Abstract
Monocyte adhesion assay, a fluorescence-based method, enables the detection and quantification of monocyte adhesion to endothelial cell (EC) monolayers in vitro and measures EC activation. We describe in this chapter a monocyte adhesion assay based on two published papers from our laboratory that can be effectively used in studying the mechanisms of both pro- and anti-inflammatory cytokines in EC activation. Endothelial cell monolayers are cultured and treated with desired drug, cytokines, or other stimuli and incubated with fluorescently labeled monocytes.
Keywords: Atherosclerosis, Endothelial cell activation, Inflammation, Monocytes, Monocyte adhesion assay, Adhesion molecules
1. Introduction
Cardiovascular diseases are the leading cause of morbidity and mortality worldwide. Atherosclerosis is one of the underlying causes of the development of cardiovascular diseases [1]. The activation and dysfunction of endothelial cells (ECs) are the earliest events and a central pathological process associated in the onset of atherosclerosis [2]. The innate immune system plays a critical role in disease development and accumulation of monocytes and macrophages is observed at the atherosclerotic lesion. Among the numerous stimuli, circulating proinflammatory cytokines bind to their receptors on the ECs, leading to the upregulation of leukocyte adhesion factors facilitating monocyte adhesion and extravasation to the subendothelial space. These monocytes differentiate into macrophages and take up oxidized low-density lipoproteins to form foam cells and fatty streaks, leading to more advanced atherosclerotic lesions [3, 4]. EC activation results in innate immune phenotypic changes of the endothelium in response to inflammatory mediators (Table 1) [2, 5–9]. EC activation is characterized by various vascular pathophysiological and immunological features, including upregulation of expression of EC adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [10], increased cytokine/chemokines secretion, activation of danger associated molecular patterns (DAMPs), upregulation of major histocompatibility complex-II and the activation of T-cell cosimulation receptors and coinhibition/immune checkpoint receptors (Table 2) [11]. Several experimental methods such as monocyte adhesion assay, Boyden chamber assay, Western blotting, real-time polymerase chain reaction (RT-PCR), flow cytometry, and RNA-sequencing have been used to examine EC activation (Table 3). We have focused here on endothelium adhesion assay, one of the most common functional EC activation assays used.
Table 1.
Different types of endothelial cell (EC) stimuli, their effects on ECs and disease models
| Type | Stimulus | Effects | Related diseases |
|---|---|---|---|
|
| |||
| Mechanical stimuli | Shear stress | The mitochondrial electron transport chain transduces signaling, leading to the release of pronociceptive mediators and causes EC activation. Lower shear rates can promote leukocyte recruitment. | Atherosclerosis |
| Chemical stimuli | Histamine | Induces the rapid mobilization of P-selectin expressed on the endothelium and enhances leukocyte rolling. | Allergy, asthma |
| Leukotrienes | Increases the expression of CD11/CD18 and transforms leukocytes from reversible adhesion into firm adhesion. | Chronic inflammation | |
| Platelet activating factor (PAF) | |||
| Cytokines and chemokines | Increases the expression of adhesion molecules [intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1)]. | Inflammation | |
| Oxidative stress | Produces reactive oxygen species (ROS) at an accelerated rate and increases leukocyte rolling, adhesion and migration. | Diabetes mellitus, metabolic syndrome | |
| Lysophosphatidylcholine (LPC) | |||
| Pathological stimuli | Lipopolysaccharide (LPS) | Causes thrombosis-enhancing effects. LPS can induce neutrophils to make neutrophil extracellular traps (NETs). | Infections |
| Antiadhesive Antiadhesive mediators | Nitric oxide (NO) | NO is an endogenous inhibitor of leukocyte adhesion. | Vasodilatation |
| Adenosine | Adenosine A2 receptor inhibits neutrophilic superoxide production. | ||
| Prostacyclin (PGI2) | Inhibits platelet aggregation. | ||
Table 2.
Six major features of endothelial cell activation
| Hallmark feature | Basic principle | Experiments | PMID |
|---|---|---|---|
|
| |||
| Upregulation of EC adhesion molecules | The adhesion of leukocytes to the vascular endothelium is a sign of EC activation. ICAM-1, VCAM-1, and PECAM-1 are immunoglobulin superfamily-like adhesion molecules expressed on the endothelium. These adhesion molecules cause leukocyte interaction with their respective receptors to mediate firm adhesion and extravasation. | Leukocyte endothelium adhesion assay | 26085094 |
| 26733204 | |||
| Photomicrograph | 19858416 | ||
| 27127201 | |||
| Flow cytometry | 26733204 | ||
| 25854700 | |||
| Secretion of cytokines and chemokines | Cytokines and chemokines are secreted proteins that usually accompany growth, activation and differentiation functions. They regulate immune responses, including immune cell trafficking. Thus, their secretion plays an important role in studying the mechanisms of EC activation. | Cytokine array | 25705917 |
| 27992360 | |||
| Cytokine and chemokine blocking assay | 26733204 | ||
| RT-PCR | 26733204 | ||
| Activation of DAMPs receptors | According to our previous data, ECs are identified as novel immune cells that perform specific innate immune functions, including in both DAMP and PAMP sensing. Increased pathogen concentrations can trigger an inflammatory response and lead to EC activation. Studying the concentration of inflammatory mediators, such as caspase-1, can indirectly show the activation of ECs. | Fluorochrome inhibitor of caspase (FLICA) kits | 26037927 |
| 25705917 | |||
| Western blots | 25705917 | ||
| Upregulation of MHC-II and cosignaling receptors | ECs can transdifferentiate into innate immune cells during inflammation and express MHC-II, DAMP receptors and T-cell costimulation/inhibition receptors on the cell surface. | RNA-sequencing | 29769317 |
| 31731100 | |||
| Database mining | 30468648 | ||
| Atherosclerosis | Atherosclerosis is one of the outcomes of EC activation. It can be used to analyze the severity of disease progression via quantification of atherosclerotic area/plaque percentage in the aortic sinus. | Histology and staining | 12506016 |
| 19858416 | |||
| Immune-tolerogenic function | EC activation can induce suppressive immune regulatory T cells (T-regs). Consequently, T-regs upregulate the expression of immune checkpoint receptors and produce anti-inflammatory cytokines to prevent further downstream inflammatory processes. | Microarray database mining | 30468648 |
| 31731100 | |||
DAMPs danger associated molecular patterns, EC endothelial cells, ICAM-1 intercellular adhesion molecule-1, MHC-II major histocompatibility class-II, PAMP pathogen-associated molecular pattern, PECAM-1 platelet endothelial cell adhesion molecule-1, RT-PCR real time polymerase chain reaction, T-regs regulatory T cells, VCAM-1 vascular cell adhesion molecule-1
Table 3.
Methods for assessing endothelial cell activation in vitro
| Technique | Description | PMID |
|---|---|---|
|
| ||
| Monocyte adhesion assay | Used to measure adhesiveness of blood monocytes into endothelial cell layer. | 26733204, 26085094, 29371247, 27127201 |
| Trans-well migration assay | Commonly used to study the migratory response of endothelial cells toward a chemoattractant. | 29511499 |
| Real-time polymerase chain reaction (RT-PCR) | Used to examine mRNA expression levels of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin. | 29371247 |
| Western blotting | Used to examine protein levels of adhesion molecules such as ICAM-1, VCAM-1, and E-selectin. | 29371247, 25705917 |
| Flow cytometry analysis | Used to examine expression of adhesion molecules such as ICAM-1 and VCAM-1 as well as monocyte migration. | 25705917, 26085094, 26733204 |
| Cytokine array | Used to determine cytokine and chemokine expression in activated endothelial cells. | 26085094, 25705917 |
| RNA sequencing | Provides information on the expression levels of adhesion molecules and other endothelial cell activation genes. | 29769317, 29371247 |
| Microarray analysis | Used to examine the expression levels of adhesion molecules and cytokines/chemokines. | 26733204, 27127201, 29371247 |
| Endothelial cell biology PCR array | Used to examine expression of 84 endothelial cell-related genes. | 27127201 |
| Enzyme-linked immunosorbent assay (ELISA) | Used to determine protein levels of different cytokine/chemokines released from activated endothelial cells. | 26085094 |
| FAM-FLICA caspase-1 assay | Utilizes the fluorescent inhibitor probe FAM-YVAD-FMK to label active caspase-1 enzyme in living cells (the inflammatory sites of the endothelium). | 25739025 |
ICAM-1 intercellular adhesion molecule-1, VCAM vascular cell adhesion molecule-1
At the functional level, EC activation is assessed by quantifying the total number of monocytes that adhere to an EC layer. This experiment is performed by either a direct count or by indirect measurement of fluorescently labeled adherent monocytes. Monocyte adhesion assay is a powerful in vitro technique used to quantify the function of adhesion molecules in leukocyte–endothelial interactions. Initially, EC adhesion was quantified by manually counting leukocytes that adhered to the EC monolayer. Over time, quantification of radiolabeled leukocytes gradually superseded the manual counting. Recently, a fluorescence-based adhesion assay has emerged and replaced the radioactive isotope technique. The fluorescence-based technique allows for a more convenient, sensitive and flexible method for studying EC adhesion with fewer disposal requirements (Fig. 1a) [9, 12, 13]. The experimental principles of the adhesion assay include: (i) activation of EC monolayers with the desired drugs or cytokines; (ii) labeling of the nonstimulated monocytes with a cell-permeable fluorescent dye; (iii) incubation of the activated EC monolayer with the fluorescently labeled monocytes; (iv) washing off unlabeled cells; and (v) quantification of the fluorescently labeled cells associated with the EC by a fluorescence plate reader (Fig. 1b) [9, 14].
Fig. 1.
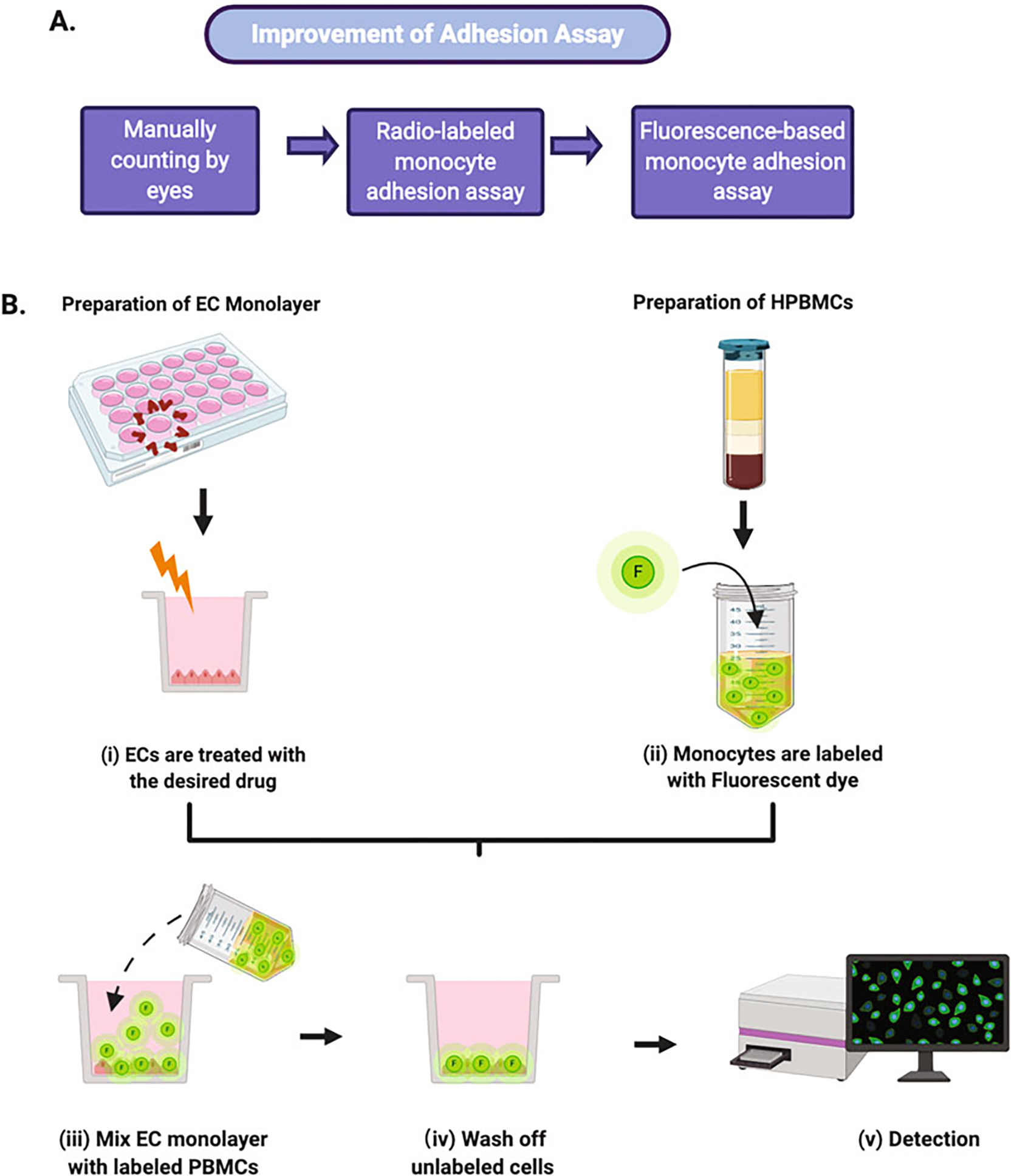
Leukocyte adhesion assay experimental workflow. (a) A brief history of the development of adhesion assay. Currently, the fluorescence-based adhesion assay is the dominant tool used to study EC adhesion. (b) A schematic diagram of the fluorescence-based adhesion assay. The process includes EC monolayer and human peripheral blood mononuclear cells (HPBMCs) preparation and treatment, mixture of treated EC monolayer and labeled HPBMCs and detection and quantitation of fluorescence intensity
In this chapter, we describe a method that allows the enumeration of adhesive cells to an endothelial monolayer. Also, we demonstrate the flexibility in using such adhesion assay for assessing both activation and inhibition of EC activation.
2. Materials
2.1. Endothelial Cell Culture
Human aortic endothelial cells (HAECs).
M1999 media.
20% fetal bovine serum (FBS): thaw FBS at room temperature (RT) or in a 37 °C water bath. Incubate in 56 °C water bath for 30 min post thaw. Cool on ice and store at −20 °C.
25 mg/mL Heparin: weigh 300 mg heparin in 15 mL tube. Add 12 mL of serum-free M199 media, mix well and filter through a 0.22 μm Nalgen filter. Store at 4 °C.
10 mg Endothelial cell growth supplement (ECGS): add 10 mL of serum free M199 media into ECGS’s vial containing 100 mg ECGS, mix well and filter through a 0.22 μm Nalgen filter. Store at −20 °C.
Penicillin/Streptomycin/Amphotericin-B (PSA): make 10 mL aliquots per tube and store at −20 °C.
Endothelial cell growth media: In 500 mL of M199 media, add 100 mL of FBS (20%), 1 mL of 25 mg/mL heparin (50 μg/mL), 3 mL of 10 mg/mL ECG (30 mg), and 5 mL of PSA (1%). Mix well and store at 4 °C.
0.2% Gelatin: weight 1 g and dissolve in a 500 mL of ultrapure water (dH2O). Autoclave and store at 4 °C.
0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) solution: 0.25% (w/v) trypsin, 0.2% (w/v) EDTA.4Na in 1 L of 1×HBSS. Store at −20 °C.
Hanks’s Balanced Salt Solution (HBSS).
0.2% gelatin-coated 75 cm2 Corning cell culture flasks.
Sterile 50 mL centrifuge tubes.
Cell culture incubator (37 °C, 5% CO2 atmosphere).
Centrifuge machine with swing-out bucket rotor.
Hemocytometer.
Falcon 24-well multiwell flat bottom TC-treated cell culture plate.
Adjustable volume micropipettes.
Micropipette tips.
Motorized Pipette Fillers.
2.2. Human Peripheral Blood Mononuclear Cell (PBMC) Isolation
Freshly collected heparinized human peripheral blood.
Ficoll Histopaque 1083 solution.
Sterile 1× phosphate-buffer saline (PBS).
Roswell Park Memorial Institute (I) 1640 media supplemented with 10% fetal calf serum (FCS).
1 mL pipette.
2.3. Monocyte-Endothelial Cell Adhesion Assay
Calcein AM fluorescence dye.
Serum-free RPMI 1640 media.
Fluorescence microplate reader.
3. Methods
All solutions and equipment coming into contact with cells must be sterile. Proper sterile technique must be used accordingly.
3.1. Preparation of Endothelial Cell Monolayer
Culture HAECs (< passage 8) in 0.2% gelatin-coated 75 cm2 Corning cell culture flasks in 20 mL of endothelial cell growth media and incubate the cells in a cell culture incubator at 37 °C with 5% CO2 and 95% humidity.
Change media every other day until cells reach 90–100% confluency.
Remove culture media and wash cell twice with 10 mL of warm HBSS.
Completely aspirate HBSS and add 2 mL of 0.2% trypsin–EDTA solution. Ensure the trypsin solution fully covers the flask surface and return the cells to the cell culture incubator for 1 min.
Once the cells have fully detached, add 8 mL of M199 media to the flask to stop the trypsinization. Gently tilt the flask and carefully pipette up and down to ensure all HAECs are detached from the flask surface.
Transfer cells to a 50 mL tube and centrifuge at 400 × g for 5 min at 4 °C to pellet the cells.
Carefully aspirate off the supernatant. Flick the tip of the conical tube with your finger to loosen the cell pellet. Resuspend the cells in 5 mL of endothelial cell growth media, gently pipetting the cells to break up any clumps.
Count the cells with a hemocytometer and adjust the cell concentration to 50,000 cells/mL.
Seed 1 mL of the cell suspension in each well of a Falcon 24-well cell culture plate coated with 0.2% gelatin and incubate in a cell culture incubator at 37 °C, 5% CO2 and 95% humidity.
Culture the cells for 48–72 h until the endothelial cells form a monolayer.
Treat the endothelial cell monolayer with the desired activator or inhibitor under investigation for 6–72 h.
3.2. Preparation of Human Peripheral Blood Mononuclear Cells (PBMC)
Add 20 mL of Histopaque 1083 solution into 50 mL centrifuge tubes (see Note 1) [15].
Add 25 mL of heparinized human peripheral blood onto Ficoll-Plaque in each tube. Layer the blood gently by allowing it to run down the side of the tube (see Note 2).
Centrifuge at 400 × g for 30 min at RT (see Note 3). After centrifugation, the human peripheral blood will be divided into five distinct layers (Fig. 2) [9, 13]. The red blood cells (RBCs) settle to the bottom of the tube and granulocytes (primarily neutrophils) serve as a “buffy coat” on top of the RBCs. Directly above the surface of the granulocytes is an intermediate phase of Ficoll, followed by the PBMC layer, and finally the plasma on the uppermost layer.
Without disturbing the PBMC layer, carefully aspirate off the plasma with plastic micropipette tips (leaving 2.5–5 mL of plasma above the PBMC layer).
Using a 1 mL pipette, carefully collect 10 mL of the PBMC layer (see Note 4).
Add 30 mL of PBS to these mononuclear cells and gently invert the tube several times to mix. Centrifuge the resuspended mononuclear cells at 250 × g for 10 min at room RT and carefully remove all the supernatant.
For platelet removal, resuspend the cell pellet in 0.5 mL of PBS. Then add another 15 mL of PBS and gently invert the tube several times to mix. Centrifuge at 350 × g for 5 min at RT and carefully remove the supernatant.
Resuspend the cell pellet in 1.5 mL of RPMI 1640 media supplemented with 10% FCS. Count the cells using a hemocytometer and adjust the cell concentration to 5 × 106 PBMCs/mL.
Fig. 2.
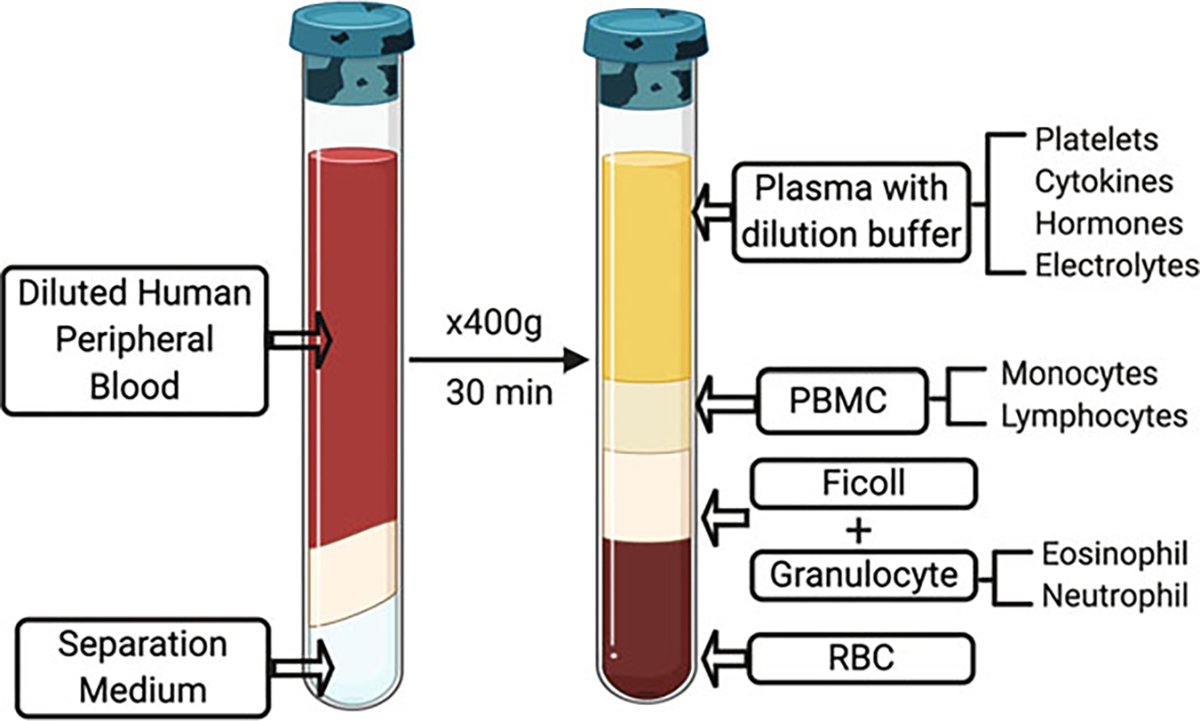
Density gradient centrifugation of human peripheral blood mononuclear cells (PBMCs). After centrifugation, PBMCs can be divided into five layers. RBC red blood cells
3.3. Monocyte-Endothelial Cell Adhesion Assay
Treat cultured HAECs in 24 well plates with desired drugs as Subheading 3.1, step 11 (see Note 5). Treatment times will vary depending on the various drugs.
Prepare PBMCs during the last 40 min of treatment as Subheading 3.2.
Stain PBMCs with 2 μM of Calcein AM fluorescent dye in serum free RPMI 1640 media at 37 °C in cell culture incubator for 30 min (see Note 6).
Keep some PBMCs in the 37 °C cell culture incubator as a nonstaining control (see Note 7).
Wash the cells from step 3 twice with 10 mL of PBS to remove any extra Calcein AM. For each wash, centrifuge cells at 250 × g for 10 min and carefully remove the supernatant.
Resuspend PBMCs in endothelial cell growth media and RPMI1640 media supplemented with 10% FCS (1:1) and adjust concentration to 1 × 106 cells/mL (see Note 8).
Remove M199 media from cultured HAECs from step 1 and wash cells twice with PBS.
Add 1 mL (1 × 106) of labeled PBMCs to each well and incubate for 1 h in a cell culture incubator at 37 °C (see Note 9).
Carefully aspirate the media from each well (do not allow the cells to dry) and gently wash three times with PBS to remove unattached monocytes. Protect stained cells from light.
Add 1 mL of PBS to each well and read the fluorescence intensity of the labeled monocytes with a fluorescence microplate reader (see Fig. 3).
Fig. 3.
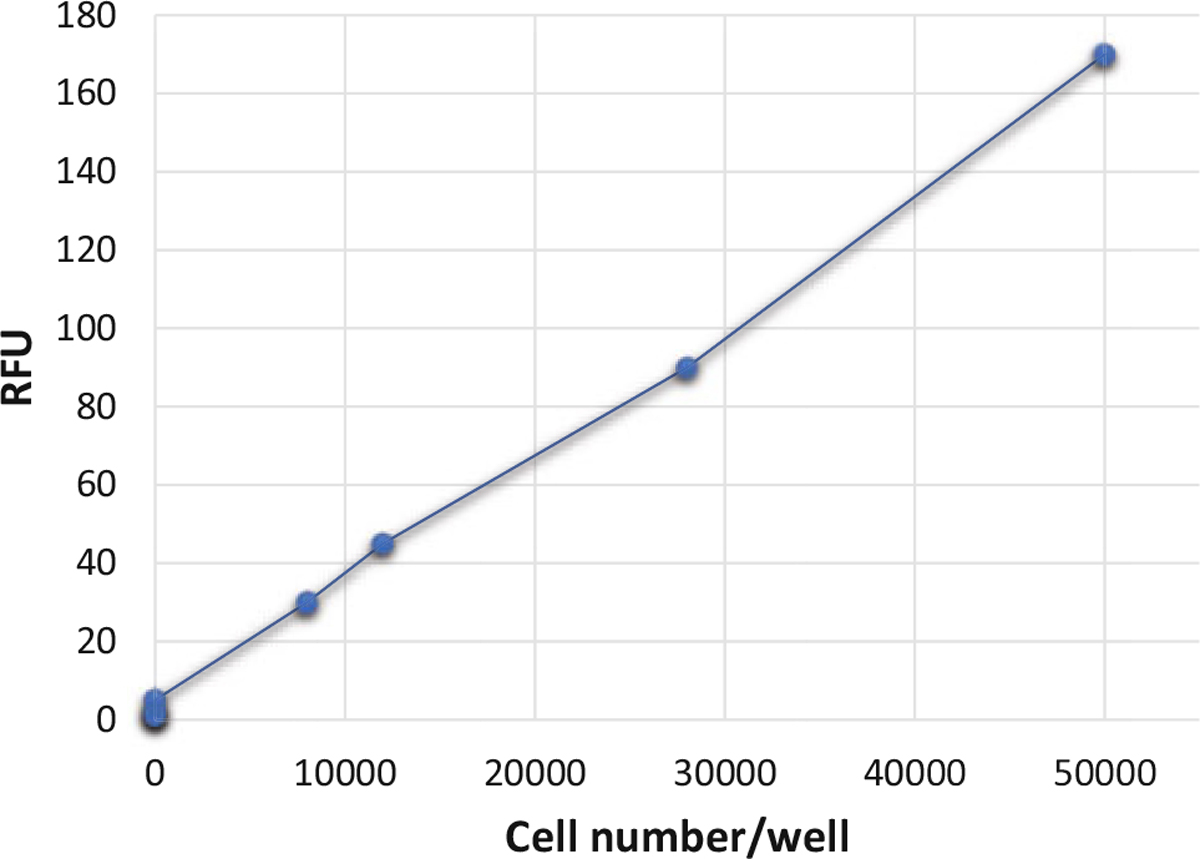
Quantitation of fluorescently labeled PBMCs adhered to activated endothelial cells. Calcein AM fluorescently labeled monocytes were titrated in 1× PBS. Fluorescence was quantified using a fluorescence plate reader and represented as relative fluorescence unit (RFU)
3.4. Example of Results
For better understanding, we have selected two published papers from our laboratory to demonstrate in detail how we implemented the monocyte adhesion assay. The fluorescence intensity was measured on a microplate fluorescence reader (FLx800, Bio-Tek, Winooski, VT) with a 494/517 nm filter set and 488 nm cutoff. The percentage of adherent monocytes to EC monolayer was represented as a percentage of the control group (Fig. 4) [13]. In the first paper, we investigated how the proinflammatory cytokine interleukin-17 (IL-17) promotes aortic EC activation [16]. The second paper [17] looks at how the anti-inflammatory cytokine interleukin-35 (IL-35) inhibits EC activation (Table 4). Interleukins (IL) have been identified as a group of cytokines that elicit a wide range of responses in cells and tissues. They are involved in several immunomodulatory functions such as adhesion, migration, maturation and proliferation. Depending on their function, ILs can be characterized as either pro- or anti-inflammatory [18]. ILs are highly expressed in leukocytes and initiate a response through binding to high-affinity receptors located on the cell surface.
Fig. 4.
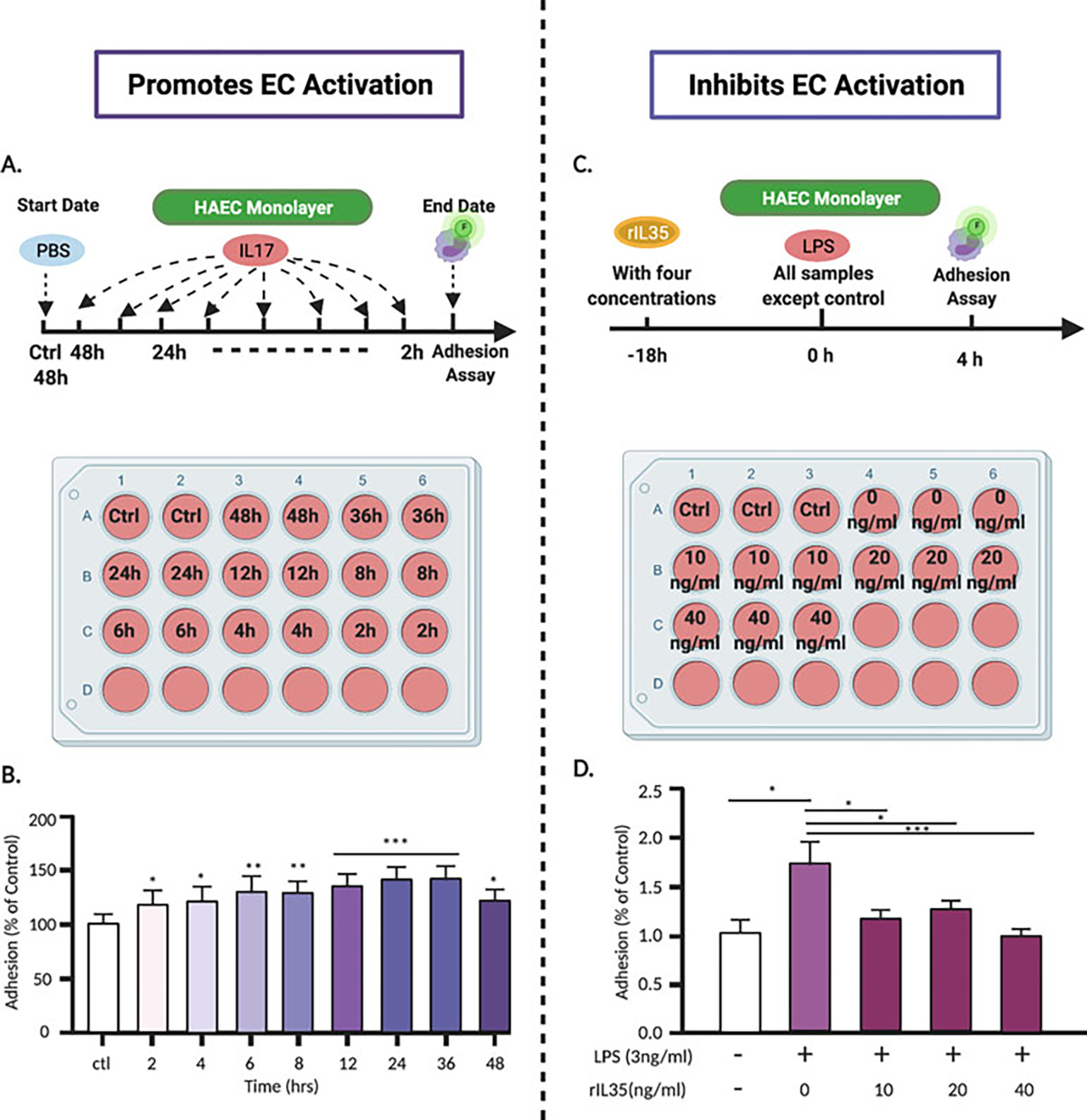
Adhesion assay experimental design for evaluating the actions of pro- and anti-inflammatory stimuli. (a) HAECs were cultured in 24-well plates and then treated with human IL-17 for various time periods. (b) Monocytes adhesion to HAECs increases by IL-17A with time: 12-, 24-, and 36-h treatment periods demonstrate a significant increase in adhesion in a time dependent manner. (c) Four different concentrations (0, 10, 20 and 40 ng/mL) of rIL35 were added 18 h prior to HAEC treatment with LPS (3 ng/mL) in 24-well plates. (d) Fluorescence-labeled human PBMCs adhesion to HAECs stimulated by LPS + rIL35 or LPS only. The results showed that after LPS treatment, the adhesion of human PBMCs to HAECs was significantly increased. However, LPS plus as low as 10 ng/ml rIL35 could dramatically inhibited the adhesion of PBMCs to LPS-activated HAECs
Table 4.
Monocyte adhesion assay.
| Treatment | IL-17A | LPS + IL-35 |
| Cells | HAECs, PBMCs | HAECs, PBMCs |
| Experimental design (trait) | Time course | Dose response |
| Result | IL-17 significantly increased monocyte adhesion in a time dependent manner and promoted EC activation | IL-35 inhibited LPS-induced leukocyte adhesion to ECs in a dose dependent manner |
| EC status | Increases EC activation | Inhibits LPS-induced EC activation |
| PMID | 26733204 | 26085094 |
EC endothelial cells, HAECs human aortic endothelial cells, IL interleukin, LPS lipopolysaccharide, PBMCs peripheral blood mononuclear cells
Therefore, ILs play a pivotal role in modulating leukocyte adhesion and EC activation. Since the specific function of a particular IL is dependent upon its interactions with receptors located on the target cells (ECs), monocyte-EC static adhesion assay is performed and are valuable in gaining insights into cytokine actions.
4. Notes
40 mL of blood is sufficient for collecting 30 × 106 PBMCs for one plate.
The blood should not penetrate deep into the Ficoll-Plaque to avoid Ficoll toxicity to the cells. The first 5 mL of layering is the most sensitive and important.
Centrifugation of 400 × g is optimal to obtain a clean buffy coat.
Do not collect more than 10 mL of cells as this will reduce yields.
- In order to achieve the different experimental aims, HAECs are treated with various drugs. As examples, we outline two experimental strategies in studying both pro- and anti-inflammatory cytokines based on our published papers.
- Study 1 [16]: To determine whether the proinflammatory cytokine IL-17 has the ability to activate ECs, HAEC monolayers were grown in 24-well plates and treated with IL-17 (100 ng/mL) at different time points (Fig. 4a) [9]. The adhesion assay was performed, and results showed that IL-17 increases the number of monocytes adhering to HAECs in a time-dependent manner, therefore activating ECs (Fig. 4b) [9]
- Study 2 [17]: To determine whether the anti-inflammatory cytokine IL-35 has a potential therapeutic ability to reverse EC activation, HAECs were stimulated with lipopolysaccharides (LPS) to induce EC activation, then treated with IL-35 (0, 10, 20 and 40 ng/mL) for 18 h (Fig. 4c) [9]. The adhesion assay was performed, and the results demonstrated that IL-35 treatment decreased the number of monocyte adhesion to HAECs, therefore inhibiting EC activation (Fig. 4d).
Calcein AM should not affect the viability of ECs and fresh Calcein AM should be used each time.
At least one well of the 24-well plate is used as a nonstaining negative control (HAECs + PBMCs in PBS).
Each stimulation is performed in triplicate for statistical analysis and the experiments should be repeated at least three times.
1 × 106 cells/mL of PBMCs in each well is sufficient for use in the adhesion assay.
Acknowledgment
This work was supported by NIH R01 grants to XY.
References
- 1.Roth GA, Johnson C, Abajobir A et al. (2017) Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 70(1):1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimbrone MA Jr, Garcia-Cardena G (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118(4):620–636. 10.1161/CIRCRESAHA.115.306301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestas J, Ley K (2008) Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18(6):228–232. 10.1016/j.tcm.2008.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P, Pasterkamp G, Crea F, Jang IK (2019) Reassessing the mechanisms of acute coronary syndromes. Circ Res 124(1):150–160. 10.1161/circresaha.118.311098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iruela-Arispe ML (2008) Endothelial cell activation. In: Angiogenesis. Springer, Boston. 10.1007/978-0-387-71518-6_3 [DOI] [Google Scholar]
- 6.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD (2013) Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci 33(7):2849–2859. 10.1523/JNEUROSCI.3229-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branco A, Yoshikawa FSY, Pietrobon AJ, Sato MN (2018) Role of histamine in modulating the immune response and inflammation. Mediat Inflamm 2018:9524075. 10.1155/2018/9524075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelesidis T, Papakonstantinou V, Detopoulou P, Fragopoulou E, Chini M, Lazanas MC, Antonopoulou S (2015) The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with HIV infection. AIDS Rev 17(4):191–201 [PMC free article] [PubMed] [Google Scholar]
- 9.Ray CJ, Abbas MR, Coney AM, Marshall JM (2002) Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544(Pt 1):195–209. 10.1113/jphysiol.2002.023440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cybulsky MI, Gimbrone MA Jr (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251(4995):788–791. 10.1126/science.1990440 [DOI] [PubMed] [Google Scholar]
- 11.Li X, Wang L, Fang P, Sun Y, Jiang X, Wang H, Yang XF (2018) Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J Biol Chem 293(28):11033–11045. 10.1074/jbc.RA118.002752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe DJ, Raj K (2015) Quantitation of endothelial cell adhesiveness in vitro. J Vis Exp 100:e52924. 10.3791/52924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.All figures were created with Biorender.com https://biorender.com/
- 14.Cell BIOLABS I (2020) Leukocyte endothelium adhesion assay
- 15.Fuss IJ (2009) Isolation of whole mononuclear cells from peripheral blood and cord blood. Curr Protoc Immunol Chapter 7:Unit7.1. 10.1002/0471142735.im0701s85 [DOI] [PubMed] [Google Scholar]
- 16.Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, Liu F, Autieri MV, Kunapuli SP, Iwakura Y, Jiang X, Wang H, Yang XF (2016) Interleukin-17A promotes aortic endothelial cell activation via transcriptionally and post-translationally activating p38 mitogen-activated protein kinase (MAPK) pathway. J Biol Chem 291(10):4939–4954. 10.1074/jbc.M115.690081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, Shan H, Jiang X, Wang H, Yang XF (2015) Interleukin-35 inhibits endothelial cell activation by suppressing MAPK-AP-1 pathway. J Biol Chem 290(31):19307–19318. 10.1074/jbc.M115.663286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V (2010) Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics 5(1): 30–55. 10.1186/1479-7364-5-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]


