Abstract
This randomized, double-blind, clinical trial was designed to compare the endurance capacity (ergogenic property) in healthy athletes after consumption of apple cider vinegar (ACV) and a commercial sports drink (CSD) before and during endurance exercise. Fourteen healthy participants were enrolled in this trial and were divided into two groups as ACV and CSD with seven participants in each. Participants were requested to consume 500 mL of either commercial ACV or CSD 1 h before endurance exercise (bicycle ergometer). Blood samples were collected at baseline, 0, 20, 40, 60 min until exhaustion to assess glucose, lactate, ammonia and non-esterified fatty acids (NEFA). Respiratory exchange rate (RER) score was measured every 15 min and the heart rate (HR) was measured every 5 min. The outcome of the present trial clearly showed that no significant differences were observed between ACV and CSD except in the blood level of ammonia (only at exhaustion time). Thus, these results show that ACV and the CSD both possessing the ergogenic property, enhanced blood glucose, NEFA, and suppress the production of lactate as well as maintains normal RER score, and HR throughout the endurance exercise. Overall this trial showcases that ACV did not significantly improve the ergogenic activity over the CSD.
Keywords: Apple vinegar, Lactate, Ammonia, RER score, Endurance, Physical performance
Introduction
Regular exercise/practice with proper nutrition has been reported to improve health status and physical performance. However, unorganized, sudden, or endurance exercise and lack of proper nutrients would trigger pain, damage muscle (soreness) and eventually results in fatigue. Fatigue is broadly classified as physical fatigue which is due to a lack of energy to muscles and mental fatigue due to lack of proper neurological response to muscles.1,2 Several reports have clearly indicated that a strong correlation exists between endurance sports and fatigue.3,4 If fatigue (especially mental fatigue) is not properly controlled, fatigue might lead to irreversible muscle damage (chronic-fatigue syndrome) or Karoshi (overwork death) and thus affect physical and mental confidence.5,6 Unfortunately, to date, no specific or established treatment for fatigue exist, but many natural supplements (nutraceuticals/functional foods) are recommended to abolish fatigue-related muscle soreness or weakness. Nevertheless, the results are controversial and hence the search for the ideal anti-fatigue and ergogenic products is in high demand. Hence, most research studies focus on developing combination products that utilize natural dietary supplements rich in protein, carbohydrates, vitamins, and minerals and are known for their synergistic anti-fatigue or ergogenic effect by improving energy production (ATP) and sparing glycogen, especially for endurance sports or exercise.7
Vinegar has been used in various cuisine as a food preservative (fermentation) and as a seasoning agent with numerous health-promoting properties including anti-obesity, anti-diabetic (improve insulin sensitivity), anti-hypertensive, anti-fatigue, anti-hyperlipidemic activities.8, 9, 10 Acetic acid is considered the major active ingredient contributing to the biological properties of vinegar. Previously, studies have shown that consumption of acetic acid beneficial impacts anti-hypertension and anti-hyperlipidemic.8,11,12 Recently, food scientists have concentrated on fruit vinegar (fermented product) as vinegars are rich in acetic acid and various phenolic compounds that can enhance the health-promoting property of vinegar.13,14 One such popular fruit vinegar is apple cider vinegar (ACV), which has been traditionally used for treating various ailments in Asia. ACV has functional ingredients other than acetic acids which include organic acids (malic and citrus acids), phenolic acid (especially chlorogenic and coumaric acids), many amino acids, minerals, and sugars.10,12,15 Because of the high amount of these functional ingredients, ACVs exhibit a wide range of biological activities such as anti-oxidant, anti-inflammatory, anti-hypercholesterolemic, immunomodulatory, anti-microbial, anti-obesity, anti-diabetic as well as providing cardioprotective, hepatoprotective, and renoprotective properties11,15,16 and thus is considered as a functional food.
Branched-chain amino acids (BCAAs) including leucine (leu), iso-leucine (Iso-leu) and valine (Val) play various crucial roles in protein anabolism via mammalian target of rapamycin; mTOR signaling pathway delaying and/or preventing protein catabolism via adenosine monophosphate-activated protein kinase (AMPK) signaling pathway. AMPK is a nutrient modulator in bioenergetics of adenosine triphosphate and ultimately ATP formation suppresses serotonin production (anti-fatigue) as well as promoting glucose homeostasis in skeletal muscle.17, 18, 19 Hence, recent studies have assessed BCAAs involvement as an anti-fatigue or muscle recovery property. Nevertheless, many of these studies did show positive results for BCAAs use during low to moderate exercise.20,21 However, the authors speculate that ACV with BCAAs might show better ergogenic or anti-fatigue activity than other sports drinks, due to the presence of acetic acid and phenolic acids. Therefore, this randomized, double-blind, cross-over designed clinical trial was designed to investigate the comparative anti-fatigue and ergogenic effects of ACV or commercial sports drink (CSD) during a single bicycle ergometer exercise session.
Materials and methods
Commercial apple cider vinegar
The experimental drink ACV was provided by Pai Chia Chen Brewery & Foods Co., Ltd, Taiwan, whereas the CSD was provided by Vitalon Food Company, Co., LTD, Taiwan. The major ingredients of ACV include apple vinegar 4% (rich in various phenolic acids-coumaric, chlorogenic, gallic and caffeic acids), starch 4%, trehalose 2.5%, branched-chain amino acids (BCAAs) 1%, sodium salt 0.1%, water (87.3%) and raw apple juice 0.5% (flavoring process), minerals (Na, K, Ca2+, Mg) and vitamins (Vit-C/E) 0.3% which is equivalent to 206 kcal with 50% carbohydrate, 24% protein and 26% fat/lipid. While, the major ingredients of CSD (Super Supau Sport drink) includes high fructose syrup 6.5%, sucrose 4%, low sodium salt 0.42%, BCAAs 1% (Leucine, Iso-Leucine, and Valine) with arginine, alanine and lysine, citric acid, Vit –C, Ca2+, Mg, Na, K (electrolytes/minerals) and water 87.38% which is equivalent to 226 kcal with 56% carbohydrate, 24% protein and 20% fat/lipid. Both ACV and CSD drinks are similar in size, shape, and color.
Recruitment of participants
Eighteen healthy subjects aged between 19 to 23 were initially recruited from the student volunteers at the Department of Physical Education, National Taichung University of Education, Taichung, Taiwan by placing advertisement/flyers and circular. All subjects underwent a basic physical examination (includes body weight and height for calculating body mass index [BMI]), vital signs and biochemical parameters (cardiac, hepatic and renal markers) to determine health status (metabolic or systemic anomalies). Family health history was requested and noted. Moreover, questionnaires pertaining to dietary supplementation, performance enhancers, medicine intake (past and present) were documented before enrolling participants. The major inclusion criteria for this trial included only young healthy athletes/cyclists and exclusion criteria were no pathological or diseased conditions, underweight, pregnant, lactating, chain smoker or alcohol or drug addict, persons taking dietary supplements or ergogenic aid in the last 3 weeks. Based on the above inclusion and exclusion criteria only 14 healthy students were enrolled in this cross-over design clinical trial.
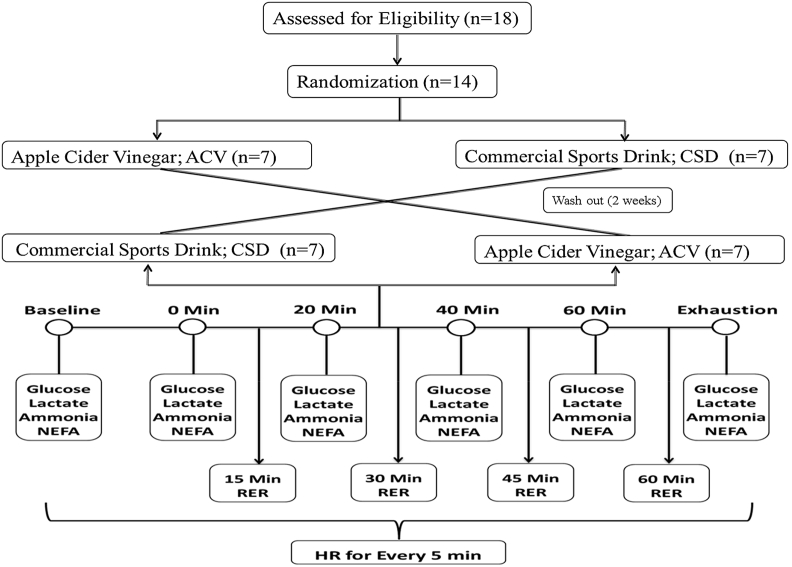
This double-blind, cross-over trial was conducted at Chung Shan Medical University, Taiwan from Jan 2016 to March 2016. This study was approved by human ethical clearance board members of Chung Shan Medical University, Taiwan (CS-04048). The protocols/procedures used in this study are carried out based on the Declaration of Helsinki (revised 1998). All study participants were asked to sign an informed consent and the details of this study (possible risk or adverse effects) were explained before starting in this trial. In addition, all the participants were given detailed instructions concerning bicycle ergometer (RER score) and other instruments and techniques employed in this trial as well as the warm-up session was conducted before a week of the real trial to document the maximum oxygen uptake (V̇O2 Max) for each participant. Two days prior to starting this experiment, participants were instructed to record the dietary and physical activity pattern and asked to follow these same patterns throughout the trial period. The flow chart/schematic representation of this trial is shown in Fig. 1.
Fig. 1.
Flow chart/Schematic representation of this current trial.
Experimental grouping
After an overnight fasted, participants were randomly divided into two groups ACV (n = 7) or CSD (n = 7) and requested to consume 500 mL (single dose) of ACV and/or CSD 1 h before endurance bicycle ergometer exercise. A two-week wash-out period cross-over study design was used. Various variables and parameters were measured before and during endurance exercise on the same day. All experiments were conducted in a sophisticated laboratory at a specific time with temperature and relative humidity were properly controlled. All participants rested over-night and were fasted at least 2–3 h before consuming ACV or CSD drink 1 h before starting the bicycle ergometer protocol. Thirty minutes before starting the endurance exercise all participants had a catheter placed in the antecubital vein. Blood samples were collected before (baseline), during exercise (every 20 min) and at the time of exhaustion.
Endurance exercise using incremental bicycle ergometer procedure
All the participants completed a bicycle ergometer (Monark Ergomedic 894e; Monark, Sweden) endurance exercise session based on progressive increase in work rate exercise test (PIET) with constant speed (60 revolutions per minute - rpm) using motion design of the oxygen uptake (i.e., 50% V̇O2 Max - Table 1) as previously described by Chang and co-workers1 with slight modifications (instead of 50 W, we used 0.5 kg load increase for every 2 min at a constant speed of 60 rpm based). During bicycle ergometer work all participants breathed through a valve connected to a pulmonary gas exchanger/analyzer (Vmax Encore PFT-29C; CareFusion, USA). Airflow rate was measured and breath by breath O2 consumption and CO2 production were determined to calculate respiratory exchange rate (RER) score. Also, HR was determined during endurance exercise using Pulsometer (Polar Accurex Plus; Finland) strapped around the chest. Determination of time to exhaustion for each participant was determined by the following criteria: Participant was not able to ride any more (End) and HR was greater than 200 minus age (HR_age), based on motion consciousness scale: Borg consciousness scale (if score was >16)22, RER score should be less than 1.10.18
Table 1.
Motion design of the peak of oxygen uptake (V̇O2 Max) by subjects.
| Stage | Duration (min) | rpm (RPM) | Resistance/Load (kg) |
|---|---|---|---|
| 1 | 0–4 | 60 | 0.5 |
| 2 | 4–6 | 60 | 1.0 |
| 3 | 6–8 | 60 | 1.5 |
| 4 | 8–10 | 60 | 2.0 |
| 5 | 10–12 | 60 | 2.5 |
| 6 | 12–14 | 60 | 3.0 |
| 7 | 14–16 | 60 | 3.5 |
| 8 | 16–18 | 60 | 4.0 |
| 9 | 18–20 | 60 | 5.0 |
Blood sample collection and analysis
Blood samples were collected before (baseline) and during exercise (every 20 min - i.e., 0, 20, 40, 60 min and exhaustion time). HR was measured every 5 min during the endurance bicycle ergometer exercise. At 15-min intervals (0 min-Baseline, 15, 30, 45, 60 min and at exhaustion) during exercise, RER scores were determined. Blood samples were collected in two different tubes; one tube coated with EDTA for plasma separation and another tube without any anticoagulant for serum preparation by centrifuging at 2000 ×g for 10 min at 4 °C and the supernatant was stored at −80 °C. Plasma glucose was measured using Glucometer (Super Glucocard II; Arkray, Japan), blood (whole) Lactose and ammonia were determined using Lactometer (Lactate Pro 2; Arkray, Japan) and commercial ammonia assay kit (bromophenol blue) using auto-analyzer (7020 clinical analyzers; Hitachi Technologies. Japan) respectively. Whereas, serum NEFA was measured using Commercial NEFA 96 well plate detection point kit from ZenBio, Inc (NC, USA).
Statistical assessment
All data in this trial were analyzed using SPSS software (Ver 17 from IBM; CA, USA). Data are presented as the mean ± standard deviation (SD). Comparisons between ACV and CSD was completed by paired t-test, whereas Student t-test was employed to check the changes in the same group from baseline to different time point/interval. The level of statistically significant was set at p < 0.05.
Results
Baseline characteristics including participants gender, age, body weight, BMI, and body fat are presented in Table 2. All values are in normal ranges and thus infers that only healthy young participants were included in this trial. Because a cross-over clinical trial designed was used, all participants consumed both the ACV and CSD drink before an endurance ergometer exercise session. Immediately after the first endurance exercise session, a two-week period was used for washout. A cross-over design does minimize the cofounding variants (counterbalance) and enhanced statistical effectiveness. Moreover, the total time to exhaustion (end) for the ACV group was 4237.5 s, and the CSD group was 4257.7 s. Analysis of this data clearly shows that no significant difference existed in total time to exhaustion between ACV and CSD group.
Table 2.
Baseline characteristics of all participants.
| Parameters/Characters | Values |
|---|---|
| Gender (Male/Female) | 14 (14/0) |
| Age (Years) | 20.4 ± 1.4 |
| Weight (kg) | 68.47 ± 8.37 |
| BMI (kg/m2) | 22.97 ± 1.63 |
| Body Fat (%) | 14.09 ± 2.05 |
Data are presented as the mean ± standard deviation (SD).
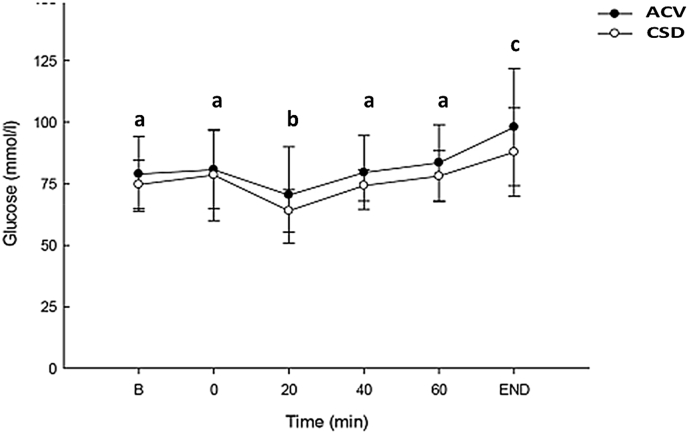
Blood glucose changes of healthy sportsperson after ACV and CSD consumption before and during endurance exercise are depicted in Fig. 2. No differences between blood glucose levels were found at baseline or at the 0-time periods. At the 20-min period of exercise, blood glucose levels for both groups were significantly lower than baseline, but after this 20-min time point, blood glucose levels increased from baseline in both the ACV and CSD groups (time-dependent manner) and were only significantly elevated at the time of exhaustion. However, no significant changes were noted between ACV and CSD groups at any time interval as both groups continuously increased blood glucose level after 20 min.
Fig. 2.
The levels of blood glucose in healthy participants after ACV and CSD consumption before and during endurance exercise. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). ACV: Apple cider vinegar.
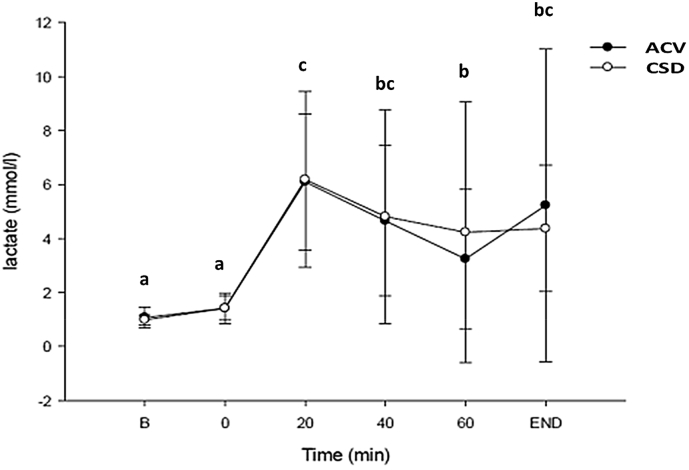
As shown in Fig. 3, blood lactate (lactic acid) levels were significantly elevated from baseline and 0-min time points to the 20-min time point for ACV and CSD groups. Blood lactate levels declined from 20 to 60-min exercise time period for both groups. Regarding the comparison between the ACV group and the CSD group, no significant differences at any time period were found between groups as both groups had similar blood lactate levels at all time points.
Fig. 3.
The levels of blood lactate (lactic acid) in healthy participants after ACV and CSD consumption before and during endurance exercise. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). ACV: Apple cider vinegar.
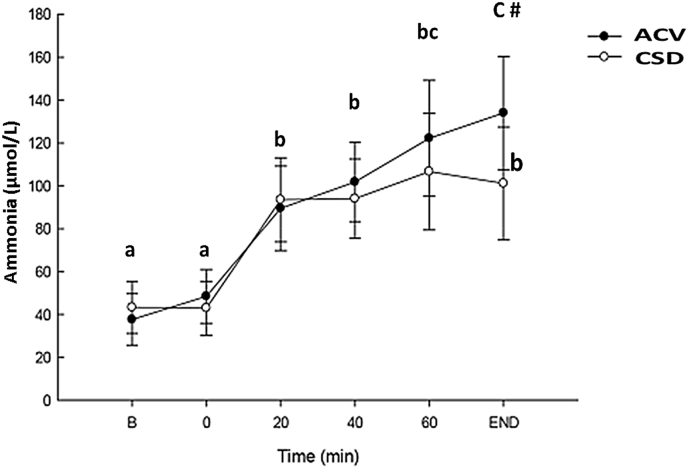
Blood ammonia concentrations after ACV and CSD consumption are found in Fig. 4. ACV and CSD groups had a parallel escalation in the blood ammonia concentration from 0 to 60 min timepoints. The concentration of blood ammonia between the ACV group and CSD group was significantly different (p < 0.05) at the time of exhaustion, but no significant differences were observed between the two groups at any other time points from baseline to 60 min.
Fig. 4.
The change in the concentration of blood ammonia in healthy participants after ACV and CSD consumption. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). Significant difference between ACV and CSD groups at different time interval are donated as # (p < 0.05). ACV: Apple cider vinegar.
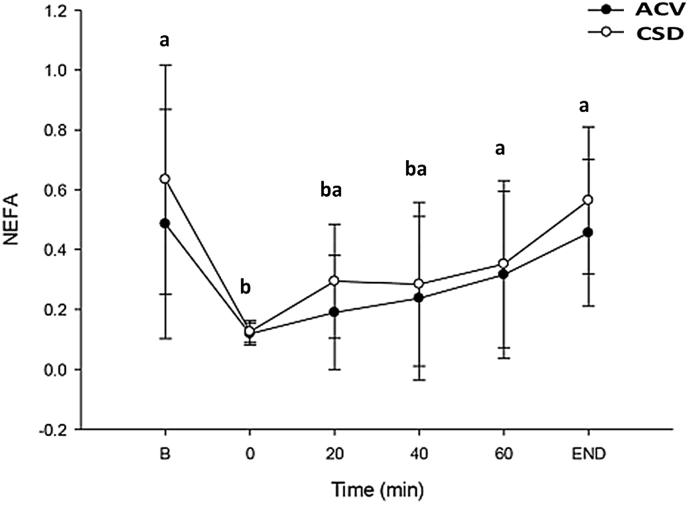
Effect of ACV or CSD on blood NEFA levels of healthy sportsperson before and during endurance exercise are presented in Fig. 5. Participants consuming either ACV or CSD before and during endurance exercise showed an increased blood NEFA level. ACV and CSD groups (ACV vs CSD) blood NEFA levels followed the same upward trend without any marked alterations between groups.
Fig. 5.
The changes in the levels of blood NEFA in healthy participants before and during endurance exercise after ACV and CSD consumption. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). ACV: Apple cider vinegar, NEFA: Non-esterified fatty acid.
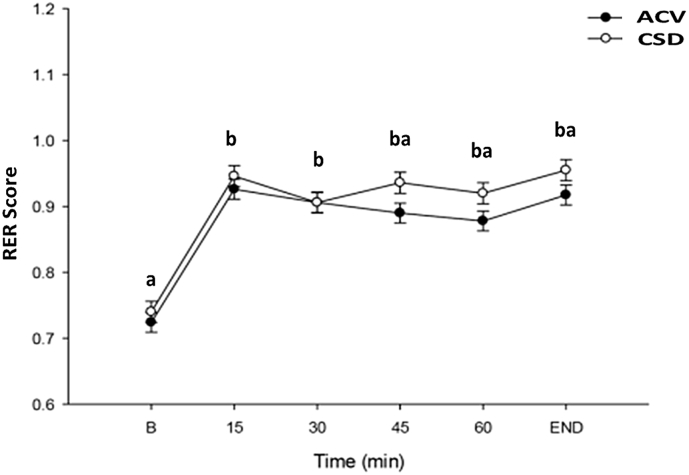
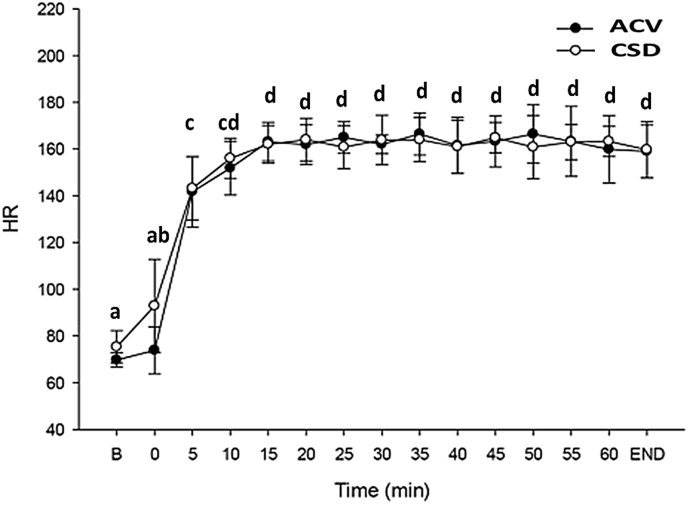
Fig. 6, Fig. 7 contain the RER scores and HRs in healthy sportsperson after consuming ACV and CSD before and during endurance exercise. RER scores and HRs increased in response to exercise. After 20 min of exercise, RER and HR values were maintained with only slight fluctuations. Both ACV and CSD groups had similar RER or HR values with no significant difference found between ACV and CSD groups at any time intervals.
Fig. 6.
The change in RER score in healthy participants after ACV and CSD consumption before and during endurance exercise. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). ACV: Apple cider vinegar, RER: Respiratory exchange rate.
Fig. 7.
The change in HR in healthy participants after ACV and CSD consumption before and during endurance exercise. Data are presented as the mean ± standard deviation (SD). Data within the same group representing different superscript letters indicate a significant difference (p < 0.05). ACV: Apple cider vinegar, HR: Heart rate.
Discussion
This novel clinical trial was framed by incorporate BCAAs in ACV and compared with BCAAs found in a CSD to investigate the anti-fatigue and ergogenic properties during endurance bicycle ergometer exercise. The outcome of this study indicates that acute exercise intervention with either ACV or CSD could significantly improve physical performance by increasing blood glucose and NEFA levels while suppressing the production of lactate (downregulate lactate dehydrogenase; LDH) as well as maintains normal RER scores and HR throughout the endurance exercise. Thus, demonstrating that both ACV and CSD possess anti-fatigue and ergogenic properties during endurance exercise.
Blood glucose has a crucial role in energy production (ATP) and facilitates normal physical activity. During endurance exercise, blood glucose levels usually decline to maintain blood glucose homeostasis. Glycogen from muscle or liver is an important fuel to facilitate continuous physical activity.23 In this study when the participants started a bicycle ergometer exercise session to exhaustion and after ACV or CSD consumption, blood glucose declined (0 to 20 min). But during exercise, blood glucose increased (time-dependent fashion) until exhaustion. This increase was likely due to increased utilization of BCAAs for ATP production as well as changes in insulin sensitivity. To support this statement, the scientific literature in this area report acetic acid present in vinegar regulates appetite, delays gastric emptying, and adipogenesis (lipogenesis), enhances insulin sensitivity, and glycogen synthesis, and promotes muscle glucose uptake.8,11 Thereby favoring aerobic glycolysis, increase blood glucose levels, and improve physical performance during endurance exercise (increase ATP production via electron transport chain). Moreover, polyphenols (phenolic acids), vitamins, and minerals also facilitate ACV to enhance insulin sensitivity and indirectly increase blood glucose level14 especially during endurance exercise and sporting events. Also, BCAAs ingestion spares glycogen depletion by supplying ATP after oxidation resulting in improved physical performance during endurance sports/exercise.18
Studies in the scientific literature confirm that during intense endurance exercise the concentration of lactate (from pyruvate via LDH) is significantly increased and is a biomarker for monitoring endurance capacity and fatigue. Elevated lactic acid significantly lowers pH and inhibits the force of muscle contraction resulting in fatigue.24,25 Subjects in this study consuming either ACV or CSD before performing an endurance bicycle ride to exhaustion showed a significant increase in the levels of blood lactate after 20 min of exercise. Nevertheless, blood lactate levels decreased from 20 to 60 min of exercise for both groups. Remember ACV and CSD both drinks contain BCAAs, which can spare glycogen utilization and reduce preferred glycolysis resulting in decreased lactic acid levels.26 Also, acetic acid found in ACV was reported to downregulate the protein expression of LDH.8,11 Moreover, the serum levels of LDH and creatine phosphokinase (CPK) were also decreased after drinking ACV and CSD but not significantly (data not shown).
Copious studies support that ammonia is one of the major indicators of muscle or physical fatigue after moderate to intense/endurance exercise.1,27 As ammonia which is toxic is produced during intense exercise (as AMP is degraded/deaminated to ammonia) due to high ATP utilization and a high protein/amino acid catabolism and contributes to mental fatigue.25 However, the level of ammonia continuously increased during exhaustive exercise in both ACV and CSD groups as they contain BCAAs that increase amino acid catabolism. The outcome reported in this study is identical to the outcome of Kim and his co-workers.18 Previously, studies have reported that BCAAs supplementation does result in hyperammonemia, and that exercise-induced hyperammonemia can lower BCAAs level especially after endurance sports or exercise participation.1,28 These results infer that ACV or CSD containing BCAAs can trigger hyperammonemia, but the specific mechanism for this triggering process is still unexplained. As stated, BCAAs inhibit serotonin production and enhances L-ornithine, and thereby lowers mental fatigue and effectively abolish mental fatigue contributed by hyperammonemia.6,18 In addition, significantly higher blood ammonia concentrations were observed at exhaustion in the ACV group compared to the CSD group. The reason for lower blood ammonia levels in the CSD group is most likely due to the presence of alanine which acts as ammonia transporter facilitating excretion via liver and kidneys.29
During moderate exercise, muscle utilizes NEFA as a major energy substrate, but during endurance exercise, glucose derived from glycogen is utilized as a pivotal energy source. However, as time in exercise progresses, blood glucose can become depleted, and NEFA may act as an energy source, sparing glycogen and improving physical performance.30 Therefore, during endurance exercise, NEFA did significantly decrease at rest as NEFA was used to spare glycogen as an energy source. Nonetheless, in this clinical trial, we found that consumption of ACV or CSD increased the concentration of blood NEFA during endurance exercise. The increased use of NEFA does spare glycogen to maintain glucose levels and protein from being utilization resulting in enhanced endurance physical performance.25 Moreover, Shimomura and his colleagues31 demonstrated that fatty acids might have a negative impact on BCAAs catabolism. Thus, preserving BCAAs levels and ultimately improving physical performance.
RER score and HR were assessed to cross-check the exercise intensity. RER score and HR increased at the beginning of bicycle ergometer endurance exercise. RER score was 0.7 at baseline and increased towards 1.0 after 15 min of exercise. The increase in RER supports glucose being used as an energy source during endurance exercise. Overall, the RER score and HR plateaued as exercise time moved from 15 to 60 min and exhaustion in both ACV and CSD groups.
This study has several limitations such as involving only a small number of participants in each group, not assessing recovery or follow-up periods, and only utilizing a single one-time consumption of ACV. To overcome all the above-indicated limitations, a large clinical trial with more than 50 athletes with ACV, CSD (standard sports drink) and BCAA group, to explore further ergogenic properties of ACV including follow-up period is needed.
Conclusion
ACV and CSD can delay muscle fatigue associated with endurance exercise while enhancing physical performance via improving blood glucose, NEFA, and suppression of the lactate production while maintaining normal RER score and HR. Taken together, ACV consumption did not significantly improve the ergogenic activity over CSD, even though ACV has phenolic acid and acetic acid, but both ACV and CSD showed an ergogenic effect. Further studies with a larger number of participants, including different exercise types such as treadmill running, swimming, and to involve different exercise intensity and exercise time duration to gain a better understanding of the ACD effect. Most important is to design these studies to better understand the mechanisms for prolonging the exercise time to exhaustion.
Submission statement
This manuscript has not been published or not under consideration for publication elsewhere.
Authors' contribution
Hui-Fang Chiu, I-Shiung Cheng and Chin-Kun Wang involved in concept and designed this clinical trial. Michael Chiang, Hui-Ju Liao, and You-Cheng Shen have conducted this study. Hui-Ju Liao and Kamesh Venkatakrishnan helped in recruiting subjects as well as involved in statistical analysis. Kamesh Venkatakrishnan, I-Shiung Cheng and Chin-Kun Wang Drafted and revised this manuscript.
Conflict of interest
No conflict of interest for this study.
Acknowledgments
The author would like to thank Chung Shan Medical University for financially supporting this trial.
Contributor Information
I-Shiung Cheng, Email: ischeng1965@mail.ntcu.edu.tw.
Chin-Kun Wang, Email: wck@csmu.edu.tw.
References
- 1.Chang C.W., Huang T.Z., Chang W.H., Tseng Y.C., Wu Y.T., Hsu M.C. Acute Garcinia mangostana (mangosteen) supplementation does not alleviate physical fatigue during exercise: a randomized, double-blind, placebo-controlled, crossover trial. Sports Nutr Rev J. 2016;13(1):20. doi: 10.1186/s12970-016-0132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nozaki S., Tanaka M., Mizuno K., et al. Mental and physical fatigue-related biochemical alterations. Nutrients. 2009;25(1):51–57. doi: 10.1016/j.nut.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Martin K., Meeusen R., Thompson K.G., Keegan R., Rattray B. Mental fatigue impairs endurance performance: a physiological explanation. Sports Med. 2018;48(9):2041–2051. doi: 10.1007/s40279-018-0946-9. [DOI] [PubMed] [Google Scholar]
- 4.Pageaux B., Lepers R. Fatigue induced by physical and mental exertion increases perception of effort and impairs subsequent endurance performance. Front Physiol. 2016;7:587. doi: 10.3389/fphys.2016.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng Y.S., Wu D. Anti-fatigue effect of green tea polyphenols (-)-Epigallocatechin-3-Gallate (EGCG) Phcog Mag. 2017;13(50):326. doi: 10.4103/0973-1296.204546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugino T., Shirai T., Kajimoto Y., Kajimoto O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr Res. 2008;28(11):738–743. doi: 10.1016/j.nutres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Goncharov N., Maevsky E., Voitenko N., et al. InNutraceuticals Academic Press; 2016. Nutraceuticals in Sports Activities and Fatigue; pp. 177–188. [DOI] [Google Scholar]
- 8.Ho C.W., Lazim A.M., Fazry S., Zaki U.K., Lim S.J. Varieties, production, composition and health benefits of vinegars: a review. Food Chem. 2017;221:1621–1630. doi: 10.1016/j.foodchem.2016.10.128. [DOI] [PubMed] [Google Scholar]
- 9.Samad A., Azlan A., Ismail A. Therapeutic effects of vinegar: a review. Curr Opin Food Sci. 2016;8:56–61. doi: 10.1016/j.cofs.2016.03.001. [DOI] [Google Scholar]
- 10.Nakamura K., Ogasawara Y., Endou K., Fujimori S., Koyama M., Akano H. Phenolic compounds responsible for the superoxide dismutase-like activity in high-Brix apple vinegar. J Agric Food Chem. 2010;58(18):10124–10132. doi: 10.1021/jf100054n. [DOI] [PubMed] [Google Scholar]
- 11.Halima B.H., Sonia G., Sarra K., Houda B.J., Fethi B.S., Abdallah A. Apple cider vinegar attenuates oxidative stress and reduces the risk of obesity in high-fat-fed male wistar rats. J Med Food. 2018;21(1):70–80. doi: 10.1089/jmf.2017.0039. [DOI] [PubMed] [Google Scholar]
- 12.Mitrou P., Petsiou E., Papakonstantinou E., et al. The role of acetic acid on glucose uptake and blood flow rates in the skeletal muscle in humans with impaired glucose tolerance. Eur J Clin Nutr. 2015;69(6):734. doi: 10.1038/ejcn.2014.289. [DOI] [PubMed] [Google Scholar]
- 13.Atik D., Atik C., Karatepe C. The effect of external apple vinegar application on varicosity symptoms, pain, and social appearance anxiety: a randomized controlled trial. Evid base Compl Alternative Med. 2016;6473678:1–8. doi: 10.1155/2016/6473678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahidi F., McDonald J., Chandrasekara A., Zhong Y. Phytochemicals of foods, beverages and fruit vinegars: chemistry and health effects. Asia Pac J Clin Nutr. 2008;17:1–8. [PubMed] [Google Scholar]
- 15.Morgan J., Mosawy S. The potential of apple cider vinegar in the management of type 2 diabetes. Int J Diabetes Res. 2016;5(6):129–134. [Google Scholar]
- 16.Omar N.A., Allithy A.N., Faleh F.M., et al. Apple cider vinegar (a prophetic medicine remedy) protects against nicotine hepatotoxicity: a histopathological and biochemical report. AJCP. 2015;3:122–127. [Google Scholar]
- 17.Bifari F., Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol. 2017;174(11):1366–1377. doi: 10.1111/bph.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D.H., Kim S.H., Jeong W.S., Lee H.Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J Exerc Nutr Biochem. 2013;17(4):169. doi: 10.5717/jenb.2013.17.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreider R.B., Wilborn C.D., Taylor L., et al. ISSN exercise & sport nutrition review: research & recommendations. Sports Nutr Rev J. 2010;7(1):7. doi: 10.1186/1550-2783-7-7. [DOI] [Google Scholar]
- 20.Rahimi M.H., Shab-Bidar S., Mollahosseini M., Djafarian K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: a meta-analysis of randomized clinical trials. Nutrients. 2017;42:30–36. doi: 10.1016/j.nut.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K., Koba T., Hamada K., Sakurai M., Higuchi T., Miyata H. Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program. J Sports Med Phys Fit. 2009;49:424–431. [PubMed] [Google Scholar]
- 22.Borg G., Ljunggren G., Ceci R. The increase of perceived exertion, aches and pain in the legs, heart rate and blood lactate during exercise on a bicycle ergometer. Eur J Appl Physiol. 1985;54(4):343–349. doi: 10.1007/BF02337176. [DOI] [PubMed] [Google Scholar]
- 23.Bagchi D., Nair S., Sen C.K., editors. Nutrition and Enhanced Sports Performance: Muscle Building, Endurance, and Strength. Academic Press; 2018. pp. 102–115. [Google Scholar]
- 24.Kantanista A., Kusy K., Zarębska E., Włodarczyk M., Ciekot-Sołtysiak M., Zieliński J. Blood ammonia and lactate responses to incremental exercise in highly-trained male sprinters and triathletes. Biomed Hum Kinet. 2016;8(1):32–38. doi: 10.1515/bhk-2016-0005. [DOI] [Google Scholar]
- 25.Yang Q., Jin W., Lv X., et al. Effects of macamides on endurance capacity and anti-fatigue property in prolonged swimming mice. Pharm Biol. 2016;54(5):827–834. doi: 10.3109/13880209.2015.1087036. [DOI] [PubMed] [Google Scholar]
- 26.Andersson-Hall U., Pettersson S., Edin F., Pedersen A., Malmodin D., Madsen K. Metabolism and whole-body fat oxidation following postexercise carbohydrate or protein intake. Int J Sport Nutr Exerc Metabol. 2018;28(1):37–45. doi: 10.1123/ijsnem.2017-0129. [DOI] [PubMed] [Google Scholar]
- 27.Brancaccio P., Maffulli N., Limongelli F.M. Creatine kinase monitoring in sport medicine. Br Med Bull. 2007;81(1):209–230. doi: 10.1093/bmb/ldm014. [DOI] [PubMed] [Google Scholar]
- 28.Falavigna G., Junior J., Rogero M., et al. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Nutrients. 2012;4(11):1767–1780. doi: 10.3390/nu4111767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coqueiro A., Raizel R., Bonvini A., et al. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients. 2018;10(2):119. doi: 10.3390/nu10020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Hall G. The physiological regulation of skeletal muscle fatty acid supply and oxidation during moderate-intensity exercise. Sports Med. 2015;45(1):23–32. doi: 10.1007/s40279-015-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimomura Y., Murakami T., Nakai N., Nagasaki M., Harris R.A. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134(6) doi: 10.1093/jn/134.6.1583S. 1583S-7S. [DOI] [PubMed] [Google Scholar]