Abstract
Photosystems I and II (PSI and PSII) are the integral components of the photosynthetic electron transport chain that utilize light to provide chemical energy for CO2 fixation. In this study, we investigated how the deficiency of PSII affects the gene expression, accumulation, and organization of thylakoid protein complexes as well as physiological characteristics of Synechocystis sp. PCC 6803 by combining biochemical, biophysical, and transcriptomic approaches. RNA‐seq analysis showed upregulated expression of genes encoding the PSII core proteins, and downregulation of genes associated with interaction between light‐harvesting phycobilisomes and PSI. Two‐dimensional separation of thylakoid protein complexes confirmed the lack of PSII complexes, yet unassembled PSII subunits were detected. The content of PsaB representing PSI was lower, while the content of cytochrome b6f complexes was higher in the PSII‐less strain as compared with control (CS). Application of oxygraph measurements revealed higher rates of dark respiration and lower PSI activity in the mutant. The latter likely resulted from the detected decrease in the accumulation of PSI, PSI monomerization, increased proportion of energetically decoupled phycobilisomes in PSII‐less cultures, and low abundance of phycocyanin. Merging the functional consequences of PSII depletion with differential protein and transcript accumulation in the mutant, in comparison to CS, identified signal transduction from the photosynthetic apparatus to the genome level.
Keywords: D1 protein, photosynthesis, photosystem II, psbA2, RNA‐seq, Synechocystis sp. PCC 6803
1. INTRODUCTION
Cyanobacteria, algae, and plants are capable of performing oxygenic photosynthesis. In cyanobacteria, light is harvested by phycobilisomes (PBS), the large pigment‐protein complexes loosely attached to the cytoplasmic side of the thylakoid membrane and functionally associated with the membrane‐embedded photosystem II (PSII) and photosystem I (PSI) complexes. The PBS comprise a central allophycocyanin (APC) subcomplex with attached peripheral rods composed of phycocyanin (PC) and connected by CpcG linker polypeptides (Kondo et al., 2009). PBS transfer energy both to PSII and PSI centers, the extent of distribution to PSII and PSI depending on the spectral composition of light in a process known as state transitions (Kondo et al., 2009).
The functional form of PSII is a dimer (Ferreira et al., 2004; Huang et al., 2021; Loll et al., 2005). Each PSII monomer of Thermosynechococcus elongatus is composed of 20 proteins, 35 chlorophyll (Chl) a molecules, and 12 β‐carotene molecules (Guskov et al., 2009). The D1 (PsbA) and D2 (PsbD) proteins together with PsbI bind all redox cofactors involved in charge separation, forming PSII reaction center complex, which is surrounded by an inner light‐harvesting antenna (CP43/PsbC and CP47/PsbB) and a number of additional small subunits (Shi & Schröder, 2004). The oxygen evolving complex is bound to PSII core complex on the lumenal side of the thylakoid membrane (Rappaport & Diner, 2008; Vinyard et al., 2019). The light energy trapped by PBS is delivered to the reaction center Chl pair (P680) of PSII resulting in primary charge separation and electron transfer from PSII to PSI via the plastoquinone (PQ) pool, cytochrome b 6 f complex (Cyt b 6 f), and the soluble electron carriers plastocyanin or cytochrome c6. In Synechocystis sp. PCC 6803 (hereafter S. 6803) the functional form of PSI is a trimer (Kłodawska et al., 2014). The core of PSI is composed of the PsaA and PsaB proteins, which are surrounded by 10 small polypeptides (Malavath et al., 2018). In addition to the reaction center Chls (P700) bound by the core proteins, PSI of Synechococcus elongatus contains 94 Chl a molecules and 22 β‐carotene molecules (Jordan et al., 2001). In the cytoplasmic side of PSI, the PsaC, PsaD and PsaE proteins form the stromal ridge, which is the site for ferredoxin (Fdx) binding (Lagoutte & Valloni, 1992; Lelong et al., 1996). The “long form” of Fdx‐NADP+ oxidoreductase (FNR) enzyme, which is bound to PBS, oxidizes Fdx and reduces NADP+ to NADPH (Shin, 2004; Tagawa & Arnon, 1962), which is further utilized in carbon assimilation and other cellular metabolism.
Several components of the cyanobacterial photosynthetic electron transfer chain (PETC) are shared with the respiratory electron transfer chain. For instance, the PQ pool, the Cyt b 6 f complex, and plastocyanin participate in electron transfer from succinate dehydrogenase or NADH dehydrogenase‐like complex type‐1 to O2 through various terminal oxidases, the quinol oxidase, alternative respiratory terminal oxidase, plastid terminal oxidase, and cytochrome c oxidase. Furthermore, protein complexes involved in cyclic electron transfer and carbon concentration mechanisms are also essential components of the thylakoid membrane (Burnap et al., 2013; Lea‐Smith et al., 2016; Selão et al., 2020).
The glucose (Glc)‐tolerant strain of S. 6803 is a valuable tool in photosynthesis research, as mutants deficient in components indispensable for photosynthesis can be grown in the presence of an external carbon source. Studies with S. 6803 mutants have revealed that assembly of PSII is a stepwise process initiated by the association of the D1 and D2 proteins and that the assembly is facilitated by a number of auxiliary factors (Komenda et al., 2011; Mulo et al., 2008). In the present study, we investigated how the deficiency of PSII affects the gene expression, accumulation, and organization of thylakoid protein complexes, and physiological characteristics of S. 6803 by combining biochemical, biophysical, and transcriptomic approaches.
2. MATERIALS AND METHODS
2.1. Strains and growth conditions
A Glc‐tolerant S. 6803 strain, with psbA1 and psbA3 genes inactivated by the insertion of an antibiotic resistance cassette within the coding region of the genes (AR strain described by Mäenpää et al., 1993), was used as a control strain (CS). As a PSII‐less strain in the AR background, we used a strain containing a spontaneous mutation (GTA ➔ TAA) forming a translation stop codon in place of Val219 of the psbA2 gene. The cells were grown on BG‐11 agar plates under continuous light at photosynthetic photon flux density of 50 μmol m−2 s−1, 32°C in the presence of 5‐mM Glc, 10‐μM 3‐(3,4‐dichlorophenyl)‐1,1‐dimethylurea (DCMU), and antibiotics (spectinomycin 10 μg/ml, streptomycin 5 μg/ml, kanamycin 5 μg/ml, and chloramphenicol 2.5 μg/ml) (Mulo et al., 1997). Liquid cultures for experiments were grown without DCMU in 50‐ml batches shaken at 90 rpm.
2.2. RNA isolation and transcriptome analysis
Total RNA was extracted with hot phenol method as described previously (Tyystjärvi et al., 2001), treated with DNase using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA), and submitted to the Finnish Functional Genomics Centre, Turku Bioscience (Finland), for single‐ended library preparation and sequencing of RNA libraries using Illumina HiSeq2500. RNA‐seq reads were aligned with the Strand NGS 2.7 software (Agilent, USA) using the reference genome and annotations from GCA_000340785.1 (GenBank assembly accession). The first three 3′ nucleotides were removed before aligning reads to the reference genome. Quantification of the aligned reads was performed using the DESeq R package. Significantly differentially expressed genes were identified by a two‐way ANOVA test using the Benjamini‐Hochberg method for false discovery rate (FDR) correction of P values. Three independent biological replicates were analyzed.
2.3. Protein extraction, gel electrophoresis and immunodetection
Total and thylakoid protein extraction was performed according to Zhang, Allahverdiyeva, et al. (2009). Proteins were separated by 12% (w/v) SDS‐PAGE containing 6‐M urea, electrotransferred to PVDF membrane (Immobilon‐P, Millipore) and immunodetected by protein‐specific antibodies. The antibodies against D1 N‐terminus, PsaB, ATPase β, Fdx, RbcL, APC, and PC were purchased from Agrisera (Vännas, Sweden). The antibody against OCP was a generous gift from Prof. D. Kirilovsky, FNR (PetH) from Prof. H. Matthijs, CpcL from Prof. M. Ikeuchi, and Cyt F from Prof. L. Zhang. Protein solubilization for blue native (BN)‐PAGE was performed as described in Zhang et al. (2012), but with no protease inhibitor in washing buffer and using 1.5% dodecyl maltoside. BN‐PAGE was performed as described by Järvi et al. (2011) and separation of proteins in the second dimension according to Mustila et al. (2016). Identification of protein spots on BN/SDS‐PAGE gels is based on Herranen et al. (2004) and Zhang et al. (2004).
2.4. Absorption, 77‐K fluorescence emission and P700 absorbance spectroscopy
Absorption spectra of the cells were measured using OLIS CLARiTY 17 spectrophotometer (Olis, USA). The spectra were double‐normalized at 440 and 750 nm (Luimstra et al., 2018). Fluorescence emission spectra were measured at 77 K during excitation with 580‐nm monochromatic light. A QEPro spectrometer (Ocean Optics, USA) was first calibrated using BG‐11 media in liquid N2, and then fluorescence emission spectra were measured in 100‐μl aliquots of 5‐min dark‐adapted cultures (7.5 μg Chl ml−1) in liquid N2. Spectra were recorded from 100 μl aliquots of culture during excitation with 580‐nm monochromatic light and normalized to 685 nm.
2.5. Respiration and photosynthetic activity
Light‐saturated PSII and PSI activities as well as respiration of the intact S. 6803 cells (OD750 = 0.4 in fresh BG‐11 medium supplemented with 5‐mM Glc and appropriate antibiotics) were measured using a Clark‐type HansaTech oxygen electrode (Waltz). O2 uptake was measured for 5 min in darkness to obtain the rate of respiration. Then, the same culture was exposed to the PPFD of 1000 μmol m−2 s−1 to measure the rate of O2 evolution in light for 5 min. PSI activity was measured as O2 uptake in 10‐μM DCMU, .1‐mM methyl viologen (MV), 50‐μM 2,6‐dichlorophenolindophenol (DCPIP), 1‐mM ascorbate, 50‐μM NaN3 for 5 min under the PPFD of 1000 μmol m− 2 s−1.
2.6. Accession numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession number SAMN26087044.
3. RESULTS
3.1. Gene expression in the PSII‐less strain
PSII is essential for the proper functioning of the PETC. To study how the loss of PSII affects the regulation of gene expression and accumulation and organization of thylakoid protein complexes, we first performed RNA‐seq analysis of the cells grown under standard conditions (i.e., in the presence of Glc under continuous light). Transcriptomic analysis revealed that the expression of the psbA2 and psbA3 genes encoding the D1 protein, and the psbD2 gene encoding the D2 subunits of PSII, was up regulated in the PSII‐less strain as compared with the CS (Table 1), which is in accordance with a previous study (Mulo et al., 1997). The slr0228 gene encoding an FtsH‐type protease, involved in PSII maintenance (Komenda et al., 2006), was also upregulated. Other genes with significantly upregulated expression encode a type IV pilin (sll1695), a peptidase (slr0646) and a 6‐phosphogluconate dehydrogenase enzyme of the oxidative pentose phosphate pathway (sll0329). The genes with most strongly down‐regulated expression in the mutant encode the CpcG2 linker found in PSI‐associated PBS (Kondo et al., 2007) and the adjacent operon sll1472 – sll1475. Expression of genes encoding a Mn2+ transporter (sll0615), a member of the PatA family of transcription regulators (slr1594) and several hypothetical proteins were also significantly downregulated (Table 1). Nevertheless, the number of significantly differentially expressed genes between the PSII‐less mutant and CS cells was surprisingly low (only 18), as shown in Table 1.
TABLE 1.
Differentially expressed genes in the PSII‐less mutant
| Gene | Description | Expression FC | FDR |
|---|---|---|---|
| slr1311 | PSII D1 protein (psbA2) | 7.8 | .0017 |
| sll1867 | PSII D1 protein (psbA3) | 6.0 | .0017 |
| slr0927 | PSII D2 protein (psbD2) | 4.5 | .0100 |
| slr0228 | FtsH protease | 2.1 | .0100 |
| sll1695 | Similar to secretion protein or type IV pilin | 2.1 | .0236 |
| slr0646 | Peptidase involved in peptidoglycan biosynthesis | 2.4 | .0450 |
| sll0329 | 6‐phosphogluconate dehydrogenase, enzyme in OPPP | 2.2 | .0100 |
| ssl0172 | Hypothetical protein | 2.3 | .0474 |
| sll0012 | Hypothetical protein | 2.1 | .0201 |
| sll1471 | Phycobilisome linker protein (cpcG2) | −5.2 | .0201 |
| sll1472* | Hypothetical protein | −4.4 | .0234 |
| sll1473* | Hik32 phytochrome | −3.4 | .0311 |
| sll1475* | Hik32 phytochrome | −2.1 | .0183 |
| slr1594 | PatA subfamily protein | −4.8 | .0197 |
| slr1593 | Hypothetical protein | −4.3 | .0345 |
| sll0615 | Mn2+ membrane transporter | −2.2 | .0240 |
| slr1152 | Hypothetical protein | −4.1 | .0100 |
| slr7057 | Hypothetical protein | −2.4 | .0228 |
Note: Genes marked with “*” are in same operon. Fold change (FC) > 2 indicates upregulation, FC < −2 indicates downregulation. False discovery rate (FDR) calculated from moderated T test p values, n = 3.
3.2. Organization of thylakoid protein complexes in the PSII‐less strain
Next, we assessed the accumulation of the key photosynthetic proteins in the PSII‐less mutant. As expected, translation stop codon within the coding region of the psbA2 gene prohibited accumulation of the D1 protein, and also the level of PsaB was found to be decreased in the PSII‐less strain (Figure 1). The level of the Cyt f subunit, in turn, was higher in the PSII‐less strain as compared with CS (Figure 1). The amounts of AtpB, Fdx, FNR, Rubisco (RbcL), and orange carotenoid protein (OCP) were similar to those in the CS, as was the flavodiiron protein Flv3, which is associated with the function of PSI (Figure 1; Helman et al., 2003; Allahverdiyeva et al., 2013). It is worth noting that CP43 and CP47 have previously been identified in the thylakoids of various PSII‐less strains (Bečková et al., 2022; Mulo et al., 1997). The accumulation of the PBS linker protein CpcL (sll1471, cpcG2) did not differ from that of CS despite strongly down‐regulated expression of the encoding gene (Table 1). However, the abundance of PC was clearly lower in the PSII‐less strain compared with CS, while the amount of APC remained unchanged (Figure 1).
FIGURE 1.
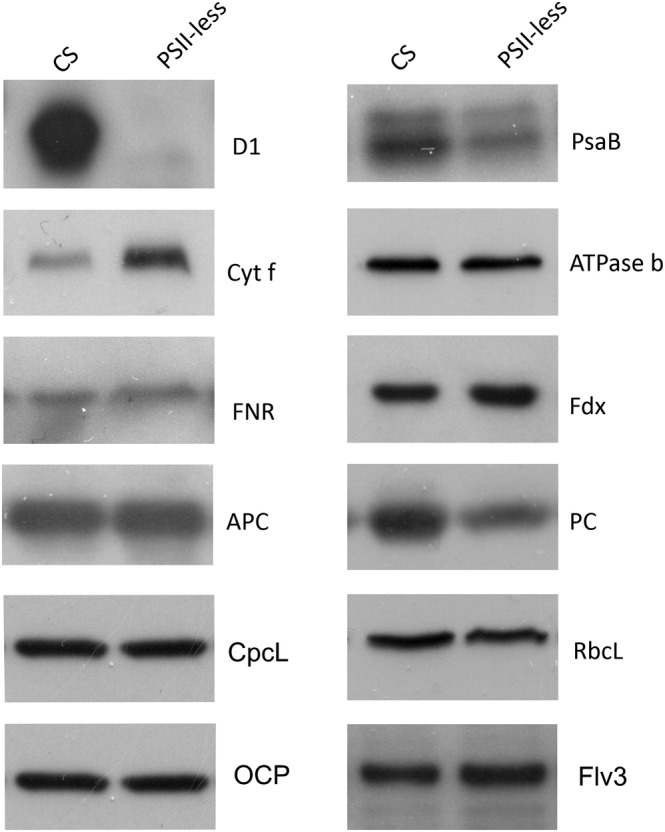
Content of photosynthetic proteins in CS and PSII‐less S. 6803. Total proteins isolated from the S. 6803 cells were separated by SDS‐PAGE and probed with antibodies to detect D1 (PSII core), PsaB (PSI core), Cyt f (Cyt b 6 f), AtpB (ATP synthase), ferredoxin (Fdx), ferredoxin: NADP+ oxidoreductase (FNR), flavodiiron 3 (Flv3), rubisco large subunit (RbcL), orange carotenoid protein (OCP), allophycocyanin (APC), phycocyanin (PC), and the PSI‐associated PC linker (CpcL). The gels were loaded based on protein concentration. The figure shows representative results from three to six independent biological replicates
We also wanted to see how the defect in the accumulation of PSII affects the organization of the thylakoid protein complexes. To that end, thylakoids were isolated and the constituent protein complexes were separated by BN‐PAGE followed by SDS‐PAGE in the second dimension. Figure 2 clearly illustrates the lack of both dimeric and monomeric PSII complexes in the mutant. Despite the lack of PSII complexes, the PSII core subunits CP43 and CP47 were abundant as unassembled proteins in the mutant (Figure 2b). 2D‐PAGE corroborated the immunoblotting results and indicated that more Cyt b 6 f complexes accumulated in the mutant as compared with CS, whereas the protein spots encompassing PC (CpcA and CpcB) and/or APC (Herranen et al., 2004) were less abundant (Figure 2b). NdhD1, NdhB, NdhA, and NdhK subunits of NDH‐1L were also detectable in PSII‐less strain as opposed to CS. Intriguingly, the ratio of PSI monomers to PSI trimers was higher in the PSII‐less mutant, and also a small proportion of PSI dimers was detected.
FIGURE 2.
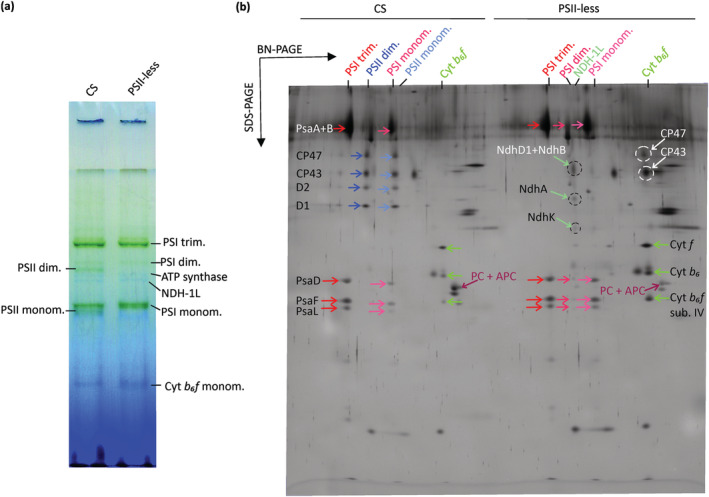
Organization and content of protein complexes in CS and PSII‐less S. 6803 thylakoids. (a) BN‐PAGE separation of protein complexes in thylakoids isolated from the CS and PSII‐less mutant strains. Bands representing visible protein complexes are indicated; 40 μg of proteins was loaded in the wells. (b) SDS‐PAGE separation of thylakoid complexes into individual protein subunits, following previous separation by BN‐PAGE in (a). Previously identified spots are labeled, including unassembled CP43, CP47, and PC and APC spots, according to identification by Herranen et al. (2004) and Zhang et al. (2004). PSI subunits are indicated in red, PSII subunits in blue, Cyt b6f subunits in green and NDH‐1L subunits in cyan. The figure shows representative results from three independent biological replicates
Next, we analyzed the pigment composition of the PSII‐less strain. Comparison of the absorption spectra of the strains revealed higher carotenoid and lower Chl content in the PSII‐less cells than in CS (Figure 3a). The abundance of PC pigments were equivalent in both strains; however, a small, but significant blue‐shift was apparent and reproducible in the peak of PC in the PSII‐less cells (621.5 nm ± 0.86) as compared with CS (623.5 nm ± 0.91; see Figure 3a, inset), which suggests differences in the composition of PBS between the PSII‐less mutant and CS.
FIGURE 3.
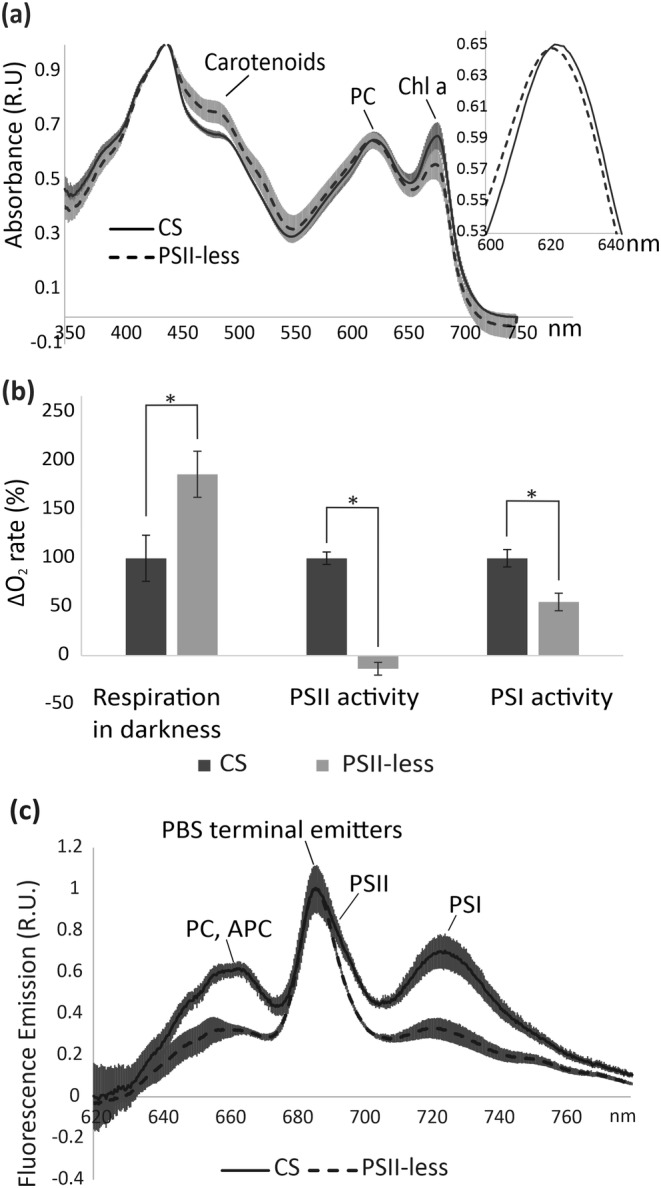
Pigment composition and energy transfer in CS and PSII‐less cells. (a) Whole cell absorption spectra of CS (solid line) and PSII‐less (dashed line) cultures grown in BG‐11 media in the presence of 5‐mM Glc. Spectra were double‐normalized at 440 and 750 nm. Peaks corresponding to carotenoids, phycocyanin (PC) and chlorophyll a (Chl a) are indicated. Spectra shown are average of three independent biological replicates, shading indicates standard deviation. Inset shows PC peak in higher resolution to highlight the peak shift. (b) Oxygen uptake/evolution rates in CS (dark gray bars) and PSII‐less strain (light gray bars) of S. 6803. Rates of change in O2 concentration were measured in darkness (respiration in darkness), under 1000 μmol m−2 s−1 light (PSII activity), and in light in the presence of DCMU, MV, 2,6‐dichlorophenolindophenol (DCPIP), ascorbate and sodium azide (NaN3) (PSI activity). Values are average of three independent biological replicates. Error bars indicate standard deviation and asterisk statistically significant difference (p < .05). (c) 77‐K fluorescence emission spectra of CS (solid line) and PSII‐less strain (dotted line) S. 6803 cells were excited at 580 nm. Spectra shown are average of three independent biological replicates. Shading indicates standard deviation
3.3. Functional consequences of the loss of PSII: Phycobilisomes, PSI activity, and respiration
To assess the functionality of the electron transfer chain, we measured O2 evolution and uptake of the cells in three different conditions. As expected, the PSII‐less strain was unable to produce any O2 and showed only 50% of the PSI activity detected in CS (Figure 3b). Moreover, PSII‐less strain showed higher O2 uptake rate in the dark as compared with CS (Figure 3b). In order to study energy transfer from PBS to PSII and PSI, we next measured the Chl a fluorescence emission at 77 K using excitation at 580 nm, which is mainly absorbed by PBS (Mullineaux, 1994; Nilsson et al., 1992; Rakhimberdieva et al., 2007). The emission peak at 690 nm, deriving from PSII Chl molecules (Andrizhiyevskaya et al., 2005; Fuhrmann et al., 2008), appeared as a shoulder to the 685 nm peak in the CS. As expected, this peak was absent in PSII‐less strain (Figure 3c), and also the fluorescence peak at 725 nm emitted from PSI was significantly lower in the PSII‐less strain than in the CS. The high peak at 685 nm, representing PBS terminal emitters (Yu et al., 1999), indicates that the proportion of energetically decoupled PBS relative to PSI is higher in the PSII‐less cultures than in CS. In S. 6803, PBS complexes contain one of two CpcG linker proteins. The PBS containing CpcL (encoded by cpcG2) are mostly associated with PSI (Kondo et al., 2007), while the CpcG1‐PBS complex transfers energy to both photosystems (Kondo et al., 2005, 2009). In the PSII‐less mutant strain, expression of the cpcG2 gene was strongly downregulated in comparison to CS (Table 1), even though no changes were detected in the accumulation of CpcL protein (Figure 1).
4. DISCUSSION
4.1. Lack of PSII affects PSI accumulation and activity
In cyanobacteria, the assembly of PSII complex starts with the formation of precursor D1‐PsbI complex (Lu, 2016; Zabret et al., 2021), and the lack of D1 prevents the formation of PSII, despite accumulation of some other PSII subunits. Indeed, earlier studies have reported accumulation of CP43, Cyt b559, 33 kDa, and 22 kDa proteins of the oxygen evolving complex as well as trace amounts of D2 and CP47 in thylakoids of various PSII‐less strains (Bittersmann & Vermaas, 1991; Mulo et al., 1997; Nilsson et al., 1990; Vermaas et al., 1988), which is in line with our results (Figures 1 and 2).
Current study revealed low PSI accumulation and activity in the PSII‐less mutant (Figures 1 and 3b) as well as an increase in the ratio of PSI monomers to PSI trimers in comparison to CS (Figure 2a,b). Usually, trimeric PSI in S. 6803 is more abundant than monomeric PSI (Figure 2b; Karapetyan et al., 1999; Tsiotis et al., 1995), whereas stress conditions such as HL increase the monomer: trimer ratio (Kopečná et al., 2012; Wang et al., 2008). Moreover, loss of HL inducible polypeptide (HliP), known to stabilize PSI trimers, results in increased PSI monomer: PSI trimer ratio and a sharp decrease of PSI activity (Wang et al., 2008). In addition to low PSI content and PSI monomerization, PSI activity may also be affected by the altered composition of the PBS, which is suggested by the blue‐shift of the PC absorption peak in the PSII‐less mutant (Figure 3a). The relatively high 685‐nm and low 720‐nm fluorescence emission peaks (Figure 3c) indicate increased proportion of energetically decoupled PBS in PSII‐less culture, and a decrease in PSI absorption cross section. An increase in the proportion of decoupled PBS has also been reported in another PSII‐less strain (Mullineaux, 1994). Decoupling of PBS also takes place in response to blue light (Luimstra et al., 2020) or HL (Tamary et al., 2012), which cause excitation imbalance and PSII photoinhibition, respectively. The observed increase in the decoupled PBS in the mutant was accompanied by lower overall PC abundance (Figures 1 and 2), further supported by a lower 650‐ to 665‐nm peak in 77‐K fluorescence emission corresponding to uncoupled PC and APC (Figure 3c) (Elanskaya et al., 2018; Puzorjov et al., 2021).
4.2. Photosynthetic signaling is altered in the absence of PSII
The expression of genes encoding PSII core protein subunits (psbA2, psbA3, and psbD2) and repair machinery (ftsH) are known to be upregulated under HL (Sakurai et al., 2012), high temperature (Kamata et al., 2005), or UV‐B light (Máté et al., 1998). These conditions result in PSII photoinhibition, where the D1 subunits of PSII are continuously damaged, degraded, and replaced. Upon PSII photoinhibition, the downstream PQ pool may be expected to become more oxidized. Indeed, the redox state of the PQ pool has been suggested to be a regulator of psbA gene transcription in multiple studies (Allen, 1995; Alfonso et al., 1999; Fujita, 1997; Li & Sherman, 2000; Meunier et al., 1997; Pfannschmidt et al., 1999), and histidine kinases might be involved in the process (Srivastava & Shukla, 2021). RppA and Hik33 (DspA) are two kinases reported to sense the redox state of PQ pool and activate the signal cascades leading to the induction of psbA2, psbA3, psbD2, and ftsH expression (Ge et al., 2017; Hsiao et al., 2004; Li & Sherman, 2000). Thus, it is plausible that the absence of PSII affects the redox state of the PQ pool with a consequent upregulation of the psbA2, psbA3, psbD2, and ftsH gene expression, possibly through the action of histidine kinases (Table 1). However, due to the post‐transcriptional regulation of gene expression shown to take place in S. 6803 (He & Vermaas, 1998; Kojima et al., 2007; Nishiyama et al., 2004; Tyystjärvi et al., 2001), changes in the accumulation of the transcripts may not be directly reflected to the accumulation of proteins and physiology of the cell. It has also been shown that in Spirulina platensis the monomerization of PSI increases in response to oxidation of the PQ pool (Zhang, Xie, et al., 2009) indicating that the increased PSI monomer to PSI trimer ratio in the PSII‐less mutant (Figure 2b) could be affected by the oxidized state of the PQ pool.
Taken together, our study reveals that in addition to preventing the ability for autotrophic growth, PSII deficiency results in reorganization of thylakoid protein complexes (i.e., changes in the relative abundance of PSI and Cyt b6f complex and PSI trimer to PSI monomer ratio), changes in gene expression, increase in respiration and decrease in PSI activity. In addition to low accumulation, PSI activity may be affected by PSI monomerization, increased proportion of energetically decoupled PBS in PSII‐less cultures, and low overall PC abundance. It is conceivable that lack of PSII and growth in the presence of Glc results in relative oxidation of PQ pool, which is a likely candidate for photosynthetic signaling providing flexibility also for the wild type cells acclimating to the fluctuations in their natural environment.
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
AUTHOR CONTRIBUTIONS
PM, EMA, and PJG designed the research and analyzed the data. MK, AL, JI, and IS performed research. MK, PJG, and PM wrote the paper and all authors revised and approved the manuscript.
CONFLICT OF INTEREST
The Authors did not report any conflict of interest.
ACKNOWLEDGMENTS
Dr. T. Tyystjärvi is thanked for sharing the PC and APC antibodies. This study was financially supported by the Jane and Aatos Erkko Foundation (MK, PJG, EMA) and the Academy of Finland (321616 for PM). The PHOTOSYN infrastructure (University of Turku; https://sites.utu.fi/photosyn/) is acknowledged for the excellent research environment.
Kılıç, M. , Gollan, P. J. , Lepistö, A. , Isojärvi, J. , Sakurai, I. , Aro, E.‐M. , & Mulo, P. (2022). Gene expression and organization of thylakoid protein complexes in the PSII‐less mutant of Synechocystis sp. PCC 6803. Plant Direct, 6(6), e409. 10.1002/pld3.409
REFERENCES
- Alfonso, M. , Perewoska, I. , & Kirilovsky, D. (1999). Redox control of psbA gene expression in the cyanobacterium Synechocystis pcc 6803. Involvement of the cytochrome b 6 /f complex. Plant Physiology, 122(2), 505–516. 10.1104/pp.122.2.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva, Y. , Mustila, H. , Ermakova, M. , Bersanini, L. , Richaud, P. , Ajlani, G. , Battchikova, N. , Cournac, L. , & Aro, E. (2013). Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proceedings of the National Academy of Sciences, 110(10), 4111–4116. 10.1073/pnas.1221194110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J. (1995). Thylakoid protein phosphorylation, state 1‐state 2 transitions, and photosystem stoichiometry adjustment: Redox control at multiple levels of gene expression. Physiologia Plantarum, 93, 196–205. 10.1034/j.1399-3054.1995.930128.x [DOI] [Google Scholar]
- Andrizhiyevskaya, E. , Chojnicka, A. , Bautista, J. , Diner, B. , van Grondelle, R. , & Dekker, J. (2005). Origin of the F685 and F695 fluorescence in photosystem II. Photosynthesis Research, 84(1–3), 173–180. 10.1007/s11120-005-0478-7 [DOI] [PubMed] [Google Scholar]
- Bečková, M. , Sobotka, R. , & Komenda, J. (2022). Photosystem II antenna modules CP43 and CP47 do not form a stable ‘no reaction centre complex’ in the cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis Research, in press. 10.1007/s11120-022-00896-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittersmann, E. , & Vermaas, W. (1991). Fluorescence lifetime studies of cyanobacterial photosystem II mutants. Biochimica et Biophysica Acta, 1098, 105–116. 10.1016/0005-2728(91)90014-F [DOI] [Google Scholar]
- Burnap, R. , Nambudiri, R. , & Holland, S. (2013). Regulation of the carbon‐concentrating mechanism in the cyanobacterium Synechocystis sp. PCC6803 in response to changing light intensity and inorganic carbon availability. Photosynthesis Research, 118(1–2), 115–124. 10.1007/s11120-013-9912-4 [DOI] [PubMed] [Google Scholar]
- Elanskaya, I. , Zlenko, D. , Lukashev, E. , Suzina, N. , Kononova, I. , & Stadnichuk, I. (2018). Phycobilisomes from the mutant cyanobacterium Synechocystis sp. PCC 6803 missing chromophore domain of ApcE. Biochimica et Biophysica Acta, 1859(4), 280–291. 10.1016/j.bbabio.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Ferreira, K. , Iverson, T. , Maghlaoui, K. , Barber, J. , & Iwata, S. (2004). Architecture of the photosynthetic oxygen‐evolving center. Science, 303(5665), 1831–1838. 10.1126/science.1093087 [DOI] [PubMed] [Google Scholar]
- Fuhrmann, E. , Gathmann, S. , Rupprecht, E. , Golecki, J. , & Schneider, D. (2008). Thylakoid membrane reduction affects the photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiology, 149, 735–744. 10.1104/pp.108.132373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y. (1997). A study on the dynamic features of photosystem stoichiometry: Accomplishments and problems for future studies. Photosynthesis Research, 53(2/3), 83–93. 10.1023/A:1005870301868 [DOI] [Google Scholar]
- Ge, H. , Fang, L. , Huang, X. , Wang, J. , Chen, W. , Liu, Y. , Zhang, Y. , Wang, X. , Xu, W. , He, Q. , & Wang, Y. (2017). Translating divergent environmental stresses into a common proteome response through the histidine kinase 33 (Hik33) in a model cyanobacterium. Molecular & Cellular Proteomics, 16(7), 1258–1274. 10.1074/mcp.M116.068080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskov, A. , Kern, J. , Gabdulkhakov, A. , Broser, M. , Zouni, A. , & Saenger, W. (2009). Cyanobacterial photosystem II at 2.9‐Å resolution and the role of quinones, lipids, channels and chloride. Nature Structural & Molecular Biology, 16, 334–342. 10.1038/nsmb.1559 [DOI] [PubMed] [Google Scholar]
- He, Q. , & Vermaas, W. (1998). Chlorophyll a availability affects psbA translation and D1 precursor processing in vivo in Synechocystis sp. PCC 6803. Proceedings of the National Academy of Sciences of USA., 95(10), 5830–5835. 10.1073/pnas.95.10.5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman, Y. , Tchernov, D. , Reinhold, L. , Shibata, M. , Ogawa, T. , Schwarz, R. , Ohad, I. , & Kaplan, A. (2003). Genes encoding A‐type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Current Biology, 13(3), 230–235. 10.1016/S0960-9822(03)00046-0 [DOI] [PubMed] [Google Scholar]
- Herranen, M. , Battchikova, N. , Zhang, P. , Graf, A. , Sirpiö, S. , Paakkarinen, V. , & Aro, E. (2004). Towards functional proteomics of membrane protein complexes in Synechocystis sp. PCC 6803. Plant Physiology, 134, 470–481. 10.1104/pp.103.032326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao, H. , He, Q. , van Waasbergen, L. , & Grossman, A. (2004). Control of photosynthetic and high‐light‐responsive genes by the histidine kinase DspA: Negative and positive regulation and interactions between signal transduction pathways. Journal of Bacteriology, 186(12), 3882–3888. 10.1128/JB.186.12.3882-3888.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, G. , Xiao, Y. , Pi, X. , Zhao, L. , Zhu, Q. , Wang, W. , Kuang, T. , Han, G. , Sui, S. , & Shen, J. (2021). Structural insights into a dimeric Psb27‐photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus . Proceedingsof the National Academy of Sciences of USA, 118(5), e2018053118. 10.1073/pnas.2018053118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvi, S. , Suorsa, M. , Paakkarinen, V. , & Aro, E. (2011). Optimized native gel systems for separation of thylakoid protein complexes: Novel super‐ and mega‐complexes. Biochemical Journal, 439, 207–214. 10.1042/BJ20102155 [DOI] [PubMed] [Google Scholar]
- Jordan, P. , Fromme, P. , Witt, H. , Klukas, O. , Saenger, W. , & Krauß, N. (2001). Three‐dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature, 411(6840), 909–917. 10.1038/35082000 [DOI] [PubMed] [Google Scholar]
- Kamata, T. , Hiramoto, H. , Morita, N. , Shen, J. , Mann, N. , & Yamamoto, Y. (2005). Quality control of photosystem II: An FtsH protease plays an essential role in the turnover of the reaction center D1 protein in Synechocystis PCC 6803 under heat stress as well as light stress conditions. Photochemical & Photobiological Sciences, 4(12), 983–990. 10.1039/b506068k [DOI] [PubMed] [Google Scholar]
- Karapetyan, N. V. , Holzwarth, A. R. , & Rögner, M. (1999). The photosystem I trimer of cyanobacteria: Molecular organization, excitation dynamics and physiological significance. FEBS Letters, 460, 395–400. 10.1016/S0014-5793(99)01352-6 [DOI] [PubMed] [Google Scholar]
- Kłodawska, K. , Kovács, L. , Várkonyi, Z. , Kis, M. , Sozer, Ö. , Laczkó‐Dobos, H. , Kóbori, O. , Domonkos, I. , Strzałka, K. , Gombos, Z. , & Malec, P. (2014). Elevated growth temperature can enhance photosystem I trimer formation and affects xanthophyll biosynthesis in cyanobacterium Synechocystis sp. PCC6803 Cells. Plant and Cell Physiology, 56(3), 558–571. 10.1093/pcp/pcu199 [DOI] [PubMed] [Google Scholar]
- Kojima, K. , Oshita, M. , Nanjo, Y. , Kasai, K. , Tozawa, Y. , Hayashi, H. , & Nishiyama, Y. (2007). Oxidation of elongation factor G inhibits the synthesis of the D1 protein of photosystem II. Molecular Microbiology, 65(4), 936–947. 10.1111/j.1365-2958.2007.05836.x [DOI] [PubMed] [Google Scholar]
- Komenda, J. , Barker, M. , Kuviková, S. , de Vries, R. , Mullineaux, C. , Tichý, M. , & Nixon, P. (2006). The FtsH protease slr0228 is important for quality control of photosystem II in the thylakoid membrane of Synechocystis sp. PCC 6803. Journal of Biological Chemistry, 281, 1145–1151. 10.1074/jbc.M503852200 [DOI] [PubMed] [Google Scholar]
- Komenda, J. , Knoppová, J. , Kopečná, J. , Sobotka, R. , Halada, P. , Yu, J. , Nickelsen, J. , Boehm, M. , & Nixon, P. (2011). The Psb27 assembly factor binds to the CP43 complex of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiology, 158, 476–486. 10.1104/pp.111.184184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo, K. , Geng, X. , Katayama, M. , & Ikeuchi, M. (2005). Distinct roles of CpcG1 and CpcG2 in phycobilisome assembly in the cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis Research, 84(1–3), 269–273. 10.1007/s11120-004-7762-9 [DOI] [PubMed] [Google Scholar]
- Kondo, K. , Mullineaux, C. , & Ikeuchi, M. (2009). Distinct roles of CpcG1‐phycobilisome and CpcG2‐phycobilisome in state transitions in a cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis Research, 99(3), 217–225. 10.1007/s11120-008-9399-6 [DOI] [PubMed] [Google Scholar]
- Kondo, K. , Ochiai, Y. , Katayama, M. , & Ikeuchi, M. (2007). The membrane‐associated CpcG2‐phycobilisome in Synechocystis: A new photosystem I antenna. Plant Physiology, 144, 1200–1210. 10.1104/pp.107.099267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopečná, J. , Komenda, J. , Bučinská, L. , & Sobotka, R. (2012). Long‐term acclimation of the cyanobacterium Synechocystis sp. PCC 6803 to high light is accompanied by an enhanced production of chlorophyll that is preferentially channeled to trimeric photosystem I. Plant Physiology, 160(4), 2239–2250. 10.1104/pp.112.207274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoutte, B. , & Valloni, O. (1992). Purification and membrane topology of PSI‐D and PSI‐E, two subunits of the photosystem I reaction center. European Journal of Biochemistry, 205(3), 1175–1185. 10.1111/j.1432-1033.1992.tb16888.x [DOI] [PubMed] [Google Scholar]
- Lea‐Smith, D. , Bombelli, P. , Vasudevan, R. , & Howe, C. (2016). Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochimica et Biophysica Acta, 1857(3), 247–255. 10.1016/j.bbabio.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Lelong, C. , Boekema, E. , Kruip, J. , Bottin, H. , Rögner, M. , & Sétif, P. (1996). Characterization of a redox active cross‐linked complex between cyanobacterial photosystem I and soluble ferredoxin. The EMBO Journal, 15(9), 2160–2168. 10.1002/j.1460-2075.1996.tb00569.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , & Sherman, L. (2000). A redox‐responsive regulator of photosynthesis gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803. Journal of Bacteriology, 182(15), 4268–4277. 10.1128/JB.182.15.4268-4277.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll, B. , Kern, J. , Saenger, W. , Zouni, A. , & Biesiadka, J. (2005). Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature, 438(7070), 1040–1044. 10.1038/nature04224 [DOI] [PubMed] [Google Scholar]
- Lu, Y. (2016). Identification and roles of photosystem II assembly, stability, and repair factors in Arabidopsis. Frontiers in Plant Science, 7, 168. 10.3389/fpls.2016.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luimstra, V. , Schuurmans, J. , Hellingwerf, K. , Matthijs, H. , & Huisman, J. (2020). Blue light induces major changes in the gene expression profile of the cyanobacterium Synechocystis sp. PCC 6803. Physiologia Plantarum, 170, 10–26. 10.1111/ppl.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luimstra, V. , Schuurmans, J. , Verschoor, A. , Hellingwerf, K. , Huisman, J. , & Matthijs, H. (2018). Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynthesis Research, 138, 177–189. 10.1007/s11120-018-0561-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpää, P. , Kallio, T. , Mulo, P. , Salih, G. , Aro, E. , Tyystjärvi, E. , & Jansson, C. (1993). Site‐specific mutations in the D1 polypeptide affect the susceptibility of Synechocystis 6803 cells to photoinhibition. Plant Molecular Biology, 22, 1–12. 10.1007/BF00038991 [DOI] [PubMed] [Google Scholar]
- Malavath, T. , Caspy, I. , Netzer‐El, S. , Klaiman, D. , & Nelson, N. (2018). Structure and function of wild‐type and subunit‐depleted photosystem I in Synechocystis . Biochimica et Biophysica Acta, 1859(9), 645–654. 10.1016/j.bbabio.2018.02.002 [DOI] [PubMed] [Google Scholar]
- Máté, Z. , Sass, L. , Szekeres, M. , Vass, I. , & Nagy, F. (1998). UV‐B‐induced differential transcription of psbA genes encoding the D1 protein of photosystem II in the cyanobacterium Synechocystis 6803. Journal of Biological Chemistry, 273(28), 17439–17444. 10.1074/jbc.273.28.17439 [DOI] [PubMed] [Google Scholar]
- Meunier, P. , Colon‐Lopez, M. , & Sherman, L. (1997). Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiology, 115(3), 991–1000. 10.1104/pp.115.3.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux, C. (1994). Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterial mutant lacking photosystem II. Biochimica et Biophysica Acta, 1184, 71–77. 10.1016/0005-2728(94)90155-4 [DOI] [Google Scholar]
- Mulo, P. , Sirpiö, S. , Suorsa, M. , & Aro, E. (2008). Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynthesis Research, 98(1–3), 489–501. 10.1007/s11120-008-9320-3 [DOI] [PubMed] [Google Scholar]
- Mulo, P. , Tyystjärvi, T. , Tyystjärvi, E. , Mäenpää, P. , & Aro, E. M. (1997). Mutagenesis of the D‐E loop of photosystem II reaction centre protein D1. Function and assembly of photosystem II. Plant Molecular Biology, 33(6), 1059–1071. 10.1023/A:1005765305956 [DOI] [PubMed] [Google Scholar]
- Mustila, H. , Paananen, P. , Battchikova, N. , Santana‐Sánchez, A. , Muth‐Pawlak, D. , Hagemann, M. , Aro, E. , & Allahverdiyeva, Y. (2016). The flavodiiron protein Flv3 functions as a homo‐oligomer during stress acclimation and is distinct from the Flv1/Flv3 hetero‐oligomer specific to the O2 photoreduction pathway. Plant and Cell Physiology, 57, 1468–1483. 10.1093/pcp/pcw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, F. , Andersson, B. , & Jansson, C. (1990). Photosystem II characteristics of a constructed Synechocystis 6803 mutant lacking synthesis of the D1 polypeptide. Plant Molecular Biology, 14, 1051–1054. 10.1007/BF00019402 [DOI] [PubMed] [Google Scholar]
- Nilsson, F. , Simpson, D. , Jansson, C. , & Andersson, B. (1992). Ultrastructural and biochemical characterization of a Synechocystis 6803 mutant with inactivated psbA genes. Archives of Biochemistry and Biophysics, 295, 340–347. 10.1016/0003-9861(92)90526-3 [DOI] [PubMed] [Google Scholar]
- Nishiyama, Y. , Allakhverdiev, S. I. , Yamamoto, H. , Hayashi, H. , & Murata, N. (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry, 43(35), 11321–11330. 10.1021/bi036178q [DOI] [PubMed] [Google Scholar]
- Pfannschmidt, T. , Nilsson, A. , & Allen, J. (1999). Photosynthetic control of chloroplast gene expression. Nature, 397(6720), 625–628. 10.1038/17624 [DOI] [Google Scholar]
- Puzorjov, A. , Dunn, K. E. , & McCormick, A. J. (2021). Production of thermostable phycocyanin in a mesophilic cyanobacterium. Metabolic Engineering Communications, 13, e00175. 10.1016/j.mec.2021.e00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhimberdieva, M. , Vavilin, D. , Vermaas, W. , Elanskaya, I. , & Karapetyan, N. (2007). Phycobilin/chlorophyll excitation equilibration upon carotenoid‐induced non‐photochemical fluorescence quenching in phycobilisomes of the cyanobacterium Synechocystis sp. PCC 6803. Biochimica et Biophysica Acta, 1767, 757–765. 10.1016/j.bbabio.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Rappaport, F. , & Diner, B. (2008). Primary photochemistry and energetics leading to the oxidation of the (Mn)4Ca cluster and to the evolution of molecular oxygen in photosystem II. Coordination Chemistry Reviews, 252(3–4), 259–272. 10.1016/j.ccr.2007.07.016 [DOI] [Google Scholar]
- Sakurai, I. , Stazic, D. , Eisenhut, M. , Vuorio, E. , Steglich, C. , Hess, W. , & Aro, E. (2012). Positive regulation of psbA gene expression by cis‐encoded antisense RNAs in Synechocystis sp. PCC 6803. Plant Physiology, 160, 1000–1010. 10.1104/pp.112.202127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selão, T. , Jebarani, J. , Ismail, N. , Norling, B. , & Nixon, P. (2020). Enhanced production of D‐lactate in cyanobacteria by re‐routing photosynthetic cyclic and pseudo‐cyclic electron flow. Frontiers in Plant Science, 10, 1700. 10.3389/fpls.2019.01700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. X. , & Schröder, W. P. (2004). The low molecular mass subunits of the photosynthetic supracomplex, photosystem II. Biochimica et Biophysica Acta, 1608(2–3), 75–96. 10.1016/j.bbabio.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Shin, M. (2004). How is ferredoxin‐NADP reductase involved in the NADP photoreduction of chloroplasts? Photosynthesis Research, 80(1–3), 307–313. 10.1023/B:PRES.0000030456.96329.f9 [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , & Shukla, P. (2021). Tightening the screws on psbA in cyanobacteria. Trends in Genetics, 37, 211–215. 10.1016/j.tig.2020.08.018 [DOI] [PubMed] [Google Scholar]
- Tagawa, K. , & Arnon, D. (1962). Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature, 195(4841), 537–543. 10.1038/195537a0 [DOI] [PubMed] [Google Scholar]
- Tamary, E. , Kiss, V. , Nevo, R. , Adam, Z. , Bernát, G. , Rexroth, S. , Rögner, M. , & Reich, Z. (2012). Structural and functional alterations of cyanobacterial phycobilisomes induced by high‐light stress. Biochimica et Biophysica Acta, 1817, 319–327. 10.1016/j.bbabio.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Tsiotis, G. , Haase, W. , Engel, A. , & Michel, H. (1995). Isolation and structural characterization of trimeric cyanobacterial photosystem I complex with the help of recombinant antibody fragments. European Journal of Biochemistry, 231(3), 823–830. 10.1111/j.1432-1033.1995.0823d.x [DOI] [PubMed] [Google Scholar]
- Tyystjärvi, T. , Herranen, M. , & Aro, E.‐M. (2001). Regulation of translation elongation in cyanobacteria: Membrane targeting of the ribosome nascent‐chain complexes controls the synthesis of D1 protein. Molecular Microbiology, 40, 476–484. 10.1046/j.1365-2958.2001.02402.x [DOI] [PubMed] [Google Scholar]
- Vermaas, W. , Ikeuchi, M. , & Inoue, Y. (1988). Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynthesis Research, 17(1–2), 97–113. 10.1007/BF00047683 [DOI] [PubMed] [Google Scholar]
- Vinyard, D. , Badshah, S. , Riggio, M. , Kaur, D. , Fanguy, A. , & Gunner, M. (2019). Photosystem II oxygen‐evolving complex photoassembly displays an inverse H/D solvent isotope effect under chloride‐limiting conditions. Proceedings of the National Academy of Sciences of USA, 116(38), 18917–18922. 10.1073/pnas.1910231116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Jantaro, S. , Lu, B. , Majeed, W. , Bailey, M. , & He, Q. (2008). The high light‐inducible polypeptides stabilize trimeric photosystem I complex under high light conditions in Synechocystis PCC 6803. Plant Physiology, 147(3), 1239–1250. 10.1104/pp.108.121087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Wu, Q. , Mao, H. , Zhao, N. , & Vermaas, W. F. (1999). Effects of chlorophyll availability on phycobilisomes in Synechocystis sp. PCC 6803. IUBMB Life, 48, 625–630. 10.1080/713803568 [DOI] [PubMed] [Google Scholar]
- Zabret, J. , Bohn, S. , Schuller, S. , Arnolds, O. , Möller, M. , Meier‐Credo, J. , Liauw, P. , Chan, A. , Tajkhorshid, E. , Langer, J. , Stoll, R. , Krieger‐Liszkay, A. , Engel, B. , Rudack, T. , Schuller, J. , & Nowaczyk, M. (2021). Structural insights into photosystem II assembly. Nature Plants, 7(4), 524–538. 10.1038/s41477-021-00895-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Allahverdiyeva, Y. , Eisenhut, M. , & Aro, E. M. (2009). Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE, 4, e5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Battchikova, N. , Jansen, T. , Appel, J. , Ogawa, T. , & Aro, E. M. (2004). Expression and functional roles of the two distinct NDH‐1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell, 16(12), 3326–3340. 10.1105/tpc.104.026526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Eisenhut, M. , Brandt, A. , Carmel, D. , Silén, H. , Vass, I. , Allahverdiyeva, Y. , Salminen, T. , & Aro, E. M. (2012). Operon flv4‐flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell, 24(5), 1952–1971. 10.1105/tpc.111.094417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Xie, J. , & Zhao, J. (2009). The mobility of PSI and PQ molecules in Spirulina platensis cells during state transition. Photosynthesis Research, 99, 107–113. 10.1007/s11120-008-9400-4 [DOI] [PubMed] [Google Scholar]


