Abstract
Increased cardiovascular fitness, O2max, is associated with enhanced endurance capacity and a decreased rate of mortality. High intensity interval training (HIIT) is one of the best methods to increase O2max and endurance capacity for top athletes and for the general public as well. Because of the high intensity of this type of training, the adaptive response is not restricted to Type I fibers, as found for moderate intensity exercise of long duration. Even with a short exercise duration, HIIT can induce activation of AMPK, PGC-1α, SIRT1 and ROS pathway as well as by the modulation of Ca2+ homeostasis, leading to enhanced mitochondrial biogenesis, and angiogenesis. The present review summarizes the current knowledge of the adaptive response of HIIT.
Keywords: High intensity interval training, Cellular adaptation, Molecular pathways, Redox signaling, Maximal oxygen uptake, Mitochondrial biogenesis
Introduction
High intensity interval training (HIIT) emerged several decades ago in response to the need for new training techniques for athletic events of high intensity and often also of long duration. In the 1920s the legendary runner “Flying Finn” Paavo Nurmi started to introduce “interval training” sessions to his annual training cycle.1 In the Nordic athletic community “natural” interval training gained popularity, was named Fartlek and was formalized by Gösta Holmér in 1937. The Fartlek method originally was developed for cross country, multi-terrain runners and consisted of a continuous distance run in which very high (sprints, or uphill runs) and low velocity running periods were integrated. In the 1950s interval training methods started to infiltrate several European athletic training programs. It became prominent probably because the four-time Olympic gold medalist Emil Zatopek (Czeckoslovakian) and other eminent runners like Vladimir Kuts (Russian), Gordon Pirie (British) or Sigfried Hermann (German) used this training method effectively during their preparation.2 Some of the first scientific papers which described HIIT in detail were published in the late 50s-early 60s by Roskamm and Reindell3 as well as Per Oløf Astrand.4 However, HIIT research gained full attention in the 1970s.5 Early protocols used the average velocity or the velocity corresponding to the personal best time of the distance of interest as reference instead of the maximal aerobic capacity. The use of maximal oxygen uptake (O2max), introduced by the Nobel laureate Archibald Vivian Hill, become widespread only after the Second World War.
Depending on the applied protocol, the exercise resembled endurance moderate intensity continuous training (MICT) or a strength-speed training adaptation. For an in depth historical review the reader is directed to the review of Véronique Billat.2
It became clear that high intensity exercise is a fundamental tool for improving peak athletic performance. It is impossible to reach the required physiological limit of athletic performance during competitions without challenging the musculoskeletal and cardiovascular systems by vigorous exercise sessions. As early as 1986 Cox and colleagues showed that after seven weeks working at 90% or higher intensity exercise induced left ventricular morphology changes in previously sedentary individuals.6 HIIT is shown to increase aerobic capacity even more efficiently than volume-based endurance training protocols.7 The time spent with doing physical exercise is also an important factor, not only for nonprofessional athletes but for professionals, as well. Recently time-efficiency became a pinnacle aspect for athletes to improve diverse skill sets. HIIT induces comparable chronic physiological adaptation with less expenditure of time than classical endurance programs,8 and the recovery time after HIIT appears to be shorter than after moderate intensity exercise of long duration.9 Moreover, some authors claim that HIIT is a superior method compared to aerobic training, in order to improve diverse physiological functions.10, 11, 12 The question that needs to be answered is “what are the differences in the adaptive response to HIIT and MICT”.
In this review we focus on investigations that applied 85–90% [taking peak power output (PPO) or PO2max vO2max] or higher loads during HIIT. This range of intensity is suggested by Wisløff et al.13 who reviewed the literature on interval training and its cardiac benefits. The duration of the high intensity load is also a matter of debate. The protocols in the following sections will be referred to as HIIT if one session does not exceed 4–5 min during the total exercise time. Sprint interval training (SIT) will be referred to as “all out” maximal effort (greater than PO2max) if the duration is less than 1 min per each sprint. Our nomenclature will follow the terminology used by MacInnis and Gibala14 in their review. The present paper aims to discuss the molecular adaptive response of HIIT and emphasizes the differences from moderate intensity exercise with long duration.
What is high intensity interval training?
Interval training can be defined simply as discontinuous, periodic (demanding) exercise loads separated by periods of recovery. However, the intensity level which defines an exercise as “high intensity” varies in different research publications. There is no accurate consensus threshold from which point the training load is categorized as ‘close to the maximal effort’. In the literature the terms “high intensity” or “vigorous” are used in a fairly diverse manner ranging from an effort as low as 65% of the peak power output15 up to 170% of the measured power at O2max.16 Even the dimension of reference intensity can be different in publications since peak power output percentage (PPO%), power (PO2max%) or velocity (vO2max%) percentage of the O2max, maximal heart rate percentage (HRmax%) and critical/maximal velocity percentage (vVmax%) are all commonly used, but, vary greatly. Using different reference dimensions can be problematic because the same numerical values may indicate a different metabolic demand with a different dimension.15 Most frequently, intensity levels are referred to the work load corresponding to the ≥90% of maximal aerobic capacity (or 90–95% of the peak HR) or maximal effort. Buchheit and Laursen noted nine characteristics which can precisely describe HIT exercise17 including interval intensity and duration, relief interval intensity and duration, exercise modality, number of reps and series, and finally between series recovery duration and intensity.
According to their publications on HIIT, protocols can be divided into two basic groups:
-
(i)
High intensity with relatively higher volume, 3–5 min with “near maximal” effort (∼80–90% intensity), separated by resting periods (but one can find protocols using more than five-minute sessions).
-
(ii)
The other form is “the all-out” or “until exhaustion” type of exercises often referred to as SIT. In the case of SIT, one sprint rarely exceeds a 30 s time interval and training intensity is termed to be “supramaximal” while exercise peak power output (PPO) levels exceed the power value recorded during O2max measurements.
HIIT and SIT are usually associated with continuous cyclic movements like running, cycling, and rowing, which can induce morphological changes such as a conversion of fiber type morphology, increased fiber type area in fast twitch fibers etc.18
Molecular effects of high intensity interval training
The pioneering work of Karlsson and Saltin19 examined the molecular effects of HIIT in skeletal muscle. Subjects were exposed to five cycling sessions with 1 min supramaximal load ∼120% of O2max followed by 5 min of passive rest. Oxygen uptake and lactate levels increased significantly during the exercise, but, interestingly enough, after the first burst of exercise, lactate values did not increase as the load progressed. At the same time creatine phosphate, glucose 6-phosphate and ATP gradually decreased in the quadriceps muscle.
When one attempts to evaluate the actual metabolic features of SIT or HIIT, in respect to MICT, a major problem emerges, because the volume and the energy usage are not always matched. It is obvious that higher power output transiently increases the energy demand but low volume or the total work performed during HIIT/SIT exercise sessions makes it difficult to access the effects of prior exercise intensity and modality on metabolic substrate turnover immediately after exercise or with recovery. Lactic acid production and post exercise levels after exercise are generally higher after HIIT20 compared to MICT. In a comparative study, where long and short interval HIIT-s and MICT were matched for the total duration, mean power showed significantly higher levels of lactic acid in peripheral blood after long duration, but were less pronounced after a short interval HIIT program.21 It is not easy to estimate how metabolically challenging a given HIT protocol is, since, as mentioned above, besides the intensity level, the work to release ratio and the release intensity (is it a passive or an active rest period?) can affect the metabolic burden. A suggested method to estimate the SIT or HIT training metabolic demand is to consider the five-minute lactic acid increment from the start of the exercise. Peripheral lactic acid may not be the perfect indicator of the exercise intensity since it can be affected by dietary status22 and it is not perfectly, but reflects the overall anaerobic environment in the exercising skeletal muscle23 and it provides sufficient information about the peripheral metabolic environment. This finding highlights the main physiological divergence between HIIT and MICT. During MICT the exercise is conducted in a physiological steady state, i.e. metabolic processes can fulfill the energy demand of skeletal muscle with no significant temporal metabolite accumulation. However, as intensity increases and the maximal lactate steady state is approached, the dominance of oxidative processes decreases, lactate accumulation takes place and, if it is too rapid, it results in quick exhaustion. In HIIT, energy need is only transiently increased in the tissue and the oxygen deficit can be countered during the resting phase, but the “metabolic perturbation” is a function of the intensity level and the recovery time. Because of the relatively short, high intensity bursts, the local metabolic capacities (CP, ATP, and mitochondrial enzymes) are able to cope with the increased demand, and the loss can be countered during recovery. Therefore, the accumulation of lactic acid is slower and the onset of fatigue can be prolonged, even at high levels of intensity.
In general, HIIT/SIT changes in substrate utilization or mobilization are believed to be steeper than during MICT. Lactate24 and plasma glucose levels25,26 increased, PCr/(Cr + PCr) ratio25 decreased during high intensity exercise, but post exercise glycogen content can be similar25,27 when compared to MICT. However, the chronic HIIT/SIT interventions seem to increase the resting intramuscular glycogen pool.28 The discrepancies, in the case of acute exercise, probably can be explained by the different energy expenditures between HIIT and MICT. Intensive exercise has a major impact on the systemic sugar homeostasis since as high as 80% of the glucose can be transposed into the tissue when insulin is present.29 The ATP turnover rate can be 100- fold higher during exercise than at rest.30 After a training period of six weeks at 90% O2 peak, 3 d/wk, glycogenolysis and lactate accumulation were decreased31 during the test phase and the protein content of MCT (monocarboxylate transporter) 1 and MCT 4 lactate transporters, fatty acid transporters FAT/CD36, FABPpm, GLUT4 glucose transporter, maximal activities of mitochondrial aspartate aminotransferase, β-hydroxyacyl CoA dehydrogenase (β-HAD) and total pyruvate dehydrogenase increased significantly, as a result of training.
Due to the different metabolic rates of HIIT and MICT there is also a difference in metabolite production. The lactate levels in blood increase during the 5 min during exhaustive exercise32 but when longer duration applied, lactate accumulation plateaued.33 Needless to say, the accumulation of lactate is dependent on the exercise intensity.34
In one elegant study, ten well-trained males completed a single bout of isoenergetic HIIT or MICT in a randomized and counterbalanced order.24 The carbohydrate oxidation was higher, whereas fat oxidation was lower during HIIT vs. MICT. From the 49 compounds measured by targeted metabolic analysis after exercise from plasma samples, 13 changes were characteristic of HIIT and only five of MCIT. Tricarboxylic acid intermediates (citric, aconitic and succinic acid) increased to a greater extent after HIIT compared to MICT. Amino acid alanine levels elevated only during HIIT, but the two BCAA compounds, valine and leucine, were significantly lower after MICT in plasma samples compared to the pre-exercise values. Despite the valuable information about HIIT-induced metabolic perturbation, the biological role of plasma TCA and other intermediaries is rather confusing since these metabolites play primal role in the working skeletal muscle.
HIIT and MICT training impact on skeletal muscle oxidative capacity
The different adaptations to exercise stimuli of skeletal muscle fiber types may help to answer the question: why is HIIT exercise capable of increasing aerobic capacity in a relative short time? Performing physical exercise at high intensity activates type II fiber contraction, obeying the size principle.35 Data suggest that mitochondrial content of Type I fibers can be increased by low intensity exercise of long duration, while in case of Type II fibers, mitochondrial biogenesis is induced by high intensity exercise.36 Since O2max is dependent on arterio-venous oxygen differences, which is influenced by mitochondrial content, it is easy to understand why HIIT-induced mitochondrial biogenesis is associated with enhanced O2max. In addition, the induction of HIF137 and VEGF37,38 have been reported, and this adaptive response may be also important to create greater arterio-venous oxygen differences and O2max. In contrast, after eight weeks of MICT39 a superior increase in capillary density was detected when compared to HIIT with similar total energy expenditure. Data with similar vascular adaptations27 is also known.
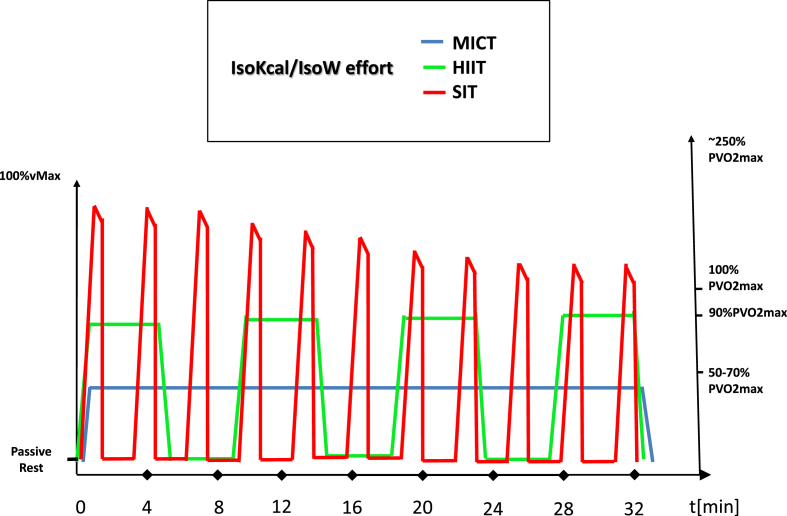
Tan and his colleagues showed that when sedentary young woman conducted a six-week low volume HIIT training (∼90% HRmax) the intervention elevated the mitochondrial COX IV in both type I and type II fibers.40 Indeed, there are numerous studies about HIIT or SIT that have demonstrated increased mitochondrial biogenesis41,42 or elevated oxidative capacity.43,44 It is also known that if HIIT and MICT are applied in an isocaloric manner, besides the improving effect on the arteriovenous difference, HIIT positively affects all the major cardiac markers (cardiac output, maximal HR, stroke volume).45 It is important to establish some common reference point where the differential adaptation pattern of HIIT/SIT and MICT can be studied, because the energy demand, the distance covered, or the work being done may be incomparable. The most widely used methods are the isocaloric/isoenergetic (work-matched) or volume/duration matched study designs. When these corrections are applied, the universal superiority of HIIT becomes less unequivocal46,47 (Fig. 1.). But generally, it seems that higher intensity exercise has the capacity to induce more pronounced beneficial changes in physiological and molecular markers.48
Fig. 1.
The energy demand of exercise with different intensities. Thesuggested energy cost of MICT, HIIT and SIT. The discrepancies of the results in various studies are due to the different energy costs of exercise training. Matched energy or power cost are required to valid evaluation.
The large energy demand of HIIT results in significant increases in cellular ADP/ATP and AMP/ATP ratios which can activate AMPK, one of the most important energy sensors of the cell.49 AMPK is made up from α, β, and γ subunits. If ATP concentration decreases and AMP levels increase as a result of muscle contraction or the lack of energy influx, then AMP bounds to Bateman domain (CBS domain) of γ-subunits as the first event in the activation of mammalian AMPK.50 Several studies showed that AMPK phosphorylation in skeletal muscle positively correlates with the exercise intensity25,41 and duration.51 AMPK phosphorylates several crucial enzymes involved in regulation of lipid and protein metabolism and glucose transport.52 The heterotrimer combination may provide an important way of adaptation to different exercise stimuli. Hence the different association of α, β and γ isoforms (α1, α2, β1, β2, γ1, γ2, γ3) can differ in fiber types.53 The activity of α subunits and increased α2 activation (as assessed by isoform specific immunoprecipitation) levels were found after 20 min of exercise only if the intensity was higher (i.e. 20 min. cycling at 50% or 70% PO2max),54 nevertheless the α1 activity levels were unaffected (the glycogen and PCr level dropped after the 70% bout). In a similar study isoform-specific AMPK activity measured by sequential immunoprecipitation after a single but of HIIT or MICT. The increase in activity of the α2β2γ3 structure/construction was 27- fold in the HIIT vastus lateralis samples compared to the 12- fold in MICT.25 On the other hand, independently of exercise trial, α1β2γ1 assembly activity decreased with exercise and α2β2γ1 AMPK activity remained unchanged. Both exercise interventions elevated AMPK (Thr172), ACC (Ser221), TBC1D1 (Ser231) and TBC1D4 (Ser704) phosphorylation similarly in total muscle lysates. However, phosphorylation of AMPK (Thr172) increased by 184% in type II fiber and only in the HIIT group. Similar observations have been made by others,55,56 underlining the role of the α2 isoform in exercise dependent AMPK regulation. The available information indicates that AMPK isoform-specific activity may play an important role in HIIT-induced metabolic adaptation, however the adative response to HIIT is very complex (Table 1).
Table 1.
HIIT induced adaptive response on humans.
| Reference | Population characteristics (n, M/F, age, O2max) | Training period | Exercise details | Main physiological and molecular changes |
|---|---|---|---|---|
| 47 | 6, 6/0, 25 ± 2.9 yr, 55.5 ± 1.3 mL/kg/min | Single bout, cycling | HIIT: ∼40–45 min 2 min at 90% and 2 min at 25% O2peak.MICT: ∼60 min of at 50% O2peak. | Postprandial fat oxidation was increased similarly in both exercise groups with a higher increase in HIIT. |
| 116 | 10, 10/0, 20 ±1yr., 52 ± 7 mL/kg/min | Single bout, running | HIIT: 3-min bouts at 90% O2maxand 3-min at 50% O2max. MICT: 50 min at 70% O2max. | Heart rate, RPE, and blood lactate was significantly higher in HIIT compared with MICT. Phosphorylation of AMPK Thr172 and p38MAPK Thr180/Tyr182 increased after exercise with no difference between exercise protocols. Muscle (vastus lateralis) PGC-1α mRNA content increased after hrs. of exercise with no difference between protocols. PGC-1α protein content was not changed at any time during the HIIT or MICT trials. |
| 24 | 10, 10/0, 33.2 ± 6.7yr., 4.8 ± 0.3 L/min (∼61 mL/kg/min) | Single bout, cycling | HIIT: 10 × 4 min cycling at 81.6 ± 3.7% O2max and 2 min with at 50 W (11.4 0.9% peak power output). MICT: cycling at 65% O2max for a time corresponds to total HIIT work. | Plasma lactate, adrenocorticotrophic hormone, cortisol, and growth hormone were all higher immediately after HIIE vs. MICT. Plasma norepinephrine and interleukin-6 increased similarly. Plasma insulin decreased during recovery in both HIIE and MICE. |
| 117 | 44,0/44, ∼25yr.,∼29 mL/kg/min | 3 times a week for 16 weeks, running/walking | HIIT: 40 min with 1 min at 80–90% O2max and 2 min at 50–60% O2max. MICT: 40 min at 60–70% O2max. | HIIT was superior in improving norepinephrine, endothelin-1 (ET-1) and (nitrite/nitrate) NOx response to exercise than MICT. HIIT and MICT were similarly effective in improving ABP and insulin sensitivity. HIIT was superior in improving cardiorespiratory fitness. |
| 118 | 16, 0/16, HIIT: 20 ± 1 yr. MICT: 19 ± 1 yr, HIT: 43.7 ± 6.8 mL/min/kg, MICT: 42.1 ± 7.2 mL/min/kg | 5 weeks 3 days per week, cycling | HIIT: 120% (week 1), 130% (weeks 2 and 3) and 140% (weeks 4 and 5) of the lactate threshold, 2 min duration, with 1 min recovery. MICT: 80% (week 1), 90% (weeks 2 and 3) and 95% (weeks 4 and 5) of the lactate threshold. Work matched to HIIT. | In HIIT group there was a significantly greater improvement in vastus lateralis muscle buffer capacity (βm in vitro) than the MICT group. O2peak increased in both group similarly. |
| 119 | 40, 40/0, 24.6 ± 3.8 yr, ∼55–60 mL/min/kg | 8 weeks 3 days per week, running | HIIT: 4 × 4 min at 90–95% HRmax with 3min active recovery at 70% HRmax. SIT: 47 × 15-s at 90–95% HRmax with 15 s active recovery at 70% HRmax. MICT: at 70% HRmax for 45 min. | After HIIT and SIT O2max and stroke volume increased significantly |
| 120 | 43, 17/26, 55–79 yr, ∼23,1–25,9 mL/min/kg | 8 weeks 4 days per week, all-extremity ergometer | HIIT: 4 × 4 min at 85–95% HRpeak with 3 min active recovery at 65–75% HRpeak. MICT: 32 min at 65–75% HRpeak. | O2peak, ejection fraction and insulin resistance (HOMA-IR) improved in HIIT. |
| 46 | 10, 10/0, 23 ± 1 yr, 46 ± 2 mL/kg/min | 2 week 6 session, One-leg cycling | HIIT: 4 × 5 min at 65% Wmax and 2.5 min recovery with 20% Wmax. MICT: 30 min with 50% Wmax | CS maximal activity and mass specific O2 flux oxidative phosphorilation capacities in HIIT vs. MICT. In whole muscle, the COXIV, NDUFA9 and mitofusin 2 (MFN2) increased similarly in both groups. |
| 121 | 9,n.d.,20–28 yr, ∼25.7–61.3 mL/kg/min | 7–8 week 3 days/week, cycling | HIIT: 5 × 4 min 2 min recovery, 101% of O2max matched by W. MICT: average 27 min, 79% of O2max. | O2max elevated only in the MICT group. SDH activity increased in both group, but no difference found in PFK levels. |
| 122 | 26, 10/16, obese,41 ± 9 yr, ∼31–36.2 mL/kg/min | 18 session 3/week, running | 4HIIT: 4 × 4 min at 85%–95%HRmax with 3 min recovery at 70% HRmax. 1HIIT: 10 × 1 min 90% HRmax with active recovery time n.d. MICT: 45 min at 70% HRmax. | O2max increased in the 4HIIT group compared to the other two groups. Calculated stroke volume increased only in the 4HIIT group. Muscle CS activity and TTE (time to exhaustion) improved in all exercise group with a difference between 4HIIT and MICT in TTE. |
| 123 | 17, 17/0, ∼24.6 ± 3 yr, 3046–3757 mL/min | 8 week 3 day/week, cycling | HIIT: 10 × 2 min at 105% with 2min recovery. 1MICT: 55 min of continuous exercise at −50%. O2max; 2MICT: 35 min at 70% O2max; | O2max and maximal exercise ventilation (VEmax) increased in all protocol with a higher increase of VEmax in HIIT to other MICT groups. |
| 124 | 13, 13/0, 19–25yr.,37–54 mL/kg/min | 4 week 5 workout/leg/week, cycling | One leg SIT: 20–30 bouts of 40–50 s all out efforts with 60–90 s recovery. One leg MICT: 30–50 min at 75% of O2max | Muscle SDH activity increased in both exercise groups. |
Calcium mediated regulation
Another important event in skeletal muscle functional activity is the muscle contraction-initiated calcium release from the sarcoplasmic reticulum by a ryanodine receptor-mediated mechanism.57 Inside skeletal muscle cells, calcium sensitive proteins evolve to mediate metabolic and electrophysiological pathways, during physical exercise. Place and colleagues showed that a 30s all-out cycling exercise (≤3 min total exercise time) with 4 min rest between bouts in recreationally active subjects caused the fragmentation of ryanodine receptor type 1 (RyR1) 24 h after the exercise stimuli.58 After SIT, only 15% of the RyR1 remained intact and ∼375, 80, and 60 kDa fragments were detected. However, this response was not present in elite well trained athletes nor in athletes preformed marathon race. The authors suggest the involvement of reactive oxygen species (ROS), since superoxide dismutase 2 (SOD2) and catalase (CAT) expression are at least two-fold higher in vastus lateralis muscle of elite athletes at baseline, indicating better antioxidant capacity. Endogenous antioxidants59,60 and Ca2+ handling61 are associated with enhanced mitochondrial function and recurrent high Ca2+, AMP and ROS levels during high intensity exercise may contribute to mitochondrial biogenesis by activating peroxisome proliferator-activated receptor-gamma coactivator (PGC)-α and the downstream nuclear respiratory factor 1–2 (NRF1- NRF2) systems.62 However, it is not clear why individuals with various levels of fitness respond differently to HIIT-like exercise stimuli. One possible explanation for this phenomenon is that training causes optimal biological responses at a distinct range of intensity for each person and, presumably, it follows a hormetic trajectory in groups with different training backgrounds.9,63
Additional interesting finding is AMPK can be regulated by the Ca2+ sensitive protein calcium/calmodulin-dependent kinase kinase β (CaMKKβ).64,65 Beside AMPK, PGC-1α gene expression is also subjected to Ca2+ dependent signaling.61,66
Mitochondrial biogenesis
It has been shown that AMPK can activate PGC-1α nuclear coactivator, which is referred to as the master regulator protein of mitochondrial biogenesis.67 PGC-1α plays an integrative role in governing mitochondrial biogenesis. In fact, it has a pivotal role in establishing the connection between cell energy sensing mechanisms68 and physiological signal conduction to nuclear transcription factors. The pinnacle aspect of PGC-1α related molecular pathways is its sensitivity to many intracellular stimuli and by co-activating NRF1, NRF2, PPARγ, ERR, YY1,69 it regulates the adaptation of the cellular oxidative metabolism. However, in a rather provocative review Islame et al. questioned the dominance of PGC-1α in coordination with mitochondrial biogenesis.70 The authors main argument is that the sequential regulation of PGC-1α, NRF1/2, TFAM, mitochondrial proteins does not always follow this temporal pattern. They propose that in humans, it is unlikely to have only one “master regulator” and they point to the possible regulatory role of PPAR-β, p53 and LRPPRC or LRP130. On the other hand, PGC-1α mRNA transcription increases with exercise intensity and the PGC-1α mRNA abundance is associated with the activation of upstream protein kinases.71 Only after high intensity exercise was induced did the downstream activating transcription factor-2 (ATF-2) phosphorylation increase.
Another level of complexity – which may contribute to the above-mentioned issue - comes from the promoter structure of the PGC-1α (PPARGC1A) gene. Alternative promoter usage coupled with alternative splicing gives rise to eight PGC-1α isoforms ranging from 257 to 797 amino acids in human samples.72 One prominent transcript from the PGC1α gene alternative promoter is marked as PGC1α4, also known as NT-PGC1α-b (Martinez-Redondo, Pettersson, & Ruas, 2015). This isoform, together with other NT (lacking the N-terminal RS and RR domains) transcripts, has been shown to be an inducible form. The recently emerged question is how these promoters are utilized and what cellular or physiological events can trigger promoter shifting.73 PGC1α4 expression has been associated with resistance training74 and it seems that during endurance exercise the transcription of PGC1α4 and other NT isoforms may be intensity-dependent.73,75 To our knowledge, there is no comparative study between isoenergetic HIIT and MICT investigating the differential promoter usage of the PPARGC1A gene.
It has been reported that 3 h after a single bout of SIT exercise, consisting of four all-out cycling sessions, AMPK α1 and α2 were phosphorylated in vastus lateralis muscle.76 Moreover, phosphorylation of ACC and p38 MAPK was also increased compared to the resting state. At the same time PGC-1α mRNA expression was increased approximately twofold with no measurable change in the protein level. Similarly, Little et al. found significant increases in PGC-1α, citrate synthase, COX II and COX IV mRNA after 3 3 h of HIIT exercise.77 Nuclear abundance of PGC-1α protein was unchanged immediately after exercise but did increase 3 h later. PGC-1α protein content in whole muscle lysates remained steady immediately after and 3 h after exercise but increased after 24 h of recovery. In a recent study it has been shown that 18 sessions of HIIT (10 × 60-sec cycling intervals at ∼90% HRmax, interspersed by 60-sec active recovery at 50 W) increased cytochrome oxidase IV levels in both type I and II muscle fibers.40 HIIT training elevated SIRT3 and COXIV protein levels more in rat soleus and rectus femoris muscle than MICT.78 It is interesting, that PGC-1a can directly be involved in the transcriptional control of lactic acid production79 by reducing the expression of LDH A and one of its regulators, the transcription factor myelocytomatosis oncogene. Fiorenza et al., in order to “induce distinct metabolic perturbation” in the myocellular environment, conducted repeated sprints (18 × 5 sec all-out effort, mean power output 902W, 30s passive recovery), speed endurance (6 × 20 s all-out effort, mean power output 669W, 120s passive recovery) and continuous, modest (50 min at 70% O2max, mean power output 218W) exercise protocol with the participation of 12 young trained men.80 All exercise protocol-induced AMPK(Thr172), ACC(Ser79) and p38 MAPK(Thr180) phosphorylation showed no significant differences between groups. NRF2, MFN2 and TFAM mRNA levels increased only in the speed endurance and continuous group.
Another crucial molecule in bioenergetics and metabolism is NAD+ and also its reduced form NADH. NADH is produced predominantly from NAD+ in the citric acid cycle as a product of oxidation. The carbon source (acetyl-CoA) derived from sugars, fatty- and amino acids, fuels the reaction cycle and provides NADH as a proton donor for the electron transport chain, to run the terminal oxidation. The NAD+/NADH ratio and its individual subcellular levels reflect the overall energy and redox status of the cell. Sirtuins are NAD + dependent enzymes81 and SIRT1 has been shown to deacetylate PGC-1α82 and control glucose homeostasis.83 During high intensity exercise NADH is produced in glycolysis and transported to the mitochondria for oxidation or, with pyruvate, it is processed by lactate dehydrogenase (LDH) to NAD+ and lactate. There is a difference of opinion and research rationale, in studies about which direction cellular NAD+/NADH changes with exercise volume and intensity.84 The calculated cytosolic NAD+/NADH ratio is reduced85 after 60 min of cycling exercise at ∼62%O2max or after The Wingate Test.86 But, according to the data of Phillips and colleagues,87 the calculated ratio was reduced after 15 min, then subsequently, returned to resting levels by 90 min of exercise at 59%O2max. Others found NAD + reduction after 70% and 100% of the O2max88 with no differences between 70% and 100% O2max values. Conversely, others have found different trajectories.89 To analyze the exercise-hypoxia- related metabolic shift in its complexity in silico methods90 were applied but results are still elusive due to the dynamic nature of redox homeostasis and experimental verifications have yet to be published. Since cytosolic NAD+ is estimated to be ∼540 times higher in skeletal muscle then NADH it is more likely that NADH is the major player in setting the cellular NAD + /NADH ratio. For a thorough overview the reader is directed to the paper of White and Schenk.84
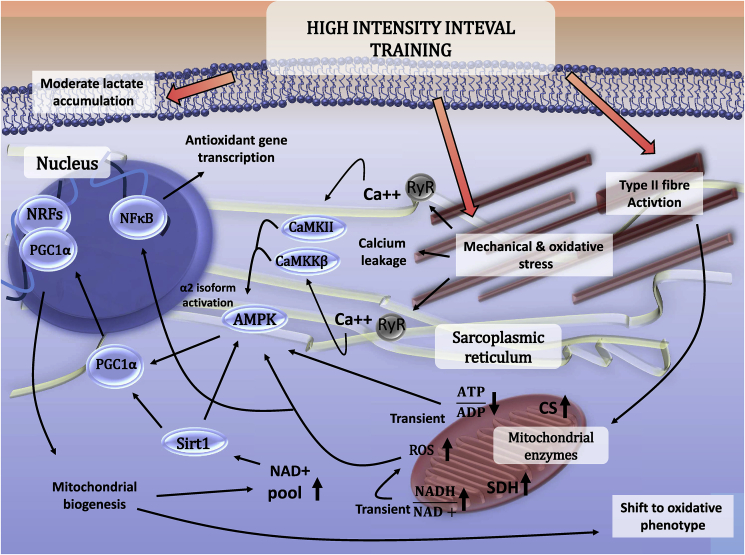
A remarkable aspect of the redox equivalent conundrum is the high NAD + level in the mitochondrial compartment.91 Early studies reported a positive relationship between resting skeletal muscle NAD + content and type I percentage in skeletal muscle.92 Although transition between cytoplasmic, nuclear and mitochondrial metabolite pools are not barrier-free, the proposed HIIT-induced oxidative shift of type II muscle fibers and its ability to induce mitochondrial biogenesis may give some clue as to why HIIT, with a huge energy demand, activates SIRT142,93 (Fig. 2).
Fig. 2.
Molecular adaptive response of skeletal muscle to HIIT. The suggested molecular pathways by which HIIT results in complex adaptive response to skeletal muscle.
Oxidative stress and the antioxidant system
Exercise-derived oxidative challenge makes a major contribution to exercise adaptation and reactive oxygen and nitrogen species are important signaling molecules in skeletal muscle.94,95 The most prominent reactive oxygen sources (and nitrogen) in skeletal muscle are nicotinamide adenine dinucleotide phosphate oxidases (NOX),96 uncoupled nitric oxide synthases (NOS),97 mitochondrial respiratory enzymes98 and xanthine oxidase (XO).99 Increased exercise intensity results in enhanced metabolic demand and generation of ROS.9 HIIT training of fourteen bouts of 20-sec swimming exercise induced H2O2 production in a rat model in permeabilized fibers of tibialis anterior and gastrocnemius but not in soleus muscle.100 We have reported a liner relationship between blood lactate levels and activity of XO after a single bout of exhausted running on the treadmill in a murine model.101 Indeed, it was also reported that high intensity exercise is associated with excess production of ROS.102 Conversely, in diseases where inactive lifestyle plays an important role, tissue specific and systemic ROS elevation is commonly detected.103,104 However, ROS production seems to be important in insulin regulated glucose uptake105,106 and GLUT4 translocation.107,108 In vitro studies found that H2O2 treatment increased PGC-1α promoter activity and mRNA expression in C2C12 cells.109 Interestingly, HIIT is found to positively regulate GLUT4 protein content.42,110 ROS are important players in exercise-associated adaptation,111 since they modulate the Ca channels and force generation,112 mitochondrial biogenesis,102 and housekeeping processes.113 Interestingly, the excess of ROS generation during exercise can be partly controlled by PGC-1α, since activation of this co-activator activates the expression of antioxidant genes such as SOD2 and GPX.114 Indeed, HIIT (5 × 4-min 75% of Wmax), SIT (4 × 30 s all-out), MICT (30 min at 50% of Wmax) both high intensity exercise, increased peak plasma hydrogen peroxide values during exercise.115 But, significant elevation of peak catalase activity was measured only in the SIT group (with a main effect of time in catalase and SOD activity). It is important to note, that exercise-associated increases in ROS generation very rarely, if ever, can reach the level which could have a negative effect on health. Mounting data support the health promoting effects of regular exercise, regardless of the intensity of exercise.
Conclusion
HIIT-associated increases in cellular metabolism of skeletal muscle results in fiber specific responses, since Type II fibers with higher recruital thresholds are fully active during HIIT. The HIIT-induced enhanced mitochondrial biogenesis is mediated by the AMPK, PGC-1α, SIRT1 and ROS pathway as well as by the modulation of Ca2+ homeostasis. The fact that the time saving HIIT associated adaptive response at least comparable or superior to MICT associated adaptation, guarantees further increases of HIIT in the preparation of elite sport and health promotion physical activities.
Conflict of interest
The authors have no conflict of interest to report.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Each Author's contributions
FT, ZG, MJ, MT, TM and ZR contributed by searching and discussions on the relevant literature. ZR drafted the final version of the paper, but all authors were involved in the correction of the original paper. FT and ZG designed and drew the figures.
References
- 1.Buchheit M., Laursen P.B. High-intensity interval training, solutions to the programming puzzle: Part I: cardiopulmonary emphasis. Sport Med. 2013;43(5):313–338. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 2.Billat L.V. Interval training for performance: a scientific and empirical practice. Special recommendations for middle- and long-distance running. Part I: aerobic interval training. Sport Med. 2001;31(1):13–31. doi: 10.2165/00007256-200131010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Roskamm H., Reindell H., Keul J. [The physiological bases of various training methods] Sportarzt Ver Sportmed. 1962;13:109–123 concl. [PubMed] [Google Scholar]
- 4.Astrand I., Astrand P.O., Christensen E.H., Hedman R. Intermittent muscular work. Acta Physiol Scand. 1960;48:448–453. doi: 10.1111/j.1748-1716.1960.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox E.L., Bartels R.L., Billings C.E., Mathews D.K., Bason R., Webb W.M. Intensity and distance of interval training programs and changes in aerobic power. Med Sci Sport. 1973;5(1):18–22. [PubMed] [Google Scholar]
- 6.Cox M.L., Bennett J.B., 3rd, Dudley G.A. Exercise training-induced alterations of cardiac morphology. J Appl Physiol. 1986;61(3):926–931. doi: 10.1152/jappl.1986.61.3.926. [DOI] [PubMed] [Google Scholar]
- 7.Weston K.S., Wisloff U., Coombes J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–1234. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 8.Burgomaster K.A., Howarth K.R., Phillips S.M., et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radak Z., Ishihara K., Tekus E., et al. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. doi: 10.1016/j.redox.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisloff U., Stoylen A., Loennechen J.P., et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 11.Milanovic Z., Sporis G., Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for O2max improvements: a systematic review and meta-analysis of controlled trials. Sport Med. 2015;45(10):1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 12.Ramos J.S., Dalleck L.C., Tjonna A.E., Beetham K.S., Coombes J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sport Med. 2015;45(5):679–692. doi: 10.1007/s40279-015-0321-z. [DOI] [PubMed] [Google Scholar]
- 13.Wisloff U., Ellingsen O., Kemi O.J. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37(3):139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 14.MacInnis M.J., Gibala M.J. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber C.E., Blissmer B., Deschenes M.R., et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sport Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 16.Tabata I., Irisawa K., Kouzaki M., Nishimura K., Ogita F., Miyachi M. Metabolic profile of high intensity intermittent exercises. Med Sci Sport Exerc. 1997;29(3):390–395. doi: 10.1097/00005768-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Buchheit M., Laursen P.B. High-intensity interval training, solutions to the programming puzzle. Part II: anaerobic energy, neuromuscular load and practical applications. Sport Med. 2013;43(10):927–954. doi: 10.1007/s40279-013-0066-5. [DOI] [PubMed] [Google Scholar]
- 18.Ross A., Leveritt M. Long-term metabolic and skeletal muscle adaptations to short-sprint training: implications for sprint training and tapering. Sport Med. 2001;31(15):1063–1082. doi: 10.2165/00007256-200131150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson J., Saltin B. Oxygen deficit and muscle metabolites in intermittent exercise. Acta Physiol Scand. 1971;82(1):115–122. doi: 10.1111/j.1748-1716.1971.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 20.Islam H., Townsend L.K., McKie G.L., Medeiros P.J., Gurd B.J., Hazell T.J. Potential involvement of lactate and interleukin-6 in the appetite-regulatory hormonal response to an acute exercise bout. J Appl Physiol. 2017;123(3):614–623. doi: 10.1152/japplphysiol.00218.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cipryan L., Tschakert G., Hofmann P. Acute and post-exercise physiological responses to high-intensity interval training in endurance and sprint athletes. J Sport Sci Med. 2017;16(2):219–229. [PMC free article] [PubMed] [Google Scholar]
- 22.Langfort J.L., Zarzeczny R., Nazar K., Kaciuba-Uscilko H. The effect of low-carbohydrate diet on the pattern of hormonal changes during incremental, graded exercise in young men. Int J Sport Nutr Exerc Metab. 2001;11(2):248–257. doi: 10.1123/ijsnem.11.2.248. [DOI] [PubMed] [Google Scholar]
- 23.Krustrup P., Mohr M., Steensberg A., Bencke J., Kjaer M., Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sport Exerc. 2006;38(6):1165–1174. doi: 10.1249/01.mss.0000222845.89262.cd. [DOI] [PubMed] [Google Scholar]
- 24.Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab. 2014;307(7):E539–E552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen D.E., Albers P.H., Prats C., Baba O., Birk J.B., Wojtaszewski J.F. Human muscle fibre type-specific regulation of AMPK and downstream targets by exercise. J Physiol. 2015;593(8):2053–2069. doi: 10.1113/jphysiol.2014.283267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marliss E.B., Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271–S283. doi: 10.2337/diabetes.51.2007.s271. [DOI] [PubMed] [Google Scholar]
- 27.Scribbans T.D., Edgett B.A., Vorobej K., et al. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgomaster K.A., Heigenhauser G.J., Gibala M.J. Effect of short-term sprint interval training on human skeletal muscle carbohydrate metabolism during exercise and time-trial performance. J Appl Physiol. 2006;100(6):2041–2047. doi: 10.1152/japplphysiol.01220.2005. [DOI] [PubMed] [Google Scholar]
- 29.Jue T., Rothman D.L., Shulman G.I., Tavitian B.A., DeFronzo R.A., Shulman R.G. Direct observation of glycogen synthesis in human muscle with 13C NMR. Proc Natl Acad Sci U S A. 1989;86(12):4489–4491. doi: 10.1073/pnas.86.12.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochachka P.W., McClelland G.B. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200(Pt 2):381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- 31.Perry C.G., Heigenhauser G.J., Bonen A., Spriet L.L. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metabol. 2008;33(6):1112–1123. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- 32.Rampinini E., Sassi A., Azzalin A., et al. Physiological determinants of Yo-Yo intermittent recovery tests in male soccer players. Eur J Appl Physiol. 2010;108(2):401–409. doi: 10.1007/s00421-009-1221-4. [DOI] [PubMed] [Google Scholar]
- 33.Dupont G., Berthoin S. Time spent at a high percentage of O2max for short intermittent runs: active versus passive recovery. Can J Appl Physiol. 2004;29(Suppl):S3–S16. doi: 10.1139/h2004-054. [DOI] [PubMed] [Google Scholar]
- 34.Billat L.V. Use of blood lactate measurements for prediction of exercise performance and for control of training. Recommendations for long-distance running. Sport Med. 1996;22(3):157–175. doi: 10.2165/00007256-199622030-00003. [DOI] [PubMed] [Google Scholar]
- 35.Conwit R.A., Stashuk D., Tracy B., McHugh M., Brown W.F., Metter E.J. The relationship of motor unit size, firing rate and force. Clin Neurophysiol. 1999;110(7):1270–1275. doi: 10.1016/s1388-2457(99)00054-1. [DOI] [PubMed] [Google Scholar]
- 36.Bishop D.J., Granata C., Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta. 2014;1840(4):1266–1275. doi: 10.1016/j.bbagen.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Taylor C.W., Ingham S.A., Hunt J.E., Martin N.R., Pringle J.S., Ferguson R.A. Exercise duration-matched interval and continuous sprint cycling induce similar increases in AMPK phosphorylation, PGC-1alpha and VEGF mRNA expression in trained individuals. Eur J Appl Physiol. 2016;116(8):1445–1454. doi: 10.1007/s00421-016-3402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilian Y., Wehmeier U.F., Wahl P., Mester J., Hilberg T., Sperlich B. Acute response of circulating vascular regulating MicroRNAs during and after high-intensity and high-volume cycling in children. Front Physiol. 2016;7:92. doi: 10.3389/fphys.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daussin F.N., Zoll J., Dufour S.P., et al. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R264–R272. doi: 10.1152/ajpregu.00875.2007. [DOI] [PubMed] [Google Scholar]
- 40.Tan R., Nederveen J.P., Gillen J.B., et al. Skeletal muscle fiber-type-specific changes in markers of capillary and mitochondrial content after low-volume interval training in overweight women. Phys Rep. 2018;6(5) doi: 10.14814/phy2.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casuso R.A., Plaza-Diaz J., Ruiz-Ojeda F.J., et al. High-intensity high-volume swimming induces more robust signaling through PGC-1alpha and AMPK activation than sprint interval swimming in m. triceps brachii. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Little J.P., Safdar A., Wilkin G.P., Tarnopolsky M.A., Gibala M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martins E.L., Ricardo J.C., de-Souza-Ferreira E., Camacho-Pereira J., Ramos-Filho D., Galina A. Rapid regulation of substrate use for oxidative phosphorylation during a single session of high intensity interval or aerobic exercises in different rat skeletal muscles. Comp Biochem Physiol B Biochem Mol Biol. 2018;217:40–50. doi: 10.1016/j.cbpb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Burgomaster K.A., Hughes S.C., Heigenhauser G.J., Bradwell S.N., Gibala M.J. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. J Appl Physiol. 2005;98(6):1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 45.Daussin F.N., Ponsot E., Dufour S.P., et al. Improvement of O2max by cardiac output and oxygen extraction adaptation during intermittent versus continuous endurance training. Eur J Appl Physiol. 2007;101(3):377–383. doi: 10.1007/s00421-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 46.MacInnis M.J., Zacharewicz E., Martin B.J., et al. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J Physiol. 2017;595(9):2955–2968. doi: 10.1113/JP272570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trombold J.R., Christmas K.M., Machin D.R., Kim I.Y., Coyle E.F. Acute high-intensity endurance exercise is more effective than moderate-intensity exercise for attenuation of postprandial triglyceride elevation. J Appl Physiol. 2013;114(6):792–800. doi: 10.1152/japplphysiol.01028.2012. [DOI] [PubMed] [Google Scholar]
- 48.Gibala M.J., Little J.P., van Essen M., et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575(Pt 3):901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burkewitz K., Zhang Y., Mair W.B. AMPK at the nexus of energetics and aging. Cell Metabol. 2014;20(1):10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao B., Heath R., Saiu P., et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449(7161):496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 51.Treebak J.T., Birk J.B., Rose A.J., Kiens B., Richter E.A., Wojtaszewski J.F. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292(3):E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman N.J., Parker B.L., Chaudhuri R., et al. Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates. Cell Metabol. 2015;22(5):922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee-Young R.S., Canny B.J., Myers D.E., McConell G.K. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009;107(1):283–289. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- 54.Fujii N., Hayashi T., Hirshman M.F., et al. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273(3):1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 55.Birk J.B., Wojtaszewski J.F. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577(Pt 3):1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jorgensen S.B., Wojtaszewski J.F., Viollet B., et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19(9):1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 57.Capes E.M., Loaiza R., Valdivia H.H. Ryanodine receptors. Skelet Muscle. 2011;1(1):18. doi: 10.1186/2044-5040-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Place N., Ivarsson N., Venckunas T., et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc Natl Acad Sci U S A. 2015;112(50):15492–15497. doi: 10.1073/pnas.1507176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koltai E., Bori Z., Osvath P., et al. Master athletes have higher miR-7, SIRT3 and SOD2 expression in skeletal muscle than age-matched sedentary controls. Redox Biol. 2018;19:46–51. doi: 10.1016/j.redox.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandauer J., Andersen M.A., Kellezi H., et al. AMP-activated protein kinase controls exercise training- and AICAR-induced increases in SIRT3 and MnSOD. Front Physiol. 2015;6:85. doi: 10.3389/fphys.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu H., Kanatous S.B., Thurmond F.A., et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296(5566):349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 62.Kelly D.P., Scarpulla R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 63.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants Redox Signal. 2013;18(10):1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawley S.A., Pan D.A., Mustard K.J., et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabol. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 65.Ye C., Zhang D., Zhao L., et al. CaMKK2 suppresses muscle regeneration through the inhibition of myoblast proliferation and differentiation. Int J Mol Sci. 2016;17(10) doi: 10.3390/ijms17101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Summermatter S., Thurnheer R., Santos G., et al. Remodeling of calcium handling in skeletal muscle through PGC-1alpha: impact on force, fatigability, and fiber type. Am J Physiol Cell Physiol. 2012;302(1):C88–C99. doi: 10.1152/ajpcell.00190.2011. [DOI] [PubMed] [Google Scholar]
- 67.Scarpulla R.C., Vega R.B., Kelly D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9):459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang H.C., Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25(3):138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scarpulla R.C., Vega R.B., Kelly D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab. 2012;23(9):459–466. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Islam H., Edgett B.A., Gurd B.J. Coordination of mitochondrial biogenesis by PGC-1alpha in human skeletal muscle: a re-evaluation. Metabolism. 2018;79:42–51. doi: 10.1016/j.metabol.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Egan B., Carson B.P., Garcia-Roves P.M., et al. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588(Pt 10):1779–1790. doi: 10.1113/jphysiol.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez-Redondo V., Pettersson A.T., Ruas J.L. The hitchhiker's guide to PGC-1alpha isoform structure and biological functions. Diabetologia. 2015;58(9):1969–1977. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 73.Tadaishi M., Miura S., Kai Y., et al. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1alpha mRNA: a role of beta(2)-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2011;300(2):E341–E349. doi: 10.1152/ajpendo.00400.2010. [DOI] [PubMed] [Google Scholar]
- 74.Ruas J.L., White J.P., Rao R.R., et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151(6):1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandt N., Dethlefsen M.M., Bangsbo J., Pilegaard H. PGC-1alpha and exercise intensity dependent adaptations in mouse skeletal muscle. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibala M.J., McGee S.L., Garnham A.P., Howlett K.F., Snow R.J., Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106(3):929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 77.Little J.P., Safdar A., Bishop D., Tarnopolsky M.A., Gibala M.J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1alpha and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1303–R1310. doi: 10.1152/ajpregu.00538.2010. [DOI] [PubMed] [Google Scholar]
- 78.Li F.H., Li T., Ai J.Y., et al. Beneficial autophagic activities, mitochondrial function, and metabolic phenotype Adaptations promoted by high-intensity interval training in a rat model. Front Physiol. 2018;9:571. doi: 10.3389/fphys.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Summermatter S., Santos G., Perez-Schindler J., Handschin C. Skeletal muscle PGC-1alpha controls whole-body lactate homeostasis through estrogen-related receptor alpha-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci U S A. 2013;110(21):8738–8743. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiorenza M., Gunnarsson T.P., Hostrup M., et al. Metabolic stress-dependent regulation of the mitochondrial biogenic molecular response to high-intensity exercise in human skeletal muscle. J Physiol. 2018;596(14):2823–2840. doi: 10.1113/JP275972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canto C., Menzies K.J., Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metabol. 2015;22(1):31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 83.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 84.White A.T., Schenk S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303(3):E308–E321. doi: 10.1152/ajpendo.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green H.J., Jones S., Ball-Burnett M., Farrance B., Ranney D. Adaptations in muscle metabolism to prolonged voluntary exercise and training. J Appl Physiol. 1995;78(1):138–145. doi: 10.1152/jappl.1995.78.1.138. [DOI] [PubMed] [Google Scholar]
- 86.Morales-Alamo D., Ponce-Gonzalez J.G., Guadalupe-Grau A., et al. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKalpha phosphorylation in human skeletal muscle. J Appl Physiol. 2013;114(5):566–577. doi: 10.1152/japplphysiol.01246.2012. [DOI] [PubMed] [Google Scholar]
- 87.Phillips S.M., Green H.J., Tarnopolsky M.A., Heigenhauser G.J., Grant S.M. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol. 1996;270(2 Pt 1):E265–E272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- 88.Lollgen H., Graham T., Sjogaard G. Muscle metabolites, force, and perceived exertion bicycling at varying pedal rates. Med Sci Sport Exerc. 1980;12(5):345–351. [PubMed] [Google Scholar]
- 89.Canto C., Gerhart-Hines Z., Feige J.N., et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y., Dash R.K., Kim J., Saidel G.M., Cabrera M.E. Role of NADH/NAD+ transport activity and glycogen store on skeletal muscle energy metabolism during exercise: in silico studies. Am J Physiol Cell Physiol. 2009;296(1):C25–C46. doi: 10.1152/ajpcell.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stein L.R., Imai S. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab. 2012;23(9):420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Graham T., Sjogaard G., Lollgen H., Saltin B. NAD in muscle of man at rest and during exercise. Pflüg Arch. 1978;376(1):35–39. doi: 10.1007/BF00585245. [DOI] [PubMed] [Google Scholar]
- 93.Gurd B.J., Perry C.G., Heigenhauser G.J., Spriet L.L., Bonen A. High-intensity interval training increases SIRT1 activity in human skeletal muscle. Appl Physiol Nutr Metabol. 2010;35(3):350–357. doi: 10.1139/H10-030. [DOI] [PubMed] [Google Scholar]
- 94.Nemes R., Koltai E., Taylor A.W., Suzuki K., Gyori F., Radak Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants. 2018;7(7) doi: 10.3390/antiox7070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594(18):5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferreira L.F., Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med. 2016;98:18–28. doi: 10.1016/j.freeradbiomed.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rush J.W. Exercising an option to prevent age related decline of vascular BH4 and uncoupling of eNOS. J Physiol. 2009;587(Pt 15):3755. doi: 10.1113/jphysiol.2009.177261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sachdev S., Davies K.J. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44(2):215–223. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 99.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramos-Filho D., Chicaybam G., de-Souza-Ferreira E., et al. High intensity interval training (HIIT) induces specific changes in respiration and electron leakage in the mitochondria of different rat skeletal muscles. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0131766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radak Z., Asano K., Inoue M., et al. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol. 1995;79(1):129–135. doi: 10.1152/jappl.1995.79.1.129. [DOI] [PubMed] [Google Scholar]
- 102.Davies K.J., Quintanilha A.T., Brooks G.A., Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 103.Radak D., Resanovic I., Isenovic E.R. Link between oxidative stress and acute brain ischemia. Angiology. 2014;65(8):667–676. doi: 10.1177/0003319713506516. [DOI] [PubMed] [Google Scholar]
- 104.Radak Z., Hart N., Sarga L., et al. Exercise plays a preventive role against Alzheimer's disease. J Alzheimer's Dis. 2010;20(3):777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 105.Henriksen E.J. Effects of H2O2 on insulin signaling the glucose transport system in mammalian skeletal muscle. Methods Enzymol. 2013;528:269–278. doi: 10.1016/B978-0-12-405881-1.00016-1. [DOI] [PubMed] [Google Scholar]
- 106.Trewin A.J., Lundell L.S., Perry B.D., et al. Effect of N-acetylcysteine infusion on exercise-induced modulation of insulin sensitivity and signaling pathways in human skeletal muscle. Am J Physiol Endocrinol Metab. 2015;309(4):E388–E397. doi: 10.1152/ajpendo.00605.2014. [DOI] [PubMed] [Google Scholar]
- 107.Contreras-Ferrat A., Llanos P., Vasquez C., et al. Insulin elicits a ROS-activated and an IP(3)-dependent Ca(2)(+) release, which both impinge on GLUT4 translocation. J Cell Sci. 2014;127(Pt 9):1911–1923. doi: 10.1242/jcs.138982. [DOI] [PubMed] [Google Scholar]
- 108.Kellogg D.L., 3rd, McCammon K.M., Hinchee-Rodriguez K.S., Adamo M.L., Roman L.J. Neuronal nitric oxide synthase mediates insulin- and oxidative stress-induced glucose uptake in skeletal muscle myotubes. Free Radic Biol Med. 2017;110:261–269. doi: 10.1016/j.freeradbiomed.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Irrcher I., Ljubicic V., Hood D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol. 2009;296(1):C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 110.Cunha V.N., de Paula Lima M., Motta-Santos D., et al. Role of exercise intensity on GLUT4 content, aerobic fitness and fasting plasma glucose in type 2 diabetic mice. Cell Biochem Funct. 2015;33(7):435–442. doi: 10.1002/cbf.3128. [DOI] [PubMed] [Google Scholar]
- 111.Radak Z., Chung H.Y., Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 112.Andrade F.H., Reid M.B., Allen D.G., Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Radak Z., Torma F., Berkes I., et al. Exercise effects on physiological function during aging. Free Radic Biol Med. 2018 doi: 10.1016/j.freeradbiomed.2018.10.444. [DOI] [PubMed] [Google Scholar]
- 114.St-Pierre J., Drori S., Uldry M., et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 115.Parker L., Trewin A., Levinger I., Shaw C.S., Stepto N.K. Exercise-intensity dependent alterations in plasma redox status do not reflect skeletal muscle redox-sensitive protein signaling. J Sci Med Sport. 2018;21(4):416–421. doi: 10.1016/j.jsams.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 116.Bartlett J.D., Hwa Joo C., Jeong T.S., et al. Matched work high-intensity interval and continuous running induce similar increases in PGC-1alpha mRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol. 2012;112(7):1135–1143. doi: 10.1152/japplphysiol.01040.2011. [DOI] [PubMed] [Google Scholar]
- 117.Ciolac E.G., Bocchi E.A., Bortolotto L.A., Carvalho V.O., Greve J.M., Guimaraes G.V. Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res. 2010;33(8):836–843. doi: 10.1038/hr.2010.72. [DOI] [PubMed] [Google Scholar]
- 118.Edge J., Bishop D., Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96(1):97–105. doi: 10.1007/s00421-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 119.Helgerud J., Hoydal K., Wang E., et al. Aerobic high-intensity intervals improve O2max more than moderate training. Med Sci Sport Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 120.Hwang C.L., Yoo J.K., Kim H.K., et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–119. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henriksson J., Reitman J.S. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97(3):392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- 122.Baekkerud F.H., Solberg F., Leinan I.M., Wisloff U., Karlsen T., Rognmo O. Comparison of three popular exercise modalities on V O2max in overweight and obese. Med Sci Sport Exerc. 2016;48(3):491–498. doi: 10.1249/MSS.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 123.Poole D.C., Gaesser G.A. Response of ventilatory and lactate thresholds to continuous and interval training. J Appl Physiol. 1985;58(4):1115–1121. doi: 10.1152/jappl.1985.58.4.1115. [DOI] [PubMed] [Google Scholar]
- 124.Saltin B., Nazar K., Costill D.L., et al. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96(3):289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]