Abstract
The fruit of Garcinia xanthochymus is consumed traditionally and is known to possess health-promoting effects. However, studies involving the characterization of phytochemicals of different parts of the fruit, and their biological activity were limited and hence warranted a comprehensive study. The proximate analyses reveal that fruit peel was rich in crude fiber. The levels of essential minerals, fatty acids, amino acids, carotenoids, organic acids, and polyphenols were significantly higher in the peel, followed by the rind, seed, and pulp. The in vitro antioxidant assays revealed that the polyphenolic extract of the peel possesses a high antioxidant effect compared to the extracts from other parts of theG. xanthochymus fruit. Furthermore, the in vitro assays reveal the antidiabetic potential of the methanol extract. This is the first comprehensive report involving the characterization and biological properties of different parts of the G. xanthochymus fruit. Hence, our study implicates the potential use of this fruit for the development of functional foods for diabetes.
1. Introduction
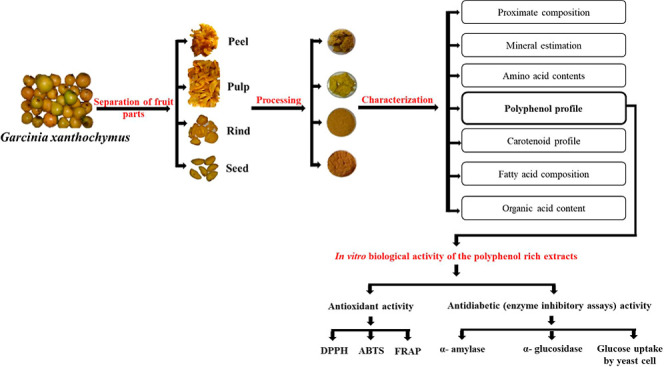
The enrichment of diet with fruits and vegetables has shown to reduce the risk of developing various metabolic disorders including diabetes, hypertension, and so forth.1 This reducing effect is attributed to the functional effect of the bioactive components present in fruits and vegetables. Many extracts and bioactives from natural sources are known to deal with several health complications. Three medicinal plants (Macaranga hurifolia, Sterculia tragacantha, and Zanthoxylum gilletii) from Africa were studied for their phytochemical profile and biological propensities. The results showed that the leaf extract of M. hurifolia had the highest phytoconstituents compared to the other two plants, whereas the highest antioxidant and enzyme inhibitory effects were exhibited by S. tragacantha and M. hurifolia, respectively, which could be attributed to the presence of phenolic components.2 Phenolic components from the Turkish medicine plant Silene salsuginea incline to support its use in managing chronic diseases such as diabetes, neurological conditions, and inflammation and also in cosmeceutical applications.3 Halophytes such as Arthrocnemum macrostachyum, Halimione portulacoides, and Salicornia europaea were evaluated for their biological and chemical fingerprints. The results showed that the ethyl acetate extract of A. macrostachyum showed the highest total phenolic and flavonoid contents, and it was found to be most potent towards antioxidant activity.4 The members of the genus Garcinia, family Clusiaceae, are as well widely used in the ayurvedic system of medicine for the treatment of various disorders.5 Approximately, 300 different species of Garcinia fruits are distributed in Asia and Africa. Among them, fruits of Garcinia cambogia and Garcinia indicia are well studied and are found to exhibit potent antioxidant, antiobesity, and antidiabetic properties.6 Another species of the same genus Garcinia xanthochymus is a perennial medium-sized tree distributed widely in China, South East Asia, and the Western Ghats of India. It is commonly known as egg tree, Himalayan Garcinia, Mysore gamboge, false mangosteen, and yellow mangosteen as its fruits are similar to mangosteen albeit different in color. Fruits are sub-globose in shape, which turn yellow upon ripening with a thin skin and berry with fleshy pulp, and two to eight large seeds are embedded in the edible pulp. Gamboge is used for the preparation of yellow dye for watercolors and fabric.7 Fruits of this plant are eaten fresh and processed to make jams, vinegar, beverages, and other food products.8 Mango peel is a rich source of bioactive components, specifically carotenoids, vitamin E, dietary fiber, polyphenols, and vitamin C.9 Mango peels contribute around 20% of total weight of the fruit, which encourages extracting pectin and other beneficial food-grade components.10 Similar to mango processing, G. xanthochymus fruits also contribute by-products to small-scale industries which can be utilized to produce pectin and dietary fiber and also to extract bioactive components such as carotenoids, polyphenols, and so forth. Traditionally, G. xanthochymus is widely used in Ayurveda for the treatment of diarrhea, dysentery, nausea, and vomiting.11 Additionally, studies have also shown its use for the therapy of metabolic disorders such as obesity.12 However, the characterization of phytochemicals in different parts of the fruit, such as the peel, pulp, and seed, is not very clear. It requires a comprehensive study to evaluate these parts for functional benefits and to utilize them for the development of functional foods. Furthermore, the functional benefits associated and therapeutic efficacy of the above-mentioned parts of the fruit in the treatment of diabetes and diabetes-induced non-alcoholic fatty liver disease are not well documented. Therefore, the aim of the study is to characterize the different parts of the fruit in relation to their antidiabetic, antioxidant, and hepatoprotective properties. Thus, the present study may help in the effective utilization of the G. xanthochymus fruit for promoting health.
2. Materials and Methods
2.1. Materials
Fruits of G. xanthochymus were obtained from M/S Alva Pharmacy, Yogaraja Arogyadhama, Mijar-574225, Dakshina Kannada district, Karnataka, India. Reverse-phase high-performance liquid chromatography (RP-HPLC) grade chemicals were procured from Sigma-Aldrich Chemicals Pvt. Ltd. (St. Louis, USA), whereas all the analytical and laboratory grade chemicals were procured from Rankem Chemicals (Bengaluru, India), Sisco Research Laboratories Pvt. Ltd. (Bengaluru, India), and Himedia Laboratories Pvt. Ltd. (Bengaluru, India). The HPLC column [Luna 5 μm C18 (2)] was procured from Phenomenex (Hyderabad, India).
2.2. Processing of Fruits
The procured fresh fruits were segregated, washed, and separated into different parts, that is, the peel, pulp, and seed. Among the separated parts, the peel/rind and pulp were lyophilized to obtain the lyophilized peel (LPe) and lyophilized pulp (LPu), respectively. The peel and seed were also dried under the sun to get the sun-dried rind (SDR) and sun-dried seed (SDS), respectively, to mimic the usage of the fruit parts by the local populations. All the processed parts were milled in a pulverizer (Pilots India, Thrissur, Kerala, India) to make a fine powder. Furthermore, the fruit parts were defatted using hexane and stored at −20 °C until the further analyses.
2.3. Proximal Composition Analysis
The fruit LPe, LPu, SDR, and SDS fractions were analyzed for moisture, protein, fat, crude ash, and crude fiber as per the best-known and most significant protocols of the Association of Official Agricultural Chemists (AOAC), methods—934.06, 960.52, 960.39, 923.03, and 962.09,13 respectively. The available carbohydrate content was estimated by difference. The dietary fiber was analyzed by the AOAC, method 991.43.14
2.4. Estimation of the Mineral Content
Micro and macromineral contents of different parts of the fruit were studied using microwave plasma-atomic emission spectroscopy according to the AOAC, method 985.01.14
2.5. Estimation of Fatty Acids
To estimate fatty acids, fatty acid methyl esters of the oil samples were prepared by trans-esterification, according to the AOAC, method 969.3314 and estimated by gas chromatography–mass spectrometry.
2.6. Estimation of Amino Acids
The amino acid profile of different parts of the fruit was determined following the method of Kamani et al.(15)
2.7. Estimation of Total and Reducing Sugars
Total sugars present in the fruit parts were estimated by Lane and Eynon methods,16 whereas the reducing sugars were analyzed by using a protocol by AOAC, method 945.66.17
2.8. Estimation of Total Ascorbic Acid
The total ascorbic acid content of fruit samples was estimated after extraction with 3% oxalic acid solution by titrating with 2,6-dichlorophenolindophenol.18
2.9. Total Acidity and pH
The total acidity and pH of the samples were analyzed as per the AOAC, method 942.15.14
2.10. Estimation of Organic Acids by HPLC
Organic acids in different parts of the fruit were identified using the RP-HPLC system, C18 column (250 × 4.6 mm) according to the established method.19
2.11. Estimation of Total Carotenoids and HPLC Analysis
Total carotenoids from all the samples were extracted with 80% ice-cold acetone and estimated according to the procedure described by Lakshminarayana et al.(20) Furthermore, 20 μL of the samples was injected into HPLC, and different carotenoids present in the samples were compared with the area of standard peaks and calculated as per Raju et al.(21)
2.12. Extraction and Estimation of Total Polyphenols, Flavonoids, and Tannins
The polyphenols, flavonoids, and tannins were extracted and estimated according to McDonald et al.,22 Chang et al.,23 and Herald et al.,24 respectively, in the defatted samples of different parts of G. xanthochymus fruits.
2.13. Quantification of Polyphenols by HPLC Analysis
The total polyphenolic content was further tested by HPLC analysis. The extracts of the fruits were subjected to HPLC analysis following the protocol of Singh et al.(25) on a reversed-phase C18 column. Different phenolic acids present in the samples were quantified by comparing them with the area of standard peaks.
2.14. Fourier-Transform Infrared Spectroscopy Analysis of Different Parts of Fruit Extracts
The potent extracts of different parts of the fruit were further subjected to Fourier-transform infrared (FTIR) analysis to determine the various functional groups present. Briefly, FTIR spectra were recorded at a resolution of 2 cm–1 at the range of 400–4000 cm–1.
2.15. Antioxidant Potential of Different Parts of Fruit Extracts
The antioxidant potential was studied by in vitro methods such as the free radical scavenging activity by 2,2-diphenyl-1-picrylhydrazyl (DPPH)26 and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)27 assay and by ferric reducing antioxidant power assay (FRAP).28
2.16. Antidiabetic Potential
The antidiabetic potential of different extracts of fruit parts was investigated by following in vitro assays such as α-amylase, α-glucosidase,29 and lipase inhibitory activity.30 The glucose uptake assay was performed using baker’s yeast as per the protocol of Harish et al.(31)
2.17. Statistical Analysis
All the experiments were performed in triplicates, and results were expressed as mean ± standard error mean (SEM), and differences between samples were determined by the multiple range test. P values at < 0.05 were regarded as significant.
3. Results
3.1. Proximate Composition of Different Parts of the Fruit
Proximate composition of food components tells about their precise content of the nutrients. Different parts of G. xanthochymus fruits were estimated for proximate composition as depicted in Table 1. The moisture content was lower in LPu (8.17 ± 0.32%) and higher in SDR (9.17 ± 0.55%); however, there was no significant difference among the samples. Low-moisture foods are safe whose microbial load is less and can be stored for longer periods. Among the different fruit parts, the SDS (28.35 ± 0.07%) showed the highest fat content followed by the SDR, LPe, and LPu (9.38 ± 0.11, 6.29 ± 0.10, and 6.08 ± 0.11%). The crude protein content varied from 2 to 4%, with LPu having slightly less than the other parts, whereas the crude fiber content was highest in LPe (16.31 ± 0.35%), followed by the SDR (12.10 ± 0.12%), LPu (7.11 ± 0.16%), and SDS (4.18 ± 0.49%). Dietary fiber plays an important role in human health; LPe possessed the highest dietary fiber content (73.85 ± 0.19%) followed by the SDR (39.12 ± 0.77%), SDS (28.81 ± 0.25%), and LPu (15.12 ± 0.29%). Insoluble and soluble dietary fibers also followed the same trend as the dietary fiber, with LPe possessing a higher content of insoluble dietary fiber (60.68 ± 0.14 and 13.18 ± 0.05%) followed by the SDR (26.81 ± 0.73 and 12.32 ± 0.39%), SDS (18.50 ± 0.12%), and LPu (9.97 ± 0.10 and 5.15 ± 0.18%). Carbohydrate was the most abundant constituent in LPu (74.36 ± 0.53).
Table 1. Proximate Composition of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| Parameters | LPe (%) | LPu (%) | SDR (%) | SDS (%) | Significance (%) |
| Moisture | 9.10 ± 0.29 | 8.17 ± 0.32 | 9.17 ± 0.55 | 8.47 ± 0.64 | NS |
| Ash | 2.53 ± 0.19a | 2.23 ± 0.09a | 3.12 ± 0.12b | 5.22 ± 0.17c | P < 0.001 |
| Crude Fat | 6.29 ± 0.10a | 6.08 ± 0.11a | 9.38 ± 0.11b | 28.35 ± 0.07c | P < 0.001 |
| Crude Protein | 2.94 ± 0.14b | 2.05 ± 0.13a | 3.64 ± 0.13c | 4.47 ± 0.14d | P < 0.001 |
| Crude Fiber | 16.31 ± 0.35d | 7.11 ± 0.16b | 12.10 ± 0.12c | 4.18 ± 0.49a | P < 0.001 |
| Total Dietary Fiber | 73.85 ± 0.19d | 15.12 ± 0.29a | 39.12 ± 0.77c | 28.81 ± 0.25b | P < 0.001 |
| Soluble Dietary Fiber | 13.18 ± 0.05d | 5.15 ± 0.18a | 12.32 ± 0.39c | 10.31 ± 0.14b | P < 0.001 |
| Insoluble ietary Fiber | 60.68 ± 0.14d | 9.97 ± 0.10a | 26.81 ± 0.73c | 18.50 ± 0.12b | P < 0.001 |
| Carbohydrate | 62.81 ± 0.83b | 74.36 ± 0.53c | 62.58 ± 0.28b | 49.31 ± 1.02a | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; NS—not significant; SDR—sun-dried rind; and SDS—sun-dried seed.
3.2. Macro and Microminerals in Different Parts of the Fruit
Minerals are vital nutrients to perform important functions in the body. They are required in minor quantities when compared to other nutrients but play a significant role in human nutrition. The macro and micromineral content of theG. xanthochymus fruit parts is shown in Table 2. LPe was rich in potassium (321.25 ± 69.43 μg/100 g), calcium (40.25 ± 7.94 μg/100 g), and magnesium (22.00 ± 3.75 μg/100 g), whereas LPu was rich in calcium (59.00 ± 2.89 μg/100 g), copper (0.75 ± 0.14 μg/100 g), and potassium (453.00 ± 6.64 μg/100 g). The SDR was rich in potassium (171.75 ± 8.52 μg/100 g), magnesium (32.25 ± 1.01 μg/100 g), and calcium (26.50 ± 0.87 μg/100 g), where the SDS was rich in iron (8.75 ± 0.43 μg/100 g), and magnesium (40.50 ± 1.44 μg/100 g) compared to other parts. In addition, LPe showed the highest amount of zinc (4.63 ± 1.38 μg/100 g) when compared to other parts of the fruit.
Table 2. Mineral Content in Different Parts of the G. xanthochymus Fruita.
| Fruit
arts |
|||||
|---|---|---|---|---|---|
| μ/100 g Sample |
|||||
| Minerals | LPe | LPu | SDR | SDS | Significance |
| Zinc | 4.63 ± 1.38b | 1.00 ± 0.29a | 1.25 ± 0.14a | 1.50 ± 0.29a | P < 0.05 |
| Calcium | 40.25 ± 7.94a,b | 59.00 ± 2.89c | 26.50 ± 0.87a | 53.75 ± 3.03b,c | P < 0.05 |
| Iron | 3.00 ± 0.58a,b | 4.25 ± 0.14b | 1.75 ± 0.43a | 8.75 ± 0.43c | P < 0.001 |
| Copper | 0.25 ± 0.14a,b | 0.75 ± 0.14c | 0.00 ± 0.00a | 0.50 ± 0.00b | P < 0.05 |
| Potassium | 321.25 ± 69.43b | 453.00 ± 6.64c | 171.75 ± 8.52a | 424.50 ± 9.24b,c | P < 0.003 |
| Manganese | 0.25 ± 0.14 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | NS |
| Magnesium | 22.00 ± 3.75a | 38.50 ± 5.77b | 32.25 ± 1.01b,c | 40.50 ± 1.44b | P < 0.05 |
| Sodium | 7.25 ± 3.03 | 1.50 ± 0.29 | 3.50 ± 0.00 | 3.25 ± 0.43 | NS |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.05) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; NS–not significant; SDR—sun-dried rind; and SDS—sun-dried seed.
3.3. Fatty Acid Composition of Different Parts of the Fruit
The fatty acid composition of different parts of the fruit was evaluated by Gas chromatography–mass spectrometry. The different parts of the G. xanthochymus fruit showed the presence of both saturated and unsaturated fatty acids (Table 3). LPe showed the highest concentrations of two primary essential fatty acids, viz., linoleic acid (8.095 ± 0.15%), and α-Linolenic acid (14.190 ± 0.32%) compared to the LPu, SDS, and SDR. Furthermore, palmitic (49.596 ± 0.09%), and oleic acids (46.228 ± 0.00%) were found to be more in the SDS followed by the LPu (32.666 ± 0.02 and 37.314 ± 0.25), SDR (29.585 ± 1.16 and 32.632 ± 0.13), and LPe (26.285 ± 0.23 and 31.167 ± 0.60).
Table 3. Fatty Acid Composition in Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| Area% |
|||||
| Fatty cids | LPe | LPu | SDR | SDS | SignificanceC |
| C10 (Capric Acid) | 0.495 ± 0.01c | 0.263 ± 0.00b | 0.00 ± 0.00a | 0.00 ± 0.00a | P < 0.001 |
| C12 (Lauric Acid) | 6.727 ± 0.12c | 2.985 ± 0.02b | 3.309 ± 0.16b | 0.00 ± 0.00a | P < 0.001C |
| C16 (Palmitic Acid) | 26.285 ± 0.23a | 32.666 ± 0.02c | 29.585 ± 1.16b | 49.596 ± 0.09d | P < 0.001 |
| C18 (Stearic Acid) | 13.041 ± 0.24c | 10.283 ± 0.07b | 13.549 ± 0.13d | 1.892 ± 0.05a | P < 0.001 |
| C18:1 (Oleic Acid) | 31.167 ± 0.60a | 37.314 ± 0.25c | 32.632 ± 0.13b | 46.228 ± 0.00d | P < 0.001 |
| C18:2 (Linoleic Acid) | 8.095 ± 0.15b | 5.735 ± 0.06b | 7.378 ± 1.91b | 46.228 ± 0.01a | P < 0.0 |
| C18:3 (α-Linolenic Acid) | 14.190 ± 0.32c | 10.755 ± 0.16b | 13.546 ± 0.34c | 46.228 ± 0.02a | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.05) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; ND—not detected; SDR—sun-dried rind; and SDS—sun-dried seed.
3.4. Essential and Non-essential Amino Acids in Different Parts of the Fruit
The amino acid content in different parts of the fruit revealed that they are rich in essential and non-essential amino acids except methionine and cysteine (Table 4). The LPe was rich in histidine (7.07 ± 0.11%) and tyrosine (5.28 ± 0.15%), whereas, LPu was rich in leucine (8.79 ± 0.01%) and lysine (8.62 ± 0.06%), while the SDR showed the high levels of threonine (5.22 ± 0.05%), valine (6.22 ± 0.05%), and phenylalanine (5.90 ± 0.06%). Arginine (8.33 ± 0.07%) was high in SDS sample.
Table 4. Amino Acid Composition in Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| Amino Acids | LPe (%) | LPu (%) | SDR (%) | SDS (%) | Significance (%) |
| Aspartate and Asparagine | 9.01 ± 0.17b | 9.92 ± 0.04c | 10.60 ± 0.21d | 7.45 ± 0.08a | P < 0.001 |
| Threonine | 4.84 ± 0.24b | 4.95 ± 0.03b | 5.22 ± 0.05b | 3.79 ± 0.07a | P < 0.001 |
| Serine | 5.87 ± 0.33b | 5.58 ± 0.02b | 5.55 ± 0.04b | 4.65 ± 0.12a | P < 0.05 |
| Glutamate and Glutamine | 12.37 ± 0.67a | 13.00 ± 0.00a | 12.10 ± 0.10a | 23.72 ± 0.22b | P < 0.001 |
| Cysteine | ND | ND | ND | ND | - |
| Proline | 6.33 ± 0.35c | 5.82 ± 0.13b,c | 5.49 ± 0.16b | 4.19 ± 0.22a | P < 0.003 |
| Glycine | 7.35 ± 0.32c | 5.88 ± 0.02a,b | 6.39 ± 0.03b | 5.53 ± 0.02a | P < 0.001 |
| Methionine | ND | ND | ND | ND | - |
| Alanine | 6.09 ± 0.09a | 7.46 ± 0.04b | 6.15 ± 0.05a | 5.98 ± 0.03a | P < 0.001 |
| Valine | 5.53 ± 0.09b | 6.12 ± 0.04c | 6.22 ± 0.05c | 3.85 ± 0.05a | P < 0.001 |
| Isoleucine | 3.82 ± 0.09 | 4.04 ± 0.03 | 4.41 ± 0.05 | 3.53 ± 0.38 | NS |
| Leucine | 8.56 ± 0.19 | 8.79 ± 0.01 | 9.17 ± 0.04 | 9.41 ± 0.48 | NS |
| Tyrosine | 5.28 ± 0.15c | 4.60 ± 0.06b | 4.97 ± 0.09c | 3.06 ± 0.06a | P < 0.001 |
| Phenylalanine | 5.70 ± 0.09b | 5.22 ± 0.02a | 5.77 ± 0.09b | 5.90 ± 0.06b | P < 0.003 |
| Histidine | 7.07 ± 0.11d | 5.90 ± 0.06b | 6.44 ± 0.23c | 3.24 ± 0.16a | P < 0.001 |
| Lysine | 8.39 ± 0.11c | 8.62 ± 0.06c | 7.56 ± 0.03b | 7.30 ± 0.17a | P < 0.001 |
| Arginine | 3.75 ± 0.09a | 3.99 ± 0.01b | 3.87 ± 0.03a,b | 8.33 ± 0.07c | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.05) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; ND—not detected; NS—not significant; SDR—sun-dried rind; and SDS—sun-dried seed.
3.5. Total and Reducing Sugars in Different Fruit Parts
The percentage of sugars was determined for different samples, and results revealed that LPu (33.42 ± 0.18%) had a high percentage of total sugars followed by the LPe (20.13 ± 0.27%) and SDR (12.13 ± 3.01%) extract, whereas the reducing sugar content was found to be high in LPe (18.13 ± 0.27%) followed by the LPu (16.55 ± 0.29%) and SDR (9.18 ± 0.17%) (Table 5).
Table 5. Total and Reducing Sugar Levels in the Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| Sugars | LPe (%) | LPu (%) | SDR (%) | SDS (%) | Significance (%) |
| Total Sugars | 20.13 ± 0.27b | 33.42 ± 0.18c | 12.13 ± 3.01a | ND | P < 0.001 |
| Reducing Sugars | 20.13 ± 0.27c | 16.55 ± 0.29b | 9.18 ± 0.17a | ND | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; ND—not detected; SDR—sun-dried rind; and SDS—sun-dried seed.
3.6. Ascorbic Acid, Total Acidity, and pH of Different Parts of the Fruit
The ascorbic acid content was highest in SDS (81.11 ± 1.84 mg/100 g) and SDR (80.18 ± 2.77 mg/100 g) extracts compared with the other samples. Furthermore, the total acidity was found to be maximum in LPu (25.00 ± 0.3%) followed by the SDR (22.69 ± 0.08%), LPe (20.65 ± 0.09%), and SDS (0.528 ± 0.02%) (Table 6).
Table 6. Ascorbic Acid, Total Acidity, and pH of Different Parts of the G. xanthochymus Fruita.
| Fruit
arts |
|||||
|---|---|---|---|---|---|
| Parameters | LPe | LPu | SDR | SDS | Significance |
| Ascorbic Acid (mg/100 g) | 57.96 ± 4.29a | 52.53 ± 1.59a | 80.18 ± 2.77b | 81.11 ± 1.84b | P < 0.001 |
| Total Acidity (%) | 20.65 ± 0.09b | 25.00 ± 0.31d | 22.69 ± 0.08c | 0.528 ± 0.02a | P < 0.001 |
| pH | 3.13 ± 0.12a | 2.93 ± 0.13a | 3.12 ± 0.12a | 5.22 ± 0.17b | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; SDR—sun-dried rind; and SDS—sun-dried seed.
3.7. Organic Acid Profile in Different Parts of the Fruit
The HPLC chromatograms of different parts of the fruit extracts were evaluated for the presence of different organic acids. Furthermore, Table 7 shows various organic acids present in different parts of the fruit. The amount of different organic acids was determined in comparison with that of the standard organic acids. The SDR extract of the fruit contained more amount of oxalic acid (17.67 ± 3.17 g/100 g), galacturonic acid (5439.48 ± 345.02 g/100 g), tannic acid (946.57 ± 76.05 g/100 g), and citric acid (462.42 ± 34.32 g/100 g), whereas succinic acid (413.14 ± 52.95 g/100 g) was high in the LPu extract, while ascorbic acid was abundant in LPe (74.23 ± 0.05 g/100 g) and SDR (73.38 ± 0.67 g/100 g) extracts, and malic acid is least in SDS extracts compared to other parts of the fruit.
Table 7. Organic Acid Content in Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| g/100 g Sample |
|||||
| Organic Acids | LPe | LPu | SDR | SDS | Significance |
| Oxalic Acid | 4.51 ± 0.11a | 5.70 ± 0.36a | 17.67 ± 3.17b | 7.29 ± 0.23a | P < 0.003 |
| Galacturonic Acid | 1725.81 ± 48.71a | 2418.88 ± 106.95b | 5439.48 ± 345.02c | 1351.76 ± 146.91a | P < 0.001 |
| Tannic Acid | 727.02 ± 53.79b | 729.04 ± 37.18b | 946.57 ± 76.05c | 238.88 ± 11.86a | P < 0.001 |
| Ascorbic Acid | 74.23 ± 0.05c | 32.99 ± 1.27b | 73.38 ± 0.67c | ND | P < 0.001 |
| Malic Acid | 133.48 ± 6.69b | 138.83 ± 1.31b | 134.78 ± 1.88b | 1.61 ± 0.33a | P < 0.001 |
| Succinic Acid | ND | 413.14 ± 52.95c | ND | 238.24 ± 19.83b | P < 0.001 |
| Citric Acid | ND | ND | 462.42 ± 34.32c | 96.33 ± 8.73b | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.003) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; ND—not detected; SDR—sun-dried rind; and SDS—sun-dried seed.
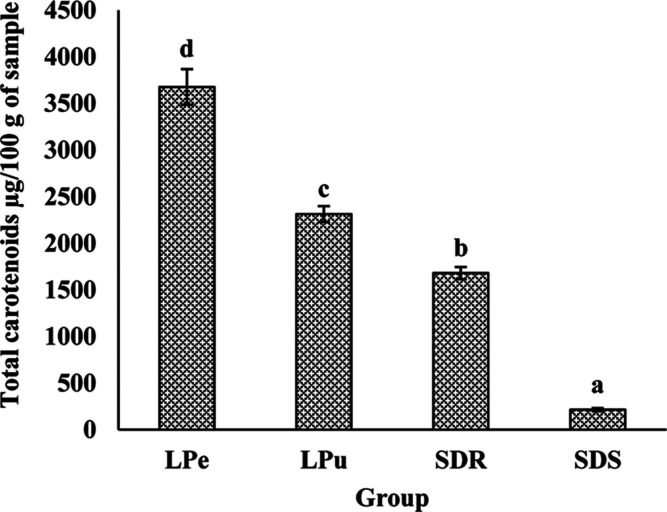
3.8. Total Carotenoids and the Carotenoid Profile in Different Parts of the Fruit
The total carotenoid content of the studied samples is presented in Figure 1 and was highest in LPe (3677.01 ± 191.13 μg/100 g) followed by LPu (2313.17 ± 86.22 μg/100 g), SDR (1680.28 ± 65.66 μg/100 g), and SDS (213.93 ± 15.44 μg/100 g) extracts. Further confirmation of different carotenoids was done by using HPLC. HPLC results of carotenoids are presented in Table 8. β-carotene (19.11 ± 0.01 mg/100 g), lutein (77.67 ± 0.05 mg/100 g), and lycopene (50.84 ± 0.01 mg/100 g) were present in LPe and SDS. Whereas, the SDR showed the presence of β-carotene (39.66 ± 0.14 mg/100 g) and lutein (113.29 ± 0.04 mg/100 g). The LPu extract contains only lutein (90.63 ± 0.05 mg/100 g). Furthermore, the lutein (77.67 ± 0.05 mg/100 g and 113.29 ± 0.04 mg/100 g) and lycopene (50.84 ± 0.01 mg/100 g and 20.41 ± 0.01 mg/100 g) content was high in LPe and SDS extracts compared to other carotenoids.
Figure 1.
Vertical bars showing the total carotenoid content of different parts of the G. xanthochymus fruit. LPe—lyophilized peel; LPu—lyophilized pulp; SDR—sundried rind ; and SDS—sundried seed. Note: All the values are mean ± SEM. Bars with different letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test.
Table 8. Different Carotenoid Content of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| mg/100 g Sample |
|||||
| Carotenoids | LPe | LPu | SDR | SDS | Significance |
| β - carotenoid | 19.11 ± 0.01c | ND | 39.66 ± 0.14d | 8.15 ± 0.04b | P < 0.001 |
| Lutein | 77.67 ± 0.05b | 90.63 ± 0.05c | 113.29 ± 0.04d | 3.29 ± 0.02a | P < 0.001 |
| Lycopene | 50.84 ± 0.01c | ND | ND | 20.41 ± 0.01b | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; ND—not detected; SDR—sundried rind; and SDS—sundried seed.
3.9. Total Phenolic Acid Content in Different Parts of the Fruit
The polyphenols were extracted and the extraction yield was measured. The results showed that the extraction yield of the crude extract was 712.05, 336.7, 284.27, and 567.04 mg/g in LPe, LPu, SDS, and SDR samples, respectively. The total phenolic acid content of different extracts of different parts of the fruit was analyzed and the results are presented in Table 9. The LPe had the maximum amount of the total polyphenols in methanol extract (1552.62 ± 6.48 mg/100 g) followed by the SDR (551.66 ± 5.74 mg/100 g) and LPu (367.19 ± 8.09 mg/100 g); the ethanol extract of the SDS (433.69 ± 2.26 mg/100 g) and the methanol extract of LPu (367.19 ± 8.09 mg/100 g) showed the maximum amount of total polyphenols, whereas the lowest amount of polyphenols was extracted from the water and ethyl acetate solvents compared to extracts of all the solvents studied. Hence, methanol was more efficient in extracting polyphenols from plant materials compared to water, ethanol, ethyl acetate, and acetone. Since the methanol extract of LPe, followed by SDR, LPu, and ethanol extract of the SDS, showed the highest total phenolic content, these extracts were used for further analyses in the present study.
Table 9. Total Phenolic Content of Different Extracts of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| mg/100 g |
|||||
| Extracts | LPe | LPu | SDR | SDS | Significance |
| Acetone | 901.12 ± 26.28d | 320.79 ± 31.23c | 51.03 ± 1.74a | 340.05 ± 9.64d | P < 0.001 |
| Ethyl Acetate | 263.03 ± 6.61b | 93.20 ± 0.78a | 142.63 ± 1.14b | 306.06 ± 1.01c | P < 0.001 |
| Ethanol | 765.65 ± 3.09c | 266.76 ± 6.32b | 526.69 ± 1.24d | 433.69 ± 2.26e | P < 0.001 |
| Methanol | 1552.62 ± 6.48e | 367.19 ± 8.09d | 551.66 ± 5.74e | 69.36 ± 0.99a | P < 0.001 |
| Water | 66.33 ± 0.56a | 128.39 ± 0.50a | 167.76 ± 1.28c | 184.49 ± 1.12b | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe—lyophilized peel; LPu—lyophilized pulp; SDR—sun-dried rind; and SDS—sun-dried seed.
3.10. Total Flavonoid and Tannin Content of Potent Extracts of Different Parts of the Fruit
The methanol extract of LPe (5943.96 ± 286.59 μg/100 g) showed significantly (P < 0.001) higher total flavonoid concentration when compared to all other extracts of the fruit (Table 10). Furthermore, the tannin content was least in LPu (5.57 ± 0.43 μg/100 g) followed by LPe (15.97 ± 0.93 μg/100 g), SDR (40.52 ± 1.97 μg/100 g), and SDS (65.88 ± 2.86 μg/100 g) suggesting that the methanol extract of LPe may have greater functional benefits compared to other extracts.
Table 10. Total Flavonoid and Tannin Content of Potent Extracts of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| μg/100 g |
|||||
| Bioactives | LPe–Methanol | LPu–Methanol | SDR–Methanol | SDS–Ethanol | Significance |
| Total Flavonoids | 5943.96 ± 286.59b | 3948.22 ± 158.41a | 3986.48 ± 155.62a | 3711.69 ± 35.90a | P < 0.001 |
| Tannin Content | 15.97 ± 0.93b | 5.57 ± 0.43a | 40.52 ± 1.97c | 65.88 ± 2.86d | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe–methanol–methanol extract of the lyophilized peel; LPu–methanol–methanol extract of the lyophilized pulp; SDR–ethanol–ethanol extract of the sun-dried rind; and SDS–methanol–methanol extract of the sun-dried seed.
3.11. Polyphenolic Profile of Potent Extracts of Different Parts of the Fruit
To identify major phenolic compounds, the HPLC analysis was carried out in the methanol extract of the LPe, SDR, and LPu and the ethanol extract of the SDS. The LPe extract showed the presence of epicatechin (575.26 ± 2.93 mg/100 g), gallic acid (149.67 ± 2.26 mg/100 g), chlorogenic acid (88.83 ± 2.19 mg/100 g), and syringic acid (10.85 ± 0.84 mg/100 g). The LPu extract showed the presence of epicatechin (113.01 ± 0.68 mg/100 g), catechin (25.25 ± 0.6 mg/100 g), coumaric acid (6.15 ± 0.09 mg/100 g), cinnamic acid (6.10 ± 0.05 mg/100 g), chlorogenic acid (5.77 ± 0.23 mg/100 g), and syringic acid (0.38 ± 0.03 mg/100 g) with epicatechin levels being in a higher amount compared to others. In the case of the SDR extract, catechin (819.49 ± 0.81 mg/100 g), epicatechin (63.35 ± 0.25 mg/100 g), gallic acid (24.09 ± 0.17 mg/100 g), and chlorogenic acid (20.43 ± 0.33 mg/100 g) were identified, and catechin was found to be the most abundant phenolic compound. On the other hand, in the SDS extract, chlorogenic acid (149.78 ± 0.27 mg/100 g) had the highest phenolic acid content, followed by sinapic acid (75.98 ± 0.38 mg/100 g), epicatechin (26.031 ± 0.08 mg/100 g), and syringic acid (7.10 ± 0.08 mg/100 g) (Table 11).
Table 11. Polyphenolic Profile of Different Extracts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| mg/100 g |
|||||
| Polyphenols | LPe–Methanol | LPu–Methanol | SDR–Methanol | SDS–Ethanol | Significance |
| Epicatechin | 575.26 ± 2.93d | 113.01 ± 0.68c | 63.35 ± 0.25b | 26.031 ± 0.08a | P < 0.001 |
| Catechin | ND | 25.25 ± 0.6b | 819.49 ± 0.81c | ND | P < 0.001 |
| Gallic acid | 149.67 ± 2.26c | ND | 24.09 ± 0.17b | ND | P < 0.001 |
| Chlorogenic acid | 88.83 ± 2.19c | 5.77 ± 0.23a | 20.43 ± 0.33b | 149.78 ± 0.27d | P < 0.001 |
| Syringic acid | 10.85 ± 0.84c | 0.38 ± 0.03a | ND | 7.10 ± 0.08b | P < 0.001 |
| Coumaric acid | ND | 6.15 ± 0.09b | ND | ND | P < 0.001 |
| Cinnamic acid | ND | 6.10 ± 0.05b | ND | ND | P < 0.001 |
| Sinapic acid | ND | ND | ND | 75.98 ± 0.38b | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. LPe–methanol–methanol extract of the lyophilized peel; LPu–methanol–methanol extract of the lyophilized pulp; ND—not detected; SDR–ethanol–ethanol extract of the sun-dried rind; and SDS–methanol–methanol extract of the sun-dried seed.
3.12. FTIR Analysis of Potent Extracts of Different Parts of the Fruit
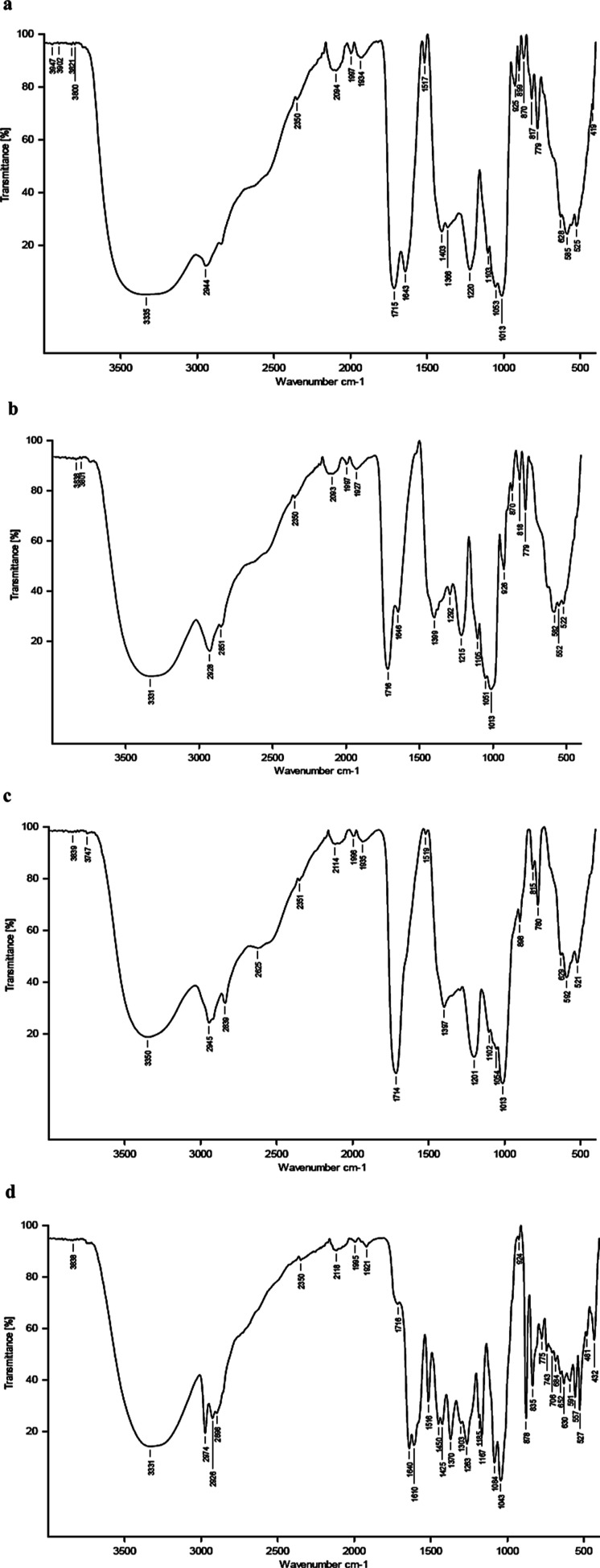
To confirm the presence of functional groups, the extracts were subjected to the FTIR analysis. The FTIR spectra of all four extracts showed a broad stretch band in the 3300 cm–1 range with LPe methanol extract showing the broader band than other extracts (LPe, 3335 cm–1; LPu and SDR, 3331 cm–1; and SDS, 3350 cm–1) indicating the presence of the OH group (Figure 2). This further confirms that fruit extracts are rich in polyphenols. Furthermore, bands at 1715 cm–1 (C=O stretching carboxylic acid dimer), 1013 cm–1 (strong C–O stretching primary alcohol), and 817 cm–1 (strong C–H bending 1,2,4-trisubstituted) were seen in LPe methanol, LPu methanol, and SDR ethanol extracts, respectively; however, the SDS methanol showed a band at 1995 cm–1 similar to other extracts and showed three bands between 1600 and 750 cm–1 different from those of other three extracts, that is, bands at 1516 cm–1 (strong N–O stretching nitro compound), 1263 cm–1 (strong C–O stretching aromatic ester), and 1084 cm–1 (strong C–O stretching primary alcohol).
Figure 2.
FTIR spectra of the (a) LPe-methanol, (b) LPu-methanol, (c) SDR-ethanol, and (d) SDS-methanol showing bands of different functional groups. LPe-methanol—methanol extract of lyophilized peel; LPu-methanol—methanol extract of lyophilized pulp; SDR-ethanol—ethanol extract of sundried rind; and SDS-methanol—methanol extract of sundried seed. Note that the band between 3600 and 3000 cm–1 suggests the presence of the −OH group in all the four potent extracts and is significantly broader in the spectra of LPe. Also, find the variation in the bands at 1500–500 cm–1 in the SDS compared to other extracts.
3.13. In Vitro Antioxidant Potential of Potent Phenolic Extracts of Different Parts of the Fruit
3.13.1. DPPH Scavenging Activity
The antioxidant capacity of all the extracts was determined by calculating the IC50 values, which denote the minimum concentration of the extract required to scavenge 50% of the DPPH free radicals. The lesser IC50 value signifies the stronger scavenging of DPPH free radicals. Among the different parts of the fruit extract, the methanol extract of LPe exhibited the significantly (P < 0.001) highest DPPH scavenging activity with the lowest IC50 value (1.58 ± 0.00 μg/mL). However, the IC50 values of SDR (methanol), SDS (ethanol) extracts, and LPu (methanol) were found to be 5.43 ± 0.02, 7.01 ± 0.03, and 7.27 ± 0.05 μg/mL, respectively (Table 12). Taken together, the DPPH scavenging activity of extracts can be represented in the following order: LPe > SDR > SDS > LPu.
Table 12. In Vitro Antioxidant Potential of Potent Polyphenolic Extracts of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
|||||
|---|---|---|---|---|---|
| IC50 Values of Antioxidant activity (μg/mL) |
|||||
| Assays | LPe–Methanol | LPu–Methanol | SDR–Ethanol | SDS–Methanol | Significance |
| DPPH | 1.58 ± 0.00a | 7.27 ± 0.05d | 5.43 ± 0.02b | 7.01 ± 0.03c | P < 0.001 |
| ABTS | 1.14 ± 0.00a | 4.14 ± 0.00c | 2.26 ± 0.00b | 5.71 ± 0.00d | P < 0.001 |
| FRAP | 0.84 ± 0.00a | 6.74 ± 0.02d | 1.67 ± 0.00b | 5.77 ± 0.02c | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.001) different as judged by Duncan’s multiple range test. ABTS—2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid; DPPH—2,2-diphenylpicrylhydrazyl; FRAP—ferric reducing/antioxidant power; LPe–methanol–methanol extract of the lyophilized peel; LPu–methanol–methanol extract of the lyophilized pulp; SDR–ethanol–ethanol extract of the sun-dried rind; and SDS–methanol–methanol extract of the sun-dried seed.
3.13.2. ABTS Assay
To verify whether the LPe–methanol extract could possess better free radical scavenging activity, ABTS assay was performed. There was a significant (P < 0.001) difference in the percentage inhibition of the different parts of fruit extracts (1–6% inhibition) (Table 12). The results of the present study showed that the LPe had substantial free radical scavenging property in the ABTS model followed by the SDR, LPu, and SDS. The results also ensured that the ABTS radical scavenging activity was directly correlated with the total phenolic content of the different parts of the fruit. The IC50 values of ABTS assay are as follows: 1.14 ± 0.00, 2.26 ± 0.00, 4.14 ± 0.00, and 5.71 ± 0.00 μg/mL for LPe, SDR, LPu, and SDS extracts, respectively. This suggested that the LPe extract possessed greater antioxidant potential compared to other extracts of the fruit used in the study.
3.13.3. Ferric Reducing Antioxidant Power
In order to confirm the antioxidant potential of the LPe extract, FRAP assay was carried out. FRAP assay data also showed that the LPe extract exhibited the significantly (P < 0.001) highest reducing power followed by the SDR, SDS, and LPu as shown in Table 12. The IC50 value for the reducing power of LPe (0.84 ± 0.00 μg/mL) was the lowest among all other parts of the fruit extract. The IC50 value of LPe was followed by that of the SDR (1.67 ± 0.00 μg/mL), SDS (5.77 ± 0.02 μg/mL), and LPu (6.74 ± 0.02 μg/mL) corroborating with other antioxidant assays studied. The above-mentioned results confirmed that the LPe extract possessed higher antioxidant activities than the other extracts from the fruit.
3.14. Antidiabetic Potential of Potent Phenolic Extracts of Different Parts of the Fruit
3.14.1. α-Amylase Inhibitory Activity
To examine whether extracts of the fruit possess antidiabetic potential, the α-amylase inhibitory assay was performed. The results revealed the ability of different parts of the fruit extracts to inhibit α-amylase in a dose-dependent manner. Furthermore, data showed that the IC50 value for the LPe (32.39 ± 0.16 μg/mL) extract was significantly (P < 0.001) lower compared to that of other extracts (Table 13). This suggested that the LPe extract possessed significant α-amylase inhibitory activity compared to other extracts and to the standard. Acarbose showed 50% α-amylase inhibitory activity with an IC50 value 84.83 ± 0.88 μg/mL, whereas the LPe, SDR, LPu, and SDS showed 50% inhibition at 32.39 ± 0.16, 111.13 ± 0.59, 149.25 ± 0.73, and 231.35 ± 0.54 μg/mL, respectively, indicating the decreasing order of their inhibitory potential.
Table 13. In Vitro Antidiabetic Potential of Potent Polyphenolic Extracts of Different Parts of the G. xanthochymus Fruita.
| Fruit
Parts |
||||||
|---|---|---|---|---|---|---|
| IC50 Values of Antidiabetic Enzymes (μg/mL) |
||||||
| Assays | LPe–Methanol | LPu–Methanol | SDR–Ethanol | SDS–Methanol | Standards | Significance |
| α-Amylase | 32.39 ± 0.16a | 149.25 ± 0.73d | 111.13 ± 0.59c | 231.35 ± 0.54e | 84.83 ± 0.88b | P < 0.001 |
| α-Glucosidase | 21.48 ± 0.02a | 152.81 ± 0.07e | 144.85 ± 0.27c | 65.50 ± 0.26b | 146.77 ± 1.03d | P < 0.001 |
| Lipase | 4.5 ± 0.02b | 19.18 ± 0.02e | 15.58 ± 0.13c | 18.57 ± 0.08d | 0.11 ± 0.00a | P < 0.001 |
Note: All the values are mean ± SEM. Mean values with the same superscript letters in the given row are not significantly different whereas those with different superscript letters are significantly (P < 0.05) different as judged by Duncan’s multiple range test. LPe–methanol–methanol extract of the lyophilized peel; LPu–methanol–methanol extract of the lyophilized pulp; SDR–ethanol–ethanol extract of the sun-dried rind; and SDS–methanol–methanol extract of the sun-dried seed.
3.14.2. α-Glucosidase Inhibitory Activity
The ability of all the extracts to inhibit α-glucosidase was also determined, and the data are presented in Table 13. All the extracts inhibited α-glucosidase significantly (P. All the extracts inhibited α-glucosidase significantly (P < 0.001). However, the LPe extract (21.48 ± 0.02 μg/mL) had higher inhibitory activity than the other phenolic extracts (SDS–65.50 ± 0.26, SDR–144.85 ± 0.27, and LPu–152.81 ± 0.07 μg/mL), including the standard acarbose (146.77 ± 1.03) (Table 13).
3.14.3. Lipase Inhibitory Activity
The results of lipase inhibitory activity revealed that all the four extracts showed significant inhibition of lipase activity (P < 0.001) but not as potent as that of the standard orlistat that showed an IC50 value of 0.11 ± 0.00 μg/mL. However, among the extracts studied, the LPe methanol extract inhibited lipase activity more effectively with an IC50 value of 4.50 ± 0.02 μg/mL, followed by SDR (15.58 ± 0.13 μg/mL), SDS (18.57 ± 0.08 μg/mL), and LPu (19.18 ± 0.02 μg/mL) extracts (Table 13).
3.14.4. Glucose Uptake by Yeast Cells
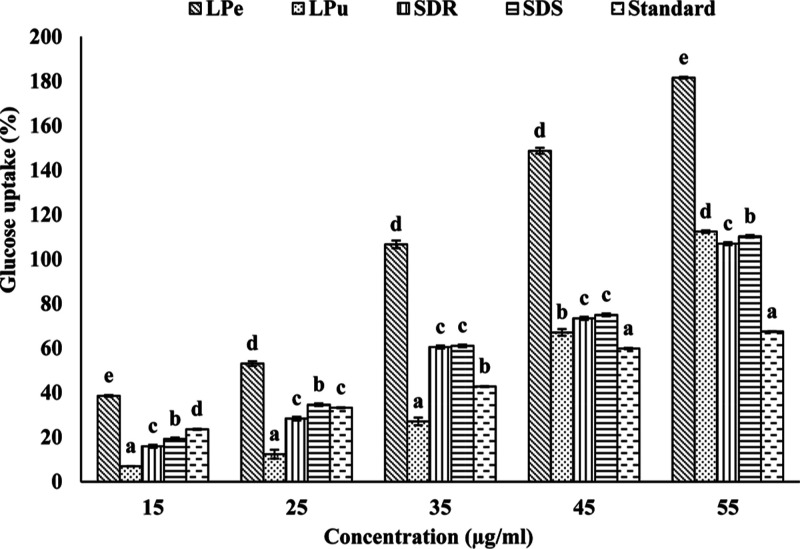
The ability of extracts to promote glucose uptake by cells was examined using yeast as a model system. The results showed that the LPe methanol extract induced significantly (P < 0.05) higher glucose uptake by the yeast cells in all the concentrations studied. The LPe methanol extract was followed by the SDS methanol, SDR ethanol, and LPu methanol extracts (Figure 3). Furthermore, all the extracts showed increased glucose uptake with a concomitant increase in their concentrations. In addition, all the extracts showed higher efficiency in inducing the glucose uptake compared to the standard metronidazole.
Figure 3.
Vertical bars showing the efficacy of potent polyphenolic extracts of different parts of the fruit in glucose uptake by yeast cells. LPe—lyophilized peel; LPu—lyophilized pulp; SDR—sundried rind; and SDS—sundried seed. Note: All the values are mean ± SEM. Bars with different letters are significantly (P < 0.05) different as judged by Duncan’s multiple range test.
4. Discussion
Various studies have shown the health beneficial effects of the fruit of G. xanthochymus.32 However, physical, chemical, and functional properties of different parts of the fruit were not well characterized. In this study, we provided the first report on the physicochemical and phytochemical characterization of different parts of the fruit. Furthermore, we also showed the antioxidative and antidiabetic effects of fruit extracts. The proximate analysis revealed that lyophilized and sun-dried parts of the fruit are rich in essential amino acids (histidine, tyrosine, threonine, valine, phenylalanine, and arginine), saturated (capric, lauric, palmitic, and stearic acids) and unsaturated fatty acids (oleic, linoleic, and α-linolenic acids), and macro and micro minerals (potassium, calcium, magnesium, copper, iron, manganese, and zinc). Compared to an earlier study by Patil and Anu-Appaiah12 on the fatty acid and amino acid composition of the rind and seed part of the fruit from the Kodagu district of Karnataka, the fruit of our study which was procured from the Mangalore district of Karnataka has shown a significantly higher amount of the stearic acid and threonine content in rind and a higher amount of histidine, lysine, and palmitic, linoleic, and α-linolenic acids in the seed part of the fruit. In addition, in our study, we have also found a good amount of the arginine content in different parts of the fruit. This may be due to a change in the geographical region or due to processing. Furthermore, different parts of the fruit were also found to be rich in phytochemicals, including polyphenols (flavonoids and tannins), carotenoids, and organic acids. The total ascorbic acid content in the SDS was found to be the highest, but it was not detected in HPLC. This could be due to the presence of different forms of ascorbic acid33 or ascorbic acid impurities in the sample.34 Different forms of ascorbic acid or ascorbic acid impurities would show different retention times. For example, dehydroascorbic acid, an oxidized form of ascorbic acid, can be detected by Miller’s method35 along with the ascorbic acid detection. The same compounds in HPLC show different retention times.36 In our study, we used only the l-ascorbic acid standard. The SDS sample would contain other forms of ascorbic acid or the ascorbic acid with impurities which need to be further studied. Several other studies have also revealed that different species of the genus Garcinia are, in general, rich in both nutritional and functional components.37 In line with other studies, our study also revealed that the G. xanthochymus fruit is a “functional food” enriched with both nutritive and health beneficial properties. Perhaps the health beneficial effects of this fruit may be attributed to these functional components. The quality and quantity of the polyphenolic content of the plant resources are dependent on the type of solvents used for extraction. According to the literature, methanol is the most effective solvent in the extraction of polyphenols from different parts of the plant.38 Similarly, in our study, methanol extracts of three different parts of the fruit, that is, the LPe, SDR, and LPu, showed a maximum extraction of total polyphenols.
The health beneficial effects of various medicinal plants or fruits are attributed to the presence of phytochemicals contained in them. Various metabolic disorders such as diabetes, obesity, cancer, and cardiovascular diseases stem from the presence of low-grade chronic inflammation and oxidative stress.39 This is the reason most studies involving phytochemicals are aimed at investigating the anti-inflammatory and antioxidative properties so that these phytochemicals can be developed into nutraceuticals for use in the prevention of metabolic disorders. Similarly, in our study, we examined the antioxidant property of different phenolic extracts by in vitro antioxidant assays. The methanol extract of LPe showed the highest DPPH scavenging activity with an IC50 value (1.58 ± 0.00) less than that of standard polyphenols. It was also found that the methanol extract of LPe showed higher antioxidant activity than the ethyl acetate fraction of leaves (IC50; 6.10 ± 0.01 μg/mL), the petroleum ether fraction of the dried fruit (IC50; 12.78 ± 0.30 μg/mL), and the n-butanol fraction of the root (IC50; 11.54 ± 0.42 μg/mL) of G. xanthochymus.40 Furthermore, the antioxidant property was confirmed by ABTS and FRAP assays, which also showed that the methanol extract of LPe showed higher antioxidant potency by both FRAP and ABTS assays. Results also indicated the direct correlation between the methanol extract of LPe with the highest total phenolic content and the potent antioxidant activity. This may be because of the presence of −OH groups in phenolic rings, which may allow them to act as a reducing agent or hydrogen donor and hence quench the free radicals.41 Several other studies have also shown that flavonoids and tannins present in fruits, vegetables, tea, and wine also contribute to the antioxidant potency.42 Therefore, the potent antioxidant effect observed in our study can be attributed to the presence of phenolic acids, flavonoids, and tannins in G. xanthochymus.
In addition to the antioxidant potency, polyphenols are also known to have the antidiabetic property.43 Hyperglycemia is the hallmark of diabetes. Various pathologies associated with diabetes are due to the durable effects of hyperglycemia.44 Therefore, similar to attenuating the oxidative stress, suppressing hyperglycemia can help in preventing the onset of diabetes and its associated complications. There are several approaches to control hyperglycemia. One of the first steps includes inhibition of hydrolyzing enzymes such as α-amylase and α-glucosidase so that there is a delay in the hydrolysis of polysaccharides in the food and subsequent production of monomers. Another approach is to promote glucose transport from the blood into cells. The blood glucose levels also increase from the non-carbohydrate substrates through the process of gluconeogenesis. Lipase breaks down the lipids into free fatty acids, which, in turn, constitute the substrate for the synthesis of glucose and further increase the blood glucose level. Hence, inhibition of lipase activity would maintain blood glucose levels under diabetic conditions. Our study showed that all the fruit extracts could inhibit the activities of both enzymes; α-amylase and α-glucosidase, and furthermore, the LPe methanol extract was more potent compared to all other extracts including standards of α-amylase and α-glucosidase, that is, acarbose and trolox, respectively. Additionally, our results also showed that all the extracts promoted the glucose uptake by yeast cells; however, the LPe extract was more efficient in improving the glucose uptake by the yeast cells. Furthermore, the LPe extract inhibited lipase activity more effectively compared to the other extracts.
It has been reported that zinc is associated with insulin production, storage, and secretion in islet cells,45 and it also improves the ability of insulin activity in vitro.46 Thus, the antidiabetic property of the G. xanthochymus fruit may also stem from the presence of 15% of the recommended dietary allowance of zinc. Several studies have shown that polyunsaturated fatty acids (PUFAs) including LA and ALA help in reducing T2DM and also improve insulin sensitivity in part through the synthesis of eicosanoids and prostaglandins which, in turn, help act against generation of low-grade chronic inflammation.47 Therefore, the presence of LA and ALA may contribute to the anti-inflammatory property of the fruit. Taken together, results indicate that the fruit extract of G. xanthochymus possesses the anti-hyperglycemic effects by inhibiting α-amylase and α-glucosidase enzyme activity, by promoting glucose transport into cells, and by inhibiting the gluconeogenesis. Additionally, its functional benefits against diabetes may also be attributed to its zinc content and its anti-inflammatory effect because of PUFAs, antioxidative effects, presence of carotenoids, and other essential amino acids.
5. Conclusions
The present study is the first report on the phytochemical characterization of different parts of the fruit of G. xanthochymus. This study also reports on therapeutic information of different parts of the non-hydroxycitric acid G. xanthochymus fruit. Fruit parts of G. xanthochymus are a rich source of dietary fiber, minerals, fatty acids, amino acids, carotenoids, organic acids, phenolic acids, and flavonoids. Extracts of the fruit parts have shown considerable therapeutic potential (antioxidative and antidiabetic) in vitro. Thus, the phytochemicals present in these fruit parts can be isolated and used for the development of functional foods for oxidative stress and diabetes. However, more detailed scientific (pre-clinical and clinical) evidence is required to establish its potency.
Acknowledgments
The authors thank the Head, Department of Biochemistry; Director, CSIR-CFTRI, for providing the infrastructure and facilities to carry out this research. The first author Janhavi P. acknowledges the Department of Science and Technology (DST) for the award of the fellowship.
Author Contributions
⊥ J.P. and S.S. both the authors contributed equally to the article.
This work was financially supported by the DST under the DST INSPIRE Fellowship scheme (no. DST/INSPIRE Fellowship/2014/IF150409).
The authors declare no competing financial interest.
References
- Bazzano L. A.; Serdula M.; Liu S. Prevention of type 2 diabetes by diet and lifestyle modification. J. Am. Coll. Nutr. 2005, 24, 310–319. 10.1080/07315724.2005.10719479. [DOI] [PubMed] [Google Scholar]
- Bibi Sadeer N.; Llorent-Martínez E. J.; Bene K.; Fawzi Mahomoodally M.; Mollica A.; Ibrahime Sinan K.; Stefanucci A.; Ruiz-Riaguas A.; Fernández-de Córdova M. L.; Zengin G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J. Pharm. Biomed. Anal. 2019, 174, 19–33. 10.1016/j.jpba.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Zengin G.; Rodrigues M. J.; Abdallah H. H.; Custodio L.; Stefanucci A.; Aumeeruddy M. Z.; Mollica A.; Rengasamy K. R. R.; Mahomoodally M. F. Combination of phenolic profiles, pharmacological properties and in silico studies to provide new insights on Silene salsuginea from Turkey. Comput. Biol. Chem. 2018, 77, 178–186. 10.1016/j.compbiolchem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Zengin G.; Aumeeruddy-Elalfi Z.; Mollica A.; Yilmaz M. A.; Mahomoodally M. F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species—A source of innovative phytopharmaceuticals from nature. Phytomedicine 2018, 38, 35–44. 10.1016/j.phymed.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Naveen G. P. A. N.; Krishnakumar G. Traditional and medicinal uses of Garcinia gummi-gutta fruit-a review. Species 2013, 4, 4–5. [Google Scholar]
- Gogoi B.; Das R. P.; Barua U. Antioxidant activity of Garcinia species of Assam. Int. J. Agric. Sci. 2016, 8, 1611. [Google Scholar]
- Muharni M.; Elfita E.; Amanda A. Biflavonoid compound from the stem bark of Gamboge (Garcinia xanthochymus). Indones. J. Chem. 2011, 11, 169–173. 10.22146/ijc.21405. [DOI] [Google Scholar]
- Rai A. K.; Anu Appaiah K. A. Application of native yeast from Garcinia (Garcinia xanthochumus) for the preparation of fermented beverage: Changes in biochemical and antioxidant properties. Food Biosci. 2014, 5, 101–107. 10.1016/j.fbio.2013.11.008. [DOI] [Google Scholar]
- Ajila C. M.; Bhat S. G.; Prasada Rao U. J. S. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. 10.1016/j.foodchem.2006.06.036. [DOI] [Google Scholar]
- Ajila C.; Naidu K.; Bhat S.; Rao U. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007b, 105, 982–988. 10.1016/j.foodchem.2007.04.052. [DOI] [Google Scholar]
- Ambasta S. P.The Useful Plants of India; Publications and Information Directorate, Council of Scientific and Industrial Research: New Delhi, 1986; p 231. [Google Scholar]
- Patil M. M.; Anu-Appaiah K. A.. Anti-obesity activity of Garcinia xanthochymus: biochemical characterization and in vivo studies in high fat diet-rat model. 18th International Conference on Obesity 2016.
- Association of Official Agricultural Chemists . Official Methods of Analysis J. Assoc. Off. Anal. Chem., 15th ed.; Arlington, VA, USA, 1990. [Google Scholar]
- Association of Official Agricultural Chemists . Official Methods of Analysis J. Assoc. Off. Anal. Chem., 17th ed.; Gaithersburg, MD, USA, 2000. [Google Scholar]
- Kamani M. H.; Martin A.; Meera M. S. Valorization of By-products derived from milled moth bean: Evaluation of chemical composition, nutritional profile and functional characteristics. Waste Biomass Valorization 2020, 11, 4895–4906. 10.1007/s12649-019-00819-2. [DOI] [Google Scholar]
- Pearson D.The Chemical Analysis of Foods, 7th ed.; Churchill Livingstone: London, 1976. [Google Scholar]
- Association of Official Agricultural Chemists . Official Methods of Analysis. J. Assoc. Off. Anal. Chem., 14th ed.; Washington, DC, USA, 1985. [Google Scholar]
- Miller D. D.Food Chem: A Laboratory Manual; Wiley: New York, 1998. [Google Scholar]
- Kelebek H.; Selli S.; Canbas A.; Cabaroglu T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. 10.1016/j.microc.2008.10.008. [DOI] [Google Scholar]
- Lakshminarayana R.; Raju M.; Krishnakantha T. P.; Baskaran V. Determination of major carotenoids in a few Indian leafy vegetables by high-performance liquid chromatography. J. Agric. Food Chem. 2005, 53, 2838–2842. 10.1021/jf0481711. [DOI] [PubMed] [Google Scholar]
- Raju M.; Varakumar S.; Lakshminarayana R.; Krishnakantha T.; Baskaran V. Carotenoid composition and vitamin A activity of medicinally important green leafy vegetables. Food Chem. 2007, 101, 1598–1605. 10.1016/j.foodchem.2006.04.015. [DOI] [Google Scholar]
- McDonald S.; Prenzler P. D.; Antolovich M.; Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. 10.1016/s0308-8146(00)00288-0. [DOI] [Google Scholar]
- Chang C. C.; Yang M. H.; Wen H. M.; Chern J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. 10.38212/2224-6614.2748. [DOI] [Google Scholar]
- Herald T. J.; Gadgil P.; Perumal R.; Bean S. R.; Wilson J. D. High-throughput micro-plate HCl–vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. 10.1002/jsfa.6538. [DOI] [PubMed] [Google Scholar]
- Singh R. S. G.; Negi P. S.; Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringaoleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. 10.1016/j.jff.2013.09.009. [DOI] [Google Scholar]
- Cheel J.; Theoduloz C.; Rodríguez J.; Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogoncitratus (DC.) Stapf.). J. Agric. Food Chem. 2005, 53, 2511–2517. 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Jeong C.-H.; Choi G. N.; Kim J. H.; Kwak J. H.; Kim D. O.; Kim Y. J.; Heo H. J. Antioxidant activities from the aerial parts of Platycodon grandiflorum. Food Chem. 2010, 118, 278–282. 10.1016/j.foodchem.2009.04.134. [DOI] [Google Scholar]
- Hemalatha P.; Bomzan D. P.; Sathyendra Rao B. V.; Sreerama Y. N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016, 199, 330–338. 10.1016/j.foodchem.2015.12.025. [DOI] [PubMed] [Google Scholar]
- McDougall G. J.; Kulkarni N. N.; Stewart D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. 10.1016/j.foodchem.2008.11.093. [DOI] [Google Scholar]
- Harish M.; Ahmed F.; Urooj A. In vitrohypoglycemic effects of Buteamonosperma Lam. leaves and bark. J. Food Sci. Technol. 2014, 51, 308–314. 10.1007/s13197-011-0496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Fan H.; Yang G.-z.; Jiang Y.; Zhong F.-f.; He H.-w. Prenylatedxanthones from the bark of Garcinia xanthochymus and their 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activities. Molecules 2010, 15, 7438–7449. 10.3390/molecules15107438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebouche C. J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Lv X.; Chen L.; Zhang H.; Zhu L.; Lu Y.; Chen X. Identification of Process-Related Impurities and Corresponding Control Strategy in Biocatalytic Production of 2-O-α-d-Glucopyranosyl-l-ascorbic Acid Using Sucrose Phosphorylase. J. Agric. Food Chem. 2022, 70, 5066. 10.1021/acs.jafc.2c00881. [DOI] [PubMed] [Google Scholar]
- Huelin F. Investigations on the stability and determination of dehydroascorbic acid. Aust. J. Biol. Sci. 1949, 2, 346–354. 10.1071/bi9490346. [DOI] [Google Scholar]
- Kim Y.; Ha N.; Kim M.-G. Simultaneous determination of L-ascorbic acid and dehydroascorbic acid in human plasma. Anal. Methods 2015, 7, 9206–9210. 10.1039/c5ay02056e. [DOI] [Google Scholar]
- Hemshekhar M.; Sunitha K.; Santhosh M. S.; Devaraja S.; Kemparaju K.; Vishwanath B. S.; Niranjana S. R.; Girish K. S. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. 10.1007/s11101-011-9207-3. [DOI] [Google Scholar]
- Uddin R.; Saha M. R.; Subhan N.; Hossain H.; Jahan I. A.; Akter R.; Alam A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014, 4, 273. 10.5681/apb.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer P. M.; Lecke S. B.; Satler F.; Morsch D. M. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015, 149, R219–R227. 10.1530/rep-14-0435. [DOI] [PubMed] [Google Scholar]
- Fu M.; Hui-Jin F.; Yu C.; De-Bin W.; Guang-Zhong Y. Antioxidant activity of Garcinia xanthochymus leaf, root and fruit extracts in vitro. Chin. J. Nat. Med. 2012, 10, 129–134. 10.3724/sp.j.1009.2012.00129. [DOI] [Google Scholar]
- Fraga C. G.; Galleano M.; Verstraeten S. V.; Oteiza P. I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. 10.1016/j.mam.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Panche A. N.; Diwan A. D.; Chandra S. R. Flavonoids: an overview. J. Nutr. Sci. 2016, 5, e47 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadoran Z.; Mirmiran P.; Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J. Diabetes Metab. Disord. 2013, 12, 43. 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat U.; Abad K.; Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm. J. 2016, 24, 547–553. 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. 10.1007/s12020-013-0032-x. [DOI] [PubMed] [Google Scholar]
- Vardatsikos G.; Pandey N. R.; Srivastava A. K. Insulino-mimetic and anti-diabetic effects of zinc. J. Inorg. Biochem. 2013, 120, 8–17. 10.1016/j.jinorgbio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Jovanovski E.; Li D.; Thanh Ho H. V.; Djedovic V.; Ruiz Marques A. C.; Shishtar E.; Mejia S. B.; Sievenpiper J. L.; de Souza R. J.; Duvnjak L.; Vuksan V. The effect of alpha-linolenic acid on glycemic control in individuals with type 2 diabetes: A systematic review and meta-analysis of randomized controlled clinical trials. Medicine 2017, 96, e6531 10.1097/MD.0000000000006531. [DOI] [PMC free article] [PubMed] [Google Scholar]