Abstract
Background and purpose
Complementary therapies, such as yoga, have been proposed to address gait and balance problems in Parkinson's disease (PD). However, the effects of yoga on gait and static balance have not been studied systematically in people with PD (PWP). Here we evaluated the effects of a 12-week long Hatha yoga intervention on biomechanical parameters of gait and posture in PWP.
Methods
We employed a pilot randomized controlled trial design with two groups of mild-to-moderate PWP (immediate treatment, waitlist control; N = 10 each; Mean Hoehn and Yahr score = 2 for each group). Baseline Unified Parkinson's Disease Rating Scale (UPDRS) motor scores, and gait and postural kinematics including postural sway path length, cadence, walking speed, and turning time were obtained. The immediate treatment group received a 60-min Hatha yoga training twice a week for 12 weeks, while the waitlisted control group received no training. After 12 weeks, gait and postural kinematics were assessed (post-test for treatment group and second-baseline for waitlist group). Then, the waitlist group received the same yoga training and was evaluated post-training.
Results
After Hatha yoga training, UPDRS motor scores improved with an 8-point mean decrease which is considered as a moderate clinically important change for mild-moderate PD. Sway path length during stance decreased significantly (mean reduction: -34.4%). No significant between-group differences or improvements in gait kinematics were observed.
Conclusion
This study showed that a 12-week Hatha yoga training can improve static balance in PWP. We found no evidence that it systematically improves gait performance in PWP.
Keywords: Hatha yoga, Parkinson's disease, Posture, Gait
Introduction
The clinical impact of altered posture and gait in Parkinson's disease (PD) is well known,1, 2, 3 leading to a reduced quality of life and an increased risk of falling.4 Reports indicate that 70% of people with PD (PWP) will fall during the disease course, often resulting in serious consequences.5 Objective measures of body sway show that antero-posterior and medio-lateral displacements of the center of pressure (COP) are markedly variable in PWP when compared to age-matched healthy controls.6,7 The neural mechanisms underlying postural dyscontrol in PD include abnormal postural reflex amplitudes, latencies,8,9 anticipatory postural adjustments,10,11 and deficits in somatosensory processing.12,13 Myogenic features such as reduced strength in leg muscles have also been described.14,15
Current treatment options such as anti-parkinsonian medication and deep brain stimulation (DBS) are known to have beneficial effects on Parkinsonian signs such as tremor, bradykinesia and rigidity.16 However, their impact on improving gait and posture is limited. For example, they do not reduce the number of postural corrections during standing.6 DBS improves asymmetry of gait, increases stride length and gait velocity, but does not restore gait that is comparable to healthy adults.17 STN DBS has been shown in recent meta-analyses to deteriorate gait.18,19
Given the limited postural improvement derived from pharmacological and neuromodulation therapies, numerous forms of complementary therapies such as treadmill training, biofeedback training, as well as ballroom dancing, tai chi and yoga have been proposed to address postural stability problems in PD.20 There is preliminary evidence that Hatha yoga positively influences balance and enhances functional joint range of motion (ROM) in PWP. A case study reported improvements in lower extremity muscle strength, reaction time, movement speed, and endpoint excursions during dynamic balance tasks after an intensive 12-week program of combined strength and yoga training.21 Other studies reported general improvements in motor function based on clinical rating scales. For example, after a 12-week yoga training, Unified Parkinson's Disease Rating Scale (UPDRS) motor scores decreased between 7-12 points22, 23, 24, 25 and Berg Balance Scores improved by 10 points,26 indicating that yoga can positively affect standing balance. It is important to note these studies reported improvements in UPDRS motor scores are moderate clinically important differences27 and improvements in Berg Balance Scores are above minimum detectable change.28
At this point, the effects of Hatha yoga on balance performance in PWP are promising, but have not been systematically evaluated. Specifically, it is unclear, whether yoga can lead to improvements in dynamic balance and gait, in addition to enhancing standing balance and functional joint range-of-motion. It is imperative to understand the effect of yoga on static as well as dynamic balance. The current study seeks to address this knowledge gap. In addition to using clinical measures, e.g. UPDRS rating scales, we obtained objective biomechanical outcome measures of standing and dynamic balance to evaluate the effects of Hatha yoga training on posture, gait and joint flexibility of the trunk and lower limbs.
Methods
Participants
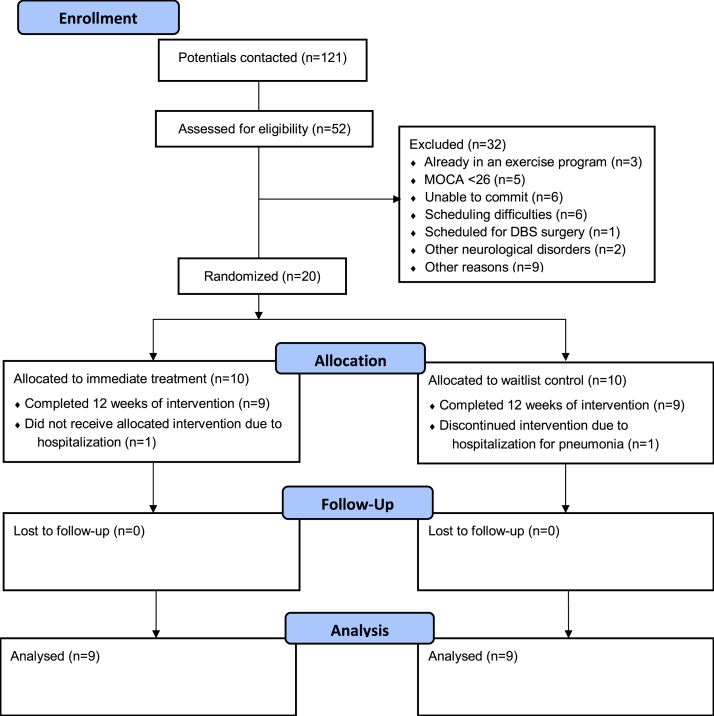
Twenty PWP were recruited for study participation (see Fig. 1) based on the following inclusion criteria: 1) A diagnosis of mild to moderate idiopathic PD (Hoehn and Yahr stages I – III), 2) age between 45-75 years, 3) stable dose of dopaminergic medication for 4 weeks prior to enrollment, 4) not having practiced any form of yoga regularly in the past 6 months, 5) not currently participating in a supervised exercise program for more than 2 days a week, 6) no other known neurological condition, 7) no history of falls in the past 3 months, 8) normal cognitive function (>/ = 26 on Montreal Cognitive Assessment), 9) stable hemodynamic function, 10) no spinal fusion or other orthopedic surgery in the past 6 months, and 11) ability to ambulate 6 m without any assistive device. Twenty people consented and 18 participants (mean age ± SD: 64.2 ± 7.5 years) completed all required study stages (see Table 1 for participant demographics). As there is no consensus on sample size for pilot and feasibility studies,29 our sample size was determined by safety concerns for class size that restricted a group of no more than 10 participants with a single yoga teacher and a research assistant. The study procedure was reviewed and approved by the Human Research Protection Program, University of Minnesota. Informed consent was obtained from all participants.
Fig. 1.
CONSORT flow diagram of study participants.
Table 1.
Participant characteristics. Group: 1 = immediate treatment; 2 = waitlist control. Levodopa equivalent dosage was determined according to Pahwa et al., 1997.32
| ID | Age (years) | Group | Gender | UPDRS-III | Hoehn & Yahr Stage | Disease Duration (years) | Levodopa Equivalent Dosage (mg) | Number of Absences in Yoga Sessions |
|---|---|---|---|---|---|---|---|---|
| 1 | 72 | 2 | M | 14 | 2 | 6 | 450 | 0 |
| 2 | 58 | 2 | F | 16 | 2 | 2 | 50 | 2 |
| 3 | 67 | 1 | F | 14 | 1 | 3 | 150 | 2 |
| 4 | 66 | 2 | F | 23 | 2 | 3.5 | 750 | 2 |
| 5∗ | 74 | 2 | M | – | 3 | 4 | 1000 | – |
| 6 | 57 | 1 | F | 14 | 3 | 13 | 1075 | 14 |
| 7 | 73 | 2 | M | 24 | 3 | 7 | 500 | 3 |
| 8 | 49 | 1 | F | 16 | 1 | 0.75 | 0 | 2 |
| 9 | 75 | 1 | M | 11 | 2 | 2.5 | 300 | 5 |
| 10 | 62 | 1 | M | 14 | 2 | 3 | 700 | 0 |
| 11 | 68 | 2 | F | 22 | 1 | 6 | 400 | 2 |
| 12 | 69 | 2 | M | 14 | 1 | 5.5 | 2386 | 3 |
| 13 | 66 | 1 | F | 13 | 2 | 4 | 300 | 0 |
| 14 | 62 | 1 | F | 23 | 3 | 1.5 | 425 | 2 |
| 15∗ | 63 | 2 | F | 28 | 3 | 10 | 800 | 17 |
| 16 | 55 | 2 | F | 34 | 3 | 5.5 | 500 | 6 |
| 17 | 55 | 1 | M | 20 | 3 | 7 | 1368 | 3 |
| 18 | 76 | 1 | M | 24 | 3 | 4 | 450 | 1 |
| 19 | 60 | 2 | M | 23 | 2 | 1.5 | 300 | 3 |
| 20 | 66 | 1 | M | 26 | 2 | 2.5 | 0 | 0 |
Note: ∗ indicates participants who dropped the study for medical reasons unrelated to this study after recruitment.
Experimental design
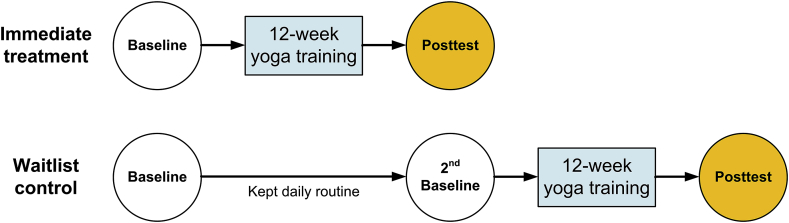
This pilot study used a randomized controlled trial design with 2-arms: an immediate treatment group and a waitlist control group (see Fig. 2). The experimental design was implemented to control for the effect of time in a neurodegenerative disease. The waitlist control group attempted to document, if gait and balance performance declined within a 12 week period due to the disease, which could mask any treatment effects. A computer-generated randomization list using pre-allocated participant IDs with an allocation ratio of 1:1 was prepared by a statistician for random group allocation. A sealed envelope with participant IDs containing group allocations and instructions about yoga intervention program was given to each participant at the end of the baseline evaluation. The researcher who collected, processed and analyzed the data was blinded to the group assignments. All participants were assessed at baseline within one week prior to the intervention program in Human Sensorimotor Control Laboratory, University of Minnesota. Participants were scheduled based on their availability and were tested in their ON medication state, typically, mid-morning, which is the time of peak antiparkinsonian medication effectiveness. For follow-up testing, care was taken to schedule the participants at the same time of the day as during baseline to control for the daily fluctuation of Parkinsonian symptoms. The immediate treatment group attended the Hatha yoga program after baseline evaluation. After 12 weeks, the immediate treatment group underwent the post-evaluation and the waitlist control group was tested for a second baseline. Upon completion of the 12-week yoga intervention program, the waitlist control group was tested again.
Fig. 2.
Experimental design. Twenty PWP were randomly assigned to 2 groups: immediate treatment and waitlist control.
Intervention and setting
A lead yoga instructor designed the intervention program based on a review of the available literature. This program was then reviewed and approved by a panel of six yoga experts who specialized in teaching people with neurological and musculoskeletal conditions. The final approved yoga intervention included: standing poses such a mountain, modified tree pose, warrior I/II, modified sun salutation; seated poses such as easy pose and seated twist; supine poses such as cat/cow, child's pose, spinal twist, and corpse pose. Seated versions of standing poses were offered to accommodate a wide variety of physical abilities within the participant pool. For the full list of exercises, see Justice et al., 2018.30 All participants progressed uniformly through the program that was administered in 60-min sessions twice every week at a local yoga studio.
Measure of disease severity
Severity of motor symptoms was assessed for all participants using the motor subsection of the UPDRS (see Table 1). The same investigator (CC), who was trained and certified to administer UPDRS, evaluated all participants throughout the study to avoid problems of inter-rater variability.
Evaluation of postural stability during stance
Postural stability during stance was evaluated in the following six conditions: 1) normal stance, arms by the side and eyes open, 2) normal stance, arms by the side and eyes closed, 3) normal stance, shoulders flexed to 90° with elbows extended and eyes open, 4) normal stance, shoulders flexed to 90° with elbows extended and eyes closed, 5) tandem stance with dominant foot front, arms by the side and eyes open, and 6) tandem stance with dominant foot front, arms by the side and eyes closed. Each participant was encouraged to maintain these positions for 20 s, while standing on a force platform. They could take a step and open their eyes (during the eyes-closed condition), if felt unstable. Two researchers stood on either side of the participant to assure safety. No inadvertent falls occurred during the course of the study. All participants were allowed only 3 trials in each of the 6 conditions, irrespective of them taking a step. If a participant took a step outside of the force platform during a trial, the data from that trial were not included in analysis. Force platform (AMTI OR6 Platform) recorded the COP changes over time at a sampling frequency of 960 Hz. The COP trajectory over the span of 20 s was used to derive two established postural sway variables: COP sway length (length of the trajectory) and COP sway area (area encompassed by the COP trajectory). These two variables served as the outcome measures for the postural stability.
Evaluation of gait kinematics
For the assessment of gait, participants walked straight from starting position from one end of the room to the other, a distance of 5.8 m, turned around, returned to the starting position, turned around and finally assumed at the initial position. Each participant completed three trials yielding a total of 32–40 steps and 6 turns. A 16-camera Vicon optical motion capture system recorded the movement at a sampling frequency of 240 Hz by tracking thirty-nine infrared reflective markers placed on specific bony landmarks on the participants' body using the Vicon's standard whole body plug-in gait marker positions. Based on the reconstructed 3D coordinate data of each marker, appropriate joint kinematics and center of mass (COM) trajectories were determined. The kinematic variables included: Maximum walking speed, average walking speed, turning time, cadence, foot clearance height and arm swing excursion. Walking speed was calculated based on the displacement of COM in the horizontal plane in all the three trials. Cadence and foot clearance height were derived from the displacement of the heel markers in the vertical plane across the trials. Arm swing was calculated as an average range of 2D shoulder flexion/extension across the trials.
Evaluation of the flexibility of the trunk and lower limbs
Each participant's joint flexibility was evaluated by a battery of standardized movements such as bending forward and backward with straight knee, side-bending, lifting each leg as high as possible by bending at hips and knees, lifting the leg laterally, and rotating the trunk on each side to look back. Each movement was repeated thrice. Movements were recorded by the optical motion capture system. Based on the motion capture data, the functional range of motion (F-ROM) of hip flexion/extension, hip abduction/adduction, trunk flexion/extension, trunk lateral flexion and rotation were derived to serve as the outcome measures of flexibility.
Statistical treatment of the variables
All variables were tested for normality using the Kolmogorov-Smirnov test. Some of the variables were found to be non-normally distributed (see Results). For consistency of reporting, we applied non-parametric Mann-Whitney U tests to determine between group differences in the demographic variables and all outcome measures at baseline. The groups were collapsed to test for within participant differences as an effect of Hatha yoga intervention. We applied non-parametric Wilcoxon signed ranks tests to determine training-related differences in all variables. All p-values were corrected for multiple comparisons using Holm's procedure.31 A hierarchical cluster analysis was conducted to understand the differences between responders and non-responders on the static postural stability measures. Mann-Whitney U tests were also applied to determine the differences between the immediate treatment and the waitlist control group after 12 weeks. All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 25.0.
Results
Results showed that several outcome measures such as COP sway length, turning time, and FROM in knee flexion, hip flexion, and trunk rotation were not normally distributed. At baseline, no systematic differences were found between the immediate treatment and the waitlist-control group in any of the measured variables (See Table 2). Consequently, this allowed both groups to be collapsed for further analyses.
Table 2.
Summary table showing the differences in the participant demographics, postural stability, gait and functional joint range of motion between the two groups of participants (Group I: Immediate Treatment & Group 2: Waitlist Control) at baseline and at 12 weeks.
| Baseline |
12 weeks |
|||||
|---|---|---|---|---|---|---|
| Group I Median (IQR) | Group II Median (IQR) | p - value | Group I Median (IQR) | Group II Median (IQR) | Effect Size, d | |
| Demographics and Disease Metrics | ||||||
| Age, years | 64.0 (12.5) | 67.0 (12.8) | 0.6 | – | – | – |
| Hoehn & Yahr Stage (1–3) | 2 (0.8) ∗ | 2 (0.8) ∗ | 0.9 | – | – | – |
| Motor UPDRS (0–108) | 25.5 (13.5) | 26.5 (11.3) | 0.6 | 15 (8.3) | 21.3 (7.8) | -0.67 |
| Sway Path Length (mm) | ||||||
| Eyes Open | ||||||
| Normal Stance | 7999.8 (3673.6) | 6471.5 (4066.4) | 0.5 | 4826.0 (1834.6) | 5823.9 (2174.2) | -0.58 |
| Stance, Flexed Arms | 7826.1 (3474.9) | 6531.5 (4004.2) | 0.8 | 5038.7 (1636.0) | 5873.3 (1769.0) | -0.34 |
| Tandem Stance | 8104.3 (2831.0) | 6889.6 (3726.9) | 0.8 | 5372.8 (1532.0) | 7017.1 (1120.9) | -0.82 |
| Eyes closed | ||||||
| Normal Stance | 7984.6 (3475.8) | 6469.8 (4202.0) | 0.7 | 4907.6 (1553.9) | 5753.2 (2235.8) | -0.27 |
| Stance, Flexed Arms | 7834.8 (3618.8) | 6557.5 (4114.4) | 0.7 | 5056.0 (1613.1) | 5808.8 (1630.9) | -0.49 |
| Tandem Stance | 8048.3 (3481.1) | 7840.8 (5569.5) | 0.9 | 5252.6 (1184.0) | 7094.7 (-) | – |
| Gait Variables | ||||||
| Foot Clearance, Left (mm) | 253.8 (33.8) | 232.3 (57.3) | 0.7 | 245.3 (25.9) | 234.8 (15.4) | 0.53 |
| Foot Clearance, Right (mm) | 241.2 (52.9) | 231.7 (50.2) | 0.6 | 247.5 (18.9) | 230.0 (15.5) | 0.79 |
| Max. Walking Speed (m/s) | 1.3 (0.2) | 1.3 (0.2) | 0.8 | 1.3 (0.2) | 1.1 (0.1) | 0.59 |
| Avg. Walking Speed (m/s) | 0.9 (0.1) | 0.9 (0.2) | 1.0 | 0.9 (0.2) | 0.9 (0.1) | 0.64 |
| Turning Time (s) | 1.1 (0.5) | 1.1 (1.1) | 0.6 | 1.1 (0.4) | 1.3 (0.4) | -0.45 |
| Cadence (steps/min) | 45.0 (5.7) | 44.6 (8.1) | 0.5 | 47.2 (6.6) | 47.7 (9.9) | 0.3 |
| Arm Swing, Left (deg) | 26.0 (20.5) | 37.6 (36.3) | 0.1 | 20.5 (12.1) | 43.5 (26.2) | -1.1 |
| Arm Swing, Right (deg) | 28.1 (30.4) | 27.3 (18.3) | 0.9 | 29.4 (23.5) | 30.1 (17.0) | -0.22 |
| Functional ROM | ||||||
| Knee Flexion, Left (deg) | 16.6 (26.9) | 26.3 (24.0) | 0.8 | 27.9 (21.5) | 36.2 (14.5) | 0.48 |
| Knee Flexion, Right (deg) | 17.6 (17.1) | 23.8 (15.5) | 0.7 | 23.0 (14.7) | 24.2 (17.7) | -0.06 |
| Hip Flexion, Left (deg) | 38.3 (11.7) | 41.4 (13.8) | 0.3 | 40.1 (10.9) | 44.8 (8.5) | 0.73 |
| Hip Flexion, Right (deg) | 46.1 (19.0) | 45.8 (14.8) | 0.6 | 46.0 (14.2) | 46.5 (8.2) | 0.45 |
| Hip Abduction, Left (deg) | 107.6 (15.3) | 98.6 (12.2) | 0.1 | 105.7 (17.1) | 102.3 (17.9) | -0.85 |
| Hip Abduction, Right (deg) | 104.1 (26.8) | 94.8 (17.3) | 0.8 | 108.9 (11.0) | 104.6 (15.9) | -0.82 |
| Trunk Flexion (deg) | 86.4 (36.7) | 95.8 (39.1) | 0.6 | 100.6 (30.7) | 99.0 (16.9) | -0.37 |
| Trunk Rotation (deg) | 70.3 (26.5) | 73.3 (44.5) | 0.5 | 64.6 (18.9) | 86.7 (22.2) | 0.67 |
Note: ∗ indicates mean (SD).
Clinical measures of motor symptom severity improved after yoga training
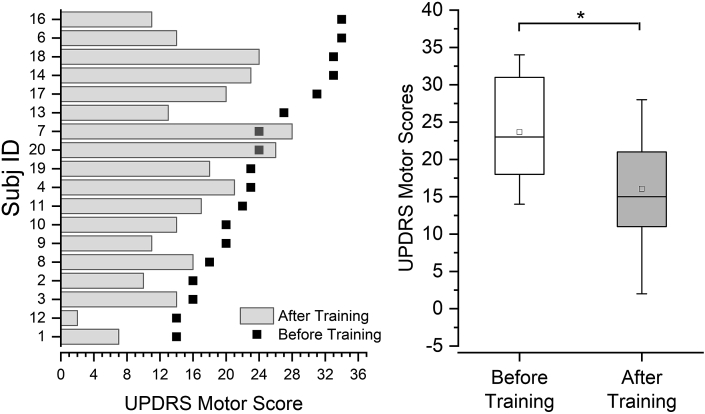
The severity of motor symptoms was evaluated using UPDRS-III. The median UPDRS-III scores decreased significantly by 8 points from 23 (range: 14–34) before training to 15 (range: 2–28) after training (Wilcoxon signed ranks test; V = 163.5, p < 0.001, a 35% reduction) indicating improvements in general motor function (see Fig. 3). 13/18 PWPs demonstrated improvements above 3 points difference which constitute as the minimum clinically important change reported in.27
Fig. 3.
A. Unified Parkinson's Disease Rating Scale (UPDRS) motor scores for each participant before and after training. All participants were arranged in an ascending order of their baseline UPDRS motor scores. Note that participants with higher UPDRS scores do not necessarily achieve greater improvements with training. B. Changes in UPDRS motor scores across all participants. ∗ indicates significant differences between before and after training scores (p < 0.001).
Measures of static postural stability show improvements after yoga training
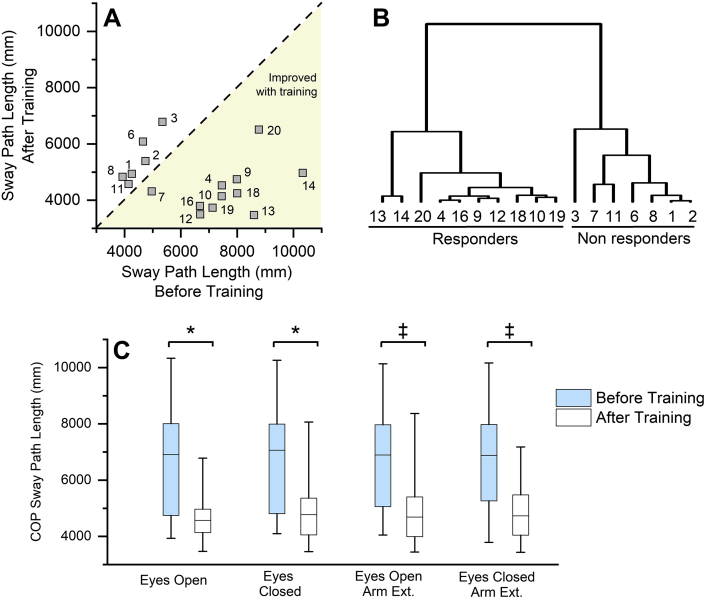
COP sway length and sway area were assessed as measures of postural stability during stance under six different conditions. Fig. 4A shows the individual sway responses of all participants to training in the normal stance - eyes open condition. Across the sample population, COP sway length was significantly reduced in all the six postural stability test conditions after training (see Table 3; Fig. 4C). No systematic differences were found in the COP sway area across all participants. The data in Fig. 4A indicate that a subgroup of participants positively responded to the training, while others showed little to no change. To better understand differences between responders and non-responders, we performed a hierarchical cluster analysis on COP sway length based on the combined sway data of five conditions (tandem stance/eyes closed condition was not included as several participants could not maintain this position and stepped off the platform, leading to incomplete data sets). Based on the hierarchical cluster analysis, a group of responders (n = 10) and non-responders (n = 5) were identified (see Fig. 4B), which matched the participants in the two clusters (seen in Fig. 4A). Quantifying the magnitude of the training effects on static sway between responders and non-responders revealed a mean reduction in COP sway length between 41.5–44.8% across all the conditions in the responders, while the non-responders maintained or showed a smaller increase in COP sway length (range: 3.3–22.1%). As a group, the responders exhibited significantly higher training-related improvements in COP sway length when compared to the non-responders (p < 0.001). We evaluated the influence of other factors such as age, the number of yoga training sessions attended, disease duration and levodopa equivalent dosage on the training related differences in the postural stability. These factors did not show any significant differences between the responders and the non-responders.
Fig. 4.
Effects of yoga training on postural stability measures. A. Training-induced center of pressure (COP) sway path length changes in each participant during the normal stance with eyes open condition. The dashed line represents the line of equality. Note that a group of participants show greater improvements in sway path length after training, while others are around the line of equality. B. A cluster dendrogram showing the results of hierarchical cluster analysis based on the combined sway path length changes during five postural stability assessment conditions. Two major clusters of responders and non-responders can be identified. Note how the participants who show improvements in the eyes-open condition (see Fig. 4A) also fall into the responder cluster across the five postural stability conditions. C. COP sway path length changes across participants in the four postural stability conditions. Note that COP sway path length improved in all the four conditions. ∗ indicates significant differences before and after training (p < 0.05). ‡ indicates significant differences before and after training (p < 0.01).
Table 3.
Summary table showing the training-induced differences in the variables of postural stability, gait and functional joint range of motion across all the participants.32
| Median |
Range |
p-value (Effect size, d) | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Sway Path Length (mm) | |||||
| Eyes Open | |||||
| Normal Stance | 6909 | 4570 | 3936–10333 | 3467–6781 | 0.012∗ (1.29) |
| Stance, Flexed Arms | 6894 | 4689 | 4048–10135 | 3445–8367 | 0.007∗ (1.15) |
| Tandem Stance | 7629 | 4967 | 3986–10552 | 3752–7997 | 0.001∗ (1.40) |
| Eyes closed | |||||
| Normal Stance | 7062 | 4773 | 4098–10262 | 3461–8064 | 0.012∗ (1.10) |
| Stance, Flexed Arms | 6878 | 4730 | 3791–10163 | 3436–7178 | 0.004∗ (1.21) |
| Tandem Stance | 8002 | 4634 | 4725–11094 | 4121–6851 | 0.001∗ (1.80) |
| Gait Variables | |||||
| Foot Clearance, Left (mm) | 244 | 243 | 205–271 | 209–299 | 0.42 (-0.30) |
| Foot Clearance, Right (mm) | 240 | 240 | 199–272 | 207–285 | 0.27 (-0.31) |
| Max. Walking Speed (m/s) | 1.20 | 1.28 | 0.91–1.60 | 0.88–1.68 | 0.07 (-0.37) |
| Avg. Walking Speed (m/s) | 0.89 | 0.96 | 0.73–1.19 | 0.67–1.27 | 0.08 (-0.32) |
| Turning Time (s) | 1.13 | 1.02 | 0.87–2.37 | 0.66–1.77 | 0.06 (0.46) |
| Cadence (steps/min) | 45.6 | 47.3 | 37.5–54.1 | 36.6–58.4 | 0.27 (-0.30) |
| Arm Swing, Left (deg) | 29.6 | 23.0 | 6.6–81.8 | 8.0–57.0 | 0.09 (0.25) |
| Arm Swing, Right (deg) | 28.1 | 28.7 | 5.1–73.3 | 5.4–50.8 | 0.35 (0.13) |
| Functional ROM | |||||
| Knee Flexion, Left (deg) | 25.55 | 26.20 | 6.1–80.1 | 4.8–43.9 | 0.45 (-0.22) |
| Knee Flexion, Right (deg) | 19.42 | 16.68 | 7.7–77.0 | 5.1–53.0 | 0.20 (-0.30) |
| Hip Flexion, Left (deg) | 42.92 | 41.50 | 30.6–54.8 | 26.0–50.4 | 0.95 (-0.03) |
| Hip Flexion, Right (deg) | 46.33 | 45.64 | 26.6–58.2 | 30.0–56.2 | 0.98 (-0.06) |
| Hip Abduction, Left (deg) | 105.09 | 99.99 | 74.9–123.9 | 75.4–137.1 | 0.62 (-0.04) |
| Hip Abduction, Right (deg) | 104.11 | 104.42 | 81.9–118.0 | 75.9–132.2 | 0.59 (0.18) |
| Trunk Flexion (deg) | 92.81 | 104.05 | 50.7–139.9 | 76.5–134.0 | 0.06 (0.48) |
| Trunk Rotation (deg) | 75.87 | 63.44 | 47.3–111.2 | 48.2–124.3 | 0.16 (-0.33) |
Note: ∗ indicates significance after Holm correction for multiple comparisons.
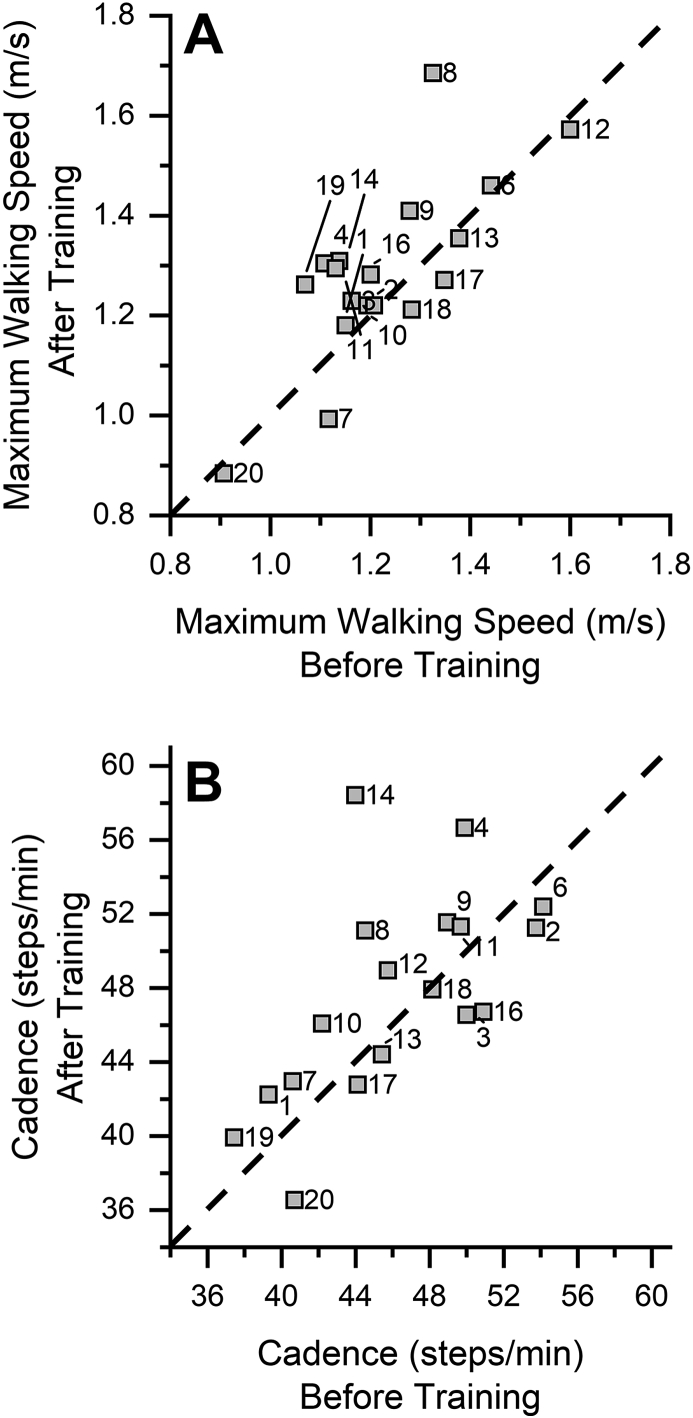
Measures of gait do not show improvements after training
The analysis of average walking speed, cadence, and turning time after yoga training did not reveal a systematic effect (see Fig. 5). Pre-post-test comparisons at the group level did not yield any significant differences for any of the gait variables as a function of yoga training (see Table 3). The effect sizes for the gait measures were small to moderate, ranging from d = 0.3–0.5. Based on these results, we performed a post-hoc power analysis and determined that a sample size of 45 or higher would be required to reach statistical significance for the observed power in any of the gait kinematic variables. Changes in the gait variables did not correlate significantly with Hoehn and Yahr staging of disease severity with r ranging from -0.3 to 0.2 (p > 0.05).
Fig. 5.
Effect of yoga training on measures of gait. A. Maximum walking speed achieved by each participant before and after training. The dashed line represents the line of equality. Note that all participants are centered around the line of equality indicating no changes in the walking speed after training. B. Cadence before and after training. The dashed line represents the line of equality. Note that participants do not show improvements after training. Unlike the postural stability measures, measures of dynamic posture did not show consistent changes in all the participants.
Functional range-of-motion in lower limbs and trunk does not change with training
To determine whether yoga training induced measurable changes in functional joint flexibility, the change in joint ROM of the trunk and lower limbs (trunk rotation/flexion, hip flexion/abduction, and knee flexion) were evaluated before and after yoga training. Largest median difference of 12° was observed in the trunk flexion across all participants. However, this difference failed to reach statistical significance across participants (for a summary of the F-ROM data, see Table 2, Table 3).
Discussion
The aim of this study was to determine the potential benefits of Hatha yoga training for improving balance and gait function in PWP. We systematically examined the effects of yoga training on parameters of both static balance and gait using clinical as well as objective, biomechanical markers of balance and locomotor performance. Oxidative stress and non-motor symptoms were evaluated in these participants as an effect of Hatha yoga intervention and is reported elsewhere.33 Prior evidence on the effects of Hatha yoga on balance performance in PWP has been inconclusive. Previous studies relied on balance rating scales that broadly assessed stationary balance (standing/sitting),22,23 or combined yoga training with resistance training,21 which made it difficult to isolate the effects of yoga. No reports contrasted balance markers during stance with markers of dynamic balance during gait to assess the comprehensive benefits of Hatha yoga for gait and standing posture in PWP. The main findings of this study are threefold: First, a 12-week Hatha yoga training program can induce significant, measurable improvements in the postural control during stance in PWP. Second, we found inconclusive evidence that yoga improves gait in PD. The observed improvements on gait performance were mild and not significant at the group level. Third, functional joint ROM did not significantly improve in our sample of PWPs. These results are discussed in detail below. Finally, no systematic differences between the groups were observed either at baseline or after 12 weeks.
Given that motor performance as measured by the UPDRS-III improved significantly in our sample indicates that PWP may benefit from regular participation in Hatha yoga. These motor scores reflect clinical features such as tremor, rigidity, bradykinesia, postural stability, and walking. The observed average improvement of 8 points (∼35% reduction) in the UPDRS motor scores constitutes a moderate clinically important difference.27 Our results corroborate findings of previous studies that reported similar rates of improvement after the same 12-week duration of yoga training.22,23
Hatha yoga improves balance during stance
A more focused look at standing balance revealed that COP postural sway length was significantly reduced across all participants after 12 weeks of Hatha yoga training. In contrast, COP sway area of the PWP did not reduce significantly – a finding also seen in earlier work.24 Based on the results, we can state that PWP sway less after practicing yoga. However, there is a caveat that the benefits of yoga did not extend uniformly across all participants. We did identify responders and non-responders. The magnitude of the reduction was substantial in those who responded to therapy (up to 45% in COP sway length). Our analysis revealed that neither participation rate, disease duration, nor the amount of anti-parkinsonian medication predicted a participant's response to yoga. However, participants with lower UPDRS motor scores seemed to respond better (see Fig. 3, Fig. 4A). In other words, those PWP who responded to yoga training with a significant decrease in COP sway path length, tended to have milder motor signs even before the onset of training. Observed improvements in COP postural sway length did not reflect in the participant perception of quality of life reported elsewhere.33
Our finding of reduced postural sway after yoga practice helps to explain previous findings showing that Berg Balance scores improve after a 12-week yoga training.26 This clinical rating scale assesses balance in a series of tasks such sitting to standing, standing unsupported, sitting unsupported, standing with eyes closed, standing with feet together, reaching forward with outstretched arm, and turning among other tasks.34 PWP with a more stable posture (i.e. less sway path length), will, in all likelihood, be more successful in these tasks. Moreover, improvements in static postural balance are known to reduce the frequency of falls in PD35 and, thereby, alleviate the serious health consequences associated with falls.36
Hatha yoga does not improve measures of gait or joint flexibility
We could neither find signs of significant improvements in gait performance, nor signs of increased functional leg and trunk flexibility after Hatha yoga training. The lack of improved joint flexibility stands in contrast to a recent report24 that evaluated a 12-week yoga intervention and found significant improvement in ankle and hip ROM. It is possible that differences in assessing joint ROM may account for these differences. While we based our joint angular measures on the optoelectronic motion capture data, the work by Colgrove and coworkers24 used hand-held goniometers that can result in a much larger measurement error (up to 10°).37 Moreover, this study intended to report changes in posture and gait after Hatha yoga training using objective biomechanical markers rather than clinical measures for its resolution, reliability and validity.
The analysis of the gait kinematic measures did not reveal any changes related to symptom relief. Indicators of bradykinesia such as step length, cadence, walking speed and turning time did not improve at the group level. At this point, one can only speculate about this failure to improve gait. One possibility is that the training dosage was insufficient. That is, to say yoga may be effective in improving gait in PWP, but training intensity, frequency and duration need to increase (i.e. 12 weeks of training are too short to evoke significant effects). While we cannot exclude this possibility, one can put forward an alternative explanation that relates to the specificity of training. That is, Hatha yoga is largely characterized by assuming and holding static postures. While the benefits of such exercise are clearly seen in tasks involving static balance, they are less relevant for improving markers of dynamic balance and performance during gait.
Conclusion
This study provides evidence that a 12-week long Hatha yoga program can improve static balance in PWP. COP sway path length, biomechanical marker of standing posture, improved by 34% on average. Improvements in gait kinematics were not observed. The frequency of falling in PWP correlates with the severity of postural instability.38
Authors’ contributions
All authors have participated in the research and/or article preparation. All authors have approved the final submitted article. Study concept and design: CC, JFW, PT, JK; Acquisition of data: NE, AM, CC; Analysis of data: NE, AM; Interpretation of data: NE, CC, AM, JFW, PT, JK; Drafting/revising the article: NE; Critique/review the article: CC, AM, JFW, PT, JK; Final approval of the article: NE, CC, AM, JFW, PT, JK.
Submission statement
The data reported in this manuscript have not been published elsewhere and the manuscript is not under consideration for publication in another journal.
Ethics statement
The study was approved by the Human Research Protection Program, University of Minnesota and registered under ClinicalTrials.gov (registration number: NCT02509611). Written informed consent was obtained from all participants before the baseline measurements.
Funding support
This work was supported by the University of Minnesota Foundation to PJT and University of Minnesota Grant-in-Aid Program to CC.
Conflict of interest
The authors have no conflicts of interest to disclose.
Acknowledgments
We sincerely thank all participants in this study who made this research possible. We also appreciate the PD support group coordinators in the Minneapolis/St. Paul metro areas for their help with recruitment, and Tarana Yoga for allowing use their yoga studio for the intervention classes.
References
- 1.Bloem B.R. Postural instability in Parkinson's disease. Clin Neurol Neurosurg. 1992;94:41–45. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen P.-H., Wang R.-L., Liou D.-J., Shaw J.-S. Gait disorders in Parkinson's disease: assessment and management. Int J Gerontol. 2013;7(4):189–193. doi: 10.1016/j.ijge.2013.03.005. [DOI] [Google Scholar]
- 3.Franzén E., Paquette C., Gurfinkel V.S., Cordo P.J., Nutt J.G., Horak F.B. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol. 2009;219(2):430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colnat-Coulbois S., Gauchard G.C., Maillard L., et al. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson's disease. Neuroscience. 2011;193:363–369. doi: 10.1016/j.neuroscience.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Bloem B.R., Steijns J.A.G., Smits-Engelsman B.C. An update on falls. Curr Opin Neurol. 2003;16(1):15. doi: 10.1097/00019052-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Bloem B.R., Beckley D.J., JGv Dijk, Zwinderman A.H., Remler M.P., Roos R.A.C. Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease. Mov Disord. 1996;11(5):509–521. doi: 10.1002/mds.870110506. [DOI] [PubMed] [Google Scholar]
- 7.Schmit J.M., Riley M.A., Dalvi A., et al. Deterministic center of pressure patterns characterize postural instability in Parkinson's disease. Exp Brain Res. 2006;168(3):357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter M.G., Allum J.H.J., Honegger F., Adkin A.L., Bloem B.R. Postural abnormalities to multidirectional stance perturbations in Parkinson's disease. J Neurol Neurosurg Psychiatr. 2004;75(9):1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cody F.W., MacDermott N., Matthews P.B., Richardson H.C. Observations on the genesis of the stretch reflex in Parkinson's disease. Brain. 1986;109(2):229–249. doi: 10.1093/brain/109.2.229. Pt 2. [DOI] [PubMed] [Google Scholar]
- 10.Horak F.B., Nutt J.G., Nashner L.M. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111(1):46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 11.Mancini M., Zampieri C., Carlson-Kuhta P., Chiari L., Horak F.B. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur J Neurol. 2009;16(9):1028–1034. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel N., Jankovic J., Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13(1):100–112. doi: 10.1016/S1474-4422(13)70213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konczak J., Corcos D.M., Horak F., et al. Proprioception and motor control in Parkinson's disease. J Mot Behav. 2009;41(6):543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 14.Inkster L.M., Eng J.J., MacIntyre D.L., Stoessl A.J. Leg muscle strength is reduced in Parkinson's disease and relates to the ability to rise from a chair. Mov Disord. 2003;18(2):157–162. doi: 10.1002/mds.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nallegowda M., Singh U., Handa G., et al. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's Disease: a pilot study. Am J Phys Med Rehabil. 2004;83(12):898. doi: 10.1097/01.phm.0000146505.18244.43. [DOI] [PubMed] [Google Scholar]
- 16.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. J Am Med Assoc. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 17.Johnsen E.L., Mogensen P.H., Sunde N.A., Østergaard K. Improved asymmetry of gait in Parkinson's disease with DBS: gait and postural instability in Parkinson's disease treated with bilateral deep brain stimulation in the subthalamic nucleus. Mov Disord. 2009;24(4):588–595. doi: 10.1002/mds.22419. [DOI] [PubMed] [Google Scholar]
- 18.George R.S., Nutt J., Burchiel K., Horak F. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010;75(14):1292–1299. doi: 10.1212/WNL.0b013e3181f61329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlenstedt C., Shalash A., Muthuraman M., Falk D., Witt K., Deuschl G. Effect of high-frequency subthalamic neurostimulation on gait and freezing of gait in Parkinson's disease: a systematic review and meta-analysis. Eur J Neurol. 2017;24(1):18–26. doi: 10.1111/ene.13167. [DOI] [PubMed] [Google Scholar]
- 20.Šumec R., Filip P., Sheardová K., Bareš M. Psychological benefits of nonpharmacological methods aimed for improving balance in Parkinson's disease: a systematic review. Behav Neurol. 2015;2015:620674. doi: 10.1155/2015/620674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriello G., Denio C., Abraham M., DeFrancesco D., Townsley J. Incorporating yoga into an intense physical therapy program in someone with Parkinson's disease: a case report. J Bodyw Mov Ther. 2013;17(4):408–417. doi: 10.1016/j.jbmt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Ni M., Mooney K., Signorile J.F. Controlled pilot study of the effects of power yoga in Parkinson's disease. Compl Ther Med. 2016;25:126–131. doi: 10.1016/j.ctim.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Sharma N.K., Robbins K., Wagner K., Colgrove Y.M. A randomized controlled pilot study of the therapeutic effects of yoga in people with Parkinson's disease. Int J Yoga. 2015;8(1):74–79. doi: 10.4103/0973-6131.146070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colgrove Y.S., Sharma N., Kluding P., et al. Effect of yoga on motor function in people with Parkinson's Disease: a randomized, controlled pilot study. J Yoga Phys Ther. 2012 doi: 10.4172/2157-7595.1000112. 02(02) [DOI] [Google Scholar]
- 25.Bega D., Stein J., Zadikoff C., Simuni T., Corcos D. Yoga versus resistance training in mild to moderate severity Parkinson's disease: a 12-week pilot study. J Yoga Phys Ther. 2016;6:222. doi: 10.4172/2157-7595.1000222. [DOI] [Google Scholar]
- 26.Nick N., Petramfar P., Ghodsbin F., Keshavarzi S., Jahanbin I. The effect of Yoga on balance and fear of falling in older adults. PM&R. 2016;8(2):145–151. doi: 10.1016/j.pmrj.2015.06.442. [DOI] [PubMed] [Google Scholar]
- 27.Shulman L.M., Gruber-Baldini A.L., Anderson K.E., Fishman P.S., Reich S.G., Weiner W.J. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol. 2010;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 28.Donoghue D., Stokes E.K. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J Rehabil Med. 2009;41(5):343–346. doi: 10.2340/16501977-0337. [DOI] [PubMed] [Google Scholar]
- 29.Hertzog M.A. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 30.Justice C., Cheung C., Samson-Burke A. Development and evaluation of a yoga intervention program for Parkinson's disease. Int. J. Yoga Ther. 2018;28(1):113–122. doi: 10.17761/2018-00015R2. [DOI] [PubMed] [Google Scholar]
- 31.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65–70. www.jstor.org/stable/4615733 [Google Scholar]
- 32.Pahwa R., Wilkinson S., Smith D., Lyons K., Miyawaki E., Koller W. High-frequency stimulation of the globus pallidus for the treatment of Parkinson's disease. Neurology. 1997;49(1):249–253. doi: 10.1212/wnl.49.1.249. [DOI] [PubMed] [Google Scholar]
- 33.Cheung C., Bhimani R., Wyman J.F., et al. Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson's disease: a pilot randomized controlled trial. Pilot Feasibility Stud. 2018;4(1):162. doi: 10.1186/s40814-018-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg K., Wood-Dauphine S., Williams J., Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41(6):304–311. doi: 10.3138/ptc.41.6.304. [DOI] [Google Scholar]
- 35.Allen N.E., Sherrington C., Paul S.S., Canning C.G. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26(9):1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 36.Burns E.R., Stevens J.A., Lee R. The direct costs of fatal and non-fatal falls among older adults - United States. J Saf Res. 2016:99–103. doi: 10.1016/j.jsr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rome K., Cowieson F. A reliability study of the universal goniometer, fluid goniometer, and electrogoniometer for the measurement of ankle dorsiflexion. Foot Ankle Int. 1996;17(1):28–32. doi: 10.1177/107110079601700106. [DOI] [PubMed] [Google Scholar]
- 38.Koller W.C., Glatt S., Vetere-Overfield B., Hassanein R. Falls and Parkinson's disease. Clin Neuropharmacol. 1989;12(2):98–105. doi: 10.1097/00002826-198904000-00003. [DOI] [PubMed] [Google Scholar]