Abstract
Evidence-based consensus suggests that physical activity and regular exercise training can reduce modifiable risk factors as well as rate of mortality and morbidity in patients with chronic diseases, such as cardiovascular disease (CVD), diabetes, obesity and cancer. Conversely, long-term exercise training and drastic increase in vigorous physical activity may also cause acute cardiovascular events (e.g. acute myocardial infarction) and deleterious cardiac remodeling, particularly when exercise is performed by unfit or susceptible individuals. There is a reversed J-shaped hormesis-like curve between the duration and intensity of exercise and level of CVD risks. Therefore, it is important for an early detection of cardiac injuries in professional and amateur athletes. Under this context, this article focuses on the use of biomarker testing, an indispensable component in the current clinical practices especially in Cardiology and Oncology. We attempt to justify the importance of using circulating biomarkers in routine practices of Sports Medicine for an objective assessment of CVD events following exercise. Special attentions are dedicated to three established or emerging cardiac biomarkers (i.e. cardiac troponins, natriuretic peptides, hypoxanthine) for myocardial tissue hypoxia/ischemia events, muscle stress, and the consequent cellular necrotic injury. Based on these focused analyses, we propose use of circulating biomarker testing in both laboratory and point-of-care settings with an increasingly broader involvement or participation of team physicians, trainers, coaches, primary care doctors, as well as educated athlete community. This diagnostic approach may improve the quality of medical surveillance and preventive measures on exercise-related CVD risks/outcomes.
Keywords: Biomarker, Cardiac troponin, Exercise, Hypoxanthine, Natriuretic peptide, Overtraining syndrome, Tissue ischemia, Risk factors
Introduction
There is an evidence-based consensus suggesting that physical activity and regular exercise training can reduce modifiable risk factors and level of mortality and morbidity in patients with major chronic diseases, such as cardiovascular disease (CVD), diabetes, obesity, and cancer.1, 2, 3, 4
In the case of CVD, two recently published large prospective cohort studies in the participants recruited from diversified urban and rural areas of 17 countries (aged 35–70 years)3 or China alone (aged 30–79 years; mean 51 years)2 convincingly demonstrated health benefits of both recreational and non-recreational physical activity in reducing occurrence and mortality of CVD during a follow-up period of 4–7 years. The multi-national study analyzed 130,843 participants without pre-existing CVD and found that higher physical activity was associated with lower risk of CVD and mortality across high-, middle-, and low-income countries.3 Compared with low physical activity (<600 metabolic equivalents [MET] × min/week), moderate (600–3000 MET × min/week) and high physical activity (>3000 MET × min/week) were associated with graded significant reduction in mortality and occurrence of major CVD.3 Similarly, another population-based prospective cohort study in 487,334 adults (59% women) from 5 urban and 5 rural areas across China with no prior CVD history demonstrated that total physical activity was inversely associated with the risk of major vascular events with a 0.77 adjusted hazard ratio between the top versus bottom quintiles of physical activity.2 Higher total occupational and nonoccupational physical activity in either males or females was associated with 6% lower risk of major vascular events and 9%, 5%, 6%, and 12% lower risk of major coronary events, ischemic stroke, intracerebral hemorrhage, and CVD death, respectively.2 In addition to its CVD prevention benefits, moderate exercise training also played an important role in the cardiac rehabilitation program following acute myocardial infarction (MI), which was supported by the evidence collected from both human clinical trials5,6 and laboratory studies using animal models of MI.7
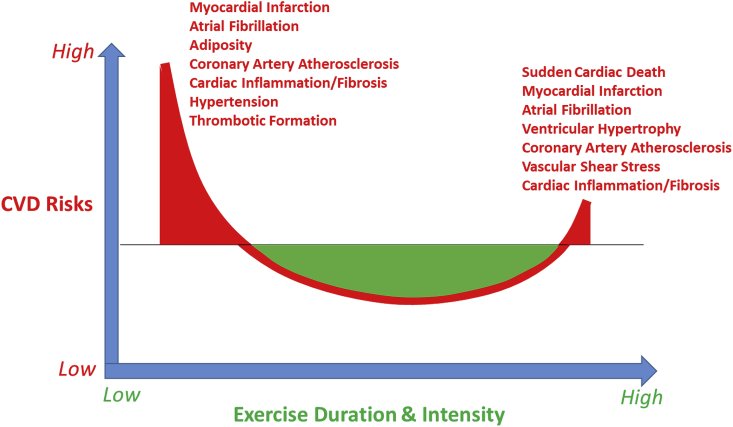
On the other hand, according to a recently published scientific statement by the American Heart Association (AHA) on exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training,8 vigorous physical activity, particularly when performed by unfit individuals, can acutely increase the risk of sudden cardiac death and acute myocardial infarction in susceptible people. As illustrated in Fig. 1 and emphasized by the AHA scientific statement, both large exercise volume and vigorous intensities are associated with potential cardiac maladaptations in a reversed J-shaped hormesis-like curve, including accelerated coronary artery calcification, exercise-induced cardiac biomarker release, myocardial fibrosis, and atrial fibrillation.8
Fig. 1.
Illustrative description of a seemingly reversed J-shaped hormesis-like curve between physical active level or exercise volume/intensity and risks for cardiovascular diseases including accelerated coronary artery atherosclerosis, hypertension, myocardial inflammation and fibrosis, atrial fibrillation, and sudden cardiac death, etc. Abbreviation: CVD – cardiovascular diseases.
The use of biomarkers has increasingly become an indispensable component in current basic and clinical research, as well as patient-care practices in major medical specialties. The term “biomarker” refers to a broad subcategory of medical signs, which are objective indicators of the patient's medical state. Two decades ago, the National Institutes of Health Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”9 In general, biomarker testing is an objective and quantifiable tool based on modern laboratory science that has already made significant impacts to medical practice. Circulating biomarker testing has emerged as a primary diagnostic tool in current evidence-based clinical medicine. Among a few widely used biomarkers, cardiac troponin (cTn) has served as a highly sensitive and specific biomarker for acute myocardial infarction since 199110,11 and prostate-specific antigen (PSA) has been recommended as a specific biomarker for early detection of prostate cancer since 1986.12,13
Contrary to the above-mentioned widespread uses of biomarker testing in major medical specialties such as Cardiology and Oncology, the utilization of biomarkers in Sports Medicine practices remain limited. Particularly, assessment of blood biomarkers for cardiac injury following intensive exercise workload has become a major issue in the field of Sports Cardiology. While exercise provides myriad benefits, it may also confer deleterious effects upon the cardiovascular system. Athletes are at particular risk of sudden cardiac arrest and myocardial damage due to cardiac adaptations typical of individuals involved in high-intensity exercise over prolonged periods of time.
Under this context, the purpose of our present review article is to provide an overview on the potential importance of using biomarkers to gain an objective assessment of health status or disease signs of humans undergoing exercise training and competitive sports events. We gave special attention to the utility of several established or emerging cardiac biomarkers that may have more important clinical impact and predictive/prognostic values in health management of amateurs and professional athletes during their exercise training and/or competition periods.
Cardiac troponin as an efficacious biomarker for detecting cardiac tissue damage following strenuous exercise
Cardiac troponin (cTn) is known to be an efficacious and reliable biomarker for diagnosis of acute coronary syndrome with excellent specificity and sensitivity, primarily because cTn is a structural component of the thin filament of myocardium and is released only from the necrotic or damaged cardiomyocytes into the bloodstream following myocardial infarction. Three types of cTn have been identified, known as cardiac troponin I (cTnI), troponin T (cTnT), and troponin C (cTnC), which together form a protein complex that regulates cardiac muscle contraction. Recent improvements in luminescence technology have developed into a series of high-sensitivity assays of cTn (hs-cTn), allowing for earlier detection of plasma cTn. cTn and hs-cTn have served as efficacious diagnostic biomarkers for acute coronary syndrome and acute myocardial infarction.14
The phenomenon of post-exercise elevation of blood cTnT levels was first reported about 25 years ago by two independent research groups – Siegel et al., in 199515 and Laslett et al., in 1996,16 approximately 5 years after cTnT was recognized as a biomarker for myocardial infarction.10,11 These early findings elicited special interests to characterize the clinical causes for such an elevation of cTnT during and after intensive exercise, a matter remains debatable today in the field of Sports Cardiology. In amateur athletes, echocardiographic changes were prevalent after marathon runs, and the incidences of cTnT or cTnI elevation were often associated with ventricular diastolic dysfunction, elevated pulmonary pressures and right ventricular dysfunction.17, 18, 19
The mechanistic explanations and clinical relevance of increased circulating cTn levels following strenuous exercise remain elusive. A comprehensive review by Koller in 2003 suggested that caution should be exercised in generalizing results to cTn-positive athletes without clinical evidence of coronary artery disease20 and that cTn may not be released following irreversible cardiac injury after vigorous exercise. In contrast to cTn-positive patients with total or partial coronary artery blockage resulting from intracoronary thrombus, there is no clear evidence in healthy athletes that a hemostatic imbalance may trigger severe acute cardiac ischemic events after strenuous exercise. Therefore, it may be premature and even dangerous to recommend pharmacologic intervention (such as low molecular weight heparins) to the cTn-positive endurance athletes exercising at sea level or high-altitude.20
As summarized in Table 1, there are several distinguishable differences in both etiology and severity of cTn release between healthy athletes after strenuous exercise and the patients with coronary artery disease during a heart attack event. There is no doubt that better characterizations of the relationship between cTn elevation and long-term effects on cardiac health in athletes are warranted. For example, an interesting study recently published by Peretti et al. investigated correlation between cardiac biomarkers and long-term cardiac health benchmarks in 21 healthy male preadolescent athletes (age 9.2 ± 1.7 years) after an intensive cycling training prolonged until muscular exhaustion.21 They reported that the short, high-intensity exercise caused a moderate elevation of cTn in 6 of the 21 boys (∼29%) at the end of exhaustive exercise, which was not associated with arrhythmias or other symptoms indicating myocardial damage. There was no correlation between cTn elevation and heart rate, age or exercise duration. There was also no indication of cardiac damage, suggesting that exercise-induced cTn release is a physiological phenomenon in response to a temporary oxygen consumption and supply imbalance in the heart.21 Nonetheless, this explanation needs further validation by studies in a wider variety of age groups, as well as a larger number of subjects. Similarly, another recent study examined the response of cardiac biomarkers to a soccer game in 22 trained adolescent (14–16 years) male soccer players.22 The results showed that serum concentration of cTnI and NT-proBNP (amino-terminal pro-brain natriuretic peptides, a marker of myocardial strain) increased immediately and 2 h after soccer game (P < 0.001). After 24 h, cTnI dropped but remained significantly higher than its baseline level (P < 0.01), whereas NT-proBNP returned to its baseline. However, at no time point did any of the biomarkers values exceed the upper reference value for MI.22 These authors suggested that the postgame elevation of cardiac biomarkers and rapid recovery are indicative of a physiological rather than pathological response.23
Table 1.
Characteristic differences of circulating cardiac troponin (cTn) elevations during strenuous exercise versus acute myocardial infarction.
| Strenuous Exercise | Myocardial Infarction | |
|---|---|---|
| Blood levels of cTn | cTnT > 0.03 ng/mL cTnI > 0.18 ng/mL |
cTnT > 0.1 ng/mL cTnI > 1.0 ng/mL |
| Time of cTn elevation | 24–48 h | Up to 72 h |
| Other clinical signs | No changes in ECG | Changes in ECG |
| Main clinical outcomes | No substantial myocardial damages | Causing acute heart failure or sudden death |
Most recently, researchers attempted to evaluate any potential cardiac muscle damage in 12 endurance runners with high-intensity intermittent exercise training (HIIT) mode as compared with the workload-matched continuous training mode. They reported that at high workload the increases for cTnI and cTnT were higher in HIIT mode as compared with continuous exercise mode, whereas such a difference was insignificant for other measured cardiac biomarkers. The results suggested that prolonged HIIT appears more damaging to cardiac muscle than continuous exercise with identical average running speed and heart rate raise in endurance runners.23 This is also corroborated by a recent meta-analysis linking exercise intensity as a strong determinant of cTn release and even one bout of high-intensity exercise could precipitate significant release of cTn.24
Natriuretic peptides as a potential cardiac muscle stress indicator during exercise
Another well-established type of cardiac biomarker are natriuretic peptides. Natriuretic peptides may also serve as an excellent indicator for cardiovascular stress. It is postulated that exercise-associated elevation of blood pressure may impact the heart walls and in turn cause the atria and ventricles to release atrial natriuretic peptides (ANP) and brain natriuretic peptides (BNP) respectively. Their baseline levels are remarkably variable, depending upon factors such as myocardial pathology and old age.25 A recent work reported that BNP concentration increased along with cTnT in the trained middle-aged half-marathon runners after their race.26 While cTnT levels normalized after 24 h, BNP concentrations returned to baseline levels after 48 h. This extended release of natriuretic peptides may suggest a sustained body response to the perturbation of systemic blood pressure by heavy endurance exercise. Another Spanish study evaluated 79 middle-aged recreational athletes of the 2016 Barcelona Marathon.27 Blood samples were collected at baseline, 1–2 h after the race and 48 h post-race. Amino-terminal pro-BNT (NT-proBNP; a marker of myocardial strain) and hs-cTnT were examined as circulating biomarkers. Immediately 1–2 h after the race, there were a 1.3-fold increase in blood levels of NT-proBNP and 16-fold elevation in hs-cTnT. They found a direct and significant relationship between the race time and increased level of hs-cTnT, indicating marathon running may have significant impact on the cardiovascular system. Furthermore, ventricular diastolic dysfunction and pulmonary pressures increase were evident during the post-race period, similar to those recently reported by Cocking et al.28 It is postulated that high-intensity athletic training and competition for prolonged periods such as marathon race can trigger release of multiple circulating cardiac biomarkers such as BNP.27
Left ventricular hypertrophy is a complication infrequently seen amongst athletes, sometimes resulting in exceedingly rare instance of sudden cardiac arrest. Onset of left ventricular hypertrophy occurs due to high oxygen demands from chronically poor cardiovascular health or recurrent bouts of extreme exercise require higher output from the heart. While heart muscle usually can adapt such an increased work demand and develop into “physiological” hypertrophy in left ventricle, a sustained or excessive stress would eventually lead to a decompensated stage of “pathological” hypertrophy with loss of blood-pumping efficiency. This can be one of the factors correlated with incidence of sudden cardiac arrest. While acute coronary syndrome and sudden cardiac death are only found in 1 of 50,000 athletes, the particularly rare incidence does not preclude warranted investigation. Rather, it indicates a deficit in understanding of the mechanism of this disease, resulting in serious deleterious effects which are still poorly understood.29
BNP is considered as a reliable diagnostic biomarker, typically used in conjunction with soluble ST2 and Gal-3, often improving prognosis of an athlete. Copeptin, ANP, and mid-regional adrenomedullin are also implemented for their predictive value, having been shown to markedly improve likelihood of early diagnosis in cardiovascular issues. cTn will often be correlated with levels of cardiac damage, but does not necessarily reflect irreversible cardiomyocyte damage or myocardial necrosis. Rather, reversible cardiomyocyte membrane damage is thought to be the driving factor in this physiological phenomenon. However, repeated high-intensity exercise will result in myocardial fibrosis, leading to malignant dysrhythmias that is sometimes associated with sudden cardiac death. Together, expression of cTn and BNP post-exercise indicates myocardial stress without long-term damage, though those without training experienced release of cardiac-associated biomarkers at higher levels should be cautiously seeking further exams by healthcare providers to identify potential underlying CVDs. Furthermore, these pathways have been shown to change based on extent and intensity of exercise, altering metabolic response to high-intensity activities. After long-term training, cardiac troponins and natriuretic peptides result in lower predispositions of severe reactions to high-intensity exercise. Subjects with increased proBNP had mean and maximal heart rates lower than children with normal natriuretic peptides levels.21
Hypoxanthine as biomarker for cardiac ischemia and exercise training status
As a vital engine for enhanced demand of blood circulation during exercise, cardiac muscle utilizes ATP as its primary fuel to perform contraction-relaxation for pumping blood. Cardiomyocytes have an abundance of mitochondria (approximately 40–50% of the cardiac cellular mass) – the powerhouse cellular organelle to produce large quantities of ATP in supporting the functional demand of heart, via aerobic oxidative phosphorylation in the mitochondrial electron transport chain. Since this crucial aerobic process is heavily oxygen-dependent, any disruption or reduction of blood flow in coronary and/or limb muscle circulation during heavy exercise can result in demand-supply imbalance of oxygen leading to major metabolic consequences in myocytes.
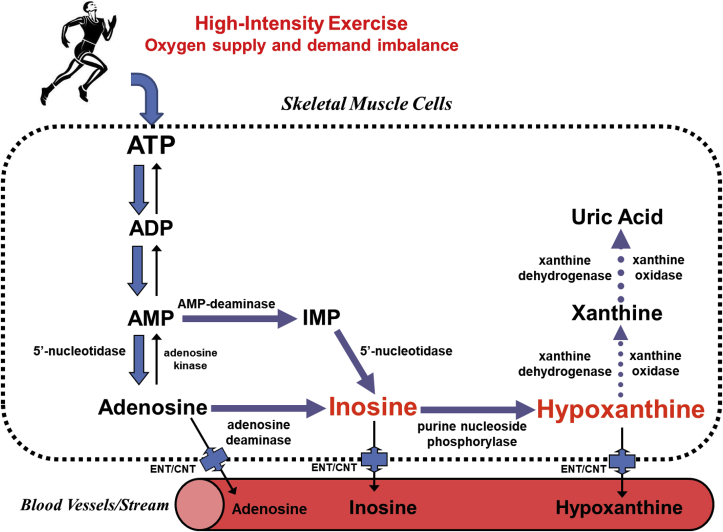
As described in our previous review,30 at the onset of cardiac ischemia/hypoxia, the high energy phosphates (creatine phosphate and ATP) are rapidly depleted and heart tissues lose 65% of ATP contents within 15 min of total ischemia.31 These ischemic events can mobilize an ATP breakdown cascade32 leading to cellular accumulation of ATP catabolic by-products, such as adenosine diphosphate (ADP), adenosine monophosphate (AMP), and activation of normally dormant enzymes, including 5′-nucleotidase, adenosine deaminase, purine nucleoside phosphorylase and xanthine oxidase, which sequentially catabolize AMP into adenosine, inosine, hypoxanthine, xanthine and uric acid (Fig. 2). Among these metabolites, hypoxanthine is a small polar substance with low molecular weight of 136 Da, which can be transported near-instantaneously by passive diffusion from affected cardiac and skeletal muscle tissues into the bloodstream, a process also facilitated by equilibrative nucleoside transporters (ENT) and/or concentrative nucleoside transporters (CNT).33 Elevated plasma hypoxanthine levels following exercise were first reported by Sahlin et al., in 1991,34 who collected blood samples from 8 men at rest or during and after cycling exercise for 6 min, at workloads of 44% and 72% of , to fatigue at 98% of . They found that hypoxanthine was significantly elevated at fatigue and was 3-fold higher 10 min post-exercise from cessation of exercise. The significant increase in plasma hypoxanthine during intensive exercise likely originated from working skeletal muscles.
Fig. 2.
Illustrative summary of major metabolic pathways of ATP degradation during myocardial ischemia and excessive exercise. Abbreviations: ATP - adenosine triphosphate; ADP - adenosine diphosphate; AMP - adenosine monophosphate; IMP - inosine monophosphate; ENT – equilibrative nucleoside transporters; CNT – concentrative nucleoside transporters.
In another study, acute effects of a single bout of high-intensive strength training on degradation of purine nucleotides were assessed with measurements of urine and serum concentrations of xanthine and hypoxanthine in 12 trained weightlifters.35 The athletes performed six sets of six times of weight-lifting, resulting in significant elevation of hypoxanthine from baseline immediately following cessation of exercise. Hypoxanthine levels then decreased following 2 h of recovery. These investigators suggested the utility of hypoxanthine as a relevant biomarker for cellular damage and/or ischemic stress associated with weightlifting exercise.35 Another group of Australian researchers studied 8 healthy male subjects and found that high-intensity intermittent cycling resulted in larger increase of purine loss than those of workload-matched continuous moderate intensity cycling.36 A greater elevation of blood lactate and hypoxanthine concentrations was observed following high-intensity intermittent exercise as compared to constant training. These results may indirectly reflect a net loss or breakdown of ATP from the exercising muscles. The authors postulated the greater loss and subsequent restoration of intramuscular ATP contents may account for the higher energy expenditures and accelerated body fat reduction observed with high-intensity intermittent exercise.36 More recently Siopi et al. noted that during HIIT, AMP is rapidly metabolized in skeletal muscle, producing inosine monophosphate, inosine, and then hypoxanthine.37
Furthermore, during the past decade Zieliński and colleagues have conducted a number of human studies and proposed an interesting new concept suggesting the utility of plasma concentration of hypoxanthine as a universal metabolic indicator of training status in competitive sports.38 It was proposed that hypoxanthine reflects exercise response and muscle adaptation, and could be more practical than the conventional cardiorespiratory and biochemical indicators of exercise.38 This group of Polish researchers have provided more data in various types of subjects and sport events to support their theories on the close relationship between hypoxanthine blood level and exercise training status.39, 40, 41, 42
Inosine and hypoxanthine have some unique features as biomarkers for cardiac ischemia, because they are small stable organic substances typically found in human plasma at low concentrations (inosine 0.75–1.49 μM, hypoxanthine 1.47–2.94 μM), resulting from purine metabolism.43 In 1994, Kock et al. studied the role of xanthine oxidase in purine metabolism in patients with myocardial ischemia.44 They evaluated hypoxanthine, xanthine and uric acid, and reported differences in hypoxanthine concentrations between healthy male subjects and patients with acute MI and ischemic heart diseases.44 Harmsen et al. reported elevated blood concentrations of hypoxanthine in the patients with ischemic heart disease undergoing atrial pacing stress testing as compared with healthy normal volunteers, whereas the levels of adenosine, inosine and xanthine were not significantly different.45 Previously our research group analyzed blood samples acquired from non-traumatic chest pain patients and found elevated plasma concentrations of inosine and/or hypoxanthine in all of the chest pain samples.46
However, there are several potential confounders in using hypoxanthine as a circulating biomarker for cardiac and skeletal muscle metabolic status or ischemic events. These confounders include: 1) genetic enzyme deficiencies (e.g. adenosine deaminase or purine nucleoside phosphorylase) can cause erroneous false negative or false positive results; 2) patients with xanthine oxidase deficiency or taking xanthine oxidase inhibitors (such as allopurinol for treatment of gout) may have elevated basal blood levels of hypoxanthine; 3) renal disease or failure may compromise elimination of hypoxanthine and lead to false positive results; and 4) high consumption of purine-containing food sources, such as organ meats, spinach, and beer or using dietary supplement of inosine for enhancing athletic performance.30
It is noteworthy that exercise-induced elevation of circulating level of hypoxanthine can result from both skeletal and cardiac muscles and exercise mode may affect the level and dynamic of hypoxanthine release. For examples, Siopi et al. recently showed that resistance exercise caused the highest change in hypoxanthine, followed by HIIT, whereas continuous moderate-level endurance exercise had minimal effect on hypoxanthine concentrations.37 In addition, Kistner et al. reported that baseline urine levels of hypoxanthine decreased after a ten-day HIIT course in amateur athletes, indicating an exercise training-induced adaptation in purine metabolism.47 Moreover, only hypoxanthine concentrations were affected in the urinary metabolome, indicating the unique niche hypoxanthine may serve as a biomarker in monitoring exercise training.47 Interestingly, the changes in hypoxanthine concentration correlated only with training profile (i.e. resistance exercise versus HIIT or aerobic endurance exercise), as opposed to skeletal muscle mass, lipidemic profiles, and other cardiovascular benchmarks.40 These data corroborate a unique capability of hypoxanthine in urine and serum profiling as an useful biomarker for training status in athletes, in addition to other classic indicators of health, such as skeletal muscle mass, resting blood pressure and heart rate, etc. Włodarczyk et al. recently reported that amateur runners experienced greater elevation in hypoxanthine concentrations immediately following cessation of exercise and returned to normal levels within 2 h.42
Taken together, a higher increase of hypoxanthine likely indicates lower physical fitness and poor compensation in purine nucleotide metabolism and exercise training may lead to a lower baseline hypoxanthine concentrations, indicating an improvement in purine metabolism.47 It should also be emphasized that hypoxanthine can be released from both skeletal muscles and myocardium during high intensity exercise and skeletal muscles are likely the main source based on the muscle mass. Therefore, it is difficult, if not impossible, to distinguish the cardiac source of hypoxanthine from those of skeletal muscle. The non-specific feature of hypoxanthine as a biomarker of cardiac damage requires careful co-examination of the athlete's medical history and physical signs (such as chest pain and arrythmia), in order to fully benefit the early ischemia-alerting power of hypoxanthine as compared with other more specific biomarkers of cardiac injuries, i.e. cTn and BNP (see Fig. 2).
Concluding remarks
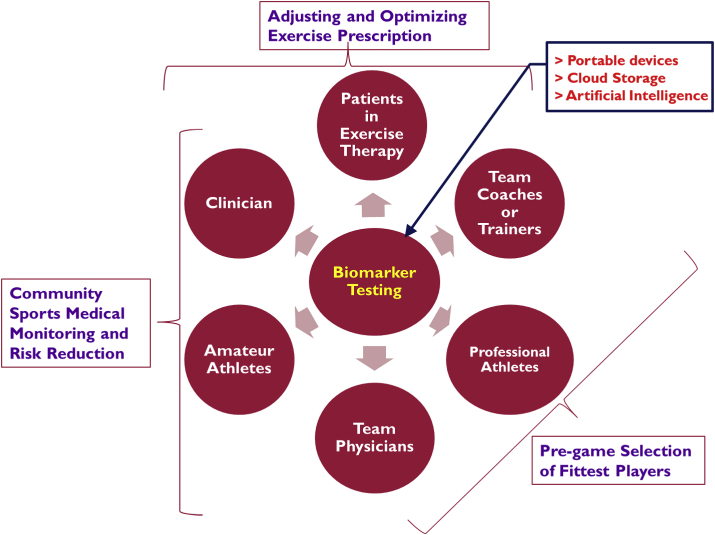
Considering the evidence discussed above, we would propose use of circulating biomarker testing in both laboratory and point-of-care settings with an increasingly broader involvement or participation of team physicians, trainers, coaches, primary care doctors, as well as educated community of professional and amateur athletes (see Fig. 3 for an illustrative description). This advanced and objective diagnostic approach may improve the quality of medical surveillance and preventive measures on exercise-related CVD risks/outcomes. As we emphasized in this review article, cTn, BNP, and hypoxanthine along with other emerging biomarker candidates (such as microRNA, galectin-3, and tumorigenicity 2)48,49 may help to gauge cardiovascular stress response, health status as well as pathological events during and after exercise training and/or competition sessions. It is notable that the diagnostic value and efficacy of the circulating cardiac biomarkers may vary in terms of accuracy and reliability. A combined test array of all the three biomarkers would be much better to ensure both sensitivity (via hypoxanthine) and specificity (via cTn and BNP) for early detection of cardiac ischemia events triggered by heavy exercise and/or cardiovascular pathology. The precise pathophysiological mechanisms and serological kinetics for their post-exercise release into blood are yet to be fully understood and require further targeted investigations in various groups of participants of physical activity, exercise and sports, in order to determine a more accurate cut-off value guidelines, as well as several other confounding factors (e.g. pre-existing CVD conditions, age, gender, history of medication/dietary supplement intake, etc.) for the cardiac biomarker testing in Sports Medicine.
Fig. 3.
Illustrative description of the potential utility of cardiac biomarker testing in exercise training and Sports Medicine practice and the important and cooperative roles played by athletes, coaches, trainers or team physicians as well as cardiologists and primary care doctors.
Submission statement
The manuscript has not been published and is not under consideration for publication elsewhere.
Authors’ contributions
LX provided the conceptual outlines, wrote the first draft and assembled the final version of this manuscript. AM wrote and edited the contents of this manuscript.
Conflict of interest
LX is a co-inventor of the United States patents (8,343,731 and 8,609,360) and European patent (EP2185926B1), which are partially related to the topics discussed in this review article.
References
- 1.Anderson E.D., Durstine J.L. Physical activity, exercise, and chronic diseases: a brief review. Sports Med Health Sci. 2019;1:3–10. doi: 10.1016/j.smhs.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett D.A., Du H., Clarke R., et al. Association of physical activity with risk of major cardiovascular diseases in Chinese men and women. JAMA Cardiol. 2017;2:1349–1358. doi: 10.1001/jamacardio.2017.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lear S.A., Hu W., Rangarajan S., et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet. 2017;390:2643–2654. doi: 10.1016/S0140-6736(17)31634-3. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 5.Ades P.A., Balady G.J., Berra K. Transforming exercise-based cardiac rehabilitation programs into secondary prevention centers: a national imperative. J Cardiopulm Rehabil. 2001;21:263–272. doi: 10.1097/00008483-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Anderson L., Oldridge N., Thompson D.R., et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Jia D., Hou L., Lv Y., Xi L., Tian Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J Cell Physiol. 2019;234:23705–23718. doi: 10.1002/jcp.28939. [DOI] [PubMed] [Google Scholar]
- 8.Franklin B.A., Thompson P.D., Al-Zaiti S.S., et al. American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Stroke Council. Exercise-related acute cardiovascular events and potential deleterious adaptations following long-term exercise training: placing the risks into perspective-an update: a scientific statement from the American Heart Association. Circulation. 2020;141(13):e705–e736. doi: 10.1161/CIR.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 9.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 10.Katus H.A., Remppis A., Neumann F.J., et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- 11.Mair J., Wieser C., Seibt I., et al. Troponin T to diagnose myocardial infarction in bypass surgery. Lancet. 1991;337:434–435. doi: 10.1016/0140-6736(91)91218-j. [DOI] [PubMed] [Google Scholar]
- 12.Seamonds B., Yang N., Anderson K., Whitaker B., Shaw L.M., Bollinger J.R. Evaluation of prostate-specific antigen and prostatic acid phosphatase as prostate cancer markers. Urology. 1986;28:472–479. doi: 10.1016/0090-4295(86)90146-9. [DOI] [PubMed] [Google Scholar]
- 13.Siddall J.K., Cooper E.H., Newling D.W., Robinson M.R., Whelan P. An evaluation of the immunochemical measurement of prostatic acid phosphatase and prostatic specific antigen in carcinoma of the prostate. Eur Urol. 1986;12:123–130. doi: 10.1159/000472596. [DOI] [PubMed] [Google Scholar]
- 14.Abela M., Sammut L. Cardiac troponin: more than meets the eye. Postgrad Med J. 2017;93:762–765. doi: 10.1136/postgradmedj-2017-134984. [DOI] [PubMed] [Google Scholar]
- 15.Siegel A.J., Lewandrowski K.B., Strauss H.W., Fischman A.J., Yasuda T. Normal post-race antimyosin myocardial scintigraphy in asymptomatic marathon runners with elevated serum creatine kinase MB isoenzyme and troponin T levels. Evidence against silent myocardial cell necrosis. Cardiology. 1995;86:451–456. doi: 10.1159/000176922. [DOI] [PubMed] [Google Scholar]
- 16.Laslett L., Eisenbud E., Lind R. Evidence of myocardial injury during prolonged strenuous exercise. Am J Cardiol. 1996;78:488–490. doi: 10.1016/0002-9149(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 17.Scharhag J., George K., Shave R., Urhausen A., Kindermann W. Exercise-associated increases in cardiac biomarkers. Med Sci Sports Exerc. 2008;40:1408–1415. doi: 10.1249/MSS.0b013e318172cf22. [DOI] [PubMed] [Google Scholar]
- 18.Scharhag J., Urhausen A., Schneider G., et al. Reproducibility and clinical significance of exercise-induced increases in cardiac troponins and N-terminal pro brain natriuretic peptide in endurance athletes. Eur J Cardiovasc Prev Rehabil. 2006;13:388–397. doi: 10.1097/01.hjr.0000219117.33038.90. [DOI] [PubMed] [Google Scholar]
- 19.Westermeyer M.L., Eilbert W.P. Elevation of troponin I in athletes: a case report in a marathon runner. J Emerg Med. 2008;34:175–178. doi: 10.1016/j.jemermed.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Koller A. Exercise-induced increases in cardiac troponins and prothrombotic markers. Med Sci Sports Exerc. 2003;35:444–448. doi: 10.1249/01.MSS.0000053736.51903.0E. [DOI] [PubMed] [Google Scholar]
- 21.Peretti A., Mauri L., Masarin A., et al. Cardiac biomarkers release in preadolescent athletes after an high intensity exercise. High Blood Pres Cardiovasc Prev. 2018;25:89–96. doi: 10.1007/s40292-017-0243-y. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini S.M., Azizi M., Samadi A., Talebi N., Gatterer H., Burtscher M. Impact of a soccer game on cardiac biomarkers in adolescent players. Pediatr Exerc Sci. 2018;30:90–95. doi: 10.1123/pes.2017-0060. [DOI] [PubMed] [Google Scholar]
- 23.Li F., Nie J., Zhang H., et al. Effects of matched intermittent and continuous exercise on changes of cardiac biomarkers in endurance runners. Front Physiol. 2020;11:30. doi: 10.3389/fphys.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donaldson J.A., Wiles J.D., Coleman D.A., Papadakis M., Sharma R., O'Driscoll J.M. Left ventricular function and cardiac biomarker release-the influence of exercise intensity, duration and mode: a systematic review and meta-analysis. Sports Med. 2019;49:1275–1289. doi: 10.1007/s40279-019-01142-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z.L., Li R., Yang F.Y., Xi L. Natriuretic peptide family as diagnostic/prognostic biomarker and treatment modality in management of adult and geriatric patients with heart failure: remaining issues and challenges. J Geriatr Cardiol. 2018;15:540–546. doi: 10.11909/j.issn.1671-5411.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassalle C., Masotti S., Lubrano V., et al. Traditional and new candidate cardiac biomarkers assessed before, early, and late after half marathon in trained subjects. Eur J Appl Physiol. 2018;118:411–417. doi: 10.1007/s00421-017-3783-x. [DOI] [PubMed] [Google Scholar]
- 27.Roca E., Nescolarde L., Lupon J., et al. The dynamics of cardiovascular biomarkers in non-elite marathon runners. J Cardiovasc Transl Res. 2017;10:206–208. doi: 10.1007/s12265-017-9744-2. [DOI] [PubMed] [Google Scholar]
- 28.Cocking S., Landman T., Benson M., et al. The impact of remote ischemic preconditioning on cardiac biomarker and functional response to endurance exercise. Scand J Med Sci Sports. 2017;27:1061–1069. doi: 10.1111/sms.12724. [DOI] [PubMed] [Google Scholar]
- 29.Pearson M.J., King N., Smart N.A. Effect of exercise therapy on established and emerging circulating biomarkers in patients with heart failure: a systematic review and meta-analysis. Open Heart. 2018;5 doi: 10.1136/openhrt-2018-000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farthing D.E., Farthing C.A., Xi L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: from bench to point-of-care. Exp Biol Med. 2015;240:821–831. doi: 10.1177/1535370215584931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jennings R.B., Hawkins H.K., Lowe J.E., Hill M.L., Klotman S., Reimer K.A. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol. 1978;92:187–214. [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings R.B., Reimer K.A., Hill M.L., Mayer S.E. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res. 1981;49:892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- 33.Molina-Arcas M., Casado F.J., Pastor-Anglada M. Nucleoside transporter proteins. Curr Vasc Pharmacol. 2009;7:426–434. doi: 10.2174/157016109789043892. [DOI] [PubMed] [Google Scholar]
- 34.Sahlin K., Ekberg K., Cizinsky S. Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand. 1991;142:275–281. doi: 10.1111/j.1748-1716.1991.tb09157.x. [DOI] [PubMed] [Google Scholar]
- 35.Atamaniuk J., Vidotto C., Kinzlbauer M., Bachl N., Tiran B., Tschan H. Cell-free plasma DNA and purine nucleotide degradation markers following weightlifting exercise. Eur J Appl Physiol. 2010;110:695–701. doi: 10.1007/s00421-010-1532-5. [DOI] [PubMed] [Google Scholar]
- 36.Gerber T., Borg M.L., Hayes A., Stathis C.G. High-intensity intermittent cycling increases purine loss compared with workload-matched continuous moderate intensity cycling. Eur J Appl Physiol. 2014;114:1513–1520. doi: 10.1007/s00421-014-2878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siopi A., Deda O., Manou V., et al. Comparison of the serum metabolic fingerprint of different exercise modes in men with and without metabolic syndrome. Metabolites. 2019;9:116. doi: 10.3390/metabo9060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zielinski J., Kusy K. Hypoxanthine: a universal metabolic indicator of training status in competitive sports. Exerc Sport Sci Rev. 2015;43:214–221. doi: 10.1249/JES.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 39.Zielinski J., Slominska E.M., Krol-Zielinska M., Krasinski Z., Kusy K. Purine metabolism in sprint- vs endurance-trained athletes aged 20-90 years. Sci Rep. 2019;9:12075. doi: 10.1038/s41598-019-48633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wlodarczyk M., Kusy K., Slominska E., Krasinski Z., Zielinski J. Changes in blood concentration of adenosine triphosphate metabolism biomarkers during incremental exercise in highly trained athletes of different sport specializations. J Strength Condit Res. 2019;33:1192–1200. doi: 10.1519/JSC.0000000000003133. [DOI] [PubMed] [Google Scholar]
- 41.Pospieszna B., Kusy K., Slominska E.M., Dudzinska W., Ciekot-Soltysiak M., Zielinski J. The effect of training on erythrocyte energy status and plasma purine metabolites in athletes. Metabolites. 2019;10 doi: 10.3390/metabo10010005. pii: E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wlodarczyk M., Kusy K., Slominska E., Krasinski Z., Zielinski J. Change in lactate, ammonia, and hypoxanthine concentrations in a 1-year training cycle in highly trained athletes: applying biomarkers as tools to assess training status. J Strength Condit Res. 2020;34:355–364. doi: 10.1519/JSC.0000000000003375. [DOI] [PubMed] [Google Scholar]
- 43.Feng J.D., Yeung P.K. A simple high-performance liquid chromatography assay for simultaneous measurement of adenosine, guanosine, and the oxypurine metabolites in plasma. Ther Drug Monit. 2000;22:177–183. doi: 10.1097/00007691-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Kock R., Delvoux B., Sigmund M., Greiling H. A comparative study of the concentrations of hypoxanthine, xanthine, uric acid and allantoin in the peripheral blood of normals and patients with acute myocardial infarction and other ischaemic diseases. Eur J Clin Chem Clin Biochem. 1994;32:837–842. doi: 10.1515/cclm.1994.32.11.837. [DOI] [PubMed] [Google Scholar]
- 45.Harmsen E., de Jong J.W., Serruys P.W. Hypoxanthine production by ischemic heart demonstrated by high pressure liquid chromatography of blood purine nucleosides and oxypurines. Clin Chim Acta. 1981;115:73–84. doi: 10.1016/0009-8981(81)90108-x. [DOI] [PubMed] [Google Scholar]
- 46.Farthing D.E., Sica D., Hindle M., et al. A rapid and simple chemiluminescence method for screening levels of inosine and hypoxanthine in non-traumatic chest pain patients. Luminescence. 2011;26:65–75. doi: 10.1002/bio.1187. [DOI] [PubMed] [Google Scholar]
- 47.Kistner S., Rist M.J., Kruger R., Doring M., Schlechtweg S., Bub A. High-intensity interval training decreases resting urinary hypoxanthine concentration in young active men-A metabolomic approach. Metabolites. 2019;9(7):137. doi: 10.3390/metabo9070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Goff C., Farre Segura J., Dufour P., Kaux J.F., Cavalier E. Intense sport practices and cardiac biomarkers. Clin Biochem. 2020;79:1–8. doi: 10.1016/j.clinbiochem.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Lombardi G., Perego S., Sansoni V., Banfi G. Circulating miRNA as fine regulators of the physiological responses to physical activity: pre-analytical warnings for a novel class of biomarkers. Clin Biochem. 2016;49:1331–1339. doi: 10.1016/j.clinbiochem.2016.09.017. [DOI] [PubMed] [Google Scholar]