurement of muscle protein FBR, the decay in enrichment of the tracer amino acid in the intracellular space is determined after cessation of tracer infusion or after a bolus injection. The shape of decay curve indicates the magnitude of dilution by total appearance of unlabeled tracee, either from inward flux and/or protein breakdown, both of which can be differentiated mathematically for the calculations of MPB [11]. The principal advantage of the FSR and FBR techniques are that they are direct reflections of the response of muscle protein metabolism. On the other hand, these methods are limited to some extent by the assumption that the responses at a single site reflect all skeletal muscle [12]. The time frame of determination of FSR and FBR are generally different, as the FSR method allows the integration of response over several hours or even the entire day, whereas the FBR is determined over a much more limited time frame. In addition, the FBR technique assumes a physiological steady state, which makes the determination of the response to a meal difficult. Further, the measurement of FBR is difficult with respect to a complicated experimental protocol and kinetic calculations. Consequently, the assessment of the maximal anabolic response has involved only the measurement of FSR, and the FBR has been assumed to not change with dietary protein intake. An alternate approach to the determination of muscle protein kinetics is to simultaneously quantify MPS and MPB with an arteriovenous balance (A–V balance) technique and stable isotope tracers [13]. This method enables quantification in skeletal muscle of all of the rates illustrated in Fig. 1, and is applicable in the non-steady state following a meal [14]. The theoretical and empirical validation of this methodology is extensive (e.g., Ref. [15]). On the other hand, the method requires femoral artery and venous catheterization in addition to muscle biopsies, and is therefore relatively invasive. In addition, the kinetic calculations require measurement of leg muscle blood flow, which can be difficult. As is the case for the measurement of FSR/FBR, the leg A–V model has not been applied to the question of the maximal anabolic response.
Fig. 1.
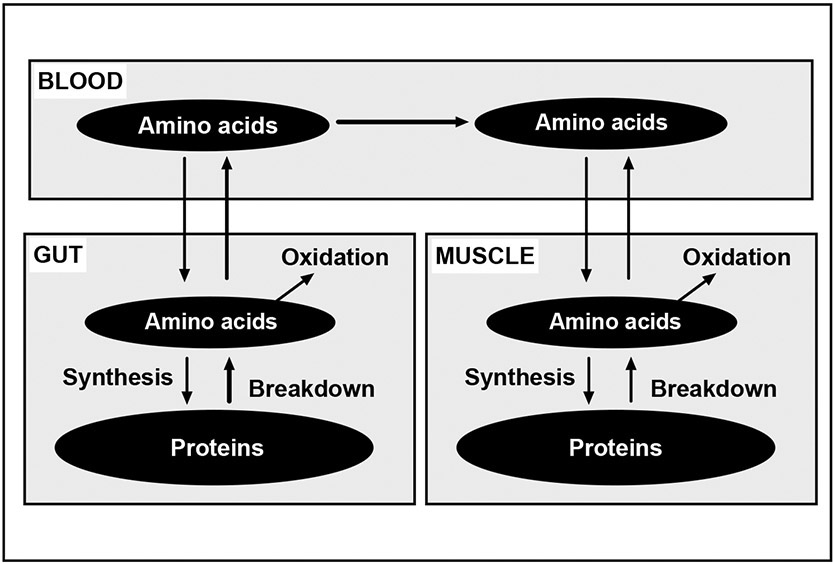
The muscle anabolic response is not only determined by protein synthesis but by the balance between protein synthesis and breakdown. Responses in the gut can also contribute to the anabolic response. Thus, the total anabolic response to protein-containing meals should be determined at whole body level in addition to at the muscle level to capture the entire response.
The whole body protein turnover approach to the measurement of protein synthesis and breakdown has advantages in the context of determining the overall maximal total anabolic response to a single meal containing protein. There are different approaches to determining whole body protein synthesis and breakdown going back close to 100 years [16]. However, the general methodology to measure dynamic changes in protein synthesis and breakdown that is most relevant to the determination of the maximal anabolic response is based on the intravenous infusion of a single or multiple essential amino acid (EAA) tracers. This approach enables the determination of the rate of appearance (Ra) into and the rate of disappearance (Rd) from plasma of a representative EAA such as phenylalanine or leucine [5]. The Ra of the unlabeled EAA corresponding to the EAA tracer (i.e., the tracee) directly reflects the rate of protein breakdown in the fasting state, since there is no endogenous production of an EAA. Rd of unlabeled tracee reflects the sum of the two possible routes of metabolic clearance: protein synthesis and oxidation. Oxidation can be measured either from the rate of labeled CO2 production, 15N production, or in the case of phenylalanine, the rate of hydroxylation of phenylalanine to tyrosine [5]. Thus, if rates of oxidation or hydroxylation are quantified, protein synthesis can be deduced from the difference between Ra and oxidation (or hydroxylation). The advantage of using a phenylalanine tracer as compared to leucine is that the determination of the isotopic enrichment and production of expired CO2 is not required with the phenylalanine tracer approach, but the underlying principles of the methods are basically the same. If unlabeled phenylalanine is ingested (i.e., consumption of a protein-containing meal), an EEA tracer with a different label than the infused one is added to the meal to quantify the contribution of the protein from the meal to the total Ra of the amino acid. Alternatively, the contribution of protein from the meal can be estimated based on the digestibility (i.e., absorption of exogenous protein as amino acids into the circulation) of different protein sources [17] as in our previous studies [18-20]. Calculation of the rates of whole body protein synthesis and breakdown requires dividing rates of the amino acid tracee flux by its fractional contribution to total body protein. The principle limitation of the whole-body approach is that tissue-specific information is not provided. On the other hand, there are several advantages of the whole body approach. First, although it is always desirable from a methodological standpoint to perform tracer studies in a physiological steady state, the whole body approach using a single amino acid tracer can dynamically measure protein synthesis and breakdown in the non-steady state following meal ingestion [18-20]. Additionally, while the anabolic response conventionally refers to the response of muscle, in fact the total whole body response is the most relevant from a nutritional standpoint, as described in the next section. The whole body approach is relatively non-invasive, since only peripheral venous catheters are required, and the methodology is particularly applicable to the determination of the response to different levels of protein intake, since both rates of protein synthesis and breakdown are determined. The method is very responsive to varying levels of protein intake [18-20].
5. Relation of the anabolic response at the muscle level to the total whole body response
The determination of the anabolic response to dietary protein intake at the muscle level is obviously important, since muscle is a major fate of EAAs absorbed from dietary protein. However, tissues other than muscle account for more than half of the total protein turnover [21,22]. Consequently, determination of the anabolic response at the muscle level could underestimate total anabolic response. Figure 1 illustrates schematically the potential role of protein turnover in the gut on the muscle anabolic response over time. In the fasted state there is a net amino acid efflux from muscle, i.e., a catabolic state. Consumption of dietary protein stimulates MPS within an hour. In addition, a significant portion of the amino acids absorbed from the meal will be retained in the splanchnic area, mainly in the gut [22,23]. The retention of amino acids in the gut may be amplified by a systemic insulin response to a mixed meal [22]. Over time EAAs released from protein breakdown in the rapidly turning-over gut tissues can be released into peripheral blood and then be incorporated into new proteins in muscle. This potential mechanism is particularly beneficial in situations in which protein intake is greater than the amount that stimulates a maximal MPS.
Our recent findings are consistent with the possibility that gut protein turnover plays a role in the anabolic response. In an acute metabolic study, we showed that MPS was stimulated to the same extent by two doses of protein intake (40 g vs. 70 g), while the higher protein intake resulted in a greater whole body protein synthesis (also net balance) [19]. In accordance with our acute study, we showed in an 8-week chronic study that even protein intake distribution over the day did not result in a higher LBM as compared with uneven protein intake distribution with dinner containing far more than the “optimal” protein. Furthermore, Bray et al. [24] demonstrated in an 8-week intervention study that LBM increased linearly with increasing amount of protein even above the “optimal” dose that has been shown to maximally stimulate MPS following increasing dose of high-quality protein [25].
6. Role of insulin in the anabolic response
The maximal anabolic response at the muscle level has been determined in many studies in response to the consumption of a pure protein [26-28]. The maximal anabolic response to dietary protein is complicated when considered in the context of a mixed meal, in part because of the impact of an increase in insulin concentration resulting from the meal intake. Ingestion of certain proteins and amino acids can stimulate insulin release, but the effect of carbohydrate intake on insulin release is more pronounced. Consumption of a mixed meal may decrease the plasma amino acid levels, even if the content of protein in the meal is relatively high [19,29]. The increase in insulin concentration in response to meal feeding is responsible for the suppression of plasma amino acid concentrations, largely because of an inhibition of protein breakdown [18-20,30]. Insulin may also stimulate the capacity of muscle to produce new protein, but the experimental protocol must be quite specific to demonstrate this effect. If insulin is infused locally into skeletal muscle at a rate low enough to avoid systemic reductions in plasma amino acids, MPS is stimulated [31]. This indicates a potential anabolic stimulatory effect of insulin on MPS, which would seemingly amplify the maximal anabolic response to dietary protein as compared to the ingestion of protein alone. However, plasma amino acids are lower after ingestion of a mixed meal than the same amount of protein alone [19,29], due to the suppressive effects of insulin on protein breakdown. Since elevated plasma amino acid concentrations are the principal driving force behind an increase in MPS following ingestion of dietary protein, the lower amino acid concentrations following a mixed meal as compared to pure protein may reduce the response of MPS. On the other hand, the suppression of breakdown due to insulin may amplify the net gain in the balance between synthesis and breakdown following a meal as compared to ingestion of protein alone. All of these considerations may vary in importance, depending on the amounts of protein, carbohydrate and fat in a mixed meal. Clearly there is no accurate way in which to predict the difference in response to a certain amount of dietary protein in a mixed meal as compared to the protein alone. Only direct experimental quantification of the anabolic responses to dietary protein intake in the context of mixed meals can definitively address this issue.
7. Role of intracellular EAA availability in the anabolic response
Although insulin suppresses protein breakdown, it is likely that there is an upper limit to this response. In accordance with this notion, it has been shown that no further suppression of leg muscle protein breakdown occurred at insulin concentration beyond 30 μ IU/ml [32]. However, it is likely that insulin cannot entirely explain the suppression of protein breakdown in response to a meal. We have previously shown that consumption of 70 g of dietary protein resulted in a greater reduction in protein breakdown at the whole-body level as compared to lower protein intake (40 g), despite similar insulin responses [19]. Regardless of the tissue in which this further suppression of breakdown occurred, it will ultimately affect the anabolic response at the muscle level. In this regard, we have previously postulated that an increase in intracellular EAA concentrations due to hyperaminoacidemia following a high protein intake leads to further suppression of protein breakdown [19]. Thus, with adequate intracellular EAA precursors to support a given rate of MPS via inward transport, provision of additional intracellular precursors from protein breakdown is no longer required. Further investigation in this issue, including identifying a mechanism of action whereby the intracellular concentration of amino acids control protein breakdown, is warranted.
8. Is there a maximal anabolic response?
We previously presented data supporting the perspective that the maximal total anabolic response increases linearly in relation to the amount of amino acid or protein intake [18,19,33-35]. These data did not show a plateauing in the gain in the net balance between protein synthesis and breakdown as the amount of intake increased. It is reasonable to assume that there must be some upper limit to the anabolic response to the amount of dietary protein in one meal. There is some level of protein intake that maximally stimulates protein synthesis, and the rate of protein breakdown can only be suppressed to a certain extent because a negative rate of protein breakdown is impossible. Thus, the short answer to the question – Is there a maximal anabolic response to dietary protein? – is must be yes. The amount of dietary protein that elicits the maximal anabolic response is the key point of relevance to protein nutrition. In order to draw quantitative conclusions, it is necessary to evaluate current data in the context of the preceding discussion of the roles of protein synthesis and breakdown in the anabolic response, as well as the methodologies that have been used to address this issue.
There is a theoretical upper limit to the anabolic response to dietary protein intake that is dictated by the maximal extent to which protein synthesis can be stimulated and protein breakdown can be suppressed. For the reasons discussed above, we will consider the whole body responses of protein synthesis and breakdown as the best reflection of the total anabolic response to dietary protein. We can make a reasonable estimate of the maximal whole body anabolic response from data in the literature. In our recent acute metabolic study, we observed that in the fasting state whole body protein synthesis was approximately 3.5 g/kg/day, and protein breakdown was approximately 4.3 g/kg/day although not shown explicitly in the paper [19]. Although it is well documented that the maximal increase in MPS in response to consumption of pure dietary protein or amino acids is approximately ~100% greater than the basal value [26-28], similar data are not available for whole body protein synthesis. Similarly, protein dose–response data for the response of MPS to a mixed meal containing protein are not available. Consequently, there is a degree of uncertainty regarding an assumed maximal stimulation of whole body protein synthesis. We will make the conservative estimate that the maximal percent increase in whole body protein synthesis is half as great as the response of MPS, or 50% greater than the basal value. This assumption is based on the fact that muscle is the primary target of dietary protein [4], and therefore muscle is more responsive to variations in intake than other tissues and organs. In our example, a 50% increase in whole body protein synthesis would be 3.5 g/kg/day × 0.50, or 1.75 g/kg/day. With regard to the maximal suppression of breakdown, we have previously shown that intake of a mixed meal containing protein suppressed breakdown by approximately 60% [19], and breakdown cannot be totally shut off, so we can be reasonably confident that whole body protein breakdown can be maximally suppressed to 20% of its basal value (80% suppression). This corresponds to a reduction in breakdown of 3.44 g/kg/day. When the maximal rate of suppression of breakdown (3.44 g/kg/day) is added to the maximal increase in synthesis (1.75 g/kg/day), the total maximal anabolic response is 5.19 g/kg/day, or 216 mg/kg/h. However, the maximal response is not sustained for an entire day in response to a single meal. If we make the conservative assumption that the response to a single meal containing a large amount of protein to elicit the maximal anabolic response lasts for 3 h, then we calculate that the theoretical total anabolic response is approximately 3 h/meal × 216 mg/kg/h = 648 mg/kg/meal. The amount of protein intake required to stimulate the maximal anabolic response depends on the quality of the protein, where quality is defined by the amount and profile of EAAs, as well as the true ileal digestibility of the protein [17,36]. For the purpose of this example, we will consider the case of a high quality protein that is extensively digested. In this case, then about 25% of dietary protein is potentially available for incorporation into protein [37]. This assumption is based on the long-standing knowledge that nitrogen retention is about 20–25% of ingested protein over a wide range of protein intake [37]. Thus, it would require approximately 2.6 g/kg/meal to achieve the maximal anabolic response at one meal. The value would be considerably higher with a lower quality protein. For a 70 kg individual it would therefore require 2.6 g/kg/meal × 70 kg = 182 g protein to achieve the maximal anabolic response. This amount of dietary protein considerably exceeds the amount of protein that would normally be eaten in a meal (10–60 g, [2]). Even if our estimates have exaggerated the actual amount of dietary protein needed to elicit maximal response by 100%, it is still highly unlikely that much protein consumption would occur in a single meal. Furthermore, it is possible that we have minimized, rather that exaggerated, the estimate of the maximal anabolic response. We assumed that the anabolic response lasts for 3 h, but with increasingly larger protein meals the time required for net protein synthesis to return to the baseline is likely to be prolonged beyond 3 h, thereby yielding an even greater anabolic response than we estimated.
Our conclusion from the calculation of the maximal anabolic response is that there is no practical limit to the response to a single meal of protein alone or in a mixed meal. This conclusion is consistent with available data in the literature. It has been recognized for more than 50 years that nitrogen balance increases linearly with increasing amounts of protein intake, even at rates of intake well beyond dietary requirements for protein [37]. Further, net protein balance increases linearly in relation to increasing amounts of dietary protein or amino acid intake when determined either by whole body protein kinetics or measurement of MPS and MPB [38]. Furthermore, it has been shown in an 8-week chronic study that LBM was increased linearly with increasing amount of protein intake encompassing 50–320g/day (0.72–4.2 g/kg/day) in young subjects [24]. The upper range of protein intake far exceeded the amount of protein that is known to stimulate a maximal MPS. This finding, in conjunction with findings from our previous studies [18,19], supports our conclusion.
The theoretical maximal anabolic response of 2.6 g/kg/meal far surpasses the value of 0.24 g (for young adults) or 0.4 g (for older adults) dietary protein/kg/meal for MPS determined in response to varying doses of whey protein isolate or egg protein [25]. There are several possible explanations for the large difference between the amount of high-quality protein needed to maximally stimulate MPS and the total anabolic response. First, the values of protein intake (i.e., 0.24 and 0.4 g/kg/meal for young and older adults, respectively) for maximizing MPS were determined in response to the intake of pure protein, whereas the maximal values in our calculations assumed the dietary protein was contained in a mixed meal that elicited an insulin response and potentially a rise in intracellular amino acids due to hyperaminoacidemia, with the associated suppression of breakdown. Second, we assumed that both the maximal synthetic effect as well as the maximal inhibition of breakdown at the whole body level could be achieved simultaneously in response to a single meal, and this may never occur in practice. On the other hand, our own study showed simultaneously significant and dose-dependent increases in whole body protein synthesis and suppression of breakdown in response to mixed meals with varying amounts of protein [18,19]. Third, while net gain in muscle protein is a primary fate of dietary protein, other tissues and organs respond to dietary protein as well, and the values mentioned above were determined for muscle only. This is a significant factor, as we showed that the whole body anabolic response exceeds the magnitude of the MPS response [18,19]. Finally, and probably most importantly, reliance on MPS excludes the contribution of the suppressive effect of protein breakdown. Our example shown above demonstrates that the potential gain in net muscle protein as a result of suppression of protein breakdown is at least as great as the potential gain as a consequence of stimulation of protein synthesis.
There was a reasonable rationale for relying on MPS as the sole criterion to determine the maximal anabolic response. Whereas the whole body is potentially involved in the anabolic response to protein ingestion, gain in muscle protein is the most relevant physiological response. Further, at lower levels of protein intake, protein breakdown is not affected as MPS is stimulated [39]. It also may be that the physiological impact of a gain in muscle mass due to stimulation of protein synthesis is greater than when the gain results from a suppression of breakdown. A stimulation of MPS may promote muscle remodeling and improved single muscle fiber function [40]. Muscle protein FSR, normalized for differences in muscle mass, is directly associated with increase strength [8] and function [3]. For example, athletic training generally results in an increase in muscle protein FSR (e.g., Ref. [39]). In contrast, no such remodeling of muscle would be anticipated as a consequence of a suppression of protein breakdown, even if muscle mass increased. Nonetheless, it is clear that assessment of the true anabolic response in terms of muscle mass must include either the measurement of protein synthesis and breakdown, or some other direct measure of muscle mass [1].
9. Does meal distribution of dietary protein matter?
Consumption of dietary protein or EAA stimulates MPS in a dose-dependent manner to a point, but at higher levels of intake the extent of stimulation of MPS plateaus (e.g., Refs. [26-28]). The minimum dose of dietary protein (eaten as pure protein) that stimulates a near maximal stimulation of MPS is 20–35 g protein [41,42] or more specifically 0.24–0.40 g/kg/meal depending on the quality of protein and individuals' age [25]. The conclusion that these values represent the maximal anabolic response has had a significant impact on recommendations for protein nutrition, as this dosage range has been promoted as an “optimal” level of protein intake per meal [1]. This recommendation is based on the fact that a greater amount of intake will not further stimulate MPS [1]. The notion that the “optimal” protein consumption at one meal is in the range of 20–35 g is significant because protein consumption with dinner in a typical American diet is between 40 and 60 g [2]. Consequently, it has been proposed that redistribution of total protein intake evenly over the day can result in a greater cumulative anabolic response than the traditional pattern of intake [1]. If true, redistribution of dietary protein intake over the day could potentially lead to improved maintenance of muscle mass and strength, as well as other functional and health benefits.
The recently postulated hypothesis regarding protein distribution throughout the day is predicated on the assumption that the amount of protein eaten with dinner exceeds the minimum amount of protein intake that achieves maximal anabolic response. However, the above discussion establishes that this rationale is faulty because MPS does not reflect the maximal anabolic response, and that to actually achieve the maximal anabolic response several fold more grams of dietary protein must be consumed. The rationale supporting an even distribution of protein throughout the day is further weakened by the so called “the muscle full” hypothesis. According to this hypothesis, stimulation of MPS following consumption of protein [29] or the intravenous infusion of amino acids [43] is diminished over time despite elevated amino acid concentrations. If so, a large amount of dietary protein at breakfast and lunch would limit the anabolic response to dietary protein at dinner. However, it is uncertain if the “muscle full” hypothesis applies in the context of mixed meals. For example, it has been shown that prior meals did not alter MPS responses, implying no role for the muscle full effect [44]. Further, we have shown that whole body net protein balance was not affected by prior meals but by the amount of protein intake in each meal [18]. In either case, the distribution hypothesis would not be supported.
Regardless of whether or not the “muscle full” effect functions physiologically, we do not find the argument favoring an even distribution of protein intake throughout the day to be compelling. In a recent acute metabolic study we did not observed any beneficial effects of distributing the same amount of dietary protein equally over three daily meals as compared to the normal American pattern of 65% of dietary protein with dinner as evaluated by measurement of whole body protein synthesis and breakdown as well as MPS [18]. The lack of impact of distribution of dietary protein was evident at two levels of dietary protein intake (0.8 and 1.5 g/kg/day), even though in the group receiving the higher amount of protein dinner contained 0.98 g/kg, which is more than twice the “optimal” value as estimated by MPS [25]. Our findings in older adults are in agreement with Murphy et al. [45]. We confirmed our findings in the acute study described above in our recent 8-week chronic study in which even distribution of protein intake throughout the day of 1.1 g protein/kg/day did not result in a higher LBM or muscle strength as compared to uneven distribution of protein intake [20]. The limitation of relying solely on MPS is illustrated by the findings of Mamerow et al. [46], who found that even distribution of protein intake (1.2 g protein/kg/day) in mixed meals resulted in a greater MPS response over 24 h in relatively young adults. This would be expected, since with the traditional distribution 0.78 g/kg/day would be consumed with dinner, which is about twice the protein dose that others have generally believed to induce a (near) maximal MPS. It is likely that had measured MPB or whole body protein synthesis and breakdown in their study, a different conclusion would have been obtained. In this regard, we have recently shown that increasing amount of protein intake resulted in a greater whole body anabolic response in a dose dependent manner (Fig. 2) [18,19]. Over a wide range of protein intake in mixed meals, encompassing 6.4–91.7 g, we observed a linear relationship between protein intake and whole body net anabolic response, extending previous findings [33,34]. Therefore, in terms of maximizing the anabolic response, the pattern of protein intake is of minimal importance as compared to the amount of protein consumed in the context of mixed meals. The practical implication of this perspective is that increasing the amount of dietary protein eaten with breakfast and lunch will benefit the overall anabolic response for the day, but in doing this there is no metabolic reason to decrease the amount of protein eaten with dinner.
Fig. 2.
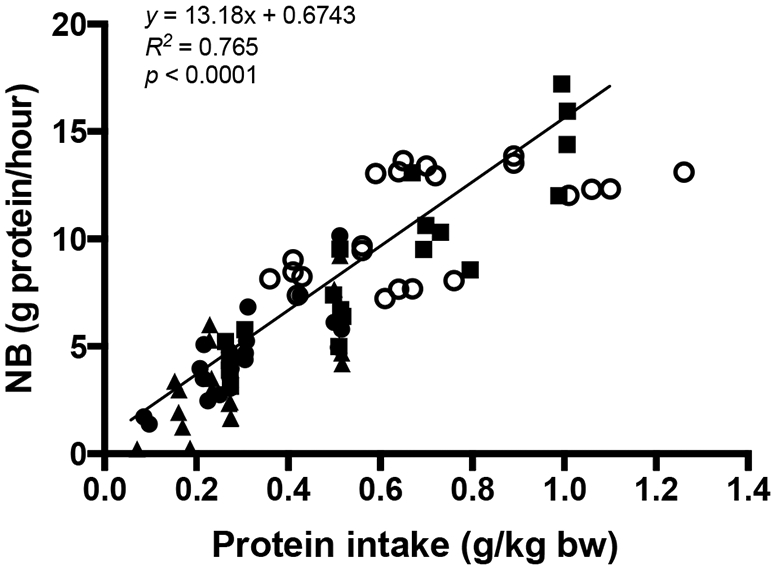
The relationship between protein intake (g per kg body weight) and whole body net anabolic response (protein synthesis – breakdown) following an individual mixed meal intake in older adults (52–75 yr): breakfast (closed triangle), lunch (closed circle), or dinner (closed rectangle) and young adults (18–40 yr, open circle). Linear regression analyses reveal that anabolic response was significantly related to dietary protein intake at levels of protein intake well beyond 0.4 g protein/kg body weight/meal: from references [18,19].
10. Summary and conclusions
The limit of the stimulation of MPS has been studied extensively, but little attention has been given to the suppression of protein breakdown at higher rates of protein intake. It has been reported that a maximal MPS response is achieved with 20–35 g of a high quality protein [41,42], or more specifically 0.24 g/kg/meal for young and 0.40 g/kg/meal for older adults [25]. This has been promoted as the maximal anabolic response [1]. However, this conclusion ignores the potential contribution of the suppression of protein breakdown to the anabolic response, as well as the fact that the whole body anabolic response may exceed the MPS response. Theoretical calculations suggest that the maximal anabolic response is in fact several folds higher than the maximal increase in MPS. Consistent with the theoretical calculations, experimental data show that the net gain in body protein in response to dietary protein intake is linear over a large range of protein intake. Consequently, it is unlikely that further gains in LBM could be expected to be achieved by altering the distribution of dietary protein intake without also significantly increasing the amount of protein consumed.
Acknowledgements
This project was financially supported by Pepper Center Grant PG30-AG-028718 and Award Number UL1-TR-000039 and KL2-TR-000063 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Conflicts of interest
Dr. Wolfe has received research grants and/or honoraria from the National Cattleman's Beef Checkoff program, Abbott Nutrition Pronutria, and PepsiCo; Dr. Deutz has received research grants and honoraria from Abbott Nutrition. Dr. Kim has no potential conflicts of interest.
References
- [1].Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 2009;12:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].USDA Agricultural Research Service. Energy intake: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, what we eat in America, NHANES 2013–2014. 2016. Available from: www.ars.usda.gov.
- [3].Ferrando AA, Paddon-Jones D, Hays NP, Kortebein P, Ronsen O, Williams RH, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr 2010;29:18–23. [DOI] [PubMed] [Google Scholar]
- [4].Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–82. [DOI] [PubMed] [Google Scholar]
- [5].Wolfe RR, Chinkes DL. Isotope tracers in metabolic research. 2nd ed. Hoboken, New Jersey: John Wiley & Sons, Inc; 2005. [Google Scholar]
- [6].Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia 1983;25:400–3. [DOI] [PubMed] [Google Scholar]
- [7].Kim I-Y, Suh S-H, Lee I-K, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med 2016;48:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab Endocr Soc 2004;89:4351–8. [DOI] [PubMed] [Google Scholar]
- [9].Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr Am Soc Nutr 2015;145:1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baumann PQ, Stirewalt WS, O'Rourke BD, Howard D, Nair KS. Precursor pools of protein synthesis: a stable isotope study in a swine model. 1994;267:E203–E209. [DOI] [PubMed] [Google Scholar]
- [11].Zhang X-J, Chinkes DL, Wolfe RR. Measurement of muscle protein fractional synthesis and breakdown rates from a pulse tracer injection. Am J Physiol Endocrinol Metab Am Physiolog Soc 2002;283:E753–64. [DOI] [PubMed] [Google Scholar]
- [12].Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, et al. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol (Lond) 2005;563:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Biolo G, Chinkes D, Zhang XJ, Wolfe RR. Harry M Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enter Nutr 1992;16:305–15. [DOI] [PubMed] [Google Scholar]
- [14].Katsanos CS, Chinkes DL, Sheffield-Moore M, Aarsland A, Kobayashi H, Wolfe RR. Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am J Physiol Endocrinol Metab Am Physiolog Soc 2005;289:E1064–70. [DOI] [PubMed] [Google Scholar]
- [15].Biolo G, Gastaldelli A, Zhang XJ, Wolfe RR. Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol 1994;267:E467–74. [DOI] [PubMed] [Google Scholar]
- [16].Schoenheimer R, Ratner S, Rittenberg D. The process of continuous deamination and reamination of amino acids in the proteins of normal animals. Sci Am Assoc Adv Sci 1939;89:272–3. [DOI] [PubMed] [Google Scholar]
- [17].Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr Am Soc Nutr 2015;145:372–9. [DOI] [PubMed] [Google Scholar]
- [18].Kim I-Y, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NEP, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab Am Physiolog Soc 2015;308:E21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim I-Y, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab Am Physiolog Soc 2016;310:E73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim I-Y, Schutzler S, Schrader AM, Spencer HJ, Azhar G, Wolfe RR, et al. Protein intake distribution pattern does not affect anabolic response, lean body mass, muscle strength or function over 8 weeks in older adults: a randomized-controlled trial. Clin Nutr 2017. 10.1016/j.clnu.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol 1988;254:E208–13. [DOI] [PubMed] [Google Scholar]
- [22].Deutz NE, Have Ten GA, Soeters PB, Moughan PJ. Increased intestinal amino-acid retention from the addition of carbohydrates to a meal. Clin Nutr 1995;14:354–64. [DOI] [PubMed] [Google Scholar]
- [23].Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. 1999; 277: E513–E520. [DOI] [PubMed] [Google Scholar]
- [24].Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA Am Med Assoc 2012;307:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 2015;70:57–62. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, et al. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 2012;108:1780–8. Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- [27].Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr Am Soc Nutr 2014;99:86–95. [DOI] [PubMed] [Google Scholar]
- [28].Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9:57. BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, et al. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr Am Soc Nutr 2010;92:1080–8. [DOI] [PubMed] [Google Scholar]
- [30].Pennings B, Groen BBL, van Dijk J-W, de Lange A, Kiskini A, Kuklinski M, et al. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr Am Soc Nutr 2013;98:121–8. [DOI] [PubMed] [Google Scholar]
- [31].Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Investig Am Soc Clin Investig 1995;95:811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab Am Physiolog Soc 2008;295:E595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Engelen MPKJ, Com G, Wolfe RR, Deutz NEP. Dietary essential amino acids are highly anabolic in pediatric patients with cystic fibrosis.J Cyst Fibros 2013;12:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jonker R, Deutz NEP, Erbland ML, Anderson PJ, Engelen MPKJ. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr 2014;33:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Engelen MPKJ, Safar AM, Bartter T, Koeman F, Deutz NEP. High anabolic potential of essential amino acid mixtures in advanced nonsmall cell lung cancer. Ann Oncol 2015;26:1960–6. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wolfe RR, Rutherfurd SM, Kim I-Y, Moughan PJ. Protein quality as determined by the digestible indispensable amino acid score: evaluation of factors underlying the calculation. Nutr Rev 2016;74:584–99. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hegsted DM. Assessment of nitrogen requirements. Am J Clin Nutr 1978;31:1669–77. [DOI] [PubMed] [Google Scholar]
- [38].Deutz NE, Wolfe RR. Is there a maximal anabolic response to protein intake with a meal? Clin Nutr 2013;32:309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cahill GF, Aoki TT. Renal gluconeogenesis and amino-acid metabolism in man. Med Clin North Am 1975;59:751–61. [DOI] [PubMed] [Google Scholar]
- [40].Fitts RH, Romatowski JG, Peters JR, Paddon-Jones D, Wolfe RR, Ferrando AA. The deleterious effects of bed rest on human skeletal muscle fibers are exacerbated by hypercortisolemia and ameliorated by dietary supplementation. Am J Physiol Cell Physiol Am Physiolog Soc 2007;293:C313–20. [DOI] [PubMed] [Google Scholar]
- [41].Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr Am Soc Nutr 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- [42].Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc 2009;109:1582–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol (Lond) 2001;532:575–9. Wiley-Blackwell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab Am Physiolog Soc 2005;288:E761–7. [DOI] [PubMed] [Google Scholar]
- [45].Murphy CH, Churchward-Venne TA, Mitchell CJ, Kolar NM, Kassis A, Karagounis LG, et al. Hypoenergetic diet-induced reductions in myofibrillar protein synthesis are restored with resistance training and balanced daily protein ingestion in older men. Am J Physiol Endocrinol Metab Am Physiolog Soc 2015;308:E734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, et al. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr Am Soc Nutr 2014;144:876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


