Abstract
Diet-induced obesity has previously been shown to occur with the concomitant rise in the expression of proinflammatory cytokines and increases in collagen deposition. While it has been known that the regenerative process of skeletal muscle is altered in obese mice following an acute muscle injury, we sought to examine differences in the expression of various markers of extracellular matrix remodeling and repair. Our laboratory has previously reported an impaired inflammatory and protein synthetic signaling in these mice that may contribute negatively to the muscle regenerative process. To expand upon this previous investigation, tissues from these animals underwent further analysis to determine the extent of changes to the regenerative response within the extracellular matrix, including transcriptional changes in Collagen I, Collagen III, and Fibronectin. Here, we show that the expression of Collagen III:I is significantly increased at 3-days post-injury in obese injured animals compared to lean injured animals (p = 0.0338), and by 28-days the obese injured animals exhibit a significantly lower Collagen III:I than their lean injured counterparts (p = 0.0035). We demonstrate an impaired response to an acute muscle injury in obese mice when compared with lean counterparts. However, further studies are required to elucidate translational consequences of these changes, as well as to determine any causative mechanisms that may be driving this effect.
Keywords: Diet induced obesity, Extracellular matrix remodeling, Skeletal muscle
Abbreviations: ECM, Extracellular Matrix; MMPs, Matrix Metalloproteinases; TIMPs, Tissue Inhibitors of Metalloproteinases; PBS, Phosphate Buffered Saline; TA, Tibialis Anterior; Ct, Cycle Threshold; MRF, Myogenic Regulatory Factor
Introduction
Obesity is an increasingly prevalent disease in the United States with approximately 33% of Americans being classified as obese.1 This disease is associated with numerous pathophysiological conditions including diabetes mellitus and cardiovascular disease. The condition has also been associated with an increased risk for the development of certain cancers and overall mortality.2 However, obesity is more commonly associated with the substantial metabolic and endocrine disruption that are commonly driven by changes in insulin signaling and chronic inflammation.2 Interestingly, as shown by our laboratory and others, there are some indications that obesity may be associated with dysregulations in the wound healing process of skeletal muscle.3,4
Obesity is commonly associated with the increased expression of proinflammatory cytokines.3, 4, 5, 6, 7 Increased TGF-β expression has been shown to correspond with the reduced cross-sectional area of muscle fibers, diminished force transduction, and an accumulation of Collagen In the extracellular matrix (ECM) of muscle.8 Collagen tends to accumulate in response to skeletal muscle damage, and increased expression of collagen markers are commonly associated with increased ECM remodeling.9 The ECM is essential for skeletal muscle growth, repair, contraction, and force transmission of skeletal muscle.10 It has been shown that the interaction between myoblasts, differentiated muscle fibers, and ECM components is of central importance for the repair of muscle tissue.11 When skeletal muscle is damaged, fiber regeneration is stimulated to occur.12 Two main components of the ECM that play a prominent role in remodeling and repair are fibronectin and collagen types I and III.13 An inflammatory response is stimulated as part of the repair process when the muscle is damaged. With this inflammation, the biosynthesis of Collagen I and III occurs.14 The matrix metalloproteinases (MMPs) are responsible for the degradation of collagens, and in skeletal muscle MMP2 and MMP9 are typically the most abundant.15 In turn, the MMPs are regulated by the aptly named tissue inhibitors of metalloproteinases (TIMPs). Both MMPs and TIMPs have been implicated in the dysregulation of muscle remodeling following acute injury.16 While the MMPs and TIMPs are regulators of ECM degradation, TGF-β acts to modulate the deposition of ECM proteins.17
It is known the extracellular matrix is a key component of the muscle regeneration process. However, little is known about the effect obesity has on ECM remodeling and repair following muscle damage. The purpose of this study was to examine the effects of obesity on the remodeling of the ECM, and to examine various gene expression markers as part of the remodeling process following acute muscle injury. Our laboratory has previously reported the regenerative process of skeletal muscle was altered following an episode of acute muscle damage between lean and obese mice.3 To build on this, we have expanded our initial investigation of the same animals, and hypothesized that the remodeling process of the extracellular matrix would be negatively altered following bupivacaine-induced muscle damage in obese animals.
Methods
Animals and housing
Forty-eight 3-week-old male C57BL/6 mice were purchased from Jackson Laboratories. Animals were housed in the University of Arkansas Central Laboratory Animal Facility. Animals were housed under a 12 h dark, 12 h light cycle, with ad libitum food and water. The overall study consisted of 2 separate animal experiments. Experiment 1 examined gene expression 3 days post-bupivacaine injection. Experiment 2 examined gene expression 28 days post-bupivacaine injection. For experiments 1 and 2, mice were randomly assigned to one of four groups: 1) lean uninjured (n = 5–9); 2) lean injured (n = 5); 3) obese uninjured (n = 4–6); 4) obese injured (n = 6–8). Mice were fed a lean diet (lean; 10% kcCals fat Research Diets, New Brunswick, New Jersey) or a high-fat diet (HFD; 60% kcals fat, Research Diets, New Brunswick, New Jersey) for 12 weeks. Independent data collected from these animals have been previously published.3 All procedures are approved by the University of Arkansas Institutional Animal Care and Use Committee (Protocol 12030).
Bupivacaine injection
At fifteen weeks of age, bupivacaine (Hospira, Lake Forest, IL) injections were performed as previously described.3 Mice were anesthetized with a subcutaneous injection of a cocktail containing ketamine hydrochloride (45 mg/kg body weight), xylazine (3 mg/kg body weight), and acepromazine (1 mg/kg body weight). Muscle damage was induced by injecting 0.03 ml of 0.75% bupivacaine (Marcaine) in the left and right tibialis anterior muscles (TA). A 25-gauge, 5/8 (0.5 × 16 mm) needle was inserted along the longitudinal axis of the muscle, and the bupivacaine was injected slowly as the needle was withdrawn. The control group was injected with 0.03 ml of phosphate-buffered saline (PBS).
Muscle and tibia extraction
Muscle and tibia extractions were performed as previously described.3,18, 19, 20, 21 Three- and 28-days post-injection, the tibialis anterior and tibias were extracted to examine the early and late regenerative response within skeletal muscle. Mice were anesthetized with a subcutaneous injection of a cocktail containing ketamine hydrochloride (90 mg/kg body weight), xylazine (3 mg/kg body weight), and acepromazine (1 mg/kg body weight). The left TA was snap-frozen in liquid nitrogen and stored at −80 °C for gene expression analysis. The right TA was cut at the midbelly, mounted in optimum cutting temperature compound (OCT), and then dropped in liquid nitrogen-cooled isopentane. After the TA was dissected out, the tibia was removed and measured with a plastic caliper (VWR, Radnor, PA, USA). Tibia measurement—a stable indicator of total body size—was used to normalize muscle weights.
RNA isolation, cDNA synthesis, and quantitative RT-PCR
RNA isolation, cDNA synthesis, and Real-Time PCR were completed as previously described.3,18, 19, 20, 21 RNA was extracted with Trizol reagent (Life Technologies, Grand Island, NY, USA). TA muscles were homogenized in Trizol. Total RNA was isolated, DNase treated, and concentration and purity were determined by fluorometry using the Qubit 2.0 (Life Technologies). cDNA was reverse transcribed from 1 μg of total RNA using the Superscript Vilo cDNA synthesis kit (Life Technologies, Carlsbad, CA, USA). Real-time PCR was performed, and results were analyzed using the StepOne Real-Time PCR system (Life Technologies, Applied Biosystems, Grand Island, NY). cDNA was amplified in a 25 μL reaction containing appropriate primer pairs and TaqMan Universal Master Mix (Applied Biosystems). Samples were incubated at 95 °C for 4 min, followed by 40 cycles of denaturation, annealing, and extension at 95 °C, 55 °C, and 72 °C respectively. TaqMan fluorescence was measured at the end of the extension step of each cycle. Fluorescence labeled probes for TGF-β (FAM dye), Collagen-I (FAM dye), Collagen-III (FAM dye), and MMP-2 (FAM dye), MMP-9 (FAM dye), Fibronectin-I (FAM dye), TIMP-1 (FAM-dye), and 18S (VIC dye) was purchased from Applied Biosystems and quantified with TaqMan Universal Master Mix. Cycle threshold (Ct) was determined, and the ΔCt value was calculated as the difference between the Ct value and the 18s Ct value. Final quantification of gene expression was calculated using the ΔΔCT method Ct = [ΔCt(calibrator) – ΔCt(sample)]. Relative quantification was then be calculated as 2−ΔΔCt.
Sirius red stain
Sirius Red Collagen staining was performed as described previously using a 0.1% Sirius Red solution (Spectrum, Gardena, Calif., USA; cat. no. S1066) into 500 mL picric acid (EM Science, Hatfield, Pa.; cat. no. px1001-1) on tissues collected at 28-days.22 Frozen TA muscle was stained in picro-sirius red for 1 h. Slides were washed with acidified water and then dehydrated with 100% ethanol. The sections were then cleared with xylene and mounted. For Sirius red staining, approximately 3-distinct digital images from stained muscle sections (10 μm) from the mid-belly of the TA at a 20x objective magnification were taken and analyzed. The intensity of Sirius red was measured using Nikon Nis-Element BR software. Slides were quantified for percent area stained with Sirius Red per image and averaged within each animal for analysis.
Statistical analysis
All data were analyzed using GraphPad Prism Version 9.1. A two-way ANOVA was performed to analyze the main effects of Injury and Diet and to determine if there are any interactions between the dependent variables at 3-days and 28-days post-bupivacaine injection. When a significant interaction was detected, differences among the individual means were assessed with Fisher's LSD post-hoc analysis. Statistical significance was set at p ≤ 0.05. All figures are presented as the mean ± the standard error of the mean.
Results
Body and muscle wet weight
Representative muscle and body weights for this research design have been published previously.3 We found a main effect of diet to increase body weight in the obese animals when compared to the lean mice at both the 3-day (∼20%) and 28-day (∼43%) time points (p < 0.05).3 In addition, we found a 16% and 36% reduction in the TA weight normalized to body weight in the obese mice at both the 3-day and 28-day time points post-bupivacaine injection, respectively.3 There was no difference in TA muscle mass to body weight ratio in the lean or obese group 3 days post-bupivacaine injection.3
Sirius red staining, collagen, and fibronectin gene expression
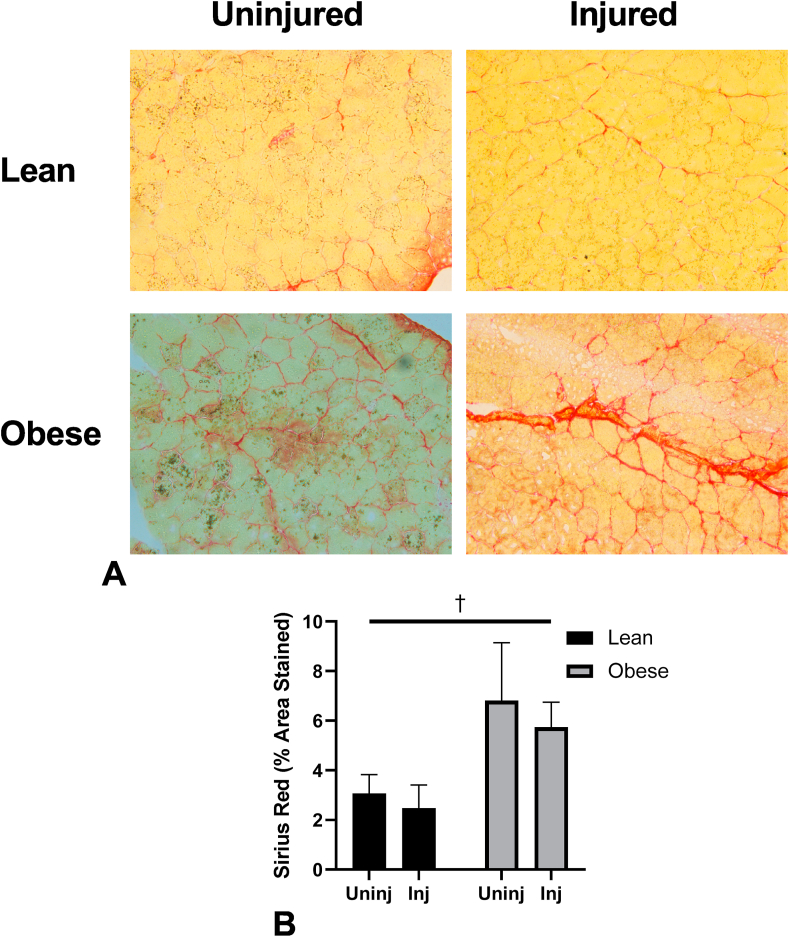
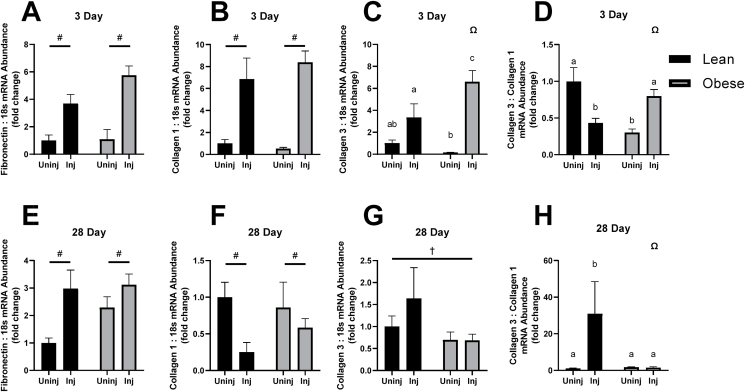
Sirius Red staining was found to have a main effect ofdiet to increase the intensity of Sirius red staining (F (1, 15) = 8.837, p = 0.0095, Fig. 1). There was a main effect of injury to increase Fibronectin expression at both time points (3-day, F (1, 16) = 33.13, p < 0.0001; 28-day, F (1, 19) = 11.06, p = 0.0036; Fig. 2A and E). The same relationship was found with Collagen 1 expression (3-day, F (1, 16) = 33.91, p < 0.0001; 28-day, F (1, 18) = 4.578, p = 0.0463; Fig. 2B and F). However, an interaction effect was found with Collagen III expression at 3-days post-bupivacaine injection (F (1, 16) = 5.064, p = 0.0389; Fig. 2C) with significant differences between Obese Injured and Lean Injured (p = 0.0172), Obese Uninjured and Obese Injured (p = 0.0001), Obese Uninjured and Obese Injured (p = 0.0299), and Lean Uninjured Obese Injured (p = 0.0003; Fig. 2C).
Fig. 1.
(A) Representative images for 28-day Sirius Red staining for collagen. (B) Sirius Red quantitation as percent area stained by group. Significant main effects of diet by †.
Fig. 2.
mRNA abundance of components of the extracellular matrix after 3 and 28 days of recovery following bupivacaine induced acute muscle injury. (A) Fibronectin mRNA abundance 3 days post bupivacaine, (B) Collagen I mRNA abundance 3 days post bupivacaine, (C) Collagen III mRNA abundance 3 days post bupivacaine, (D) Collagen III:I, 3 days post bupivacaine. (E) Fibronectin mRNA abundance 28 days post bupivacaine, (F) Collagen I mRNA abundance 28 days post bupivacaine, (G) Collagen III mRNA abundance 28 days post bupivacaine, (H) Collagen III:I, 28 days post bupivacaine. Significant main effects of injury indicated by # and diet by †. Interaction indicated by Ω.
There was a main effect of diet to decrease Collagen III expression by 28 days (F (1, 17) = 5.008, p = 0.0389). Taking collagen expression as the ratio of Collagen III:I, an interaction effect was found for both the 3-Day (F (1, 16) = 20.30, p = 0.0004) and 28-Day (F (1, 13) = 5.975, p = 0.0295; Fig. 2D and H). Within the 3-Day timepoint, Lean Injured was significantly lower than Lean Uninjured (p = 0.0392), Obese Uninjured was significantly lower than Lean Uninjured (p = 0.0011), Obese Uninjured was significantly lower than Obese Injured (p = 0.001), and Obese Injured was significantly higher than Lean Injured (p = 0.0338, Fig. 1D). Within the 28-Day timepoint, Lean Injured was significantly higher than Lean Uninjured (p = 0.0060), Obese Uninjured (p = 0.0104), and Obese Injured (p = 0.0035, Fig. 2H).
MMP2, MMP9, TIMP1, TGF-β gene expression
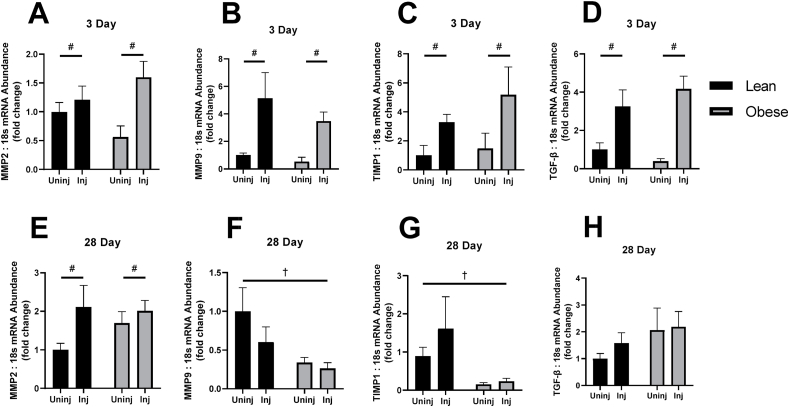
A main effect of Injury was found at the 3-Day timepoint to increase MMP2 (F (1, 16) = 6.892, p = 0.0184, Fig. 3A), MMP9 (F (1, 16) = 11.54, p = 0.0037, Fig. 3B), TIMP1 (F (1, 16) = 5.058, p = 0.0389, Fig. 3C), and TGF-β (F (1, 16) = 23.05, p = 0.0002, Fig. 3D). By 28-Days, MMP2 exhibited a similar main effect of Injury to increase expression (F (1, 24) = 5.340, p = 0.0297, Fig. 3E) with respect to 3-day MMP2. However, MMP9 and TIMP1 did not exhibit a main effect of Injury at 28 days, but rather a main effect of diet decreased MMP9 and TIMP1 expression (F (1, 22) = 5.466, p = 0.0289, Fig 3F; F (1, 22) = 9.325, p = 0.0058, Fig. 2G, respectively). TGF-β did not have any significant interaction or main effects by 28 Days (Fig. 3H).
Fig. 3.
mRNA abundance of markers of extracellular matrix remodeling and turnover after 3 and 28 days of recovery following bupivacaine induced acute muscle injury. (A) Matrix Metalloproteinase 2 (MMP2) mRNA abundance 3 days post bupivacaine, (B) MMP9 mRNA abundance 3 days post bupivacaine, (C) Tissue Inhibitors of Metaloproteinases 1(TIMP1) mRNA abundance 3 days post bupivacaine, (D) TGF-β, 3 days post bupivacaine. (E) MMP2 mRNA abundance 28 days post bupivacaine, (F) MMP9 mRNA abundance 28 days post bupivacaine, (G) TIMP1 mRNA abundance 28 days post bupivacaine, (H) TGF-β, 28 days post bupivacaine. Significant main effects of injury indicated by # and diet by †.
Discussion
Remodeling of the ECM directly affects the ability of the skeletal muscle to regenerate following injury. The amount and type of collagen deposition, as well as the composition of the entire ECM play a crucial role in the ECM remodeling response. Our laboratory has previously reported altered skeletal muscle regenerative responses following an episode of acute muscle damage between lean and obese mice.3 While the primary goal of that study was to examine how obesity alters the regenerative capacity of muscle, this investigation directly builds upon that work by specifically exploring changes in the remodeling of the ECM of skeletal muscle. The initial hypothesis of these studies was that obese mice would exhibit reduced myogenic regulatory factor (MRF) expression, as well as reduced markers of inflammation and protein synthesis following acute muscle damage. To that end, we previously demonstrated a blunted inflammatory response via TNF-α in obese mice following an acute injury, with a concomitant effect of impaired IL-6 signaling and decreases in markers of protein synthetic signaling.3 Building upon that work and expanding the scope of the initial investigation to include markers of skeletal muscle ECM remodeling, we hypothesized that these same animals would exhibit markers consistent with an impaired remodeling of the extracellular matrix. Here, we demonstrate an impaired regenerative response in the ECM following an acute skeletal muscle injury, possibly through impaired extracellular matrix remodeling. However, the current findings only extend to an altered transcriptional response, and further investigations will be required to ascertain the extent of translational outcomes beyond Sirius Red staining.
Following an acute skeletal muscle injury, the migration of inflammatory cells and muscle satellite cells along with the extracellular matrix to the site of injury is imperative to an efficient regenerative response,23 and it has been demonstrated previously that the integrity of the extracellular matrix is crucial in facilitating this response.24 While the deposition of Collagen I can be beneficial in wound repair, it's spatial orientation and covalent cross-linking with other Collagen I fibrils contribute to a very high tensile strength and is associated with stiffer areas of scarring.25,26 Increased overall collagen deposition is associated with increased stiffness of surrounding skeletal muscle and has been shown to impair muscle progenitor cell proliferative activity.27 This is consistent with our previous findings where we demonstrated altered myogenic proliferative markers consistent with reduced regenerative ability.3 In contrast, Collagen III forms a looser network and is better suited for the maintenance of structural integrity and distensibility of the region.28 There have been previous reports of increased collagen deposition with obesity29 and our results are consistent with that finding. By 3 days following acute muscle injury, lean and obese animals exhibited large increases in the expression of Collagen I, but obese injured animals exhibited greater expression of Collagen III when compared to all other groups (Fig. 2B and C). Increases in Collagen III gene expression are a common finding in models of multiple injuries.30 However, 28 days following acute injury, there is a marked reversal of the Collagen III:I ratios in all groups. While the lean injured animals have a significantly lower Collagen III:I ratio compared to the lean uninjured animals, the obese injured and obese uninjured animals were not statistically different from each other. This could be due in part to an impaired expression of Collagen III leading up to 28 days in the obese injured group, although there was no interaction effect nor main effects detected due to high variability in samples. There have been reports of increases in the Collagen III to I ratio during later timepoints of regeneration,28,31 and our data support these findings.
A recent study demonstrated that type I collagen promotes migration and differentiation of C2C12 cells.32 During skeletal muscle regeneration, migration of these cells to the damaged site is critical for proper repair. Therefore, the increased Collagen III to I ratio could negatively affect the migration of myoblasts and result in delayed skeletal muscle regeneration. Additionally, at the later time point, the obese mice had no change in the Collagen III to I ratio. The normal upregulation of the Collagen III to I ratio at later regenerative timepoints supports myoblast differentiation contributing to optimal skeletal muscle regeneration.33 Therefore, the lack of Collagen III to I gene expression response in obese mice could potentially delay myoblast differentiation thus contributing to the delay in skeletal muscle regeneration associated with high-fat feeding.
Typically, ECM remodeling occurs rapidly at the onset of skeletal muscle regeneration. While there were no interaction effects found in any of the tested markers of ECM remodeling enzymes, MMP2, MMP9, TGF-β, and TIMP1 showed increased expression at 3 days following acute muscle injury irrespective of diet, consistent with previous reports of bupivacaine induce muscle injury.34 Interestingly, the blunted expression of TIMP1, a matrix metalloproteinase inhibitor, is associated with a decreased expression of MMP9 at 28 days post-acute muscle injury. While this alone seems to indicate a decreased inhibition of the matrix metalloproteinases that may aid in the digestion and turnover of collagen molecules, the Sirius Red staining indicates that the cumulative effect is that of collagen deposition in obese animals. There have been previous reports that indicate that MMP2 is maximally activated between 3 and 10 days following acute muscle injury, but can remain elevated for up to 30 days,34 and we corroborate those findings. Furthermore, Kherif et al. found that MMP9 expression is elevated early, but returns to basal levels by day 10, which is also corroborated by our findings.34 There is some evidence that MMP9 is produced predominantly by inflammatory cells and activated satellite cells, and in the context of obesity, could explain the reduction in MMP9 gene expression seen in obese mice at 28 days post bupivacaine injection. These findings are consistent with our previous report of decreased cyclin D1 gene expression at 28 days.3
In summary, we are among the first to describe how obesity modulates the ECM remodeling response during skeletal muscle regeneration following acute injury. While many of the alterations observed early can be attributed exclusively to the bupivacaine-induced muscle injury, the changes in Collagen III expression indicate an obesity mediated dysregulation of the regenerative process. However, altered expression of MMP9 and TIMP1 by 28 days indicates a more complex dysregulation that may be occurring after our 3-day timepoint, but before the 28-day timepoint. We clearly demonstrate an altered response to acute muscle injury between obese and lean mice, and these results indicate that further study is necessary to elucidate a causative mechanism driving this effect.
Submission statement
This manuscript is an original work that has not been previously published, nor will it be under consideration for publication by any other journal before a decision has been made by Sports Medicine and Health Science. If accepted, this manuscript will not be published elsewhere. All contributing authors are represented in the list of authors appearing on the manuscript, and all authors approve of this manuscript and agree with submission for consideration of publication in Sports Medicine and Health Science.
Ethical approval statement
All procedures are approved by the University of Arkansas Institutional Animal Care and Use Committee (Protocol 12030). All animals were housed at the University of Arkansas Central Laboratory Animal Facility according to the guidelines set forth by the Institutional Animal Care and Use Committee.
Authors' contributions
TAW and NPG synthesized the experimental design. TAW, LAB, and RAP assisted with animal experiments. JWD, LAB, WAH, MER, and MAT collected and analyzed data. JWD, ERS, LAB, WAH, RAP, MER, MAT, NPG and TAW edited and revised the manuscript. TAW acquired funding.
Conflict of interest
The authors have no conflicts of interest to report.
Acknowledgements
The authors would like to thank the Human Performance Laboratory staff for administrative contributions and Alyssa Papineau and Dameon Smith for their technical assistance. Support has been provided in part by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
References
- 1.Kumanyika S., Dietz W.H. Solving population-wide obesity—progress and future prospects. N Engl J Med. 2020;383(23):2197–2200. doi: 10.1056/NEJMp2029646. [DOI] [PubMed] [Google Scholar]
- 2.Lauby-Secretan B., Scoccianti C., Loomis D., et al. Body fatness and cancer — viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown L.A., Lee D.E., Patton J.F., et al. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. 2015;215(1):46–57. doi: 10.1111/apha.12537. [DOI] [PubMed] [Google Scholar]
- 4.Akhmedov D., Berdeaux R. The effects of obesity on skeletal muscle regeneration. Front Physiol. 2013;4:371. doi: 10.3389/fphys.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellanger T.M., Bray G.A. Obesity related morbidity and mortality. J La State Med Soc. 2005;157(1):S42–S49. quiz 49. [PubMed] [Google Scholar]
- 6.de Heredia F.P., Gomez-Martinez S., Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 7.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 8.Mendias C.L., Gumucio J.P., Davis M.E., et al. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45(1):55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackey A.L., Brandstetter S., Schjerling P., et al. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle. Faseb J. 2011;25(6):1943–1959. doi: 10.1096/fj.10-176487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csapo R., Gumpenberger M., Wessner B. Skeletal muscle extracellular matrix – what do we know about its composition, regulation, and physiological roles? A narrative review. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedesco F.S., Dellavalle A., Diaz-Manera J., et al. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120(1):11–19. doi: 10.1172/jci40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren G.L., Summan M., Gao X., et al. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582(2):825–841. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Souza T.O., Fedri, Mesquita D.A., et al. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Laser Med Sci. 2011;26(6):803–814. doi: 10.1007/s10103-011-0951-9. [DOI] [PubMed] [Google Scholar]
- 14.Alexakis C., Partridge T., Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293(2):C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 15.Goetsch S.C., Hawke T.J., Gallardo T.D., et al. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genom. 2003;14(3):261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomidis J.S., Hendrick J.W., Parkhurst A.M., et al. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005;288(1):H149–H158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 17.Verrecchia F., Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118(2):211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown J.L., Rosa-Caldwell M.E., Lee D.E., et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(6):926–938. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene N.P., Nilsson M.I., Washington T.A., et al. Impaired exercise-induced mitochondrial biogenesis in the obese Zucker rat, despite PGC-1α induction, is due to compromised mitochondrial translation elongation. Am J Physiol Endocrinol Metab. 2014;306(5):E503–E511. doi: 10.1152/ajpendo.00671.2013. [DOI] [PubMed] [Google Scholar]
- 20.Washington T.A., Healey J.M., Thompson R.W., et al. Lactate dehydrogenase regulation in aged skeletal muscle: regulation by anabolic steroids and functional overload. Exp Gerontol. 2014;57:66–74. doi: 10.1016/j.exger.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Washington T.A., White J.P., Davis J.M., et al. Skeletal muscle mass recovery from atrophy in IL-6 knockout mice. Acta Physiol. 2011;202(4):657–669. doi: 10.1111/j.1748-1716.2011.02281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa-Caldwell M.E., Brown J.L., Lee D.E., et al. Hepatic alterations during the development and progression of cancer cachexia. Appl Physiol Nutr Metabol. 2020;45(5):500–512. doi: 10.1139/apnm-2019-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keely P., Nain A. Capturing relevant extracellular matrices for investigating cell migration. F1000Res. 2015;4 doi: 10.12688/f1000research.6623.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg K., Ward C.L., Rathbone C.R., et al. Transplantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue Res. 2014;358(3):857–873. doi: 10.1007/s00441-014-2006-6. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Chen J., Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25(1):9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Delgado L.M., Bayon Y., Pandit A., et al. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng B Rev. 2015;21(3):298–313. doi: 10.1089/ten.TEB.2014.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacraz G., Rouleau A.J., Couture V., et al. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J.T., Kasukonis B.M., Brown L.A., et al. Recovery from volumetric muscle loss injury: a comparison between young and aged rats. Exp Gerontol. 2016;83:37–46. doi: 10.1016/j.exger.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang L., Ayala J.E., Lee-Young R.S., et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60(2):416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceafalan L.C., Dobre M., Milanesi E., et al. Gene expression profile of adhesion and extracellular matrix molecules during early stages of skeletal muscle regeneration. J Cell Mol Med. 2020;24(17):10140–10150. doi: 10.1111/jcmm.15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg K., Corona B.T., Walters T.J. Losartan administration reduces fibrosis but hinders functional recovery after volumetric muscle loss injury. J Appl Physiol. 1985;117(10):1120–1131. doi: 10.1152/japplphysiol.00689.2014. 2014. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Gao Y., Long X., et al. Type I collagen promotes the migration and myogenic differentiation of C2C12 myoblasts via the release of interleukin-6 mediated by FAK/NF-kappaB p65 activation. Food Funct. 2020;11(1):328–338. doi: 10.1039/c9fo01346f. [DOI] [PubMed] [Google Scholar]
- 33.Alexakis C., Partridge T., Bou-Gharios G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293(2):C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kherif S., Lafuma C., Dehaupas M., et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205(1):158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]