Abstract
Adolescence is an important stage for brain maturation. To investigate the effect of different exercise doses on inhibitory control in adolescents aged 12 to 14-year old, an after-school exercise program was offered 5 days per week for 12 weeks during a school semester. Thirsty-four adolescents (17 boys) from the first six classes were randomly divided into low-dose exercise group (LE group, one 30-min aerobic exercise bout per day, n = 16) and high-dose exercise group (HE group, two 30-min aerobic exercise bouts per day, n = 18), while 23 adolescents (10 boys) in the control group (CON, zero 30-min exercise bout, n = 23) were from the last two classes. All the participants in different classes received the same physical education with the same contents, duration, and intensity at class. All the participants completed flanker tests and cardiorespiratory tests before and after exercise intervention. The HE group showed more significant improvements on inhibitory control and than CON (p < 0.05). Changes in physical activity (PA) were significantly correlated with changes in interference scores (Spearman rho = -0.30, p < 0.05), (Spearman rho = 0.31, p < 0.05), and BF percentage (Spearman rho = -0.32, p < 0.05). This study demonstrated that effect of exercise on inhibitory control in adolescents is dose-dependent, which highlights the need to focus on the exercise dose in daily life for improving cognition among adolescents.
Keywords: Long-term exercise interventions, Cardiorespiratory fitness, Dose-response, Adolescents, Inhibitory control, Cognition, Physical activity
Abbreviations
- ANOVA
One-way analysis of variance
- BDNF
Brain-derived neurotrophic factor
- BF
Body fat
- BMI
Body mass index
- CON
Control group
- FFM
Free fat mass
- HE
High-dose exercise
- LE
Low-dose exercise
- MVPA
Moderate-to-vigorous physical activity
- PA
Physical activity
- PAQ-A
Physical Activity Questionnaire for Adolescents
- RCT
Randomized controlled trial
- RPE
Rate of perceived exertion
- rpm
Round per minute
- RT
Reaction time
Introduction
Physical activity (PA) has been established as a beneficial factor for health during adolescence, and is known to improve cardiorespiratory and muscular fitness, body composition, bone development, and cardiometabolic health.1 A growing body of evidence suggests that PA is also associated with improvement on cognition,2 and brain structure and function3 in children. However, according to a global survey report in 2016,4 84.3% of students (80.1% of boys and 89.1% of girls) aged 11–17 years did not perform sufficient PA in China. Like the findings in other countries, most adolescents in China did not meet current PA guidelines,4 which recommend 60 min or more of daily PA of moderate-to-vigorous intensity.1 Studies evaluating preschoolers,5 preadolescent children,6 adolescents7 and older adult people8 have shown positive impact of exercise on cognition, but some other studies reported different results as well.9
Exercise can increase cognition and brain function throughout life.8 Evidence indicates that brain structures like the prefrontal cortex,3 as well as the hippocampal volumes,10 and white matter integrity11 may show disproportionate plasticity due to exercise. However, the speed of cognition development varies with age. Executive control is a component of cognitive function, which is very important for success in academic achievement, vocation, and daily life.12 Executive control is a complex cognitive process involving inhibitory control, working memory and cognitive flexibility.13 One study showed that the development of inhibitory control occurred most rapidly at 7–8 years of age, dropped at 9–12 years, and slightly improved after 13–15 years.14 Watach et al. reported that PA showed no significant relationship with executive control in adolescents aged 11–17 years,15 and some randomized trials reported that exercise interventions did not lead to inhibitory control improvement in children.16 However, a meta-analysis of exercise studies in children showed a positive association between PA and inhibitory control among children and adolescents.17 Moreover, the existing studies on exercise psychology did not focus on evaluating the effects of different exercise doses on cognition in adolescents. To our knowledge, only one study evaluated the dose-response benefits of exercise on cognitive and achievement results in overweight children aged 7–11 years.18 However, a recent study on adolescents reported no effects on executive control and working memory after acute exercise, over durations of 10, 20, or 30 min.19 Thus, knowledge of the influence of long-term exercise interventions with different exercise doses on adolescents’ cognitive responses is still quite limited.
To address the hypothesis that exercise promotes cognition in adolescents, we administered an after-school exercise program with a modified flanker test to examine whether an aerobic exercise intervention could improve the inhibitory control in adolescents aged 12-14. The secondary hypothesis was that a higher dose of exercise training would lead to a greater effect on inhibitory control in adolescents.
Material and methods
Participants
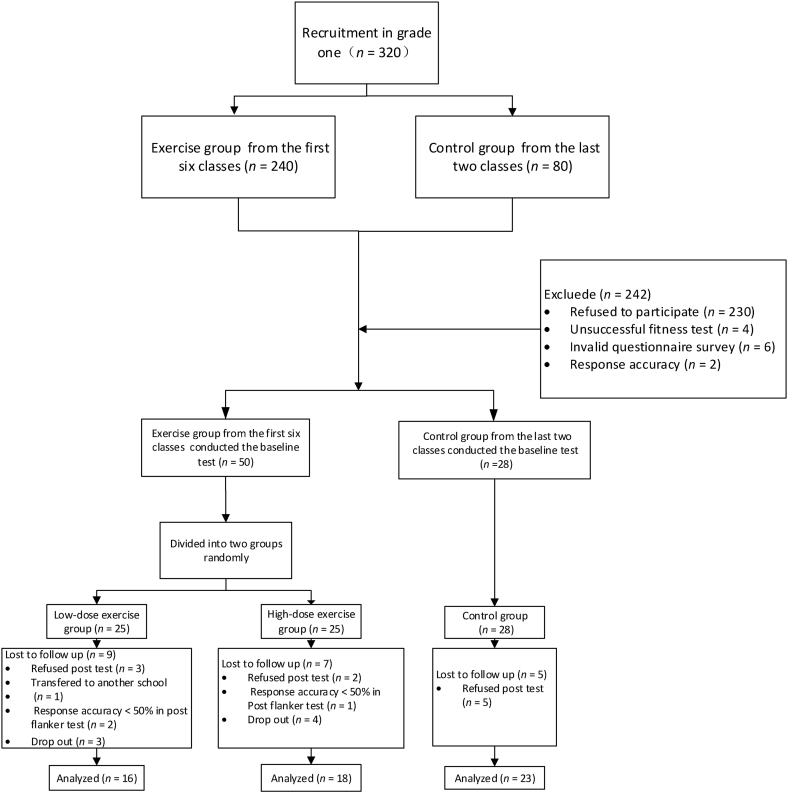
Participants were recruited from a junior high school in Beijing, China during 2014–2015 for a trial evaluating the effects of aerobic exercise on adolescents’ health. A total of 320 teenagers in eight classes were recruited from among the first-grade students. The first six classes were on the first floor, and the last two classes were on the second floor. To avoid mutual interference between the two experimental groups and the control group, the two exercise groups were from the first six classes, while adolescents in the control group were from the last two classes. After the baseline test, 78 eligible adolescents were included in the study. Fifty adolescents (25 males and 25 females) were in exercise groups, while 28 adolescents (13 males and 15 females) in the control group. All participants were required to be right-handed with normal uncorrected or corrected visual acuity and no history of nervous and mental diseases. Exclusion criteria for all participants were as follows:(i) heart disease or other chronic diseases, (ii) bone and joint disorders affecting exercise, and (iii) resting blood pressure over 180/110 mmHg. Lack of time for this after-school exercise program was the most important reason for refusal to participate in the study. Since adolescents in China have to deal with a lot of homework and tuition classes after a school day, over 70% (n = 230) of the students refused to enter our exercise program. Twelve teenagers were excluded for other reasons, including an unsuccessful fitness test (n = 4), invalid questionnaire survey (n = 6) or response accuracy < 50% (n = 2). The school administration provided permission for the study, and the study protocol was approved by the Ethics Committee of the Beijing Sport University (China). All participants were provided a written informed consent form to be completed by their parents. Testing and intervention were conducted at this school. The participant flow diagram is presented in Fig. 1.
Fig. 1.
Experimental flow diagram.
Evaluation of cognitive inhibition (flanker test)
A modified Eriksen flanker task20,21 was used to measure the inhibitory control of adolescents. All the tests were completed on the computer with the Prime 2.0 software. Before the test, the researchers, who had received unified training, explaining the experimental requirements to the participants with similar sentences. During the task, participants were asked to respond to the symbols on a screen. Each stimulus consisted of 5 arrows in a row (one target at the center and two flankers on either side). These two response-eliciting stimuli were flanked by an array of other arrows that were congruent (>>>>> or <<<<<), or incongruent (>><>>or <<><<) to the centrally placed target stimulus. An arrow pointing to the right “>” required the participant to press “j” with the right hand, and an arrow pointing to the left “<” required the participant to press “f” with the left hand on the keyboard. Targets pointed to the left or the right appeared randomly with equal probability. Participants completed one practice block of 20 trials to identify any problem they had in understanding and performing the test. If this test did not yield satisfactory results, another practice block was completed, and this was repeated until the tester was certain that the participant had understood the task correctly. This was followed by two blocks consisting of 50 trials each. The total reaction time was 1350 ms (ms), including a 120-ms stimulus presentation interval. Participants were instructed to respond as quickly and as accurately as possible. The outcomes for the test were reaction time (RT) to the stimulus of the arrow on screen (ms) and accuracy (percentage of possible trials where the correct response was selected). To test the effects of exercise on interference control, we defined the interference score (RTincongruent- RTcongruent) as the primary outcome measure. Since the interference score is a summary measure and exercise can affect several components of executive functioning (e.g., attention and inhibition processes) as well as general processing speed, we also defined RT in the congruent and incongruent trials separately as primary outcome measures.22 If the accuracy of the responses was less than 50%, the participant's data were excluded. A pilot study was conducted prior to the baseline testing on the study age group, and the response accuracy was over 80% in the incongruent trials.
Fitness testing
Fitness testing was conducted using a cycle ergometer ( EC3000, CUSTO Med; Germany), an electronic, rate-independent ergometer. Participants performed maximal exercise tests by cycling at 50 W (25 W for girls) at the beginning, followed by increases of 25 W every 2 min until they could no longer maintain a pedal cadence of 50 rpm. Respiratory gas levels were measured using a gas metabolism analyzer (METALYZER-3B, Cortex; Germany). Volume and gas calibrations were conducted before each test. Output from the gas analyses (, CO2 production, ventilation, and respiratory exchange ratio) was averaged at 30 s-intervals. Heart rate was measured directly from the electrocardiogram monitoring system. Rate of perceived exertion (RPE) values were obtained using the Cart and Load Effort Rating scale. was determined as the average value over the last 30 s during the maximal exercise test. Maximal effort was defined when at least two of the following criteria were met: (i) an oxygen plateau (with increasing work rate, increase in < 2 mL/kg/min); (ii) heart rate>200 beats per minute; (iii) respiratory exchange ratio >1.0; and (iv) participants asked to stop or an emergency appeared.
Physical activity level survey
The subjective amount of PA during the last 7 days was assessed using the China form of the Physical Activity Questionnaire for Adolescents (PAQ-A). The PAQ-A is a 7-day recall questionnaire containing 8 items that are used to assess general PA levels during the school year. The PAQ-A was designed for large-sample studies and can be completed in a classroom setting. It takes approximately 8–10 min for a student to complete the PAQ-A, and each item is scored on a 5-point scale. The mean score of these 8 items is the final PAQ-A activity summary score (PA score). A score of 1 indicates low PA, whereas a score of 5 indicates high PA. The China form of PAQ-A showed good internal consistency and acceptable validity, and was been widely used in China.23
Body composition assessment
We assessed several body composition parameters, including body mass index (BMI), body fat (BF) percentage determined by the bioelectrical impedance method (J20, Inbody; Korea), and fat free mass (FFM) (total weight minus fat weight).
Experimental procedure
A random number table method was used to divide the 50 adolescents into a low-dose exercise group (LE group, 30 min of aerobic exercise per day, one 30-min exercise bouts) and a high-dose exercise group (HE group, 60 min of aerobic exercise per day, two 30-min exercise bouts). Adolescents assigned to the control condition were asked to continue their usual activities (CON, zero 30-min exercise bouts). All the participants in different classes received the same physical education with the same contents, durations and intensity at class. The adolescents' participation in the experiment and the final completion of the intervention process are presented in Fig. 1.
Exercise intervention program
The aerobic exercise program was offered 5 days per week after school for 12 weeks during a school semester. The exercise conditions were equivalent in intensity and types, but differed only in the duration of daily exercise. Participants in the two exercise groups started an exercise class each afternoon after school. The activities were determined in advance. The emphasis was on intensity, enjoyment, variety, and safety. The exercise program mostly consisted of active games involving running and chasing, such as modified football, basketball, and volleyball games, rope ladder practice, dancing, and jumping rope. During the first 30-min bout, adolescents in the two exercise groups would exercise together. Then, the LE group ended the exercise training and returned to their regular schedule, while the HE group started the next 30-min bout after a 10-min break. Exercise intensity was evaluated by using a heart rate monitor (RX800CX, Polarsportstester; Finland) and subjective sensation. In each training class, six students in each group were selected to wear a heart rate monitor during exercise in order to ensure the intensity was moderate to vigorous, i.e., over 50% of the heart rate reserve for each student. A variety of small gifts, such as pens and notebooks, were used to encourage students to keep exercising. The exercise intervention program was led by professional researchers. Parents and teachers also cooperated with the researchers to motivate students to exercise.
Statistical analyses
Statistical analysis was performed using SPSS version 16.0 for Windows (SPSS, Chicago, IL). An α level of 0.05 was used as the criterion for statistical significance. Normality characteristics were determined via the Kolmogorov–Smirnov test. Within-group analysis was performed using a paired t-test. One-way analysis of variance was used to compare the differences in changes (Post minus Pre) among the groups for normally distributed data, and Kruskal-Wallis test was used for non-normally distributed data. Spearman correlation coefficients (rho) were used to explore the correlations between increments of PA level and increments in inhibitory control.
Results
Demographic data
Participant characteristics are depicted in Table 1. The participants in different groups showed no significant differences in age, height, weight, body mass index, BF percentage, PA score, and gender composition at baseline (p > 0.05). Participants in the two exercise groups also showed no significant difference in attendance rates (p > 0.05). However, the PA increase per day in the LE group was significantly lower than in the HE group (t = -4.23, p < 0.01).
Table 1.
Participant characteristics.
| Variables | CON (n=23) | LE (n =16) | HE (n =18) | Statistics | p-value |
|---|---|---|---|---|---|
| Age (years) | 12.70±0.56 | 12.50±0.52 | 12.94±0.73 | F=2.31 | 0.11 |
| Gender (F/M) | 13/10 | 8/8 | 9/9 | X2=0.23 | 0.89 |
| ANTHROPOMETRICS | |||||
| Height (cm) | 162.03±5.72 | 162.11±8.41 | 161.87±6.99 | F=0.01 | 0.99 |
| Weight (kg) | 51.54±9.10 | 51.69±8.01 | 56.02±8.65 | F=1.60 | 0.21 |
| BMI (kg/m2) | 19.62±3.26 | 19.61±2.28 | 21.36±2.89 | F=2.24 | 0.12 |
| BF (%) | 21.23±8.18 | 21.64±7.52 | 24.86±9.39 | F=1.05 | 0.36 |
| FFM (kg) | 40.22±5.73 | 40.35±6.39 | 41.83±6.91 | F=0.38 | 0.69 |
| FITNESS | |||||
| PA score | 2.63±0.65 | 2.50±0.97 | 2.56±0.78 | F=0.09 | 0.92 |
| V̇O2peak (ml/kg/min) | 34.58±8.57 | 32.60±7.64 | 31.61±5.60 | F=0.85 | 0.44 |
| FLANKER TEST | |||||
| Accuracy, congruent (%) | 100.0 (88.0,100) | 98.0 (92.0,100) | 100.0(94.0,100) | X2=2.65 | 0.27 |
| RT, congruent (ms) | 443.02±63.30 | 387.20±60.67 | 404.79±43.70 | F=4.96 | 0.011∗ |
| Accuracy, incongruent (%) | 92.0 (74.0,100.0) | 82.0 (52.0,96.0) | 90.0(60.0,100.0) | X2=7.88 | 0.019∗ |
| RT, incongruent (ms) | 499.35±51.12 | 459.96±60.49 | 497.58±73.76 | F=2.27 | 0.11 |
| interference score (ms) | 56.33±33.82 | 72.75±24.96 | 92.79±36.12 | F=6.39 | 0.003∗∗ |
| attendance (%) | / | 57.2±22.7 | 53.5±20.1 | t=0.51 | 0.61 |
| change in PA (min per day) | 0 | 12.53 (5.26,18.60) | 23.05(10.51,41.24) | X2=48.07 | <0.001∗∗ |
∗∗p < 0.01.
, peak oxygen consumption evaluated by the maximal exercise test.
Normally distributed data: mean ± standard deviation (SD), one-way analysis of variance (ANOVA).
Non-normally distributed data: median (min, max), KruskalWallis test. BMI, body mass index; BF, body fat; FFM, fat free mass; PA, physical activity; RT, reaction time.
Effects of exercise on behavior in the flanker task
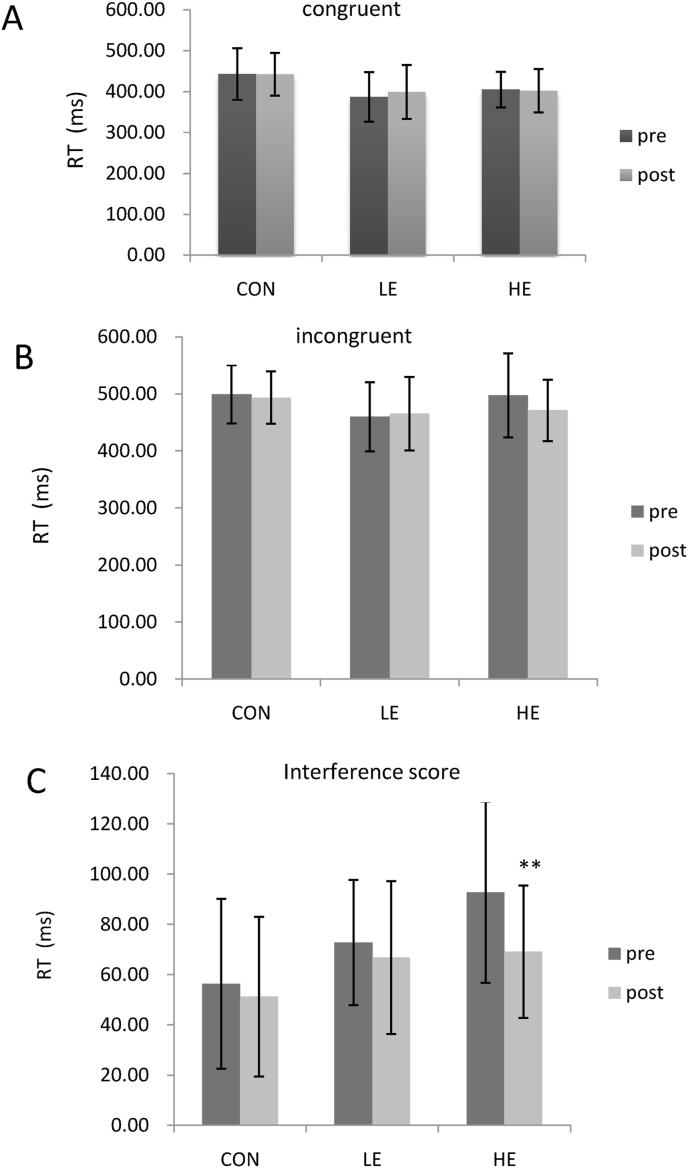
Fig. 2 presents the findings for the primary outcome in the flanker task pre- and post-exercise intervention. The interference score significantly decreased only in the HE group (t = 3.22, p < 0.01) after the exercise intervention. No significant within-group differences were observed in the RTs for congruent and incongruent conditions (p > 0.05).
Fig. 2.
Effects of group on primary outcome measures and reaction times (RTs) in (A) congruent and (B) incongruent trials, and the (C) interference score. Error bars represent standard deviations of the mean. ∗∗p < 0.01 (refers to paired -t tests). CON, control group; LE, low-dose exercise group; HE, high-dose exercise group.
Table 2 presents the changes in primary outcomes in the flanker task from baseline to 12 weeks in the three groups. Data were computed by determining the difference between post-exercise and pre-exercise measurements. The mean changes in the interference scores for the three groups were significantly different (F = 3.29, p = 0.045). In the post-hoc tests, the HE group showed a more significant drop than the control group (p = 0.022) and LE group (p = 0.043), while the LE group did not show a significant difference from the control group. The RTs in the congruent and incongruent conditions did not show significant between-group differences.
Table 2.
Change from baseline to 12 weeks in the flanker task (mean ± SD).
| variables | CON (n=23) | LE (n=16) | HE (n=18) | F | p-value |
|---|---|---|---|---|---|
| change in interference score (ms) | -5.14±23.83 | -5.99±17.17 | -23.70±31.26∗# | 3.29 | 0.045 |
| change in RT, congruent (ms) | −0.59±49.09 | 11.55±46.27 | −2.80±40.68 | 0.48 | 0.620 |
| change in RT, incongruent (ms) | −5.73±48.90 | 5.56±46.26 | −26.50±44.99 | 2.07 | 0.136 |
| change in BMI (kg/m2) | −0.26±0.49 | −0.61±0.39 | −0.38±0.69 | 2.05 | 0.14 |
| change in BF (%) | −0.87±2.84 | −2.19±3.42 | −2.58±2.30 | 2.02 | 0.14 |
| change in FFM (kg) | 0.33±1.76 | 0.38±1.72 | 1.40±1.69 | 2.26 | 0.11 |
| change in (mL/kg/min) | 2.05±4.39 | 6.08±5.07@ | 5.18±5.45& | 3.70 | 0.03 |
RT, reaction time; SD, standard deviations of the mean; BMI, body mass index; BF, body fat; FFM, fat free mass.
p values are for group differences assessed by one-way analysis of variance (ANOVA). For a given outcome measure, when ANOVA showed statistically significant findings (p < 0.05), all pairwise comparisons among groups were tested for statistical significance using the least significant difference test. The pairwise comparison that was significantly different is indicated in the following footnote.
∗ CON vs HE, p = 0.022.
# LE vs HE, p = 0.043.
@ CON vs LE, p =0.015.
& CON vs HE, p =0.049.
The mean changes in the values for the 3 groups were significantly different (F = 3.70, p = 0.03). The post-hoc tests suggested that the LE (p = 0.015) and HE (p = 0.049) groups showed more significant improvements than the control group. However, the other variables showed no significant differences between the control and the two exercise groups.
Correlates of change
Table 3 shows significant intercorrelations of change scores for the main outcome variables. Changes in PA were significantly correlated with changes in interference scores (Spearman rho = -0.30, p < 0.05), (Spearman rho = 0.31, p < 0.05) and BF percentage (Spearman rho = -0.32, p < 0.05). In addition, changes in were significantly correlated with changes in BF percentage (r = -0.45, p < 0.01).
Table 3.
Bivariate correlations between changes (Δ) in the outcome variables from baseline to 12 weeks.
| ΔPA | Δ interference score | ΔRT, incongruent | ΔRT, congruent | Δ | ΔBMI | ΔBF% | |
|---|---|---|---|---|---|---|---|
| ΔPA | 1 | -0.30∗ | −0.16 | 0.02 | 0.31∗ | −0.17 | −0.32∗ |
| Δ interference score | -0.30∗ | 1 | 0.36∗∗ | -0.19 | -0.14 | 0.03 | 0.03 |
| ΔRT, incongruent | −0.16 | 0.36∗∗ | 1 | 0.85∗∗ | −0.05 | −0.01 | −0.12 |
| ΔRT, congruent | 0.02 | -0.19 | 0.85∗∗ | 1 | 0.03 | −0.02 | −0.14 |
| Δ | 0.31∗ | -0.14 | −0.05 | 0.03 | 1 | −0.35∗∗ | −0.45∗∗ |
| ΔBMI | −0.17 | 0.03 | −0.01 | −0.02 | −0.35∗∗ | 1 | 0.36∗∗ |
| ΔBF% | −0.32∗ | 0.03 | −0.12 | −0.14 | −0.45∗∗ | 0.36∗∗ | 1 |
∗p< 0.05.
∗∗p < 0.01.
ΔPA: spearman correlation; PA, physical activity.
Δ interference score; ΔRT, incongruent; ΔRT, congruent; Δ; ΔBMI; ΔBF%: Pearson correlation; RT, reaction time; BMI, body mass index; BF, body fat.
Discussion
The existing literature contains very little information regarding the influence of different exercise doses on adolescents’ cognitive responses. This study showed that healthy adolescents aged 12–14 who participated in an after-school exercise program over 12 weeks showed more favorable changes in cognitive function and cardiovascular fitness than did youths who maintained a regular lifestyle. Moreover, the findings indicated a dose-response relationship between the exercise dose and changes in inhibitory control in adolescents.
Overall, these findings are consistent with those describing obvious behavior changes due to long-term exercise interventions in children.24, 25, 26 They also add evidence of the dose-dependent nature of these responses, which is rare in exercise trials with children and adolescents. In our study, the biggest drop in the interference score was observed in the high-dose group. Another randomized, controlled trial also showed similar results highlighting a dose-response relationship between exercise and cognition.18 Furthermore, in that study, 3 months of aerobic exercise intervention (40 min per day) improved Planning scores in overweight 7- to 11-year-old children, but the post-intervention cognitive scores in the low-dose (20 min per day) group did not show significant differences from the control group.18
The current study supports a positive association between increased exercise amounts and improvements in inhibitory control. A cross-sectional study also found that adolescents’ attention capacity was positively associated with longer time spent in moderate or moderate-to-vigorous PA (MVPA) in free-living conditions.27 Another study including 4755 participants with both longitudinal and cross-sectional analyses observed that MVPA may be beneficial for attention processes in adolescence, especially in males.28 A randomized controlled trial also reported that exercise duration (minutes exercised over 26 weeks) was correlated with changes in visuospatial processing in people aged 65 years, but this relationship was fully mediated by the changes in cardiorespiratory fitness.29
Several facts require consideration when interpreting the findings. Brain-derived neurotrophic factor (BDNF) may play a central role in brain neuroplasticity by mediating changes in neurogenesis30 and synaptic density.31 One study showed that the BDNF may mediate the relationship between exercise and cognitive function.32 In animals, exercise has been consistently shown to induce upregulation of BDNF. Muscles secrete myokines that contribute to the regulation of hippocampal function.33 A recent review showed that exercised skeletal muscle could cleave and secrete FNDC5 and myokine cathepsin B, which mediated muscle-brain crosstalk and resulted in the upregulation of BDNF, resulting in beneficial effects on neurogenesis, learning, and memory.34 In humans, exercise has been suggested to promote an increase in serum BDNF levels.35 A meta-analytic review also indicated that acute exercise or regular exercise could increase BDNF levels (Hedges’ g = 0.28–0.58, p < 0.01).36 Furthermore, exercise could induce hippocampal cell proliferation and cell survival in adults and seniors, resulting in better learning and memory.37,38 Imaging techniques like MRI can also provide evidence for the influence of exercise on brain functional remodeling. Chaddock-Heyman L et al. found that 7–9-year-old children who participated in a 9-month after-school exercise intervention showed increased white matter microstructure in the genu of the corpus callosum, which plays a role in cognition,39 and that children in this exercise program also showed a reduction in the fMRI activation in the right anterior prefrontal cortex during incongruent flanker trials from pre-test to post-test.40 Moreover, evidence from an RCT also showed that aerobic exercise training could increase hippocampal volume and improve memory in older adults.41
Limitations
This study had several limitations. First, to reduce the mutual interference between the experimental group and the control group, we used different class floors as a means of spatial isolation instead of a RCT design, but this choice reduced the validity of our interpretation of the results. Second, only 57 adolescents were analyzed and the average attendance rate was only 50%, which greatly reduced the effect of exercise intervention. Third, we did not explore the mechanism underlying the dose-dependent relationship between exercise and inhibitory control. Further studies to evaluate these mechanisms are, therefore, warranted.
Conclusions
The findings of the present study support the results of previous reports and add evidence for a dose-response relationship between exercise and inhibitory control in healthy adolescents. Meanwhile, a certain amount of exercise also promotes the fitness of adolescents. In conclusion, for adolescents aged 12–14 years, the effect of exercise on cognitive function might be dose-dependent, highlighting the need to focus on the exercise dose in daily life to improve cognitive health.
Submission statement
This manuscript has not been published and is not under consideration for publication elsewhere. The publication of this manuscript has been approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, the manuscript will not be published elsewhere including electronically in the same form, in English or any other language, without the written consent of the copyright-holder.
Ethics statement
The study was conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and all procedures were approved by the ethics committee of Beijing Sport University. All participants received a written informed consent to be completed by their parents. Parents, students and teachers all agreed to participate in the study.
Authors’ contributions
ZZW and YW conceived the study and designed the experiment. XL managed communication with the schools, handled data collection, conducted final data analysis, and wrote the draft of the manuscript. XTL and DFL developed the adolescents’ assessment pre- and post-exercise interventions and led and monitored the adolescents during the exercise interventions. All the authors participated in refining the data analysis by means of group discussions, added sections to the manuscript, and revised the whole text up until final approval.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the contribution of Chun SUN (Department of Physical Education, Shandong Normal University, China) and Mengdie WANG (Beijing Sport University, China) who helped monitor the adolescents doing exercise. The authors also thank Dr. Xijuan LUO (Department of Physical Education, Sun Yat-sen University, China) for her advice on the statistical analyses, and Dr. Di CUI (Department of Physical Education, Hunan Univeristy, China) for her editorial contributions. The authors would also like to gratefully acknowledge the contributions of the adolescents and teachers in conducting this study. This work was supported by National Key Research and Development Program Major Prevention and Control Research on Chronic Non-communicable Diseases (2016YFC1300202).
References
- 1.Committee PAGA . US Department of Health and Human Services; Washington, DC: 2018. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. [Google Scholar]
- 2.Donnelly J.E., Hillman C.H., Castelli D., et al. Physical activity, fitness, cognitive function, and academic achievement in children: a systematic review. Med Sci Sports Exerc. 2016;48(6):1197–1222. doi: 10.1249/MSS.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herting M.M., Chu X. Exercise, cognition, and the adolescent brain. Birth Defects Res. 2017;109(20):1672–1679. doi: 10.1002/bdr2.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guthold Regina, Stevens Gretchen A., Riley Leanne M., C Bull Prof Fiona. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1·6 million participants. Lancet Child Adolesc Health. 2019;4(1):23–35. doi: 10.1016/S2352-4642(19)30323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Z., Lee J.E., Zeng N., Pope Z.C., Zhang Y., Li X. Home-Based Exergaming on preschoolers' energy expenditure, cardiovascular fitness, body mass index and cognitive flexibility: a randomized controlled trial. J Clin Med. 2019;8(10):1745. doi: 10.3390/jcm8101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai Z. Effects of soccer exercise and stop practice on executive function of school age children. J Chengdu Sport Univ. 2020;46:109–113. doi: 10.15942/j.jcsu.2020.05.017. [DOI] [Google Scholar]
- 7.Ludyga S., Gerber M., Herrmann C., Brand S., Pühse U. Chronic effects of exercise implemented during school-break time on neurophysiological indices of inhibitory control in adolescents. Trends Neurosci Edu. 2018;10:1–7. doi: 10.1016/j.tine.2017.11.001. [DOI] [Google Scholar]
- 8.Hillman C., Erickson K.A. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 9.Tarp J., Domazet S.L., Froberg K., Hillman C.H., Andersen L.B., Bugge A. Effectiveness of a school-based physical activity intervention on cognitive performance in Danish adolescents: LCoMotion—learning, cognition and motion – a cluster randomized controlled trial. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0158087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaddock L., Erickson K.I., Prakash R.S., et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaddock-Heyman L., Erickson K.I., Holtrop J.L., et al. Aerobic fitness is associated with greater white matter integrity in children. Front Hum Neurosci. 2014;8:584. doi: 10.3389/fnhum.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond A., Barnett W.S., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaddock L., Hillman C.H., Pontifex M.B., Johnson C.R., Raine L.B., Kramer A.F. Childhood aerobic fitness predicts cognitive performance one year later. J Sports Sci. 2012;30(5):421–430. doi: 10.1080/02640414.2011.647706. [DOI] [PubMed] [Google Scholar]
- 14.Levin Harvey S. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7(3):377–395. doi: 10.1080/87565649109540499. [DOI] [Google Scholar]
- 15.Watach A.J., Radcliffe J., Xanthopoulos M.S., Novick M.B., Sawyer A.M. Executive function impairments in adolescents with obesity and obstructive sleep apnea syndrome. Biol Res Nurs. 2019;21(4):377–383. doi: 10.1177/1099800419846411. [DOI] [PubMed] [Google Scholar]
- 16.Ludyga S., Koutsandréou F., Reuter E., Voelcker-Rehage C., Budde H. A randomized controlled trial on the effects of aerobic and coordinative training on neural correlates of inhibitory control in children. J Clin Med. 2019;8(2):184. doi: 10.3390/jcm8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue Y., Yang Y., Huang T. Effects of chronic exercise interventions on executive function among children and adolescents: a systematic review with meta-analysis. Br J Sports Med. 2019;53(22):1397–1404. doi: 10.1136/bjsports-2018-099825. [DOI] [PubMed] [Google Scholar]
- 18.Davis C.L., Tomporowski P.D., McDowell J.E., et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30(1):91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Berg V., Saliasi E., Jolles J., de Groot R.H.M., Chinapaw M.J.M., Singh A.S. Exercise of varying durations: No acute effects on cognitive performance in adolescents. Front Neurosci-Switz. 2018;12:672. doi: 10.3389/fnins.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–149. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- 21.Chaddock L., Erickson K.I., Prakash R.S., et al. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev Neurosci. 2010;32(3):249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehren A., Özyurt J., Lam A.P., et al. Acute effects of aerobic exercise on executive function and attention in adult patients with ADHD. Front Psychiatr. 2019;10:132. doi: 10.3389/fpsyt.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Wang Y., Li X.T., et al. Reliability and validity of physical activity questionnaire for adolescents (PAQ-A) in Chinese version. J Beijing Sport Univ. 2015;38(5):63–67. doi: 10.19582/j.cnki.11-3785/g8.2015.05.012. [DOI] [Google Scholar]
- 24.Hillman C.H., Pontifex M.B., Castelli D.M., et al. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics. 2014;134(4):e1063–e1071. doi: 10.1542/peds.2013-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S.R., Tseng C.L., Kuo S.Y., Chang Y.K. Effects of a physical activity intervention on autonomic and executive functions in obese young adolescents: a randomized controlled trial. Health Psychol. 2016;35(10):1120–1125. doi: 10.1037/hea0000390. [DOI] [PubMed] [Google Scholar]
- 26.Drollette E.S., Pontifex M.B., Raine L.B., et al. Effects of the FITKids physical activity randomized controlled trial on conflict monitoring in youth. Psychophysiology. 2017;55(3) doi: 10.1111/psyp.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanhelst J., Béghin L., Duhamel A., et al. Physical activity is associated with attention capacity in adolescents. J Pediatr Urol. 2016;168:126–131. doi: 10.1016/j.jpeds.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Booth J.N., Tomporowski P.D., Boyle J.M., et al. Associations between executive attention and objectively measured physical activity in adolescence: findings from ALSPAC, a UK cohort. Mental Health Phys Activity. 2013;6(3):212–219. doi: 10.1016/j.mhpa.2013.09.002. [DOI] [Google Scholar]
- 29.Vidoni E.D., Johnson D.K., Morris J.K., et al. Dose-Response of aerobic exercise on cognition: a community-based, pilot randomized controlled trial. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharfman H., Goodman J., Macleod A., Phani S., Antonelli C., Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Zagrebelsky M., Korte M. Form follows function: BDNF and its involvement in sculpting the?function and structure of synapses. Neuropharmacology. 2014;76:628–638. doi: 10.1016/j.neuropharm.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Erickson K.I., Miller D.L., Roecklein K.A. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrann C.D., White J.P., Salogiannnis J., et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metabol. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bente, Klarlund, Pedersen Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 35.Ferris L.T., Williams J.S., Shen C.L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 36.Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Praag H.V., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 38.Van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaddock-Heyman L., Erickson K.I., Kienzler C., et al. Physical activity increases white matter microstructure in children. Front Neurosci. 2018;12:950. doi: 10.3389/fnins.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaddock-Heyman L., Erickson K.I., Voss M.W., et al. The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Front Hum Neurosci. 2013;7:72. doi: 10.3389/fnhum.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson K.I., Voss M.W., Prakash R.S., et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]