Abstract
Cancer cachexia is a progressive disorder characterized by body weight, fat, and muscle loss. Cachexia induces metabolic disruptions that can be analogous and distinct from those observed in cancer, obscuring both diagnosis and treatment options. Inflammation, hypogonadism, and physical inactivity are widely investigated as systemic mediators of cancer-induced muscle wasting. At the cellular level, dysregulation of protein turnover and energy metabolism can negatively impact muscle mass and function. Exercise is well known for its anti-inflammatory effects and potent stimulation of anabolic signaling. Emerging evidence suggests the potential for exercise to rescue muscle's sensitivity to anabolic stimuli, reduce wasting through protein synthesis modulation, myokine release, and subsequent downregulation of proteolytic factors. To date, there is no recommendation for exercise in the management of cachexia. Given its complex nature, a multimodal approach incorporating exercise offers promising potential for cancer cachexia treatment. This review's primary objective is to summarize the growing body of research examining exercise regulation of cancer cachexia. Furthermore, we will provide evidence for exercise interactions with established systemic and cellular regulators of cancer-induced muscle wasting.
Keywords: Physical activity, Anabolic resistance, Inflammation, IL-6, Metabolic dysfunction, Protein turnover
Introduction
Cancer-induced wasting, or cancer cachexia, is a progressive disorder associated with a terminal disease characterized by severe body weight, fat, and skeletal muscle loss.1 Cachexia is multifactorial and induces nutritional and metabolic abnormalities that can be analogous and distinct from the patient's underlying condition, thus obscuring both diagnosis and treatment options.2 Cachectic patients may experience poor disease prognosis, increased treatment-related toxicities, fatigue, reduced physical well-being, and overall quality of life. Skeletal muscle comprises 40%–50% of body mass and is maintained through an ongoing balance of protein synthesis and degradation, which are tightly regulated by systemic and local environmental stimuli. Inflammation, hypogonadism, malnutrition, insulin resistance, and sedentary behavior can disrupt muscle energy metabolism and protein turnover during cancer cachexia3 (see Fig. 1). Suppressed muscle protein synthesis has been reported in preclinical cancer models and cancer patients; however, interventions directed toward increasing protein synthesis (i.e., amino acids, protein administration) alone do not fully counteract cancer-induced wasting. Therefore, a multimodal intervention approach may be necessary.4, 5, 6, 7, 8 The inability to stimulate protein synthesis, termed “anabolic resistance,” is observed in models of aging and wasting disorders.3,9, 10, 11 However, mechanisms governing this phenomenon are complex and not yet fully understood.
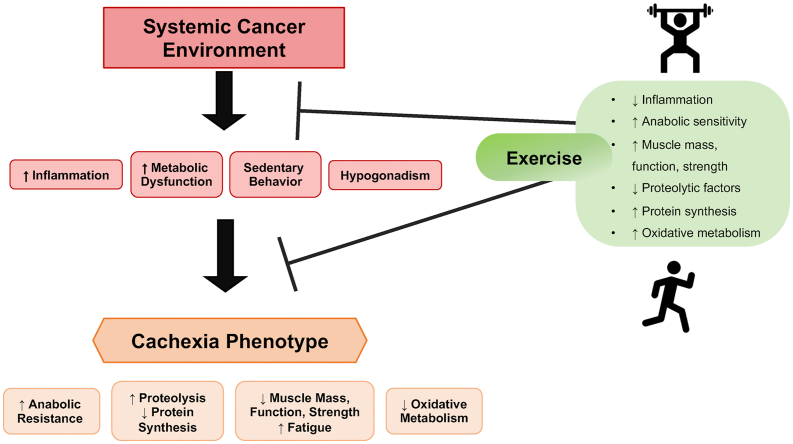
Fig. 1.
Exercise Regulation of Cancer-Induced Cachexia. The systemic cancer environment induces whole body alterations including chronic inflammation, metabolic dysfunction, sedentary behavior, hypogonadism, endocrine disruption, insulin resistance, and malnutrition. These systemic factors contribute to the development and progression of the cachectic phenotype. Cachexia can induce a metabolic shift in which skeletal muscle develops resistance to anabolic stimuli (e.g., nutrients, physical activity, growth hormones), altered protein turnover, decreased oxidative metabolism, and an overall loss of muscle mass, strength and function. Regular physical exercise (e.g., walking, running, cycling, resistance training) can benefit patients by improving skeletal muscle function, strength, and metabolic homeostasis, reducing muscle mass loss, and suppressing systemic and cellular signaling associated with cancer-induced wasting.
Regular physical exercise has proven to benefit individuals with chronic diseases by improving muscle metabolic homeostasis and suppressing intrinsic signaling associated with wasting12, 13, 14 (see Fig. 1). According to the 2020 ASCO guidelines,15 no recommendation can be made for exercise-based interventions in the management and treatment of cachexia due to the lack of clinical trial evidence. However, a promising intervention remains a combination of exercise with other therapies.16 There has been a rapidly growing interest in understanding how exercise can interact with cancer cachexia's development and progression, with over 450 publications consisting of both literature reviews and original research articles being listed on PubMed related to cancer cachexia and exercise. Amazingly, more than 290 of these have been published since 2015. Preservation of muscle mass and improving physical performance are essential goals of cachexia therapy. The anti-inflammatory nature of exercise and its capacity to induce positive metabolic alterations provide a strong premise for further mechanistic investigations for related to improving the cachectic condition. While cancer cachexia dramatically reduces muscle strength and endurance, the mechanistic underpinnings of these functional changes are not fully understood.17 Skeletal muscle is highly adaptable and responsive to muscle contraction and loading, which mediate increases in anabolic signaling.18 Adaptations to physical activity are dependent on exercise mode, intensity, duration, and frequency.19 Although increased activity is beneficial for cancer patients, prescribing regimens that are well adhered to is difficult. The current recommended dose to elicit health benefits is 150–300 min/week of moderate-intensity aerobic exercise (i.e., walking, running, cycling, swimming) and strength training ∼2 days a week. However, these “minimal dose” guidelines relate to disease prevention rather than treatment.20
Interestingly, epidemiological studies and clinical trials show that improved prognosis in physically active cancer patients is more closely associated with exercise performed after diagnosis as opposed to exercise habits prior.21, 22, 23 Sedentary behavior and muscle disuse can exacerbate disease and treatment-related disruptions. Therefore, even small doses of physical activity or the use of exercise mimetics (i.e., neuromuscular electrical stimulation, pharmacological agents24,25) may provide critical physiological benefits to the patient.26, 27, 28, 29 This review will summarize the growing body of research examining how exercise can either prevent or treat cancer cachexia. Furthermore, we will provide evidence and support for how exercise can interact with established systemic and cellular regulators of cancer-induced muscle wasting.
Systemic mediators of cancer-induced muscle wasting
Inflammation
To recognize and treat cancer cachexia, an understanding of its pathophysiology is imperative. Systemic inflammation is a significant contributor to cancer-induced cachexia through the increased production of related pro-inflammatory cytokines, tumor factors, and hormones resulting in metabolic alterations in patients.30,31 Inflammatory mediators promote activation of wasting related pathways in both adipose tissue and skeletal muscle. Chronic inflammation is prevalent in both clinical and preclinical models of cachexia. For example, mouse models of cachexia have demonstrated elevated interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), TNF-like inducer of apoptosis (TWEAK), TNF receptor (TNFR)-associated factor 6 (TRAF6), interferon-gamma (INF-γ), and leukemia inhibitory factor (LIF).31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 The activated cytokines can act on multiple pathways, including the nuclear factor-κB (NF-κB) pathway, p38 mitogen-activated protein kinase (MAPK) pathway, and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. These signaling cascades are associated with increased activity of the ubiquitin-proteasome system (UPS), mitochondrial dysfunction, increased oxidative stress, and dysfunctional hypothalamic-pituitary-adrenal (HPA) axis signaling (e.g., cortisol response).42, 43, 44, 45, 46 Despite evidence for the involvement of several inflammatory mediators during cachexia development in preclinical models, we have a limited understanding of how these signaling pathways interact, form regulatory networks, or of their involvement in redundant signaling cascades with different cancers. This complexity appears to be a significant barrier for targeting a single signaling cascade or regulatory process to preserve skeletal muscle mass in the cancer patient.
Hypogonadism
Hypogonadism, a sex hormone deficiency, is often associated with the cachectic condition.47 However, understanding its role in cancer cachexia and potential interaction with other well studied drivers of cachexia such as inflammation is currently underdeveloped. Estrogens and androgens are established regulators of growth and maturation and affect many adult tissues targeted by cachexia, including bone, skeletal muscle, and adipose tissue.48 Sex hormones can also regulate processes involving central nervous system function, immune function, and metabolism, which impact cancer cachexia. Hypogonadism can result from normal aging, gonad dysfunction, and many chronic disease states and has been linked to declines in muscle mass and function.49, 50, 51, 52 Cancer is often diagnosed after 65 years of age, and the age-related decline in circulating sex steroids could be a factor contributing to the cachectic environment.53 Skeletal muscle exhibits sexual dimorphism in overall mass, fiber size, metabolic enzymes, expression of different myosin isoforms, fatiguability, and gene expression.54, 55, 56 Both testosterone and estrogen target skeletal muscle gene expression, metabolism, and protein turnover and can be released in response to muscle contraction.57,58 Notably, 70% of male patients with cancer cachexia have low testosterone levels.59,60 In males, low testosterone levels lead to decreased muscle mass and strength, and testosterone replacement therapy effectively attenuates these deficits.59,61, 62, 63 Testosterone increases muscle insulin-like growth factor 1 (IGF-1) and protein synthesis through activation of Akt/mTORC1 signaling64 and can decrease systemic inflammatory cytokines such as IL-6 and TNF-α in humans.64, 65, 66 The mechanistic role of sex hormones in cancer cachexia's progression and treatment, particularly in females, is not well understood but has been recently reviewed.67,68 Female sex hormones may contribute to an attenuation of inflammation by inhibiting IL-6 transcription and associated signaling.32,69 17 β-Estradiol – the most concentrated form of circulating estrogen – favorably affects skeletal muscle contractility independent of physical activity in preclinical models.70 Estrogen can also induce IGF-1 signaling.71, 72, 73 Decreased circulating estrogen may alter the cachectic phenotype through dysregulation of protein turnover driven by increased inflammation and autophagy and altered anabolic signaling.74 Taken together, hypogonadism during cancer negatively affects survival and patient quality of life.59 Further research is needed to determine the efficacy and mechanisms of sex hormones' role during cancer cachexia.
Inactivity and disuse
Many cancer patients suffer from chronic fatigue, malnutrition, and limited ability to perform physical activity due to disease progression along with anti-cancer treatment.17,75,76 Phenotypically, cachexia may appear similar to starvation, however in mice and humans, cachexia often precedes decreases in food intake, and cachexia can occur with or without the presence of anorexia.77,78 While malnutrition does occur in cancer patients, metabolic disruptions, and altered resting energy expenditure may also be significant contributors to wasting.79, 80, 81 Preclinical models have shown that mice with cachectic phenotypes display low volitional physical activity levels before cachexia development.82, 83, 84 Muscle disuse, similar to disease-induced atrophy (i.e., cachexia), negatively affects metabolism by decreasing protein synthesis, increasing degradation, and promoting resistance to anabolic stimuli (i.e., IGF-1, nutrients, physical activity).26,85,86 More specifically, skeletal muscle atrophied by disuse exhibits decreased Akt/mTORC1 signaling and suppressed muscle protein synthesis, which is similar to cachexia.86,87 Prolonged inactivity is also associated with increased reactive oxygen species (ROS), promoting muscle protein breakdown, and has been extensively reviewed.88 There is a strong rationale for further examination into the impact of disuse and sedentary behavior on cachectic muscle responses to other anabolic therapies.
Cellular regulation of cancer-induced muscle wasting
Increased inflammatory signaling
Cellular mechanisms driving cancer-induced skeletal muscle wasting concentrate on disrupted protein turnover regulation, mitochondrial dysfunction,89,90 and an emerging area of impaired muscle regeneration.91 Cellular inflammatory signaling, specifically IL-6 family members, have been widely investigated as regulators of muscle protein turnover in some cancer types.92 IL-6 is a pleiotropic cytokine involved in processes spanning immune-inflammatory response to skeletal muscle's response to exercise.40 Cachectic patients demonstrate increased plasma levels of IL-6 compared to non-cachectic patients.93 IL-6 exerts effects on target cells by forming a heterodimer at the cell surface with glycoprotein 130 (gp130) and the IL-6 receptor,37,92,94 which activates intracellular signaling involving JAK/STAT and ERK1/2. The cancer environment can promote skeletal muscle STAT3 phosphorylation, and STAT3 inhibition prevents cachexia in some preclinical cancer models.37,38 Notably, STAT3 also seems to play a part in the blockage of autophagy pathways by altering the beclin-1 complex.95 In ApcMin/+ cachectic mice, activation of these pathways via IL-6 is associated with decreased muscle protein synthesis by mTORC1 signaling and induced muscle protein breakdown.35,49 Moreover, IL-6 overexpression in pre-cachectic ApcMin/+ mice accelerates body weight loss and muscle wasting and can suppress basal protein synthesis in tumor and non-tumor bearing mice.96 Furthermore, in cultured myotubes, IL-6 suppression of mTORC1 activity is dependent on AMPK activation and independent of STAT3 signaling.34 Circulating tumor-derived factors can disrupt mitophagy and mitochondrial remodeling.97 IL-6 has been studied extensively as a potential inflammatory driver for muscle wasting in cancer cachexia. However, the interaction between physical activity and IL-6 signaling requires further study to determine if this regulatory network is a viable therapeutic target for reversing cachexia.
Dysregulation of muscle protein turnover
Disruptions to the homeostatic regulation of muscle protein turnover can have detrimental consequences on cellular metabolism, muscle function, and growth. Muscle atrophy involves the breakdown and net loss of intracellular proteins and organelles, resulting in smaller myofibers. Studies have shown that cancer patients exhibit higher whole-body protein turnover with reduced protein synthesis, and patients with low muscle protein generally present poorer clinical outcomes.6,98,99 Many factors can disrupt protein turnover and muscle wasting in cancer (for reviews see Refs. 100,101); however, both hypo-anabolism and hyper-catabolism still create a unique challenge to discovering therapeutics. During cachexia, protein breakdown and muscle catabolism can occur even with adequate nutrient intake.102 Reduced protein synthesis and an impaired response to anabolic stimuli - anabolic resistance - must also be overcome to treat cancer-induced wasting.3,103,104 The IGF-1/PI3K/Akt/mTORC1 signaling pathway integrates various stimuli to activate protein synthesis and growth-related pathways. Reduced IGF-1 levels occur in preclinical cancer cachexia models and could have a role in the pathophysiology of muscle wasting.105 Several experimental cachexia models have reported mTORC1 suppression.34,106,107 Muscle protein degradation in cancer cachexia primarily involves activation of the UPS and autophagy lysosomal systems, which normally function to clean up damaged proteins under physiological conditions, but cause excessive protein degradation in diseased states.101 Glucocorticoids, cytokines (i.e., TNF-⍺, NF-κB), activins, myostatin, proteolytic enzymes, ROS, and tumor released factors108, 109, 110, 111 can all regulate muscle protein degradation. Mitochondrial dysfunction can increase myonuclear apoptosis in skeletal muscle, further contributing to cachexia.112,113 In fact, activation of apoptotic factors such as B cell leukemia/lymphoma 2 (BCL2)-associated X protein (BAX) and presence of DNA fragmentation has been described in wasting muscles from either cancer patients or tumor-bearing mice.82,114, 115, 116 Under atrophic conditions, UPS is responsible for the breakdown of larger myofibrillar proteins, while autophagy contributes to the breakdown of long-lived proteins and organelles.117 UPS and autophagy activation exacerbate muscle loss in tumor-bearing animals.101,118,119 A better understanding of cancer-induced mechanisms regulating anabolic plasticity in patients may elucidate valuable treatment options for muscle wasting and improve responsiveness to anabolic therapies.
Muscle metabolic dysfunction
Mitochondrial dysfunction is an established regulator of cancer-induced muscle wasting.97,120,121 Skeletal muscle plays a critical role in regulating systemic metabolism and displays plasticity to adapt to demands such as nutrient availability, activity, and the systemic environment.122 Interestingly, nutritional interventions, such as diets high in fat and low in carbohydrates (i.e., ketogenic diet), may reduce tumor growth and improve treatment efficacy by altering cancer cell and systemic metabolism (for review see:123). Emphasis has been placed on ketone bodies' anticatabolic effects during inflammation-related muscle atrophy.124, 125, 126 In vitro, ketone bodies can attenuate tumor conditioned media induced myotube E3 ligase expression.127 In C26-tumor-bearing mice, a ketogenic diet partially attenuated muscle and body weight loss.128 Furthermore, a ketogenic diet can impact the gut microbiome and reduce inflammation129; ketone bodies' impact on metabolic dysfunction in cachexia warrants further investigation. Cancer-induced alterations involve increased oxidative stress, decreased mitochondrial biogenesis, and elevated mitophagy. These changes can result in mitochondrial loss and dysfunction,90 which is evident in tumor-bearing mice before muscle wasting.130 Skeletal muscle mitochondrial dysfunction also induces functional changes resulting in increased muscle fatigability and weakness.97 Declines in mitochondrial health with severe wasting are apparent in both oxidative and glycolytic muscle and is associated with increased circulating IL-6.131 There is a strong premise for examining how the hypogonadal state contributes to cancer-induced mitochondrial dysfunction. Estrogen can regulate muscle mitochondria biogenesis and mitophagy.70 It is interesting to consider whether an improvement in the hypogonadal condition could positively impact cachectic muscle mitochondrial dysfunction. Muscle disuse atrophy involves increased oxidative stress and disrupted mitochondrial quality control.132 Moreover, models of cancer cachexia display disrupted mitochondrial dynamics (see review 97) and suppressed PGC-1α expression.107 Data is equivocal regarding PGC-1α in disuse as some studies report increases,133 decreases,134 or no change.135 Interestingly, PGC-1α overexpression does not protect against disuse-induced atrophy but does attenuate E3 ubiquitin ligase expression.136 Increasing metabolic demand in muscle during exercise exerts positive effects on mitochondrial function and activates genes responsible for mitochondrial biogenesis, while sedentary behavior is associated with decreased mitochondrial health.137, 138, 139 Current data does not fully describe if improved mitochondrial health can overcome anabolic resistance in skeletal muscle.
A role for exercise in the prevention and treatment of cachexia
A role for exercise
Increased physical activity can benefit cancer patients by positively impacting muscle mass, function, and metabolism and decreasing treatment-related toxicity. Physical activity elicits systemic anti-inflammatory effects acting to reduce protein degradation and increase protein synthesis; moreover, training can improve oxidative metabolism and maximize substrate utilization to combat metabolic dysfunction.140,141 Additionally, ketone bodies, organic compounds derived from lipids, are oxidized during prolonged exercise and utilized as fuel sources.142 Some types of ketone bodies may function to maintain redox homeostasis in response to metabolic stress, reduce inflammation, and improve exercise performance.143,144 A single bout of exercise in healthy individuals activates muscle signaling pathways linked to energy metabolism and, when repeated over-time (i.e., training) can elicit beneficial metabolic adaptations.145,146 Hence, exercise's potential therapeutic role in preventing or treating cancer cachexia should examine both the acute response and chronic adaptations to exercise. However, tumor-derived factors and increased metabolic stress can interfere with muscle mechanical signaling. For example, in vitro models using tumor-derived culture media impair the mechanical stretch activation of myotube protein synthesis.147 Additionally, in severely cachectic mice, the anabolic signaling response to muscle contraction is disrupted.139 Muscle disuse atrophy and aging, variables to consider with cancer patients, can impair the muscle's response to anabolic stimuli (e.g., amino acids, insulin).148, 149, 150 Understanding physiological skeletal muscle signaling in response to exercise and mechanical stimuli is critical when designing interventions to treat cancer-induced wasting. Interestingly, mechanical stimuli can activate muscle protein synthesis through mTORC1, independent of Akt signaling.151 Acute bouts of muscle contraction increase mTORC1 signaling and phosphorylation of its downstream effector p70.152 In fact, increased muscle mass after chronic mechanical stimulation is strongly associated with p70 phosphorylation.153,154 Mitogen-activated protein kinase (MAPK) signaling cascades, including ERK1/2 and p38, are increased during exercise and myofiber mechanical stretch.155,156 ERK-dependent mTORC1 activation is involved in muscle mass regulation,157 but mechanical stimulation can induce mTORC1 signaling independent of ERK, which may involve phosphatidic acid signaling.158,159 Mechanical activation of protein synthesis pathways combined with other anabolic therapies may provide a means to circumvent anabolic resistance to nutrients and growth factors. In fact, exercise performed with nutritional interventions can improve muscle mass and reduce tumor growth in animal models.160,161 However, further studies examining muscle sensitivity to different exercise types and nutrition status are needed to elucidate reliable interventions.
In pre-clinical models, exercise performed before severe cachexia development can reduce indices of cachexia and mitigate treatment-related toxicities. Voluntary aerobic exercise in colon-26 (C26) mice can prevent muscle mass loss and improve function by modulating autophagy flux.21 Moderate treadmill exercise (1 h/d, 6 d/week, 5% grade) attenuates IL-6-dependent cachexia in ApcMin/+ mice.162 Moreover, myokines released during aerobic exercise have shown the potential for cachexia therapy through the downregulation of proteolytic factors.163 In a mouse model of prostate cancer, long-term voluntary wheel running (20wks) was sufficient to preserve muscle mass and function.164 In mice, exercise performed before cachectic tumor inoculation can prevent muscle loss and Akt/mTOR suppression.165 In vitro electrical stimulation or mechanical stretch can prevent chemotherapy-induced myotube atrophy.166 Increased muscle metabolic demand can increase mitochondrial biogenesis. For example, cachectic tumor-bearing mice subjected to an acute bout of low-frequency stimulation display increased activity of genes responsible for biogenesis: PGC1-α, NRF-1, and Tfam.139 High-frequency electrical stimulation can attenuate cachexia induced muscle loss, improve oxidative capacity, and activate mTOR signaling.167,168 Cancer cachexia induces muscle ERK1/2 and p38 MAPKs,169 and ERK signaling inhibition can promote anabolism.170 For example, inhibition of either ERK1/2 or p38 signaling rescues the mechanical stretch induction of myotube protein synthesis in the presence of LLC media.147 Resistance exercise increases muscle protein synthesis through mTORC1 and can improve mitochondrial function and muscle mass in tumor-bearing mice (see review11). Repeated bouts of eccentric contractions can increase muscle mass and oxidative metabolism in cachectic mice, and these changes coincide with reduced AMPK activity.3,168 Mechanically stimulated pathways and their regulation in cachexia induced muscle wasting in response to mechanical stimuli require further investigation as they could play a role in mTORC1 mediated protein synthesis and autophagy regulation. Combined exercise and nutritional interventions have shown improvements in patient physical function and quality of life. These multimodal approaches deserve further investigation for the promotion of anabolism and attenuated wasting in cancer patients.
Exercise modulation of oxidative stress during cancer cachexia
Increased pro-inflammatory cytokines and dysfunctional mitochondria contribute to muscle oxidative stress during cachexia.97 Understanding exercise's role in redox homeostasis modulation is essential, as excessive mitochondrial ROS production can promote tumorigenesis, disrupt cellular processes, and exacerbate declines in skeletal muscle mass and function (see reviews171,172). Muscle oxidative stress and mitochondrial dysfunction are observed early in cachexia progression, preceding muscle wasting.130 Many cachectic cancer patients and cachectic mice cannot perform standard exercise training paradigms.173 However, preventative therapeutics, such as exercise performed before muscle wasting and fatigue, may offset adverse outcomes by improving oxidative metabolism. Acute exercise promotes ROS production, which may serve as a necessary adaptive response, and chronic exercise training upregulates antioxidant defenses and can reduce inflammation and oxidative stress.174, 175, 176, 177 In C26-tumor-bearing mice, moderate aerobic exercise improved muscle mass and function and was associated with an improved redox balance.178 Aerobic interval training in tumor-bearing rats did not restore muscle mass but improved muscle function, overall survival, and reduced oxidative stress.179 Furthermore, in cancer patients, exercise reduced cancer-related fatigue, increased circulating antioxidants, and decreased blood markers of oxidative stress.180 Recently, the emphasis on oxidative stress during cancer progression has been directed towards understanding and preventing chemotherapeutics' toxic effects.181 However, we still lack knowledge of whether chemotherapy exacerbates oxidative stress during cancer cachexia progression and if exercise is sufficient to offset this response. Cancer patients may likely benefit from therapies that improve redox homeostasis (e.g., antioxidant supplementation, exercise, exercise mimetics). Whether these combined interventions serve to reinforce muscle antioxidant defenses and reduce oxidative stress remains an open question.
Exercise and muscle anabolic resistance
Unlike healthy muscle, wasting conditions can cause muscle responsiveness to anabolic stimuli to be reduced.10,85,182 The inability to stimulate protein synthesis in response to anabolic factors (e.g., anabolic resistance) has been observed in aging and cancer. It may play a central role in muscle function decrements during cachexia.9,11,103,183 Evidence for anabolic resistance has been observed in cancer patients with moderate weight loss and apparent systemic inflammation via impaired glucose uptake in response to insulin104. Unfortunately, our current mechanistic understanding of muscle protein turnover does not account for an inherent hour to hour physiological regulation of anabolic stimuli, such as feeding, fasting, and daily physical activity.84 A recent study reported that the administration of an HDAC inhibitor suppressed muscle IL6/STAT signaling and could improve anabolic sensitivity to androgen based therapy.184 Combining pharmacological agents and anabolic treatment has the potential to restore muscle responsiveness to these stimuli (i.e., androgens, exercise, nutrients) in cancer patients. In severe cachexia, IL-6/STAT signaling seems to have a role in the initial development of anabolic resistance. Determining mechanisms of anabolic plasticity in cancer patients may elucidate valuable treatment options and improve responsiveness to anabolic therapies. Since nutritional support cannot fully reverse cachexia, the suppressed responsiveness to nutrient supplementation may be related to dysfunctional protein turnover regulation. Cachectic cancer patients exhibit exacerbated whole-body protein turnover rates in response to feeding.185,186 Interestingly, cachectic pancreatic cancer patients did not increase whole-body protein synthesis after eating like healthy individuals, pointing to impaired anabolic plasticity in cancer.187 Williams et al. further showed that cachectic colorectal cancer patients did not increase muscle protein synthesis in response to feeding.188
Summary
The complex interplay of systemic and cellular disruptions in cancer cachexia has delayed the development of reliable treatment interventions to improve skeletal muscle mass, function, and metabolism. Skeletal muscle is crucial for movement, posture, breathing, and whole-body metabolism; consequently, altered muscle homeostasis intensifies cancer-induced systemic disruptions, worsening disease prognosis. Although exercise is not typically the first line of clinical therapy, its ability to reduce systemic inflammation and promote anabolic processes warrant continued study to treat cancer-induced wasting. Therefore, we reviewed the published research examining exercise and cancer cachexia. Overall, there is evidence that increased physical activity can help attenuate cancer cachexia progression in tumor-bearing mice. To date, there is not a recommended dose of exercise for cachectic cancer patients due to a lack of clinical trials. Results from clinical studies examining exercise are inconclusive (see review189), potentially due to poor cachexia diagnostic criteria and heterogeneity in patient cohorts (i.e., cancer type, sex, degree of cachexia, patient age) and variable muscle sample collection sites (i.e., rectus abdominis, quadriceps, diaphragm). Overall, human studies primarily focus on gastrointestinal cancer patients and suggest altered skeletal muscle morphology, increased proteolysis, systemic inflammation, and mitochondrial dysfunction.31,189 A multimodal treatment approach to cachexia management in cancer patients involving some type of physical activity can positively affect the rate of cachexia progression and functional decline. However, the achievable threshold of physical activity or exercise needed for these positive effects is not well understood. Published studies have demonstrated the ability of exercise to reduce systemic inflammation, reduce muscle mass loss, and improve muscle mitochondrial function. Exercise can stimulate muscle protein synthesis and, in combination with anti-catabolic agents, may have promise for ameliorating cancer-induced anabolic resistance in muscle. Research in clinical settings will provide evidence for the effectiveness of these strategies for improving mortality and quality of life. Well-controlled and characterized exercise studies in cancer patients and complementary mechanistic preclinical cachexia studies should provide further insight into the molecular signaling mechanisms and potential exercise benefits. This knowledge will help delineate the exercise dose that can attenuate cancer cachexia progression.
Submission statement
This manuscript has not been published and is not under consideration for publication elsewhere.
Authors’ contributions
All authors listed on the manuscript have made substantial contributions to the literature review and writing.
Conflict of interest
The authors have no conflict of interest to report.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 CA-121249 (National Cancer Institute) and R21 CA-231131 to JAC.
References
- 1.Muscaritoli M., Anker S.D., Argiles J., Aversa Z., Bauer J.M., Biolo G., et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG)“cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Baracos V.E., Martin L., Korc M., Guttridge D.C, C H Fearon K., et al. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 3.Hardee J.P., Montalvo R.N., Carson J.A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism. Oxid Med Cell Longev. 2017;2017:8018197. doi: 10.1155/2017/8018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworzak F., Ferrari P., Gavazzi C., Maiorana C, Bozzetti F., et al. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer. 1998;82(1):42–48. doi: 10.1002/(Sici)1097-0142(19980101)82:1<42::Aid-Cncr5>3.0.Co;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Smith K.L., Tisdale M.J. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br J Canc. 1993;67(4):680–685. doi: 10.1038/bjc.1993.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery P.W., Edwards R.H., Rennie M.J., Souhami R.L., Halliday D., et al. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J. 1984;289(6445):584–586. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoun S., Raynard B. Muscle protein anabolism in advanced cancer patients: response to protein and amino acids support, and to physical activity. Ann Oncol. 2018;29(suppl_2):ii10–ii17. doi: 10.1093/annonc/mdx809. [DOI] [PubMed] [Google Scholar]
- 8.Gullett N.P., Mazurak V.C., Hebbar G., Ziegler T.R., et al. Nutritional interventions for cancer-induced cachexia. Curr Probl Canc. 2011;35(2):58–90. doi: 10.1016/j.currproblcancer.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.S Fry C., B Rasmussen B. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 2011;4(3):260–268. doi: 10.2174/1874609811104030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson J.M., Volpi E., Rasmussen B.B. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41(4):216–223. doi: 10.1097/JES.0b013e3182a4e699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montalvo R.N., Hardee J.P., VanderVeen B.N., Carson J.A., et al. Resistance exercise's ability to reverse cancer-induced anabolic resistance. Exerc Sport Sci Rev. 2018;46(4):247–253. doi: 10.1249/JES.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell C.J., Churchward-Venne T.A., Bellamy L., Parise G., Baker S.K., Phillips S.M., et al. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PloS One. 2013;8(10) doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara R., Fujita S., Hornberger T.A., et al. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep. 2016;6:31142. doi: 10.1038/srep31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardee J.P., Porter R.R., Sui X., et al. The effect of resistance exercise on all-cause mortality in cancer survivors. Mayo Clin Proc. 2014;89(8):1108–1115. doi: 10.1016/j.mayocp.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roeland E.J., Bohlke K., Baracos V.E., et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38(21):2438–2453. doi: 10.1200/JCO.20.00611. [DOI] [PubMed] [Google Scholar]
- 16.Grande A.J., Silva V., Maddocks M. Exercise for cancer cachexia in adults: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2015;6(3):208–211. doi: 10.1002/jcsm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Argilés J.M., Busquets S., López-Soriano F.J., Costelli P., Penna F., et al. Are there any benefits of exercise training in cancer cachexia? Journal of cachexia, sarcopenia and muscle. 2012;3(2):73–76. doi: 10.1007/s13539-012-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West D.W.D., Burd N.A., Staples A.W., Phillips S.M., et al. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42(9):1371–1375. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Booth F.W., Tseng B.S., Flück M., Carson J.A. Molecular and cellular adaptation of muscle in response to physical training. Acta Physiol Scand. 1998;162(3):343–350. doi: 10.1046/j.1365-201X.1998.0326e.x. [DOI] [PubMed] [Google Scholar]
- 20.Piercy K.L., Troiano R.P., Ballard R.M., et al. The physical activity guidelines for Americans. Jama. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pigna E., Berardi E., Aulino P., et al. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci Rep. 2016;6:26991. doi: 10.1038/srep26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin M.L., Smith A.W., McTiernan A., et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyerhardt J.A., Giovannucci E.L., Holmes M.D., et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis-Gomar F., Lopez-Lopez S., Romero-Morales C., Maffulli N., Lipipi G., Pareja-Galeano H., et al. Neuromuscular electrical stimulation: a new therapeutic option for chronic diseases based on contraction-induced myokine secretion. Front Physiol. 2019;10:1463. doi: 10.3389/fphys.2019.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan W., Evans R.M. Exercise mimetics: impact on health and performance. Cell Metabol. 2017;25(2):242–247. doi: 10.1016/j.cmet.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandarian S.C., Stevenson E.J. Molecular events in skeletal muscle during disuse atrophy. Exerc Sport Sci Rev. 2002;30(3):111–116. doi: 10.1097/00003677-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Malavaki C.J., Sakkas G.K., Mitrou G.I., et al. Skeletal muscle atrophy: disease-induced mechanisms may mask disuse atrophy. J Muscle Res Cell Motil. 2015;36(6):405–421. doi: 10.1007/s10974-015-9439-8. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa A., Natio T., Sugiyama M., et al. Impact of cancer cachexia on hospitalization-associated physical inactivity in elderly patients with advanced non-small-cell lung cancer. Asia Pac J Oncol Nurs. 2018;5(4):377–382. doi: 10.4103/apjon.apjon_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardee J.P., Counts B.R., Carson J.A. Understanding the role of exercise in cancer cachexia therapy. Am J Lifestyle Med. 2019;13(1):46–60. doi: 10.1177/1559827617725283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleckner I.R., Jatoi A., Schwarz E.M., Dunne R.F., et al. The role of systemic inflammation in cancer-associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM clinical reports. 2018;3(2):1–19. [PMC free article] [PubMed] [Google Scholar]
- 31.Argiles J.M., Lopez-Soriano F.J., Busquets S. Mediators of cachexia in cancer patients. Nutrition. 2019;66:11–15. doi: 10.1016/j.nut.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Hetzler K., Hardee J.P., Puppa M.J., et al. IL-6 signaling during cancer cachexia progression: the female response. Faseb J. 2015;29(1_supplement):1038. doi: 10.1016/j.bbadis.2014.12.015. 11. [DOI] [Google Scholar]
- 33.Patel H.J., Patel B.M. TNF-alpha and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56–63. doi: 10.1016/j.lfs.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 34.White J.P., Puppa M.J., Gao S., Sato S., Welle S.L., Carson J.A., et al. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304(10):E1042–E1052. doi: 10.1152/ajpendo.00410.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White J.P., Puppa M.J., Gao S., Sato S., et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the Apc>Min mouse. Skeletal Muscle. 2012;2(1):14. doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakovenko A., Cameron M., Trevino J.G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J Gastrointest Surg. 2018;10(9):95–106. doi: 10.4240/wjgs.v10.i9.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonetto A., Aydogdu T., Jin X., et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303(3):E410–E421. doi: 10.1152/ajpendo.00039.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmers T.A., Fishel M.L., Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41. doi: 10.1016/j.semcdb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dogra C., Changoua H., Wedhas N., Qin X., Wergedal J.E., Kumar A., et al. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. Faseb J. 2007;21(8):1857–1869. doi: 10.1096/fj.06-7537com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Narsale A.A., Carson J.A. Role of IL-6 in cachexia–therapeutic implications. Curr Opin Support Palliat Care. 2014;8(4):321. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilir C., Engin H., Can M., Temi Y.B., Demirtas D., et al. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol. 2015;32(3):56. doi: 10.1007/s12032-015-0497-y. [DOI] [PubMed] [Google Scholar]
- 42.McClung J.M., Judge A.R, Powers S.K., Yan Z., et al. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298(3):C542–C549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moresi V., Adamo S., Berghella L. The JAK/STAT pathway in skeletal muscle pathophysiology. Front Physiol. 2019;10:500. doi: 10.3389/fphys.2019.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.G uttridge D.C., Mayo M.W., Madrid L.V., Wang C., Baldwin Jr A.S., et al. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289(5488):2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 45.Moore-Carrasco R., et al. Both AP-1 and NF-κB seem to be involved in tumour growth in an experimental rat hepatoma. Anticancer Res. 2009;29(4):1315–1317. [PubMed] [Google Scholar]
- 46.Seruga B., Zhang H., Bernstein L.J, Tannock I.F., et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Canc. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 47.Dev R., Del Fabbro E., Dalal S. Endocrinopathies and cancer cachexia. Curr Opin Support Palliat Care. 2019;13(4):286–291. doi: 10.1097/SPC.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 48.Carson J.A., Manolagas S.C. Effects of sex steroids on bones and muscles: similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67–78. doi: 10.1016/j.bone.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White J.P., Puppa M.J., Narsale A., Carson J.A., et al. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biology open. 2013;2(12):1346–1353. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horstman A.M., Dillon E.L., Urban R.J., Sheffield-Moore M., et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140–1152. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner J.L., Fukuda D.H., Rossetti M.L., Hoffman J.R., Gordon B.S., et al. Castration alters protein balance after high-frequency muscle contraction. J Appl Physiol. 2017;122(2):264–272. doi: 10.1152/japplphysiol.00740.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClung J.M., Davis J.M., Wilson M.A., Goldsmith E.C, Carson J.A., et al. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol. 2006;100(6):2012–2023. doi: 10.1152/japplphysiol.01583.2005. [DOI] [PubMed] [Google Scholar]
- 53.Lin H., Gu X., Zhang S., et al. Analysis on incidence and mean age at diagnosis for Global Cancer. Zhonghua zhong liu za zhi [Chinese journal of oncology] 2018;40(7):543–549. doi: 10.3760/cma.j.issn.0253-3766.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Haizlip K.M., Harrison B.C., Leinwand L.A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology. 2015;30(1):30–39. doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maher A.C., Fu M.H., Isfort R.J., Varbanov A.R., Qu X.A., A M., et al. Sex differences in global mRNA content of human skeletal muscle. PloS One. 2009;4(7):e6335. doi: 10.1371/journal.pone.0006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welle S., Tawil R., Thornton C.A. Sex-related differences in gene expression in human skeletal muscle. PloS One. 2008;3(1):e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Razzak Z.A., Khan A.A., Farooqui S.I. Effect of aerobic and anaerobic exercise on estrogen level, fat mass, and muscle mass among postmenopausal osteoporotic females. Int J Health Sci. 2019;13(4):10. [PMC free article] [PubMed] [Google Scholar]
- 58.Daly W., Seegers C.A, Rubin D.A, Dobridge J.D, Hackney A.C., et al. Relationship between stress hormones and testosterone with prolonged endurance exercise. Eur J Appl Physiol. 2005;93(4):375–380. doi: 10.1007/s00421-004-1223-1. [DOI] [PubMed] [Google Scholar]
- 59.Burney B.O., Hayes T.G., Smiechowska J., et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97(5):E700–E709. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 60.Chlebowski R.T., Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Canc Res. 1982;42(6):2495–2498. [PubMed] [Google Scholar]
- 61.Antonio J., Wilson J.D., George F.W. Effects of castration and androgen treatment on androgen-receptor levels in rat skeletal muscles. J Appl Physiol. 1999;87(6):2016–2019. doi: 10.1152/jappl.1999.87.6.2016. [DOI] [PubMed] [Google Scholar]
- 62.Bhasin S., Storer T.W., Berman N., et al. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997;82(2):407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 63.Wright T.J., Dillon E.L., Durham W.J., et al. A randomized trial of adjunct testosterone for cancer-related muscle loss in men and women. J Cachexia Sarcopenia Muscle. 2018;9(3):482–496. doi: 10.1002/jcsm.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White J.P., Gao S., Puppa M.J., Sato S., Welle S.L., Carson J.A., et al. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365(2):174–186. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burckart K., Beca S., Urban R.J., Sheffield-Moore M., et al. Pathogenesis of muscle wasting in cancer cachexia: targeted anabolic and anti-catabolic therapies. Curr Opin Clin Nutr Metab Care. 2010;13(4):410. doi: 10.1097/MCO.0b013e328339fdd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serra C., Sandor N.C., Jang H., et al. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology. 2013;154(12):4594–4606. doi: 10.1210/en.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montalvo R.N., Counts B.R., Carson J.A. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. 2018;12(4):394–403. doi: 10.1097/SPC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhong X., Zimmers T.A. Sex differences in cancer cachexia. Curr Osteoporos Rep. 2020:1–9. doi: 10.1007/s11914-020-00628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hetzler K.L., Hardee J.P., LaVoie H.A., Murphy E.A., Carson J.A., et al. Ovarian function's role during cancer cachexia progression in the female mouse. Am J Physiol Endocrinol Metab. 2017;312(5):E447–E459. doi: 10.1152/ajpendo.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spangenburg E.E., Geiger P.C., Leinwand L.A., Lowe D.A., et al. Regulation of physiological and metabolic function of muscle by female sex steroids. Med Sci Sports Exerc. 2012;44(9):1653–1662. doi: 10.1249/MSS.0b013e31825871fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hetzler K.L., Hardee J.P, Puppa M.J., et al. Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim Biophys Acta. 2015;1852(5):816–825. doi: 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kjobsted R., Hingst J.R., Fentz J., et al. AMPK in skeletal muscle function and metabolism. Faseb J. 2018;32(4):1741–1777. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollanen E., Ronkainen P.H.A., Horttanainen M., et al. Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Hormone IGF Res. 2010;20(5):372–379. doi: 10.1016/j.ghir.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Counts B., Fix D., Carson J. The effect of estradiol administration on muscle mass loss and cachexia progression in female ApcMin/+ mice. Front Endocrinol. 2019;10:720. doi: 10.3389/fendo.2019.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hopkinson J.B. Psychosocial impact of cancer cachexia. J Cachexia Sarcopenia Muscle. 2014;5(2):89–94. doi: 10.1007/s13539-014-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopkinson J. Psychosocial support in cancer cachexia syndrome: the evidence for supported self-management of eating problems during radiotherapy or chemotherapy treatment. Asia Pac J Oncol Nurs. 2018;5(4):358–368. doi: 10.4103/apjon.apjon_12_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tisdale M.J. Cancer anorexia and cachexia. Nutrition. 2001;17(5):438–442. doi: 10.1016/s0899-9007(01)00506-8. [DOI] [PubMed] [Google Scholar]
- 78.Jeejeebhoy K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care. 2012;15(3):213–219. doi: 10.1097/MCO.0b013e328352694f. [DOI] [PubMed] [Google Scholar]
- 79.Ravasco P., Monteiro-Grillo I., Vidal P.M, Camilo M.E., et al. Cancer: disease and nutrition are key determinants of patients' quality of life. Support Care Canc. 2004;12(4):246–252. doi: 10.1007/s00520-003-0568-z. [DOI] [PubMed] [Google Scholar]
- 80.Ryan A.M., Power D.G., Daly L., Cushen S.J., Bhuachalla Ē.N, Prado C.M., et al. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199–211. doi: 10.1017/S002966511500419X. [DOI] [PubMed] [Google Scholar]
- 81.Argiles J.M. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S39–S50. doi: 10.1016/j.ejon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Baltgalvis K.A., Berger F.G., Peña M.M.O., Davis J.M., White J.P., Carson J.A., et al. Activity level, apoptosis, and development of cachexia in Apc Min/+ mice. J Appl Physiol. 2010;109(4):1155–1161. doi: 10.1152/japplphysiol.00442.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toledo M., Busquets S., Sirisi S., et al. Cancer cachexia: physical activity and muscle force in tumour-bearing rats. Oncol Rep. 2011;25(1):189–193. [PubMed] [Google Scholar]
- 84.Counts B.R., Hardee J.P., Fix D.K., VanderVeen B.N., Montalvo R.N., Carson J.A., et al. Cachexia disrupts diurnal regulation of activity, feeding, and muscle mechanistic target of rapamycin complex 1 in mice. Med Sci Sports Exerc. 2020;52(3):577–587. doi: 10.1249/MSS.0000000000002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drummond M.J., Dickinson J.M, Fry C.S., et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302(9):E1113–E1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelleher A.R., Kimball S.R., Dennis M.D., Schilder R.J., Jefferson L.S., et al. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab. 2012;304(2):E229–E236. doi: 10.1152/ajpendo.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bodine S.C., Stitt T.N., Gonzalez M., et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 88.Powers S.K., Smuder A.J., Judge A.R. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012;15(3):240–245. doi: 10.1097/MCO.0b013e328352b4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Argilés J., Busquets S., López-Soriano F. Muscle protein kinetics in cancer cachexia. The Molecular Nutrition of Amino Acids and Proteins. 2016:133–144. doi: 10.1097/SPC.0b013e328359e6dd. [DOI] [Google Scholar]
- 90.Carson J.A., Hardee J.P., VanderVeen B.N. The emerging role of skeletal muscle oxidative metabolism as a biological target and cellular regulator of cancer-induced muscle wasting. Semin Cell Dev Biol. 2016;54:53–67. doi: 10.1016/j.semcdb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Talbert E.E., Guttridge D.C. Impaired regeneration: a role for the muscle microenvironment in cancer cachexia. Semin Cell Dev Biol. 2016;54:82–91. doi: 10.1016/j.semcdb.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carson J.A., Baltgalvis K.A. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38(4):168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han J., Lu C., Meng Q., Halim A., Yean T., Wu G., et al. Plasma concentration of interleukin-6 was upregulated in cancer cachexia patients and was positively correlated with plasma free fatty acid in female patients. Nutr Metab. 2019;16(1):80. doi: 10.1186/s12986-019-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puppa M.J., Gao S., Narsale A.A., Carson J.A, et al. Skeletal muscle glycoprotein 130's role in Lewis lung carcinoma-induced cachexia. Faseb J. 2014;28(2):998–1009. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamada E., Bastie C.C, Koga H., Wang Y., Cuervo A.M., Pessin J.E., et al. Mouse skeletal muscle fiber-type-specific macroautophagy and muscle wasting are regulated by a Fyn/STAT3/Vps34 signaling pathway. Cell Rep. 2012;1(5):557–569. doi: 10.1016/j.celrep.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hardee J.P., Fix D.K., Wang X., Goldsmith E.C., Koh H., Carson J.A., et al. Systemic IL-6 regulation of eccentric contraction-induced muscle protein synthesis. Am J Physiol Cell Physiol. 2018;315(1):C91–C103. doi: 10.1152/ajpcell.00063.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.VanderVeen B.N., Fix D.K., Carson J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: a role for inflammation. Oxid Med Cell Longev. 2017:3292087. doi: 10.1155/2017/3292087. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deutz N.E.P., Safar A., Schutzler S., et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011;30(6):759–768. doi: 10.1016/j.clnu.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pin F., Couch M.E., Bonetto A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care. 2018;12(4):420–426. doi: 10.1097/Spc.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baracos V.E. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. 2000;16(10):1015–1018. doi: 10.1016/s0899-9007(00)00407-x. [DOI] [PubMed] [Google Scholar]
- 101.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45(10):2121–2129. doi: 10.1016/j.biocel.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fearon K., Arends J., Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90–99. doi: 10.1038/nrclinonc.2012.209. [DOI] [PubMed] [Google Scholar]
- 103.Cuthbertson D., et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 104.Winter A., MacAdams J., Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31(5):765–773. doi: 10.1016/j.clnu.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Costelli P., Muscaritoli M., Bossola M., et al. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R674–R683. doi: 10.1152/ajpregu.00104.2006. [DOI] [PubMed] [Google Scholar]
- 106.Lantier L., Mourner R., Leclerc J., Pende M., Foretz M., Viollet B., et al. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. Faseb J. 2010;24(9):3555–3561. doi: 10.1096/fj.10-155994. [DOI] [PubMed] [Google Scholar]
- 107.White J.P., Baynes J.W., Welle S.L., et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the ApcMin/+ mouse. PloS One. 2011;6(9) doi: 10.1371/journal.pone.0024650. e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Braun T.P., Marks D.L. The regulation of muscle mass by endogenous glucocorticoids. Front Physiol. 2015;6:12. doi: 10.3389/fphys.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hentila J., Nissinen T.A, Korkmaz A., et al. Activin receptor ligand blocking and cancer have distinct effects on protein and redox homeostasis in skeletal muscle and liver. Front Physiol. 2019;9:1917. doi: 10.3389/fphys.2018.01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jackman R.W., Cornwell E.W., Wu C., Kandarian S.C., et al. Nuclear factor-kappaB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol. 2013;98(1):19–24. doi: 10.1113/expphysiol.2011.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jackman R.W., Kandarian S.C. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287(4):C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 112.de Castro G.S., Simoes E., Lima J.D.C.C., et al. Human cachexia induces changes in mitochondria, autophagy and apoptosis in the skeletal muscle. Cancers. 2019;11(9):1264. doi: 10.3390/cancers11091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Du J., Wang X., Miereles C., et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113(1):115–123. doi: 10.1172/Jci200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Busquets S., et al. Apoptosis is present in skeletal muscle of cachectic gastro-intestinal cancer patients. Clin Nutr. 2007;26(5):614–618. doi: 10.1016/j.clnu.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 115.Belizario J.E., Lorite M.J., Tisdale M.J. Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Canc. 2001;84(8):1135–1140. doi: 10.1054/bjoc.2001.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Figueras M., Busquets S., Carbó N., et al. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569(1-3):201–206. doi: 10.1016/j.febslet.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 117.Moarbes V., Mayaki D., Huck L., et al. Differential regulation of myofibrillar proteins in skeletal muscles of septic mice. Phys Rep. 2019;7(20) doi: 10.14814/phy2.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Penna F., Ballarò R., Martinez-Cristobal P., et al. Autophagy exacerbates muscle wasting in cancer cachexia and impairs mitochondrial function. J Mol Biol. 2019;431(15):2674–2686. doi: 10.1016/j.jmb.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 119.Baracos V.E., et al. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995;268(5 Pt 1):E996–E1006. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- 120.van der Ende M., Grefte S., Plas R., et al. Mitochondrial dynamics in cancer-induced cachexia. Biochim Biophys Acta Rev Canc. 2018;1870(2):137–150. doi: 10.1016/j.bbcan.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 121.Halle J.L., Pena G.S., Paez H.G., et al. Tissue-specific dysregulation of mitochondrial respiratory capacity and coupling control in colon-26 tumor-induced cachexia. Am J Physiol Regul Integr Comp Physiol. 2019;317(1):R68–R82. doi: 10.1152/ajpregu.00028.2019. [DOI] [PubMed] [Google Scholar]
- 122.Baskin K.K., Winders B.R., Olson E.N. Muscle as a “mediator” of systemic metabolism. Cell Metabol. 2015;21(2):237–248. doi: 10.1016/j.cmet.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weber D.D., Aminzadeh-Gohari S., Tulipan J., Catalano L., Feichtinger R.G., Kofler B., et al. Ketogenic diet in the treatment of cancer–where do we stand? Molecular metabolism. 2020;33:102–121. doi: 10.1016/j.molmet.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomsen H.H., et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–867. doi: 10.1093/ajcn/nqy170. [DOI] [PubMed] [Google Scholar]
- 125.Koutnik A.P., D'Agostino D.P., Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metabol. 2019;30(4):227–229. doi: 10.1016/j.tem.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 126.Fearon K.C, Borland W., Preston T., Tisdale M.J., Shenkin A., Calman K.C., et al. Cancer cachexia: influence of systemic ketosis on substrate levels and nitrogen metabolism. Am J Clin Nutr. 1988;47(1):42–48. doi: 10.1093/ajcn/47.1.42. [DOI] [PubMed] [Google Scholar]
- 127.Shukla S.K., Gebregiworgis T., Purohit V., et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Canc Metabol. 2014;2(1):18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamura K., Tonouchi H., Sasayama A., Ashida K., et al. A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients. 2018;10(2):206. doi: 10.3390/nu10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ang Q.Y., Alexander M., Newman J.C., et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263–1275. doi: 10.1016/j.cell.2020.04.027. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown J.L., Rosa-Caldwell M.E, Lee D.E., et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017;8(6):926–938. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White J.P., Baltgalvis K.A., Puppa M.J., Sato S., Baynes J.W., Carson J.A., et al. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R201–R211. doi: 10.1152/ajpregu.00300.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cannavino J., Brocca L., Sandri M., Grassi B., Bottinelli R., Pellegrino M.A., et al. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593(8):1981–1995. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wagatsuma A., Kotake N., Kawachi T., Shiozuka M., Yamada S., Matsuda R., et al. Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol Cell Biochem. 2011;350(1-2):1–11. doi: 10.1007/s11010-010-0677-1. [DOI] [PubMed] [Google Scholar]
- 134.Mazzatti D.J., Smith M.A., Oita R.C., Lim F, White A.J., Reid M.B., et al. Muscle unloading-induced metabolic remodeling is associated with acute alterations in PPARδ and UCP-3 expression. Physiol Genom. 2008;34(2):149–161. doi: 10.1152/physiolgenomics.00281.2007. [DOI] [PubMed] [Google Scholar]
- 135.Nagatomo F., Fujino H., Kondo H., et al. PGC-1alpha and FOXO1 mRNA levels and fiber characteristics of the soleus and plantaris muscles in rats after hindlimb unloading. Histol Histopathol. 2011;26(12):1545–1553. doi: 10.14670/HH-26.1545. [DOI] [PubMed] [Google Scholar]
- 136.Rosa-Caldwell M.E., Lim S., Haynie W.S, et al. Altering aspects of mitochondrial quality to improve musculoskeletal outcomes in disuse atrophy. J Appl Physiol. 1985:2020. doi: 10.1152/japplphysiol.00407.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lira V.A., Benton C.R., Yan Z., Bonen A., et al. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299(2):E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wright D.C., Han D., Garcia-Roves P.M., Geiger P.C.., Jones T.E., Holloszy J.O., et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282(1):194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 139.Puppa M.J., Murphy E.A., Fayad R., Hand G.A., Carson J.A., et al. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol. 2014;116(8):1078–1087. doi: 10.1152/japplphysiol.01270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A., et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 141.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabol. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 142.Pinckaers P.J., Churchward-Venne T.A., Bailey D., Loon L.J.C.v., et al. Ketone bodies and exercise performance: the next magic bullet or merely hype? Sports Med. 2017;47(3):383–391. doi: 10.1007/s40279-016-0577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rojas-Morales P., Pedraza-Chaverri J., Tapia E. Ketone bodies, stress response, and redox homeostasis. Redox Biol. 2020;29:101395. doi: 10.1016/j.redox.2019.101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Evans M., Cogan K.E., Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595(9):2857–2871. doi: 10.1113/JP273185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu S., Niu Y., Fu L. Metabolic adaptations to exercise training. Journal of Science in Sport and Exercise. 2020;2(1):1. doi: 10.1007/s42978-019-0018-3. [DOI] [Google Scholar]
- 146.Rockl K.S., Witczak C.A., Goodyear L.J. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60(3):145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao S., Carson J.A. Lewis lung carcinoma regulation of mechanical stretch-induced protein synthesis in cultured myotubes. Am J Physiol Cell Physiol. 2016;310(1):C66–C79. doi: 10.1152/ajpcell.00052.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dardevet D., Sornet C., Balage M., Grizard J., et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130(11):2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 149.Gao Y., Arfat Y., Wang H., Goswami N., et al. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front Physiol. 2018;9:235. doi: 10.3389/fphys.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li M., Li C., Parkhouse W.S. Age-related differences in the des IGF-I-mediated activation of Akt-1 and p70 S6K in mouse skeletal muscle. Mech Ageing Dev. 2003;124(7):771–778. doi: 10.1016/s0047-6374(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 151.Figueiredo V.C., Markworth J.F. Mechanisms of protein synthesis activation following exercise: new pieces to the increasingly complex puzzle. J Physiol. 2015;593(21):4693–4695. doi: 10.1113/JP271431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nader G.A., Esser K.A. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol. 2001;90(5):1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 153.Parkington J.D., LeBrasseur N.K., Siebert A.P., Fielding R.A., et al. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;97(1):243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- 154.Baar K., Esser K. Phosphorylation of p70S6kcorrelates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276(1):C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 155.Kumar A., Chaudhry I., Reid M.B., Boriek A.M., et al. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J Biol Chem. 2002;277(48):46493–46503. doi: 10.1074/jbc.M203654200. [DOI] [PubMed] [Google Scholar]
- 156.Widegren U., Ryder J.W., Zierath J.R. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172(3):227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- 157.Carriere A., Romeo Y., Acosta-Jaquez H.A., et al. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J Biol Chem. 2011;286(1):567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin S.S., Liu Y.W. Mechanical stretch induces mTOR recruitment and activation at the phosphatidic acid-enriched macropinosome in muscle cell. Frontiers in Cell and Developmental Biology. 2019;7:78. doi: 10.3389/fcell.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.You J.S., Frey J.W., Hornberger T.A. Mechanical stimulation induces mTOR signaling via an ERK-independent mechanism: implications for a direct activation of mTOR by phosphatidic acid. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0047258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Penna F., Busquets S., Pin F., et al. Combined approach to counteract experimental cancer cachexia: eicosapentaenoic acid and training exercise. J Cachexia Sarcopenia Muscle. 2011;2(2):95–104. doi: 10.1007/s13539-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salomao E.M., Toneto A.T., Silva G.O., Gomes-Marcondes M.C.C., et al. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in Walker tumor-bearing rats. Nutr Canc. 2010;62(8):1095–1104. doi: 10.1080/01635581.2010.492082. [DOI] [PubMed] [Google Scholar]
- 162.Puppa M.J., White J.P, Velázquez K.T., et al. The effect of exercise on IL-6-induced cachexia in the Apc Min/+ mouse. Journal of cachexia, sarcopenia and muscle. 2012;3(2):117–137. doi: 10.1007/s13539-011-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Re Cecconi A.D., Forti M., Chiappa M., et al. Musclin, A myokine induced by aerobic exercise, retards muscle atrophy during cancer cachexia in mice. Cancers. 2019;11(10):1541. doi: 10.3390/cancers11101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patel D.I., Abuchowski K., Sheikh B., Rivas P., Musi N., Kumar A.P., et al. Exercise preserves muscle mass and force in a prostate cancer mouse model. European Journal of Translational Myology. 2019;29(4) doi: 10.4081/ejtm.2019.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanaka M., Sugimoto K., Fujimoto T., et al. Differential effects of pre-exercise on cancer cachexia-induced muscle atrophy in fast- and slow-twitch muscles. Faseb J. 2020;34(11):14389–14406. doi: 10.1096/fj.202001330R. [DOI] [PubMed] [Google Scholar]
- 166.Guigni B.A., Fix D.K., Bivona 3rd J.J., Palmer B.M, Carson J.A., Toth M.J., et al. Electrical stimulation prevents doxorubicin-induced atrophy and mitochondrial loss in cultured myotubes. Am J Physiol Cell Physiol. 2019;317(6):C1213–C1228. doi: 10.1152/ajpcell.00148.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sato S., Gao S., Puppa M.J., Kostek M.C., Wilson L.B., Carson J.A., et al. High-frequency stimulation on skeletal muscle maintenance in female cachectic mice. Med Sci Sports Exerc. 2019;51(9):1828–1837. doi: 10.1249/MSS.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hardee J.P., Mangum J.E., Gao S., et al. Eccentric contraction-induced myofiber growth in tumor-bearing mice. J Appl Physiol. 2016;120(1):29–37. doi: 10.1152/japplphysiol.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barreto R., Mandili G., Witzmann F.A., Novelli F., Zimmers T.A, Bonetto A., et al. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472. doi: 10.3389/fphys.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Quan-Jun Y., Yan H., Yong-Long H., et al. Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Canc Therapeut. 2017;16(2):334–343. doi: 10.1158/1535-7163.Mct-16-0324. [DOI] [PubMed] [Google Scholar]
- 171.Penna F., Ballaro R., Costelli P. The redox balance: a target for interventions against muscle wasting in cancer cachexia? Antioxidants Redox Signal. 2020;33(8):542–558. doi: 10.1089/ars.2020.8041. [DOI] [PubMed] [Google Scholar]
- 172.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Canc. 2014;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wasley D., Gale N., Roberts S., et al. Patients with established cancer cachexia lack the motivation and self-efficacy to undertake regular structured exercise. Psycho Oncol. 2018;27(2):458–464. doi: 10.1002/pon.4512. [DOI] [PubMed] [Google Scholar]
- 174.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future. J Physiol. 2016;594(18):5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ji L.L. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222(3):283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 176.Miyazaki H., Oh-ishi S., Ookawara T., et al. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84(1-2):1–6. doi: 10.1007/s004210000342. [DOI] [PubMed] [Google Scholar]
- 177.He F., Li J., Liu Z., Chuang C., Yang W., Zuo L., et al. Redox mechanism of reactive oxygen species in exercise. Front Physiol. 2016;7:486. doi: 10.3389/fphys.2016.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ballaro R., Penna F., Pin F., Gómez-Cabrera M.C., Viña J., Costelli P., et al. Moderate exercise improves experimental cancer cachexia by modulating the redox homeostasis. Cancers. 2019;11(3):285. doi: 10.3390/cancers11030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alves C.R.R., Neves W.d, Almeida N.R.d., et al. Exercise training reverses cancer-induced oxidative stress and decrease in muscle COPS2/TRIP15/ALIEN. Mol Metab. 2020;39:101012. doi: 10.1016/j.molmet.2020.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Repka C.P., Hayward R. Effects of an exercise intervention on cancer-related fatigue and its relationship to markers of oxidative stress. Integr Canc Ther. 2018;17(2):503–510. doi: 10.1177/1534735418766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Smuder A.J. Exercise stimulates beneficial adaptations to diminish doxorubicin-induced cellular toxicity. Am J Physiol Regul Integr Comp Physiol. 2019;317(5):R662–R672. doi: 10.1152/ajpregu.00161.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Burd N.A., Gorissen S.H., van Loon L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–173. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- 183.Breen L., Phillips S.M. Skeletal muscle protein metabolism in the elderly: interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab. 2011;8(1):68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liva S.G., Tseng YC., Dauki AM., et al. Overcoming resistance to anabolic SARM therapy in experimental cancer cachexia with an HDAC inhibitor. EMBO Mol Med. 2020;12(2) doi: 10.15252/emmm.201809910. e9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Borzotta A.P., Clague M.B., Johnston I.D. The effects of gastrointestinal malignancy on whole body protein metabolism. J Surg Res. 1987;43(6):505–512. doi: 10.1016/0022-4804(87)90123-5. [DOI] [PubMed] [Google Scholar]
- 186.O'Keefe S.J.D., Ogden J., Ramjee G., et al. Contribution of elevated protein turnover and anorexia to cachexia in patients with hepatocellular carcinoma. Canc Res. 1990;50(4):1226–1230. [PubMed] [Google Scholar]
- 187.van Dijk David P.J., van de Poll Marcel C.G., Moses Alastair G.W., et al. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2015;6(3):212–221. doi: 10.1002/jcsm.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Williams J.P., Phillips B.E., Smith K., et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96(5):1064–1070. doi: 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- 189.Dolly A., Dumas J.F., Servais S. Cancer cachexia and skeletal muscle atrophy in clinical studies: what do we really know? J Cachexia, Sarcopenia Muscle. 2020 doi: 10.1002/jcsm.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]