Abstract
To examine the contralateral repeated bout effect (CL-RBE) on muscle damage markers and motor unit (MU) control strategies, seventeen healthy adults performed two bouts of 60 eccentric contractions with elbow flexor (EF group; n = 9) or index finger abductor (IA group; n = 8) muscles, separated by 1 week. All participants randomly performed eccentric exercise on either the right or left arm or hand muscles, and muscle damage markers and submaximal trapezoid contraction tests were conducted pre, post, 1- and 2-day post eccentric protocol. One week after the first bout, the same exercise protocol and measurements were performed on the contralateral muscles. Surface electromyographic (EMG) signals were collected from biceps brachii (BB) or first dorsal interosseous (FDI) during maximal and submaximal tests. The linear regression analyses were used to examine MU recruitment threshold versus mean firing rate and recruitment threshold versus derecruitment threshold relationships. EMG amplitude from BB (bout 1 vs. bout 2 = 65.71% ± 22.92% vs. 43.05% ± 18.97%, p = 0.015, d = 1.077) and the y-intercept (group merged) from the MU recruitment threshold versus derecruitment threshold relationship (bout 1 vs. bout 2 = −7.10 ± 14.20 vs. 0.73 ± 16.24, p = 0.029, d = 0.513) at 50% MVIC were significantly different between two bouts. However, other muscle damage markers did not show any CL-RBE in both muscle groups. Therefore, despite changes in muscle excitation and MU firing behavior, our results do not support the existence of CL-RBE on BB and FDI muscles.
Keywords: Muscle damage, Repeated bout effect, Cross-over effect, Motor unit behavior
Abbreviation
- ANOVA
Analysis of Variance
- BB
Biceps Brachii
- CL-RBE
Contralateral Repeated Bout Effect
- CNS
Central Nervous System
- CSA
Cross Sectional Area
- EF
Elbow Flexor
- EMG
Electromyography
- FDI
First Dorsal Interosseous
- FPB
Flexor Pollicis Brevis
- IA
Index Finger Abductor
- MCP
Metacarpophalangeal
- MU
Motor Unit
- MVIC
Maximal Voluntary Isometric Contraction
- RBE
Repeated Bout Effect
- RMS
Root Mean Square
- ROM
Range of Motion
- RPE
Rating of Perceived Exertion
- SD
Standard Deviation
- VA
Voluntary activation
- VAS
Visual Analog Scale
Introduction
Muscle-damaging eccentric exercise can provide potential acute adaptation (e.g., protective effect) that attenuates changes in the indirect muscle damage markers in the similar subsequent exercise bout.1, 2, 3 This is referred to as the repeated bout effect (RBE), and it is characterized by the dampened muscle damage and faster recovery of indirect muscle damage markers in the exercised muscles after the second exercise bout.2,4,5 In addition, the RBE has been investigated with different exercise types and muscles,6 intensities,7 exercise volumes,8 and age groups9,10 to identify the potential underlying mechanisms.
In the recent decade, several studies revealed that this protective effect after eccentric exercise can also be transferred to the contralateral limb muscles (contralateral RBE; CL-RBE) which is not exercised during the initial muscle-damaging eccentric exercise.11, 12, 13 One of the possible mechanisms for CL-RBE is neural adaptation.5 Tsuchiya et al.12 reported that transverse relaxation time (T2), which reflects activation of muscle fibers, was longer in contralateral elbow flexors after the second bout when compared to the initial bout, suggesting that greater muscle activation can be one of the underpinned mechanisms for CL-RBE. In addition, Starbuck and Eston11 reported that electromyographic (EMG) median frequency decreased on the contralateral arm muscles after the second bout, suggesting a shift from the recruitment of high-threshold motor unit (MU) to the low-threshold MU and/or increased MU synchronization to protect subsequent muscle damage,2 during the second bout of eccentric exercise. Moreover, muscle-damaging eccentric exercise results in enhancements in MU synchronization14 and descending drive from the central nervous system (CNS).15 Thus, modulated CNS after eccentric exercise could alter MU behavior to protect from subsequent muscle-damaging exercise. However, the exact mechanisms for CL-RBE are still unclear.
Interestingly, the CL-RBE was mostly investigated in elbow flexors8,11,12,16,17 and knee flexors/extensors18, 19, 20 in previous studies, and one study examined CL-RBE on the flexor pollicis brevis (FPB).21 Ochi and colleague21 revealed that eccentric exercise can lead to dysfunction of nerve function and muscle damage, but did not induce any CL-RBE on hand muscle. To the best of our knowledge, only one study20 compared the CL-RBE of two different muscle groups (e.g., elbow flexors vs. knee flexors), and revealed that CL-RBE was smaller for the knee flexor muscles than for the elbow flexor muscles, suggesting that the magnitude of the CL-RBE may be different among muscle groups. In addition, there are differences in the physiological properties between biceps brachii (BB) and first dorsal interosseous (FDI) muscles. The BB is a relatively larger muscle, and it has distinct MU control strategies for generating force when compared to the FDI muscle, a relatively smaller muscle.22, 23, 24, 25 Furthermore, Stål et al.26 revealed that the BB muscle has a greater and lower percentage of type Ⅱ (type Ⅱa + type Ⅱb) and type Ⅰ muscle fibers than the FDI muscle does, respectively. Different proportions of muscle fiber types could influence MU firing properties, such as a less negative relationship between MU recruitment vs. mean firing rate in individuals with a higher percentage of type I MHC isoform content, when compared to individuals with a higher percentage of type II MHC isoform content.27 Thus, comparison of CL-RBE on arm and hand muscles may provide insight into the differences in the neural mechanisms of CL-RBE. Therefore, the purposes of this study were 1) to examine the CL-RBE on indirect muscle damage markers after a subsequent muscle-damaging exercise bout in BB and FDI muscles and compare the magnitude of CL-RBE between these muscles, 2) to identify the neural mechanisms for CL-RBE with motor unit firing properties. We hypothesized that 1) the changes in indirect muscle damage markers would be attenuated in the contralateral BB and FDI muscles after the second bout, and 2) the magnitude of CL-RBE and motor unit control strategies would be different between BB and FDI muscles.
Materials and methods
Subjects
Seventeen healthy men (n = 13, mean ± SD: age = 25.5 ± 4.6 years, height = 178.7 ± 6.2 cm, weight = 81.4 ± 9.3 kg) and women (n = 4, mean ± SD: age = 21.5 ± 2.4 years, height = 169.9 ± 6.5 cm, weight = 59.8 ± 7.5 kg) participated in this study. All the subjects were physically-active but did not have a consistent training routine (aerobic or resistance exercise training). They also did not have neuromuscular, musculoskeletal, or cardiovascular diseases or disorders within six months prior to the investigation. The University Institutional Review Board (protocol number: 19–079) approved the experimental procedures, and all experimental procedures were in conformity with the policy statement regarding the use of human subjects by the Helsinki Declaration of 1975, as revised in 2008. Before any experimental procedures, each subject completed a consent form and a pre-exercise health and exercise status questionnaire. During the consenting process, the subjects were asked to keep their normal activity regarding food intake, hydration status, and sleep, and to avoid vigorous physical activities or training during the entire study.
Experimental design
This study used a between-group design to compare the magnitude of the potential CL-RBE of the BB and FDI muscles. Seven separate visits to the laboratory were required to complete this investigation. The subjects were randomly assigned into the elbow flexion exercise group (EF group; n = 9) and the index finger abductor exercise group (IA group; n = 8). After the first visit as the familiarization, all the subjects performed submaximal eccentric exercise (six sets of 10 eccentric contractions) with a randomly chosen (dominant or non-dominant) elbow flexor or index finger muscles (Visit 2). Before (Pre), immediately after (Post), 1 day (24-h post: Visit 3), and 2 days (48-h post: Visit 4) after the exercise, the examinations of the responses of the indirect muscle damage markers were conducted. With a rest interval of one week, all subjects performed the exact same exercise protocols with the contralateral muscle groups (Visit 5), and the same indirect muscle damage markers were measured at the same time points (Visits 6 to 7) as during the first bout.
Procedures
All subjects visited the laboratory to be introduced to the procedures of the study, and they were familiarized with the maximal voluntary isometric contraction (MVIC) test, submaximal isometric trapezoid contractions at 30% and 50% MVIC, muscle soreness measure (visual analog scale: VAS), Borg 6 to 20 Rating of Perceived Exertion (RPE) scale, ROM testing, as well as the muscle-damaging eccentric exercise during the familiarization. At least 24 h after the familiarization visit, the subjects returned to the laboratory (Visit 2), and they performed three MVICs with the designated muscle group (EF group or IA group). For the EF group, the subjects were seated in an upright position in a chair. The elbow was placed onto a square-shaped pad, which made the elbow joint angle 90°, and the wrist was wrapped with a cuff connected to a steel frame with a force transducer (Model SM-500; Interface, Scottsdale, Arizona, USA). With this isometric setup, the subjects were instructed to perform isometric contraction at about 50% of the perceived maximal effort to warm-up followed by three, 5-s isometric contraction as hard as they could (Fig. 1a). For the IA group, the subjects sat in a chair. Both forearms and wrists were secured with the Velcro® straps to avoid the involvement of other muscles and were placed in the position directly in front of the body. In addition, both hands were placed onto a custom-made index finger abduction device with a neutral hand position (palms facing each other). The mid, ring, and little fingers were tied together and placed away from the index finger to avoid involvement in FDI muscle MVIC testing. The thumb was secured at an approximately 90° angle to the index finger, and the index finger was located under a brass tube attached perpendicularly to a Mini Beam force transducer (Model MB-100; Interface, Scottsdale, AZ, USA). Thus, during the MVIC, the subjects used an index finger to push up three times for 5-s against the brass tube as hard as they could (Fig. 1b). During the MVIC testing, the investigator verbally encouraged the subjects, and at least 1-min of rest was provided between each trial. The highest 1-sec portion of the MVICs was selected as the BB or FDI muscle's maximal strength. To compare the strength level between two muscles, the relative isometric strength values from BB and FDI muscles at the Post, 24-h post, and 48-h post muscle-damaging eccentric exercise were calculated as the percentages of the Pre-value (100%).
Fig. 1.
The set up for maximal isometric testing of (a) biceps brachii (BB) and (b) first dorsal interosseous (FDI) muscles.
Following the MVIC testing, all subjects performed submaximal isometric trapezoid contractions of the EF or IA at 30% and 50% of the pre-determined MVIC. With the same setup as the MVIC testing, the subjects contracted the designated muscle group against the immovable setup in a “ramp-up, hold, and ramp-down” manner. A computer monitor was provided to display the target force template, as well as the subjects’ real-time force output. Specifically, the subjects gradually increased the force output from 0% (rest) to 30% MVIC for 3 s (10% MVIC per second), held it for 10 s, and then gradually decreased it to 0% MVIC for 3 s. The same force increasing/decreasing rate was used when they performed isometric trapezoid contraction at 50% MVIC.
At least 5 min after the submaximal isometric trapezoid contractions, all subjects then performed 6 sets of 10 muscle-damaging eccentric exercises with the load equivalent to 50% of the pre-MVIC. For the EF group, the subjects sat in front of a preacher curl bench with the selected elbow placed onto the elbow support, and flexed their elbow (starting position: 130° of elbow angle) with the hand in a supinated position. Prior to the eccentric exercise, the subjects were provided an auditory tempo from a smartphone app (Metronome; Gismart, London, United Kingdom) with a 2-s up/2-s down rhythm. Following the tempo, the investigator released the weight to the subject's hand, and the subject gradually extended their elbow (0° of elbow angle) eccentrically in a controlled manner. Then, the weight was lifted by the investigator to the starting position. For the IA group, the subjects were seated in a chair with the forearm and wrist secured with the Velcro® straps. The exercised hand with a pronated position was placed onto a custom-made eccentric IA device, and the non-exercised hand was placed and relaxed on the subjects' thighs. The mid, ring, and little fingers were strapped together, and the thumb was secured at an approximately 40° angle relative to the index finger using the Velcro® straps and a bolt. The index finger was placed in an abduction position (starting position: 50° of metacarpophalangeal [MCP] joint angle), and was wrapped with a finger splint (Finger Splint Brace, Tuanyue, Hebei, China), connecting to a load from a custom-built pulley system (Fig. 2). For the index finger eccentric exercise, 1.5-s abduct/1.5-s adduct tempo was provided because of the smaller ROM of the MCP joint. Following the tempo, as the load was released, subjects tried to adduct their index fingers toward the mid finger direction (0° of MCP joint angle) in 1.5 s against the load in a controlled manner. Then, the investigator lifted the load so the subject's index finger returned to the starting position without the load. After each exercise set, the subjects were asked for the RPE using a Borg 6 to 20 RPE scale.28 At least 2-min was provided between consecutive sets during both eccentric EF and IA exercises, and the subjects were verbally encouraged during exercise.
Fig. 2.
The demonstration of eccentric exercise for first dorsal interosseous (FDI) muscle.
Measurements
Force
The force output was calculated by the tension applied to the force transducer during both maximal and submaximal isometric contraction testing. The force signals were then digitized with a 16-bit analog to digital converter (Model USB-6259; National Instruments, Austin, TX) and stored in the laboratory computer (Dell XPS 8900; Dell, Inc., Round Rock, TX, USA).
Muscle soreness (visual analog scale: VAS)
Both EF and IA groups recorded muscle soreness at Pre, Post, 24-h post, and 48-h post time points using a VAS. The VAS is a 100 mm line rating from “Not sore at all” at 0 mm to “Unbearable sore” at 100 mm. All subjects were asked to passively extend (or adduct) and flex (or abduct) the exercised elbow joint by the investigator several times and then subjectively indicate their overall soreness by a marking vertical line on the 100 mm line. The level of muscle soreness was quantified by measuring the distance from 0 mm to the vertical line marked by participants on the scale.
Range of motion (ROM)
Elbow joint and index finger MCP joint ROM were measured using an 8-inch goniometer (EMI Plastic Goniometer; Elite Medical Instruments, Inc., Fullerton, CA, USA) and a 6-inch goniometer (EMI 180-degree Goniometer, Elite Medical Instruments, Inc., Fullerton, CA, USA), respectively. The elbow joint ROM was determined as the difference in the joint angles between voluntary maximal flexion and extension of the elbow in the upright standing position. For the index finger MCP joint ROM, the subjects were asked to sit down, and the hand was placed on a table in a pronated position. The ROM was then measured as the difference between voluntary maximal abduction and the normal position of the index finger. At least three trials were performed. If there was more than two degrees difference between any two trials, additional ROM testing was conducted.29
Surface EMG acquisition and data analyses
For the EF group, the surface EMG signals were recorded using a bipolar surface electrode (DE 2.1 single differential surface EMG sensor, 10-mm interelectrode distance, Delsys, Inc., Natick, MA). This sensor was placed over the BB muscle belly based on the recommendations from SENIAM.30 In addition, a decomposition surface EMG sensor (dEMG sensor, Delsys, Inc., Natick, MA) consisting of 5 pins located on the corner and the center was also attached to the BB muscle belly. For the IA group, only the 5-pin decomposition surface EMG sensor was used due to the relatively small FDI surface muscle area. The reference electrode (Model USX2000; Axelgaard, Fallbrook, CA) was then attached to the seventh cervical vertebrae (C7). All electrodes were firmly fixed with medical tape. Prior to the application of the electrode on the designated muscle, skin surface was shaved and cleaned with rubbing alcohol. The collected bipolar EMG signals from maximal and submaximal isometric contractions were amplified (gain = 1000), and filtered with a high and low pass (20–450 Hz) filter using a Bagnoli 16-channel EMG system (Delsys, Inc., Natick, MA). Then, the filtered signals were digitized at a sampling rate of 20,000 Hz with a 12-bit analog-to-digital converter (Model USB-6259; National Instruments, Austin, TX). The amplitude of the surface EMG signal for BB and FDI muscles was calculated as the root-mean-square (RMS) of the selected mid-6-s plateau portion of the submaximal trapezoid contraction. All EMG RMS were normalized as the percentages of the highest EMG values from the maximal isometric strength tests.
Following the acquisition of the surface EMG signals, the four separated EMG signals from the 5-pin dEMG sensor were decomposed into constituent MU action potential trains using the dEMG analysis program (dEMG 1.1 Analysis, Delsys, Inc., Natick, Massachusetts). The signals from the mid-6-s plateau portion of the submaximal trapezoid contractions were selected to calculate the mean firing rate of each MU. In addition, the MU recruitment threshold and derecruitment threshold were defined as the first and last firing instances that occurred of the decomposed MU, respectively, and were identified with the percentage of the MVIC (%MVIC). Moreover, examinations were conducted to ensure the first and last firing instances were true firing events of all the decomposed MUs were performed using Decompose-Synthesize-Decompose-Compare (DSDC) test. Linear regression analyses were then used to calculate the linear slope coefficient and y-intercept of the MU recruitment threshold vs. mean firing rate relationship, as well as MU recruitment threshold vs. derecruitment threshold relationship during each 30% and 50% trapezoid contraction. The exclusion criterion included < 90% accuracy of decomposed MUs, and a low coefficient of determination (r2 < 0.60). The Pre and Post values of the normalized EMG RMS, and the linear slope coefficients, y-intercepts of recruitment threshold vs. mean firing rate and MU recruitment threshold vs. derecruitment threshold relationship were selected for further statistical analysis.
Statistical analyses
Assumptions of dependent variables for normality of distribution were confirmed with the Shapiro-Wilk test. Paired sample t-test was used to examine the Pre values of all dependent variables for the first and second bouts of eccentric exercise. A three-way (Set [1 vs. 2 vs. 3 vs. 4 vs. 5 vs. 6] × Bout [1st bout vs. 2nd bout] × Group [EF group vs. IA group]) mixed factorial analysis of variance (ANOVA) was used to compare the RPE during two bouts of eccentric exercise in both groups. To compare the isometric strength, muscle soreness, and ROM between two bouts in EF and IA groups, separate three-way (Time [pre vs. post vs. 24-h post vs. 48-h post] × Bout [1st bout vs. 2nd bout] × Group [EF group vs. IA group]) mixed factorial ANOVAs were used. Additionally, separate three-way (Time [pre vs. post] × Bout [1st bout vs. 2nd bout] × Group [EF group vs. IA group]) mixed factorial ANOVAs were used to examine the normalized EMG amplitude, linear slope coefficients, y-intercepts of the MU recruitment threshold vs. mean firing rate and MU recruitment threshold vs. derecruitment threshold relationships at 30% and 50% trapezoid contractions. The partial statistics were provided for all repeated measure comparisons, with values of 0.01, 0.06, and 0.14 corresponding to small, medium, and large effect sizes, respectively.31 In addition, Cohen's d was calculated for paired comparisons, with 0.2, 0.5, and 0.8 corresponding to small, medium, and large effect size, respectively.31 All data are reported as mean ± standard deviation (SD), and all statistical analyses were conducted using SPSS 24.0 (IBM SPSS statistics 24.0, IBM, Armonk, NY) with alpha set < 0.05.
Results
Pre-measurement of dependent variables
The baseline of dependent variables and comparisons are presented in Table 1. The paired sample t-test indicated no significant differences between the Pre-values of the first and second bouts for maximal isometric strength, ROM, and muscle soreness in both EF and IA groups.
Table 1.
Baseline differences between bout 1 and bout 2 in EF and IA groups.
| Pre – Bout 1 | Pre – Bout 2 | p | d | |
|---|---|---|---|---|
| Strength (N) | ||||
| EF group | 337.21 ± 118.27 | 332.79 ± 127.90 | 0.345 | 0.036 |
| IA group | 209.70 ± 57.18 | 207.45 ± 63.73 | 0.440 | 0.037 |
| ROM (°) | ||||
| EF group | 122.51 ± 6.44 | 121.61 ± 7.83 | 0.384 | 0.126 |
| IA group | 40.31 ± 8.37 | 40.38 ± 9.26 | 0.487 | 0.008 |
| Muscle soreness (mm) | ||||
| EF group | 0.56 ± 1.67 | 0.11 ± 0.3 | 0.233 | 0.374 |
| IA group | 0.38 ± 1.06 | 1.00 ± 2.02 | 0.201 | 0.384 |
Values are means ± SD. ROM: Range of motion, EF: elbow flexion, IA: index finger abductor, N: newtons.
RPE during eccentric exercise
The three-way ANOVA showed no three-way (p = 0.592), two-way (p > 0.05) interactions, or main effects for bout (p = 0.675) and group (p = 0.291), but there was a significant main effect for set (F = 36.44, p < 0.001, partial = 0.708). The follow-up pairwise comparisons showed significant RPE differences between any two sets, except exercise sets 4 vs. 5 and sets 4 vs. 6, indicating that the RPE gradually increased throughout exercise sets.
Changes in the dependent variables
Isometric strength
The three-way ANOVA for the absolute strength showed no time × bout × group interaction (p = 0.987), but there was a significant time × group interaction (F = 8.861, p < 0.001, partial = 0.371). The follow-up pairwise comparisons showed that isometric strength was significantly lower at each time point (Post, 24-h Post, and 48-h Post) when compared to Pre, and the strength value at 48-h Post was significantly greater than that at 24-h Post for the EF group. However, there was no difference between each time point (Pre, Post, 24-h Post, and 48-h Post) for the IA group. In addition, independent sample t-tests showed the EF group only had significantly greater absolute isometric strength than the IA group during the Pre. For better illustration, the changes in relative isometric strength in both groups were presented in Fig. 3.
Fig. 3.
The responses of relative isometric strength (% MVIC) before (Pre), after (Post), 1 day post (24-h Post), and 2 days post (48-h Post) eccentric exercise in elbow flexion (EF) group and index finger abductor (IA) group. ∗ Significant difference from Pre. # Significant difference between EF group and IA group.
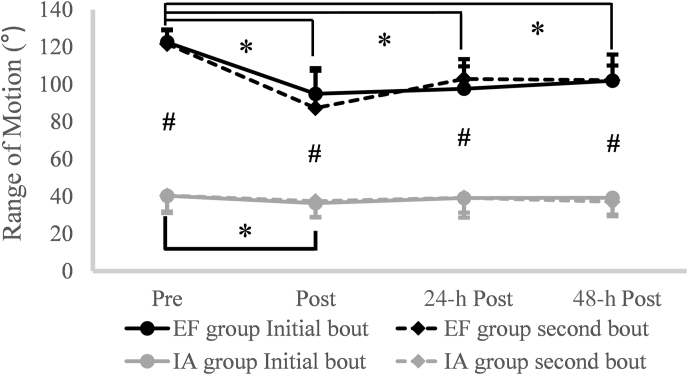
Range of motion (ROM)
Fig. 4 shows the changes in both groups’ ROM following two bouts of damaging eccentric exercise. The result from the three-way ANOVA showed no significant time × bout × group interaction (p = 0.116), but there was a significant time × group interaction (F = 27.823, p < 0.001, partial = 0.650). The follow-up pairwise comparisons showed that the ROM was significantly lower at each time point (Post, 24-h Post, and 48-h Post) when compared to Pre, and the ROM at 24-h Post and 48-h Post were significantly greater than that during the Post for the EF group. For the IA group, only the Post showed significantly less value than the Pre. In addition, independent sample t-tests showed the absolute ROM values were always significantly greater for the EF group than those for the IA group at each time point.
Fig. 4.
The responses of range of motion (ROM) at Pre, Post, 24-h Post, 48-h Post following eccentric exercise in elbow flexion (EF) group and index finger abductor (IA) group. ∗ Significant difference from Pre. # Significant difference between EF group and IA group.
Muscle soreness
Fig. 5 shows the responses of both groups’ muscle soreness values following two bouts of damaging eccentric exercise. For the changes in muscle soreness, the result from the three-way ANOVA indicated no time × bout × group interaction (p = 0.147), but there was a significant time × group interaction (F = 8.72, p < 0.001, partial = 0.368). The follow-up pairwise comparisons showed that soreness was significantly greater at each time point (Post, 24-h Post, and 48-h Post) when compared to Pre for both EF and IA groups. Additionally, when compared to the Post, the soreness values at both 24 Post and 48 Post significantly increased for the EF group. Lastly, independent sample t-tests revealed that the EF group had significantly greater muscle soreness values at 24-h post (EF group vs. IA group = 44.69 ± 10.27 vs. 22.88 ± 18.65, p = 0.004, d = 1.48) and 48-h post (EF group vs. IA group = 55.25 ± 10.41 vs. 30.06 ± 23.87, p = 0.006, d = 1.40) than those for the IA group.
Fig. 5.
The responses of muscle soreness (Visual Analog Scale: VAS) before (Pre), after (Post), 1 day post (24-h Post), and 2 days post (48-h Post) eccentric exercise in elbow flexion (EF) group and index finger abductor (IA) group. ∗ Significant difference from Pre. # Significant difference between EF group and IA group.
EMG amplitude during submaximal isometric trapezoid contractions
Table 2 shows the responses of both groups’ EMG parameters following two bouts of damaging eccentric exercise. For the EMG amplitude during 30% MVIC contraction intensity, the result from the three-way ANOVA indicated a significant time × bout × group interaction (F = 6.87, p = 0.019, partial = 0.314). The follow-up two-way (time × bout at each group) repeated measures ANOVA showed a significant time × bout interaction (F = 12.74, p = 0.007, partial = 0.614) for the EF group, but only significant time main effect (F = 8.35, p = 0.023, partial = 0.544) for the IA group. The pairwise comparisons revealed a significant increase in EMG amplitude from Pre to Post for the IA group. For the EF group, the follow-up paired sample t-test indicated significant increases in EMG amplitude from Pre to Post during bout 1 (Pre-vs. Post = 24.15% ± 9.68% vs. 65.71% ± 22.92%, p < 0.001, d = 2.362) and during bout 2 (Pre-vs. Post = 28.47% ± 9.01% vs. 43.05% ± 18.97%, p = 0.031, d = 0.982). In addition, EMG amplitude at Post was significantly lower during bout 2 than during bout 1 (bout 1 vs. bout 2 = 65.71% ± 22.92% vs. 43.05% ± 18.97%, p = 0.015, d = 1.077).
Table 2.
Before and after muscle-damaging eccentric exercise, changes in muscle activation and motor unit behavior during submaximal trapezoid contractions test at 30% and 50% MVIC.
| Initial bout |
Second bout |
||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| EMG amplitude (30%) | |||||
| (V) | EF group | 0.24 ± 0.10 | 0.66 ± 0.23# | 0.28 ± 0.09 | 0.43 ± 0.19∗# |
| IA group | 0.40 ± 0.05 | 0.49 ± 0.13 | 0.38 ± 0.16 | 0.51 ± 0.21 | |
| EMG amplitude (50%) | |||||
| (V) | EF group | 0.47 ± 0.15 | 0.82 ± 0.24 | 0.61 ± 0.19 | 0.90 ± 0.23 |
| IA group | 0.67 ± 0.12 | 0.71 ± 0.16 | 0.64 ± 0.20 | 0.72 ± 0.20 | |
| Slope coefficient (30%) | |||||
| (RT vs. MFR) | EF group | −0.52 ± 0.37 | −1.11 ± 0.42 | −0.73 ± 0.37 | −1.02 ± 0.26 |
| (pps) | IA group | −0.63 ± 0.33 | −0.93 ± 0.32 | −0.53 ± 0.28 | −0.88 ± 0.40 |
| y-intercept (30%) | |||||
| (RT vs. MFR) | EF group | 24.52 ± 8.75 | 35.46 ± 13.26 | 29.04 ± 10.08 | 35.73 ± 8.02 |
| (pps) | IA group | 20.74 ± 7.57 | 24.26 ± 6.67 | 18.92 ± 5.65 | 23.39 ± 2.80 |
| Slope coefficient (50%) | |||||
| (RT vs. MFR) | EF group | −0.63 ± 0.22 | −1.00 ± 0.59 | −0.65 ± 0.20 | −0.73 ± 0.32 |
| (pps) | IA group | −0.55 ± 0.37 | −0.65 ± 0.15 | −0.59 ± 0.35 | −0.45 ± 0.17 |
| y-intercept (50%) | |||||
| (RT vs. MFR) | EF group | 33.61 ± 6.51 | 37.85 ± 13.21 | 37.14 ± 10.37 | 32.73 ± 9.90 |
| (pps) | IA group | 24.79 ± 9.87 | 25.74 ± 6.77 | 26.53 ± 12.01 | 22.77 ± 6.48 |
| Slope coefficient (50%) | |||||
| (RT vs. DT) | EF group | 1.26 ± 0.45 | 1.51 ± 0.66 | 1.80 ± 0.65 | 1.16 ± 0.63 |
| (% MVIC DT) | IA group | 1.08 ± 0.40 | 1.22 ± 0.48 | 0.84 ± 0.17 | 0.86 ± 0.32 |
| y-intercept (50%) | |||||
| (RT vs. DT) | EF group | −16.73 ± 15.09 | −12.45 ± 15.26 | −38.73 ± 29.95 | −5.03 ± 16.39 |
| (% MVIC DT) | IA group | −3.65 ± 11.24 | 0.93 ± 7.95 | 3.37 ± 8.29 | 9.37 ± 12.63 |
Values are means ± SD. MVIC: maximal voluntary isometric contraction, EF: elbow flexion, IA: index finger abductor, EMG: electromyography, RT: recruitment threshold, MFR: mean firing rate, DT: derecruitment threshold, V: volte, pps: pulses per second. ∗ indicated significant difference between bout 1 and bout 2 at Post. # indicated significant difference between Pre and Post.
For the EMG amplitude during 50% MVIC contraction intensity, the result from the three-way ANOVA did not show time × bout × group interaction (p = 0.339), but there was a significant time × group interaction (F = 10.04, p = 0.006, partial = 0.401). The follow-up paired sample t-test indicated a significant increase in EMG amplitude from Pre to Post for the EF group (Pre-vs. Post-value = 54.01% ± 14.05% vs. 86.08% ± 17.09%, p = 0.001, d = 2.050) but not for IA group (Pre-vs. Post-value = 65.46% ± 8.66% vs. 71.79% ± 14.67%, p = 0.093, d = 0.526). In addition, the result from the independent sample t-test showed the EF group was lower in EMG amplitude at Pre than IA group (EF group vs. IA group = 54.01% ± 14.05% vs. 65.46% ± 8.66%, p = 0.033, d = 0.967), and was greater in EMG amplitude at Post than IA group (EF group vs. IA group = 86.08% ± 17.09% vs. 71.79% ± 14.67%, p = 0.043, d = 0.892).
Motor unit firing properties
Table 2 shows the responses of both groups’ MU firing properties following two bouts of damaging eccentric exercise. For the linear slope coefficients and y-intercepts for the MU recruitment threshold and mean firing rate relationship at 30% MVIC, a total of thirteen subjects were included for linear regression analysis, due to the quality of data. The three-way ANOVA for slope coefficients only showed a significant main effect for time (F = 34.66, p < 0.001, partial = 0.759), indicating that the slope coefficients were significantly more negative in Pre than in Post. For the y-intercepts, the three-way ANOVA revealed no three-way (p = 0.546) or two-way (time × group p = 0.266, bout × group p = 0.431, time × bout p = 0.701) interactions, but there was main effect for time (F = 9.72, p = 0.010, partial = 0.469), suggesting that the y-intercept was significantly greater in Post than in Pre-values.
For the linear slope coefficients and y-intercepts for the MU recruitment threshold and mean firing rate relationship during 50% MVIC, a total of fourteen subjects were included for linear regression analysis. The results from the three-way ANOVAs for slope coefficients revealed no three-way (p = 0.869) or two-way (time × group p = 0.133, bout × group p = 0.839, time × bout p = 0.081) interactions, as well as no main effects for time (p = 0.195), bout (p = 0.367), and group (p = 0.086). In addition, the results from the three-way ANOVAs for y-intercepts showed no three-way (p = 0.625) or two-way (time × group p = 0.743, bout × group p = 0.976, time × bout p = 0.115) interactions, as well as no main effects for time (p = 0.712) and bout (p = 0.804), but there was a singificant main effect for group (F = 8.555, p = 0.013, partial = 0.416), indicating that the y-intercepts were greater in the EF group than the IA group.
The slope coefficients and y-intercepts for the MU recruitment threshold and derecruitment threshold relationship during 30% MVIC were not analyzed due to the poor quality of MU data from both groups. For the linear slope coefficients and y-intercepts from the MU recruitment threshold and derecruitment threshold relationship at 50% MVIC, two subjects were excluded due to the poor quality of data. Thus, a total of fifteen subjects were included for linear regression analysis. The three-way ANOVA for slope coefficients showed no three-way (p = 0.178) or two-way (time × group p = 0.442, bout × group p = 0.101, time × bout p = 0.079) interactions, as well as no main effects for time (p = 0.762), bout (p = 0.363), but a significant main effect for group (F = 11.474, p = 0.005, partial = 0.469), indicating that the slope coefficients were greater in the EF group than the IA group. For the y-intercepts, the result from the three-way ANOVA indicated no three-way interaction (p = 0. 068), but there was a significant time × bout interaction (F = 4.83, p = 0.047, partial = 0.271). The follow-up paired sample t-tests revealed that the y-intercept was significantly lower in Pre than in Post during bout 2 (Pre-vs. Post-value = −21.89 ± 31.51 vs. 0.73 ± 16.24, p = 0.005, d = 0.902), and the y-intercept was significantly greater during bout 2 than during bout 1 at the Post time point (bout 1 vs. bout 2 = −7.10 ± 14.20 vs. 0.73 ± 16.24, p = 0.029, d = 0.513) (Fig. 6).
Fig. 6.
Change in y-intercept from relationship between motor unit recruitment threshold (RT) and decruitment threshold (DT) during 50% maximal voluntary isometric contraction (MVIC) of submaximal isometric trapezoid contraction collapsed across group at Pre and Post eccentric exercise during the initial and second bout. ∗ Significant difference between bout 1 and bout 2. # Significant difference between pre and post during bout 2.
Discussion
The main findings of this study were: (1) the first bout of eccentric exercise on BB and FDI muscles resulted in muscle damage, as evidenced by the changes in indirect muscle damage markers (i.e., strength loss, decreased ROM, and elevated muscle soreness); (2) muscle excitation (EMG amplitude) from the BB muscle was significantly different between the first and second bouts, but no differences in strength, ROM, and muscle soreness between two bouts; (3) the y-intercept of recruitment threshold vs. derecruitment threshold relationship at 50% MVIC was significantly different between two bouts after eccentric exercise; and (4) for the IA group, there were no differences between the two bouts for all indirect muscle damage markers.
In the current study, the results showed strength loss, decreased ROM, and increased muscle soreness after the first bout of eccentric exercise, which were generally in agreement with previous studies4,32,33 that examined indirect muscle damage markers after eccentric exercise. The eccentric exercise induces high stress to the skeletal muscle fibers and muscle damage due to the characteristics of eccentric contraction including; generating greater force, lower energy expenditure per unit, and recruiting fewer MUs and faster-twitch MUs when compared with concentric contraction.34 Such impairment in skeletal muscle is accompanied by changes in indirect muscle damage markers.4 The results of the current study showed that eccentric exercise-induced muscle damage in both BB and FDI muscles. However, the magnitude of muscle damage was greater in the BB muscle than the FDI muscle. The difference in the magnitude of muscle damage between two muscles could be due to the properties of the muscle fibers. Jones et al.35 examined morphological changes from BB and calf muscles after eccentric exercise, and they revealed that type Ⅱ muscle fibers from the calf muscle were more severely damaged by eccentric exercise when compared to type Ⅰ muscle fibers. These results indicate that type Ⅰ muscle fibers are less susceptible to muscle damage by muscle-damaging eccentric exercise. Since the FDI muscle has a greater proportion of type Ⅰ muscle fibers than the BB muscle,26 the difference in the proportion of muscle fiber could affect the magnitude of muscle damage.
The CL-RBE has been extensively investigated.8,12,13,20 Generally speaking, skeletal muscles adapt to damaging eccentric exercise and provide protective mechanisms on the exercised muscle in a subsequent muscle-damaging bout, but this protective effect can transfer to the related contralateral limb muscles by systemic mechanisms (e.g., neural and inflammatory adaptations).5 Tsuchiya et al.12 revealed that there was RBE for strength, ROM, muscle soreness, T2, and cross-sectional area (CSA) on contralateral BB muscle after two bouts of 30 eccentric EF exercises separated by 2 weeks. The current study showed that EMG amplitude in the BB muscle was significantly lower during submaximal isometric trapezoid contraction at 30% MVIC after bout 2 than after bout 1, indicating less muscle excitation in the contralateral BB muscle after the second bout of muscle-damaging eccentric exercise when compared to the initial exercise. In addition, the y-intercept of MU recruitment threshold vs. derecruitment threshold relationship at 50% MVIC (group merged) was significantly greater in the second bout than that of the first bout, indicating that MUs were derecruited at higher force levels than which they were recruited after the second bout. Eccentric exercise results in mechanical changes (e.g., sarcomere structure and optimal length, etc.),36 as well as elevated central fatigue (i.e. reduced voluntary activation [VA])37 and brain cytokines.38 These changes in the CNS may be involved to alter motor control strategies during and/or after exercise,39 and may affect MU behavior in the related contralateral limb. In addition, ipsilateral changes caused by exercise (e.g., concentric and eccentric exercise) in the skeletal muscle can be transferred to the contralateral side by neural adaptation (i.e., cross-education).40 The exact mechanism is still unclear, but the signal pathways of interhemispheric connections and/or cross-limb cortical interaction sites could help contribute to changes in muscle activation and MU control strategies in contralateral limb muscles.41
However, despite the changes in the neural activities on the contralateral BB muscle, other indirect muscle damage markers (strength, ROM, and muscle soreness) were not shown in the current study. This may be due to the magnitude of the neural adaptation after the first eccentric exercise bout at 50% MVIC. It has been reported that muscle-damaging eccentric exercise at 100% MVIC on one muscle in the initial bout produced a partial protective effect (approximately 50%) on the contralateral limb muscle against muscle damage during the second bout of exercise.20 However, in the current study, we performed 50% MVIC for muscle damaging eccentric exercise in the initial bout, and the relatively low intensity of exercise may be insufficient to provide the contralateral protective effect on other indirect muscle damage markers, despite changes in the neural factors. In fact, the current study showed about 40% and 20% strength loss for EF and IA group, respectively, after the initial bouts, whereas other studies showed at least a 45% decrease in strength.42,43 Thus, the intensity of the initial bout may not be enough to utilize the mechanism for each indirect muscle damage marker for inducing the protective effect. In addition, Xin et al.19 found the CL-RBE on strength and NF-kB activation after two bouts of 100 maximal eccentric knee extension repetitions separated by 4 weeks, but no CL-RBE on creatine kinase and muscle soreness. The results suggest that each indirect muscle damage marker may apply different mechanisms to produce the protective effect. The current study indicated no CL-RBE on indirect muscle damage markers with changes in the neural activities in the subsequent bout. Thus, it is possible that MU activity and other markers have different mechanisms for inducing the CL-RBE and/or change in MU activity may not be related to the CL-RBE.
It is interesting to note that there was no CL-RBE on indirect muscle damage markers for the FDI muscle. This may be due to the proportion of muscle fiber type in FDI muscle. The FDI muscle has a greater percentage of type Ⅰ muscle fiber when compared to the BB muscle,26 and the type Ⅰ muscle fibers are less susceptible to muscle damage.35 Previous studies9,44 reported that the non-protective effect may be due to insufficient muscle damage from the initial bout. In addition, Ochi et al.21 demonstrated no CL-RBE on hand muscle after two bouts of maximal eccentric exercise. Thus, rather than the intensity of muscle-damaging eccentric exercise (50% MVIC of eccentric exercise in the current study), the proportion of type Ⅰ muscle fibers in the FDI muscle could be a plausible reason for why no CL-EBE was shown.
Although this study had some interesting findings, it is necessary to mention that there are some limitations. First, we examined indirect muscle damage markers, but inflammatory responses after two bouts of exercise were not measured. Since the results provide evidence for the changes in neural factors, examining circulating factors (e.g., inflammatory responses) may provide further insight into the systemic effects of protective effects. Second, although our results showed that MU control strategies were changed in the subsequent bout, we cannot speculate any further because we were not able to examine the neural factors from spinal or supraspinal levels. Thus, future studies may be required to examine the central neuromuscular indices for CL-RBE. Third, in the current study, eccentric muscle action was conducted for 2 s and 1.5 s up (or abduct) and down (or adduct) tempo for BB and FDI muscles, respectively. Kuiper45 reported that speed of muscle contraction (e.g., lengthening) can be one of the factors that alter the magnitude of muscle damage. Thus, differences in contraction speed during eccentric exercise for CL-RBE could be explored in future research. Lastly, no CL-RBE may be due to the insufficient muscle damage from the initial bout of eccentric exercise at 50% MVIC in the current study. Ochi et al.21 also demonstrated no contralateral protective effect in hand muscle after maximal eccentric contraction. Since the magnitude of muscle damage can be one of the factors for CL-RBE, future studies may be required to perform supramaximal intensity for muscle-damaging exercise.
In conclusion, the current study showed that no evidence of the CL-RBE was shown in the contralateral BB muscle for indirect muscle damage markers including strength, ROM, and muscle soreness, in spite of changes in MU control strategies. This may help further understand the importance of the magnitude of muscle damage from the initial bout for CL-RBE: insufficient muscle damage may not provide a protective effect for the contralateral limb muscle, even if neurological factors are altered. In addition, our results indicated that the FDI muscle did not show any CL-RBE. This is probably due to the relatively low muscle damage from the initial bout caused by the proportion of the muscle fiber type. Thus, it can be considered to have a weaker or non-protective effect on muscles with a higher percentage of type 1 muscle fibers.
Conflict of interest
The current research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors. There were no conflicts of interest declared from authors for the completion of this project and manuscript.
Submission statement
The work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere including electronically in the same form, in English or any other language, without the written consent of the copyright-holder.
Authors' contributions
SJ and XY contributed to the design of the work; SJ, WMM, JSS, and XY contributed to the acquisition, analysis, and interpretation of data; SJ, WMM, JSS, and XY contributed to the draft of the work and revise it critically for important intellectual content; SJ, WMM, JSS, and XY approved the version to be submitted, and agreed to be accountable for all aspects of the work in ensuring that the questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval statement
The experimental procedures from this work were in accordance with the ethical standards of 1964 Helsinki declaration and its later amendments or comparable ethical standards. This research was approved by the University of Mississippi Institutional Review Board (protocol number: 19–079). Lastly, written informed consents were collected from participants.
Acknowledgments
Thanks to all authors who took time out of their schedules to help with this project.
Contributor Information
Sunggun Jeon, Email: sujeon@okstate.edu.
Xin Ye, Email: xye@hartford.edu.
References
- 1.Thompson H., Clarkson P., Scordilis S. The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand. 2002;174(1):47–56. doi: 10.1046/j.1365-201x.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- 2.McHugh M.P. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports. 2003;13(2):88–97. doi: 10.1034/j.1600-0838.2003.02477.x. [DOI] [PubMed] [Google Scholar]
- 3.Nosaka K., Newton M.J., Sacco P. Attenuation of protective effect against eccentric exercise-induced muscle damage. Can J Appl Physiol. 2005;30(5):529–542. doi: 10.1139/h05-139. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson P.M., Hubal M.J. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(11):S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Hyldahl R.D., Chen T.C., Nosaka K. Mechanisms and mediators of the skeletal muscle repeated bout effect. Exerc Sport Sci Rev. 2017;45(1):24–33. doi: 10.1249/JES.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 6.Chen T.C., Yang T.J., Huang M.J., et al. Damage and the repeated bout effect of arm, leg, and trunk muscles induced by eccentric resistance exercises. Scand J Med Sci Sports. 2019;29(5):725–735. doi: 10.1111/sms.13388. [DOI] [PubMed] [Google Scholar]
- 7.Chen T.C., Nosaka K., Sacco P. Intensity of eccentric exercise, shift of optimum angle, and the magnitude of repeated-bout effect. J Appl Physiol. 2007;102(3):992–999. doi: 10.1152/japplphysiol.00425.2006. [DOI] [PubMed] [Google Scholar]
- 8.Howatson G., Van Someren K., Hortobagyi T. Repeated bout effect after maximal eccentric exercise. Int J Sports Med. 2007;28(7):557–563. doi: 10.1055/s-2007-964866. [DOI] [PubMed] [Google Scholar]
- 9.Lavender A.P., Nosaka K. Responses of old men to repeated bouts of eccentric exercise of the elbow flexors in comparison with young men. Eur J Appl Physiol. 2006;97(5):619–626. doi: 10.1007/s00421-006-0224-7. [DOI] [PubMed] [Google Scholar]
- 10.Škarabot J., Ansdell P., Temesi J., et al. Neurophysiological responses and adaptation following repeated bouts of maximal lengthening contractions in young and older adults. J Appl Physiol. 2019;127(5):1224–1237. doi: 10.1152/japplphysiol.00494.2019. [DOI] [PubMed] [Google Scholar]
- 11.Starbuck C., Eston R.G. Exercise-induced muscle damage and the repeated bout effect: evidence for cross transfer. Eur J Appl Physiol. 2012;112(3):1005–1013. doi: 10.1007/s00421-011-2053-6. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya Y., Nakazato K., Ochi E. Contralateral repeated bout effect after eccentric exercise on muscular activation. Eur J Appl Physiol. 2018;118(9):1997–2005. doi: 10.1007/s00421-018-3933-9. [DOI] [PubMed] [Google Scholar]
- 13.Jeon S., Kang M., Ye X. Contralateral protective effect against repeated bout of damaging exercise: a meta-analysis. Res Sports Med. 2021:1–20. doi: 10.1080/15438627.2021.1954512. [DOI] [PubMed] [Google Scholar]
- 14.Dartnall T.J., Nordstrom M.A., Semmler J.G. Adaptations in biceps brachii motor unit activity after repeated bouts of eccentric exercise in elbow flexor muscles. J Neurophysiol. 2011;105(3):1225–1235. doi: 10.1152/jn.00854.2010. [DOI] [PubMed] [Google Scholar]
- 15.Duclay J., Martin A., Robbe A., et al. Spinal reflex plasticity during maximal dynamic contractions after eccentric training. Med Sci Sports Exerc. 2008;40(4):722–734. doi: 10.1249/mss.0b013e31816184dc. [DOI] [PubMed] [Google Scholar]
- 16.Newton M.J., Sacco P., Chapman D., et al. Do dominant and non-dominant arms respond similarly to maximal eccentric exercise of the elbow flexors? J Sci Med Sport. 2013;16(2):166–171. doi: 10.1016/j.jsams.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen T.C., Chen H.L., Lin M.J., et al. Contralateral repeated bout effect of eccentric exercise of the elbow flexors. Med Sci Sports Exerc. 2016;48(10):2030–2039. doi: 10.1249/mss.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 18.Connolly D.A., Reed B.V., McHugh M.P. The repeated bout effect: does evidence for a crossover effect exist? J Sports Sci Med. 2002;1(3):80. PMID 24701128. [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L., Hyldahl R.D., Chipkin S.R., et al. A contralateral repeated bout effect attenuates induction of NF-κB DNA binding following eccentric exercise. J Appl Physiol. 2014;116(11):1473–1480. doi: 10.1152/japplphysiol.00133.2013. [DOI] [PubMed] [Google Scholar]
- 20.Chen T.C., Lin M.J., Chen H.L., et al. Contralateral repeated bout effect of the knee flexors. Med Sci Sports Exerc. 2018;50(3):542–550. doi: 10.1249/mss.0000000000001470. [DOI] [PubMed] [Google Scholar]
- 21.Ochi E., Ueda H., Tsuchiya Y., et al. Eccentric exercise causes delayed sensory nerve conduction velocity but no repeated bout effect in the flexor pollicis brevis muscles. Eur J Appl Physiol. 2021;121(11):3069–3081. doi: 10.1007/s00421-021-04773-7. [DOI] [PubMed] [Google Scholar]
- 22.Kukulka C.G., Clamann H.P. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219(1):45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- 23.De Luca C.J. Control properties of motor units. J Exp Biol. 1985;115(1):125–136. doi: 10.1242/jeb.115.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Seki K., Narusawa M. Firing rate modulation of human motor units in different muscles during isometric contraction with various forces. Brain Res. 1996;719(1-2):1–7. doi: 10.1016/0006-8993(95)01432-2. [DOI] [PubMed] [Google Scholar]
- 25.Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol. 2005;30(3):341–351. doi: 10.1139/h05-126. [DOI] [PubMed] [Google Scholar]
- 26.Stål P., Eriksson P.O., Eriksson A., et al. Enzyme-histochemical differences in fibre-type between the human major and minor zygomatic and the first dorsal interosseus muscles. Arch Oral Biol. 1987;32(11):833–841. doi: 10.1016/0003-9969(87)90011-2. [DOI] [PubMed] [Google Scholar]
- 27.Trevino M.A., Herda T.J., Fry A.C., et al. Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. J Neurophysiol. 2016;116(2):552–562. doi: 10.1152/jn.01021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borg G. Human kinetics; Champaign, IL, USA: 1998. Borg’s Perceived Exertion and Pain Scales. [Google Scholar]
- 29.Killen B.S., Zelizney K.L., Ye X. Crossover effects of unilateral static stretching and foam rolling on contralateral hamstring flexibility and strength. J Sport Rehabil. 2019;28(6):533–539. doi: 10.1123/jsr.2017-0356. [DOI] [PubMed] [Google Scholar]
- 30.Hermans H., Freriks B., Merletti R., et al. European recommendations for surface electromyography: results of the SENIAM project. Roessingh Res Dev. 1999;48:51–52. [Google Scholar]
- 31.Cohen J. Academic Press Books-Elsevier; Cambridge: 1988. Statistical Power Analysis for the Behavior Science: Lawrance Eribaum Association. [Google Scholar]
- 32.Hunter A.M., Galloway S.D., Smith I.J., et al. Assessment of eccentric exercise-induced muscle damage of the elbow flexors by tensiomyography. J Electromyogr Kinesiol. 2012;22(3):334–341. doi: 10.1016/j.jelekin.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Philippou A., Koutsilieris M., Maridaki M. Changes in kinematic variables at various muscle lengths of human elbow flexors following eccentric exercise. J Muscle Res Cell Motil. 2012;33(3-4):167–175. doi: 10.1007/s10974-012-9314-9. [DOI] [PubMed] [Google Scholar]
- 34.Howatson G., Van Someren K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38(6):483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Jones D., Newham D., Round J., et al. Experimental human muscle damage: morphological changes in relation to other indices of damage. J Physiol. 1986;375(1):435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brockett C.L., Morgan D.L., Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001;33(5):783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Prasartwuth O., Taylor J., Gandevia S. Maximal force, voluntary activation and muscle soreness after eccentric damage to human elbow flexor muscles. J Physiol. 2005;567(1):337–348. doi: 10.1113/jphysiol.2005.087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmichael M.D., Davis J.M., Murphy E.A., et al. Role of brain IL-1β on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1344–R1348. doi: 10.1152/ajpregu.00141.2006. [DOI] [PubMed] [Google Scholar]
- 39.Taylor J.L., Amann M., Duchateau J., et al. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc. 2016;48(11):2294. doi: 10.1249/MSS.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kidgell D.J., Frazer A.K., Rantalainen T., et al. Increased cross-education of muscle strength and reduced corticospinal inhibition following eccentric strength training. Neuroscience. 2015;300:566–575. doi: 10.1016/j.neuroscience.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 41.Carroll T.J., Herbert R.D., Munn J., et al. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101(5):1514–1522. doi: 10.1152/japplphysiol.00531.2006. [DOI] [PubMed] [Google Scholar]
- 42.Chen T.C., Lin M.J., Lai J.H., et al. Low-intensity elbow flexion eccentric contractions attenuate maximal eccentric exercise-induced muscle damage of the contralateral arm. J Sci Med Sport. 2018;21(10):1068–1072. doi: 10.1016/j.jsams.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Chen T.C., Lin M.J., Chen H.L., et al. Muscle damage protective effect by two maximal isometric contractions on maximal eccentric exercise of the elbow flexors of the contralateral arm. Scand J Med Sci Sports. 2018;28(4):1354–1360. doi: 10.1111/sms.13042. [DOI] [PubMed] [Google Scholar]
- 44.McHugh M.P., Pasiakos S. The role of exercising muscle length in the protective adaptation to a single bout of eccentric exercise. Eur J Appl Physiol. 2004;93(3):286–293. doi: 10.1007/s00421-004-1196-0. [DOI] [PubMed] [Google Scholar]
- 45.Kuipers H. Exercise-induced muscle damage. Int J Sports Med. 1994;15(3):132–135. doi: 10.1055/s-2007-1021034. [DOI] [PubMed] [Google Scholar]