Conspectus
The asymmetric synthesis of heavily substituted benzylic stereogenic centers, prevalent in natural products, therapeutics, agrochemicals, and catalysts, is an ongoing challenge. In this Account, we outline our contribution to this endeavor, describing our discovery of a series of new reactions that not only have synthetic applicability but also present significant mechanistic intrigue. The story originated from our longstanding interest in the stereochemistry and reactivity of functionalized organolithiums. While investigating the lithiation chemistry of ureas (a “Cinderella” sister of the more established amides and carbamates), we noted an unexpected Truce–Smiles (T-S) rearrangement involving the 1,4-N → C transposition of a urea N′-aryl group to the α-carbanion of an adjacent N-benzyl group. Despite this reaction formally constituting an SNAr substitution, we found it to be remarkably tolerant of the electronic properties of the migrating aryl substituent and the degree of substitution at the carbanion. Moreover, in contrast to classical SNAr reactions, the rearrangement was sufficiently rapid that it took place under conditions compatible with configurational stability in an organolithium intermediate, enabling enantiospecific arylation at benzylic stereogenic centers. Experimental and computational studies confirmed a low kinetic barrier to the aryl migration arising from the strong preference for a trans arrangement of the urea N′-aryl and carbonyl groups, populating a reactive conformer in which spatial proximity was enforced between the carbanion and N′-aryl group, hugely accelerating ipso-substitution.
This discovery led us to uncover a whole series of conformationally accelerated intramolecular N → C aryl transfers using different anilide-based functional groups, including a diverse range of urea, carbamate, and thiocarbamate-substituted anions. Products included enantioenriched α-tertiary amines (including α-arylated N-heterocycles) and alcohols, as well as rare α-tertiary thiols. Synthetically challenging diarylated centers with differentiated aryl groups featured heavily in all product sets. The absolute enantiospecificity (retention versus inversion) of the reaction was dependent on the heteroatom α to the lithiation site: the origin of this stereodivergence was probed both experimentally and computationally. Asymmetric variants of the rearrangement were realized by enantioselective deprotonation, and connective strategies were developed in which an intermolecular C–C bond-forming event preceded the anionic rearrangement. Substrates where the N′-nucleofuge (at the aryl ipso position) was tethered to the migrating arene allowed us to use the rearrangement as a ring expansion method to generate 8- to 12-membered medium-ring N-heterocycles from very simple precursors. Stabilized carbon nucleophiles such as alkali metal enolates also readily promoted intramolecular N → C aryl transfer in N′-arylureas, opening up access to biologically relevant hydantoins, and enabling a “chiral memory” approach for the (hetero)arylation of chiral α-amino acids with programmable retention or inversion of configuration. Collectively, our studies of electronically versatile T-S rearrangements in anilide-based systems have culminated in a practical and general strategy for transition metal-free C(sp3)-arylation. More broadly, our results highlight the power of conformational activation to achieve unprecedented reactivity in the construction of challenging C–C bonds.
Key References
Leonard D. J.; Ward J. W.; Clayden J.. Asymmetric α-Arylation of Amino Acids. Nature 2018, 562, 105–109.1Arene-tethered enolates derived from chiral α-amino acids undergo N′ → Cα aryl transfer with both electron-rich and -poor arenes. The process is formally enantiospecific and can be engineered for either retention or inversion of configuration.
Clayden J.; Dufour J.; Grainger D. M.; Helliwell M.. Substituted Diarylmethylamines by Stereospecific Intramolecular Electrophilic Arylation of Lithiated Ureas. J. Am. Chem. Soc. 2007, 129, 7488–7489.2The first report of urea tethers in conformationally accelerated SNAr reactions of carbon(sp3) nucleophiles, including enantiospecific C-arylation.
1. Introduction
Asymmetric carbon–carbon bond formation is a central theme in organic chemistry, with the stereoselective introduction of aromatic rings to carbon frameworks of particular importance due to the prevalence of benzylic stereocenters in functional organic molecules (e.g., 1–9, Figure 1).
Figure 1.
Valuable compounds containing a benzylic stereocenter.
Benzylic stereocenters are typically built either by asymmetric polar or radical additions to unsaturated carbon-based functionality (Scheme 1, strategy 1a/b) or by modification at an existing tertiary or quaternary (sp3)-carbon (strategy 2a/b). In these approaches, the aryl ring may be introduced during the reaction (substrategy a) or alternatively may already present as a substituent (substrategy b). Although asymmetric methods within each of these conceptual frameworks have been developed,3−13 the construction of fully substituted benzylic stereocenters with control of absolute stereochemistry remains a general challenge, especially in acyclic systems.14
Scheme 1. SNAr Reaction and the Construction of Benzylic Stereocenters.
Arylation of carbon-(sp3) (pro)nucleophiles is most commonly achieved using transition metal-catalyzed reactions of aryl halides.15,16 But from a conceptual standpoint, a nucleophilic aromatic substitution (SNAr) reaction between a carbon (pro)nucleophile 12 and an aryl electrophile 11 also appears a viable strategy for the direct arylation of acidic C(sp3)–H bonds (Scheme 1, strategy 2a′). However, even for intramolecular variants that use tethered substrates 13 (strategy 2a″), the so-called Truce–Smiles (T-S) rearrangement,17−19 these reactions have traditionally been confined to typically SNAr-reactive substrates in which the aryl electrophile carries anion stabilizing groups.20−26
In this Account, we outline the discovery and development of a new class of transition metal-free, intramolecular C(sp3)-arylations that, although formally SNAr reactions, offer remarkably broad scope and utility for the preparation of quaternary benzylic stereocenters, including full control of absolute configuration. The electronic versatility of these reactions, which do not require electron-deficient aryl electrophiles, arises from substrate activation by an often underappreciated but crucial determiner of molecular reactivity: conformational control (Scheme 1, strategy 2a″).
Conformational acceleration in intramolecular SNAr reactions of 13 requires an appropriate tether to enforce the spatial proximity of the carbon nucleophile and the aryl electrophile (Scheme 1). We have found that N-alkyl anilides and their congeners are universally effective as tethers, owing to the trans conformational preference of their carbonyl and aromatic (or other π-electron-rich) groups,27−29 which organizes them into a conformation primed for a T-S-like intramolecular SNAr reaction.30 This concept is illustrated in Scheme 2 for the general substrate 13a, where a carbon pronucleophile is tethered to an arene via a fully substituted anilide nitrogen atom. The conformational preference of 13a is dictated by unfavorable steric and electronic interactions in cis-13a, enforcing a strong bias for the trans conformer. Steric interactions are further relieved by rotation about the N–aryl bond in trans-13a, disrupting conjugation as the aryl ring twists perpendicular to the carbonyl plane. The “reactive” conformer trans-13a′ is thus favored, with the arene π-system directly available for attack by the adjacent carbon nucleophile.
Scheme 2. Conformationally Accelerated SNAr.

The conformational predisposition of 13a– lowers the activation energy for SNAr sufficiently to allow N → C aryl transfer by mechanisms that apparently bypass discrete Meisenheimer intermediates and, instead, follow partially concerted trajectories.31−34 As a result, these reactions break free of the normal requirement for arene activation by electron-withdrawing groups. SNAr reactions of 13a– involving highly nucleophilic carbanions are rapid enough to enable benzylic and allylic tertiary C–H bonds to be deprotonated and arylated enantiospecifically, even with electron-rich arenes.
Several additional features contribute to the general utility of these “nonclassical” T-S rearrangements: (1) The electrophilic arylating agents are formally inexpensive and readily available anilines, rather than halogenated arenes. (2) The substrates 13a are readily prepared by classical methods that make use of commercially available isocyanate or carbamoyl chloride derivatives. (3) The remains of the tether is readily cleaved after rearrangement, providing an overall “traceless” method. (4) The conformational preferences described extend across ureas, carbamates, and thiocarbamates, allowing arylation α to nitrogen, oxygen, or sulfur (“X” in Scheme 2).
2. N → C Aryl Migration in Ureas: Synthesis of α-Tertiary Amines
2.1. Reaction Discovery and Mechanistic Studies
In connection with our work on N,N′-diarylureas as conformational controllers in molecular communication devices,35 we had cause to investigate the functionalization of urea 15 by lithiation chemistry (Scheme 3a).36,37 To determine the preferred site of lithiation, 15 was treated with excess s-BuLi, aiming to methylate with MeI. Remarkably, the dearomatized derivative 16 was the major (though unstable) product.2 We were immediately intrigued that 16 had apparently been formed via a sequence of events that involved intramolecular nucleophilic attack of benzyllithium 15-Li′ (Scheme 3b) on the adjacent 2,6-dimethylphenyl ring (colored green), resulting in a 1,4-N → C aryl transfer. In support of this proposal, quenching the reaction with NH4Cl instead of MeI gave a T-S rearrangement product 17 in excellent yield (Scheme 3a), presumably via N- and α-protonation of 17-2Li.2 Considered alongside conventional SNAr reactivity, the fact that the arene in 15 was electron-rich, sterically encumbered, and prone to migration even at low temperature made the discovery of this T-S rearrangement all the more remarkable.38
Scheme 3. Discovery of N → C Aryl Migration.
Secondary benzylic ureas related to 15 underwent analogous rearrangement with high efficiency.2 The fact that complete aryl migration routinely occurred within 30 min at −78 °C led us to question whether such a process might be stereospecific with tertiary benzylic organolithiums. Indeed, with the addition of N,N′-dimethylpropyleneurea (DMPU) to accelerate carbanion arylation, a range of enantiopure α-methylbenzylureas 18 were rearranged to diarylalkylureas 19 with high enantiospecificity and net stereoretention (Scheme 4),2 including products bearing electron-rich (19b), electron-poor (19c), and sterically demanding arenes (19d, 19e). The corresponding diarylamine derivatives 20 were accessible by hydrolysis of 19 after initial activation by N-nitrosylation (although we later discovered simpler conditions for this reaction; see below).
Scheme 4. Enantiospecific Aryl Migration in α-Methylbenzylureas.
A more complete mechanistic picture of the conversion of 18 into 19 emerged from density functional theory (DFT) computational studies (Scheme 5).39 First, carbonyl-directed benzylic lithiation provides 18-Li, which undergoes rotation about the indicated N–CO bond to give reactive conformer 18-Li′, which is primed for intramolecular SNAr. Migration of the solvated lithium cation between the aryl rings in 18– leaves behind a delocalized benzylic carbanion, which retains transient axial chirality about the C––N bond due to its perpendicular carbanion and urea planes. The reaction then passes through a low barrier, spirocyclic transition state (TS-ret-N) where the lithium cation stabilizes the building negative charge on the N′-aryl ring, resulting in 1,4-N → C aryl transfer with retention of configuration. The role of excess organolithium (RLi, ≥2 equiv) is, first, to promote decomplexation of the intramolecular O–Li interaction in 18-Li through steric and electronic influence, which in turn promotes N–CO bond rotation, and, second, to stabilize the developing negative charge on the urea oxygen in TS-ret-N. In a complementary manner, DMPU (or THF) assists the rotation of 18-Li to 18-Li′ and stabilizes 18– by coordination to the lithium cation. NMR and IR reaction monitoring failed to detect a dearomatized Meisenheimer intermediate between 18-Li and 19-Li, except in special cases where the migrating arene was a 1-naphthyl group.2,39
Scheme 5. Mechanism of Stereoretentive Aryl Migration in α-Methylbenzylureas Supported by Computational Studies.

2.2. Other Lithiated N-Benzyl-, N-Allyl-, and N-Vinylureas: Expanding Scope
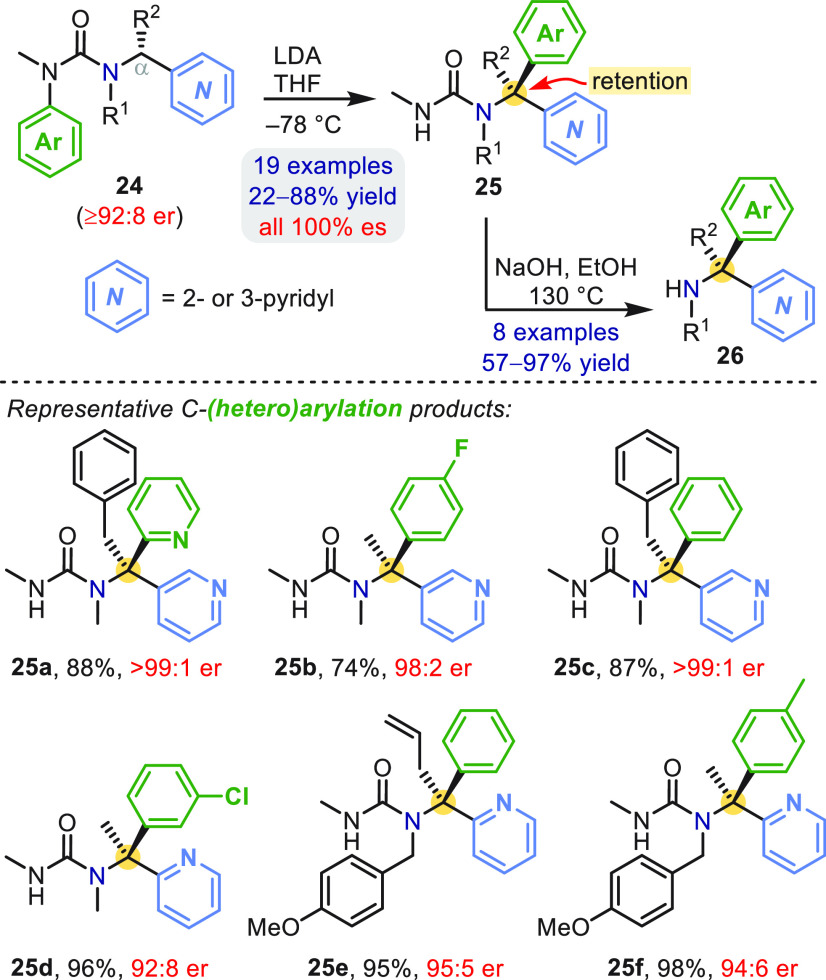
A simple modification of our original conditions, using LDA as base instead of s-BuLi to avoid direct nucleophilic addition to the pyridine ring, enabled 2-, 3-, and 4-pyridyl groups to be transferred to the benzylic stereocenter in 21 with near complete enantiospecificity (Scheme 6).40 Solvolysis of the desired products 22 with n-BuOH revealed the corresponding amines 23 in representative cases: this method is equally applicable to other ureas and is now our method of choice for releasing the amine products.
Scheme 6. N → C Pyridyl Migration in α-Methylbenzylureas.
Enantioenriched α-pyridyl benzylamines 25 may also be made by stereoretentive aryl migration using pyridines as the anion stabilizing group (Scheme 7).41 The increased C–H acidity α to the pyridine ensured complete site selectivity in the initial deprotonation, even with allyl, benzyl, and p-methoxybenzyl (PMB) groups as R1 or R2. Even 2-pyridyl substrates 24 reacted enantiospecifically, despite the intermediacy of an aza-enolate-like species, which must preserve homochirality on the time scale of the aryl transfer.41 Amine derivatives 26 were isolated in good yields after urea cleavage, this time with hydroxide.
Scheme 7. Aryl Migration in N-Pyridinylmethylureas.
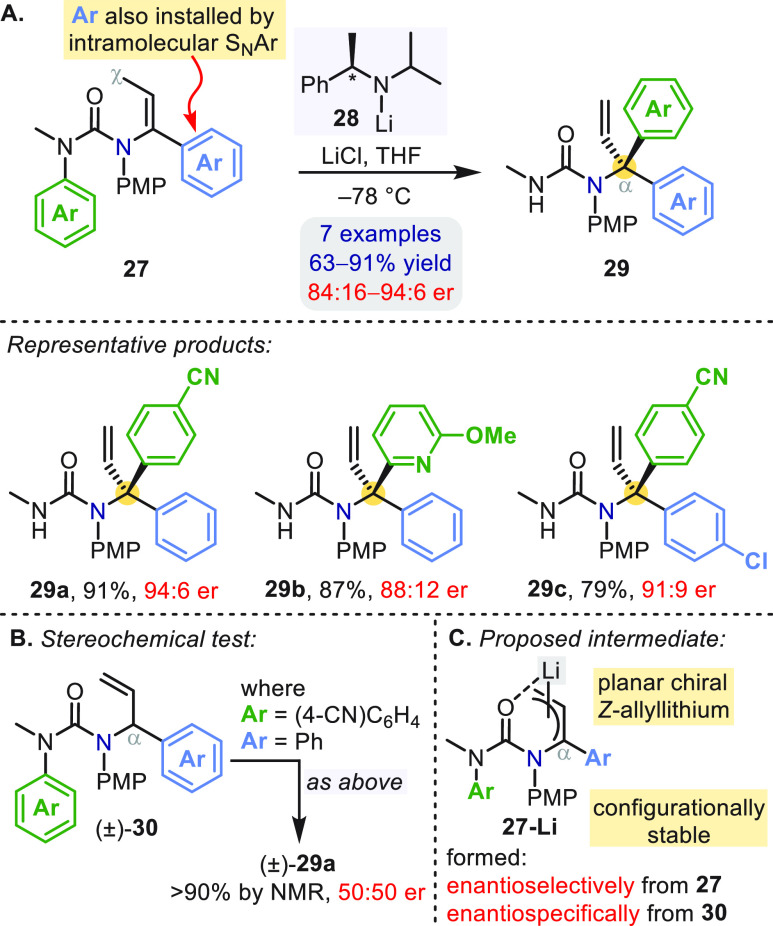
With allyllithiums as the carbon nucleophiles in the intramolecular SNAr reactions, two different aryl groups could be transferred in succession to the α-carbon of N-allyl ureas.42 LDA-promoted N′ → Cα aryl migration was followed by a palladium-catalyzed N-arylation of the urea to give the intermediate ureas 27 (Scheme 8a). A second Cα-arylation (involvingthe green aryl ring) was effected by the chiral lithium amide 28 to give enantioenriched α,α-diaryl allylic amine derivatives 29. To probe the enantiodetermining step in the conversion of 27 to 29, an isomeric substrate (±)-30 (Scheme 8b) was used as an alternative starting point for the reaction via the same allyllithium intermediate. The racemic product (±)-29a ruled out the interconversion of diastereomeric allyllithium·28 complexes as the stereodetermining step. δ-Deprotonation of 27 by 28 presumably gives directly an enantioenriched, configurationally stable planar chiral allyllithium 27-Li (Scheme 8c), which undergoes stereospecific α-arylation.
Scheme 8. Enantioselective Aryl Migration in N-Vinylureas.
An unexpected discovery was made during hydrolysis attempts using Na2CO3/EtOH or NaH/DMF (Scheme 9a): with an electron-deficient arene in 29, “reverse” 1,4-aryl migration (C → N) occurred to return vinyl ureas 27.4329-Na presumably exists in a rapid but unfavorable equilibrium with 27-Na by C → N aryl migration, and irreversible γ-protonation of 27-Na by the solvent or remaining 29 drives the reverse rearrangement to completion.
Scheme 9. Reversible Aryl Migrations.
Yields for methods A and B are NMR yields.
This newly uncovered reactivity made possible some remarkable molecular reorganizations using a cycle of N → C → N or C → N → C aryl shuttling (Scheme 9b), exchanging the arene constitution in vinyl ureas 27g/27d or inverting the configuration of urea 29a.43
In substrates 31, the nucleophilic allylic or benzylic α-carbon is part of a five- or six-membered carbocycle (Scheme 10).44,45 These give α-aryl exocyclic amine derivatives 32 containing electron-rich (32b) and electron-poor (32f) arenes, as well as structures (32d and 32e) closely related to the anesthetic ketamine (4, Figure 1). Similarly, heterocyclic N′-aryl ureas 34 underwent efficient α-arylation when treated with base (Scheme 11a).45,46 High regioselectivity in the formation of 35e–35g arises from the kinetic preference of deprotonation by the bulky base (α-allylic > α′-benzylic > γ-allylic), rather than differing reactivities of equilibrating organolithiums. This is supported by the preservation of enantiopurity in 35f and 35g, confirming that no proton exchange occurred at the existing benzylic α′-stereocenter before migration of the second (green) arene.
Scheme 10. Aryl Migration in Ureas Derived from Exocyclic Amines.
Scheme 11. Aryl Migration in Ureas Derived from Endocyclic Amines.
Rearrangements involving the five- and six-membered cyclic systems 31 and 34 were not stereospecific at the lithiation site: enantioenriched samples of 31c or 34e gave racemic 32c and 35e (Schemes 10 and 11a).45,46 Slower rearrangement when the carbon nucleophile is within such a ring as a consequence of the more strained bicyclic transition state appears to allow racemization of the organolithium to outcompete C–C bond formation. By contrast, enantiospecificity of the arylation is restored on moving to seven-membered ring systems 37 (Scheme 11b), which give α,α-diaryl azepanes 38 in enantiopure form.47
With the migrating N′-aryl electrophile embedded in a heterocyclic system, migration leads to a three-atom ring expansion, giving medium rings 41 (Scheme 12).48 Urea derivatives 40 of a variety of common N-heterocycles (e.g., indoline, tetrahydroquinoline, benzomorpholine, benzoazepine) provided starting materials for a practical synthesis of 8- to 12-membered heterocycles. The established attributes of the T-S rearrangements described previously are exhibited by this ring expanding variant: unactivated arenes function as migrating groups, the carbon nucleophile may be cyclic (41b) or acyclic, and the reaction is both highly diastereoselective (41e, 41f) and enantiospecific (41h, 41i).
Scheme 12. Ring-Expanding Aryl Migration.
In an interesting synthetic application (not shown), eight- to ten-membered heterocycles 41 underwent an acid-promoted SN1-like rearrangement at the benzylic carbon, in which the proximal urea NMe nitrogen was displaced by the distal urea NH.49 Overall, a three-atom ring expansion (Scheme 12) and ensuing two-atom ring contraction constitute the formal insertion of a benzylic carbon into the Caryl–N bond of a nitrogen heterocycle.
2.3. Enolates and Metalated Nitriles: Synthesis of Hydantoins and Quaternary Amino Acids
The success of intramolecular SNAr processes with carbanions as nucleophiles encouraged us to investigate rearrangements of other, less basic carbon nucleophiles such as enolates. For example, treating amino acid-derived ureas 42 with LDA and LiCl likewise led to Cα-arylation under mild conditions (Scheme 13a).50In situ IR reaction monitoring showed lithium carboxylate 42-Li, dianionic enolate 42-2Li, and dianion 43 as the sole reaction intermediates; spontaneous cyclization of 43 to 44 occurs upon quenching with MeOH. Cleavage of the PMB group in 44f facilitated alkaline hydrolysis to the corresponding α-quaternary amino acid.50
Scheme 13. Aryl Migration in Ureas Derived from Amino Acids and Nitriles.
Hydantoins 44 also form from amino nitrile-derived ureas 45 (Scheme 13b).50 The iminohydantoin products of the rearrangement 46 hydrolyzed to 44 with acid. Related nitrile-stabilized carbanions 47– allowed ring expansion of heterocycles 47 to iminohydantoin-bridged eight- to ten-membered N-heterocycles 49 (Scheme 14).51 When X was (or was part of) a pronucleophile (e.g., CO or NBoc as in 49b), a second transannular exo-cyclization onto the C=N bridge formed even more complex caged structures.51
Scheme 14. Ring-Expanding Aryl Migration to Give Hydantoin-Bridged Medium Rings.
The synthesis of α-aryl hydantoins by enolate arylation was streamlined into a one-pot sequential α-amination and α-arylation of silyl ketene acetals (Scheme 15).52 AgOTf-catalyzed α-amination of the masked ketene by 50 gives α-amino ester 51, from which potassium hexamethyldisilazide (KHMDS) triggers N-desilylation to an ester enolate to which the aryl group migrates, releasing the urea, which cyclizes onto the ester to give hydantoins 52. The overall transformation in Scheme 15 may be considered a formal (3 + 2)-cycloaddition, where 50 serves as a latent “N––C(=O)–N+” 1,3-dipole.
Scheme 15. Hydantoins by Tandem α-Amination/α-Arylation of Silyl Ketene Acetals.
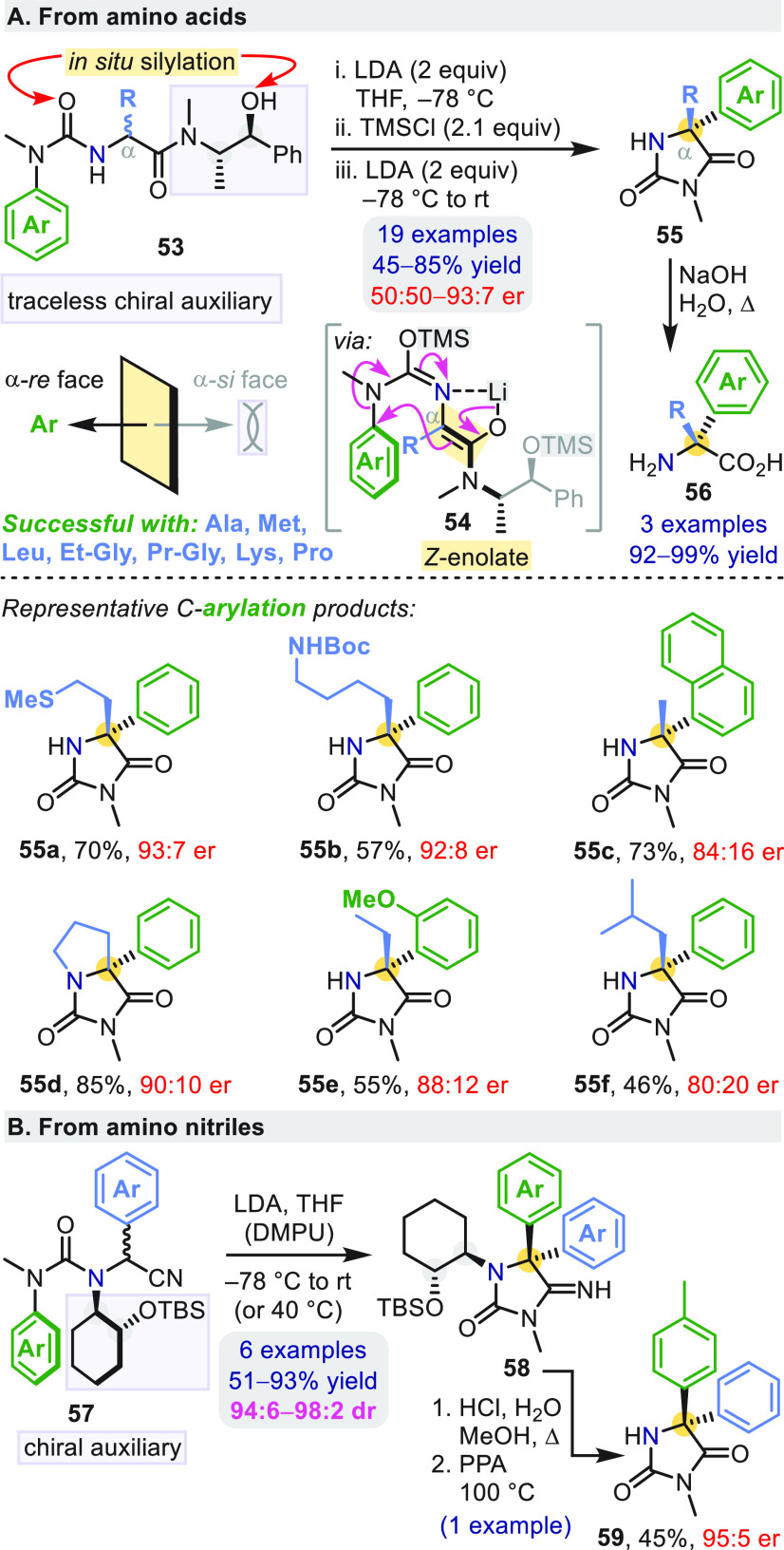
Asymmetric enolate arylation was initially achieved using chiral auxiliaries, among which pseudoephedrine proved most effective (Scheme 16a).53 Trisubstituted urea 53 was silylated in situ (steps i and ii) prior to generation of enolate 54 (step iii). Arylation was followed by spontaneous cyclization of the anionic urea, expelling the recyclable pseudoephedrine auxiliary and providing enantioenriched quaternary hydantoins 55.
Scheme 16. Chiral Auxiliary-Directed Arylation of Amino Acids and Nitriles.
Enolates of phenylglycine-derived substrates were too unreactive under these conditions, so a chiral cyclohexyl auxiliary was instead appended to the nitrogen of an amino nitrile-derived substrate, 57 (Scheme 16b).54 This modified approach enabled the enantioselective synthesis of the previously elusive chiral (imino)phenytoin analogues 58 and 59.
Stereocenters within a urea substrate also direct the facial selectivity of enolate arylation (Scheme 17). Enantioenriched ureas 60 with defined backbone chirality were treated with KHMDS to generate a set of α-aryl proline derivatives 61 and 62 as single diastereomers in most cases.55 Unlike related heterocycles 35f and 35g (Scheme 11), the α-carbon in 61 is fully substituted, so epimerization by further deprotonation is not possible and the diastereoenrichment in 61 must reflect the kinetic selectivity of C-arylation. It is therefore noteworthy that high substrate-controlled diastereoselectivity was observed regardless of whether the directing stereocenter was at the 3-, 4-, or 5-position of the heterocycle backbone (61a–61d).
Scheme 17. Diastereoselective Aryl Migration in Proline-Derived Ureas.
The diastereocontrol displayed by cyclic enolates found its most useful application in an asymmetric arylation of acyclic amino acids based on Seebach’s “self-regeneration of stereocenters” concept (Scheme 18a).1,56 Starting from amino acid derivatives 63, either the anti or syn epimer of the required imidazolidinone could be formed, ultimately enabling both α-arylated enantiomers of 56 to be prepared from l-amino acids. α-Deprotonation of urea anti-65, formed in situ, or syn-65 transiently erases the α-stereocenter to give a pair of enantiomeric enolates ent-65– (shown) and 65– that undergo diastereoselective arylation anti to the t-Bu directing group. The configuration of the newly arylated α-stereocenter is thus inverted in ent-66 and retained in 66.
Scheme 18. Asymmetric Arylation of Amino Acids by Self Regeneration of Stereocenters.

One exception was a valine-derived substrate, which gave 91:9 dr using Et2NLi (stereoretentive route).
An exception was phenylglycine-derived substrates via the stereoretentive route, which gave 81:19–87:13 er values due to partial racemization during conversion of 67 to syn-65.
The broad utility of this Cα-arylation (Scheme 18a) was demonstrated by 58 examples of the synthesis of 66 and ent-66 from a pool of eight different amino acid precursors (listed) and 16 different migrating (hetero)arenes exhibiting the full range of electronic characters (Scheme 18b).1 A Hammett kinetic analysis of the conversion of syn-65 to 66, followed by in situ IR spectroscopy, revealed that enolate formation was rate-determining for electron-poor arenes, while enolate arylation was rate-determining for electron-rich arenes. In the latter domain, a ρ value of +4.5 was obtained, consistent with a partially concerted SNAr mechanism.31−34 Enantiopure α-aryl quaternary amino acids ent-56 were formed by a straightforward sequence of N-methylation (required to avoid hydantoin formation) and acidic hydrolysis (Scheme 18a).
A different “chiral memory” approach to the asymmetric α-arylation of a group of amino acids with bulky side chains was reported by Kawabata using urea-substituted axially chiral enolates (Scheme 19a).57 Deprotonation of amino esters 68 at −60 °C resulted in a stereoinvertive α-arylation to give hydantoins 69. Mechanistically, selective deprotonation of 68″ (over diastereomeric conformer 68′) was proposed as a result of its antiperiplanar Cα–H and urea N–CO bonds (Scheme 19b), forming an enantioenriched Z-enolate 68-M that must attack the aryl ring from its α-Si face due to restricted rotation about the Cα–N bond. Complete enantiospecificity was observed for electron-poor arenes (69b, 69c) but slower arylation rates with less activated rings (69a) compromised the enantiopurity (Scheme 19a). Nonetheless, the fact that enolate 68-M was arylated faster than racemization in certain cases is remarkable and reinforces the powerful conformationally activating effect of the urea tether.
Scheme 19. Arylation of Amino Acids via Memory of Chirality.
3. N → C Aryl Migration in Carbamates: Synthesis of Tertiary Alcohols
Like their urea congeners, N-aryl-N-alkyl carbamates exhibit a strong preference for a conformation in which the N-aryl and carbonyl groups lie trans. As a consequence, α-lithiation of N-aryl carbamates 70 likewise triggers a N → C transfer of a variety of aryl groups to give rearranged products 71 (Scheme 20a).58 The corresponding α,α-diaryl alcohols 72 may then be returned by hydrolysis with NaOH.
Scheme 20. Aryl Migration in O-Benzylcarbamates.
The rearrangement of enantioenriched carbamates 70 (R = Me) likewise gave enantioenriched arylation products (S)-71c–71e (Scheme 20a);58−60 but only moderate enantiospecificity (50–83% es) arose, due to racemization of the organolithium on the time scale of the arylation. Nonetheless, (S)-71c (84:16 er) provided a key intermediate in the first enantioselective synthesis of the antihistamine clemastine (Scheme 20b).59
A distinctive feature of the lithiated carbamates was the stereochemical course of their C-arylation: (S)-71c–71e were formed with inversion of configuration (Scheme 20a),58−60 in contrast to the stereochemical retention of related ureas (Schemes 4–7). DFT calculations of the T-S rearrangement of carbamate 70 (R = Me, both Ar = Ph) illuminated the origin of this effect. Scheme 21 shows two possible trajectories identified from 70-Li: an energetically favorable “inversion” pathway, and a higher energy “retention” pathway58,60 analogous to that taken by ureas (Scheme 5).39 These pathways (Scheme 21) differ primarily in the direction that the lithium cation takes during charge separation from the carbanion and importantly they lead to opposite enantiomers because they start from different conformers of the benzyllithium (70-Li′ or 70-Li″). The lowest energy pathway proceeds from 70-Li″ to 70–(b) where the lithium cation migrates to the α oxygen’s available lone pair. The opposite face of the carbanion is now available to attack the arene through the low barrier TS-inv-O (ΔG⧧ = 16.7 kJ mol–1), giving 71 with net configurational inversion. The (disfavored) retentive pathway for the carbamates is higher in energy than the analogous pathway calculated for ureas by ∼40 kJ mol–1,39 showing that carbamates give stereochemical inversion both by disfavoring the retention pathway and by opening up an inversion pathway unavailable to ureas, which lack a electron pair orthogonal to the C=O π bond.
Scheme 21. Stereoinvertive Aryl Migration in Lithiated Carbamates.
O-Cinnamyl-, O-propargyl- and O-vinylcarbamates (74, 76, and 78) all underwent T-S rearrangements when treated with lithium diisopropylamide (LDA) (Scheme 22).60 In the case of propargyl substrates 76 bearing a terminal phenyl group (R2 = Ph), the aryl migration was followed by spontaneous 5-exo-dig cyclization of the urea nucleofuge to give oxazolidinones 77b and 77c.
Scheme 22. Aryl Migration in O-Cinnamyl-, O-Propargyl-, and O-Vinylcarbamates.
4. N → C Aryl Migration in S-Thiocarbamates: Synthesis of Tertiary Thiols
Chiral, nonracemic tertiary thiols are challenging synthetic targets, but conformationally activated T-S rearrangements also provide an enantioselective entry to this compound class. As summarized in Scheme 23, the addition of lithium tetramethylpiperidide (LiTMP) to enantioenriched S-benzylic thiocarbamates 80 resulted in N → C migration of electronically diverse arenes to give tertiary thiol derivatives 81 with generally high stereospecificity (≥87% es) and ≥91:9 er.61 Only when the nucleophilicity of the intermediate benzyllithium was attenuated (blue Ar = 3-CF3C6H4) was the enantiopurity of 81 compromised by significant racemization. The corresponding enantioenriched α,α-diaryl thiols 82 were obtained simply by stirring 81 with NaOH in EtOH at room temperature for 15 min.
Scheme 23. Enantiospecific Aryl Migration in α-Alkylbenzylthiocarbamates.
As with benzylic ureas, the configuration of the stereocenter in S-thiocarbamates 80 was retained upon arylation (Scheme 23).61 Nonetheless, a unique mechanistic trajectory for the T-S rearrangement of 80 (R = Me, both Ar = Ph) was identified by DFT calculations (Scheme 24).62 Previous experimental and theoretical investigations of the T-S rearrangements of both ureas and carbamates showed that population of the reactive organolithium conformation for SNAr requires coordination of exogenous lithium base to drive X–CO bond rotation (Schemes 5 and 21).39,60 Evidently, this is not the case for S-thiocarbamates 80 (Scheme 24); instead, the stabilizing effect of sulfur on the α carbanion allows C–Li bond cleavage to occur directly from the intramolecularly complexed 80-Li, accompanied by a 1,4-shift of the lithium cation to the carbonyl oxygen. The consequence is that S–CO bond rotation in 80– on route to the reactive conformer (80–)′, which lacks a C–Li bond, is now energetically favorable. Meanwhile, partial π-character to the C––S bond in the planar carbanion 80– provides a form of chiral memory, preventing C––S bond rotation and stereochemical scrambling. As such, the same face of the carbanion (80–)′ is presented for attack on the arene as originally occupied by lithium, resulting in retention of configuration via TS-ret-S (ΔG⧧ = 48.3 kJ mol–1).62
Scheme 24. Stereoretentive Aryl Migration in Lithiated Thiocarbamates.
Tertiary allylic thiols were synthesized by way of enantioenriched thiocarbamates 83 (Scheme 25a), formed by enantioselective (R2 = H)63 and enantiospecific (R2 = Cy)64 [3,3]-sigmatropic rearrangements of O-allylic thiocarbamates. Intramolecular C(sp3)-arylation gave thiol derivatives 84 with retention of configuration.63,64 The synthetic utility of the thiols 85 (Scheme 25a) was demonstrated by ring-closing metathesis of 86, giving enantioenriched dihydrothiophenes 87 bearing an α-quaternary stereocenter (Scheme 25b).64
Scheme 25. Enantiospecific Aryl Migration in Allylthiocarbamates.
5. Connective Routes to α-Tertiary Amines, Alcohols, and Thiols
The intramolecular SNAr reactions of N-aryl ureas and (thio)carbamates described above all involve direct deprotonation to provide the required carbon nucleophile for the N → C aryl migration. An alternative connecting approach forms the α-carbanion by umpolung β-addition of a carbon nucleophile to α,β-unsaturated substrates, allowing an additional C–C bond to be formed in tandem with the T-S rearrangement.
With N-alkenyl ureas as starting materials (Scheme 26a), we found that an α-aryl substituent was needed to promote clean carbolithiation. Enamine derivatives 88 and an organolithium reagent gave the products 89 of successive “umpolung” β-addition and α-arylation.65 The process was completely regioselective and could be triggered by arylation, vinylation, or alkylation at the β-position (89a–89c). Reactions of geometrically defined substrates (R2 = Me) were diastereoselective: β-branched products (89d and 89e) were formed with >20:1 dr from E-88, while exchanging E-88 for the corresponding Z-isomer provided both diastereomers of a given product (89d and epi-89d) with equally high levels of stereoenrichment; the fact that β-methyl, Z-vinyl ureas followed the carbolithiation/rearrangement pathway was notable in itself because of their known susceptibility to γ-deprotonation (Scheme 8).42 Cyclic substrates likewise reacted with complete stereospecificity (89g, 89h).46
Scheme 26. Carboarylation of N-Vinyl Ureas.
The relative stereochemistry of products arising from β-substituted substrates 88 (linear and cyclic) was consistent with syn carbolithiation followed by stereoretentive α-arylation (Scheme 26b).46,65 Free amines 90 were revealed by solvolysis in refluxing n-BuOH (Scheme 26a).46,65
Analogous β-alkylation/α-arylation was also possible with vinyl carbamates 92, where an N-isopropyl group was needed to enforce chemoselective carbolithiation of the “enolate” alkene over direct attack at C=O (Scheme 27).66 The rearrangement to 93 (X = Li) was followed by in situ (CO)–O bond cleavage, either by reaction with the excess organolithium or, if required, by converting the remaining lithiated carbamate to the base-labile nitroso derivative (X = NO) before workup. This procedure conveniently afforded a range of tertiary alcohols 94.
Scheme 27. Carboarylation of O-Vinylcarbamates.
Using toluene as solvent and TMEDA as an additive.
This “umpolung” connective approach was further applied to S-vinyl thiocarbamates 95 (Scheme 28) to give a set of hindered tertiary thiols 97 carrying branched carbon chains:67 complete stereospecificity was observed in most cases.
Scheme 28. Carboarylation of S-Vinyl Thiocarbamates.
Starting material had an N-Et substituent.
An asymmetric variant of the carboarylation of vinyl ureas was developed using (−)-sparteine as a ligand (Scheme 29a).68 The enhanced reactivity of the (−)-sparteine-complexed organolithium made possible a facially selective carbolithiation of 98, producing an enantioenriched benzyllithium that, upon addition of DMPU, underwent enantiospecific T-S rearrangement2 to deliver 99. The key to good enantioselectivity was the use of the noncoordinating solvent cumene, which allowed complete carbolithiation within 1 h at −50 °C and enhanced the configurational stability of the resultant organolithium. Products of opposite absolute configuration were obtained by exchanging the position of the aryl groups in 98 or, in some cases, by using the (+)-sparteine surrogate 100 as the chiral ligand in THF (Scheme 29b).68O-Vinyl carbamates 92 and S-vinyl thiocarbamates 95 gave enantioenriched products using (−)-sparteine or 100 only with modest enantioselectivity.66,69
Scheme 29. Enantioselective Carboarylation of N-Vinyl Ureas.
Configuration of 99 is corrected from original paper.68b
Carbolithiation has limited compatibility with reactive functional groups, but vinyl ureas 98 underwent more versatile photoredox-based carboarylation by way of the addition of carbon-centered radicals (Scheme 30a).70 A readily available organic dye (4CzIPn) was used to initiate a radical-polar crossover process, providing products 102 of tandem β-fluoroalkylation and α-arylation. The photoredox cycle involved oxidation of a sulfinate anion to release electrophilic fluoroalkyl radicals that underwent a polarity-matched β addition to 98. Reduction of the resulting benzylic radical 101• by [4CzIPn]•– closes the photoredox cycle and generates α-anion 101–, which traps a variety of N′-aryl groups.
Scheme 30. Photocatalytic Carboarylation of N-Vinyl Ureas.
The standard potential of redox pair 101•/101– was challenging to measure directly, but compelling evidence of 101– as a viable intermediate was obtained by submitting modified N′,N′-dialkyl urea 104 to the standard conditions with D2O added as an anion quencher (Scheme 30b); significant α-deuteration (76–83%) of addition product 105 was observed in three different solvents with higher pKa values and lower (C–H) bond dissociation energies than D2O.70 Furthermore, repeating the standard reaction of 98b (to form 102b) but with added D2O predominantly returned the carbodeuteration product (not shown), ruling out the possibility of a radical-based T-S rearrangement.71 Vinyl urea 98g (Scheme 30c) also accepts P-centered radicals to give arylphosphonylation products 106 without any modifications to the standard conditions,70 suggesting that this photoredox approach holds wider promise for the construction of α,β-functionalized amines.
6. Conclusion and Outlook
The inherent conformational bias in acylated N-alkyl anilide congeners enforces spatial proximity between an N-aryl group and tethered carbanions and leads to remarkably versatile intramolecular SNAr reactions. These stereocontrolled C-arylations transfer (hetero)aryl groups of diverse electronic and steric nature to an sp3 carbon without the use of transition metals. Our strategy not only enables unprecedented SNAr reactivity for a wide range of nonstabilized and stabilized carbon nucleophiles but opens up access to rare or previously elusive compound classes such as tertiary thiols, α-aryl azepanes, and α-aryl quaternary amino acids.
Looking ahead, with conformational preorganization firmly established as a means of accelerating simple electrophilic arylation, we are seeking to apply the approach to the asymmetric arylation of enantiotopic secondary C–H bonds, and the catalytic, enantioselective arylation of stabilized carbanions. Amide tethers as conformational controllers72,73 also hold promise for the assembly of benzylic stereocenters.74,75 In addition, we are using the conformational preference of anilide congeners to develop other types of intramolecular transition metal-free couplings, including C-alkenylations.76−79
Our work demonstrates that the design of substrates where conformational bias predisposes intramolecular reactivity allows chemists to break free of the limitations of “classical” reactivity. Molecular conformation, whether by opportunity or design, is sure to continue to play a central role in the discovery of new ways to construct challenging C–C and C–X bonds.
Acknowledgments
The corresponding author is indebted to the many former co-workers who carried out the research described in this Account, along with industrial collaborators and funding agencies, especially the ERC and EPSRC, who have supported the work financially.
Biographies
Steven M. Wales obtained his Ph.D. in 2010 from the University of Wollongong under Prof. Paul Keller. He was an American Australian Association Fellow with Prof. Jeffrey Johnson at the University of North Carolina before continuing postdoctoral studies with Prof. Christopher Moody and Prof. Hon Lam at the University of Nottingham. In 2019, he joined the laboratory of Prof. Jonathan Clayden, investigating new molecular communication mechanisms based on conformational control.
Rakesh K. Saunthwal received his Ph.D. in 2017 with Prof. Akhilesh Verma from the University of Delhi, before taking up a SERB Overseas Postdoctoral Fellowship with Prof. Jonathan Clayden. Since 2019, he has continued his research in the Clayden laboratory as a Marie Curie Postdoctoral Fellow, investigating asymmetric Truce–Smiles and photochemical rearrangements.
Jonathan Clayden completed a Ph.D. in 1992 at the University of Cambridge with Dr. Stuart Warren. After postdoctoral work with Prof. Marc Julia at the École Normale Supérieure in Paris, he began his independent research career in 1994 at the University of Manchester, before moving to Bristol in 2015. His research interests lie broadly in the areas of dynamic molecular shape and function.
Author Contributions
§ S.M.W. and R.K.S. contributed equally. The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
References
- Leonard D. J.; Ward J. W.; Clayden J. Asymmetric α-Arylation of Amino Acids. Nature 2018, 562, 105–109. 10.1038/s41586-018-0553-9. [DOI] [PubMed] [Google Scholar]
- Clayden J.; Dufour J.; Grainger D. M.; Helliwell M. Substituted Diarylmethylamines by Stereospecific Intramolecular Electrophilic Arylation of Lithiated Ureas. J. Am. Chem. Soc. 2007, 129, 7488–7489. 10.1021/ja071523a. [DOI] [PubMed] [Google Scholar]
- Bonet A.; Odachowski M.; Leonori D.; Essafi S.; Aggarwal V. K. Enantiospecific sp2-sp3 Coupling of Secondary and Tertiary Boronic Esters. Nat. Chem. 2014, 6, 584–589. 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Han S. J.; Liu W. B.; Stoltz B. M. Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res. 2015, 48, 740–751. 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. H.; Zhang F. G.; Meng X. G.; Duan S. W.; Xiao W. J. Enantioselective Michael Reactions of β,β-Disubstituted Nitroalkenes: A New Approach to β2,2-Amino Acids with Hetero-Quaternary Stereocenters. Org. Lett. 2009, 11, 3946–3949. 10.1021/ol901572x. [DOI] [PubMed] [Google Scholar]
- Bartlett S. L.; Keiter K. M.; Johnson J. S. Synthesis of Complex Tertiary Glycolates by Enantioconvergent Arylation of Stereochemically Labile α-Keto Esters. J. Am. Chem. Soc. 2017, 139, 3911–3916. 10.1021/jacs.7b00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Cobb K. M.; Tan T.; Watson M. P. Stereospecific Cross Couplings to Set Benzylic, All-Carbon Quaternary Stereocenters in High Enantiopurity. J. Am. Chem. Soc. 2016, 138, 12057–12060. 10.1021/jacs.6b08075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillion E.; Wilsily A. Asymmetric Synthesis of All-Carbon Benzylic Quaternary Stereocenters via Cu-Catalyzed Conjugate Addition of Dialkylzinc Reagents to 5-(1-Arylalkylidene) Meldrum’s Acids. J. Am. Chem. Soc. 2006, 128, 2774–2775. 10.1021/ja056692e. [DOI] [PubMed] [Google Scholar]
- Stymiest J. L.; Bagutski V.; French R. M.; Aggarwal V. K. Enantiodivergent Conversion of Chiral Secondary Alcohols into Tertiary Alcohols. Nature 2008, 456, 778–783. 10.1038/nature07592. [DOI] [PubMed] [Google Scholar]
- Sha W.; Deng L.; Ni S.; Mei H.; Han J.; Pan Y. Merging Photoredox and Copper Catalysis: Enantioselective Radical Cyanoalkylation of Styrenes. ACS Catal. 2018, 8, 7489–7494. 10.1021/acscatal.8b01863. [DOI] [Google Scholar]
- Robak M. T.; Herbage M. A.; Ellman J. A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- Mei T. S.; Werner E. W.; Burckle A. J.; Sigman M. S. Enantioselective Redox-Relay Oxidative Heck Arylations of Acyclic Alkenyl Alcohols Using Boronic Acids. J. Am. Chem. Soc. 2013, 135, 6830–6833. 10.1021/ja402916z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y. J.; Hu X. S.; Zhou Y.; Zhou J.; Yu J. S. Catalytic Enantioselective α-Arylation of Carbonyl Enolates and Related Compounds. ACS Catal. 2020, 10, 955–993. 10.1021/acscatal.9b04480. [DOI] [Google Scholar]
- Feng J.; Holmes M.; Krische M. J. Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev. 2017, 117, 12564–12580. 10.1021/acs.chemrev.7b00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; Mao E.; Millet A.; Oakley J. v.; Reed N. L.; Sakai H. A.; Seath C. P.; MacMillan D. W. C. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. 10.1021/acs.chemrev.1c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana R.; Pathak T. P.; Sigman M. S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-Organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. R. P.; Kosowan J. R.; Wood T. E. The Truce-Smiles Rearrangement and Related Reactions: A Review. Can. J. Chem. 2017, 95, 483–504. 10.1139/cjc-2016-0594. [DOI] [Google Scholar]
- Snape T. J. A Truce on the Smiles Rearrangement: Revisiting an Old Reaction-the Truce-Smiles Rearrangement. Chem. Soc. Rev. 2008, 37, 2452–2458. 10.1039/b808960d. [DOI] [PubMed] [Google Scholar]
- Holden C. M.; Greaney M. F. Modern Aspects of the Smiles Rearrangement. Chem.—Eur. J. 2017, 23, 8992–9008. 10.1002/chem.201700353. [DOI] [PubMed] [Google Scholar]
- Bella M.; Kobbelgaard S.; Jørgensen K. A. Organocatalytic Regio- and Asymmetric C-Selective SNAr Reactions-Stereoselective Synthesis of Optically Active Spiro-Pyrrolidone-3,3′-Oxoindoles. J. Am. Chem. Soc. 2005, 127, 3670–3671. 10.1021/ja050200g. [DOI] [PubMed] [Google Scholar]
- Dey C.; Katayev D.; Ylijoki K. E. O.; Kündig E. P. Aza-Oxindole Synthesis via Base Promoted Truce-Smiles Rearrangement. Chem. Commun. 2012, 48, 10957–10959. 10.1039/c2cc36068c. [DOI] [PubMed] [Google Scholar]
- Shirakawa S.; Koga K.; Tokuda T.; Yamamoto K.; Maruoka K. Catalytic Asymmetric Synthesis of 3,3′-Diaryloxindoles as Triarylmethanes with a Chiral All-Carbon Quaternary Center: Phase-Transfer- Catalyzed SNAr Reaction. Angew. Chem., Int. Ed. 2014, 53, 6220–6223. 10.1002/anie.201403046. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Zhang X.; Ma Y.; Ma C. An Efficient C-C Bond Formation Reaction for the Synthesis of α-Arylated Ketones Under Mild Conditions. Tetrahedron Lett. 2013, 54, 402–405. 10.1016/j.tetlet.2012.11.024. [DOI] [Google Scholar]
- Shirakawa S.; Yamamoto K.; Tokuda T.; Maruoka K. Phase-Transfer-Catalyzed Asymmetric α-Arylation of α-Amino Acid Derivatives. Asian J. Org. Chem. 2014, 3, 433–436. 10.1002/ajoc.201400004. [DOI] [Google Scholar]
- Kondoh A.; Aoki T.; Terada M. Organocatalytic Arylation of α-Ketoesters Based on Umpolung Strategy: Phosphazene-Catalyzed SNAr Reaction Utilizing [1,2]-Phospha-Brook Rearrangement. Chem.—Eur. J. 2018, 24, 13110–13113. 10.1002/chem.201803218. [DOI] [PubMed] [Google Scholar]
- Ameen D.; Snape T. J. Developing the Scope of O→C Aryl Migrations: Exploring Amide Substrates as Potential Precursors for Asymmetric Reactions. Eur. J. Org. Chem. 2014, 2014, 1925–1934. 10.1002/ejoc.201301716. [DOI] [Google Scholar]
- Yamaguchi K.; Matsumura G.; Kagechika H.; Azumaya I.; Ito Y.; Itai A.; Shudo K. Aromatic Architecture. Use of the N-Methylamide Structure as a Molecular Splint. J. Am. Chem. Soc. 1991, 113, 5474–5475. 10.1021/ja00014a060. [DOI] [Google Scholar]
- Itai A.; Toriumi Y.; Saito S.; Kagechika H.; Shudo K. Preference of Cis-Amide Structure in N-Acyl-N-Methylanilines. J. Am. Chem. Soc. 1992, 114, 10649–10650. 10.1021/ja00052a078. [DOI] [Google Scholar]
- Clayden J.; Lemiègre L.; Pickworth M.; Jones L. Conformation and Stereodynamics of 2,2′-Disubstituted N, N′-Diaryl Ureas. Org. Biomol. Chem. 2008, 6, 2908–2913. 10.1039/b802673d. [DOI] [PubMed] [Google Scholar]
- We may draw some analogy here to the well appreciated Thorpe–Ingold effect on accelerating cyclization reactions, where backbone substitution causes ground state destabilization of unreactive anti conformers, resulting in an increase in the population of reactive gauche conformers in which the nucleophile and electrophile are close in space, effectively lowering the activation energy to cyclization; see:; Jung M. E. Substituent and Solvent Effects in Intramolecular Diels-Alder Reactions. Synlett 1990, 1990, 186–190. 10.1055/s-1990-21028. [DOI] [Google Scholar]
- Lennox A. J. J. Meisenheimer Complexes in SNAr Reactions: Intermediates or Transition States?. Angew. Chem., Int. Ed. 2018, 57, 14686–14688. 10.1002/anie.201809606. [DOI] [PubMed] [Google Scholar]
- Rohrbach S.; Smith A. J.; Pang J. H.; Poole D. L.; Tuttle T.; Chiba S.; Murphy J. A. Concerted Nucleophilic Aromatic Substitution Reactions. Angew. Chem., Int. Ed. 2019, 58, 16368–16388. 10.1002/anie.201902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann C. N.; Hooker J. M.; Ritter T. Concerted Nucleophilic Aromatic Substitution with 19F–- and 18F–-. Nature 2016, 534, 369–373. 10.1038/nature17667. [DOI] [PubMed] [Google Scholar]
- Kwan E. E.; Zeng Y.; Besser H. A.; Jacobsen E. N. Concerted Nucleophilic Aromatic Substitutions. Nat. Chem. 2018, 10, 917–923. 10.1038/s41557-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J.; Lemiègre L.; Helliwell M. Synthesis and Stacked Conformations of Symmetrical and Unsymmetrical Oligo-Ureas of Metaphenylenediamine. J. Org. Chem. 2007, 72, 2302–2308. 10.1021/jo061989w. [DOI] [PubMed] [Google Scholar]
- Clayden J.; Turner H.; Pickworth M.; Adler T. Ring-Selective Functionalization of N, N′-Diarylureas by Regioselective N-Alkylation and Directed Ortho Metalation. Org. Lett. 2005, 7, 3147–3150. 10.1021/ol0508025. [DOI] [PubMed] [Google Scholar]
- Clayden J.; Dufour J. Lateral Lithiation of N, N′-Diaryl Ureas. Tetrahedron Lett. 2006, 47, 6945–6946. 10.1016/j.tetlet.2006.07.134. [DOI] [Google Scholar]
- For a related example of a T-S rearrangement of a trisubstituted urea, see:; Dannecker W.; Fariborz M. Rearrangements of Metallated Organosubstituted Ureas, II. Formation of N,α-Diorganylbenzylamines from Dimetallated N-Benzyl-N,N′-Diorganylureas. Z. Naturforsch 1974, 29b, 578–580. 10.1515/znb-1974-7-832. [DOI] [Google Scholar]
- Grainger D. M.; Campbell Smith A.; Vincent M. A.; Hillier I. H.; Wheatley A. E. H.; Clayden J. The Mechanism of the Stereospecific Intramolecular Arylation of Lithiated Ureas: The Role of Li+ Probed by Electronic Structure Calculations, and by NMR and IR Spectroscopy. Eur. J. Org. Chem. 2012, 731–743. 10.1002/ejoc.201101475. [DOI] [Google Scholar]
- Clayden J.; Hennecke U. α-Pyridylation of Chiral Amines via Urea Coupling, Lithiation and Rearrangement. Org. Lett. 2008, 10, 3567–3570. 10.1021/ol801332n. [DOI] [PubMed] [Google Scholar]
- Maury J.; Zawodny W.; Clayden J. Stereospecific Intramolecular Arylation of 2- and 3-Pyridyl Substituted Alkylamines via Configurationally Stable α-Pyridyl Organolithiums. Org. Lett. 2017, 19, 472–475. 10.1021/acs.orglett.6b03603. [DOI] [PubMed] [Google Scholar]
- Tetlow D. J.; Hennecke U.; Raftery J.; Waring M. J.; Clarke D. S.; Clayden J. Sequential Double α-Arylation of N-Allylureas by Asymmetric Deprotonation and N→C Aryl Migration. Org. Lett. 2010, 12, 5442–5445. 10.1021/ol102155h. [DOI] [PubMed] [Google Scholar]
- Tetlow D. J.; Vincent M. A.; Hillier I. H.; Clayden J. Reversible Aryl Migrations in Metallated Ureas: Controlled Inversion of Configuration at a Quaternary Carbon Atom. Chem. Commun. 2013, 49, 1548–1550. 10.1039/c2cc38704b. [DOI] [PubMed] [Google Scholar]
- Tait M. B.; Ottersbach P. A.; Tetlow D. J.; Clayden J. Synthesis of 1-Arylcycloalkenamines by Intramolecular Arylation of Lithiated Ureas. Org. Process Res. Dev. 2014, 18, 1245–1252. 10.1021/op500173q. [DOI] [Google Scholar]
- Bach R.; Clayden J.; Hennecke U. α-Arylation of Cyclic Amines By Aryl Transfer in Lithiated Ureas. Synlett 2009, 421–424. 10.1055/s-0028-1087543. [DOI] [Google Scholar]
- Tait M. B.; Butterworth S.; Clayden J. 2,2- and 2,6-Diarylpiperidines By Aryl Migration Within Lithiated Urea Derivatives of Tetrahydropyridines. Org. Lett. 2015, 17, 1236–1239. 10.1021/acs.orglett.5b00199. [DOI] [PubMed] [Google Scholar]
- Zawodny W.; Montgomery S. L.; Marshall J. R.; Finnigan J. D.; Turner N. J.; Clayden J. Chemoenzymatic Synthesis of Substituted Azepanes by Sequential Biocatalytic Reduction and Organolithium-Mediated Rearrangement. J. Am. Chem. Soc. 2018, 140, 17872–17877. 10.1021/jacs.8b11891. [DOI] [PubMed] [Google Scholar]
- Hall J. E.; Matlock J. v.; Ward J. W.; Gray K. v.; Clayden J. Medium-Ring Nitrogen Heterocycles through Migratory Ring Expansion of Metalated Ureas. Angew. Chem., Int. Ed. 2016, 55, 11153–11157. 10.1002/anie.201605714. [DOI] [PubMed] [Google Scholar]
- Hill J. E.; Matlock J. v.; Lefebvre Q.; Cooper K. G.; Clayden J. Consecutive Ring Expansion and Contraction for the Synthesis of 1-Aryl Tetrahydroisoquinolines and Tetrahydrobenzazepines from Readily Available Heterocyclic Precursors. Angew. Chem., Int. Ed. 2018, 57, 5788–5791. 10.1002/anie.201802188. [DOI] [PubMed] [Google Scholar]
- Atkinson R. C.; Leonard D. J.; Maury J.; Castagnolo D.; Volz N.; Clayden J. Intramolecular Arylation of Amino Acid Enolates. Chem. Commun. 2013, 49, 9734–9736. 10.1039/c3cc46193a. [DOI] [PubMed] [Google Scholar]
- Millward M. J.; Ellis E.; Ward J. W.; Clayden J. Hydantoin-Bridged Medium Ring Scaffolds by Migratory Insertion of Urea-Tethered Nitrile Anions into Aromatic C-N Bonds. Chem. Sci. 2021, 12, 2091–2096. 10.1039/D0SC06188C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunthwal R. K.; Cornall M. T.; Abrams R.; Ward J. W.; Clayden J. Connective Synthesis of 5,5-Disubstituted Hydantoins by Tandem α-Amination and α-Arylation of Silyl Ketene Acetals. Chem. Sci. 2019, 10, 3408–3412. 10.1039/C8SC05263H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson R. C.; Fernández-Nieto F.; Mas Rosellõ J.; Clayden J. Pseudoephedrine-Directed Asymmetric α-Arylation of α-Amino Acid Derivatives. Angew. Chem., Int. Ed. 2015, 54, 8961–8965. 10.1002/anie.201502569. [DOI] [PubMed] [Google Scholar]
- Mas-Roselló J.; Okoh M.; Clayden J. Enantioselectively Functionalised Phenytoin Derivatives by Auxiliary-Directed N to C Aryl Migration in Lithiated α-Amino Nitriles. Chem. Commun. 2018, 54, 10985–10988. 10.1039/C8CC06833J. [DOI] [PubMed] [Google Scholar]
- Maury J.; Clayden J. α-Quaternary Proline Derivatives by Intramolecular Diastereoselective Arylation of N-Carboxamido Proline Ester Enolates. J. Org. Chem. 2015, 80, 10757–10768. 10.1021/acs.joc.5b01912. [DOI] [PubMed] [Google Scholar]
- Seebach D.; Sting A. R.; Hoffmann M. Self-Regeneration of Stereocenters (SRS)—Applications, Limitations, and Abandonment of a Synthetic Principle. Angew. Chem., Int. Ed. 1996, 35, 2708–2748. 10.1002/anie.199627081. [DOI] [Google Scholar]
- Tomohara K.; Yoshimura T.; Hyakutake R.; Yang P.; Kawabata T. Asymmetric α-Arylation of Amino Acid Derivatives by Clayden Rearrangement of Ester Enolates via Memory of Chirality. J. Am. Chem. Soc. 2013, 135, 13294–13297. 10.1021/ja406653n. [DOI] [PubMed] [Google Scholar]
- Clayden J.; Farnaby W.; Grainger D. M.; Hennecke U.; Mancinelli M.; Tetlow D. J.; Hillier I. H.; Vincent M. A. N to C Aryl Migration in Lithiated Carbamates: α-Arylation of Benzylic Alcohols. J. Am. Chem. Soc. 2009, 131, 3410–3411. 10.1021/ja808959e. [DOI] [PubMed] [Google Scholar]
- Fournier A. M.; Brown R. A.; Farnaby W.; Miyatake-Ondozabal H.; Clayden J. Synthesis of (−)-(S, S)-Clemastine by Invertive N → C Aryl Migration in a Lithiated Carbamate. Org. Lett. 2010, 12, 2222–2225. 10.1021/ol100627c. [DOI] [PubMed] [Google Scholar]
- Fournier A. M.; Nichols C. J.; Vincent M. A.; Hillier I. H.; Clayden J. Lithium Choreography: Intramolecular Arylations of Carbamate-Stabilised Carbanions and Their Mechanisms Probed by in Situ IR Spectroscopy and DFT Calculations. Chem.—Eur. J. 2012, 18, 16478–16490. 10.1002/chem.201201761. [DOI] [PubMed] [Google Scholar]
- MacLellan P.; Clayden J. Enantioselective Synthesis of Tertiary Thiols by Intramolecular Arylation of Lithiated Thiocarbamates. Chem. Commun. 2011, 47, 3395–3397. 10.1039/c0cc04912c. [DOI] [PubMed] [Google Scholar]
- Vincent M. A.; Maury J.; Hillier I. H.; Clayden J. Lithium Choreography Determines Contrasting Stereochemical Outcomes of Aryl Migrations in Benzylic Carbamates, Ureas and Thiocarbamates. Eur. J. Org. Chem. 2015, 2015, 953–959. 10.1002/ejoc.201403572. [DOI] [Google Scholar]
- Mingat G.; Maclellan P.; Laars M.; Clayden J. Tertiary Thiols from Allylic Thiocarbamates by Tandem Enantioselective [3,3]-Sigmatropic Rearrangement and Stereospecific Arylation. Org. Lett. 2014, 16, 1252–1255. 10.1021/ol5002522. [DOI] [PubMed] [Google Scholar]
- Mingat G.; McDouall J. J. W.; Clayden J. Dihydrothiophenes Containing Quaternary Stereogenic Centres by Sequential Stereospecific Rearrangements and Ring-Closing Metathesis. Chem. Commun. 2014, 50, 6754–6757. 10.1039/C4CC02596B. [DOI] [PubMed] [Google Scholar]
- Clayden J.; Donnard M.; Lefranc J.; Minassi A.; Tetlow D. J. Tandem β-Alkylation-α-Arylation of Amines by Carbolithiation and Rearrangement of N-Carbamoyl Enamines (Vinyl Ureas). J. Am. Chem. Soc. 2010, 132, 6624–6625. 10.1021/ja1007992. [DOI] [PubMed] [Google Scholar]
- Fournier A. M.; Clayden J. Tertiary Alcohols by Tandem β-Carbolithiation and N→C Aryl Migration in Enol Carbamates. Org. Lett. 2012, 14, 142–145. 10.1021/ol2029355. [DOI] [PubMed] [Google Scholar]
- Castagnolo D.; Foley D. J.; Berber H.; Luisi R.; Clayden J. Carbolithiation of S-Alkenyl-N-Aryl Thiocarbamates: Carbanion Arylation in a Connective Route to Tertiary Thiols. Org. Lett. 2013, 15, 2116–2119. 10.1021/ol400570r. [DOI] [PubMed] [Google Scholar]
- a Tait M.; Donnard M.; Minassi A.; Lefranc J.; Bechi B.; Carbone G.; O’Brien P.; Clayden J. Amines Bearing Tertiary Substituents by Tandem Enantioselective Carbolithiation-Rearrangement of Vinylureas. Org. Lett. 2013, 15, 34–37. 10.1021/ol3029324. [DOI] [PubMed] [Google Scholar]; Correction of absolute stereochemistry of products:; b Tait M.; Donnard M.; Minassi A.; Lefranc J.; Bechi B.; Carbone G.; O’Brien P.; Clayden J. Amines Bearing Tertiary Substituents by Tandem Enantioselective Carbolithiation–Rearrangement of Vinylureas. Org. Lett. 2013, 15, 974–976. 10.1021/ol4002009. [DOI] [PubMed] [Google Scholar]
- Castagnolo D.; Degennaro L.; Luisi R.; Clayden J. Enantioselective Carbolithiation of S-Alkenyl-N-Aryl Thiocarbamates: Kinetic and Thermodynamic Control. Org. Biomol. Chem. 2015, 13, 2330–2340. 10.1039/C4OB02329C. [DOI] [PubMed] [Google Scholar]
- Abrams R.; Clayden J. Photocatalytic Difunctionalization of Vinyl Ureas by Radical Addition Polar Truce-Smiles Rearrangement Cascades. Angew. Chem., Int. Ed. 2020, 59, 11600–11606. 10.1002/anie.202003632. [DOI] [PubMed] [Google Scholar]
- Hervieu C.; Kirillova M. S.; Suárez T.; Müller M.; Merino E.; Nevado C. Asymmetric, Visible Light-Mediated Radical Sulfinyl-Smiles Rearrangement to Access All-Carbon Quaternary Stereocentres. Nat. Chem. 2021, 13, 327–334. 10.1038/s41557-021-00668-4. [DOI] [PubMed] [Google Scholar]
- Costil R.; Dale H. J. A.; Fey N.; Whitcombe G.; Matlock J. v.; Clayden J. Heavily Substituted Atropisomeric Diarylamines by Unactivated Smiles Rearrangement of N-Aryl Anthranilamides. Angew. Chem., Int. Ed. 2017, 56, 12533–12537. 10.1002/anie.201706341. [DOI] [PubMed] [Google Scholar]
- Costil R.; Lefebvre Q.; Clayden J. Medium-Sized-Ring Analogues of Dibenzodiazepines by a Conformationally Induced Smiles Ring Expansion. Angew. Chem., Int. Ed. 2017, 56, 14602–14606. 10.1002/anie.201708991. [DOI] [PubMed] [Google Scholar]
- Abrams R.; Jesani M. H.; Browning A.; Clayden J. Triarylmethanes and Their Medium-Ring Analogues by Unactivated Truce-Smiles Rearrangement of Benzanilides. Angew. Chem., Int. Ed. 2021, 60, 11272–11277. 10.1002/anie.202102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunthwal R. K.; Mortimer J.; Orr-Ewing A. J.; Clayden J. Enantioselective One-Carbon Expansion of Aromatic Rings by Simultaneous Formation and Chromoselective Irradiation of a Transient Coloured Enolate. Chem. Sci. 2022, 13, 2079–2085. 10.1039/D1SC06684F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc J.; Fournier A. M.; Mingat G.; Herbert S.; Marcelli T.; Clayden J. Intramolecular Vinylation of Secondary and Tertiary Organolithiums. J. Am. Chem. Soc. 2012, 134, 7286–7289. 10.1021/ja301591m. [DOI] [PubMed] [Google Scholar]
- Corbet B. P.; Matlock J. V.; Mas-Roselló J.; Clayden J. Intramolecular Vinylation of Carbanions Using N-Acyl Benzomorpholines as Masked Vinylureas and Vinylcarbamates. C. R. Chim. 2017, 20, 634–642. 10.1016/j.crci.2017.01.006. [DOI] [Google Scholar]
- Mas-Roselló J.; Hachisu S.; Clayden J. Geometry-Retentive C-Alkenylation of Lithiated α-Aminonitriles: Quaternary α-Alkenyl Amino Acids and Hydantoins. Angew. Chem., Int. Ed. 2017, 56, 10750–10754. 10.1002/anie.201704908. [DOI] [PubMed] [Google Scholar]
- Abas H.; Mas-Roselló J.; Amer M. M.; Durand D. J.; Groleau R. R.; Fey N.; Clayden J. Asymmetric and Geometry-Selective α-Alkenylation of α-Amino Acids. Angew. Chem., Int. Ed. 2019, 58, 2418–2422. 10.1002/anie.201813984. [DOI] [PubMed] [Google Scholar]