Abstract
Accumulating evidence show that exercise and diet interventions are associated with improved sleep quality. Studies investigating the effects of exercise and dieting on circulating metabolomics in people with sleep disorders, particularly insomnia, are scarce. This 6-month randomized study aimed to assess the effects of exercise and dietary interventions on serum metabolites in men with insomnia symptoms. Seventy-two Finnish men (age: 51.6 ± 10.1 years) with chronic insomnia symptoms who were assigned to different intervention groups completed this study (exercise, n = 24; diet, n = 27; and control, n = 21). The Shapiro-Wilk W-test, Levene test, Spearman correlation analysis, and analysis of variance were used for data analysis. We found that exercise and diet intervention were associated with improved sleep quality and with a number of metabolites across different biochemical pathways. Although we could not show causality, our findings provide new insight into the biological mechanisms underlying the health effects of physical activity, diet, and sleep quality. Further investigation is needed to better understand the link among lifestyle, sleep quality, and metabolic health.
Keywords: Diet, Exercise, Insomnia, Metabolomics
Abbreviations: SOL, sleep onset latency; BMI, body mass index; TST, total sleep time; SE, sleep efficiency; GC, gas chromatography; MS, mass spectrometry; NIST, National Institute of Standards and Technology; (O)PLS-DA, (orthogonal) partial least-squares-discriminant analysis; VIP, variable importance in the projection; FM, fat mass; KIC, alpha-ketoisocaproic acid; 3-HB, 3-hydroxybutric acid; BCAA, branched chain amino acid; Q2, quartile 2
Introduction
Insomnia, the most prevalent sleep disorder, is common in modern society and has become an important public health issue.1 The prevalence of insomnia symptoms is 21.4% in adults from the United States, as assessed by criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition.2 Furthermore, sleep loss or sleep disruptions induced by insomnia symptoms are also associated with an increase in all-cause mortality and can lead to detrimental effects on neuroendocrine systems.3 To date, the studies concerning the effect of exercise interventions on sleep have focused on good sleepers,4,5 fit athletes,6,7 or patients with sleep apnea.8,9 The effects of exercise on sleep in an untrained population and insomnia have yet to be established.10 We have shown that 6 months of exercise and diet intervention shortened objective sleep onset latency (SOL) and improved subjective sleep quality in overweight and obese men with insomnia symptoms, independent of weight loss.11,12 However, these studies did not investigate the metabolic changes associated with improved sleep quality.
The results of dietary interventions targeting patients with sleep disorders are conflicting,13 with two studies showing that isocaloric high fat intake is associated with significantly better sleep than other diets in humans.11,14 A large cross-sectional study from China reported an association between decreased sleep duration and increased fat intake in 2828 adults.15 However, the biological mechanisms underlying the associations between exercise/diet and sleep disorders largely remain unclear.
Serum high-throughput metabolomics profiling is a feasible method to study the characteristic changes in small molecular metabolites in a pathophysiological state. Studies have shown that levels of glucose, amino acids, fatty acids and tricarboxylic acid cycle intermediates differed in individuals with sleep disorders compared with healthy people16, 17, 18 and in animal studies.19,20 However, no study has examined the effect of either exercise or diet intervention on circulating metabolomics in patients with insomnia symptoms. We hypothesize that exercise and dietary interventions affect the sleep quality of patients with insomnia symptoms through different metabolic pathways. Therefore, we conducted the present study to assess the associations between serum metabolites and sleep quality by conducting randomized controlled 6-month exercise or diet intervention in men with insomnia symptoms.
Material and methods
Ethics statements
The study was approved by the Ethics Committee of the Central Finland Health Care District (7/2011 OTE) and registered at www.controlled-trials.com (ISRCTN77172005). Written informed consent was obtained from all participants before the baseline measurements, and copies of the signed consent forms were archived.
Study design and population
This study was part of a 6-month randomized lifestyle intervention in middle-aged Finnish men with sleep disorders (including insomnia and sleep apnea).21 For this report, we included 72 men aged 30–65 years who self-reported suffering from chronic insomnia lasting 3 months or longer and completed the 6-month intervention.21 The participants further completed a Vitalmed sleep questionnaire, which consists of all the questions in the Basic Nordic Sleep Questionnaire and Epworth Sleepiness Scale, and a physician reviewed their responses. Of the qualified participants who completed the intervention, 24 participants were randomized to the exercise group, 27 to the diet group, and 21 to the control group.
Exercise intervention
Exercise was prescribed according to the recommendations of the American College of Sports Medicine.22 Nordic walking combined with other aerobic exercises was performed for 30–60 min per session, 3 to 5 sessions per week, at the intensity level of 60–75% of the estimated maximum heart rate. Two experienced exercise trainers were responsible for the instruction and supervision of exercise training once a week and in the other sessions, the participants exercised independently. At the beginning of each week, the trainer informed the participant of the duration and intensity (heart rate interval) of each exercise session.12
Diet intervention
A specific individualized diet program was developed after baseline assessments of each participant's current dietary intakes (based on a 3-day food diary) and body weight were performed. The suggested diet contained the following relative macronutrient composition of energy intake: 40% carbohydrates with <5% sucrose, 40% fat (saturated fatty acid 10%, monounsaturated fatty acid 15–20%, and polyunsaturated fatty acid 10%), and 20% protein. Overweight/obese participants were advised to moderately reduce their total energy intake (by 300–500 kcal per day for the first 3 months) with guidance on the proportion of macronutrients to be consumed. The target was to reduce their body weight by 3 kg in the first 3 months of the intervention. Detailed information was given in previous paper.11
During the intervention, an online diet and nutrition counseling service (Meal Tracker; Wellness Foundry Holding Ltd., Helsinki, Finland) was utilized to supervise participants’ dietary intake and provide diet suggestions. Participants were instructed to photograph all daily dietary intakes (including drinks) by using a smartphone or a digital camera, and upload all photographs to the server 1–3 days per week during the intervention. The average of photo uploading in diet group was 26 images per month (37 for month 1, 35 for month 2, 26 for month 3, 21 for month 4, 24 for month 5 and 14 for month 6).
Control group
Participants in the control group were instructed to maintain their habitual, pre-recruitment lifestyle during the intervention. They were invited to a lecture that explained the purpose of their group. After a 6-month study period, they were given an opportunity to participate in the exercise plus dietary intervention program for 3 months.
Background information and anthropometric measurements
All lifestyle and medical history information was collected by self-administered questionnaires at the Laboratory of Sport and Health Sciences, University of Jyväskylä. Anthropometric measurements were performed after overnight fasting (12 h). Height was measured using a fixed wall scale device to the nearest 0.1 cm. Weight was determined to the nearest 0.1 kg using an electronic scale and calibrated before each measurement session. Body mass index (BMI) was calculated as weight (kg) per height2 (m2).
Objective measurements of sleep
Seven night sleep measurements before and after the intervention were performed for all participants by using a non-contact sleep monitoring system at their homes (Beddit sleep tracker; Beddit, Espoo, Finland)23 and automatically analyzed using the Beddit server via the Internet. The system included a piezoelectric bed sensor. Ballistocardiographic signals were sampled by the piezoelectric sensor at 140 Hz and simultaneously uploaded to a web server via the Internet, where sleep/wake status was classified in 30-s epochs based on heart rate variability, respiratory rate variability, and a binary actigram. Total sleep time (TST), SOL (determined as the duration from being present in bed with lights out to the first 5 min of consecutive sleep), wakefulness after sleep onset, and sleep efficiency (SE) (commonly defined as the ratio of a person's TST to time spent in bed, which refers to the amount of time a person is actually asleep during the time spent trying to sleep) were obtained for each night. Average values over the nights measured were used in the analyses. The possible conditions that may affect the measurement, such as children and pets in the bedroom were recorded in the participant's sleep diary. A research assistant visited each participant's home to set up the system before measurements were started. The sleep data were validated by the piezoelectric system against the two-night polysomnography measurement (31 participants with complaints of insomnia, mean age: 51.8 ± 8.4 years, mean BMI:30.9 ± 4.8 kg/m2).12
Measurement of the maximal oxygen uptake
The 2-km walk test (UKK Institute, Tampere, Finland) was used to determine each participant's fitness level. Participants were instructed to walk 2 km as fast as possible at a steady pace. The walk time duration and heart rate immediately upon finishing the walk were measured to estimate the maximal aerobic power (estimated maximal oxygen uptake). An exercise watch with a heart rate monitoring belt (M5; Suunto Ltd., Vantaa, Finland) was used to determine participants' heart rate. The tests were conducted at on an outdoor athletic field and considered/proven safe for the overweight and obese adults who met our inclusion criteria.24
Serum sample collection
Blood samples were collected from all subjects in the morning between 7:00 and 9:00 a.m. after overnight fasting at baseline and after the 6-month intervention. Serum was extracted by centrifugation and stored immediately at −80 °C until analyzed.
Metabolomics assessment
Sample preparation
The serum sample (100 μL) was first thawed at room temperature, then mixed with a methanol-chloroform solvent (300 μL 3:1, v/v) and L-2-chlorophenylalanine (10 μL, 0.3 mg/mL in water) for metabolites extraction, and kept at −20 °C for 10 min. After centrifugation at 15 000 g for 10 min, 300 μL of supernatant was obtained and dried completely under nitrogen. Next, 80 μL of methoxyamine (15 mg/mL in pyridine) was added to the vial, vortexed for 30 s and kept at 37 °C for 90 min followed by the addition of 80 μL N,O-Bis(trimethylsilyl)trifluoroacetamide (1% trimethylchlorosilane) and 20 μL of n-hexane at 70 °C for 60 min.
Quality control samples for serum were prepared by pooling equal aliquots of each sample and then they were pretreated as serum samples.
Pegasus 4-dimensional gas chromatography time-of-flight mass spectrometry analysis
Each 1 μL aliquot of the derivatized solution was injected in splitless mode into the Pegasus 4-dimensional gas chromatography (GC) time-of-flight mass spectrometry (MS) system (LECO Corporation, St Joseph, MI, USA). Separation was performed on a non-polar DB-5 capillary column (30 m × 250 μm I.D., J&W Scientific, Folsom, CA, USA), with high purity helium as the carrier gas at a constant flow rate of 1.0 mL/min. The GC temperature programming was set to begin at 90 °C and hold for 0.2 min, followed by 10 °C/min oven temperature ramps to 180 °C, 30 °C/min to 240 °C, 20 °C/min to 280 °C, and a final 11-min maintenance at 280 °C. The temperatures of the injection and ion source was set to 280 °C and 220 °C, respectively. Electron impact ionization (−70 eV) at full scan mode (m/z 30–600) was used (solvent delay: 7.6 min), with an acquisition rate of 100 spectrum/second in the MS setting.
The acquired MS data from GC−MS were analyzed using the ChromaTOF software (version 4.34, LECO, St Joseph) combined with National Institute of Standards and Technology (NIST) mass spectral libraries (NIST 14), LECO/Fiehn Metabolite mass spectral library (version 1.00) and our in-house library. Based on the retention time of a series of fatty acid methyl esters (C6–C24), the retention index was calculated by the Retention Index Method function of the ChromaTOF software.25
Data processing and statistics
SPSS statistical software version 24 (IBM Corp., Armonk, NY, USA) was used to perform statistical analysis. All data were checked for normality by using the Shapiro-Wilk W-test and for homogeneity by using the Levene test before each analysis. Natural logarithm transformations were performed on non-normally distributed data. Spearman correlation analysis was performed with the change percentage of metabolites and change percentage of parameters related to sleep. Between-group differences were evaluated by repeated measures analysis of variance, followed by Sidak corrections for multiple comparisons. All tests were two-sided with a significance level set at p < 0.05.
To account for heteroscedasticity, data were normalized by log2 transformation. Multivariate statistical analysis and univariate analysis were used for metabolomics data analysis.
Data sets resulting from GC–MS were separately imported into the SIMCA-P+ 14.0 software package (Umetrics, Umea, Sweden). Principle component analysis and (orthogonal) partial least-squares-discriminant analysis ((O)PLS-DA) were performed with SIMCA-P, and differential metabolites were based on variable importance in the projection (VIP) > 1.0 and p < 0.05 according to the Student t-test. In the present study, a 7-fold cross validation procedure and 200 random permutations tests were performed to avoid overfitting of the supervised (O)PLS-DA models. The intensity of each metabolite was used as the final result.
Results
Characteristics of the study participants are shown in Table 1. During the 6-month intervention period, body weight and fat mass (FM) were significantly increased in the control group, while no significant changes in body weight and FM were observed in the intervention groups. However, compared to the control group, the diet group had significantly low body weight and FM (p = 0.005 and p = 0.027, respectively), whereas in the exercise group did not show such differences.
Table 1.
Characteristics of subjects (mean ± SD).
| Exercise (n = 24) |
Diet (n = 27) |
Control (n = 21) |
Between-group |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | 1 vs 3 | 2 vs 3 | |
| Age (years) | 51.2(9.7) | – | 51.7(9.3) | – | 50.7(9.3) | – | ||
| Weight (kg) | 92.3(14.6) | 92.5(13.9) | 93.8(11.8) | 92.7(11.9) | 93.1(17.2)c | 94.4(17.8) | 0.176 | 0.005 |
| BMI | 29.3(4.0) | 29.4(3.8) | 29.4(3.7) | 29.0(3.7) | 29.2(4.4) | 29.4(4.6) | 0.612 | 0.047 |
| Total fat mass (kg) | 27.3(9.5) | 27.2(9.0) | 27.4(8.4) | 26.8(8.6) | 28.0(9.7)c | 28.9(10.7) | 0.180 | 0.027 |
| Triglycerides (mmol/L) | 1.7(0.7) | 1.8(0.9) | 1.6(0.6) | 1.5(0.6) | 1.5(0.7) | 1.4(0.6) | 0.104 | 0.867 |
| Energy intake (kcal) | 2285.2(561.6) | 2043.1(643.6) | 1981.2(372.1) | 1877.1(487.9) | 2223.5(410.1)c | 1920.9(463.7) | 0.588 | 0.606 |
| VO2max | 29.3(9.3)a | 31.4(9.3) | 27.2(9.6)b | 29.6(10.8) | 30.9(7.9) | 30.4(8.0) | 0.086 | 0.053 |
| Total energy expenditure (MET min/day) | 2349.9(224.8) | 2335.5(235.3) | 2346.1(215.9) | 2398.8(234.9) | 2341.1(242.8) | 2322.6(233.7) | 0.887 | 0.282 |
a: exercise 0 m compare with exercise 6 m; b: diet 0 m compare with diet 6 m; c: control 0 m compare with control 6 m, p < 0.05.
Between-group (1: exercise, 2: diet, 3: control) differences were evaluated by repeated measures ANOVA, followed with Sidak corrections for multiple comparisons.
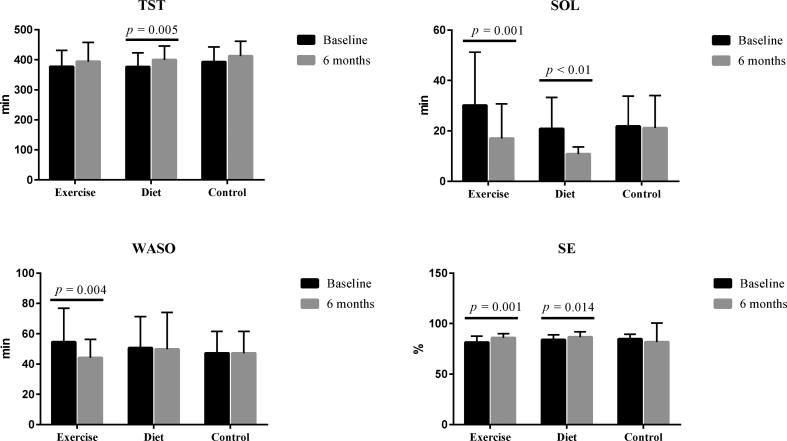
Of the sleep quality related variables, SOL decreased (p < 0.05) after the exercise intervention, and SE increased (p < 0.05) after the diet intervention. No significant changes in sleep-related parameters were found in the control group (Fig. 1).
Fig. 1.
Changes of sleep parameters after the interventions. TST, total sleep time; SOL, sleep onset latency; WASO, wakefulness after sleep onset; SE, sleep efficiency.
Two hundred ten known metabolites in serum were observed among the different groups. The results showed a clear separation between the baseline and follow-up findings (exercise group R2Y = 0.959, quartile 2 [Q2] = 0.675; diet group R2Y = 0.969, Q2 = 0.823; and control group R2Y = 0.981, Q2 = 0.828) (Supplementary Fig. S1). Figs. S1B and S1D, and S1F show that the calculated R2 and Q2 values in permutation tests were lower than the original ones, and the Q2 intercept on the vertical axis was < 0. Therefore, the model was considered valid.
The significant metabolites in serum were screened according to the VIP value larger than 1 and p-value (p < 0.05) from the Student t-test adjusted by the false discovery rate. Twenty-one metabolites were affected by the exercise intervention, 33 metabolites were affected by the diet intervention, and 5 were affected in the control group (VIP > 1, p < 0.05). All metabolites, except acetanilide in the exercise and control groups, showed greater than 2-fold change between baseline values at and 6 months (Table S1). The significantly altered metabolites in all groups are shown in Fig. 2A, B and 2C. Fig. 2D shows 2 unique metabolites that were altered in the exercise group and 14 metabolites that were altered in the diet group.
Fig. 2.
The significantly altered metabolites after the intervention and Venn figure of these metabolites in the three groups. A, significantly altered metabolites in exercise group; B, significantly altered metabolites in diet group; C, significantly altered metabolites in control group; D, common and different metabolites in the three groups. Y-axis, standard data after log2 transformation.
Next, we assessed whether these metabolites were associated with changes in sleep parameters (Fig. 3 and Table 2). We found that decreased alpha-ketoisocaproic acid (KIC) was correlated with improved SE in the exercise group (r = −0.52, p = 0.026). Additionally, 67% of participants showed increased phosphate levels after the exercise intervention, although the association between phosphate and SE was not statistically significant. In the diet group, change of 3-hydroxybutric acid (3-HB) (r = −0.47, p = 0.025) and d-glucopyranose (r = −0.54, p = 0.006) correlated negatively with SE. However, in the same group, oxalic acid (r = 0.49, p = 0.021), d-glucopyranose (r = 0.43, p = 0.048), 4-deoxyerythronic acid (r = 0.60, p = 0.004) and tagatose (r = 0.51, p = 0.016) correlated positively with change of SOL, and 2-keto-isovaleric acid (r = 0.45, p = 0.029) correlated with TST.
Fig. 3.
The intensity of significantly changed metabolites in individuals between baseline and the 6-month follow-up in the exercise and diet groups. Each line represents one individual.
Table 2.
Correlations between significantly changed metabolites and sleep parameters.
| Group | Metabolites | SE |
SOL |
TST |
|||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | ||
| Exercise | alpha-ketoisocaproic acid | −0.52 | 0.026 | ||||
| Diet | 3-hydroxybutric acid | −0.47 | 0.025 | ||||
| d-glucopyranose | −0.54 | 0.006 | 0.43 | 0.048 | |||
| Tagatose | 0.51 | 0.016 | |||||
| Oxalic acid | 0.49 | 0.021 | |||||
| 4-deoxyerythronic acid | 0.60 | 0.004 | |||||
| 2-keto-isovaleric acid | 0.45 | 0.029 | |||||
r: correlation coefficient; p: p value; SE: sleep efficiency; SOL: sleep onset latency; TST: total sleep time.
Discussion
In this study, 21 metabolites were altered significantly after the exercise intervention and 33 metabolites were altered after the dietary intervention. Five metabolites changed significantly in the control group during the same period.
SE is commonly defined as the ratio of a person's TST to time spent in bed.26 Earlier studies using self-administered questionnaires showed that sleep timing is associated with metabolic alterations in different pathways.27 Herein, we used an objective measure of sleep and a non-contact sleep monitoring system at participants' homes. We found that the significantly altered metabolites after the exercise intervention were mainly related to amino acids metabolism (isoleucine, l-allothreonine, and glutamine), carbohydrates metabolism (4-deoxyerythronic acid, erythrose, tagatose, fucose, d-glucopyranose, and trehalose-6-phosphate) and lipids metabolism (butyric acid, 2-keto-isovaleric acid, monoolein, beta-glycerophosphoric acid, and 2-monopalmitin). We found that decreased KIC was correlated with improved SE. KIC is readily aminated to leucine via leucine dehydrogenase and/or branched chain amino acid (BCAA) transferase at the expense of NH3 in various tissues including skeletal muscle,28 which may ultimately decrease fatigue and preserve skeletal muscle force production during intense exercise. However, to our knowledge no previous study has linked exercise and KIC with sleep quality.29
BCAAs contribute to energy metabolism during exercise as energy sources and substrates to expand the pool of citric acid-cycle intermediates, thus allowing more efficient mitochondrial energy production from lipids and thereby better metabolic health.30 A study conducted in overweight dysglycemic and normal weight normoglycemic men found that insulin sensitivity increased similarly in dysglycemic and normoglycemic men after 12 weeks of exercise, in parallel to similar increases in the concentration of plasma glutamine.31 However, other previous studies showed that a decrease in the molar sum of circulating BCAAs was the best predictor of improvement in insulin sensitivity induced by a combined resistance and aerobic exercise intervention in overweight individuals.32 Long-term leisure-time physical activity was associated with low circulating BCAAs concentrations.33 Because of the discrepancy between our study results and the results of other studies, more studies are needed to better understand how exercise affects amino acid metabolism related to insomnia symptoms. On the other hand, the effect of exercise and sleep on carbohydrates metabolism34,35 and lipids metabolism36, 37, 38 have been reported previously. Exercise appears to enhance the ability of skeletal muscles to utilize lipids as opposed to glycogen, thus reducing plasma lipid levels.39 Several studies also showed that poorer or longer sleep duration were associated with higher risks of abnormal serum lipid profiles.40, 41, 42 Further studies are needed to explore whether the alterations of energy-related amino acids, carbohydrates, and lipids metabolisms associated with exercise can translate to improved sleep quality.
The dietary intervention was associated with altered concentrations of 33 metabolites. Among those metabolites, 7 were correlated with improved sleep quality. These metabolites are mainly related to carbohydrates metabolism (4-deoxyerythronic acid, tagatose, and d-glucopyranose), lipids metabolism (2-keto-isovaleric acid) and organic acids metabolism (3-HB, and oxalic acid). Increased oxalic acid, d-glucopyranose, 4-deoxyerythronic acid and tagatose were associated with improved SOL. Oxalic acid (oxalate) is derived from diet, the degradation of ascorbate, and produced by the liver and erythrocytes. Oxalic acid has been shown to be reduced in rats and humans following sleep restriction, and recover to near baseline levels after sleep restriction.43 Moreover, the 3-HB concentration increased after the dietary intervention. Serum 3-HB, which is a ketone body,44,45 has been shown to increase in subcutaneous adipose after sleep loss in humans.46 We also found that d-glucopyranose increased after the diet intervention. d-glucopyranose (glucose) is a monosaccharide containing 6 carbon atoms and an aldehyde group and is therefore referred to as an aldohexose, which is a primary source of energy for living organisms. Abnormal glucose metabolism have been linked to disturbances of different aspects of sleep, including sleep duration and quality, respiratory function during sleep, and circadian timing.47 Short and long sleep durations were associated with worse glucose control in people with diabetes.48 Recently, Kay et al. showed that good sleepers, but not patients with insomnia, had lower whole-brain glucose metabolism during recovery non-rapid eye movement sleep compared to baseline findings and individuals with insomnia.49 We also found increased tagatose and KIV after the diet intervention. Tagatose (d-tagatose) has been associated with improved glucose control, weight loss, and an elevated high-density lipoprotein cholesterol level.50 The observed results of the dietary intervention induced alteration of purine, glucose, and galactose metabolisms, and research is needed to whether these alterations are attributed to improved sleep quality.
The diet intervention group failed to reduce body weight and FM significantly during the study, and this was probably because the diet program focused on the relative macronutrient composition and some subjects were unable to reduce their total energy intake. These results suggest two points. First, in addition to short sleep duration,51 chronic insomnia symptoms also contribute to weight gain.52 Second, the altered energy-balance is likely to play a role in diet induced sleep alteration.51 Nevertheless, further studies are needed to elucidate the underlying mechanisms.
Our study has strengths and limitations. With regard to the strengths, first, the study participants were randomly assigned to the intervention and control groups, and sleep was measured using a non-contact sleep monitoring system. Second, the exercise training program was prescribed according to the recommendations of the American College of Sports Medicine and was supervised by experienced trainers. Lastly, the diet intervention was individualized according the participants’ body weight and caloric intake, and durations of the exercise and diet interventions were relatively long. The limitations include the small number of participants and a relatively high drop-out rate in the control group, which may have affected the study results. In addition, we should have added another group which is exercise plus dieting group, this would be better to distinguish the effects of exercise, diet, and exercise plus diet.
Conclusion
In conclusion, the exercise and diet interventions were associated with improved sleep quality and circulating metabolites across different biochemical pathways. The effect of exercise on sleep is mainly related to amino acids, carbohydrates and lipids metabolisms, whereas the effect of diet on sleep is related to carbohydrates, lipids and organic acids metabolisms. Although our study results could not show whether the alterations of metabolic were causally linked to lifestyle interventions or improved sleep quality, our findings may provide new insight into the biological mechanisms underlying the metabolic effects of physical activity, diet, and sleep quality. Further studies are needed to better understand the role of lifestyle and sleep quality in metabolic regulation.
Authors’ contributions
The authors’ contributions are as follows: SC is the principal investigator; she designed the study and oversaw the implementation of the project, trained the researchers, supervised the doctoral students, and participated in the data collection, analyses and interpretation, and editing of the manuscript. XZ, XW, and PW drafted the manuscript. SL, XO, and XT participated in the data analyses, and interpretation and discussion of the manuscript.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Ethical approval
The study was approved by the Ethics Committee of the Central Finland Health Care District (7/2011 OTE) and registered at www.controlled-trials.com (ISRCTN77172005). Written informed consent was obtained from all participants before the baseline measurements were performed, and copies of the signed consent forms were obtained.
Conflict of interest
The authors have no conflicts of interest to report.
Acknowledgments
The authors are grateful to Shumei Cheng, Tuija M. Mikkola, Samu Martinmäki, Jarkko Tenhunen, Satu Pekkala, EveliinaMunukka, and Aki Rahikainen, for their contributions to the data collection or exercise intervention and diet intervention.
This study was supported financially by the Finnish Funding Agency for Technology and Innovation (TEKES2206/31/2010), 111 Project (B17029) of Shanghai Jiao Tong University, Shanghai Jiao Tong University Zhiyuan Foundation (grant CP2014013), and China Postdoc Scholarship Council (201806230001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.smhs.2020.05.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
References
- 1.Yazdi Z., Sadeghniiat-Haghighi K., Loukzadeh Z., Elmizadeh K., Abbasi M. Prevalence of sleep disorders and their impacts on occupational performance: a comparison between shift workers and nonshift workers. Sleep Disord. 2014;2014:870320. doi: 10.1155/2014/870320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budhiraja R., Roth T., Hudgel D.W., Budhiraja P., Drake C.L. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai X.H., Xie Y.P., Li X.C., et al. The prevalence and associated risk factors of sleep disorder-related symptoms in pregnant women in China. Sleep Breath. 2013;17(3):951–956. doi: 10.1007/s11325-012-0783-2. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro C.M., Warren P.M., Trinder J., et al. Fitness facilitates sleep. Eur J Appl Physiol Occup Physiol. 1984;53(1):1–4. doi: 10.1007/BF00964680. [DOI] [PubMed] [Google Scholar]
- 5.Meintjes A.F., Driver H.S., Shapiro C.M. Improved physical fitness failed to alter the EEG patterns of sleep in young women. Eur J Appl Physiol Occup Physiol. 1989;59(1-2):123–127. doi: 10.1007/BF02396589. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery I., Trinder J., Paxton S.J. Energy expenditure and total sleep time: effect of physical exercise. Sleep. 1982;5(2):159–168. doi: 10.1093/sleep/5.2.159. [DOI] [PubMed] [Google Scholar]
- 7.Edinger J.D., Morey M.C., Sullivan R.J., et al. Aerobic fitness, acute exercise and sleep in older men. Sleep. 1993;16(4):351–359. doi: 10.1093/sleep/16.4.351. [DOI] [PubMed] [Google Scholar]
- 8.Aiello K.D., Caughey W.G., Nelluri B., Sharma A., Mookadam F., Mookadam M. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med. 2016;116:85–92. doi: 10.1016/j.rmed.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Kline C.E., Crowley E.P., Ewing G.B., et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driver H.S., Taylor S.R. Exercise and sleep. Sleep Med Rev. 2000;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- 11.Tan X., Alen M., Wang K., et al. Effect of six-month diet intervention on sleep among overweight and obese men with chronic insomnia symptoms: a randomized controlled trial. Nutrients. 2016;8(11) doi: 10.3390/nu8110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan X., Alen M., Wiklund P., Partinen M., Cheng S. Effects of aerobic exercise on home-based sleep among overweight and obese men with chronic insomnia symptoms: a randomized controlled trial. Sleep Med. 2016;25:113–121. doi: 10.1016/j.sleep.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Knowlden A.P., Hackman C.L., Sharma M. Systematic review of dietary interventions targeting sleep behavior. J Alternative Compl Med. 2016;22(5):349–362. doi: 10.1089/acm.2015.0238. [DOI] [PubMed] [Google Scholar]
- 14.Lindseth G., Murray A. Dietary macronutrients and sleep. West J Nurs Res. 2016;38(8):938–958. doi: 10.1177/0193945916643712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Z., McEvoy M., Luu J., Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes. 2008;32(12):1835–1840. doi: 10.1038/ijo.2008.191. [DOI] [PubMed] [Google Scholar]
- 16.Giskeodegard G.F., Davies S.K., Revell V.L., Keun H., Skene D.J. Diurnal rhythms in the human urine metabolome during sleep and total sleep deprivation. Sci Rep. 2015;5:14843. doi: 10.1038/srep14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell L.N., Kilkus J.M., Booth J.N., 3rd, Bromley L.E., Imperial J.G., Penev P.D. Effects of sleep restriction on the human plasma metabolome. Physiol Behav. 2013;122:25–31. doi: 10.1016/j.physbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H., Zheng X., Jia W., Yin S. Chromatography/mass spectrometry-based biomarkers in the field of obstructive sleep apnea. Medicine (Baltim) 2015;94(40) doi: 10.1097/MD.0000000000001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan S., Wu Y., Sun P., Lin H., Zhu Y., Han X. Decrease in circulating fatty acids is associated with islet dysfunction in chronically sleep-restricted rats. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sengupta A., Rhoades S.D., Kim E.J., et al. Sleep restriction induced energy, methylation and lipogenesis metabolic switches in rat liver. Int J Biochem Cell Biol. 2017;93:129–135. doi: 10.1016/j.biocel.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Tan X., Saarinen A., Mikkola T.M., et al. Effects of exercise and diet interventions on obesity-related sleep disorders in men: study protocol for a randomized controlled trial. Trials. 2013;14:235. doi: 10.1186/1745-6215-14-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garber C.E., Blissmer B., Deschenes M.R., et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 23.Paalasmaa J., Waris M., Toivonen H., Leppakorpi L., Partinen M. Unobtrusive online monitoring of sleep at home. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:3784–3788. doi: 10.1109/EMBC.2012.6346791. [DOI] [PubMed] [Google Scholar]
- 24.Oja P., Laukkanen R., Pasanen M., Tyry T., Vuori I. A 2-km walking test for assessing the cardiorespiratory fitness of healthy adults. Int J Sports Med. 1991 Aug;12(4):356–362. doi: 10.1055/s-2007-1024694. [DOI] [PubMed] [Google Scholar]
- 25.Kind T., Wohlgemuth G., Lee D.Y., et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81(24):10038–10048. doi: 10.1021/ac9019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed D.L., Sacco W.P. Measuring sleep efficiency: what should the denominator be? J Clin Sleep Med. 2016;12(2):263–266. doi: 10.5664/jcsm.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Q., Derkach A., Moore S.C., et al. Habitual Sleep and human plasma metabolomics. Metabolomics. 2017;13(5) doi: 10.1007/s11306-017-1205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews D.E., Bier D.M., Rennie M.J., et al. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981;214(4525):1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- 29.Banister E.W., Cameron B.J. Exercise-induced hyperammonemia: peripheral and central effects. Int J Sports Med. 1990;11(Suppl 2):S129–S142. doi: 10.1055/s-2007-1024864. [DOI] [PubMed] [Google Scholar]
- 30.Kainulainen H., Hulmi J.J., Kujala U.M. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev. 2013;41(4):194–200. doi: 10.1097/JES.0b013e3182a4e6b6. [DOI] [PubMed] [Google Scholar]
- 31.Lee S., Olsen T., Vinknes K.J., et al. Plasma sulphur-containing amino acids, physical exercise and insulin sensitivity in overweight dysglycemic and normal weight normoglycemic men. Nutrients. 2018;11(1) doi: 10.3390/nu11010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn E.L., Piner L.W., Huffman K.M., et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia. 2015;58(10):2324–2335. doi: 10.1007/s00125-015-3705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kujala U.M., Makinen V.P., Heinonen I., et al. Long-term leisure-time physical activity and serum metabolome. Circulation. 2013;127(3):340–348. doi: 10.1161/CIRCULATIONAHA.112.105551. [DOI] [PubMed] [Google Scholar]
- 34.Mul J.D., Stanford K.I., Hirshman M.F., Goodyear L.J. Exercise and regulation of carbohydrate metabolism. Prog Mol Biol Transl Sci. 2015;135:17–37. doi: 10.1016/bs.pmbts.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Cauter E., Spiegel K., Tasali E., Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papandreou C., Camacho-Barcia L., Garcia-Gavilan J., et al. Circulating metabolites associated with objectively measured sleep duration and sleep variability in overweight/obese participants: a metabolomics approach within the SATIN study. Sleep. 2019;42(5) doi: 10.1093/sleep/zsz030. [DOI] [PubMed] [Google Scholar]
- 37.Spriet L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014;44(Suppl 1):S87–S96. doi: 10.1007/s40279-014-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seelig E., Keller U., Klarhofer M., et al. Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: a prospective case-control study. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earnest C.P., Artero E.G., Sui X., Lee D.C., Church T.S., Blair S.N. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc. 2013;88(3):259–270. doi: 10.1016/j.mayocp.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuula L., Pesonen A.K., Kajantie E., et al. Sleep and lipid profile during transition from childhood to adolescence. J Pediatr. 2016;177:173–178. doi: 10.1016/j.jpeds.2016.06.026. e1. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Y., Chen R., Yu J. Sleep duration and abnormal serum lipids: the China Health and Nutrition Survey. Sleep Med. 2014;15(7):833–839. doi: 10.1016/j.sleep.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Kerkhofs M., Boudjeltia K.Z., Stenuit P., Brohee D., Cauchie P., Vanhaeverbeek M. Sleep restriction increases blood neutrophils, total cholesterol and low density lipoprotein cholesterol in postmenopausal women: a preliminary study. Maturitas. 2007;56(2):212–215. doi: 10.1016/j.maturitas.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Weljie A.M., Meerlo P., Goel N., et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc Natl Acad Sci U S A. 2015;112(8):2569–2574. doi: 10.1073/pnas.1417432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zivin J.A., Snarr J.F. An automated colorimetric method for the measurement of 3-hydroxybutyrate concentration. Anal Biochem. 1973;52(2):456–461. doi: 10.1016/0003-2697(73)90048-1. [DOI] [PubMed] [Google Scholar]
- 45.Brashear A., Cook G.A. A spectrophotometric, enzymatic assay for D-3-hydroxybutyrate that is not dependent on hydrazine. Anal Biochem. 1983;131(2):478–482. doi: 10.1016/0003-2697(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 46.Cedernaes J., Schonke M., Westholm J.O., et al. Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv. 2018;4(8):eaar8590. doi: 10.1126/sciadv.aar8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reutrakul S., Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann N Y Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355. [DOI] [PubMed] [Google Scholar]
- 48.Alnaji A., Law G.R., Scott E.M. The role of sleep duration in diabetes and glucose control. Proc Nutr Soc. 2016;75(4):512–520. doi: 10.1017/S002966511600063X. [DOI] [PubMed] [Google Scholar]
- 49.Kay D.B., Karim H.T., Hasler B.P., et al. Impact of acute sleep restriction on cerebral glucose metabolism during recovery non-rapid eye movement sleep among individuals with primary insomnia and good sleeper controls. Sleep Med. 2019;55:81–91. doi: 10.1016/j.sleep.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Y., Levin G.V., Donner T.W. Tagatose, a new antidiabetic and obesity control drug. Diabetes Obes Metabol. 2008;10(2):109–134. doi: 10.1111/j.1463-1326.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 51.Shechter A., O'Keeffe M., Roberts A.L., Zammit G.K., RoyChoudhury A., St-Onge M.P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R883–R889. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swift D.L., Johannsen N.M., Lavie C.J., Earnest C.P., Church T.S. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56(4):441–447. doi: 10.1016/j.pcad.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.