Abstract
Limb-loaded walking using ankle weights has been widely applied to increase exercise intensity in older adults. Examining changes in the activation pattern between proximal (RFP) and distal (RFD) regions of the rectus femoris (RF) muscle is key to clarifying gait deficits in older adults. The aim of this study was to determine regional neuromuscular regulation within the RF muscle following three-month limb-loaded walking in older adults. The study participants were 22 healthy older adults (69 ± 6.3 years) who walked at least 160 min per month. Surface electromyography (EMG) and motion capture were used to measure the neuromuscular activities of RFP and RFD and generate kinematic data on the left lower extremity on walking for 240 s at the preferred gait speed on a treadmill at pre- and post-intervention, respectively. Averaged rectified values (ARV) of RFP and RFD were normalized by maximum values of ARV during a gait cycle within ten consecutive gait cycles. Normalized ARV of RFP was greater than RFD at 30%–40% and 70%–90% of the gait cycle and hip joint flexion at 0%–100%, and the walking speed and swing timing at post-were greater than at pre-intervention (p < 0.05). No significant differences were noted in the RFP to RFD activity ratio (RFP/RFD ratio) between pre- and post-intervention, and there was no correlation between the RFP activity level and hip flexion angle in the swing phase (p > 0.05). The activity of RFP compared with RFD and hip joint flexion were increased following limb-loaded walking intervention in older adults.
Keywords: Older adults, Limb-loaded walking, Surface electromyography, Proximal to distal region activity ratio, Hip joint angle, Swing phase
Highlights
-
•
Hip joint flexion was increased after 3 months walking with load in older adults.
-
•
The activity of proximal region of rectus femoris muscle was more than distal region.
-
•
Swing timing and walking speed were increased after limb-loaded walking intervention.
-
•
Changes of regional activity of rectus femoris muscle and hip flexion no correlated.
-
•
Using load during walking can improve gait problems in older adults.
List of abbreviations
- RF
rectus femoris
- RFP
proximal region of the rectus femoris
- RFD
distal region of the rectus femoris
- EMG
electromyography
- ARV
averaged rectified values
- RFP/RFD
ratio proximal to distal region activity ratio of rectus femoris
- ASIS
anterior superior iliac spine
Introduction
Age-associated gait pattern problems, such as slow gait speed, short swing and long stance timing, motor control impairment, rectus femoris (RF) muscle weakness, and repetitive falling, limit the daily activities of older adults.1, 2, 3 Neuromuscular activation of the proximal region of the RF (RFP) muscle is reduced in older compared with young adults.4 It was considered that the reduced activity of RFP may impair subsequent kinematics of lower-extremity movement and essential toe clearance in the swing phase.4 Aging also decreases the maximum knee extension torque and hip flexion moment during walking.5
RF muscle activity in different joint positions within two regions is not uniform.6 The neuromuscular activities of RFP and distal region of the RF (RFD) muscle during knee extension are equal, but the activity of the proximal region during hip flexion is more than distal.7 RFP shows complex innervation8 and plays a more important role in hip flexion compared with the RFD during gait.6 RFP contracts concentrically to move the lower extremities forward, similar to a pendulum at the start of a swing, while RFD contracts eccentrically to control excessive knee flexion.9 Therefore, optimal neuromuscular regulation between these two regions of the RF muscle may facilitate smooth hip and knee flexion movement from pre-to mid-swing.4
Neuromuscular regulation of the RF muscle during the swing phase is affected by the history of falls in older adults.10 According to previous studies, gait training decreased the risk of falling and increased the gait speed in chronic stroke patients.11 Loaded gait training increased the dynamic stability and functional performance of stroke patients.12 Limb-loading during knee flexion and extension movements on the ground also increased electromyography (EMG) activity of the RF muscle,13 and limb-loaded walking increased RF muscle activity during walking on a treadmill in older adults.14
Regarding reduced RFP activity during walking in older adults in previous studies.4,7 We followed the recovery of decreased neuromuscular regulation within the RF muscle. We aimed to detect regional neuromuscular regulation within the RF muscle after three months of limb-loaded walking in older adults in this study. Since the RFP muscle exhibited a superior hip flexor role in the swing phase6 with increased RF muscle activity during limb-loaded walking in older adults,14 and the muscle activity ratio showed an association between muscles activities,15 we hypothesized that RFP compared with RFD activity and the RFP to RFD activity ratio (RFP/RFD ratio) would increase in the swing phase after intervention. We also hypothesized that hip joint flexion in the swing phase would be greater after than before intervention in older adults, and that changes of RFP activity in the swing phase would be correlated with hip and thigh flexion angles.
Methods
Participants
Twenty-two older adults (age: 69 ± 6.3 years, height: 158 ± 8.5 cm, weight: 54.2 ± 9.9 kg, body mass: 21.6 ± 2.5 kg/m2) were recruited in this study. The participants provided written informed consent following a detailed explanation of the purpose, potential benefits, and participation risks. The participants had no history of musculoskeletal or neurological disorders or limitations advised by a physician about performing the exercise. The study procedures were conducted according to the Declaration of Helsinki and research code of Ethics of Chukyo University, and approved by the Committee on Human Experiments of Chukyo University (2019–002).
Experimental design
All participants were trained to walk with loads attached to both legs in at least two 20-min sessions per week, and such limb loading was deemed appropriate if the participants walked at least 160 min per month for the three months. The participants were instructed to mark the times of limb-loaded walking per week over the 3-month period in a custom-designed table. After examining the preliminary trials, selection of the load amount was based on the capability of older adults to walk with a normal posture using loads made up of weights of 0.5, 0.8, 1, and 1.5 kg (1.67 ± 0.38% of body weight).
We recorded demographic characteristics and the normal preferred gait speed of participants over a distance of 10 m on flat ground at pre-intervention. Also, we measured the preferred gait speed of participants at post-intervention to compare changes in walking speed between pre- and post-intervention. Before the start of the experiment, the participants were familiarized with walking on a treadmill. The participants walked while wearing a safety harness on the treadmill (TAKEI S-16075, Takei Kiki Kogyo Co., LTD, Japan) at their preferred gait speed, determined at pre-intervention, for 240 s at pre- and post-intervention, while the left lower extremity was used to record the neuromuscular activity of RFP and RFD and lower-extremity joint kinematics. Muscle activity and joint kinematic data were recorded 30 s after starting into the treadmill walking. Also, Synchronization between EMG and motion capture system was determined as 60 s after the starting.
Lower-extremity kinematics
We used a three-dimensional motion-capture system with six cameras coordinated by reflective markers set on the left lower extremity in the sagittal plane with a sampling rate of 100 Hz (Vicon Bonita3, Vicon Motion Systems Ltd., Oxford, United Kingdom). We also attached seven reflective markers on the left acromion, greater trochanter, lateral femoral condyle, lateral malleolus, fifth metatarsal, toe, and center of the heel. Vertical coordinates of the toe and heel were measured using toe and heel markers to detect heel contact and toe-off timing before walking with a normal standing posture. The start timing of toe-off was the start of the swing phase, and heel contact was the start of the stance phase minus vertical coordinates. Also, determined coordinates for each marker in the sagittal plane were filtered using a fourth-order Butterworth low-pass filter (6 Hz), and EMG and motion capture data were synchronized using an infrared light-emitting diode with electrical signals.
Surface EMG recording
We attached bipolar surface Ag/Agcl EMG electrodes with a 20-mm inter-electrode distance (Dual electrodes, 15,770 N, Greenway-Hayden Loop, Scottsdale, AZ 85260, Noraxon USA, Inc.) along a straight line between the anterior superior iliac spine (ASIS) and the midpoint of the superior pole of the patella to record RF muscle activity of the left lower extremity. The skin site where electrodes were placed was shaved and cleaned with alcohol. Electrodes for RFP were placed at 20% and those for RFD at 50% from ASIS in a straight line, with a common reference electrode on the left iliac crest of the pelvis.16 Locations of electrodes and muscles activities were confirmed with isometric contractions of the RF muscle. EMG signals were filtered with a fourth-order Butterworth filter (bandwidth: 20–400 Hz) (FA-DL-141, 4 assist, Tokyo, Japan) with a sampling rate of 1000 Hz.
Data analysis
The angles of hip, knee, and ankle joints in the sagittal plane and neuromuscular activity levels of the RFP and RFD at pre- and post-intervention were dependent variables. We analyzed the data from 90 s after synchronization between EMG and motion capture systems for ten consecutive gait cycles of the left lower extremity and averaged rectified values (ARV) of RFP and RFD at pre- and post-intervention. We also normalized ARV of RFP and RFD by peak values of ARV during a gait cycle and calculated the mean ARV for each 5% of proportions in ten gait cycles. We defined the hip joint angle as that between the greater trochanter of the femur and trunk, knee joint angle as that between the thigh and shank, and ankle joint angle as that between the condyle of the fifth metatarsus and lateral malleolus. In addition, we defined the thigh angle as that between the horizontal axis of the body and the thigh, and the trunk angle as that between trunk alignment and the vertical axis. The RFP/RFD ratio was determined as the association between neuromuscular activities of RFP and RFD. We detected the difference average of neuromuscular activity of RFP, and RFD and the RFP/RFD ratio from the increased hip joint torque at 30% until decreased at 70% of the gait cycle and, the difference average of hip and thigh angles in the swing phase from toe-off timing to the end of the gait cycle.17,18
We assessed the distribution of data using the Kolmogorov-Smirnov test and analyzed the data with non-parametric tests using SPSS software (version 20, Tokyo, Japan). We also compared RFP and RFD activity levels, hip, knee, ankle, trunk, and thigh motions, and the RFP/RFD ratio with the Wilcoxon rank sum test at pre- and post-intervention. The Spearman correlation test was used to assess the association between changes in neuromuscular activity of RFP and RFD, the RFP/RFD ratio, and hip and thigh flexion. Each gait cycle was considered to be from 0% to 100% and was divided into twenty 5% proportions. The significance level was 0.05.
Results
Gait speed significantly increased, and toe-off timing significantly decreased between pre- and post-intervention (p < 0.05) (Table 1).
Table 1.
Gait parameters pre- and post-intervention.
| Pre | Post | p-Value | |
|---|---|---|---|
| Cadence (Step number/min) | 130.16 ± 7.32 | 128.89 ± 8.84 | 0.46 |
| Toe-off timing (% gait cycle) | 63.57 ± 2.20 | 62.73 ± 2.08 | 0.002∗ |
| Preferred walking speed (km/h) | 4.65 ± 0.70 | 5.21 ± 0.45 | 0.002∗ |
NOTE: p < 0.05 is significant∗.
The average duration of limb-loading during intervention was 1203.63 ± 457.68 min, the load was 0.90 ± 0.26 kg, and the preferred gait speed during walking on a treadmill was 4.68 ± 0.62 (km/h).
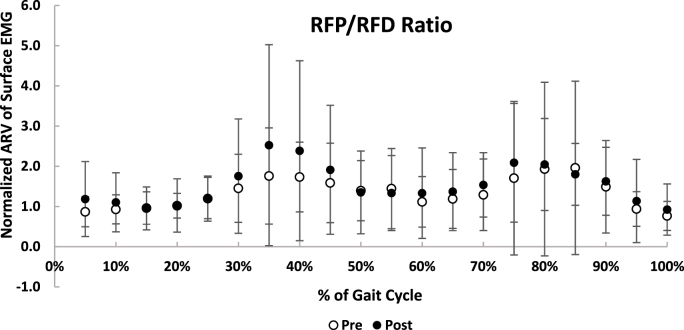
The mean ARV of RFP in 45% (0.31 ± 0.18) and 50% (0.21 ± 0.1) of the gait cycle at post-was lower than at pre-intervention (45% = 0.41 ± 0.22; 50% = 0.32 ± 0.18) (p < 0.05). The mean ARV of RFD in 70% (0.35 ± 0.2) of gait cycle at post-was lower than at pre-intervention (0.45 ± 0.25) (p < 0.05). The mean ARV of RFP at 30%–40%, 80%–85%, and 100% of the gait cycle was higher than that of RFD at pre-intervention, and the mean ARV of RFP at 30%–40% and 70%–90% was higher than that of RFD at post-intervention (p < 0.05) (Fig. 1A and B). There was no significant difference in the mean ARV of the RFP/RFD ratio between pre- and post-intervention (p > 0.05) (Fig. 2).
Fig. 1.
Mean normalized averaged rectified values (ARV) of surface electromyography (EMG) (Mean and Standard deviation) of the proximal (RFP) and distal (RFD) regions of the rectus femoris muscle pre- (A) and post- (B) intervention.
∗ indicates p < 0.05.
Fig. 2.
Mean normalized averaged rectified values (ARV) of surface electromyography (EMG) (Mean and Standard deviation) of proximal to distal region activity ratio of the rectus femoris muscle (RFP/RFD ratio) pre- and post-intervention.
Averages of hip joint and knee joint angles at 0%–100% at post-were lower than those at pre-intervention during each gait cycle (p < 0.05) (Fig. 3A and B). There was no significant difference in the ankle joint angle between pre- and post-intervention (p > 0.05) (Fig. 3C). Average thigh angle changes at 0%–100% of the gait cycle at post-intervention were lower than those at pre-intervention (p < 0.05). Also, average trunk angle changes at 10%–70% and 100% at post-intervention were higher than those at pre-intervention (p < 0.05). There were no significant correlations between the difference in the average neuromuscular activity of RFP and RFD or the RFP/RFD ratio at 30%–70% of the gait cycle and difference in the average thigh and hip angles in the swing phase at pre- and post-intervention (p > 0.05) (Fig. 4A–F).
Fig. 3.
Hip (A), knee (B), and ankle (C) joint range of motion (degrees) in the sagittal plane during walking pre- and post-intervention. ∗ indicates p < 0.05.
Fig. 4.
Correlation between the difference in normalized averaged rectified values (ARV) of surface electromyography (EMG) of proximal (RFP) (A and B) and distal (RFD) (C and D) regions of the rectus femoris muscle, proximal to distal region activity ratio of the rectus femoris muscle (RFP/RFD ratio) (E and F) at 30%–70% of the gait cycle, and difference in the average thigh and hip joint flexion in the swing phase, between pre- and post-intervention. The correlation coefficient is provided (r).
Discussion
This study compared the neuromuscular activation of RFP and RFD after three months of intervention involving limb-loaded walking in older adults. Our results showed that three-month limb-loaded walking increased the neuromuscular activity of RFP in relation to RFD in specific proportions of stance and swing phases, with greater proportions during the swing phase at post-intervention (Fig. 1). Previous studies showed that the maximum activity of the RF muscle is around toe-off and in the terminal swing phase.19 Contrary to this, our results confirm those of previous studies on the major function of RFP during hip flexion in the swing phase.7 In addition, the RFP activity level in older adults was lower than that of young adults in the swing phase during walking at the preferred gait speed, while RFD activity was similar between the two age groups.7 The central nervous system with the selective activation of RFP mainly flexes the hip joint, and RFD is not fully activated during hip flexion.7,20 Thus, RFP plays a key role in elevating the lower extremity during hip flexion in the mid-swing phase.4 According to our results, limb-loaded walking increased neuromuscular activity of RFP more markedly in the mid-swing phase compared with RFD. This finding is informative when considering rehabilitative exercise programs to improve the RFP activity level in the swing phase in older adults.
The neuromuscular activity level of RFP at 45% and 50% of the gait cycle and activity level of RFD at 70% were decreased at post-compared with pre-intervention. A load of 1–3 kg increased knee flexor muscle activity during walking on a treadmill in spinal cord injury patients.21 Backpack loads of more than 30 kg increased RF muscle activity during slope walking in young males.22 Also, the acute application of stable and unstable loads totaling 15% of the body mass during walking on a treadmill increased RF muscle activity in healthy older adults.14 We applied a load of less than 1 kg to each leg (each load was 1.7% of the body weight) based on the ability of older adults to walk for 20 min with two loads. Decreases of RFP and RFD activity levels without any decrease in the range of motion of lower-extremity joints are known to signify improved performance with reduced energy consumption, and this may often be employed to design assistive equipment,22 but the significant decrease of RFP activity levels in two segments and RFD in one segment of the gait cycle after intervention are insufficient to understand this. Therefore, we suggest that RFP and RFD activity levels did not show clear individual changes on limb-loading in older adults.
The functional independent activation from proximal to distal fibers of the RF muscle was determined as intra-muscular sequencing within the muscle.23 The researchers hypothesized that the base of efficient movement is intra-muscular co-ordination that expresses the relation between the activities of RF muscle fibers.23 Thus, the RFP/RFD ratio may determine the association between RFP and RFD activity levels based on the concept of the intra-muscular activity balance and/or intra-muscular co-ordination within the RF muscle. Our results did not show a significant difference in the RFP/RFD ratio between pre- and post-intervention. Although the RFP compared with RFD activity level was higher in specific proportions of the swing phase, there was no significant difference in RFP and RFD activity levels individually at pre- and post-intervention (Fig. 2). Regarding the lack of RFP/RFD ratio studies, we recommend that future studies assess the RFP/RFD ratio within the RF muscle as a bi-articular muscle to clarify the effect of limb-loaded walking on regional activity balance within the RF muscle.
Applying loads of 1–3 kg increased knee flexor muscle activity in the swing phase, but RF muscle activity was not significantly increased.21 Also, 12-week resistance exercise intervention involving the quadriceps muscle increased hip and knee joint flexion and decreased knee joint extension in older adults with knee osteoarthritis.24 According to these studies, our results showed that limb-loaded walking increased hip joint flexion and decreased knee joint extension (Fig. 3). During a dynamic movement such as walking, since hip joint flexion is increased with lower-extremity elevation, increasing knee joint flexion is necessary, and the RF muscle has a role in controlling knee joint extension in the swing phase.19 Our results along with those of previous studies highlight the function of RFP to elevate the lower extremity and RFD to control knee extension during the swing phase.4,6,10,19 Lower-extremity elevation against gravity necessitates an increase in the neuromuscular activity of hip and knee flexor muscles. Hence, our results confirm that the main effects of limb-loaded walking are increasing hip flexion and decreasing knee extension in older adults. A positive correlation between decreasing hip joint flexion and increasing falling was noted in older adults during walking.25 Also, toe vertical displacement was enhanced with increasing hip flexion torque during walking.4,7 We suggest that the increase in hip joint flexion after intervention in this study may decrease the risk of falling during walking in older adults. Although our results did not show a significant change in ankle joint motion at post-compared with pre-intervention, the adequate activity of the RF muscle leads to optimal positioning of the foot to avoid obstacles during stepping and prevents falling (Fig. 3).19 Also, the toe-off timing was decreased and the speed of walking increased after three months of limb-loaded walking in this study (Table 1). Therefore, we agree with previous researchers that limb-loaded walking increases the swing timing and speed of walking, and that it is useful to improve gait deficits in older adults.26
According to our results, thigh flexion was increased in all of the gait cycles at post-compared with pre-intervention, and trunk flexion at 10%–70% and 100% of the gait cycle at post-was greater than at pre-intervention. Therefore, limb-loaded walking intervention may modify trunk and hip kinematics based on the contribution of trunk and hip flexor muscles during walking. There were no significant correlations between the difference average of neuromuscular activity of RFP and RFD and RFP/RFD ratio at 30%–70% of the gait cycle at pre- and post-intervention and difference average of thigh and hip flexion in the swing phase (Fig. 4). Previous studies showed that the RF muscle contributes only 25% of full hip flexion torque.27 Although, the increasing hip flexion is determined with the increasing RF and iliopsoas muscle activity, the contribution of the iliopsoas muscle is greater than that of other hip flexors during hip flexion.28 Previous studies found that limb-loaded walking on a treadmill increased RF muscle activity, but hip and knee joint angles were not significantly changed in older adults.14 According to our results, the RFP activity level was not increased after intervention, and the increase in hip flexion was independent of RFP muscle activity. Previous researchers estimated that the flexed position of the hip joint increases active knee flexor torque during isometric contractions29 and decreases the passive knee extensor torque.30 Thus, the regional activity of the RF muscle was not necessarily correlated with hip and thigh flexion values (Fig. 4), and it may be related to the contribution of other hip flexors.
Limitations
One limitation of this study concerned the load that the older adults used during intervention. We suggest that additional load timing or progressive load intervention is required to clearly change the regional activity of the RF muscle. Since additional loading for older adults is difficult, we recommend examining the effect of limb-loaded walking with various load timings during the intervention to identify the optimal load and optimize the neuromuscular activity of RFP and RFD in older adults. Moreover, there was the limitation that we compared the effect of limb-loaded walking after three months of intervention while the comparison with a control group to strengthen these results may be more useful. Also, regarding the use of fine wire electrodes to detect the activity level of deep muscles such as the psoas muscle, the determination of the contribution of psoas muscle activity during hip flexion in older adults was limited. The determination of psoas and trunk muscles activity on limb-loaded walking intervention may explain the respective changes in the regional activity of the RF muscle. Future studies are recommended to investigate in young subjects during walking, examining psoas and trunk muscles activity associated with the regional activity of the RF muscle on loading.
Conclusions
We investigated the effect of the limb-loaded walking intervention on regional neuromuscular regulation within the RF muscle in older adults. In summary, load application during walking increased the neuromuscular activity of RFP compared with RFD in specific proportions of stance and swing phases, with greater activity during the swing. Also, limb-loaded walking increased hip joint flexion, swing timing, and the speed of walking. Furthermore, the increasing hip joint flexion was not affected by the neuromuscular regional activity of the RF muscle. These results may help guide clinician rehabilitative therapists to design training methods of limb-loaded walking intervention to improve gait patterns in older adults.
Ethical approval statement
All procedures in this study were according to the Declaration of Helsinki and research code of Ethics of Chukyo University, and approved by the Committee on Human Experiments of Chukyo University (2019–002). All participants provided informed consent.
Submission statement
This work described has not been published previously, that is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or in any other language, without the written consent of the copyright-holder.
Authors’ contribution
SN carried out the experiments, data collection and statistical analysis, writing the original draft, review and editing. HA was involved in funding acquisition, supervision, design and coordination and revising the manuscript. KW administrated the study, design and coordination, and helped to data analysis and draft the manuscript, review and editing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Funding
This research was supported by AMED (Japan Agency for Medical Research and Development) [Grant number JP19le0110012].
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Spinoso D.H., Bellei N.C., Marques N.R., et al. Quadriceps muscle weakness influences the gait pattern in women with knee osteoarthritis. Adv. rheumatol. 2018;58:26. doi: 10.1186/s42358-018-0027-7. [DOI] [PubMed] [Google Scholar]
- 2.Marques N.R., Hallal C.Z., Spinoso D.H., et al. Age-related alterations in the activation of trunk and lower limb muscles during walking. J Back Musculoskelet Rehabil. 2016;29(2):295–300. doi: 10.3233/BMR-150628. [DOI] [PubMed] [Google Scholar]
- 3.Jahn K., Zwergal A., Schniepp R. Gait disturbances in old age. Dtsch Arztebl Int. 2010;107(17):306–316. doi: 10.3238/arztebl.2010.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe K., Kouzaki M., Moritani T. Regional neuromuscular regulation within human rectus femoris muscle during gait in young and elderly men. J Biomech. 2016;49(1):19–25. doi: 10.1016/j.jbiomech.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Devita P., Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe K., Kouzaki M., Moritani T. Non-uniform surface EMG responses to change in joint angle within rectus femoris muscle. Muscle Nerve. 2014;50(5):794–802. doi: 10.1002/mus.24232. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K., Kouzaki M., Moritani T. Task-dependent spatial distribution of neural activation pattern in human rectus femoris muscle. J Electromyogr Kinesiol. 2012;22(2):251–258. doi: 10.1016/j.jelekin.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Sung D.H., Jung J.Y., Kim H.D., et al. Motor branch of the rectus femoris: anatomic location for selective motor branch block in stiff-legged gait. Arch Phys Med Rehabil. 2003;84(7):1028–1031. doi: 10.1016/S0003-9993(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 9.Whittle M.W. Butterworth-Heinemann; Edinburgh: 2008. Gait Analysis: An Introduction. [Google Scholar]
- 10.Watanabe K. Relationship between toe clearance strategy and regional regulation of rectus femoris muscle during swing phase in prolonged walking in young and older adults. Front. Physiol. 2018;9:1–8. doi: 10.3389/fphys.2018.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki L., Bigongiari A., Franciulli P.M., et al. The efPhys. Ther. Rehabil. Sci.fect of gait training and exercise programs on gait and balance in post-stroke patients. Med Dent J. 2015:1–5. doi: 10.5935/MedicalExpress.2015.04.01. [DOI] [Google Scholar]
- 12.Shin S.H., Lee M.Y. Effect of gait training with additional weight on balance and gait in stroke patients. Phys. Ther. Rehabil. Sci. 2014;3:55–62. doi: 10.14474/ptrs.2014.3.1.55. [DOI] [Google Scholar]
- 13.Santos I.N.P., Mendes I.D.S., Lima M.O., et al. Muscle electrical activity during exercises with and without load executed on dry land and in an aquatic environment. Res. Biomed. Eng. 2015;31(1):19–25. doi: 10.1590/2446-4740.0380. [DOI] [Google Scholar]
- 14.Walsh G.S., Low D.C., Arkesteijn M. Effect of stable and unstable load carriage on walking gait variability, dynamic stability and muscle activity of older adults. J Biomech. 2018;73:18–23. doi: 10.1016/j.jbiomech.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Nimphius S, McBride Jeffrey M, et al. Comparison of Quadriceps and Hamstring Muscle Activity during an Isometric Squat between Strength-Matched Men and Women. Journal of Sports Science and Medicine. 2019;18(1):101–108. [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe K. Effect of taping and its conditions on electromyographic responses of knee extensor muscles. Hum Mov. Sci. 2019;63:148–155. doi: 10.1016/j.humov.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee M., Kim J., Son J., et al. Kinematic and kinetic analysis during forward and backward walking. Gait Posture. 2013;38(4):674–678. doi: 10.1016/j.gaitpost.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Fukuchi C.A., Fukuchi R.K., Duarte M. A public dataset of over ground and treadmill walking kinematics and kinetics in healthy individuals. Peer. J. 2018;6 doi: 10.7717/peerj.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frigo C.A., Wyss C., Brunner R. The effects of the rectus femoris muscle on knee and foot kinematics during the swing phase of normal walking. Appl Sci. 2020;10(21):7881. doi: 10.3390/app10217881. [DOI] [Google Scholar]
- 20.Miyamoto N., Wakahara T., Kawakami Y. Task dependent inhomogeneous muscle activities within the bi articular human rectus femoris muscle. Plos One. 2012;7(3):1–5. doi: 10.1371/journal.pone.0034269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam T., Wirz M., Lunenburger L., et al. Swing phase resistance enhances flexor muscle activity during treadmill locomotion in incomplete spinal cord injury. Neurorehabi. Neural. Rep. 2008;22(5):438–446. doi: 10.1177/1545968308315595. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Qiang L., Song Q., et al. Effects of Backpack loads on leg muscle activation during slope walking. Appl Sci. 2020;10(14):4890. doi: 10.3390/app10144890. [DOI] [Google Scholar]
- 23.Von Laßberg C., Julia A.S., Graf D., et al. Longitudinal sequencing in intramuscular coordination: a new hypothesis of dynamic functions in the human rectus femoris muscle. Plos One. 2017;17:1–23. doi: 10.1371/journal.pone.0183204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguiar G.C., Rocha S.G., Rezende G.A.S., et al. Effects of resistance training in individuals with knee osteoarthritis. Fisioter. Mov. 2016;29(3):589–596. doi: 10.1590/1980-5918.029.003.AO17. [DOI] [Google Scholar]
- 25.Burnfield J.M., Josephson K.R., Powers C.M., et al. The influence of lower extremity joint torque on gait characteristics in elderly men. Arch Phys Med Rehabil. 2000;81(9):1153–1157. doi: 10.1053/apmr.2000.7174. [DOI] [PubMed] [Google Scholar]
- 26.Demura T., Demura S. Relationship among gait parameters while walking with varying loads. J Physiol Anthropol. 2010;29(1):29–34. doi: 10.2114/jpa2.29.29. [DOI] [PubMed] [Google Scholar]
- 27.Landin D., Thompson M., Reid M. Actions of two Bi-articular muscles of the lower extremity: a review article. J Clin Med Res. 2016;8(7):489–494. doi: 10.14740/jocmr2478w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiroumaru T., Kurihara T., Isaka T. Measurement of muscle length-related electromyography activity of the hip flexor muscles to determine individual muscle contributions to the hip flexion torque. SpringerPlus. 2014;3:624. doi: 10.1186/2193-1801-3-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guex K., Gojanovic B., Millet G.P. Influence of hip-flexion angle on hamstrings isokinetic activity in sprinters. J Athl Train. 2012;47(4):390–395. doi: 10.4085/1062-6050-47.4.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellis E. Biceps femoris fascicle length during passive stretching. J Electromyogr Kinesiol. 2018;38:119–125. doi: 10.1016/j.jelekin.2017.11.015. [DOI] [PubMed] [Google Scholar]