Abstract
Glycemic variability is a more sensitive assessment of glycemic health as opposed to traditional clinical measurements. It considers all blood glucose concentrations over a given period to better account for glucose oscillations that occur and provides clinicians with insight into how individuals regulate and/or maintain their glycemic health. The advancement of continuous glucose monitoring (CGM) allows for the measurement of free-living glucose concentrations while providing a more reliable assessment of treatment of dysregulated glycemic. CGM coupled with management of lifestyle behavioral factors, such as reduced sedentary behavior and increased physical activity and regular exercise, potentially offers a previously untapped method for promoting improved glycemic health through greater regulation of glucose concentrations. The aim of this review is to critically evaluate the evidence regarding the measurement of glycemic variability and summarize the current understanding of the relationship between glycemic variability, sedentary behavior, physical activity, the influence of a single exercise session or repeated exercise sessions, and exercise training. This review considers information pertaining to the strengths and limitations for measuring glycemic variability and provides insight into future study designs aimed at evaluating the relationship between sedentary behavior and physical activity with, as well as the influence of exercise on, glycemic variability as a primary outcome.
Keywords: Continuous glucose monitoring (CGM), Exercise, Glycemic control, Glycemic variability, Physical activity
Abbreviations
- %CV
Percentage coefficient of variation
- AUC
Area under the curve
- CGM
Continuous glucose monitor
- CINAHL
Cumulative Index of Nursing and Allied Health Literature
- CMI/CMIE
Continuous moderate-intensity exercise
- CONGA
Continuous overlapping net glycemic action
- HbA1c
Hemoglobin A1c
- HIIEfast
Fasted state high-intensity interval exercise
- HIIEfed
Post-breakfast high-intensity interval exercise
- HIIE
High-intensity interval exercise
- HIIT
High-intensity interval training
- LW
Light-intensity walking
- LV-HIIE
Low volume high-intensity interval exercise
- MAGE
Mean amplitude of glycemic excursion
- mg/dL
Milligrams per deciliter
- MICEfast
Fasted state moderate-intensity continuous exercise
- MICEfed
Post-breakfast moderate-intensity continuous exercise
- MODD
Mean of daily difference
- OGTT
Oral glucose tolerance test
- REHIIT
Reduced-exercise high-intensity interval training
- SD
Standard deviation
- SIT
Uninterrupted sitting
- SRA
Simple resistance activities
Introduction
Glycemic variability accounts for glucose fluctuations and is considered a sensitive measure of glycemic health and has become a readily available assessment tool due to advancements in continuous glucose monitor (CGM) technology.1,2 Glycemic variability has been shown to be greater in overweight or obese adults compared to normal-weight adults, as well as in type 1 and type 2 diabetics compared to adults without diabetes.3 Decreased sedentary time and increased physical activity or exercise of any intensity are beneficial for better glycemic health.4,5 A single exercise session or repeated exercise sessions over a short duration (< 1week) reduce glycemic variability in healthy and metabolically compromised adults, while studies in type 2 diabetic adults have noted improvements in glycemic variability following exercise training, including aerobic, resistance, and high-intensity interval training (HIIT).6 Therefore, the aim of this review is to highlight the importance of glycemic variability, evaluate the relationship of glycemic variability with sedentary behavior and physical activity, and evaluate how a single exercise session or repeated exercise sessions and exercise training influence glycemic variability.
Importance of glycemic variability
Evaluation of glycemic control has historically involved evaluation of hemoglobin A1c (HbA1c) levels, which accounts for the average volume of glucose bound to the hemoglobin of circulating red blood cells during a 3-month period.7 Type 1 and type 2 diabetic patients typically have HbA1c measured every 3-month to determine whether glycemic control targets are obtained and/or maintained.8 Impaired glycemic control assessed by HbA1c is a strong predictor for diabetes onset and diabetic complications.9, 10, 11 Thus, glycemic control assessed as HbA1c is a useful tool for a point-of-care opportunity between healthcare providers and patients in optimizing glycemic management to deter the progression of diabetic complications.
However, using HbA1c as a sole measure of glycemic health to assess glycemic control has limitations, as the cut-point of HbA1c from the diagnostic perspective is still controversial.12 Additionally, the HbA1c test is considered an indirect measure of average glycemia as HbA1c variability often results from variations in red blood cell numbers brought on by cell turnover, anemia, blood transfusions, and pregnancy.13,14 Additionally, HbA1c differences exist regarding ethnicity/race, sex, and age. For example, greater HbA1c values are observed in African Americans compared to Caucasians, males compared to females, and increases with age in all groups.15 These factors can potentially lead to misclassification in specific populations.
HbA1c measurement is traditionally accompanied with self-monitoring of blood glucose concentrations in addition to traditional clinical examinations of glycemic health in a fasting and/or glucose-challenged state.7 Mean glucose concentration utilizing CGM technology is strongly correlated with HbA1c.16 However, the relationship between CGM-assessed glucose concentrations and HbA1c can vary within an individual, due to individual-level non-modifiable factors (i.e., age) and modifiable factors (i.e., body weight status).17 CGM technology allows for further insight into daily fluctuations and day-to-day glucose concentration variability. This is especially true as a strong relationship between CGM-assessed 24-h mean glucose concentration and HbA1c are not often reflected on an individual level.17 Therefore, clinical use of CGM in conjunction with HbA1c evaluation allows for a greater understanding of individual-level glycemic control. With the advent of new CGM technology from established companies, specifically Dexcom (San Diego, CA), Abbott Laboratories (Chicago, IL), Medtronic (Minneapolis, MN), and Senseonics Holdings (Germantown, MD), CGM accuracy and affordability is evolving rapidly while allowing for evaluation of free-living glucose concentrations and glycemic variability.
Methods
The present brief review sought to summarize recent literature on the specified topic and provide future directions for use and implementation by better understanding the importance of glycemic variability, relationship with sedentary behavior and physical activity, and the influence of exercise. To address the study purpose, a search of electronic databases PubMed, Google Scholar, Web of Science, BioMed Central, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and SCOPUS was completed up to December 2020. A secondary search strategy included scanning bibliographies of retrieved publications. The initial manuscript selection was based on title and abstract. Glycemic variability is commonly referred to as glycemic excursions and glucose or glycemic control, and therefore, these terms were included as search terms. Only studies using CGM to assess glycemic variability were included, specifically, studies utilizing CGM devices previously tested for accuracy against blood glucose concentrations were included in this analysis. Studies examining the relationship between sedentary behavior and exercise on glycemic variability regardless of study design were included. Sedentary behavior, defined as any waking behavior ≤ 1.5 metabolic equivalents, and time spent sedentary was included as search terms. Physical activity variables measured subjectively by questionnaires or objectively utilizing accelerometry and/or pedometers were included in this analysis. How a single exercise session or repeated exercise sessions and exercise training affects glycemic variability were also examined only if exercise training was monitored.
Determining glycemic variability
Glycemic variability measurements range in sensitivity and specificity, with each having its own considerations depending on the method of calculation, number of readings necessary, and respective clinical implications.18 These considerations include establishing intra- and inter-day glycemic variability utilizing CGM technology.19 Glycemic variability measurements include the standard deviation (SD) of the mean for glucose concentration and the percent coefficient of variation (%CV). Further comprehensive analyses, which allow for a more sensitive measurement of glycemic variability, have been adopted, including the mean amplitude of glycemic excursions (MAGE), continuous overlapping net glycemic action over an n-h period (CONGA-n), and the mean of daily differences (MODD) which is the average of the difference between blood glucose values measured at the same time on consecutive days. In the following section, the most frequently used measurements of glycemic variability are discussed with strengths and limitations listed (Table 1).
Table 1.
Frequently used measurements of glycemic variability.
| Glycemic variability measurement | Calculation definition | Strengths | Limitations | Author (publication date) |
|---|---|---|---|---|
| Standard deviation (SD) | SD of all glucose concentrations in a distribution. | Simple, classical statistical method. | Does not account for skewed distributions or outliers. | Rodbard D (2009)25; Hirsch IB & Brownlee M (2005)2; Rodbard D (2018)18 |
| Percent coefficient of variation (%CV) | (SD÷mean) × 100 SD and mean value of all glucose concentrations in a distribution. |
Simple, classical statistical method. Incorporates both the SD and mean value of a distribution. |
Does not account for skewed distributions or outliers. | Rodbard D (2009)25; Hirsch IB & Brownlee M (2005)2; Rodbard D (2018)18 |
| Mean amplitude of glycemic excursions (MAGE) | Average amplitude of upstrokes or downstrokes with a magnitude > 1 SD above the mean value for all glucose concentrations. | Account for physiological fluctuations due to events throughout the day. e.g. meals, exercise |
Less efficient to calculate than SD, while providing similar outcomes. | Service FJ et al. (1970)22; Service FJ & Nelson RL (1980)23; Service et al. (1987)24 |
| Continuous overlapping net glycemic action over n-h (CONGA-n) | The SD of the difference between 2 glucose concentrations obtained exactly n h apart. | Potential to address a variety of clinical questions. CONGA-1 to CONGA-4 valid and reliable when accounting for corresponding times between different activities. |
Validity and reliability decrease once time frame > 4 h in a controlled setting. | McDonnell CM et al. (2005)26; Nathan DM et al. (2008)27; Kuenen JC et al. (2011)28 |
| Mean of daily differences (MODD) | Mean of absolute differences between glucose values obtained at the same time of day on 2 consecutive days under standardized conditions. Mean of absolute differences in glucose values over > 2 days between any value and the value exactly 24 h later. |
Describes between-day variability. Ability to permit use of data from > 2 unstructured days. |
Originally defined for 2 consecutive days assuming similar meals, activities, and therapy on both days. | Service FJ & Nelson RL (1980)23; Service et al. (1987)24 |
Table 1 provides frequently used measurements of glycemic variability, which includes the calculation definition of each glycemic variability measurement with further consideration for strengths and limitations of each glycemic variability measurement.
SD = standard deviation; %CV = percentage coefficient of variation; MAGE = mean amplitude of glycemic excursions; CONGA-n = continuous overlapping net glycemic action over n-h; MODD = mean of daily differences.
The SD of mean glucose concentration and %CV were the initial primary procedures utilized.20 Statistically, SD and %CV are often thought of as the best variation measure, because these measures are based on all measurements.21 Additionally, %CV is potentially more inclusive and sensitive when compared to SD alone, as %CV incorporates both the mean and SD of the distribution.20
One of the initial avenues for determining intra-day glycemic variability is MAGE,22 which is calculated by taking the arithmetic mean of the blood glucose increases or decreases (from blood glucose nadirs to peaks or vice versa) when both ascending and descending segments exceed the value of 1 SD of the mean blood glucose for the same 24-h period.23 MAGE does not account for time spent in euglycemia or periods of low-level hypo- or hyper-glycemia.24 This calculation does create ambiguity as to where or when each glycemic excursion occurs and potentially limits the ability to measure the magnitude of individual glycemic excursions.25 However, MAGE provides insight into the extent that glycemic excursions occur, specifically accounting for postprandial hyperglycemia and fasted-state hypoglycemia.
Consideration for time-dependent glycemic variability utilizing the continuous nature of CGM is determined with other measurements. CONGA-n was formulated to account for each observation after the base measurement and the n-h of observations after the base measurement, where n equals the defined hour time-point between observations.26 The difference between the base observation and the observation n-h is calculated with CONGA-n defined as the SD of the differences.26 CONGA-n is calculated from 1-h up to 24-h; but is mostly utilized for 1-h to 4-h intervals to better account for time between various daily activities.26, 27, 28 For example, CONGA-1 was initially created as a measurement of glycemic variability to account for glycemic excursions throughout a 24-h period, while CONGA-2 accounts for the time between snacks, and CONGA-4 as the variability observed between standard meals.26 CONGA-n continues as a more promising measure of intra-day glycemic variability than SD or %CV and provides more flexibility in the evaluation of intra-day glycemic variability.26
In addition to intra-day glycemic variability, CGM allows for the analysis of inter-day glycemic variability. The MODD measure of inter-day glycemic variability is designed as a meticulous protocol occurring over 2 consecutive days during in-house clinical testing.23,24 MODD is traditionally calculated as the mean of the absolute value of the difference between glucose concentrations measured at the same time intervals over a 24-h period on 2 consecutive days23,24 and is calculated continuously over an extended free-living period (> 2 days).23,24 This use of standardized conditions procedure is strongly correlated with MODD over the initial 2 consecutive day period.25
With the advancement and incorporation of CGM technology, the ability to design and implement more sensitive and time-specific measures of glycemic variability are viable and improves the ability to delineate glycemic health. In addition to the SD and %CV of measured glucose concentrations over an extended period, the ability to measure inter- and intra-day variabilities are further advanced by incorporating MAGE, CONGA-n, and MODD results.
Results
Relationship of glycemic variability with sedentary behavior and physical activity
Sedentary time and physical inactivity are independently associated with elevated fasting glucose concentrations, impaired glucose metabolism, and glucose tolerance in non-diabetic and diabetic adults.4,5,29, 30, 31, 32, 33, 34 Adults diagnosed with type 2 diabetes have increased sedentary time, which is shown to predict significant increases in time spent in hyperglycemia.35 These findings highlight the relationship between sedentary time and physical activity time with glucose concentrations and glucose tolerance. However, few studies have examined the relationship between sedentary behavior and/or physical activity with glycemic variability (Table 2).
Table 2.
Relationship between sedentary behavior and physical activity with glycemic variability.
| Author (publication date) | Study design Number of participants |
Primary findings | Conclusion | Strengths and limitations |
|---|---|---|---|---|
| Non-diabetic | ||||
| Gude F et al. (2017)40 |
Cross-sectional; n = 622 |
No relationship found between physical activity status with any glycemic variability indices in non-diabetic adults. |
Physical activity status may not relate to glycemic variability indices in non-diabetic adults. |
Strengths: Large diverse sample of adults Limitations: Cross-sectional design; subjective assessment of physical activity status |
| Type 1 diabetes | ||||
| Martyn-Nemeth P et al. (2017)36 |
Prospective repeated-measures design; n = 35 |
Total physical activity minutes did not relate to glycemic variability assessed as the SD of the 24-h mean glucose concentration. |
Increases in total physical activity performed throughout the day may not relate to lower glycemic variability in type 1 diabetic adults. |
Strengths: Actigraphy-assessed physical activity Limitations: Small sample size; physical activity was not a primary outcome of this study |
| Type 2 diabetes | ||||
| Paing AC et al. (2018)37 | Cross-sectional; n = 37 | Sedentary time negatively and breaks in sedentary time positively associated with time spent in euglycemia. | Decreasing sedentary time, breaking up sedentary time, or a combination of these sedentary behaviors potentially influence time spent in euglycemia in type 2 diabetic adults. |
Strengths: Actigraphy-assessed sedentary time Limitations: Cross-sectional design; small sample size |
| Paing AC et al. (2020)38 | Longitudinal; n = 37 | Increased sedentary time positively associated with higher glucose concentrations and time spent in-range. | Reducing sedentary time and promoting breaks in sedentary time could improve glucose regulation in type 2 diabetes adults. |
Strengths: Actigraphy-assessed sedentary time Limitations: Cross-sectional design; small sample size |
| McMillan KA et al. (2020)39 | Longitudinal; n = 37 | No association between total sedentary time and mean glucose Sedentary bout duration was positively associated with mean glucose and glucose SD |
Sedentary bout duration but not sedentary time was associated with mean glucose and glucose variability. |
Strengths: Actigraphy-assessed sedentary time; individual level analysis Limitations: Cross-sectional design; small sample size |
Table 2 presents studies that provided information regarding the association between sedentary time and physical activity with glycemic control and glycemic variability in non-diabetic, as well as type 1 and type 2 diabetic adults.
The table includes: 1) author information; 2) study design; 3) findings related to the association between sedentary time and physical activity with glycemic control and glycemic variability; 4) conclusions derived from the findings between the relationship between sedentary time and physical activity with glycemic control and glycemic variability; 5) strength and limitations of each study.
SD = standard deviation; mg/dL = milligrams per deciliter.
Using a repeated-measures prospective study design, Martyn-Nemeth et al.36 evaluated young adults (18–35 years of age) diagnosed with type 1 diabetes and found no relationship between total physical activity minutes and the SD of the 24-h mean glucose concentration.36 Paing et al.37 examined the associations between objectively measured sedentary time with 24-h glycemic control in adult type 2 diabetics and found that increased sedentary time was positively associated with poor CGM-assessed glycemic control expressed as hours per day spent in hyperglycemia (> 7.8 mmol/L; 140 mg/dl).37 Subsequently, spending more time sedentary reduced time spent in euglycemia. However, breaks in sedentary time were positively associated with time spent in euglycemia. Therefore, decreasing sedentary time and breaking up time spent sedentary positively impacts glycemic variability, independent of increases in physical activity.36,37
More recently, secondary data analyses performed by Paing et al.38 and McMillan et al.39 further support these findings as sedentary time was negatively associated with time-in-range,38 with time-in-range defined as any glucose concentration between 70 and 180 mg/dL.7 Breaks in sedentary time were positively associated with time-in-range and sedentary time was positively correlated with the SD of mean glucose concentration.38,39 Gude et al.40 utilized a cross-sectional design to analyze a subsample of non-diabetic and diabetic adults (12% diagnosed type 1 and 2 diabetics) who were participants in the A Estrada Glycation and Inflammation Study and completed the International Physical Activity Questionnaire and had valid CGM monitoring data.40 No relationship was found between physical activity status with glycemic variability measures including the SD of 24-h glucose concentration, MAGE, and CONGA-1 in the non-diabetic adults. No findings were reported for the diabetic population.40
These studies suggest the potential importance of sedentary behavior and physical activity with glycemic control and variability assessed by CGM in a variety of populations. Further, they provide insight into how CGM is incorporated as a measure of glycemic control and variability in addition to traditional clinical measures. However, when examining CGM-assessed glycemic variability, further consideration for other lifestyle factors, including free-living dietary patterns or structured physical activity, such as exercise, could provide information on how to further improve glycemic variability outside of decreasing time spent sedentary and increasing physical activity.
Influence of single or repeated exercise sessions on glycemic variability
Exercise is known to beneficially influence insulin resistance and glucose tolerance and is commonly utilized as a treatment for both non-insulin and insulin-dependent type 2 diabetes.41,42 Even a single exercise session beneficially impacts glucose homeostasis, whole-body glucose disposal, and skeletal muscle glucose uptake.43, 44, 45, 46 The effects on glucose uptake and insulin sensitivity persist for several hours after exercise completion.47, 48, 49
Previous studies examining the influence of a single exercise or repeated exercise sessions on CGM-assessed glycemic control and variability in non-diabetic, normal weight, and overweight or obese adults report comparable and consistent results (Table 3).50, 51, 52 Figueira et al.52 found that a single session of moderate-intensity aerobic exercise or eccentric resistance exercise provided similar decreases in glycemic variability when compared to the pre-exercise control period.52 Little et al.50 found that the postprandial glycemic response decreased following 1 session of high-intensity interval exercise (HIIE) when compared to continuous moderate-intensity exercise and control conditions.50 Parker et al.51 observed that the 24-h average glucose concentration and hyperglycemic response decreased following either low-volume HIIE or continuous moderate-intensity exercise in overweight or obese normoglycemic adults.51 In these studies glycemic variability and glycemic control were measured in a variety of ways including 24-h mean glucose concentration, SD and %CV of the 24-h mean glucose concentration, and postprandial glycemic excursions. These studies highlight that a single exercise session or repeated single exercise sessions, regardless of exercise modality or duration, provide beneficial and lasting effects on 24-h glycemic control and variability in adults having normal weight, overweight, or obesity.
Table 3.
Influence of a single bout of exercise or repeated bouts of exercise on glycemic variability.
| Author (publication date) | Study design | Primary findings | Conclusion | Strengths and limitations |
|---|---|---|---|---|
| Non-diabetic | ||||
| Figueira FR et al. (2019)52 | Randomized crossover trial design; n = 15 2 experimental sessions; aerobic cycle ergometry; eccentric resistance exercise |
Glucose variance and glucose %CV and SD were lower post-exercise compared to pre-exercise. | Acute aerobic and eccentric exercise promotes comparable reductions in glycemic variability. |
Strengths: Controlled laboratory setting Limitations: Small sample size; exercise was of moderate to high intensity over an extended period |
| Little JP et al. (2014)50 | Randomized counterbalance trial design; n = 10 Two 3-day experimental exercise testing periods; continuous moderate-intensity (CMI) exercise; high-intensity interval exercise (HIIE) |
Absolute PPG spike following standardized meals were significantly lower following HIIT exercise compared to no-exercise. | A single session of HIIE exercise improved overall postprandial glycemia in overweight or obese adults. |
Strengths: Controlled laboratory setting. Limitations: Small sample size; inclusion of adults with impaired fating glucose |
| Parker L et al. (2017)51 |
Randomized clinical trial; n = 27 4-day experimental design; low volume high-intensity interval exercise (LV-HIIE); continuous moderate-intensity exercise (CMIE) |
LV-HIIE resulted in lower mean glucose and peak glucose concentration, area under the curve, and time spent hyperglycemic compared to pre-exercise control. |
LV-HIIE improves glycemic control similarly to CMIE in overweight and obese adults. |
Strengths: Controlled laboratory setting Limitations: Small sample size; participants were not blinded to real-time CGM readings |
| Type 1 diabetes | ||||
| van Dijk JW et al. (2016)54 | Observational during Nijegen Four Day Marches; n = 10 40–50 km walked per day over 4 days |
CONGA-1 and CONGA-2 measures of glycemic variability were greater during walking event compared to habitual physical activity. | Prolonged continuous walking compared to habitual physical activity increased glycemic variability in type 1 diabetics. |
Strengths: Examined a prolonged exposure to increase in physical activity Limitations: Small sample size; non-standard exercise modality |
| Manohar C et al. (2012)53 |
Center-based clinical trial; n = 24 Increase daily energy expenditure 3-fold from measured basal metabolic rate over 3 monitored days |
No change in %CV was noted in type 1 diabetic adults following meals with physical activity. Post-meal glycemic excursions were observed to be lower type 1 diabetics following meals with physical activity. |
Performing low-intensity physical activity after meals, such as taking a short walk, potentially benefit type 1 diabetics by lowering postprandial glucose excursions. |
Strengths: Age- and sex-matched healthy controls and type 1 diabetics; controlled laboratory setting Limitations: Small sample size; type 1 diabetics received insulin boluses prior to their meals |
| Type 2 diabetes | ||||
| Farabi SS et al. (2015)58 | Center-based randomized clinical cross-over trial; n = 37 Two 3-day experimental trials both in morning; sedentary for 30 min; 30-min exercise session |
Daytime CONGA-1 significantly decreased following exercise compared to sedentary trial. | A single bout of early morning moderate-intensity exercise reduced daytime glycemic variability in type 2 diabetic and/or impaired glucose tolerant obese adults. |
Strengths: Controlled laboratory setting Limitations: Inclusion of adults with impaired glucose tolerance in the same group |
| van Dijk JW et al. (2013)57 | Randomized crossover trial; total n = 60; non-insulin treated n = 37; insulin treated = 23 Two 3-day intervention periods separated by a week; sedentary protocol; 45–60 min of continuous cycling |
24-h mean glucose concertation, time spent hyperglycemic, and CONGA-1, CONGA-2, and CONGA-4 measures of glycemic variability were all lower following a single bout of exercise. | A single bout of moderate-intensity exercise reduces hyperglycemia and glycemic variability throughout the subsequent day following exercise. |
Strengths: Use of CGM; inclusion of insulin and non-insulin treated type 2 diabetics Limitations: Only inclusion of males |
| Praet SF et al. (2006)55 | Intervention-based clinical trial; n = 11 Resistance exercise and aerobic exercise |
Time spent hyperglycemic was significantly lower during the subsequent 24 h following exercise. | A single bout of exercise reduces the prevalence of hyperglycemia in insulin-treated, type 2 diabetic male adults. |
Strengths: Implementation of resistance and aerobic exercise Limitations: Small sample size; large inter-subject variability |
| Figueria FR et al. (2013)56 | Randomized crossover design performed 7 days apart; n = 14 Aerobic exercise; aerobic plus resistance exercise |
Changes in glycemic variability were noted in the aerobic plus resistance training group only. | Conventional analyses of glycemic variability may lack sensitivity to account for minor oscillations in glucose concentrations observed using non-conventional analyses. |
Strengths: Implementation of resistance and aerobic exercise; use of conventional and non-conventional methods Limitations: Small sample size; no resistance exercise only group |
| Haxhi J et al. (2016)60 | Randomized crossover trial performed 7 days apart; n = 9 Control; 40-min split exercise (20-min pre-lunch, 20-min post-lunch); 40-min continuous exercise immediately post-lunch |
Split exercise resulted in less time spent in hyperglycemia after lunch compared to continuous exercise. Continuous exercise reduced hyperglycemic time after breakfast consumed the morning after the exercise session. |
Splitting an exercise session into 2 bouts, pre- and post-lunch, affects the glycemic response to lunch, while a single-continuous isoenergetic session exerts its effect later in the 24-h period. |
Strengths: Implementation of a randomized crossover design; Evaluation of multiple measures of free-living glycemia. Limitations: Small sample size. |
| Myette-Cȏté É et al. (2016)59 | Randomized crossover design; n = 10 Morning-evening doses of metformin (no exercise); morning-evening doses of metformin with exercise; evening dose of metformin with exercise; morning dose of metformin with exercise |
Morning-evening doses of metformin with exercise increased the average 2-h postprandial incremental AUC following standardize meals but did not affect daily mean or fasting glucose concentration. | The addition of a bout of exercise to metformin led to an increase in postprandial glucose levels without affecting mean glucose concentrations. |
Strengths: Implementation of a randomized crossover design; Evaluation of metformin dosing with addition of exercise. Limitations: Small sample size; No exercise only group. |
| Terada T et al. (2016)61 | Randomized, controlled, crossover design; n = 10 Fasted state high-intensity interval exercise (HIIEfast); post-breakfast HIIE (HIIEfed); fasted state moderate-intensity continuous exercise (MICEfast); post-breakfast MICE (MICEfed); no exercise control |
Compared to the control condition, HIIEfast lowered 24-h mean glucose, fasting, overall postprandial glycemic increment, glycemic variability, and time spent in hyperglycemia. | HIIE is effective in lowering nocturnal/fasting glycemia. Exercise performed in the fasted state reduces postprandial glycemic increments. |
Strengths: Implementation of a randomized crossover design; Evaluation of multiple modalities of exercise and comparing fasted versus fed state. Continual monitoring past the exercise or control condition. Limitations: Small sample size. |
| Dempsey PC et al. (2016)62 | Randomized cross-over trial; n = 24 8-h conditions on 3 separate days with 6–14 day washout period Uninterrupted sitting (control; SIT); sitting plus 3-min bouts of light-intensity walking (LW) every 30 min; sitting plus 3-min bouts of simple resistance activities (SRA) every 30 min |
Compared with SIT, both activity-break conditions (LW and SRA) significantly attenuated incremental AUCs for glucose concentrations. | Interrupting prolonged sitting with brief bouts of LW or SRA attenuates acute postprandial glucose concentration responses in adults with type 2 diabetes mellitus. |
Strengths: Implementation of a randomized crossover design; Evaluation of multiple modalities of exercise that are easily incorporated into everyday life. Limitations: Small sample size; no continuous exercise implementation to compare to breaks in sedentary time with exercise. |
| Dempsey PC et al. (2017)63 | Randomized cross-over trial; n = 24 8-h conditions on 3 separate days with 6–14 day washout period Uninterrupted sitting (control; SIT); sitting plus 3-min bouts of light-intensity walking (LW) every 30 min; sitting plus 3-min bouts of simple resistance activities (SRA) every 30 min |
Compared with SIT, both LW and SRA reduced 22-h glucose and nocturnal mean glucose concentrations. | Interrupting prolonged sitting time with either LW or SRA reduced 22-h hyperglycaemia. |
Strengths: Implementation of a randomized crossover design; Evaluation of multiple modalities of exercise that are easily incorporated into everyday life. Limitations: Small sample size; no continuous exercise implementation to compare to breaks in sedentary time with exercise. |
| Metcalfe RS et al. (2018)64 | Randomized, four-trial crossover study; n = 11 No exercise (Con); 30 min of continuous exercise (MICT); 10 × 1 min ∼90% HRmax of high-intensity interval training (HIIT); 2 × 20 s maximal exertion sprinting reduced-exertion HIIT (REHIIT) |
Compared to CON, mean 24-h glucose concentration was lower following REHIIT, but not HIIT. Observed a lower glycaemic response to dinner AUC following both REHIIT and MICT but not HIIT. |
REHIIT may offer a genuinely time-efficient exercise option for improving 24-h glycaemia in men with type 2 diabetes and warrants further study. |
Strengths: Implementation of a randomized crossover design; Evaluation of multiple modalities of exercise. Limitations: Small sample size. |
Table 3 presents studies that provided information regarding the influence of a single bout of exercise or following repeated bouts of exercise on glycemic control and glycemic variability in non-diabetic, as well as type 1 and type 2 diabetic adults. The table includes: 1) author information; 2) study design; 3) findings related to the alterations in glycemic control and glycemic variability; 4) conclusions derived from the findings on changes in glycemic control and glycemic variability; 5) strength and limitations of each study.
SD = standard deviation; %CV = percentage coefficient of variation; CMI = continuous moderate-intensity exercise; HIIE = high-intensity interval exercise; LV-HIIE = low volume high-intensity interval exercise; CMIE = continuous moderate-intensity exercise; CGM = continuous glucose monitor; CONGA-1 = continuous overlapping net glycemic action over 1-h; CONGA-2 = continuous overlapping net glycemic action over 2-h; CONGA-4 = continuous overlapping net glycemic action over 4-h; HIIEfast = fasted state high-intensity interval exercise; HIIEfed = post-breakfast high-intensity interval exercise; MICEfast = fasted state moderate-intensity continuous exercise; MICEfed = post-breakfast moderate-intensity continuous exercise; SIT = uninterrupted sitting; LW = light-intensity walking; SRA = simple resistance activities; REHIIT = reduced-exercise high-intensity interval training; AUC = area under the curve.
Improving glycemic control and variability with exercise in individuals who rely on lifestyle self-management techniques to control glucose levels, such as adults diagnosed with type 1 diabetes, has profound implications. Previous research under free-living or clinic-based conditions has found that increasing structured physical activity or exercise has differential effects on glycemic control and variability in type 1 diabetic adults.53,54 Mixed findings are not uncommon, as 1 study noted differential changes in free-living glycemic control or glycemic variability following repeated exercise sessions,53,54 while clinic-based exercise influenced glycemic control and variability in type 1 diabetic adults. van Dijk et al.54 observed that glycemic variability increased during a 4-day walking event compared to a habitual physical activity group in type 1 diabetic adults. However, insulin dosage decreased and total energy intake, specifically calories composed of carbohydrates, increased during the 4-day walking event when compared to the habitual physical activity group.54 Yet, under controlled clinic-based assessment, Manohar et al.,53 found that intermittent sessions of low-volume and low-intensity walking decreased the post-meal glucose area under the glucose tolerance curve and glycemic response compared to inactive healthy control and type 1 diabetic adults.53 Additionally, the free-living assessment provides more pertinent information than clinic-based assessment, as adults diagnosed with type 1 diabetes rely on lifestyle management techniques compared to non-diabetic and non-insulin-treated type 2 diabetic adults to control blood glucose concentrations.
Evidence obtained from adults diagnosed with impaired glucose tolerance and type 2 diabetes in the presence and absence of pharmaceutical treatments using insulin-sensitizing drugs or exogenous insulin, supports improvements in glycemic control and variability regardless of exercise modality.55, 56, 57, 58, 59, 60, 61, 62, 63, 64 Farabi et al.58 examined adults with impaired glucose tolerance or type 2 diabetes and found that a single 30-min session of moderate-intensity exercise significantly decreased diurnal CONGA-1. In addition, differentially decreased glucose tolerance changes from pre-to post-exercise in non-diabetic adults were also described.58 van Dijk et al.57 noted decreased CONGA-1, CONGA-2, and CONGA-4 following a single 45–60 min session of moderate-intensity aerobic exercise in non-insulin and insulin-treated type 2 diabetic adults.57 Praet et al.55 observed decreases in hyperglycemic responses following a single session of low-weight/high-volume upper- and lower-body and abdominal resistance exercise in insulin-treated type 2 diabetic adults.55 However, despite comparable decreases in glucose concentrations following either a single 40-min session of continuous moderate-intensity aerobic exercise or combined aerobic plus whole-body resistance exercise, Figueira et al.56 found no changes in 24-h glucose variance or %CV in type 2 diabetic adults.56 Findings from these studies differ based on glycemic control and variability evaluation method used, and suggest that only intra-day, but not inter-day, glycemic variability changes following a single exercise session or repeated exercise sessions. Moreover, studies extending the exercise recovery time into the subsequent day found no persistent changes in glycemic control or glycemic variability beyond the initial 24-h period.55,56 Additionally, incorporating breaks in sedentary time with short periods of walking or simple resistance activities, or altering the time of feeding when exercising, such as fasted versus postprandial exercise, or the use of short duration reduced-exertion HIIE are effective strategies for improving glycemic response to standard meals, and in a controlled or “free-living” environments.59, 60, 61, 62, 63, 64 When attempting to improve glycemic profiles through targeted exercise prescription, these studies highlight the importance of a single exercise session or repeated exercise sessions, regardless of exercise modality and lasting for at least 24-h post-exercise, to improve glycemic responses and glycemic variability in type 2 diabetic adults.
Influence of exercise training on glycemic variability
In the previous section, a single exercise session or repeated exercise sessions were shown to improve the glycemic profile, the glycemic response, and glycemic variability that occur throughout the day. Additional long-term adaptations and enduring improvements in glucose metabolism occur due to regular/habitual/chronic exercise participation. Exercise training-induced improvements in glycemic control are primarily explained by increases in glucose disposal and insulin sensitivity.65 Yet, these physical activity and exercise adaptations are reversible, and the effects of detraining begin within 5–10 days after cessation of exercise.49 Therefore, general involvement and, more importantly, continued regular exercise participation are necessary to impact long-term glycemic health.
Epidemiological studies show that regular exercise participation reduces the risk of developing non-insulin-dependent diabetes.66, 67, 68 More than 35 years ago Holloszy et al.69 published data showing that a decline in glucose tolerance and insulin sensitivity is prevented by performing regular exercise. Further, prolonged and frequent exercise participation has been shown to normalize glucose tolerance by decreasing insulin resistance in individuals having impaired glucose tolerance.69 However, to date, few published studies have evaluated changes in glycemic variability following exercise training as opposed to general exercise participation. Because limited published findings are available, exercise training interventions of a week or greater are included in this section (Table 4).
Table 4.
Influence of exercise training on glycemic variability.
| Author (publication date) | Study design | Primary findings | Conclusion | Strengths and limitations |
|---|---|---|---|---|
| Type 2 diabetes | ||||
| Mikus CR et al. (2012)70 | Clinical controlled trial; n = 13 7-day moderate-intensity continuous aerobic exercise training program |
Glucose concentrations and number of glucose excursions decreased over the final 3 days following the 7-day exercise training compared to 3 days of habitual activity. | 7 days of aerobic exercise training reduces postprandial glucose and glycemic control in free-living individuals with type 2 diabetes. |
Strengths: Controlled exercise setting Limitations: Small sample size; volume of exercise performed was above recommended guidelines |
| Kartstoft K et al. (2013)71 | Randomized clinical trial performed over 4 months (16 weeks); total n = 32; control group n = 8; continuous-walking group n = 12; interval-walking group n = 12 Habitual daily activity; continuous-walking; interval-walking group |
24-h mean, and minimum glucose concentrations increased in the control group, while 24-h mean, and maximum glucose concentrations decreased in the interval-walking group only. | Continuous-walking exercise may offset the deleterious effects of no exercise, while interval-walking exercise may superiorly improve measures of glucose concentrations in type 2 diabetic adults. |
Strengths: Extended exercise intervention; inclusion of a control group; applicable to a free-living condition for exercise and glucose control Limitations: Small sample size; limited to type 2diabetic adults |
| Francois ME et al. (2017)72 | Proof-of-concept, double-blind, randomized clinical trial; n = 53 3 days per week for 12 weeks of high-intensity interval training (HIIT); resistance and aerobic-based exercised |
There was a significant decrease in glycemic control (HbA1c), as well as 24-h mean glucose concentration, SD of the 24-h mean glucose concentration, and MAGE. | Twelve weeks of low-volume HIIT improved glycemic control and glycemic variability. |
Strengths: Standardized 12-week HIIT exercise program Limitations: Older sample limited to type 2 diabetic adults; unable to account for participant characteristics differences |
Table 4 presents studies that provided information regarding the influence of exercise training on glycemic control and glycemic variability in type 2 diabetic adults. The table includes: 1) author information; 2) study design; 3) findings related to the alterations in glycemic control and glycemic variability; 4) conclusions derived from the findings on changes in glycemic control and glycemic variability; 5) strength and limitations of each study.
PPG = postprandial glucose; OGTT = oral glucose tolerance test; HIIT = high-intensity interval training; HbA1c = hemoglobin A1c; SD = standard deviation; MAGE = mean amplitude of glycemic excursions.
Despite the limited number of exercise training studies, the evidence remains consistent with previous literature observing the influence of a single exercise session or repeated exercise sessions in non-diabetic and type 1 and type 2 diabetic adults on glycemic variability. Mikus et al.70 found that 7 days of moderate-intensity aerobic exercise training (∼60 min per exercise session) positively influenced glycemic control and variability in adult type 2 diabetics. Presently, an absence of evidence exists in the literature regarding the impact of regular exercise on glycemic variability.70 Additionally, studies incorporating extended (> 1 week) exercise programs found alterations in glycemic control and variability were exercise intensity-dependent rather than due to exercise participation. These studies found that HIIE and interval walking, as opposed to low-intensity continuous exercise are more beneficial for glycemic control and variability in type 2 diabetic adults.71,72 Karstoft et al.71 found that CGM-assessed glycemic control worsened in a non-exercise control group, improved following 4 months of 60 min per day of interval walking, and did not change following 4 months of 60 min per day of continuous walking.71 Further, Francois et al.72 found that 12 weeks of prescribed HIIE decreased glycemic variability measured as SD of 24-h mean glucose concentration and MAGE compared to pre-exercise training values in type 2 diabetic adults.72
These findings provide evidence that high-intensity or interval exercise improves glycemic control and variability in type 2 diabetic adults. The literature also supports using supervised aerobic and resistance exercise, or free-living walking interventions of varying modalities to improve glycemic control and variability in adults with type 2 diabetes. This literature also supports that implementing regular physical activity and exercise in type 1 diabetics, overweight or obese, and otherwise healthy adults improves metabolic health in addition to traditional treatment of metabolically compromised individuals.
Discussion
General discussion
Findings from studies highlighting the importance of the relationships that exist between sedentary behavior and physical activity with glycemic control and variability assessed by CGM in a variety of populations were consistent. Decreasing sedentary behavior and increasing physical activity improve glycemic control and variability. Further, the discussed studies provide insight into how CGM is incorporated as a measure of glycemic control and variability in addition to traditional clinical measures. However, when examining CGM-assessed glycemic variability, further consideration for other lifestyle factors, including free-living dietary patterns or structured physical activity, such as exercise, could provide information on how to further improve glycemic variability outside of decreasing time spent sedentary and increasing physical activity. This was exemplified when examining the influence of a single bout or repeated bouts of exercise on glycemic control and variability. When attempting to improve glycemic profiles through targeted exercise prescription, these studies highlight the importance of a single exercise session or repeated exercise sessions, regardless of exercise modality and lasting for at least 24-h post-exercise, to improve glycemic responses and glycemic variability in type 2 diabetic adults. When examining the influence of chronic exercise training on glycemic control and variability, the presented studies provided evidence that high-intensity or interval exercise improves glycemic control and variability in type 2 diabetic adults. The literature also supports using supervised aerobic and resistance exercise, or free-living walking interventions of varying modalities to improve glycemic control and variability in adults with type 2 diabetes. This literature also supports that implementing regular physical activity and exercise in type 1 diabetics, overweight or obese, and otherwise healthy adults improves metabolic health in addition to traditional treatment of metabolically compromised individuals.
Mechanistic considerations for the relationship between sedentary behavior and physical activity, the influence of an acute bout or repeated bouts of exercise, and adaptation to chronic exercise training on glycemic variability
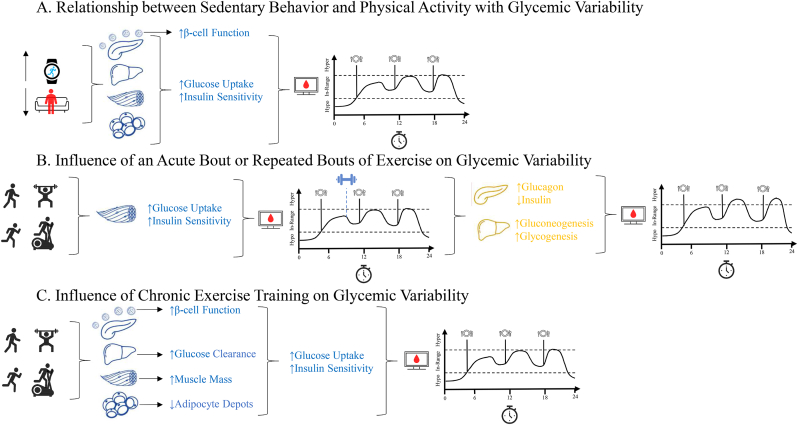
As highlighted in Figure Panel 1, there are several mechanistic considerations that influence glycemic variability that remain to be addressed. When seeking to understand the relationship between sedentary behavior and physical activity with glycemic variability (Fig. 1A) there are specific considerations to be drawn. The maintenance of lower levels of sedentary behaviors and higher levels of physical activity improve pancreatic β-cell function and insulin sensitivity,73 while also promoting systemic and central insulin sensitivity and glucose uptake.74 These physiological adaptations that occur over time allow for enhanced glycemic control by increasing time spent in-range (70–180 mg/dL)75 and decreasing the prevalence of extreme peaks and nadirs in glucose concentrations throughout the day thereby decreasing glycemic variability.
Figure Panel 1.
Figure Panel 1. Mechanistic Considerations for Glycemic Variability. Figure Panel 1 includes 3 figures to discuss the mechanistic considerations for the 1A) Relationship between Sedentary Behavior and Physical Activity with Glycemic Variability, 1B) Influence of an Acute Bout of Exercise on Glycemic Variability, and 1C) Influence of Chronic Exercise Training on Glycemic Variability.
Unlike Fig. 1A highlighting the mechanistic considerations for the relationship between sedentary behavior and physical activity with glycemic variability, the influence of an acute bout of exercise on glycemic variability is primarily driven by systemic skeletal muscle glucose uptake necessary for fueling skeletal muscle contraction during exercise (Fig. 1B).76 However, despite this initial enhanced insulin sensitivity allowing for increased glucose uptake, the benefits of an acute bout of exercise may be abolished by central mechanisms aimed at the maintenance of glucose concentrations, via decreased insulin secretion and increased glucagon secretion from the pancreas and increased gluconeogenesis and glycogenesis in the liver.77 As such, without the maintenance of an exercise routine, the subsequent 48–72 h may either return to normal or have exacerbated glycemic responses in a free-living condition and potentially maintain or increase glycemic variability compared to pre-exercise.
Similar to Fig. 1A highlighting the mechanistic considerations for the relationship between sedentary behavior and physical activity with glycemic variability and building upon mechanistic considerations made in Fig. 1B describing the influence of an acute bout of exercise, the influence of chronic exercise on glycemic variability is driven by physiological adaptations to both central and systemic mechanisms which regulate glycemic control (Fig. 1C).77 As with the maintenance of higher levels of physical activity, there are noted improvements in pancreatic β-cell function and insulin sensitivity.78,79 In addition to these mechanistic considerations, the continual engagement in exercise specifically targets increases in skeletal muscle mass contributing to increased skeletal muscle glucose uptake,80 and decreased adipocyte depots embedded within adipose tissue and reducing insulin resistance and thereby increasing insulin sensitivity.81 Unlike an acute bout of exercise, the continual engagement in exercise elicits chronic physiological adaptations in the liver to enhance glucose metabolism and decreases insulin clearance which reduces the prevalence of peaks and nadirs in glucose concentrations.82 Collectively, these mechanistic adaptations allow for enhanced regulation of glucose concentrations and limiting glycemic excursions thereby decreasing glycemic variability, which may be maintained for an extended period post-exercise.
Understanding the similar findings between studies, evaluation methods, and modalities of exercise: Implications for future research studies
Understanding the relationship between sedentary behavior and physical activity, the influence of a single bout or repeated bouts of exercise, and the adaption to chronic exercise training on glycemic variability remains complex. On the surface, the overarching findings suggest that decreasing sedentary behavior and increasing physical activity, as well as engaging in structured physical activity, known as exercise, positively influences glycemic variability regardless of the basic principles of exercise prescription, including frequency, intensity, time, and type.83 However, recently published secondary data analyses have provided evidence that individual-level differences exist when evaluating the relationship between sedentary behavior and physical activity with CGM-assessed glucose concentration and glycemic variability.84 Similarly, past research has identified that there exists the colloquially identified “responders” and “non-responders” to exercise based on modifiable and non-modifiable factors, such as genetic, physiological, and environmental contributors.85, 86, 87
The future of precision medicine to target glycemic control and variability warrants individual-level prescription based on these factors. To effectively improve this measure of glycemic health, clinicians need to understand those who are most at-risk for impaired glycemic health (i.e., increased glycemic variability) and whether a specific type of intervention that needs to be implemented. In April and May 2021, The National Institutes of Health (NIH) released Notices of Special Interest on the development and testing of multilevel physical activity interventions to improve health and well-being in a variety of populations that include novel assessments of cardiometabolic health (NOT-OD-21-087; NOT-OD-21-120). Therefore, based on these considerations and evidence provided in the present review, researchers need to better understand how to incorporate sensitive measurement techniques, such as glycemic variability assessment using CGM, in conjunction with appropriate approaches to enhance physical activity.
Conclusion
Although physical activity and exercise are important treatment strategies to improve glycemic variability, the impact of exercise training on free-living glycemic control and variability remains largely unexplored in most populations. The introduction and advancement of CGM technology have enabled researchers and practitioners to assess how lifestyle factors impact glycemic health in a free-living environment. Current literature has demonstrated that relationships exist between glycemic control and variability with sedentary time and physical activity. Furthermore, a single exercise session or repeated exercise sessions, and exercise training do beneficially impact glycemic control and variability immediately following exercise, especially in adults diagnosed with type 2 diabetes. Future research studies should consider individual-level factors, as well as the modality of physical activity and exercise in the therapeutic treatment aimed to improve glycemic variability across all populations. The future of precision medicine to prevent the onset of impaired glycemic health requires an appropriate prescription. As such, attention should be placed on the individual, as well as the group of participants as a whole to effectively target reducing disease risk.
Submission statement
All authors have read and agree with manuscript content and while this manuscript is being reviewed for this journal, the manuscript will not be submitted elsewhere for review and publication.
Ethical approval statement
All studies included in this review were ensured to have obtained informed consent from each participant, and that the study was reviewed by the affiliated institution(s) and received approval to implement the study.
Conflict of interest
The authors have no conflicts of interest to disclose.
Authors’ contributions
JRS conceptualized and drafted the manuscript. EEK, MAS, and JLD critically reviewed the manuscript. JMD and PWG reviewed the manuscript. XW conceptualized and critically reviewed the manuscript.
Acknowledgements
We would like to thank the past and present members of the Human Metabolism Laboratory at the University of South Carolina for their hard work and dedication.
References
- 1.Monnier L., Colette C., Owens D.R. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2(6):1094–1100. doi: 10.1177/193229680800200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch I.B., Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabet Complicat. 2005;19(3):178–181. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Buscemi S., Cosentino L., Rosafio G., et al. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin Nutr. 2013;32(3):346–352. doi: 10.1016/j.clnu.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Høstmark A.T., Ekeland G.S., Beckstrøm A.C., et al. Postprandial light physical activity blunts the glucose increase. Prev Med. 2006;42(5):369–371. doi: 10.1016/j.ypmed.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard H., Grindaker E., Rønnestad B.R., et al. Long-term effects of daily postprandial physical activity on blood glucose: A randomized controlled trial. Appl Physiol Nutr Metab. 2017;42(4):430–437. doi: 10.1139/apnm-2016-0467. [DOI] [PubMed] [Google Scholar]
- 6.Blankenship J.M., Granados K., Braun B. Effects of sitting versus adding exercise on glycemic control and variability in sedentary office workers. Appl Physiol Nutr Metab. 2014;39(11):1286–1293. doi: 10.1139/apnm-2014-0157. [DOI] [PubMed] [Google Scholar]
- 7.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028/-/DC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei N., Zheng H., Nathan D.M. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care. 2014;37(4):1048–1051. doi: 10.2337/dc13-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton I.M., Adler A.I., Neil H.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little R.R., Rohlfing C.L., Sacks D.B., National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: From chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205–214. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 11.Laiteerapong N., Ham S.A., Gao Y., et al. The legacy effect in type 2 diabetes: Impact of early glycemic control of future complications (The Diabetes & Aging Study) Diabetes Care. 2019;42(3):416–426. doi: 10.2337/dc17-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherwani S.I., Khan H.A., Ekhzaimy A., et al. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:90–104. doi: 10.4137/BMI.S38440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovič L., Savas H., Mehta M., et al. Frequent monitoring of A1c during pregnancy as a treatment tool to guide therapy. Diabetes Care. 2019;34(1):53–54. doi: 10.2337/dc10-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck R.W., Connor C.G., Mullen D.M., et al. The fallacy of average: How using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(80):994–999. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q., Liu H., Xiang G., et al. Association between glycated hemoglobin A1c levels with age and gender in Chinese adults with no prior diagnosis of diabetes mellitus. Biomed Rep. 2016;4(6):737–740. doi: 10.3892/br.2016.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch I.B., Welsh J.B., Calhoun P., et al. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36(12):1637–1642. doi: 10.1111/dme.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chehregosha H., Khamesh M.E., Malek M., et al. A view beyond HbA1c: Role of continuous glucose monitoring. Diabetes Ther. 2019;10(3):853–863. doi: 10.1007/s13300-019-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodbard D. Glucose variability: A review of clinical applications and research developments. Diabetes Technol Therapeut. 2018;20(S2):S25–S215. doi: 10.1089/dia.2018.0092. [DOI] [PubMed] [Google Scholar]
- 19.Suh S., Kim J.H. Glycemic variability: How do we measure it and why is it important? Diabetes Metab J. 2015;39(4):273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovatchev B., Anderson S., Heinemann L., et al. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160–1164. doi: 10.2337/dc07-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman D.G., Bland J.M. Standard deviations and standard errors. BMJ (Clinical research ed.) 2005;331(7521):903. doi: 10.1136/bmj.331.7521.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Service J.F., Molnar G.D., Rosevear J.W., et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 23.Service F.J., Nelson R.L. Characteristics of glycemic stability. Diabetes Care. 1980;3(1):58–62. doi: 10.2337/diacare.3.1.58. [DOI] [PubMed] [Google Scholar]
- 24.Service F.J., O’Brien P.C., Rizza R.A. Measurements of glycemic control. Diabetes Care. 1987;10(2):225–237. doi: 10.2337/diacare.10.2.225. [DOI] [PubMed] [Google Scholar]
- 25.Rodbard D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes Technol Therapeut. 2009;11(Supplement 1):S55–S67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell C.M., Donath S.M., Vidmar S.I., et al. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Therapeut. 2005;7(2):253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 27.Nathan D.M., Kuenen J., Borg R., et al. Translating the A1c assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuenen J.C., Borg R., Kuik D.J., et al. Does glucose variability influence the relationship between mean plasma glucose and HbA1c levels in type 1 and type 2 diabetic patients? Diabetes Care. 2011;34(8):1843–1847. doi: 10.2337/dc10-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmerhorst H.J., Wijndaele K., Brage S., et al. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58(8):1776–1779. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunstan D.W., Barr E.L., Healy G.N., et al. Television viewing time and mortality: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 31.Ford E.S., Zhao G., Li C. Pre-diabetes and the risk for cardiovascular disease. Diabetes Care. 2010;55(13):1310–1317. doi: 10.2337/dc19-1074. [DOI] [PubMed] [Google Scholar]
- 32.Thorp A.A., Healy G.N., Owen N., et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care. 2010;33(2):327–334. doi: 10.2337/dc09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veerman J.L., Healy G.N., Cobiac L.J., et al. Television viewing time and reduced life expectancy: A life table analysis. Br J Sports Med. 2012;46(13):927–930. doi: 10.1136/bjsports-2011-085662. [DOI] [PubMed] [Google Scholar]
- 34.Lahjibi E., Heude B., Dekker J.M., et al. Impact of objectively measured sedentary behavior on changes in insulin resistance and secretion over 3 years in the RISC study: interaction with weight gain. Diabetes Metab. 2013;39(3):217–225. doi: 10.1016/j.diabet.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Fritschi C., Park H., Richardson A., et al. Association between daily time spent in sedentary behavior and duration of hyperglycemia in type 2 diabetes. Biol Res Nurs. 2016;18(2):160–166. doi: 10.1177/1099800415600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martyn-Nemeth P., Quinn L., Penckofer S., et al. Fear of hypoglycemic: Influence on glycemic variability and self-management behavior in young adults with type 1 diabetes. J Diabet Complicat. 2017;31(4):735–741. doi: 10.1016/j.jdiacomp.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paing A.C., McMillan K.A., Kirk A.F., et al. The associations of sedentary time and breaks in sedentary time with 24-hour glycaemic control in type 2 diabetes. Prev Med Rep. 2018;12:94–100. doi: 10.1016/j.pmedr.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paing A.C., McMillan K.A., Kirk A.F., et al. Impact of free-living pattern of sedentary behaviour on intra-day glucose regulation in type 2 diabetes. Eur J Appl Physiol. 2020;120(1):171–179. doi: 10.1007/s00421-019-04261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillan K.A., Paing A.C., Kirk A.F., et al. Measuring group and individual relationship between patterns in sedentary behaviour and glucose in type 2 diabetes adults. Practical Diabetes. 2020;37(1):13–18c. doi: 10.1002/pdi.2254. [DOI] [Google Scholar]
- 40.Gude F., Díaz-Vidal P., Rúa-Pérez C., et al. Glycemic variability and its association with demographics and lifestyles in a general adult population. J Diabetes Sci Technol. 2017;11(4):780–790. doi: 10.1177/1932296816682031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joslin E.P., Root H.F., White P., Marble A. 5. Lea & Febiger; Philadelphia: 1935. The Treatment of Diabetes Mellitus; pp. 299–301. [Google Scholar]
- 42.Goodyear L.J., Kahn B.B. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 43.Pruett E.D.R., Oseid S. Effect of exercise on glucose and insulin response to glucose infusion. Scand J Clin Lab Invest. 1970;26(3):277–285. doi: 10.3109/00365517009046234. [DOI] [PubMed] [Google Scholar]
- 44.Bogardus C., Thuillex P., Ravussin E., et al. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest. 1983;72(5):1605–1610. doi: 10.1172/jci111119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter E.A., Mikines K.J., Galbo H., et al. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol. 1989;66(2):876–885. doi: 10.1152/jappl.1989.66.2.876. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Patterson B.W., Smith G.I., et al. A ∼60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women. J Appl Physiol. 2013;114(11):1563–1568. doi: 10.1152/japplphysiol.01364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devlin J.T., Horton E.S. Effect of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes. 1985;34(10):973–979. doi: 10.2337/diab.34.10.973. [DOI] [PubMed] [Google Scholar]
- 48.Devlin J.T., Hirshman M.F., Horton E.S., Horton E.D. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36(4):434–439. doi: 10.2337/diab.36.4.434. [DOI] [PubMed] [Google Scholar]
- 49.Mikines K.J., Sonne B., Farrell P.A., et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254(3 Part 1):E248–E259. doi: 10.1152/ajpendo.1988.254.3.E248. [DOI] [PubMed] [Google Scholar]
- 50.Little J.P., Jung M.E., Wright A.E., et al. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Phsysiol Nutr Metab. 2014;39(7):835–841. doi: 10.1139/apnm-2013-0512. [DOI] [PubMed] [Google Scholar]
- 51.Parker L., Shaw C.S., Banting L., et al. Acute low-volume high-intensity interval exercise and continuous moderate-intensity exercise elicits a similar improvement in 24-hour glycemic control in overweight or obese adults. Front Physiol. 2017;7:661. doi: 10.3389/fphys.2016.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueira F.R., Umpierre D., Bock P.M., et al. Effect of exercise on glucose variability in healthy subjects: Randomized crossover trial. Biol Sport. 2019;36(2):141–148. doi: 10.5114/biolsport.2019.83006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manohar C., Levine J., Nandy D.K., et al. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care. 2012;35(12):2493–2499. doi: 10.2337/dc11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Dijk J.-W., Eijsvogels T.M., Nyakayiru J., et al. Glycemic control during consecutive days with prolonged walking exercise in individuals with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2016;117:74–81. doi: 10.1016/j.diabres.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 55.Praet S.F., Manders R.J., Lieverse A.G., et al. Influence of acute exercise on hyperglycemia in insulin-treated type 2 diabetes. Med Sci Sports Exerc. 2006;38(12):2037–2044. doi: 10.1249/01.mss.0000235352.09061.1d. [DOI] [PubMed] [Google Scholar]
- 56.Figueira F.R., Umpierre D., Casali K.R., et al. Aerobic and combined exercise sessions reduce glucose variability in type 2 diabetes: Crossover randomized trial. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0057733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dijk J.-W., Manders R.J., Canfora E.E., et al. Exercise and 24-hour glycemic control: Equal effects for all type 2 diabetes patients? Med Sci Sports Exerc. 2013;45(4):628–635. doi: 10.1249/MSS.0b013e31827ad8b4. [DOI] [PubMed] [Google Scholar]
- 58.Farabi S.S., Carley D.W., Smith D., et al. Impact of exercise on diurnal and nocturnal markers of glycaemic variability and oxidative stress in obese individuals with type 2 diabetes or impaired glucose tolerance. Diab Vasc Dis Res. 2015;12(5):381–385. doi: 10.1177/1479164115579003. [DOI] [PubMed] [Google Scholar]
- 59.Myette-Côté É, Terada T., Boulé N.G. The Effect of Exercise with or Without Metformin on Glucose Profiles in Type 2 Diabetes: A Pilot Study. Can J Diabetes. 2016;40(2):173–177. doi: 10.1016/j.jcjd.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Haxhi J., Leto G., di Palumbo A.S., et al. Exercise at lunchtime: effect on glycemic control and oxidative stress in middle-aged men with type 2 diabetes. Eur J Appl Physiol. 2016;116(3):573–582. doi: 10.1007/s00421-015-3317-3. [DOI] [PubMed] [Google Scholar]
- 61.Terada T., Wilson B.J., Myette-Côté E., et al. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism. 2016;65(5):599–608. doi: 10.1016/j.metabol.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Dempsey P.C., Larsen R.N., Sethi P., et al. Benefits for type 2 Diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39(6):964–972. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- 63.Dempsey P.C., Blankenship J.M., Larsen R.N., et al. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia. 2017;60(3):499–507. doi: 10.1007/s00125-016-4169-z. [DOI] [PubMed] [Google Scholar]
- 64.Metcalfe R.S., Fitzpatrick B., Fitzpatrick S., et al. Extremely short duration interval exercise improves 24-h glycaemia in men with type 2 diabetes. Eur J Appl Physiol. 2018;118(12):2551–2562. doi: 10.1007/s00421-018-3980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheider S.H., Amorosa L.F., Khachadurian A.K., et al. Studies on the mechanism of improved glucose control during regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1984;26(5):355–360. doi: 10.1007/BF00266036. [DOI] [PubMed] [Google Scholar]
- 66.Manson J.E., Rimm E.B., Stampfer M.J., et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 67.Manson J.E., Nathan D.M., Krolewski A.S., et al. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;268(1):63–67. doi: 10.1001/jama.1992.03490010065031. [DOI] [PubMed] [Google Scholar]
- 68.Helmrich S.P., Ragland D.R., Leung R.W., et al. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Eng J Med. 1991;325(3):147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 69.Holloszy J.O., Schultz J., Kusnierkiewicz J., et al. Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Med Scand Suppl. 1986;711:55–65. doi: 10.1111/j.0954-6820.1986.tb08932.x. [DOI] [PubMed] [Google Scholar]
- 70.Mikus C.R., Oberlin D.J., Libla J., et al. Glycaemic control is improved by 7 days of aerobic exercise training in patients with type 2 diabetes. Diabetologia. 2012;55(5):1417–1423. doi: 10.1007/s00125-012-2490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karstoft K., Winding K., Knudsen S.H., et al. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2013;36(2):228–236. doi: 10.2337/dc12-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francois M.E., Durrer C., Pistawka K.J., et al. Combined interval training and post-exercise nutrition in type 2 diabetes: A randomized control trial. Front Physiol. 2017;8:528. doi: 10.3389/fphys.2017.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colberg S.R., Sigal R.J., Yardley J.E., et al. Physical activity/exercise and diabetes: A position statement from the American Diabetes Association. Diabetes Care. 2016;39(11):2065–2079. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bird S.R., Hawley J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2(1) doi: 10.1136/bmjsem-201-000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei N., Zheng H., Nathan D.M. Empirically established blood glucose targets to achieve HbA1c goals. Diabetes Care. 2014;37(4):1048–1051. doi: 10.2337/dc13-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93(3):993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 77.Thyfault J.P., Bergouignan A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia. 2020;63:1464–1474. doi: 10.1007/s00125-020-05177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slentz C.A., Tanner C.J., Bateman L.A., et al. Effects of exercise training intensity on pancreatic β-cell function. Diabetes Care. 2009;32(10):1807–1811. doi: 10.2337/dc09-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iaccarino G., Franco D., Sorriento D., et al. Modulation of insulin sensitivity by exercise training: Implications for cardiovascular prevention. J Cardiovasc Transl Res. 2021;14:256–270. doi: 10.1007/s12265-020-10057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans P.L., McMillin S.L., Weyrauch L.A., et al. Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients. 2019;11(10):2432. doi: 10.3390/nu11102432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Honkala S.M., Motiani P., Kivelä R., et al. Exercise training improves adipose tissue metabolism and vasculature regardless of baseline glucose tolerance and sex. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2019-000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.DiMenna F.J., Arad A.D. The acute vs. chronic effect of exercise on insulin sensitivity: Nothing lasts forever. Cardiovasc Endocrinol Metab. 2020;10(3):149–161. doi: 10.1097/XCE.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.American College of Sports Medicine . 10th ed. Lippincott Williams & Wilkins; Philadelphia (PA): 2017. ACSM’s Guidelines for Exercise Testing and Prescription; pp. 195–202. [Google Scholar]
- 84.McMillan K.A., Paing A.C., Kirk A.F., et al. Measuring group and individual relationship between patterns in sedentary behaviour and glucose in type 2 diabetes adults. Practical Diabetes. 2020;37(1):13–18c. doi: 10.1002/pdi.2254. [DOI] [Google Scholar]
- 85.Pickering C., Kiely J. Do non-responders to exercise exist-and if so, what should we do about them? Sports Med. 2019;49:1–7. doi: 10.1007/s40279-018-01041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maturana F.M., Soares R.N., Murias J.M., et al. Responders and non-responders to aerobic exercise training: beyond the evaluation of V̇O2max. Physiol Rep. 2021;9(16) doi: 10.14814/phy2.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hrubeniuk T.J., Bouchard D.R., Gurd B.J., et al. Can non-responders be ‘rescued’ by increasing exercise intensity? A quasi-experimental trial of individual responses among humans living with pre-diabetes or type 2 diabetes mellitus in Canada. BMJ Open. 2021;11(4) doi: 10.1136/bmjopen-2020-044478. [DOI] [PMC free article] [PubMed] [Google Scholar]