Abstract
A PCR identification method in which four primers that recognize homologous conserved regions in the Sinorhizobium meliloti genome are used was developed and tested. The regions used for identification were the nodbox 4 locus, which is located in one of the symbiotic megaplasmids, and the mucR gene, which is located in the chromosome. The new method was used to establish a collection of S. meliloti strains from polluted soils.
Sinorhizobium meliloti forms a nitrogen-fixing symbiosis with plants of the genera Medicago, Melilotus, and Trigonella, including the crop alfalfa. During this symbiosis, the bacterium promotes the formation of a new plant organ, the root nodule, invades this organ, and differentiates into a nitrogen-fixing form, the bacteroid (15). Although isolation of S. meliloti from soil or nodule macerates is relatively easy, final identification of an isolate requires reisolation of the strain from nodules induced under axenic conditions, a time-consuming process which can take up to 3 weeks. Direct identification is hampered by the lack of a selective medium for this bacterium and by the presence of saprophytic bacteria in nodules, especially deteriorated nodules, which are typically found in plants collected in the field. An additional problem is the pleomorphism of the species; mucoid and dry strains are isolated frequently (12, 16).
PCR has proven to be an easy and reliable technique for identification of bacteria. However, in most studies of members of the Rhizobiaceae the workers have concentrated on identification of individual strains previously assigned to a species. In this type of work enterobacterial repetitive intergenic consensus (ERIC)-PCR and repetitive extragenic palindromic PCR have been used very successfully (8, 14). In a recent report Niemann et al. showed that the sequence of an ERIC amplicon is highly conserved in S. meliloti laboratory strains and proposed that this amplicon could be used for identification of the species by PCR (13). However, this amplicon encodes a 2-hydroxyacid dehydrogenase which is a widely distributed enzyme and has been tested with only a few laboratory strains and field isolates. Other primers used for identification of S. meliloti have targeted the nodH gene (4) and the insertion sequence element ISRm5 (5). In order to develop a method for rapid identification of S. meliloti field isolates, we investigated identification by PCR in which the following two regions of the S. meliloti genome were used: the nodbox 4 promoter (1) and the mucR gene (10). Both of these regions are specific for S. meliloti and are highly conserved and ubiquitous in the species (1, 12). Furthermore, they are located in different replicons; the nodbox 4 region is located in one of the symbiotic megaplasmids (1), and mucR is located in the chromosome (10). Simultaneous detection of both regions should increase the specificity of the method compared to methods based on use of a single primer pair.
The nodbox 4 region was amplified by using primers nodbox1 and nodbox2 (Table 1), which were derived from the previously published sequence of S. meliloti AK631 (1). PCR yielded a 138-bp band from the genomic DNA of three unrelated S. meliloti strains, strains AK631 (1), Rm2011 (6), and EFB1(11). The PCR products were cloned in the pCR2.1 vector and sequenced, and they exhibited 100% identity in the three strains. The sequence obtained differed at two places from the previously published sequence for the AK631 nodbox 4 region. Conservation of these mismatches in the three strains suggested that there was a sequencing mistake in the previously published AK631 sequence. A search performed with the BLAST program did not reveal homology with any other sequences in databases, indicating that the nodbox 4 region is specific for S. meliloti and not conserved in other rhizobia. The PCR product amplified with primers nodbox1 and nodbox2 might lead to confusing results due to its small size. For this reason a third primer, primer nodbox3 (Table 1), was designed for the adjacent nodM coding region. Amplification with nodbox1 and nodbox3 yielded a 646-bp fragment with the DNA of the three strains tested.
TABLE 1.
Primers used for PCR amplification
| Primer | Sequence (5′-3′) | Melting temp (°C) |
|---|---|---|
| nodbox1 | TCTTTTCTTATCCATAGGGTGG | 57.28 |
| nodbox2 | GAAATAATCTAGGCGCACGAGT | 58.4 |
| nodbox3 | ACGGATCGTCCTCGAAG | 57.10 |
| mucRf | ATGACAGAGACTTCGCTCGGT | 59.8 |
| mucRr | TCACTTGCCGCGACGCTT | 58.2 |
mucR codes for a regulatory zinc finger protein implicated in regulation of exopolysaccharides in S. meliloti (10). We have previously shown that the mucR gene is present in all strains irrespective of colony mucoidy (12). The mucR genes from Rm2011 and EFB1 have been sequenced and exhibit 96% identity (12). Homologues of mucR have been found in other members of the Rhizobiaceae, although with much lower levels of homology (2, 3, 7, 9). These characteristics make mucR a good candidate for PCR identification. When genomic DNA from laboratory S. meliloti strains were amplified by using primers mucRf and mucRr (Table 1), a 431-bp fragment was obtained.
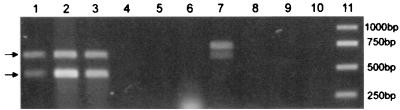
Figure 1 shows the products amplified from genomic DNA of laboratory strains of S. meliloti and other related or soil bacteria with primers nodbox1, nodbox3, mucRf, and mucRr used simultaneously. PCR was performed with 1 U of Tth polymerase (Biotools, Madrid, Spain) in a 25-μl (final volume) reaction mixture. The conditions used were as follows: 95°C for 30 s, 53°C for 45 s, and 72°C for 30 s for 25 cycles, followed by a 7-min elongation step at 72°C. Each primer was added at a concentration of 0.4 μM. Two discrete bands at 646 and 431 bp were amplified from all of the S. meliloti strains but not from other bacteria, showing that amplification of mucR and nodbox 4 can be used for rapid identification of S. meliloti.
FIG. 1.
PCR amplification of genomic DNA from different soil bacteria when primers nodbox1, nodbox3, mucRf, and mucRr were used simultaneously. The arrows indicate the positions of the 431-bp mucR amplicon and the 646-bp nodbox4 amplicon. Lane 1, S. meliloti EFB1; lane 2, S. meliloti 2011; lane 3, S. meliloti GR4; lane 4, Rhizobium etli CE3; lane 5, Rhizobium leguminosarum 3841; lane 6, R. leguminosarum STR6; lane 7, Sinorhizobium fredii HH103; lane 8, Rhizobium sp. strain NGR234; lane 9, Pseudomonas fluorescens F113; lane 10, negative control without DNA; lane 11, molecular weight markers.
We used this approach to identify S. meliloti strains from nodules of Medicago sp. plants collected in fields around Madrid (Spain). Nodules were surface sterilized with 70% ethanol and crushed in a saline solution (0.9% NaCl), and dilutions were plated onto TY medium supplemented with 10 μg cycloheximide per ml to prevent fungal growth. A total of 240 colonies were isolated irrespective of colony morphology or pigmentation and were tested with the PCR. For each PCR a small amount of bacteria was collected with a toothpick from a fresh culture on a TY plate and resuspended in 6 μl of MilliQ water. The cells were broken by incubation at 94°C for 20 min, and a PCR was performed as described above. Fourteen colonies resulted in amplification of two bands with the appropriate sizes for nodbox 4 and mucR amplicons. None of the colonies produced a single band. ERIC-PCR experiments showed that the 14 positive isolates represented 13 independent strains (data not shown). Alfalfa nodulation assays were performed with the 13 strains by using Leonard jar systems containing perlite as the solid substrate and FP as the mineral medium (11). All of the PCR-positive strains induced root nodules within 3 weeks.
We also used this method to establish a collection of S. meliloti strains from hydrocarbon-polluted environments. Three sites with a history of contamination with polychlorinated biphenyls and/or polycyclic aromatic hydrocarbons in the Madrid region were sampled. The following strategies were used to isolate putative S. meliloti strains: (i) nodule macerates from Medicago sp. plants were directly plated onto TY medium, and colonies were isolated; (ii) nodule macerates were used to inoculate trap Medicago sativa plants in Leonard jars, and the nodules obtained were crushed and plated onto TY for colony isolation; and (iii) soil and soil infusions were inoculated onto trap M. sativa plants as described above. Sixty-eight colonies with morphology consistent with S. meliloti colony morphology were tested with PCR, and 46 positive strains were identified. Two isolates yielded a single band at ca. 700 bp that was considered nonspecific, and these isolates were considered negative. All of the isolates were inoculated onto M. sativa plants in Leonard jars, and only the PCR-positive strains induced nodule formation within 3 weeks. The relatively high number of negative isolates probably reflected the presence in the nodules of saprophytic nonrhizobial bacteria. It should be noted that in contrast to nodules induced with axenic cultures of S. meliloti, the nodules obtained often yielded more than one type of colony when they were crushed and plated onto TY medium.
The results presented here show that PCR amplification of the nodbox 4 and mucR loci is a reliable and rapid method for identification of S. meliloti strains. The method that we developed is useful for identification of S. meliloti, especially when high numbers of other bacteria are expected to be present in nodules. Therefore, this method can be used to generate a strain collection from field samples with reduced laboratory effort in a short period of time. Furthermore, the two amplicons used in this work add new probes to those already available for identification of S. meliloti. Optimization of this PCR procedure allowed simultaneous detection of the two loci with a single PCR. Redundant strains can eventually be detected by established ERIC or repetitive extragenic palindromic PCR methods.
Acknowledgments
We are grateful to F. Fernández-Piñas, L. Bolaños, M. Redondo, and R. I. Oruezabal for their help with sampling and plating.
This work was supported by grant 07M-0170-1997 from Comunidad Autónoma de Madrid and by grant BIO4-CT97-2227 from the E.U.
REFERENCES
- 1.Baev N, Endre G, Petrovics G, Banfalvi Z, Kondorosi A. Six nodulation genes of nod box locus 4 in Rhizobium meliloti are involved in nodulation signal production: nodM codes for d-glucosamine synthetase. Mol Gen Genet. 1991;228:113–124. doi: 10.1007/BF00282455. [DOI] [PubMed] [Google Scholar]
- 2.Bittinger M A, Milner J L, Saville B J, Handelsman J. rosR, a determinant of nodulation competitiveness in Rhizobium etli. Mol Plant-Microbe Interact. 1997;10:180–186. doi: 10.1094/MPMI.1997.10.2.180. [DOI] [PubMed] [Google Scholar]
- 3.Brightwell G, Hussain H, Tiburtius A, Yeoman K H, Johnston A W. Pleiotropic effects of regulatory ros mutants of Agrobacterium radiobacter and their interaction with Fe and glucose. Mol Plant-Microbe Interact. 1995;8:747–754. doi: 10.1094/mpmi-8-0747. [DOI] [PubMed] [Google Scholar]
- 4.Bromfield E S P, Wheatcroft R, Barran L R. Medium for direct isolation of Rhizobium meliloti from soil. Soil Biol Biochem. 1994;26:423–428. [Google Scholar]
- 5.Bromfield E S P, Barran L R, Wheatcroft R. Relative genetic structure of a population of Rhizobium meliloti isolated directly from soil and from nodules of alfalfa (Medicago sativa) and sweet clover. Mol Ecol. 1995;4:183–188. [Google Scholar]
- 6.Casse F, Boucher C, Julliot J S, Michel M, Dénarie J. Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J Gen Microbiol. 1979;113:229–242. [Google Scholar]
- 7.Cooley M B, D'Souza M R, Kado C I. The virC and virD operons of the Agrobacterium Ti plasmid are regulated by the ros chromosomal gene: analysis of the cloned ros gene. J Bacteriol. 1991;173:2608–2616. doi: 10.1128/jb.173.8.2608-2616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bruijn F J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992;58:2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature. 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 10.Keller M, Roxlau A, Weng W M, Schmidt M, Quandt J, Niehaus K, Jording D, Arnold W, Pühler A. Molecular analysis of the Rhizobium meliloti mucR gene regulating the biosynthesis of the exopolysaccharides succynoglycan and galactoglucan. Mol Plant-Microbe Interact. 1995;8:267–277. doi: 10.1094/mpmi-8-0267. [DOI] [PubMed] [Google Scholar]
- 11.Lloret J, Bolaños L, Lucas M M, Peart J, Brewin N J, Bonilla I, Rivilla R. Ionic and osmotic pressure induce different alterations in the lipopolysaccharide of a Rhizobium meliloti strain. Appl Environ Microbiol. 1995;61:3701–3704. doi: 10.1128/aem.61.10.3701-3704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martín M, Lloret J, Sánchez-Contreras M, Bonilla I, Rivilla R. MucR is necessary for galactoglucan production in Sinorhizobium meliloti EFB1. Mol Plant-Microbe Interact. 2000;13:129–135. doi: 10.1094/MPMI.2000.13.1.129. [DOI] [PubMed] [Google Scholar]
- 13.Niemann S, Dammann-Kalinowski T, Nagel A, Pühler A, Selbitschka W. Genetic basis of enterobacterial repetitive intergenic consensus (ERIC)-PCR fingerprint pattern in Sinorhizobium meliloti and identification of S. meliloti employing PCR primers derived from an ERIC-PCR fragment. Arch Microbiol. 1999;172:22–30. doi: 10.1007/s002030050735. [DOI] [PubMed] [Google Scholar]
- 14.Niemann S, Pühler A, Tichy H V, Simon R, Selbitschka W. Evaluation of the resolving power of three different DNA fingerprinting methods to discriminate among isolates of a natural Rhizobium meliloti population. J Appl Microbiol. 1997;82:477–484. doi: 10.1046/j.1365-2672.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 15.Schulte M, Kondorosi A. Regulation of symbiotic root nodule development. Annu Rev Genet. 1998;32:33–57. doi: 10.1146/annurev.genet.32.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Zevenhuizen L P, Faleschini P. Effect of the concentration of sodium chloride in the medium on the relative proportions of poly- and oligo-saccharides excreted by Rhizobium meliloti strain YE-2SL. Carbohydr Res. 1991;209:203–209. doi: 10.1016/0008-6215(91)80157-i. [DOI] [PubMed] [Google Scholar]