Abstract
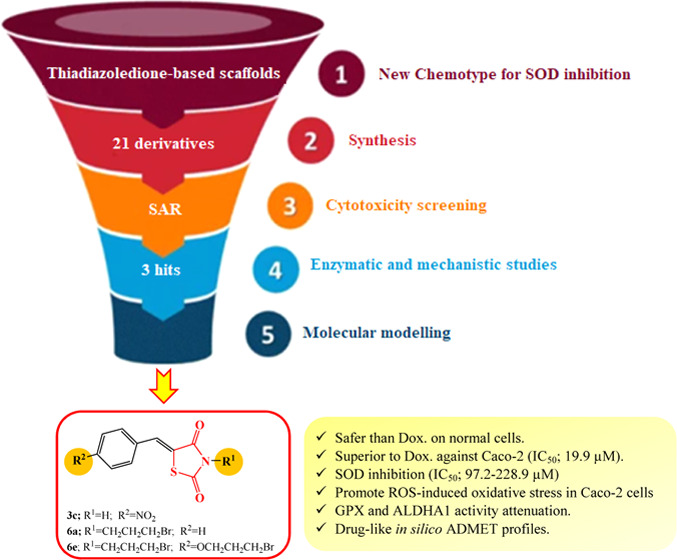
Based on the “canonical” view of reactive oxygen species’ (ROS) contribution to carcinogenesis, ROS induce oxidative stress and promote various tumor progression events. However, tumor cells also need to defend themselves against oxidative damage. This “heresy” was supported by several recent studies underlining the role of cellular antioxidant capacity in promoting metastasis and resistance to chemotherapy. Accordingly, harnessing the ROS-induced oxidative stress via selective suppression of the cancer antioxidant defense machinery has been launched as an innovative anticancer strategy. Within this approach, pharmacological inhibition of superoxide dismutases (SODs), the first-line defense antioxidant enzymes for cancer cells, selectively kills tumor cells and circumvents their acquired resistance. Various SOD inhibitors have been introduced, of which some were tolerated in clinical trials. However, the hit SOD inhibitors belong to diverse chemical classes and lack comprehensive structure–activity relationships (SAR). Herein, we probe the potential of newly synthesized benzylidene thiazolidinedione derivatives to inhibit SOD in colorectal cancer with special emphasis on their effects on correlated antioxidant enzymes aldehyde dehydrogenase 1 (ALDH1) and glutathione peroxidase (GPx). This may possibly bring a new dawn for utilizing thiazolidinediones (TZDs) in cancer therapy through SOD inhibition mechanisms. The preliminary 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed that all of the evaluated TZDs exhibited excellent safety profiles on normal human cells, recording an EC100 of up to 47.5-folds higher than that of doxorubicin. Compounds 3c, 6a, and 6e (IC50 = 4.4–4.7 μM) were superior to doxorubicin and other derivatives against Caco-2 colorectal cancer cells within their safe doses. The hit anticancer agents inhibited SOD (IC50 = 97.2–228.8 μM). Then, they were selected for further in-depth evaluation on the cellular level. The anticancer IC50 doses of 3c, 6a, and 6e diminished the antioxidant activities of SOD (by 29.7, 70.1, and 33.3%, respectively), ALDH1A (by 85.92, 95.84, and 86.48%, respectively), and GPX (by 50.17, 87.03, and 53.28%, respectively) in the treated Caco-2 cells, elevating the Caco-2 cellular content of ROS by 21.42, 7.863, and 8.986-folds, respectively. Docking simulations were conducted to display their possible binding modes and essential structural features. Also, their physicochemical parameters and pharmacokinetic profiles formulating drug-likeness were computed.
1. Introduction
Dysregulation of the cell energetics is a well-known hallmark of carcinogenesis.1 Tumor cells produce more reactive oxygen species (ROS),2 causing oxidative stress and promoting tumor progression.3 Hence, various anticancer agents were introduced to halt oxidative stress via scavenging ROS, defending against their destructive action or even inhibiting their production.4−8 While various antioxidants appear to be chemopreventive agents, the clinical outcomes of antioxidant-based cancer therapy are nonconclusive, if not disappointing. This could be attributed to the fact that ROS also mediate natural defense mechanisms against tumor propagation. Recently, harnessing oxidative stress via selective suppression of the cancer antioxidant defense mechanisms has been adopted as an innovative strategy for cancer therapy.9 In this regard, the mitochondrial antioxidant enzymes superoxide dismutases (SODs) have received growing attention as survival factors for cancer cells.10 SODs are ubiquitous components of the normal cellular antioxidant system. As firstly reported by McCord and Fridovich, SODs protect the cellular machinery from free radical-induced damage by catalyzing superoxide ions’ disproportionation to oxygen and hydrogen peroxide,11 which is then detoxified to water and oxygen via glutathione peroxidase (GPx).12 Several families of SODs have been identified. The catalytic activity of each family relies upon specific redox-active metal ions, which are manganese, iron, copper, zinc, or nickel ions. The accentuations of their normal functions are associated to several diseases and malignancies.13 The strongest connection between SODs and abnormalities is found for the copper and zinc-dependent forms.14
Despite the fact that SODs can supress tumor incidence, several lines of evidence have disclosed the unexpected oncogenic potential of SODs. It is clear that SOD is crucial for cancer cell resistance to cytotoxic agents and prevention of the oncogene-induced apoptosis triggered by p53, as evidenced by its elevated levels in different stages of various neoplasms, in a fashion that correlates with the malignancy degree.15−19 SODs enhance metastasis and the invasiveness of cancers via promoting epithelial–mesenchymal transition of pancreatic cancer cells prior to activation of the H2O2/ERK/NF-κB axis.20 Interestingly, halting angiogenesis by disulfiram, a potential Cu/Zn SOD inhibitor, also raises the possibility that attenuation of SOD activity may be adopted in the treatment of angiogenesis-dependent pathologies.21,22 Furthermore, SOD suppression was found to be a promising strategy to reduce the stemness and tumorigenicity of breast cancer cells expressing aldehyde dehydrogenase 1-positive (ALDH1+).23 This finding highlights a possible correlation between SOD and ALDH that catalyzes reactive aldehyde oxidation to maintain cellular homeostasis. However, overexpression of ALDH isozymes has been reported in various cancers and associated with relapses; thus, various inhibitors of ALDH enzymes have been developed as potential anticancer agents.24
Review of the literature revealed various anticancer SOD inhibitors of diverse chemical classes, such as dithiocarbamate derivatives (e.g., diethylthiocarbamate salts and disulfiram) and the bis-choline tetrathiomolybdate ATN-224, an orally bioavailable inhibitor of SOD (IC50 = 330 nM), via copper chelation, which attenuates angiogenesis and tumor proliferation. Interestingly, ATN-224 was well tolerated in phase I and II anticancer clinical trials.25,26 Further investment in tailoring efficient copper chelators can lead to the introduction of LD100, a potent SOD inhibitor.27
4,5-Dichloro-2-(3-tolyl)pyridazin-3(2H)-one (LCS-1) is another SOD inhibitor discovered from high-throughput screening of promising anticancer agents that preferentially inhibits lung adenocarcinoma cell growth.28,29 LCS-1 binds to and inhibits SOD in vitro. Mechanistic studies have revealed that the sensitivity of tumor cells to LCS-1 is closely related to SOD expression level.29 Motivated by such approach, the current study portrays the design, synthesis, and evaluation of new SOD inhibitors as potential anticancer agents for colorectal cancer given the association between SOD levels and colorectal cancer staging and grade of differentiation.15
2. Design Rationale
As illustrated (Figure 1), the reported SOD inhibitors belong to diverse chemical classes with limited structure–activity relationship (SAR) data. The introduction of new inhibitors with privileged scaffold will pave the way for further studies towards establishing comprehensive SAR against SOD. Herein, thiazolidinedione (TZD), the widely represented pharmacophore,30−32 was elected as a new core for introducing SOD inhibitors. The adopted design strategy relied on probing the potential of TZD to inhibit Cu/Zn SOD, inspired by its intrinsic property to coordinate the active site Zn of various metalloenzymes such as histone deacetylase 4,33 and carbonic anhydrases.34 In this regard, a series of 5-arylidene-2,4-thiazolidinediones were synthesized and derivatized to introduce various functionalities for enriching the SAR.
Figure 1.
Reported SOD inhibitors and target thiazolidinedione.
All of the synthesized derivatives (Scheme 1) were initially screened for their cytotoxic effect on normal fibroblasts to assess their safety profiles, followed by evaluating their anticancer activities against human colon cancer (Caco-2) cells via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously reported.35−37 The most promising derivatives were subjected to SOD inhibition assay. The study was extended to explore the inhibitory activities of the hit TZDs against the correlated antioxidant enzymes ALDH1 and GPX in Caco-2 cells; then, cellular ROS was quantified after treatment of Caco-2 cells with the studied derivatives. Docking simulations were conducted to predict their binding modes and pharmacophoric features contributing to interactions with the receptor sites. Finally, their physicochemical parameters and pharmacokinetic profiles were computed.
Scheme 1. Synthesis of N-Alkyl-5-arylidene-2,4-thiazolidinedione 4–7.

Reagents and conditions: (a) anhydrous sodium acetate, EtOH, reflux 3–15 h; (b) alkyl halide, anhydrous K2CO3, dimethylformamide (DMF), ultrasound irradiation for 20–60 min at room temperature.
3. Results and Discussion
3.1. Chemistry
Synthesis of 2,4-thiazoldinedione (TZD) was attempted by the reaction of chloroacetic acid and thiourea in the presence of HCl under reflux for 10–12 h. Structure modification of the TZD core has been carried out using substitution at both the NH and/or the methylene groups. 5-Arylidene-2,4-thiazolidinediones have been synthesized through Knoevenagel condensation of aromatic aldehydes with TZD using various catalysts.38 Thus, reaction of 2,4-thiazolidinedione 1 with aromatic aldehydes 2a–e in the presence of anhydrous sodium acetate under reflux for 3–15 h afforded the respective 5-arylidene derivatives 3a–e in 56–86% yield. Use of ultrasound irradiation in organic synthesis has become a more efficient, safe, convenient, and simple method. Ultrasound irradiation reactions are much more advantageous than traditional thermal methods in terms of reaction rates, yield, purity of the products, and selectivity.39−41 Thus, alkylation of the 5-arylidene-2,4-thiazolidinedione 3a–e with different alkyl halides was established under ultrasound irradiation in DMF in the presence of anhydrous potassium carbonate at room temperature in order to afford the respective N-alkyl-5-arylidene-2,4-thiazolidinedione analogues 4–7 with good yield within 20–60 min (Scheme 1).
Accordingly, treatment of 5-arylidene-2,4-thiazolidinediones 3a–e with allyl bromide in DMF in the presence of potassium carbonate under ultrasound irradiation for 25–30 min at room temperature afforded the respective N-allyl derivatives 4a–e in 52–64% yield. The structures of compounds 4a–d were elucidated from their spectral data. The 1H NMR spectra showed the disappearance of a characteristic signal corresponding to the N–H proton in the downfield region and instead, the allyl proton signals were assigned. The N-CH2 protons were assigned as a doublet at δH 4.37–4.40 ppm, whereas the multiplet peak resonated at δH 5.83–5.93 ppm was corresponding to the CH=CH2 proton. The terminal CH=CH2 protons were assigned as two doublets at δH 5.23–5.36 ppm with Jgem 12.0–16.0 Hz. The benzylidene proton was assigned as a singlet peak at δH 7.85–7.94 ppm. In addition, the characteristic carbon signals of the allyl moiety were assigned at δC 130.6–134.7, 118.5–119.5, and 43.7–44.2 ppm, corresponding to CH2=CH, CH2=CH, and N-CH2, respectively, whereas the benzylidene carbon was assigned at δC 134.0–149.0 ppm. The carbonyl groups of the thiazolidinedione ring were assigned at the downfield region at δC 166.3–168.1 ppm, while C-4 was resonated at δC 112.5–126.0 ppm. On the other hand, the reaction of allyl bromide with 5-(4-hydroxybenzylidene)-2,4-thiazolidinedione 3e under the same reaction conditions gave the O-,N-diallyl derivative 4e. The structure of 4e was confirmed from the absence of both the NH of the thiazolidine ring and the phenolic OH protons in the 1H NMR spectrum, and instead, two allyl groups were present. The two doublets corresponding to the NCH2 and OCH2 protons were assigned at δH 4.37 and 4.63 ppm, respectively, whereas their respective carbons were resonated at δC 43.78 and 68.96 ppm, respectively. Moreover, the CH2=CHCH2N and CH2=CHCH2O protons were assigned as three doublet of doublets at δH 5.25–5.48 ppm with J < 1 Hz, 4 Hz and Jgem = 12 Hz; these protons were correlated to the carbons assigned at δC 118.3 and 118.8, respectively. The CH2=CHCH2N and CH2=CHCH2O protons were resonated as multiplet signals at δH 5.83–5.93 and 6.03–6.12 ppm, respectively, correlated to the carbon signals assigned at δC 130.3 and 132.4 ppm, respectively.
Moreover, the reaction of the 5-arylidene derivatives 3a, 3c, and 3d with ethyl bromoacetate in DMF and in the presence of anhydrous potassium carbonate under the influence of ultrasound irradiation for 30–60 min at room temperature afforded the ethyl N-acetate derivatives 5a, 5c, and 5d, respectively, whereas the 4-hydroxybenzylidene derivatives 3e afforded the respective O-,N-derivative 5e as a result of the double alkylation on both the NH and OH groups; this agreed with Ionut et al.42 and Marc et al.,43 who reported the synthesis of the O-,N-dialkylated derivative under microwave irradiation and conventional heating. However, Nawale et al.44 reported that only the phenolic OH was alkylated with ethyl chloroacetate in acetone under reflux.
The characteristic signals indicating the alkylation with the ethylacetate group were assigned from the 1H and 13C NMR spectra of the synthesized compounds 5a and 5c-e. Thus, the NCH2 protons were resonated as a singlet at δH 4.43–4.50 ppm, which correlated with its carbon assigned at δC 37.3–42.1 ppm. The ethyl protons were assigned as a triplet (CH3CH2-) and a quartet (CH3CH2-) peak at δH 1.24–1.32 ppm and δH 4.20–4.27 ppm, respectively, whereas their carbons were assigned at δC 14.1 and 62.0–62.4 ppm, respectively. On the other hand, the NMR of compound 5e showed the presence of a quartet pattern at δH 1.30–1.35 ppm as a result of the overlap of the two triplets that were assumed for the methyl protons. Alternatively, the CH2CH3 protons were also overlapped and resonated as two quartet patterns at δH 4.24–4.34 ppm. Other protons and carbons are detailed and reported in the experimental section.
Alternatively, the reaction of 3a, 3c, and 3d with 1,3-dibromopropane in the presence of anhydrous potassium carbonate in DMF for 20–40 min afforded the N-(3-bromopropyl)-2,4-thiazolidinone derivatives 6a, 6c, and 6d, respectively, as verified from the spectral data of these compounds. Their 1H NMR spectra showed three characteristic signals for the propyl moiety that resonated as two triplets and a quintet at δH 3.92–3.96, 3.43–3.44, and 2.27–2.29 ppm corresponding to NCH2, CH2Br, and NCH2CH2CH2Br, respectively. The N-(3-bromopropyl) derivatives 6a, 6c, and 6d were further confirmed from the assignment of a singlet integrating proton at δH 7.86–7.95 ppm corresponding to the arylidene CH=C proton. Moreover, their 13C NMR spectra showed two carbonyl signals at δC 167.9–165.9 and one carbon signal corresponding to the benzylidene carbon CH=C resonated at δC 134.0–152.0 ppm.
On the other hand, the reaction of 5-(4-hydroxybenzylidene)-2,4-thiazoldinedione 3e with 1,3-dibromopropane afforded the O-,N-dialkylated derivative 6e, as confirmed from the NMR spectra. The 1H NMR spectra of 6e showed two quintets, each of them integrating two protons at δH 2.37 and 2.27 ppm corresponding to OCH2CH2CH2Br and NCH2CH2CH2Br, whereas the OCH2 and NCH2 protons were resonated as two triplets at δH 4.20 and 3.92 ppm, respectively. The CH2Br protons were resonated at a lower frequency as two triplets at δH 3.43 and 3.63 ppm. Alternatively, the 13C NMR spectrum of 6e showed the presence of six signals corresponding to the propyl carbon moieties, which were assigned at δC 29.5, 29.7 ppm (2 CH2CH2CH2Br), 30.8, 32.1 (2 CH2Br), 40.6 (NCH2), and 65.5 ppm (OCH2). Moreover, only two carbonyl signals corresponding to the thiazolidine-2,4-dione moiety were resonated at δC 166.4 and 168.1 ppm.
Alternatively, the reaction of 3a, 3c, and 3d with 3-chloropropanol under ultrasound irradiation for 25–45 min at room temperature in DMF and in the presence of anhydrous potassium carbonate afforded the respective 3-(3-hydroxypropyl)-5-(arylidene)-2,4-thiazolidinediones 7a, 7c, and 7d, respectively. The characteristic signals for the 3-hydroxypropyl moiety were elucidated from their 1H and 13C NMR spectra. Thus, the NCH2 protons were assigned as a triplet at δH 3.96 ppm and correlated to the carbon signal at δC 38.5 for compound 7a (measured in CDCl3). However, the NCH2 signals for 7c and 7d (measured in DMSO-d6) were masked under the solvent peak. The CH2OH protons for compound 7a were assigned as a triplet at δH 3.65, whereas those for compounds 7c and 7d were assigned as a quartet at δH 3.47 and 3.45 ppm, respectively, and were correlated with their carbon signals at δC 58.9 ppm. The quintet corresponding to NCH2CH2CH2OH was assigned at δH 1.92, 1.75, and 1.72 ppm for 7a, 7c, and 7d, respectively, whereas its carbon was resonated at δC 30.6–30.9 ppm. The hydroxyl proton was resonated as an exchangeable broad singlet at δH 2.15 for 7a, while for 7c and 7d it resonated as a triplet at 4.61 and 4.58 ppm, respectively.
3.2. Biological Evaluation
3.2.1. Cytotoxicity Screening on Normal Human Cells
Firstly, the newly synthesized thiazolidine-2,4-dione derivatives were screened via MTT assay for their cytotoxic activities against normal human cells (Wi-38) to assess their safety profiles. Thus, the safe concentration (EC100), at which 100% of normal cell viability is attained, was calculated using the percentage of cell viability at serial concentrations of the studied derivatives, and compared to the reference chemotherapeutic drug (Table 1). Based on the high EC100 indicating a high safety on cells, the results revealed notable promising safety profiles of the synthesized series, where all compounds were safer than the reference chemotherapy doxorubicin (DOX). Compound 4a came at the top of the series with the highest detected EC100 (58.92 μM) being approximately 47.5-folds safer than DOX, followed by 5a, 4e, and 4d (EC100 30.97–28.97 μM). 3d (EC100 25.35 μM) and 1 (EC100 22.05 μM) were of relatively moderate safety. Other derivatives exhibited lower EC100 values ranging from 19.68 to 5.62 μM.
Table 1. Cytotoxicity of the Thiazolidine-2,4-dione Derivatives 4–7 on Normal Human Cells (Wi-38), Expressed as EC100 (μM).
| compound | EC100 (μM)a |
|---|---|
| 1 | 22.047 ± 0.434 |
| 3a | 19.679 ± 3.105 |
| 3b | 5.621 ± 0.071 |
| 3c | 6.0653 ± 0.127 |
| 3d | 25.345 ± 0.655 |
| 3e | 6.727 ± 0.040 |
| 4a | 58.916 ± 10.698 |
| 4b | 9.902 ± 2.360 |
| 4c | 7.078 ± 0.136 |
| 4d | 28.971 ± 2.121 |
| 4e | 30.971 ± 0.971 |
| 5a | 30.456 ± 0.456 |
| 5c | 8.573 ± 0.409 |
| 5d | 16.179±6.066 |
| 5e | 8.417 ± 0.004 |
| 6a | 18.938 ± 0.975 |
| 6c | 13.424 ± 1.130 |
| 6d | 14.776 ± 1.125 |
| 6e | 10.683 ± 2.384 |
| 7a | 18.923 ± 1.959 |
| 7c | 11.439 ± 0.927 |
| 7d | 12.073 ± 3.577 |
| DOX | 1.239 ± 0.285 |
All values are expressed as mean ± standard error of mean (SEM).
3.2.2. Anticolorectal Cancer Activity
The studied compounds were subjected to anticancer evaluation via MTT assay against human colon cancer Caco-2 cells and compared to DOX as reference chemotherapy (Table 2). To accurately assess the growth inhibitory potential of these compounds, the half-maximal inhibitory concentration (IC50), at which Caco-2 growth rate is inhibited by 50% relative to the untreated cells, was estimated. The low value of this dose was used as an indicator of high anticancer potential. All derivatives were superior to the reference (IC50; 19.89 μM) against Caco-2, except 3a, within their safe doses (EC100). Compounds 6e (IC50; 4.41 μM), 3c (IC50; 4.71 μM), and 6a (IC50; 4.73 μM) exhibited the highest anticancer potentials among the evaluated derivatives. These compounds (3c, 6a, and 6e) caused severe shrinkage and collapse of Caco-2 cells, as shown in Figure 2. The remainder compounds were relatively moderate, recording IC50 ranging from 6.51 to 13.75 μM. Accordingly, they (3c, 6a, and 6e) were selected as our hit thiazolidine-2,4-dione derivatives for further mechanistic studies.
Table 2. Cytotoxicity of the Thiazolidine-2,4-Dione Derivatives 4–7 on Human Colon Cancer (Caco-2), Expressed as IC50 (μM).
| compound | IC50 (μM)a |
|---|---|
| 1 | 12.054 ± 3.253 |
| 3a | 74.627 ± 8.310 |
| 3b | 7.028 ± 0.315 |
| 3c | 4.718 ± 0.602 |
| 3d | 8.954 ± 0.631 |
| 3e | 8.478 ± 0.599 |
| 4a | 11.989 ± 1.358 |
| 4b | 13.755 ± 0.789 |
| 4c | 6.517 ± 0.077 |
| 4d | 7.062 ± 0.005 |
| 4e | 12.204 ± 3.124 |
| 5a | 8.201 ± 0.135 |
| 5c | 6.668 ± 0.118 |
| 5d | 8.241 ± 2.589 |
| 5e | 8.571 ± 0.942 |
| 6a | 4.739 ± 0.021 |
| 6c | 10.407 ± 0.499 |
| 6d | 7.940 ± 0.599 |
| 6e | 4.413 ± 0.546 |
| 7a | 10.152 ± 0.770 |
| 7c | 6.765 ± 0.096 |
| 7d | 7.118 ± 0.010 |
| DOX | 19.894 ± 2.370 |
All values are expressed as mean ± standard error of mean (SEM).
Figure 2.
Morphological changes of Caco-2 cells after 72 h treatment with 3c, 6a, and 6e compared to doxorubicin (DOX).
3.2.3. SOD Inhibition
SOD inhibition profiles of the selected anticancer hits 3c, 6a, and 6e were detected and compared to the reference sodium diethyldithiocarbamate (DDC) using the superoxide dismutase kit from R&D Systems according to the manufacturer’s instructions. In the assay, superoxide anions generated by xanthine oxidase (XOD) convert nitrobluetetrazolium (NBT) to NBT-diformazan, which absorbs light at λ = 550 nm. SOD reduces the superoxide anion concentration and thereby lowers the rate of NBT-diformazan formation. SOD activity was determined based on the difference between the control and test. The results (Table 3) showed that 5-(4-(3-bromopropoxy)benzylidene)-3-(3-bromopropyl)thiazolidine-2,4-dione 6e was the most potent among the groups, followed by the 5-benzylidene derivative 6a and 5-(4-nitrobenzylidene)thiazolidine-2,4-dione 3c, respectively.
Table 3. SOD Inhibitory Activities of the Selected Thiazolidine-2,4-diones 3c, 6a, and 6ea.
| compound no. | IC50 (μM) |
|---|---|
| 3c | 228.87 ± 6.75 |
| 6a | 154.42 ± 5.92 |
| 6e | 97.24 ± 3.84 |
| DDC | 23.04 ± 1.62 |
All values are expressed as mean ± SEM.
3.2.4. Inhibition of the Antioxidant Enzymes in Colorectal Cancer Cells (Caco-2)
The most promising TZDs 3c, 6a, and 6e were selected for further in-depth evaluation, wherein mechanistic studies were conducted to study their potential to inhibit the antioxidant activities on a cellular level compared to the chemotherapeutic agent DOX owing to its prooxidant activities. The results showed that the IC50 doses of these TZDs diminished the intracellular SOD by 70.14, 29.74, and 33.35%, respectively, compared to 30.78% for DOX (Figure 3A) in the treated Caco-2 cells. The compounds were also tested for their inhibitory activities against ALDH1A and GPX being directly correlated to the cellular ROS content. Interestingly, compounds 3c, 6a, and 6e inhibited Caco-2 ALDH1A activity by 85.92, 95.84, and 86.48%, respectively (Figure 3B). Meanwhile, DOX revealed the lowest ALDH1A inhibition potency of about 4.800%. Regarding the activity of glutathione peroxidase (GPx), these compounds and DOX suppressed it by 50.17, 87.03, 53.28, and 40.31% in Caco-2 cells after 72 h of incubation, respectively, as demonstrated in Figure 3C.
Figure 3.
Inhibitory potency of the most effective anticancer compounds (3c, 6a, and 6e) as well as DOX on the activities of antioxidant enzymes: (A) SOD, (B) ALDH1A, and (C) GPX after 72 h of incubation with Caco-2 cells.
3.2.5. Determination of Cellular ROS in Colorectal Cancer (Caco-2)
In light of the aforementioned results, the cellular ROS in Caco-2 was determined after treatment with the studied derivatives 3c, 6a, and 6e by utilizing their anticolorectal cancer IC50 (Table 2). The results (Figure 4) revealed that the Caco-2 cellular content of ROS increased by 7.863, 21.42, and 8.986-folds in the treated Caco-2 cells relative to the untreated cells after incubation with the investigated compounds. Also, Figure 4 shows that the reference chemotherapeutic drug (DOX) had a lower ROS generation potency than the studied derivatives because DOX increased the cellular ROS level by only 4.065-folds compared to the untreated Caco-2 cells.
Figure 4.
Fold increment in cellular ROS level in Caco-2 cells treated with 3c, 6a, and 6e as well as DOX relative to the untreated cells.
3.3. Molecular Modelling
3.3.1. Docking of the Active TZDs 3c, 6a, and 6e into SOD
Docking simulations were performed by MOE 2015.10,45 to predict the probable binding modes of the hit TZDs into the SOD active site and to possibly justify the rationalized design strategy. The crystal structure of SOD1 retrieved from the protein data bank (PDB ID: 6FOI)46 was prepared by eliminating unwanted residues, ligands, and water molecules, and then subjected to the default “Structure preparation” module settings, where hydrogen atoms were added, hydrogen bonds were optimized, and atomic clashes were removed. The studied TZDs were built in silico, energy minimized, and optimized using the default MOE settings, then located in the active site, specifically in the vicinity of the enzyme Zn. The docking protocol was conducted by employing the Triangular matcher algorithm as the ligand placement method and α HB as the scoring function, generating the top 10 nonredundant poses of the conformers with the lowest binding energies. Among the top-ranked poses according to docking scores and molecular interactions (Figure 5), the TZD localization into SOD1 can be stabilized mainly via both coordination to the SOD Zn as well as hydrogen bonds and other interactions with key SOD1 residues, particularly those normally coordinating the enzyme Zn. Interestingly, the TZD core C2 carbonyl oxygens of the hit TZDs 3c, 6a, and 6e were predicted to coordinate Zn, supporting the design rationale. The nitrobenzylidene TZD 3c (ΔG = −2.99 kcal/mol) displayed H−π interactions with His46 and His80, through the TZD core NH and the p-nitrophenyl ring. The relatively more active SOD inhibitor TZD derivative 6a (ΔG = −1.11 kcal/mol) posed additional hydrogen bonding and π–π interactions linking the TZD core and enzymes Thr137 and His80, respectively. The N-bromopropyl appendage displayed hydrogen bond with Gly72 and H−π bond with His46. Furthermore, hydrogen bond interaction between the benzylidene CH and the key residue His63 (normally coordinating Zn) was observed. Concerning the active SOD inhibitor 6e (ΔG = −4.47 kcal/mol), the TZD core sulfur was able to interact with the SODs Val47 and Pro62 via hydrogen bonding interactions.
Figure 5.
(A) 3D binding mode of 3c (green sticks), (B) two-dimensional (2D) binding mode of 3c, (C) 3D binding mode of 6a (cyan sticks), (D) 2D binding mode of 6a, (E) 3D binding mode of 6e (cyan sticks), and (F) 2D binding mode of 6e into SOD (PDB ID: 6FOI)46 Zn domain.
Taking all together, the illustrated binding modes of the investigated derivatives (Figure 5) were nearly correlated with their in vitro and cell-based SOD inhibitory profiles (Table 3), supporting the design rationale and highlighting the potential of TZDs as a privileged scaffold for introducing SOD inhibitors.
3.3.2. In Silico Physicochemical Properties, ADMET, and Drug-Likeness
Recently, the medicinal chemistry sector of drug discovery research adopts in silico ADMET prediction and computational drug-likeness studies as reliable tools for lead identification. Herein, various physicochemical properties formulating important ADME and drug-likeness parameters of the most promising 2,4-thiazolidinedione derivatives were computed by employing SwissADME(47) software (Table 4). Interestingly, the three studied compounds were in full accordance to Lipinski’s,48 Veber’s,49 Muegge’s,50 and Ghose’s51 bioavailability parameters. The compounds displayed predicted drug-like TPSA (<140–150 A2), high intestinal absorption, and acceptable aqueous solubility. Notably, 3c recorded excellent predicted aqueous solubility. They were also predicted to lack cytochrome P450 (CYP3A and CYP2D6) inhibition, except 6e regarding CYP3A. All compounds displayed high predicted intestinal absorption. Both 6a and 6e were predicted to cross the blood–brain barrier, while 3c was predicted to be devoid of any CNS side effects. PROTOX,52 the toxicity predictor program, predicted the average lethal dose (LD50) of the studied compounds in rodents and classified it according to the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) as class IV concerning acute oral toxicity. Interestingly, the compounds were also devoid of any predicted hepatotoxicity. In light of the abovementioned data, the synthesized 2,4-thiazolidinedione derivatives could be considered druggable.
Table 4. In Silico Prediction of Physicochemical Properties, ADMET, and Drug-Likeness of the Selected Derivatives.
| physiochemical
parameters |
ADMET |
bioavailability
& drug-likeness |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | Log Pa | M.Wtb | HBAc | HBDd | NROTBe | TPSAf | Sg | HIAh | BBBi | CYP3A4 inhibitor | CYP2D6 inhibitor | LD50j | hepatotoxicity | Lipiniskik | Veberl | Mueggem | Ghosen | PAINSo |
| 3c | 1.04 | 250.23 | 4 | 1 | 2 | 117.29 | 654 | high | no | no | no | 1000 | no | yes | yes | yes | yes | 0 |
| 6a | 2.08 | 326.21 | 2 | 0 | 4 | 62.68 | 9.76 | high | yes | no | no | 1000 | no | yes | yes | yes | yes | 0 |
| 6e | 2.99 | 463.18 | 3 | 0 | 8 | 71.91 | 0.28 | high | yes | yes | no | 1000 | no | yes | yes | yes | yes | 0 |
Log P: logarithm of the compound partition coefficient between n-octanol and water.
M.Wt: molecular weight.
HBA: number of hydrogen bond acceptors.
HBD: number of hydrogen bond donors.
NROTB: number of rotatable bonds.
TPSA: polar surface area. Drug-like TPSA < 140–150 A2.
S: aqueous solubility (mg/L).
HIA: human intestinal absorption.
BBB: blood–brain barrier penetration.
LD50: the median lethal dose (mg/kg). Toxicity classes according to GHS are Class I: fatal if swallowed (LD50 ≤ 5), Class II: fatal if swallowed (5 < LD50 ≤ 50), Class III: toxic if swallowed (50 < LD50 ≤ 300), Class IV: harmful if swallowed (300 < LD50 ≤ 2000), Class V: may be harmful if swallowed (2000 < LD50 ≤ 5000), and Class VI: nontoxic (LD50 > 5000).52
Lipinski rule: log P ≤ 5, M.Wt ≤ 500 Da, HBA ≤ 10, and HBD ≤ 5.48
Veber rule: NROTB ≤ 10 and TPSA ≤ 140.49
Muegge rule: −2 ≤ log P ≤ 5, 200 ≤ M.Wt ≤ 600 Da, TPSA ≤ 150, Num. rings ≤ 7, Num. carbons> 4, Num. heteroatom > 1, NROTB ≤ 15, HBA ≤ 10, and HBD ≤ 5.50
Ghose rule: 160 ≤ M.Wt ≤ 480 Da, −0.4 ≤ log P ≤ 5.8, 20 ≤ atoms ≤ 70, 40 ≤ MR ≤ 130.51
3.4. Structure–Activity Relationship
In light of the aforementioned assays, the structure–activity relationship of the synthesized derivatives can be deduced. The general cytotoxic activity pattern reflects promising anticancer potential, as displayed by the designed TZD scaffold. However, the anticancer profile of each derivative regarding safety and activity was a function of the terminal benzylidene and N3-substitution. The N3-ally-substituted benzylidene derivative 4a exhibited the highest safety profile. Allyloxy 4e or dimethylamino 4d substitution on the benzylidene ring slightly affected the scaffold’s safety. Similarly, N3-substitution with ethylacetate 5a showed a slightly lower safety than the N3-ally-substituted analogue 4a. Deletion of N3-substitution 3d also lowered the derivative’s safety. Other substitutions showed lower and comparable EC100 values. Obviously, the TZD derivatives bearing 4-nitrobenzylidene 3c, N3-3-bromopropyl 6a, or terminal 3-bromopropoxy together with substituting the TZD N3 with 3-bromopropyl 6e conferred the highest anticancer activity towards Caco-2 cells. On the other hand, the unsubstituted benzylidenethiazolidine-2,4-dione derivative 3a was relatively devoid of antiproliferative activity. However, diversifying the terminal benzylidene substitution restored considerable anticancer potency and controlled selectivity, as seen in the case of N3-substitution with 3-allyl 4c, 3-ethylacetate 5c, 3-hydroxypropyl 7c, or 3-bromopropyl 6c groups, whereas other derivatives and the parent unsubstituted thiazolidine-2,4-dione 1 showed moderate anticancer activities against the screened cell line with relatively slight differences in activities when compared.
Mechanistic studies revealed that the N3-3-bromopropyl TZD 6a exhibited the highest inhibitory potentials of SOD, ALDHA1, and GPX among the elected hits. The TZD derivatives bearing 4-nitrobenzylidene 3c and the 5-(4-(3-bromopropoxy)benzylidene)-3-(3-bromopropyl) thiazolidine-2,4-dione 6e were nearly equipotent. These results were correlated with the TZD derivatives’ potential to induce cellular ROS content following Caco-2 cells’ treatment with IC50 doses. However, in vitro evaluation of SOD showed that the (3-bromopropoxy)benzylidene derivative 6e was slightly more potent than the N3-3-bromopropyl TZD 6a precursor.
4. Conclusions
The current study portrays the utility of TZD-based SOD inhibitors endowed with ALDH and GPX inhibitory potentials to harness oxidative stress for combating colorectal cancer. Interestingly, all of the designed TZDs were safe on normal human cells (EC100 approaching 47.5-folds higher than doxorubicin). The hit derivatives 3c, 6a, and 6e (IC50 = 4.4–4.7 μM) were superior to doxorubicin against Caco-2 cells. They exhibited modest SOD inhibition (IC50 = 97.2–228.8 μM). On a cellular level, the IC50 doses of the hit derivatives 3c, 6a, and 6e promoted ROS-induced oxidative stress by 21.42, 7.863, and 8.986-folds via suppression of the antioxidant activities of SOD, ALDH1A, and GPX up to 70.1, 95.84, and 87.03%, respectively, in the treated Caco-2 cells. Docking simulations displayed their possible binding modes and essential structural features. Their computed physicochemical parameters and pharmacokinetic profiles highlighted acceptable drug-like properties. Accordingly, the oxidative stress-based anticancer therapeutics launch an opportunity to improve our ability to introduce novel strategies for combating cancer.
5. Experimental Section
5.1. Chemistry
5.1.1. General Methods and Instruments
Melting points were uncorrected and observed using the Mel-Temp apparatus. Reactions were monitored using thin-layer chromatography (TLC) (aluminum plates of silica gel; Kiesel gel G, Merk). Ultraviolet light (UV 254 nm) was used in visualization. Sonication was carried out using an Ultrasonic Cleaner model UD50SH-2LQ. IR spectra were determined using a Bruker Tensor 37 FTIR spectrophotometer. NMR spectroscopic analysis was carried out using JEOL ECA 500, NMR unit, Mansoura University, Egypt. Chemical shifts are reported in ppm and coupling constants (J) in Hz (Hertz) (abbreviations used in the spectra: s = singlet, d = doublet, t = triplet, q = quartet, m = multiple, dd = double of doublets). Microanalysis was established at the Faculty of Science, El Azhar University, Egypt.
5.2. General Procedure for the Preparation of 5-Arylidine-2,4-thiazolidinediones
A mixture of 1 (0.085 mol) and sodium acetate (0.69 g, 0.085 mol) in ethanol (30 mL) was refluxed with aromatic aldehydes (0.085 mol) for 3–15 h. The precipitate obtained was filtered off and washed with methanol.
5.2.1. 5-Benzylidenethiazolidine-2,4-dione (3a)
It was obtained as a pale yellow powder, in 64% yield; mp = 238 °C (Lit. mp53 = 240–241 °C).
5.2.2. 5-(3-Chlorobenzylidene)thiazolidine-2,4-dione (3b)
It was obtained as a white crystal, in 60% yield; mp = 272 °C (Lit. mp53 = 270–271 °C).
5.2.3. 5-(4-Nitrobenzylidene)thiazolidine-2,4-dione (3c)
It was obtained as a pale yellow powder, in 71% yield; mp = 220 °C (Lit. mp54 = 220–223 °C).
5.2.4. 5-(4-(Dimethylamino)benzylidene)thiazolidine-2,4-dione (3d)
It was obtained as an orange powder, in 86% yield; mp = 279 °C (Lit. mp55 = 281–282 °C).
5.2.5. 5-(4-Hydroxybenzylidene)thiazolidine-2,4-dione (3e)
It was obtained as a yellow crystal, in 56% yield; mp = 278 °C (Lit. mp53 = 280–281 °C).
5.3. General Procedure for the Synthesis of N-Alkyl 5-arylidene-2,4-thiazolidinediones
A mixture of 5-arylidine-2,4-thiazoldinedione 7–11 (1 mmol) and anhydrous potassium carbonate (0.49 g, 1.2 mmol) in DMF (15 mL) was stirred for 15 min, then the alkyl halide (1.2 mmol) was added. The reaction mixture was irradiated under ultrasound irradiation for 20 to 60 min till the end of the reaction, as monitored by TLC. The mixture was poured on to crushed ice and the precipitate obtained was filtered off, washed with water, dried, and recrystallized from ethanol.
5.3.1. 3-Allyl-5-(substituted benzylidene)thiazolidene-2,4-diones (4)
5.3.1.1. 3-Allyl-5-benzylidenethiazolidine-2,4-dione (4a)
It was obtained as a white powder, in 64% yield, mp = 98 °C (Lit. mp40 = 101–103 °C), Rf = 0.69 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1733 (C=O), 1685 (C=O), 1607 (C=C), 1115 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.94 (s, 1 H, Ph-CH=C), 7.55–7.44 (m, 5 H, Ar-H), 5.93–5.83 (m, 1 H, CH2CH=CH2), 5.35, 5.30 (2 d, 2 H, J = 4.0 Hz, J = 12.0 Hz, J = 16.0 Hz, HC=CH2), 4.39 (d, 2 H, J = 8.0 Hz, CH2-N); Figure S1. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.5, 165.9 (2 C=O), 134.0 (Ph-CH=C), 133.2 (Ar-C), 130.6 (CH=CH2), 130.2, 129.2 (Ar-C), 121.4 (CH=C), 119.0 (HC=CH2), 43.8 (N-CH2); Figure S2. Anal. calcd for C13H11NO2S: C, 63.65; H, 4.98; N, 5.32; S, 13.07. Found C, 63.62; H, 4.50; N, 5.35; S, 13.09.
5.3.1.2. 3-Allyl-5-(3-chlorobenzylidene)thiazolidine-2,4-dione (4b)
It was obtained as pale yellow needles, yield (60%), mp = 210 °C, Rf = 0.86 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1736 (C=O), 1675 (C=O), 1606 (C=C), 1110 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.85 (s, 1 H, Ar-CH=C), 7.50 (s, 1 H, Ar-H), 7.43 (m, 3 H, Ar-H), 5.92–5.83 (m, 1 H, CH=CH2), 5.35, 5.30 (2 d, 2 H, HC=CH2), 4.38 (d, 2 H, CH2-N); Figure S3. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.0, 165.6 (2 C=O), 135.3 (Ph-CH=C), 134.9 (Ar-C), 132.2 (CH=CH2), 130.5, 130.4, 130.0, 127.9 (Ar-C), 123.1 (CH=C), 119.2 (HC=CH2), 43.9 (N-CH2); Figure S4. Anal. calcd for C13H10ClNO2S: C, 55.82; H, 3.60; N, 5.01; S, 11.46. Found C, 55.81; H, 3.57; N, 5.11; S, 11.44.
5.3.1.3. 3-Allyl-5-(4-nitrobenzylidene)thiazolidine-2,4-dione (4c)
It was obtained as a yellow powder, 52% yield, mp = 159 °C, Rf = 0.79 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1738 (C=O), 1678 (NC=O), 1606 (C=C), 1515, 1345 (NO2), 1111 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 8.36 (d, 2 H, J = 8 Hz Ar-H), 7.95 (s, 1 H, Ar-CH=C), 7.70 (d, 2 H, Ar-H), 5.93–5.83 (m, 1 H, CH2CH=CH2), 5.36–5.29 (m, 2 H, HC=CH2), 4.40 (d, 2 H, J = 4 Hz, CH2-N); Figure S5. 13C NMR (CDCl3, 100 MHz) δC ppm; 166.3, 165.3 (2× C=O), 148.0 (Ar-C), 139.2 (Ph-CH=C), 130.66 (CH=CH2), 130.65, 129.7 (Ar-C), 126.07 (CH=C), 124.41 (Ar-C), 119.5 (HC=CH2), 44.2 ((N-CH2); Figure S6. Anal. calcd for C13H10N2O4S: C, 53.79; H, 3.47; N, 9.65; S, 11.04. Found C, 53.75; H, 3.46; N, 9.62; S, 11.07.
5.3.1.4. 3-Allyl-5-(4-(dimethylamino)benzylidene)thiazolidine-2,4-dione (4d)
It was obtained as a yellow crystal, 58% yield, mp = 228 °C, Rf = 0.54 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1728 (C=O), 1672 (NC=O), 1590 (C=C), 1110 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.85 (s, 1 H, Ar-CH=C), 7.44–7.42 (d, 2 H, J = 8 Hz, Ar-H), 6.78–6.76 (d, 2 H, J = 8 Hz, Ar-H), 5.93–5.83 (m, 1 H, CH2CH=CH2), 5.32, 5.23 (2 d, 2 H, HC=CH2), 4.37 (d, 2 H, J = 8 Hz, CH2-N), 3.09 (s, 6 H, 2 CH3); Figure S7. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.1, 166.4 (2 C=O), 151.3 (Ar-C), 134.8 (Ph-CH=C), 132.5(N-CH=CH2), 132.1, 130.5, 121.1 (Ar-C), 118.6 (HC=CH2), 118.5 (CH=C), 114.5, 113.3 (Ar-C), 112.2 (N-CH2), 43.6, 40.2 (2 CH3); Figure S8. Anal. calcd for C15H16N2O2S: C, 62.48; H, 5.59; N, 9.71; S, 11.12. Found C, 62.43; H, 5.56; N, 9.69; S, 11.11.
5.3.1.5. 3-Allyl-5-(4-(allyloxy)benzylidene)thiazolidine-2,4-dione (4e)
It was obtained as a white powder, 65% yield, mp = 81 °C, Rf = 0.73 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1739 (C=O), 1685 (NC=O), 1595 (C=C), 1181.1 (C-O), 1110 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.88 (s, 1 H, Ar-CH=C), 7.49–7.47 (d, 2 H, J = 8 Hz, Ar-H), 7.03–7.01 (d, 2 H, J = 8Hz, Ar-H), 6.11–6.03 (m, 1 H, OCH2CH=CH2), 5.91–5.83 (m, 1 H, NCH2CH=CH2), 5.48, 5.43, 5.36, 5.25 (4 d, 4 H, O-, N-HC=CH2), 4.63 (d, 2 H, J = 4 Hz, OCH2), 4.37 (d, 2 H, J = 4 Hz, NCH2); Figure S9. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.7, 166.1 (2 C=O), 160.5 (Ar-C), 133.8 (Ph-CH=C), 132.4 (OCH2-CH=CH2), 132.3 (Ar-C), 130.3 (NCH2-CH=CH2), 125.9 (Ar-C), 118.8 (OCH2-CH=CH2), 118.4 (HC=CH2), 118.3 (NCH2-CH=CH2), 115.5 (Ar-C), 68.9 (OCH2CH=CH2), 43.8 (NCH2CH=CH2); Figure S10. Anal. calcd for C16H15NO3S: C, 63.77; H, 5.02; N, 4.65; S, 10.64. Found C, 63.74; H, 5.06; N, 4.64; S, 10.62.
5.3.2. Synthesis of Ethyl-2-(5-substituted benzylidene-2,4-dioxothiazolidin-3-yl)acetate (5)
5.3.2.1. Ethyl-2-(5-benzylidene-2,4-dioxothiazolidin-3-yl)acetate (5a)
It was obtained as colorless crystals, 79% yield, mp = 92 °C (Lit. mp56 75–77 °C), Rf = 0.61 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1741 (C=O), 1684 (NC=O), 1605 (C=C), 1219 (C-N), 1152 (C-O). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.96 (s, 1 H, Ph-CH=C), 7.55–7.47 (m, 5 H, Ar-H), 4.50 (s, 2 H, NCH2), 4.27 (q, 2 H, OCH2CH3), 1.32 (t, 3 H, OCH2CH3); Figure S11. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.5, 166.2, 165.6 (3× C=O), 134.7 (Ph-CH=C), 133.1, 130.8, 130.3, 129.3 (Ar-C), 121.0 (-CH=C), 62.2 (OCH2CH3), 42.1 (NCH2), 14.1 (OCH2CH3); Figure S12. Anal. calcd for C14H13NO4S: C, 57.72; H, 4.50; N, 4.81; S, 11.01. Found C, 57.76; H, 4.48; N, 4.80; S, 10.98.
5.3.2.2. Ethyl-2-(5-(4-nitrobenzylidene)-2,4-dioxothiazolidin-3-yl)acetate (5c)
It was obtained as yellow needles, 62% yield, mp = 139 °C, Rf = 0.71 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1734 (C=O), 1697 (NC=O), 1609 (C=C), 1535, 1344 (NO2), 1235 (C-N), 1149 (C-O). 1H NMR (CDCl3, 400 MHz) δH ppm; 8.28 (d, 2 H, J = 8 Hz, Ar-H), 7.89 (s, 1 H, Ph-CH=C), 7.62 (d, 2 H, Ar-H), 4.43 (s, 2 H, NCH2), 4.20 (q, 2 H, J = 8 Hz, OCH2CH3), 1.24 (t, 3 H, J = 8 Hz, OCH2CH3); Figure S13. 13C NMR (CDCl3, 100 MHz) δC ppm; 166.3, 166.0, 165.0 (3× C=O), 148.1 (Ar-C), 139.1 ((Ph-CH=C), 131.3, 130.7, 125.6 (Ar-C), 124.4 (-CH=C), 62.4 (OCH2CH3), 44.3 (NCH2), 14.1 (OCH2CH3); Figure S14. Anal. calcd for C14H12N2O6S: C, 50.00; H, 3.60; N, 8.33; S, 9.53. Found C, 49.97; H, 3.65; N, 8.31; S, 9.55.
5.3.2.3. Ethyl-2-(5-(4-(dimethylamino)benzylidene)-2,4-dioxothiazolidin-3-yl)acetate (5d)
It was obtained as orange crystals, yield (79%), mp = 170 °C (Lit. mp56 = 170–171 °C), Rf = 0.5 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1729 (C=O), 1676 (NC=O), 1589 (C=C), 1220 (C-N), 1147 (C-O). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.87 (s, 1 H, Ar-CH=C), 7.45 (d, 2 H, J = 8.8 Hz, Ar-H), 6.78 (d, 2 H, J = 12 Hz, Ar-H), 4.48 (s, 2 H, NCH2), 4.26 (q, 2 H, J = 7.2 Hz, OCH2CH3), 3.09 (s, 6 H, N, N-2× CH3), 1.31 (t, 3 H, J = 7.2 Hz, OCH2CH3); Figure S15. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.1, 166.5, 166.0 (3 C=O), 151.4 (Ar-C), 135.5 (Ph-CH=C), 132.6, 120.9 (Ar-C), 114.0 (Ph-CH=C), 112.2 (Ar-C), 62.0 (OCH2CH3), 42.0 (NCH2), 40.2 (2× NCH3), 14.1 (OCH2CH3); Figure S16. Anal. calcd for C16H18N2O4S: C, 57.47; H, 5.43; N, 8.38; S, 9.59. Found C, 57.46; H, 5.33; N, 8.41; S, 9.55.
5.3.2.4. Ethyl-2-(5-(4-(2-ethoxy-2-oxoethoxy)benzylidene)-2,4-dioxothiazolidin-3-yl)acetate (5e)
It was obtained as yellow crystals, 51% yield, mp = 86 °C (Lit. mp42 = 115 °C), Rf = 0.47 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1741 (C=O), 1688 (NC=O), 1592 (C=C), 1209 (C-N), 1148.25 (C-O). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.90 (s, 1 H, Ar-CH=C), 7.51 (d, 2 H, J = 8 Hz, Ar-H), 7.03 (d, 2 H, J = 8 Hz, Ar-H), 4.70 (s, 2 H, O-CH2), 4.49 (s, 2 H, N-CH2), 4.33–4.24 (qq, 4 H, O-CH2-CH3), 1.35–1.30 (dd, 6 H, 2× CH3); Figure S17. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.2, 167.5, 166.3, 165.7 (4× C=O), 159.6 (Ar-C-O), 134.2 (Ph-CH=C), 132.33 (Ar-C), 126.71 (Ar-C), 118.7 (Ph-CH=C), 115.4 (Ar-C), 65.2 (O-CH2), 62.1 (O-(O-CH2-CH3)), 61.6 (N-(O-CH2-CH3)), 42.1 (N-CH2), 14.19 (O-(O-CH2-CH3)), 14.11 (N-(O-CH2-CH3)); Figure S18. Anal. calcd for C18H19NO7S: C, 54.95; H, 4.87; N, 3.56; S, 8.18. Found C, 54.92; H, 4.86; N, 3.55; S, 8.16.
5.3.3. Synthesis of 3-(3-Bromopropyl)-5-(substituted benzylidene)thiazolidine-2,4-dione (6)
5.3.3.1. 5-Benzylidene-3-(3-bromopropyl)thiazolidine-2,4-dione (6a)
It was obtained as colorless crystals, 60% yield, mp = 118 °C, Rf = 0.68 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1745 (C=O), 1684 (C=O), 1609.(C=C), 1232 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.95 (s, 1 H, Ph-CH=C), 7.54–7.46 (m, 5 H, Ar-H), 3.94 (t, 2 H, NCH2), 3.44 (t, 2 H, NCH2-CH2-CH2), 2.28 (quintet, 2 H, CH2-CH2-CH2,); Figure S19. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.92, 166.29, (2 C=O), 134.22, (CH=C), 133.16, 130.68, 130.28, 129.28, (Ar-C), 121.20, (CH=C), 40.69 (NCH2), 30.8 (NCH2-CH2-CH2), 29.48 (CH2-CH2-CH2); Figure S20. Anal. calcd for C23H18N2O4S2: C, 61.32; H, 4.03; N, 6.22; S, 14.23. Found C, 61.31; H, 4.01; N, 6.21; S, 14.22.
5.3.3.2. 3-(3-Bromopropyl)-5-(4-nitrobenzylidene)thiazolidine-2,4-dione (6c)
It was obtained as a yellow powder, 49% yield, mp = 128 °C, Rf = 0.60 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1748 (C=O), 1691 (NC=O), 1606 (C=C), 1516–1346 (NO2), 1130 (C-N), 685 (C-Br). 1H NMR (CDCl3, 400 MHz) δH ppm; 8.37 (d, 2 H, J = 12 Hz, Ar-H), 7.95 (s, 1 H, Ph-CH=C), 7.70 (d, 2 H, J = 8 Hz, Ar-H), 3.96 (t, 2 H, NCH2), 3.44 (t, 2H, BrCH2), 2.29 (pentet, 2 H, CH2CH2CH2); Figure S21. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.5, 165.9 (2× C=O), 148.03 (CH=C), 139, 131.4, 130.4 (Ar-C), 126.5 (CH=C), 124.83 (Ar-C), 40.9 (NCH2), 32.1 (BrCH2), 30.7 (N-CH2-CH2-CH2), Figure S22. Anal. calcd for C13H11BrN2O4S: C, 42.06; H, 2.99; N, 7.55; S, 8.64. Found C, 42.11; H, 2.94; N, 7.54; S, 8.66.
5.3.3.3. 3-(3-Bromopropyl)-5-(4-(dimethylamino)benzylidene)thiazolidine-2,4-dione (6d)
It was obtained as yellow crystals, 63% yield, mp = 160 °C, Rf = 0.62 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1724 (C=O), 1674 (C=O), 1581 (C=C),1189 (C-N), 520 (C-Br). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.86 (s, 1 H, Ar-CH=C), 7.46 (d, 2 H, J = 8 Hz, Ar-H), 6.86–6.81 (m, 2 H, Ar-H), 3.92 (t, 2 H, NCH2), 3.43 (t, 2 H, BrCH2), 3.1 (s, 6 H, 2× N-CH3), 2.27 (quintet, 2 H, CH2-CH2-CH2); Figure S23. 13C NMR (DMSO-d6, 100 MHz) δC ppm; 168.1, 166.3 (2× C=O), 152.0 (Ar-C), 134.3 (CH=C), 134.3, 132.8, 130.5, 120.2 (Ar-C), 113.8 (CH=C), 112.5 (Ar-C), 58.9 (N-CH2), 40.09, 40.17 (2× CH3), 32.2 (CH2-CH2-CH2), 30.8 (CH2-CH2-CH2); Figure S24. Anal. calcd for C15H17BrN2O2S: C, 48.79; H, 4.64; N, 7.59; S, 8.68. Found C, 48.75; H, 4.66; N, 7.55; S, 8.65.
5.3.3.4. 5-(4-(3-Bromopropoxy)benzylidene)-3-(3-bromopropyl)thiazolidine-2,4-dione (6e)
It was obtained as a yellow precipitate, 35% yield, mp = 92 °C, Rf = 0.49 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 1738 (C=O), 1680 (NC=O), 1598 (C=C), 1258 (C-O), 1127 (C-N).1H NMR (CDCl3, 400 MHz) δH ppm; 7.88 (s, 1 H, CH=C), 7.50 (d, 2 H, J = 8 Hz, Ar-H), 7.03 (d, 2 H, Ar-H), 4.20 (t, 2 H, O-CH2CH2CH2), 3.92 (t, 2 H, N-CH2CH2CH2), 3.63 (t, 2 H, N-CH2CH2CH2), 3.43 (t, 2 H, O-CH2CH2CH2), 2.37 (quintet, 2 H,O-CH2CH2CH2), 2.27 (quintet, 2 H, N-CH2CH2CH2); Figure S25. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.1, 166.4 (2× C=O), 160.6 (Ar-C), 134.0 (CH=C), 132.3 (Ar-C), 126.0 (Ar-C), 115.30 (Ar-C), 114.8 (CH=C), 65.5 (OCH2) 40.6 (NCH2), 32.1, 30.8 (2× CH2Br), 29.7, 29.5 (CH2CH2CH2); Figure S26. Anal. calcd for C26H23BrN2O6S2: C, 51.75; H, 3.84; N, 4.64; S, 10.62. Found C, 51.73; H, 3.81; N, 4.65; S, 10.65.
5.3.4. 3-(3-Hydroxypropyl)-5-(substituted benzylidene)thiazolidine-2,4-dione (7)
5.3.4.1. 5-Benzylidene-3-(3-hydroxypropyl)thiazolidine-2,4-dione (7a)
It was obtained as colorless crystals, 65% yield, mp = 110 °C, Rf = 0.34 (ethylacetate/n-hexane, 1:3). IR (KBr) υ (cm–1): 3428 (OH), 1757 (C=O), 1698 (NC=O), 1610 (C=C), 1112 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.95 (s, 1 H, CH=C), 7.55–7.47 (m, 5 H, Ar-H), 3.96 (t, 2 H, N-CH2CH2CH2), 3.65 (t, 2 H, CH2CH2CH2-OH), 2.15 (bs, 1 H, OH, D2O exchangeable H), 1.92 (quintet, 2 H, CH2CH2CH2); Figure S27. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.5, 167.0 (2× C=O), 134.4 (CH=C), 133.1, 130.7, 130.3, 129.5, 129.3 (Ar-C), 121.1 (CH=C), 58.9 (CH2OH), 38.5 (NCH2), 30.6 (CH2CH2CH2); Figure S28. Anal. calcd for C13H13NO3S: C, 59.30; H, 4.89; N, 5.32; S, 12.18. Found C, 59.28; H, 4.88; N, 5.28; S, 12.13.
5.3.4.2. 3-(3-Hydroxypropyl)-5-(4-nitrobenzylidene)thiazolidine-2,4-dione (7c)
It was obtained as a brown powder, 55% yield, mp = 170 °C, Rf = 0.17 (ethylacetate:n-hexane, 1:3). IR (KBr) υ (cm–1): 3451 (OH), 1738 (C=O), 1676 (NC=O), 1608 (C=C), 1510, 1345 (NO2), 1106 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 8.37 (d, 2 H, J = 8 Hz, Ar-H), 8.04 (s, 1 H, CH=C), 7.91 (d, 2 H, Ar-H), 4.61 (t, 1 H, OH, D2O exchangeable H), 3.74 (t, 2H, NCH2), 3.47 (q, 2 H, CH2OH), 1.75 (quintet, 2 H, CH2CH2CH2); Figure S29. 13C NMR (CDCl3, 100 MHz) δC ppm; 167.3, 165.8 (2× C=O), 148.0 (Ar-C), 139.8 (CH=C), 131.5, 130.4 126.4 (CH=C), 124.8 (Ar-C), 58.9 (OCH2), 30.6 (CH2-CH2-CH2); Figure S30. Anal. calcd for C13H12N2O5S: C, 50.64; H, 3.92; N, 9.09; S, 10.40. Found C, 50.59; H, 3.91; N, 9.04; S, 10.37.
5.3.4.3. 5-(4-(Dimethylamino)benzylidene)-3-(3-hydroxypropyl)thiazolidine-2,4-dione (7d)
It was obtained as a brown precipitate, yield (85%), mp = 154 °C, Rf = 0.43 (ethylacetate/n-hexane, 1:3). IR(KBr) υ (cm–1): 3452 (OH), 1715.22 (C=O), 1645 (NC=O), 1585 (C=C), 1129 (C-N). 1H NMR (CDCl3, 400 MHz) δH ppm; 7.79 (s, 1 H, CH=C), 7.48 (d, 2 H, J = 12 Hz, Ar-H), 6.84 (d, 2 H, Ar-H), 4.58 (t, 1 H, OH, D2O exchangeable H), 3.70 (t, 2 H, NCH2), 3.45 (q, 2 H, CH2OH), 3.03 (s, 6 H, 2× CH3), 1.72 (quintet, 2 H, CH2CH2CH2); Figure S31. 13C NMR (CDCl3, 100 MHz) δC ppm; 168.0, 166.3 (2× C=O), 152.0 (Ar-C), 134.3 (CH=C), 132.8, 120.2 (Ar-C), 113.8 (CH=C), 112.5 (Ar-C), 58.95 (CH2OH), 40.06, 40.15 (NCH3) under a solvent peak, 30.9 (CH2CH2CH2); Figure S32. Anal. calcd for C15H18N2O3S: C, 58.80; H, 5.92; N, 9.14; S, 10.46. Found C, 58.79; H, 5.91; N, 9.11; S, 10.44.
5.4. Biological Evaluation
5.4.1. Cytotoxicity Screening on Normal Human Cells
The normal human lung fibroblast Wi-38 cell line was used to detect the cytotoxicity of the studied compounds via MTT assay as detailed in the Supporting Information.
5.4.2. Anticancer Screening
The anticancer effect of the abovementioned compounds was assayed using the colon cancer cell line Caco-2 via MTT assay as described in the Supporting Information.
5.4.3. SOD Inhibition Assay
SOD inhibition was assayed as detailed in the Supporting Information using the superoxide dismutase kit from R&D Systems according to the manufacturer’s instructions.
5.4.4. Inhibition of the Antioxidant Enzymes in Colorectal Cancer Cells (Caco-2)
After 72 h of incubation of Caco-2 cells with IC50 of the most effective anticancer compounds, the antioxidant enzyme activities (SOD, ALDH1, and GPX) were determined as previously reported,57−59 as described in the Supporting Information.
5.4.5. Determination of Cellular Reactive Oxygen Species in Colorectal Cancer (Caco-2)
The cellular reactive oxygen species (ROS) was determined by incubating the untreated and the most effective anticancer compounds-treated Caco-2 with 2′,7′-dichlorofluorescein diacetate (DCFDA), as detailed in the Supporting Information.
5.5. Molecular Modeling Studies
5.5.1. Docking
Docking simulations were performed by employing the Molecular Operating Environment (MOE) software package version 2015.1045 as described in the Supporting Information.
5.5.2. In Silico Prediction of Physicochemical Properties, ADMET, and Drug-Likeness Parameters
Physicochemical properties, ADME. and drug-likeness were computed by SwissADME(47) software. Toxicity was predicted by PROTOX.52
Acknowledgments
The authors thank Alexandria University for its support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02410.
1H- and 13C NMR spectra of all the synthesized compounds are included, also, detailed biological evaluation and docking studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Azad M. B.; Chen Y.; Gibson S. B. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid. Redox Signaling 2009, 11, 777–790. 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- Sosa V.; Moliné T.; Somoza R.; Paciucci R.; Kondoh H.; Leonart M. E. Oxidative stress and cancer: an overview. Ageing Res. Rev. 2013, 12, 376–390. 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Sashi Papu John A. M.; Ankem M. K.; Damodaran C. Oxidative Stress: A Promising Target for Chemoprevention. Curr. Pharmacol. Rep. 2016, 2, 73–81. 10.1007/s40495-016-0052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa D.; Ghosh R. Antioxidants for prostate cancer chemoprevention: Challenges and opportunities. Biochem. Pharmacol. 2012, 83, 1319–1330. 10.1016/j.bcp.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Mohsenzadegan M.; Farhad S.; Mohammad M. F.; Majid K. Anti-Oxidants as Chemopreventive Agents in Prostate Cancer: A Gap Between Preclinical and Clinical Studies. Recent Pat. Anti-Cancer Drug Discovery 2018, 13, 224–239. 10.2174/1574892813666180213164700. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Chemoprevention of cancer. Cancer Res. 1985, 45, 1–8. [PubMed] [Google Scholar]
- Piccolella S.; Pacifico S. Plant-derived polyphenols: a chemopreventive and chemoprotectant worth-exploring resource in toxicology. Adv. Mol. Toxicol. 2015, 161–214. 10.1016/B978-0-12-802229-0.00005-0. [DOI] [Google Scholar]
- Postovit L.; Widmann C.; Huang P.; Gibson S. B. Harenssing Oxidative Stress as an Innovative Target for Cancer Therapy. Oxid. Med. Cell. Longevity 2018, 2018, 6135739 10.1155/2018/6135739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani G.; Colavitti R.; Bedogni B.; Fusco S.; Ferraro D.; Borrello S.; Galeotti T. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr. Med. Chem. 2004, 11, 1299–1308. 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- McCord J. M.; Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. 10.1016/S0021-9258(18)63504-5. [DOI] [PubMed] [Google Scholar]
- Margis R.; Dunand C.; Teixeira F. K.; Margis-Pinheiro M. Glutathione peroxidase family–an evolutionary overview. FEBS J. 2008, 275, 3959–3970. 10.1111/j.1742-4658.2008.06542.x. [DOI] [PubMed] [Google Scholar]
- Younus H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [PMC free article] [PubMed] [Google Scholar]
- Culotta V. C.; Yang M.; O’Halloran T. V. Activation of superoxide dismutases: putting the metal to the pedal. Biochim. Biophys. Acta, Mol. Cell Res. 2006, 1763, 747–758. 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irawan B.; Labeda I.; Lusikooy R. E.; Sampetoding S.; Kusuma M. I.; Uwuratuw J. A.; Syarifuddin E.; Faruk M.; et al. Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: a cross-sectional study. Ann. Med. Surg. 2020, 58, 194–199. 10.1016/j.amsu.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Fu L.; Tian T.; Deng L.; Li H.; Xiao W.; Gong Q. Disrupting SOD1 activity inhibits cell growth and enhances lipid accumulation in nasopharyngeal carcinoma. Cell Commun. Signaling 2018, 16, 28 10.1186/s12964-018-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder T. P.; Verspaget H. W.; Janssens A. R.; De Bruin P. A.; Griffioen G.; Lamers C. B. Neoplasia-related changes of two copper (Cu)/zinc (Zn) proteins in the human colon. Free Radical Biol. Med. 1990, 9, 501–506. 10.1016/0891-5849(90)90128-6. [DOI] [PubMed] [Google Scholar]
- Skórska K. B.; Płaczkowska S.; Prescha A.; Porębska I.; Kosacka M.; Pawełczyk K.; Zabłocka-Słowińska K. Serum Total SOD Activity and SOD1/2 Concentrations in Predicting All-Cause Mortality in Lung Cancer Patients. Pharmaceuticals 2021, 14, 1067 10.3390/ph14111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A.; Janssen L.; Bosman C. B.; van Duijn W.; Oostendorp-van de Ruit M. M.; Kubben F. J.; Griffioen G.; Lamers C. B.; Han J.; Van Krieken J. M.; van de Velde C. J. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin. Cancer Res. 2000, 6, 3183–3192. [PubMed] [Google Scholar]
- Li W.; Cao L.; Han L.; Xu Q.; Ma Q. Superoxide dismutase promotes the epithelial-mesenchymal transition of pancreatic cancer cells via activation of the H2O2/ERK/NF-κB axis. Int. J. Oncol. 2015, 46, 2613–2620. 10.3892/ijo.2015.2938. [DOI] [PubMed] [Google Scholar]
- Marikovsky M.; Nevo N.; Vadai E.; Harris-Cerruti C. Cu/Zn superoxide dismutase plays a role in angiogenesis. Int. J. Cancer 2002, 97, 34–41. 10.1002/ijc.1565. [DOI] [PubMed] [Google Scholar]
- Mendes F.; Pereira E.; Martins D.; Tavares-Silva E.; Pires A. S.; Abrantes A. M.; Figueiredo A.; Botelho M. F. Oxidative stress in bladder cancer: an ally or an enemy?. Mol. Biol. Rep. 2021, 48, 2791–2802. 10.1007/s11033-021-06266-4. [DOI] [PubMed] [Google Scholar]
- Arumsari S.; Noor D.; Dewi S.; Syahrani R.; Wanandi S. In vitro transfection of manganese superoxide dismutase small interfering RNA suppresses stemness of human breast cancer stem cells (aldehyde dehydrogenase 1-positive): Focus on OCT4 mRNA expression and mammosphere-forming capacity. J. Nat. Sci., Biol. Med. 2019, 10, S82–S87. 10.4103/jnsbm.JNSBM_113_19]. [DOI] [Google Scholar]
- Koppaka V.; Thompson D. C.; Chen Y.; Ellermann M.; Nicolaou K. C.; Juvonen R. O.; Petersen D.; Deitrich R. A.; Hurley T. D.; Vasiliou V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012, 64, 520–539. 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes S. A.; Adams A.; Timms A.; Fisher N.; Smythe J.; Watt S. M.; Joel S.; Donate F.; Hayward C.; Reich S.; Middleton M.; Mazar A.; Harris A. L. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors Clin. Cancer Res. 2008, 14, 7526–7534. 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- Lin J.; Beer T. M.; Ryan C. J.; Mathew P.; Wilding G.; Morris M.; Callahan J. A.; Gordon G.; Reich S.; Carducci M. A. A randomized, phase II study of ATN-224 in patients with biochemically relapsed, hormone-naive prostate cancer: a DOD/PCF Prostate Cancer Clinical Trials Consortium trial. J. Clin. Oncol. 2009, 27, 5135 10.1200/jco.2009.27.15_suppl.5135. [DOI] [Google Scholar]
- Dong X.; Zhang Z.; Zhao J.; Lei J.; Chen Y.; Li X.; Chen H.; Tian J.; Zhang D.; Liu C.; Liu C. The rational design of specific SOD1 inhibitors via copper coordination and their application in ROS signaling research. Chem. Sci. 2016, 7, 6251–6262. 10.1039/C6SC01272H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwar R.; Shum D.; Djaballah H.; Varmus H. Identification and preliminary characterization of novel small molecules that inhibit growth of human lung adenocarcinoma cells. J. Biomol. Screen. 2009, 14, 1176–1184. 10.1177/1087057109350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somwar R.; Erdjument-Bromage H.; Varmus H. E.; et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 16375–16380. 10.1073/pnas.1113554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminskyy D.; Kryshchshyn A.; Lesyk R. 5-Ene-4-Thiazolidionones-An efficient tool in medicinal chemistry. Eur. J. Med. Chem. 2017, 140, 542–594. 10.1016/j.ejmech.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim M. J.; Alam M. J.; Ahmed S.; Nawaz F.; Shrivastava N.; Sahu M.; Alam O. Therapeutic journey of 2,4-thiazolidinedions as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250. 10.1016/j.ejmech.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Munwar S.; Lango K. Thiazolidinediones (TZD) as a versatile scaffold in medicinal chemistry biological importance: A Review. Int. J. Pharm. Sci. Rev. 2020, 63, 58–70. [Google Scholar]
- Schweipert M.; Jänsch N.; Upadhyay N.; Tilekar K.; Wozny E.; Basheer S.; Wurster E.; Müller M.; CS R.; Meyer-Almes F. J. Mechanistic Insights into Binding of Ligands with Thiazolidinedione Warhead to Human Histone Deacetylase 4. Pharmaceuticals 2021, 14, 1032 10.3390/ph14101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. L.; Chrysanthopoulos P. K.; Halili M. A.; Hepburn C.; Nebl T.; Supuran C. T.; Nocentini A.; Peat T. S.; Poulsen S. A. The Glitazone Class of Drugs as Carbonic Anhydrase Inhibitors: A Spin-Off Discovery from Fragment Screening. Molecules 2021, 26, 3010 10.3390/molecules26103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Lotfy G.; Abdel Aziz Y. M.; Aziz Y. M. A.; Said M. M.; El Ashry E. S. H.; El Tamany E. S. H.; Abu-Serie M. M.; Abu-Serie M. M.; Teleb M.; Teleb M.; Dömling A.; Dömling A.; Barakat A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 117, 105427 10.1016/j.bioorg.2021.105427. [DOI] [PubMed] [Google Scholar]
- Aziz Y. M. A.; Lotfy G.; Said M. M.; El Ashry E. S. H.; El Tamany E. S. H.; Soliman S.; Abu-Serie M. M.; Teleb M.; Yousuf S.; Dömling A. Design, synthesis, chemical and biochemical insights on to novel hybrid spirooxindoles-based p53-MDM2 inhibitors with potential Bcl2 signaling attenuation. Front. Chem. 2021, 9, 735236 10.3389/fchem.2021.735236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadla N.; Bahia M. S.; Kaur M.; Silakari O. Thiazolidin-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorg. Med. Chem. 2015, 23, 2953–2974. 10.1016/j.bmc.2015.03.071. [DOI] [PubMed] [Google Scholar]
- Lo Fiego M. J.; Lorenzetti A. S.; Silbestri G. F.; Domini C. E. The use of ultrasound in the South Cone region. Advances in organic and inorganic synthesis and in analytical methods. Ultrason. Sonochem. 2021, 80, 105834 10.1016/j.ultsonch.2021.105834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thari F. Z.; Tachallait H.; El Alaoui N-E.; Talha A.; Arshad S.; Álvarez E.; Karrouchi K.; Bougrin K. Ultrasound-assisted one-pot green synthesis of new N- substituted-5-arylidene-thiazolidine-2,4-dione-isoxazoline derivatives using NaCl/Oxone/Na3PO4 in aqueous media. Ultrason. Sonochem. 2020, 68, 105222 10.1016/j.ultsonch.2020.105222. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Priya A.; Kaur M.; Singh A.; Kaur G.; Banerjee B. Ultrasound-assisted synthesis of bioactive S-heterocycles. Synth. Commun. 2021, 51, 3209–3236. 10.1080/00397911.2021.1970775. [DOI] [Google Scholar]
- Marc G.; Ionut I.; Pirnau A.; Vlase L.; Vodnar D. C.; Duma M.; Tiperciuc B.; Oniga O. Microwave assisted synthesis of 3,5-disubstituted thiazolidine-2,4-diones with antifungal activity. Design, synthesis, virtual and in vitro antifungal screening. Farmacia 2017, 65, 414–422. [Google Scholar]
- Marc G.; Stana A.; Pirnau A.; Vlase L.; Vodnar D. C.; Duma M.; Tiperciuc B.; Oniga O. 3,5-Disubstituted thiazolidine-2,4-diones: Design, Microwave- Assisted synthesis, Antifungal activity and ADMET Screening. SLAS Discovery 2018, 23, 807–814. 10.1177/2472555218759035. [DOI] [PubMed] [Google Scholar]
- Nawale S. L.; Avinash S. D. Synthesis and evaluation of novel thiazolidinedione derivatives for antibacterial activity. Der Pharma Chem. 2012, 4, 2270–2277. [Google Scholar]
- C.C.G. Molecular Operating Environment (MOE), Montreal, Canada, 2014. http://www.chemcomp.com.
- Sala F. A.; Wright G. S. A.; Antonyuk S. V.; Garratt R. C.; Hasnain S. S. Molecular recognition and maturation of SOD1 by its evolutionarily destabilized cognate chaperone hCCS. PLoS Biol. 2019, 17, e3000141 10.1371/journal.pbio.3000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A.; Michielin O.; Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- Veber D. F.; Johnson S. R.; Cheng H.-Y.; Smith B. R.; Ward K. W.; Kopple K. D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- Muegge I.; Heald S. L.; Brittelli D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. 10.1021/jm015507e. [DOI] [PubMed] [Google Scholar]
- Ghose A. K.; Viswanadhan V. N.; Wendoloski J. J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- Banerjee P.; Eckert A. O.; Schrey A. K.; Preissner R. ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, 257–263. 10.1093/nar/gky318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D.; Narwal S.; Sandhu J. S. Catalyst-free synthesis of highly biologically active 5-arylidene rhodanine and 2,4-thiazolidinedione derivatives using aldonitrones in polyethylene glycol. Int. J. Med. Chem. 2013, 2013, 273534 10.1155/2013/273534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva I. M.; Filho J. S.; Santigo P. B. G. S.; Do Egito M. S.; De Souza C. A.; Gouveia F. L.; Ximenes R. M.; Gouveia K. X. F. L.; De Sena K. X. F. R.; De Faria A. R.; Brondani D. J.; De Albuquerque J. F. C. Synthesis and antimicrobial activities of 5-arylidene-thiazolidine-2,4-dione derivatives. BioMed Res. Int. 2014, 2014, 316082 10.1155/2014/316082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirupathi G.; Venkatanarayana M.; Dubey P. K.; Kumari Y. B. Facile and green syntheses of substituted-5-arylidene-2,4-thiazolidinediones. Der Pharma Chem. 2012, 4, 2009–2013. [Google Scholar]
- Bhat B. A.; Ponnala S.; Sahu D. P.; Tiwari P.; Tripathi B. K.; Srivastava A. K. Synthesis and antihyperglycemic activity profiles of novel thiazolidinedione derivatives. Bioorg. Med. Chem. 2004, 12, 5857–5864. 10.1016/j.bmc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Graham C. E.; Brocklehurst K.; Pickergill R. W.; Warren M. J. Characterization of retinaldehyde dehydrogenase 3. Biochem. J. 2006, 394, 67–75. 10.1042/BJ20050918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotruck J. T.; Pope A. L.; Ganther H. E.; Swanson A. B.; Hafeman D. G.; Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Marklund S.; Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







