Abstract
OBJECTIVE.
The objective of this study was to assess to the role of contrast-enhanced digital mammography (CEDM) as a screening tool in women at intermediate risk for developing breast cancer due to a personal history of lobular neoplasia without additional risk factors.
MATERIALS AND METHODS.
In this institutional review board-approved, observational, retrospective study, we reviewed our radiology department database to identify patients with a personal history of breast biopsy yielding lobular neoplasia who underwent screening CEDM at our institution between December 2012 and February 2019. A total of 132 women who underwent 306 CEDM examinations were included. All CEDM examinations were interpreted by dedicated breast imaging radiologists in conjunction with a review of the patient’s clinical history and available prior breast imaging. In statistical analysis, sensitivity, specificity, NPV, positive likelihood ratio, and accuracy of CEDM in detecting cancer were determined, with pathology or 12-month imaging follow-up serving as the reference standard.
RESULTS.
CEDM detected cancer in six patients and showed an overall sensitivity of 100%, specificity of 88% (95% CI, 84–92%), NPV of 100%, and accuracy of 88% (95% CI, 84–92%). The positive likelihood ratio of 8.33 suggested that CEDM findings are 8.3 times more likely to be positive in an individual with breast cancer when compared with an individual without the disease.
CONCLUSION.
CEDM shows promise as a breast cancer screening examination in patients with a personal history of lobular neoplasia. Continued investigation with a larger patient population is needed to determine the true sensitivity and positive predictive value of CEDM for these patients.
Keywords: atypical lobular hyperplasia, contrast-enhanced digital mammography, lobular carcinoma in situ, lobular neoplasia, screening
Breast cancer accounted for the largest number of new cancer diagnoses and the second largest number of cancer deaths in women in 2019 [1]. Although mammography is currently the only breast imaging modality proven to reduce mortality, the examination has limitations. Despite its widespread availability and relatively low cost, the limited sensitivity and specificity of mammography, especially in women with dense breasts, illustrates the need for improved or supplemental screening [2, 3].
The risk of developing breast cancer varies among women. Certain groups of women are classified as having a high risk (≥ 20% lifetime risk), including women with deleterious gene mutations and their untested first-degree relatives, women with a history of chest irradiation between ages 10 and 30 years old, women with an extensive family history of breast cancer without an identified mutation, and women with a personal history of breast cancer who also have dense breasts or a personal history of breast cancer diagnosed before age 50 years old. For women with a high risk, supplemental screening with annual contrast-enhanced breast MRI in addition to mammography is recommended. Other women are classified as having an intermediate risk (15% to < 20% lifetime risk), including women with a personal history of breast cancer diagnosed after age 50 years old; those with a family history of breast cancer; and those who have had biopsy-proven high-risk lesions, including lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH), collectively referred to as lobular neoplasia. These high-risk lesions confer a 4–10% increased risk of developing breast cancer compared with individuals without specific risk factors, thus placing these women in an intermediate risk group [4–8]. Given the predictable increased risk of developing breast cancer, screening tools with higher sensitivity may be justified for women with an intermediate risk. Currently, standard recommendations for women at intermediate risk have not been established.
Screening MRI is a highly sensitive examination that allows the concurrent evaluation of both morphologic and functional information through the imaging of tumor neovascularity and perfusion. Studies have shown that in approximately 45% of patients at intermediate to high risk for developing breast cancer, the cancer is occult on mammography and/or ultrasound and is detected only on MRI [9, 10]. However, MRI is associated with high false-positive rates, which can lead to unnecessary patient stress and anxiety and additional biopsies. In addition, MRI is expensive and time-consuming, making accessibility an issue for certain patient populations [11, 12].
Contrast-enhanced digital mammography (CEDM) is an emerging breast imaging technique that, similar to MRI, combines both structural and functional information by showing evidence of neovascularity. The examination is less time-consuming than MRI and significantly less expensive. Because CEDM involves a hardware and software addition to many standard mammography units, it can potentially be offered to a wider range of patient populations. CEDM has already been shown to be effective in the diagnostic setting, in evaluating abnormal screening mammograms and symptomatic patients [13], and in staging known cancers both before surgery and before and after neoadjuvant chemotherapy [14–16]. Early studies suggest CEDM may be of value in screening women at increased risk, some women with dense breasts, and women at high risk who cannot undergo MRI [17–19].
Given the diverse groups of women who are at increased risk for developing breast cancer, a screening paradigm that incorporates individual risk assessment and optimizes the sensitivity and specificity of the breast imaging examination for individual indications is of paramount importance. Recommendations tailored to specific risk groups, such as women with a history of lobular neoplasia, are necessary. The purpose of this study was to assess the role of CEDM as a screening tool in women at intermediate risk for developing breast cancer due to a personal history of lobular neoplasia without any additional risk factors.
Materials and Methods
Patients
Our institutional review board approved this HIPAA-compliant retrospective study, and the need for patient consent was waived.
We retrospectively reviewed the radiology department database to identify patients who had an intermediate risk of developing breast cancer due to a personal history of breast biopsy yielding lobular neoplasia, who underwent screening CEDM at our institution between December 2012 and February 2019, and who had at least 1 year of imaging follow-up. This resulted in 132 women who underwent 306 CEDM examinations. Patients with additional risk factors including personal or family history of breast cancer (in a first- or second-degree relative), deleterious gene mutation, or personal history of mantle radiation were excluded.
Institutional electronic medical records were then reviewed to confirm pathologic tissue diagnosis of LCIS and/or ALH, age at diagnosis, age at the screening examination, and breast density.
Of the 132 women in the study, 52 were previously reported in the literature: 43 in a study that compared screening contrast-enhanced mammography and breast MRI [19] and nine in a study evaluating CEDM in women with a higher-than-average risk of breast cancer [17]. Whereas the prior studies included women with variable risk factors that resulted in their higher-than-average lifetime risk (with many women classified as having a high risk), the current study was designed to be specifically limited to women at intermediate risk with a history of lobular neoplasia and no other risk factors.
CEDM Examination
All CEDM examinations were performed on a dual-energy mammography system (Senographe Essential, GE Healthcare). Each patient received iohexol (Omnipaque 350, GE Healthcare) at a dose of 1.5 mL/kg through IV power injection at a rate of 3 mL/s up to a maximum dose of 150 mL. The contrast injection was followed by a delay of approximately 2.5 minutes so the patient could be positioned for CEDM. Craniocaudal and mediolateral oblique views of each breast were obtained. Each view was imaged with two almost simultaneous exposures, a low-energy exposure (26–30 kVp) and a high-energy exposure (45–49 kVp), to straddle the K-edge of iodine. Low-energy images are the equivalent of a 2D full-field digital mammogram [20]. Iodine images recombine the low- and high-energy images by using a proprietary algorithm to elucidate areas of contrast enhancement.
Imaging Interpretation
All CEDM examinations were interpreted by dedicated breast imaging radiologists in conjunction with a review of the patient’s clinical history and available prior breast imaging studies. Reports from prior examinations were reviewed to determine breast density. Reviewers also determined whether CEDM findings were seen on low-energy images, iodine images, or both.
Any abnormalities seen on either low-energy images or iodine images were further evaluated at the time of CEDM with additional mammographic views and/or ultrasound according to the routine standard of care at our institution. For patients with areas of enhancement on CEDM without a mammographic or sonographic correlate, breast MRI was recommended for further characterization and potential biopsy because a Food and Drug Administration–approved CEDM biopsy device for sampling lesions seen only on iodine images does not currently exist. A final American College of Radiology BI-RADS category was assigned for each patient on the basis of all imaging findings [21].
Reference Standard
Outcomes were determined based on either pathology or negative 12-month imaging follow-up. Pathology records of biopsies performed as a result of a CEDM finding were reviewed. Biopsy results were used to categorize the lesions as benign, high risk, or malignant. The number of cancers detected and the method of detection were also evaluated.
Statistical Evaluation
Epidemiologic parameters such as sensitivity, specificity, NPV, positive likelihood ratio, and accuracy of CEDM were determined using the PROC SURVEYFREQ statement in SAS (version 9.4, SAS Institute). The correlation between successive diagnostic measurements in the same patient was accounted for by using the CLUSTER option in PROC SURVEYFREQ, thereby producing reasonable and robust estimates of the aforementioned parameters. We estimated 95% CIs for sensitivity and specificity using the Clopper-Pearson method for binomial proportions [22]. The 95% CIs of NPV were estimated as standard logit CIs [23].
Results
Patient Characteristics
Within the study population of 132 women who underwent 306 CEDM examinations, the median number of CEDM examinations performed per patient was 2 (range, 1–6). Table 1 summarizes the patient characteristics. The median age at diagnosis of lobular neoplasia was 47 years (range, 27–66 years; data were missing for one patient), and the median age at initial CEDM examination was 52 years (range, 35–70 years). The median interval between diagnosis of lobular neoplasia and initial CEDM examination was 2 years (range, 0–20 years).
TABLE 1:
Clinical Characteristics of Women Included in the Study
| Characteristic | Value |
|---|---|
| No. of patients | 132 |
| No. of examinations | 306 |
| Examinations per patient | 2 (1–6) |
| Age (y) | |
| At initial CEDM screening | 52 (35–70) |
| At diagnosis of lobular neoplasia | 47 (27–66)a |
| Breast density | |
| Dense | 116 (88) |
| Nondense | 16 (12) |
| BI-RADS examinations | |
| Category 1 or 2 | 264 (86) |
| Category 0, 3, or 4 | 42 (14) |
| Lobular neoplasia subtype | |
| LCIS | 77 (58) |
| ALH | 23 (17) |
| LCIS and ALH | 32 (24) |
Note—Values are the median with the range in parentheses or the number of patients or examinations with the percentage in parentheses.
CEDM = contrast-enhanced digital mammography, LCIS = lobular carcinoma in situ, ALH = atypical lobular hyperplasia.
Data were missing for one patient.
Of the 132 women, 77 had a personal history of LCIS, 23 had a personal history of ALH, and 32 had a personal history of both LCIS and ALH. Thirteen women (10%) had extremely dense breasts, 103 (78%) had heterogeneously dense breasts, 15 (11%) had scattered fibroglandular densities, and 1 (< 1%) had predominately fatty breasts.
Benign Findings on CEDM
Of the 306 CEDM examinations, 264 (86%) found lesions classified as BI-RADS category 1 or 2. At least 1 year of imaging follow-up with negative findings occurred after all of these examinations. No interval cancers were detected.
Abnormal Findings on CEDM
Because this was a retrospective study, classifications for abnormal CEDM findings included BI-RADS categories 0, 3, and 4 (no examinations were classified as BI-RADS category 5). Altogether, 42 of the 306 examinations showed abnormal results, yielding six patients with cancer (Table 2).
TABLE 2:
Clinical and Imaging Features of Patients Diagnosed With Breast Cancer
| Age (y) | |||||
|---|---|---|---|---|---|
| Patient No. | At Lobular Neoplasia Diagnosis (y) | At Cancer Diagnosis (y) | Detection Method | Imaging Finding | Pathologic Finding |
| 1 | 41 | 45 | CEDM | Nonmass enhancement | IDC |
| 2 | 42 | 46 | CEDMa | Calcifications without enhancement | DCIS |
| 3 | 47 | 52 | CEDM | Calcifications without enhancement, ultrasound mass | IDC |
| 4 | 45 | 48 | CEDM | Nonmass enhancement | DCIS |
| 5 | 65 | 66 | CEDM | Nonmass enhancement | IDC |
| 6 | 46 | 49 | CEDM | Enhancing mass | ILC |
Note—CEDM = contrast-enhanced digital mammography, IDC = invasive ductal carcinoma, DCIS = ductal carcinoma in situ, ILC = invasive lobular carcinoma.
Low energy only.
Of the 306 CEDM examinations, 14 (5%) in 14 patients were classified as BI-RADS category 0. None of these examinations had a mammographic or sonographic correlate for the enhancement seen on CEDM. Therefore, further evaluation with MRI was recommended for all 14 patients. Of the 14 patients, six had a negative MRI result and were given a final classification of BI-RADS category 1 or 2, whereas seven had an MRI correlate and underwent MRI-guided biopsy, one yielding ductal carcinoma in situ, one yielding invasive ductal carcinoma, and the other five yielding benign results. An additional patient opted not to undergo MRI evaluation but had a negative result on standard 2D mammography at 2-year follow-up.
BI-RADS category 3 was assigned to 14 of the 306 examinations (5%) in 14 patients. Of the 14 patients, seven underwent MRI within 1 month of the CEDM examination for further evaluation of the enhancement seen on CEDM. Of these seven patients, five had a negative MRI result and were given a final classification of BI-RADS category 1 or 2, whereas two had an MRI correlate and underwent MRI-guided biopsy (one patient had invasive ductal carcinoma [Fig. 1] and one had a benign result). Six of the 14 patients were monitored with short-interval imaging for 2 years without a change or development of malignancy. Finally, one of the 14 patients underwent 6-week follow-up imaging for a probable hematoma, which had resolved at follow-up. Therefore, the BI-RADS category 3 malignancy rate was one of 14 (7%).
Fig. 1—
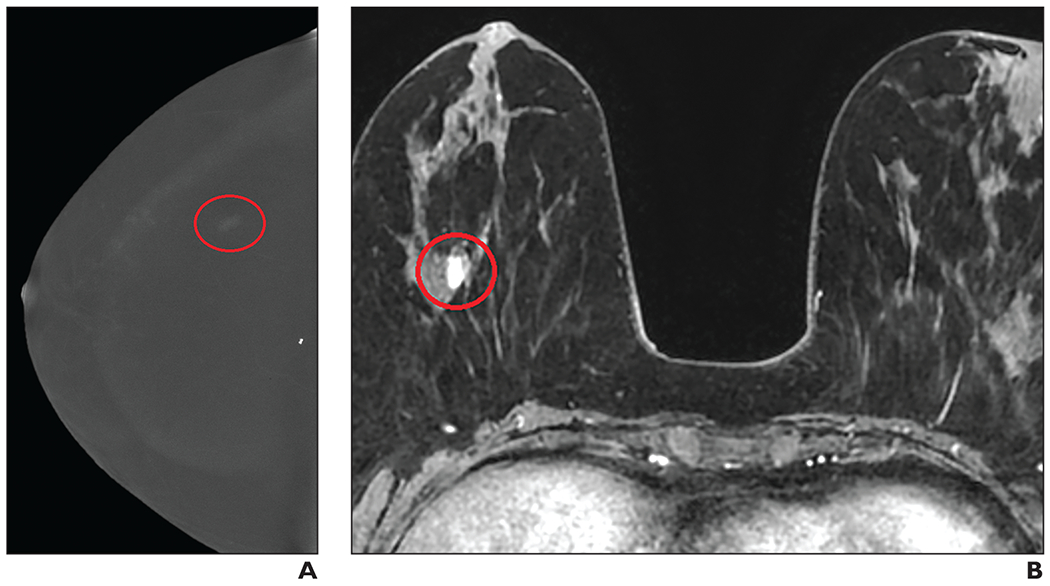
58-year-old woman with right breast atypical lobular hyperplasia and lobular carcinoma in situ that was diagnosed on core biopsy in 2002. A, Contrast-enhanced digital mammography (CEDM) image obtained during screening evaluation in January 2019 shows focal nonmass enhancement (oval) in right breast. Examination was classified as BI-RADS category 3, and MRI was recommended. B, MR image shows correlate to CEDM enhancement (circle). MRI-guided biopsy was performed, yielding invasive ductal carcinoma.
Finally, 14 of the 306 examinations (5%) in 14 patients were classified as BI-RADS category 4. A sonographic correlate was identified and biopsied in five patients, with four patients having benign results and one having invasive ductal carcinoma. MRI was recommended in five patients, all of whom had a correlative finding that required MRI-guided biopsy; four of these five patients had benign results and one had invasive lobular carcinoma. Another four patients had 2D mammography findings without associated enhancement; biopsy was recommended for these patients, with one having DCIS and three having benign pathology. No additional cancers were detected on MRI that were not seen on CEDM.
Performance of CEDM
Table 3 presents the overall sensitivity, specificity, NPV, and accuracy of CEDM in this intermediate-risk patient population. PPV could not be accurately reported because of the low prevalence of breast cancer in this cohort; the positive likelihood ratio, defined as the ratio of sensitivity to false-positive rate, was reported as a surrogate [23, 24]. The positive likelihood ratio incorporates both the sensitivity and specificity of the examination to determine the extent to which a positive result increases the odds of having a disease. In our study, the positive likelihood ratio was 8.33, meaning that CEDM results are 8.3 times more likely to be positive in an individual with breast cancer when compared with an individual without breast cancer.
TABLE 3:
Statistical Analysis Results of Contrast-Enhanced Digital Mammography in Detecting Breast Cancer
| Parameter | Value | 95% CI |
|---|---|---|
| Positive likelihood ratio | 8.33 | 6.62–9.90 |
| Sensitivity | 100 | NA |
| Specificity | 88 | 84–92 |
| NPV | 100 | NA |
| Accuracy | 88 | 84–92 |
Note—Values are percentages. NA = not applicable.
Discussion
The lifetime risk of developing breast cancer is known to vary widely depending on several individual risk factors. Yearly MRI screening in addition to mammography is recommended for high-risk women (lifetime risk of ≥ 20%) [25]; however, no screening guidelines have been established for intermediate-risk patients (lifetime risk of 15% to < 20%). Individuals with a personal history of lobular neoplasia without additional risk factors are considered to have an intermediate risk and represent a unique subgroup of patients who likely require supplemental screening for breast cancer. In this study, we assessed the role of CEDM in women at intermediate risk for developing breast cancer who have a personal history of lobular neoplasia without any additional risk factors.
Previously, studies investigating the value of MRI in screening women with a personal history of lobular neoplasia have shown inconclusive results. Friedlander et al. [26] examined the role of MRI screening in women with a personal history of lobular neoplasia without additional risk factors and found that MRI performs similarly in these women compared with high-risk women. In another study, Sung et al. [27] investigated the value of MRI screening in patients with a history of LCIS without additional risk factors and found a 4.5% incremental cancer detection rate when MRI was used in addition to mammography. The authors noted that MRI should be used in addition to and not in place of mammography in this population, because the sensitivity to detect breast cancer was highest when MRI and mammography were combined in patients with atypical hyperplasia and/or LCIS [27]. Port et al. [28] studied 182 patients with a history of LCIS or atypical hyperplasia, including both lobular and ductal subtypes, who underwent screening MRI in addition to mammography; although 4% of patients with LCIS had MRI-detected breast cancer, MRI added no value for patients with a history of atypical hyperplasia. Port et al. excluded patients with high-risk genetic mutations, though those with a concomitant family history of breast cancer were included. A prospective study by King et al. [29] that included 776 patients with a history of LCIS found that screening MRI did not improve the cancer detection rate or the diagnosis of cancer at an earlier stage; patients with a known BRCA mutation and personal history of breast malignancy were excluded. Given the inconclusive results and variable risk factors of the patients evaluated in these studies, the optimal screening paradigm for this intermediate-risk patient population is still under investigation.
To date, studies on CEDM have focused on its use in the diagnostic setting; reports on the use of CEDM for screening are limited. A study by Sung et al. [17] found CEDM to be promising as a screening modality in high-risk women but did not focus specifically on those with an intermediate risk secondary to lobular neoplasia. In our study, we found that the positive likelihood ratio of detecting breast cancer on screening CEDM was 8.33 in the subgroup of patients with a personal history of lobular neoplasia without additional risk factors, meaning that CEDM is 8.3 times more likely to be positive in an individual with breast cancer in this subgroup when compared with an individual without breast cancer. The positive likelihood ratio serves as a valuable tool to assess the diagnostic accuracy of a positive test result, especially when the prevalence of disease is very low (approximately 2% in this cohort), allowing it to be used as a surrogate for PPV [23, 24]. CEDM had a similarly high sensitivity (100% vs 88%), specificity (88% vs 94%), and negative predictive value (100% vs 99.7%) in detecting breast cancer in our specific subgroup of intermediate-risk patients compared with the high-risk patients studied by Sung et al. The slightly lower specificity in our study may be partly due to the smaller sample size and overall lower cancer risk in this limited population.
Our study has several limitations. This was a single-institution retrospective study. Specific guidelines addressing the workup of intermediate risk patients have not been established; therefore, the decision to perform screening examinations was at the discretion of the referring physician and the patient, which may have introduced selection bias. The sample size was small with a low number of cancers, thus limiting our ability to distinguish between incidence and prevalence of disease. Finally, we observed substantial variation in the number of CEDM examinations performed per patient and in the number of clinical and imaging follow-up examinations, although all patients had at least 1 year of follow-up available.
Conclusion
CEDM shows promise as a screening examination for the detection of breast cancer in patients with a personal history of lobular neoplasia. Continued investigation with a larger patient population is needed to determine the true sensitivity and PPV of CEDM for these patients.
Acknowledgment
We thank Joanne Chin for her help in editing this article.
Supported by research grants from Hologic and GE Healthcare to J. Sung and in part by the NIH National Cancer Institute Cancer Center Support Grant (P30 CA008748).
Footnotes
The authors declare that they have no disclosures relevant to the subject matter of this article.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69:7–34 [DOI] [PubMed] [Google Scholar]
- 2.Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:268–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambinder EB, Harvey SC, Panigrahi B, Li X, Woods RW. Synthesized mammography: the new standard of care when screening for breast cancer with digital breast tomosynthesis? Acad Radiol 2018; 25:973–976 [DOI] [PubMed] [Google Scholar]
- 4.Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet 2003; 361:125–129 [DOI] [PubMed] [Google Scholar]
- 5.Bodian CA, Perzin KH, Lattes R. Lobular neoplasia: long term risk of breast cancer and relation to other factors. Cancer 1996; 78:1024–1034 [DOI] [PubMed] [Google Scholar]
- 6.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast: risk assessment and management options. N Engl J Med 2015; 372:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses’ Health Study. Cancer 2007; 109:180–187 [DOI] [PubMed] [Google Scholar]
- 8.Chuba PJ, Hamre MR, Yap J, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol 2005; 23:5534–5541 [DOI] [PubMed] [Google Scholar]
- 9.Lo G, Scaranelo AM, Aboras H, et al. Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology 2017; 285:36–43 [DOI] [PubMed] [Google Scholar]
- 10.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008; 26:3248–3258 [DOI] [PubMed] [Google Scholar]
- 11.Brekelmans CT, Seynaeve C, Bartels CC, et al. ; Rotterdam Committee for Medical and Genetic Counseling. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001; 19:924–930 [DOI] [PubMed] [Google Scholar]
- 12.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol 2009; 27:6124–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobbes MB, Lalji U, Houwers J, et al. Contrast-enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014; 24:1668–1676 [DOI] [PubMed] [Google Scholar]
- 14.Iotti V, Ravaioli S, Vacondio R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res 2017; 19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel BK, Garza SA, Eversman S, Lopez-Alvarez Y, Kosiorek H, Pockaj BA. Assessing tumor extent on contrast-enhanced spectral mammography versus full-field digital mammography and ultrasound. Clin Imaging 2017; 46:78–84 [DOI] [PubMed] [Google Scholar]
- 17.Sung JS, Lebron L, Keating D, et al. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 2019; 293:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR 2018; 211:[web]W267–W274 [DOI] [PubMed] [Google Scholar]
- 19.Jochelson MS, Pinker K, Dershaw DD, et al. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol 2017; 97:37–43 [DOI] [PubMed] [Google Scholar]
- 20.Francescone MA, Jochelson MS, Dershaw DD, et al. Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM). Eur J Radiol 2014; 83:1350–1355 [DOI] [PubMed] [Google Scholar]
- 21.D’Orsi CJSE, Mendelson EB, Morris EA, et al. ACR BI-RADS atlas: Breast Imaging Reporting and Data System. American College of Radiology, 2013 [Google Scholar]
- 22.Mercaldo ND, Lau KF, Zhou XH. Confidence intervals for predictive values with an emphasis to case-control studies. Stat Med 2007; 26:2170–2183 [DOI] [PubMed] [Google Scholar]
- 23.Zhou XH, Obuchowski N, McClish D. Statistical methods in diagnostic medicine. Wiley, 2002 [Google Scholar]
- 24.Mandrekar J, Mandrekar S. Statistical methods in diagnostic medicine using SAS software. SAS Institute website. support.sas.com/resources/papers/proceedings/proceedings/sugi30/211-30.pdf. Accessed June 15, 2020 [Google Scholar]
- 25.Mainiero MB, Moy L, Baron P, et al. ; Expert Panel on Breast Imaging. ACR Appropriateness Criteria breast cancer screening. J Am Coll Radiol 2017; 14(11 suppl):S383–S390 [DOI] [PubMed] [Google Scholar]
- 26.Friedlander LC, Roth SO, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology 2011; 261:421–427 [DOI] [PubMed] [Google Scholar]
- 27.Sung JS, Malak SF, Bajaj P, Alis R, Dershaw DD, Morris EA. Screening breast MR imaging in women with a history of lobular carcinoma in situ. Radiology 2011; 261:414–420 [DOI] [PubMed] [Google Scholar]
- 28.Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol 2007; 14:1051–1057 [DOI] [PubMed] [Google Scholar]
- 29.King TA, Muhsen S, Patil S, et al. Is there a role for routine screening MRI in women with LCIS? Breast Cancer Res Treat 2013; 142:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]


