Abstract
We examined bone mineral density (BMD) measurements made by dual-energy-xray-absorptiometry (DEXA) taken from 100 patients (♂46/♀54, 66±6yr) who previously underwent single total-knee arthroplasty (TKA) to determine if automated software-based artifact detection (ASAD) adequately removes implant artifact from the DXA image before analysis and if potential inaccuracies could be overcome through manual artifact correction (MAC). We also sought to determine if software-based inaccuracies would result in fracture risk misclassification (Low-BMD/Osteopenia = Young-Adult T-Score < −1). Select Results: When using ASAD, limbs with implants had higher BMD (+12.0 ± 1.7%, p < 0.001) compared to control limbs resulting in a 2.5 ± 0.2% overestimation of total-body BMD (single implant). Consequently, the prevalence of osteopenia in 95% of patients who would have been observed to have low leg BMD (18/19 patients) and 80% of those found to have low total-body BMD (4/5 patients) would have gone un-diagnosed. This overestimation was eliminated when using MAC. These results reveal a potential issue with ASAD for total-body DEXA scans in TKA patients and highlight the importance of careful review and MAC in those with joint replacements before making diagnostic decisions.
Keywords: Dual-energy-xray-absorptiometry, DXA, DEXA, Bone density, Total joint replacement, Arthroplasty, Osteopenia, Osteoporosis
Abbreviations
- BMD
Bone Mineral Density (g/cm2)
- DEXA
Dual-Energy-Xray-Absorptiometry
- MAC
Manual Artifact Correction
- ASAD
Automated software-based artifact detection
- THA
Total Hip Arthroplasty
- TKA
Total Knee Arthroplasty
Introduction
Osteopenia and osteoporosis are important modifiable health factors in older adults. With a reported 10.3% prevalence of osteoporosis and 43.9% prevalence of osteopenia, it is estimated that in 2010, 10.2 million adults had osteoporosis and 43.4 million adults had low bone mass.1 Dual-energy-xray absorptiometry (DEXA) allows accurate diagnosis of osteoporosis, estimation of fracture risk.2 The World Health Organization has established DEXA as the best densitometric technique for assessing bone mineral density (BMD) in postmenopausal women and based the definitions of osteopenia and osteoporosis on its results.3 While site-specific scans of the spine, forearm, and femur have classically been used in clinic for such diagnoses,4 total-body DEXA scans have grown in popularity in wellness centers and clinics due to the utility of a single short duration (∼5–10min) scan that simultaneously provides information on BMD and regional body composition and soft tissue distribution.5 In these instances, those observed to have low BMD via total scan measures can be referred for a clinical follow-up site-specific scan to confirm diagnosis of osteopenia or osteoporosis.
In the same population group that osteoporosis and osteopenia present (older adults), patients are also most likely to receive total joint replacements. Most patients who receive a total hip (THA) and knee arthroplasty (TKA) are between 65 and 85 years of age.6 Current total THA and TKA volume in the United States is approximately one million/year, and is expected to grow to ∼1.5 million/year by the year 2030.7 For those receiving total-body DEXA scans to track body composition and BMD, automated software-based artifact detection (ASAD) is relied upon by radiologists to remove image artifact of non-tissue origin. However, there is little (if any) information reported on the efficacy of ASAD artifact removal that may have a significant impact on DEXA measures and interpretation. This is of particular importance for total-body scans whereby measurement error associated with joint implants may result in mis-classification of those who may be at an elevated fracture risk. Therefore, the purpose of this study was to examine total-body BMD measurements taken via DEXA from older patients with a single primary TKA implants to determine if ASAD adequately removes implant artifact from the DXA image before analysis and if potential inaccuracies could be overcome through manual artifact correction (MAC). We hypothesized that ASAD would remove some, but not all, implant artifact resulting in an overestimation of BMD.
Material and methods
The procedures to follow were approved by our organization's institutional review board for research involving human subjects and all participants provided informed consent for their data to be used for research purposes prior to any measures being taken.
Study sample
One hundred older adults (m = 46, 65±6yr, 177.1 ± 7.1 cm, 99.6 ± 21.0 kg, BMI 31.6 ± 5.6 kg m2 |f = 54, 66±6yr, 163.6 ± 7.9 cm, 84.3 ± 20.4 kg, BMI 31.3 ± 6.4 kg m2) who previously underwent primary TKA for a single limb within the past year from a single hospital location in Houston, Texas consented to have their BMD assessed.
Outcome measures
On the day of measurement, total-body and regional BMD was assessed using a total-body DEXA scan (iDXA, GE®, Boston Massachusetts, USA). Scans were then analyzed by a trained technician using enCORE.v16 software (GE®) and standardized procedures from the manufacturer. To determine the regional effect of TKA implants on BMD analysis of the legs by DEXA, BMD was recorded individually for the CONTROL (no implant) and TKA (with implant) limbs. To determine the effect of the TKA implants on total-body measures, total-body BMD was analyzed cumulatively as well as on the non-TKA side only. Total and regional T-scores were calculated for all measures using a general population normative data base8,9 defined as the difference between study participant BMD and the mean BMD of a young population reference group, divided by the population standard deviation.10 T-scores were then interpreted as follows: >-1, normal; −1 to −2.5, osteopenia; <-2.5, osteoporosis.10 Within the analysis software, implant materials were automatically detected and removed as artifact via ASAD. To examine the effectiveness of ASAD for removing implant artifact from the analysis, additional measures were taken and scored when artifact was manually removed from the images by the technician (Manual artifact correction, MAC, Fig. 1). This procedure was performed within the analysis software using a brush/eraser-like tool to edit what is referred to in the software as “point-typing” by removing remaining implant hardware not automatically excluded via ASAD (Fig. 1). During MAC, the technician took care to only remove (paint out) remaining implant hardware (and not bone) from the image.
Fig. 1.
Image showing the DEXA control image with no implant present, automated artifact detection and removal (showing undetected implant hardware remaining), and manual artifact correction (MAC – requiring manual editing of scan image to remove remaining artifact).
Statistical analysis
All data were analyzed using SPSS (V.23, IBM Statistics®, Armonk, New York, USA). A mixed-model ANOVA was used to compare BMD and T-scores between the control limb, the TKA limb following ASAD, and the TKA limb following MAC. The same procedure was then performed comparing total-body BMD following ASAD, total-body following MAC, and BMD assessed from the non-TKA side only. All detected significant interactions were followed by a Tukey's post hoc test for pairwise comparisons. For all significant comparisons, effect size was calculated using either a Cohen's d (t-test) or Phi (Chi-square) statistic and was interpreted as follows: 0–0.1 (negligible, N); 0.1–0.3 (small, S); 0.3–0.5 (moderate, M); 0.5–0.7 (large, L); >0.7 (very large, VL).11 Chi-square analysis was also used to determine the effect of TKA implants on BMD risk classification frequencies for both regional and total body measures. Type I error was set as α = 0.05 for all analyses.
Results
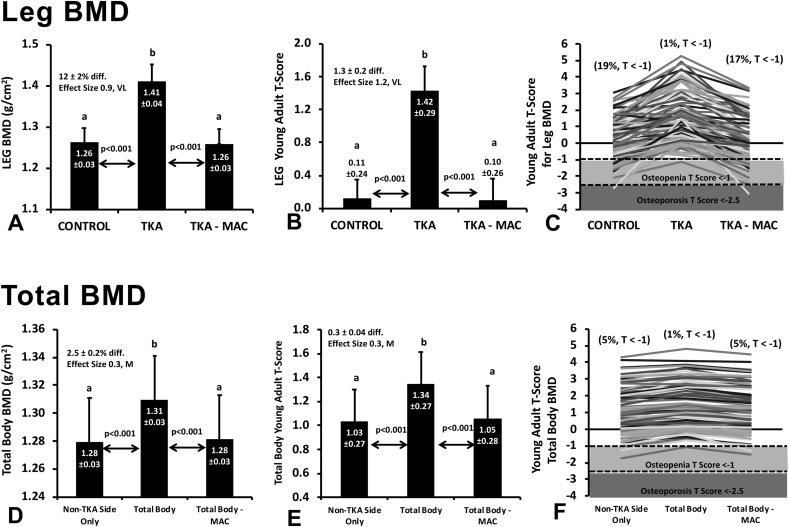
Results of our analysis are presented in Fig. 2(A-F).
Fig. 2.
Impact of knee implant on DEXA measures. Data are presented as means ± 95CI for bone mineral density (BMD, g/cm2) of the legs and total body (A & D) with corresponding T-scores calculated for each measure (C & E). Regional limb comparison (A & B): CONTROL, limb with no implant present; TKA, limb with TKA implant assessed using standard iDXA software analysis; TKA-MAC, limb with TKA implant following manual artifact correction. Total body comparison (D & E) Total body, total body BMD with implant included assessed suing standard iDXA software analysis; Non-TKA Side Only, BMD assessed from the lateral side of the body with no implant; Total Body – MAC, total body BMD analyzed following manual artifact removal. Individual patient data are shown for those classified as normal, osteopenic (T < −1), or osteoporotic (T < −2.5) regionally (E) and for the total body across each analysis comparison (F).
Limbs containing TKA implants were found to have significantly higher BMD [12.0 ± 1.7% difference, p < 0.001, ES 7.24(VL)] when analyzed following ASAD compared to control limbs that did not have an implant (Fig. 2A). This resulted, on average, in a 2.5 ± 0.2% overestimation of total body BMD [for a single implant, Fig. 2D, p < 0.001, ES 1.76(VL)] relative to the contralateral side. When analyzing legs, 19 of the 100 patients (19%) were found to have low BMD falling into the osteopenic (T-score < -1) or osteoporotic (T-score < -2.5) categories (Fig. 2B and C). This was masked in the TKA limb whereby only 1 of 100 (1%) patients were observed to have a T-score below −1 indicating that the implant resulted in an overestimation of BMD. With regards to the impact of a single knee implant on the total-body analysis when ASAD was used (Fig. 2E and F), only 1 of 100 (1%) patients were observed to be osteopenic whereas 5 of 100 patients (5%) were observed to be osteopenic when analyzing the non-TKA side only. MAC was found to successfully ameliorate the overestimation of BMD and under-classification of osteopenia for both regional and total-body measures (Fig. 2A–F).
Discussion
In the present study, we determined that standard artifact removal via ASAD typically used in total body DEXA analysis results in overestimation of BMD in older adults with total knee implants that may result in misclassification of bone health and risk for low BMD, osteopenia, and osteoporosis. These findings are of clinical importance for those (physicians, radiologists, wellness professionals) who treat or evaluate total-body DEXA results from patients with joint implants. Patients who are misclassified as having a normal BMD when they in fact have an at-risk or overtly osteopenic BMD may, as a result, not receive appropriate interventions to prevent progression such as vitamin D and calcium supplementation, light physical activity, and in some cases, bisphosphonate or other medical treatment.12 Consequently, this occurrence may unknowingly also contribute to a continued elevated risk for fragility fractures in those with joint replacements.13
Fragility fractures are an increasingly burdensome problem within our aging population. Current cost in treating the immediate fracture amounts to $14 billion per year in the United States alone.14 That is not including the eventual medical sequela that can result from sustaining a fragility fracture. Identification and treatment of patients having osteoporosis and being at risk for fragility fractures with appropriate medical intervention (i.e. bisphosphonates) has been shown to decrease the incidence of fragility fractures by as much as 50%.15 Our present data showed that total-body BMD was overestimated by about 2.5% per knee implant and limb specific BMD was overestimated by 12% (Fig. 2A,D). This resulted in misclassifying 4% of total-body scans and 18% of leg specific scans as showing normal BMD in cases where the contralateral side without the implant were found to be osteopenic (Fig. 2C,F). Regarding total-body BMD assessment, we find it probable that the more joint replacements a person has, the greater the overestimation of BMD if artifact is not manually corrected. If scaled up nationally, this could result in a high number of missed diagnoses in populations who may stand to benefit from interventions. Therefore, it is important that individuals with joint implants seeking a total-body DEXA scan for tracking of BMD and body composition as well as personal physicians should be aware of the present issues with measurement inaccuracies due to implant artifact.
Importantly, MAC was observed to correct for DEXA software overestimation of BMD in this population. For technicians and radiologists, this finding highlights the importance of close monitoring of whether or not automated software artifact removal is adequate and when manual correction of the image is needed prior to providing patients and physicians with results that indicate T-score associated fracture risk. Additionally, these data highlight the importance of physicians viewing DEXA images in addition to the results output to ensure that proper implant artifact removal has been performed. Crucially, any portion of the implant not removed from the image will be interpreted in the analysis as native bone for these types of scans. In the hands of experienced technicians, limb and total-body BMD analyzed on the non-TKA side only closely matched our manually corrected values when including the implanted limb. While this may solve the present artifact issue, it is worth noting that MAC can be time consuming (taking upwards of 10–15 min per implant on average in our investigation), and subjective based on the technician training and experience.16,17
This study evaluated total-body and regional DEXA measures from a single total-body scan rather than using separate isolated site-specific central (example: spine/hip/pelvis) or peripheral (example: forearm/hand) scans commonly used for final formal diagnosis of osteopenia or osteoporosis.18, 19, 20 While central DEXA scans are commonly used for the formal diagnosis of osteopenia and osteoporosis,19 total-body scans are commonly used in a variety of clinics and health centers at a reduced cost as part of general health-based screening programs as they can be performed in a reduced amount of time while also providing other valuable health information such as body composition and visceral fat analysis.21, 22, 23 Therefore, while the findings presented in the present study are reflective of total-body scans only, we find it likely that there remains a high volume of patients with implants being under-diagnosed. For example, patients living in smaller rural centers without access to central DEXA scans may have incorrect screenings performed if corrections are not made. For practical clinical application, if a physician, therapist, trainer, or other professional health practitioner were to view a total-body DEXA report whereby MAC had not been performed to remove all implant artifact (and may not be readily reanalyzed), it may be preferred to recalculate T-score values and draw interpretation from the side of the body that does not have implants. However, this may not be possible in the event of bilateral implants at one or more locations. Further study would need to determine whether or not it would be a valid alternative to recalculate BMD values and T-scores by mathematically correcting for implant error.
In addition to the primary findings of this study, we considered if software design and brand made a difference in our results. All participants in this study underwent DEXA analysis from the same model of DEXA. However, we did observe this error to be present in a single case scan performed using a separate DEXA model (Horizon, Hologic, San Diego, CA) where the overestimation of BMD was present similar to the scans in this investigation (Fig. 3). Therefore, more research is needed to determine the extent to which other software designs have similar issues with artifact removal.
Fig. 3.
Single case bone mineral density analysis with demonstrated automated artifact correction error observed in a different DEXA make and model (Horizon A, Hologic, San Diego, CA).
Limitations of the study include the inclusion of only participants with total knee replacements. While not able to confirm, we find it likely that the present observations would hold true for any large, metal-based in-situ implants in a patient (example: total hip, shoulder, ankle or elbow). Additionally, the lack in diversity of software used may make it difficult to draw conclusions to other makes and models of DEXA scanners, however due to our small observed irregularity mentioned earlier, we find it highly likely that the issue of implant artifact error likely extends beyond the model of scanner used in this study alone. Lastly, validation studies remain needed for non-software-based calculations for correcting BMD overestimation in this population in the event that images are unable to be reanalyzed.
Conclusion
Conventional means for evaluating bone health via total body DEXA overestimates limb and overall BMD in older adults with in situ total knee replacements, and therefore under-identifies low BMD, osteopenia, and osteoporosis in at-risk populations. Those who have joint implants should be flagged by technicians and physicians as such for closer scrutiny, and potentially manual correction, of total body DEXA images or automatically be referred for central or peripheral scans rather than total-body scans. In the case of any scan, it is critical that all implant artifact be removed (manually or by automated software) prior to final analysis and diagnosis. Normalization of values can occur through thorough manual artifact removal, or by applying correction factors described in this study for the purposes of screening. Future research should focus on overall national prevalence of this phenomenon and which types of patients or implant types are most at-risk for having incorrect DEXA study results.
Submission statement
We confirm that we have given due consideration to the protection of intellectual property associated with this work and there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Authors’ contributions
Katharine D. Harper assisted in data analysis, primary literature review, and was the primary manuscript writer. Terry A. Clyburn and Stephen J. Incavo provided the primary source of patients within the database used for analysis. Both also contributed to manuscript development. Bradley S. Lambert was responsible for facility and equipment support, data collection, analysis, statistics, generation of figures and tables, assistance in manuscript writing.
Ethical approval
The procedures were approved by the institutional review board for research involving human subjects (IRB PRO00015628), and all volunteers signed a written informed consent prior to participating in the experimental procedures.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Wright N.C., Looker A.C., Saag K.G., et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Maghraoui A., Roux C. DXA scanning in clinical practice. QJM. 2008;101(8):605–617. doi: 10.1093/qjmed/hcn022. [DOI] [PubMed] [Google Scholar]
- 3.Kanis J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int. 1994;4(6):368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 4.Yedavally-Yellayi S., Ho A.M., Patalinghug E.M. Update on osteoporosis. Prim Care. 2019;46(1):175–190. doi: 10.1016/j.pop.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Denton N., Karpe F. Measuring body composition and regional fat mass accurately. Practical Diabetes. 2016;33(7):224–226. [Google Scholar]
- 6.Fang M., Noiseux N., Linson E., Cram P. The effect of advancing age on total joint replacement outcomes. Geriatr Orthop Surg Rehabil. 2015;6(3):173–179. doi: 10.1177/2151458515583515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan M., Premkumar A., Sheth N.P. Projected volume of primary total joint arthroplasty in the US, 2014 to 2030. J Bone Joint Surg. 2018;100(17):1455–1460. doi: 10.2106/JBJS.17.01617. [DOI] [PubMed] [Google Scholar]
- 8.Borrud L.G., Flegal K.M., Looker A.C., Everhart J.E., Harris T.B., Shepherd J.A. Body composition data for individuals 8 years of age and older: US population, 1999–2004. Vital and health statistics. Series 11. Data from the national health survey. 2010;(250):1. [PMC free article] [PubMed] [Google Scholar]
- 9.Looker A., Borrud L., Hughes J., Fan B., Shepherd J., Sherman M. Total body bone area, bone mineral content, and bone mineral density for individuals aged 8 years and over: United States, 1999-2006. Vital and health statistics. Series 11. Data from the National Health Survey. 2013;(253):1–78. [PubMed] [Google Scholar]
- 10.Leib E.S., Lewiecki E.M., Binkley N., Hamdy R.C. Official positions of the international society for clinical densitometry. J Clin Densitom. 2004;7(1):1–5. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 11.Ialongo C. Understanding the effect size and its measures. Biochem Med. 2016;26(2):150–163. doi: 10.11613/BM.2016.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddy D., Cc J.J., Cummings S., et al. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Status report. Osteoporos Int. 1998;8(suppl 4) [PubMed] [Google Scholar]
- 13.Elvey M., Pugh H., Schaller G., Dhotar G., Patel B., Oddy M. Failure in the application of fragility fracture prevention guidelines. Ann R Coll Surg Engl. 2014;96(5):381–385. doi: 10.1308/003588414X13946184901164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blume S.W., Curtis J.R. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011;22(6):1835–1844. doi: 10.1007/s00198-010-1419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad N., Sunderamoorthy D., Martin J., Murray J. Secondary prevention of fragility fractures: are we following the guidelines? Closing the audit loop. Ann R Coll Surg Engl. 2006;88(5):470–474. doi: 10.1308/003588406X116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg M., Kharb S. Dual energy X-ray absorptiometry: pitfalls in measurement and interpretation of bone mineral density. Indian J Endocrinol Metab. 2013;17(2):203. doi: 10.4103/2230-8210.109659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewiecki E.M., Lane N.E. Common mistakes in the clinical use of bone mineral density testing. Nat Clin Pract Rheumatol. 2008;4(12):667–674. doi: 10.1038/ncprheum0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelmohsen A.M. Comparison of central and peripheral bone mineral density measurements in postmenopausal women. J Chiropr Med. 2017;16(3):199–203. doi: 10.1016/j.jcm.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake G.M., Fogelman I. Peripheral or central densitometry: does it matter which technique we use? J Clin Densitom. 2001;4(2):83–96. doi: 10.1385/jcd:4:2:083. [DOI] [PubMed] [Google Scholar]
- 20.Hans D.B., Shepherd J.A., Schwartz E.N., et al. Peripheral dual-energy X-ray absorptiometry in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11(1):188–206. doi: 10.1016/j.jocd.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Mattila V.M., Tallroth K., Marttinen M., Pihlajamäki H. Body composition by DEXA and its association with physical fitness in 140 conscripts. Med Sci Sports Exerc. 2007;39(12):2242–2247. doi: 10.1249/mss.0b013e318155a813. [DOI] [PubMed] [Google Scholar]
- 22.Zanzonico P. Nuclear Medicine Textbook. Springer; 2019. Technical aspects of dual-energy X-ray absorptiometry and quantitative computed tomography for assessment of bone mineral density and body composition; pp. 1085–1098. [Google Scholar]
- 23.Moreno B., Crujeiras A.B., Bellido D., Sajoux I., Casanueva F.F. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine. 2016;54(3):681–690. doi: 10.1007/s12020-016-1050-2. [DOI] [PubMed] [Google Scholar]