Abstract
This study sought to address the complex interplay between both biological and psychological perceptions of stress and sleep in the acute stages following a mild traumatic brain injury. A secondary goal was to identify potential targets for intervention. Eleven acutely injured youth (mean age 12 years) were studied at home with overnight actigraphy, salivary cortisol and melatonin assays, and subjective ratings of stress and fatigue (injured group). Nine matched control youth also were assessed (control group). Results suggested longer sleep latencies (time to fall asleep) and higher levels of fatigue in the injured group exist (p = 0.025 and p = 0.004, respectively). In the injured group, stress and sleep onset were significantly related with most subjects meeting criteria for Acute Stress Disorder. Melatonin levels were lower at bedtime in the injured group. Saliva samples were collected via passive drool at three time points: ∼1 h before bed (“bedtime” or T1), immediately upon waking (time 2: T2), and 30 min post-waking (time 3: T3). Overnight increases in cortisol (T1 to T2) were greater for the injured group; however, post-sleep changes in cortisol (T2 to T3) were reversed with control concentrations increasing. These findings are unique in using actigraphy and salivary hormone levels in an acutely injured youth while in their homes. The differences in sleep latency and the presence of injury-related stress point to potential treatment targets in acute concussion.
Keywords: Sleep, Mild traumatic brain injury, Fatigue, Actigraphy, Youth, Salivary hormones, Stress, Concussion
Introduction
Concussion, sleep, fatigue, and stress
Sleep disruption is a commonly reported problem in children with traumatic brain injury (TBI), including mild TBI.1 Fatigue is likewise frequently reported, even after considerable time from inuury in moderate severe TBI.2 In pediatric TBI, sleep-wake disturbances (SWD) have been noted to complicate treatment across the spectrum of injury severities.2 SWD are defined as changes in nighttime sleep resulting in daytime impairments.3 Studies in the adult population demonstrate that SWD are extremely prevalent in the acute post-injury phase.4 In moderate to severe TBI, the combination of affective disturbance and sleep disruption related to melatonin production has been documented up to 2-years post injury.5
In youth, poor sleep is related to prolonged recovery from mild traumatic brain injury (mTBI).6 In adults, sleep disruption and fatigue are well-known consequences of TBI across the spectrum of severity; however, the co-occurrence of sleep and fatigue seem to be present at all levels of brain injury severity.7 Fatigue is recognized as a multidimensional concept by the World Health Organization's International Classification of Functioning, Disability and Health.8 According to Wylie and Flashman, fatigue interacts with brain injury or disease to affect bodily functions and structure, as well as actions and movement of the injured individual.9
In adults, SWD and fatigue following TBI are well documented, but this is not the case for youth populations. A serious concern exists given that adequate sleep is paramount for cognitive, behavioral, and socioemotional functioning during childhood and adolescence.10 Sleep is disrupted in a number of ways following brain injury.11 Longer relative nocturnal wakefulness and shorter relative REM sleep have been reported.5,12 REM, or rapid eye movement, is a stage of sleep responsible for many tasks such as the consolidation of emotional memory.12 These outcomes were associated with an altered balance of systems dominant in wakefulness (e.g., norepinephrine and serotonin systems) and those dominant in REM sleep (e.g., acetylcholine neuronal systems). Therefore, additional research is necessary to better understand the nature of mTBI within a shorter time window of the initial injury in order to better understand the immediate effects on sleep and physiology, as well as self-reported symptoms, in youth with mild TBI (mTBI), preferably in a more contextually accurate recovery setting.
Pediatric mTBI and actigraphy
Due to the invasive challenges of obtaining such detailed sleep data, less invasive measures are often applied. Frequently, parent reports are used to assess a child's sleep. However, several criticisms surround this method of data collection, including their subjective nature.13, 14, 15 For example, unless in the same room as the child, parents may not accurately report their child's sleep behavior each night (e.g., number of night awakenings).
Use of actigraphs has become common in pediatric sleep research as these devices provide a more objective measure than parent-report.16 This non-intrusive tool employs activity-based monitoring to estimate sleep-wake patterns. Commonly calculated sleep variables include sleep onset, number of night awakenings, sleep efficiency (proportion of time spent in bed asleep), and sleep latency.17
Previous findings in younger populations using actigraphy have produced conflicting results. Sumpter et al. reported no significant differences between groups of seven to twelve-year-olds with and without head injury on actigraph measures for sleep efficiency or wake time.17 Conversely, other studies have reported longer sleep latency and poorer sleep efficiency for a head injured group compared to a healthy group, indicating head injury is associated with sleep inefficiency in the form of sleep onset and maintenance problems.17 Additionally, Kaufman et al. reported that adolescents still demonstrate lower sleep efficacy and more minutes of wake time for as long as three years after initial injury.18
The impact that stress has on sleep is unclear and is complicated by factors such as definition and measurement of such a broad concept as “stress.” Stress is often measured by subjective surveys,20, 21, 22 whereas other studies incorporate physiological markers such as salivary cortisol.14,15 In order to increase ecological validity, stress is often observed in naturalistic environments specific to the population of interest. Adding this environmental setting information allows for the attribution of physiological cascades observed to the stressor of interest, rather than to the novelty of a laboratory setting.23
Objective and subjective sleep latency measures have been related to decreases in cortisol response to stress, sleep-wake problems, increased sleep latencies and increased daytime sleepiness.24,25
In the current study, we conceptualize TBI as an acute stressor on the biology of the human body via a physical trauma to the brain. Additionally, we utilize the terms “subjective stress” to characterize self-reported psychological status, post-injury, and “cortisol actigraphy” as objective (i.e., biological) measures of stress and sleep.
The goals of the current study were to add to the understanding of the relationships between mTBI, stress, and sleep by evaluating sleep in an ecologically valid setting (home), utilizing both objective and subjective measures. Identifying potential treatment targets was a secondary goal. The hypothesis was that concussed youth would show differences in sleep, in comparison to non-injured youth, as indexed by fatigue, actigraphy and melatonin, and that stress would contribute to sleep metrics as indexed by ratings and cortisol concentrations.
Materials and methods
Participants
Eleven concussed youth (injured group) were recruited from either an urban Emergency Department or Sports Medicine clinic. Concussions were verified by the attending physician. Activities of injury included sports (n = 8) and accident (n = 3). Nine age-, race- and sex-matched non-injured individuals were approached by research staff through authors' children's school outreach (control group). This research was approved by the Institutional Review Board, informed assent and consent were obtained for all participants and parents, and participants received a stipend for participation. Participant characteristics appear in Table 1.
Table 1.
Demographic information and self-report measures.
| Characteristic/Measure | Injured (n = 11) | Control (n = 9) |
|---|---|---|
| Age | 13.36 (1.57) | 13.33 (1.73) |
| Sex | ||
| Male | 5 | 4 |
| Female | 6 | 5 |
| Race | ||
| White | 7 | 5 |
| Black | 4 | 4 |
| GSC at diagnosis | Mdn: 38; Interquartile range: 29; Range:18-103 | N/A |
| Mean (s.d.) | ||
| Interval Since Injury | 5.91 (3.96) | N/A |
| ASC-Kids | 24.72 (10.71) | N/A |
| PED-SF | 20.18 (11.11) | 11.11 (1.79) |
Note: GSC – Graded Symptom Checklist; Mdn = median; Interval Since Injury = Number of days between concussion and evaluation; ASC-Kids = Acute Stress Checklist for Kids.
PED-SF = Pediatric Fatigue Scale Short Form.
Procedure
Injured youth and their parents were approached by the attending physician and given general information about the study. Youth and parents interested in study participation signed a consent to contact form. That form was forwarded to the research team who then contacted parents and made arrangements for the study. The comparison participants in the community were approached by research staff.
With parental approval, a member of the research team made arrangements to arrive at the participant's home 1 h before typical bedtime. On arrival, demographic forms and behavioral rating scales were completed, and the actigraph was explained and fitted to the participant. Parents and participants were both trained for saliva data collection. Parents were given step-by-step instructions and watched as the researcher took the first saliva sample. The parents and participant were specifically guided through each individual step of the study protocol. Researchers took needed time to review the study protocol with the parents and participant to ensure understanding and to answer any questions. An instruction sheet was left with parents and participants to review the collection steps as needed.
The researcher returned to the home at a pre-arranged time the next morning. Upon awakening, the parent collected the next saliva sample. At this point, the researcher reviewed the sleep log, collected the actigraph, and took the final saliva sample.
Measures
Salivary biomarkers
Saliva samples were collected via passive drool at three time points: ∼1 h before bed (“bedtime:” T1), immediately upon waking (T2), and 30 min post-waking (T3). Participants were instructed to swallow all saliva then allow new saliva to pool in their mouth. Lastly, saliva was to flow from their mouth into a Saliva Collection Aid into a polypropylene cryovial (SalivaBio). Participants collected approximately 0.75 mL of saliva for analysis; saliva samples were placed in a freezer within 30 min of collection. All samples were transported on ice to the University of Nebraska-Lincoln Salivary Bioscience Laboratory. Samples were assayed in duplicate for salivary melatonin and cortisol via ELISA (Salimetrics, Inc., State College, PA). Intra-assay coefficient of variations (CVs) for salivary cortisol and melatonin were 3.72% and 6.67%, and inter-assay CVs were 2.34% and 9.20%, respectively. Salivary cortisol and salivary melatonin concentration levels are measured in μg/dL and pg/mL, respectively.
Actigraphy
Micro Mini Motion Loggers, from Ambulatory Monitoring Inc. in Ardsley, New York, were used to collect actigraph data. Actigraph readings were validated against sleep logs for accuracy by comparing actigraph readings to the log kept by each participant. Calculations derived from actigraphs were: sleep latency (minutes to the start of a continuous block of at least 30-min of sleep), number of awakenings, length of time of awakenings, total sleep time and sleep efficiency. Sleep efficiency was defined as the percentage of time children spent in bed asleep. This percentage was calculated by dividing the total time asleep (minus any night awakenings) by the total time spent in bed.
Subjective rating scales
The Acute Stress Checklist for Children (ASC-Kids) is a 29-item self-report scale that purports to measure the impact of a recent stressful event. Note that this checklist was only administered to the injured participants. This measurement has strong test-retest reliability (α = 0.83) and internal consistency (α = 0.85), as well as concurrent and predictive validity with other traumatic stress measures (e.g., Child and Adolescent Trauma Survey Symptom Scale). Internal consistency was α = 0.91. ASC-Kids is used as a diagnostic tool for Acute Stress Disorder based on specific scoring criteria. A subset of items from the ASC inquires about symptoms the participant is currently experiencing. A separate score of those items was also used for analysis.
The PedsQL Multidimensional Fatigue Scale is an 18-item self-report scale designed to measure fatigue in pediatric patients and is comprised of the General Fatigue Scale (6 items), Sleep/Rest Fatigue Scale (6 items), and Cognitive Fatigue Scale (6 items). Results with a group of rheumatology patients obtained internal consistency reliability for the PedsQL Multidimensional Fatigue Scale Total Score (α = 0.95 child, 0.95 parent report), General Fatigue Scale (α = 0.93 child, 0.92 parent), Sleep/Rest Fatigue Scale (α = 0.88 child, 0.90 parent), and Cognitive Fatigue Scale (α = 0.93 child, 0.96 parent). These scores are considered excellent for group and individual comparisons. The child short form was used (8-items) and the internal consistency was α = 0.88.
Sleep logs
As is common practice for use in verifying actigraph data, parents and participants were instructed to complete a sleep log, and how to do so properly. Questions included the participant's time in bed, when the participant was assumed to fall asleep (as parent was instructed to check in on their child), if any night waking occurred and if so, what time the participant returned to bed (which could be corroborated by parent if also awakened), what time the participant awoke in the morning, and if the actigraph was still in place upon awakening. Additionally, parents and participants were asked about any additional comments regarding sleep (e.g., difficulty falling asleep, staying asleep, etc.).
Statistical analysis
The subjective stress measure (ASC-Kids) was only administered to the injured group. Changes (differences) in mean cortisol and melatonin concentrations from T1(bedtime) to T2 (wake), and T2 (wake) to T3 (30-min later) per sampling protocol were calculated. The difference in concentrations from T1 to T2 and the difference from T2 to T3 were multiplied to create an interaction terms (cortisol and melatonin). These calculated interaction terms were used to complete Spearman correlation analyses and t-tests.
Results
Demographics
No differences in age, sex or race between groups existed. A wide range of days from injury to assessment in the injured group did exist. Table 1 presents the demographic information of the subjects.
Actigraphy
No differences between sex or race groups on any of the actigraph variables were found. Between-group MANOVA of all five primary sleep metrics (number of awakenings, awake duration, sleep time, latency and efficiency) was significant: F(5,13) = 6.593, p < 0.001, η2 = 0.711. Follow-up comparisons revealed a significant group difference in sleep latency (time to fall asleep): F(1,16) = 30.571, p = 0.001, η2 = 0.622, where the mTBI group took almost 40 min longer to fall asleep on average. All other metrics indicated slightly better but non-significant sleep for the comparison group. Table 2 displays descriptive statistics for the mTBI group and control group, respectively.
Table 2.
Actigraphy metrics between TBI and control groups.
| Group | Statistic | Number of Awakenings | Awake Duration | Sleep Time | Latencya | Sleep Efficiency (%) |
|---|---|---|---|---|---|---|
| mTBI | n | 10 | 10 | 10 | 10 | 10 |
| Mean | 1.40 | 17.20 | 477.30 | 46.30 | 96.34 | |
| Median | 1 | 11 | 482.50 | 50.50 | 97.64 | |
| Std. Dev. | 1.2649 | 16.21 | 70.49 | 18.84 | 3.53 | |
| Control | n | 8 | 8 | 8 | 8 | 8 |
| Mean | 0.50 | 8.25 | 479.25 | 8 | 98.16 | |
| Median | 0 | 0 | 465.50 | 6.50 | 100 | |
| Std. Dev. | 0.76 | 12.88 | 73.73 | 5.55 | 3.13 |
Note: Awake duration, sleep time, and latency were measured in minutes; Std. Dev = standard deviations.
a. p < 0.001.
Salivary hormones
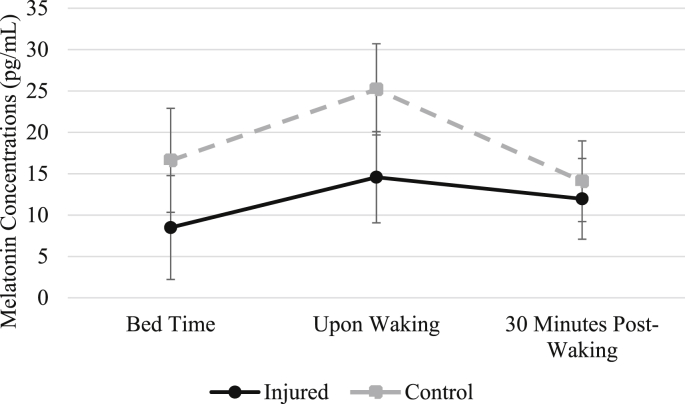
Melatonin. Overall, melatonin concentrations showed an expected pattern of increase from T1 (bedtime) to T2 (wake), and then decreased at T3 (30 min later). One injured individual did not collect saliva samples during T1 (at night) and T2 (immediately upon waking), and one control subject's saliva sample was unusable at T3. One injured individual was removed from analysis due to self-reported use of exogenous melatonin on the night of sampling, and another injured individual was removed for self-reported caffeine intake before bedtime. A 2 (groups) by 3 (time) repeated-measures ANOVA was completed. A significant multivariate effect of time, F(2,11) = 10.33, p = 0.003, Wilks' λ = 0.35, and a multivariate effect approaching significance for melatonin by group over the three time points, F(2,11) = 2.98, p = 0.09, Wilks' λ = 0.35 were found. In post-hoc univariate between-group analyses, no significant differences in individual time point concentrations were found although T2 (wake) approached a significant difference (p = 0.08). Fig. 1 contains the melatonin concentration changes over the three time points. The interaction term of differences (T1 to T2 and T2 to T3) were not different by group.
Fig. 1.
Changes in Salivary Melatonin over Three Time Points
Note: Error bars represent standard error values.
Cortisol. One salvia sample was unusable at T1 for a control subject, and five individuals were removed from analysis due to a discrepancy in self-reported morning sampling (within a few minutes of waking) and actigraph time of awakening. As expected, (due to diurnal changes in cortisol), a significant multivariate effect on cortisol across the three time points was found, F(2,9) = 30.90, p < 0.001, Wilks' λ = 0.35. However, no group differences across the three time points were observed, F(2,9) = 1.99, p = 0.19, Wilks’ λ = 0.69. The cortisol samples demonstrated a nonsignificant difference pattern between groups; however, the waking levels were somewhat higher in the mTBI group. Fig. 2 contains changes in salivary cortisol over the three time points.
Fig. 2.
Changes in Salivary Cortisol over Three Time Points
Note: Error bars represent standard error values.
The interaction terms of differences (T1 to T2 and T2 to T3) were not significantly different by group. Although cortisol differences at each time-point were not significantly different by group, plotting cortisol differences showed a nonsignificant interaction of changes from T1 to T2 and then T2 to T3 with the slopes reversing (Fig. 3). For the control group, the increase in cortisol from T1 to T2 was 0.119 μg/dL, whereas the cortisolincrease for the injured group was 0.210 μg/dL. Cortisol in the control group increased from T2 to T3 was 0.222 μg/dL, whereas the injured group's cortisol increase was 0.149 μg/dL.
Fig. 3.
Differences in Cortisol Production by Group, Time 1 to Time 2, and Time 2 to Time 3
Note. T1 T2 and T2 T3 difference are the differences in cortisol values from time-1 to time-2 and time-2 to time-3, respectively.
Rating scales
The pediatric fatigue scale was administered to both groups. One subject did not complete the NSFQ. A significant difference by group was found, F(1,17) = 14.49, p = 0.001, η2 = 0.46, where the injured group had higher fatigue scores which is associated with greater fatigue. A significant effect of the post-sleep cortisol concentrations on the fatigue scale was found for all subjects (T2 to T3): rs = 0.606, p = 0.008.
Salivary hormones and sleep metrics
Although the differences in bedtime melatonin were not significantly different between groups (both n = 8), a graph of melatonin concentrations and sleep latency indicates an interaction by group (see Fig. 4).
Fig. 4.
Sleep Latency and Melatonin Differences between Groups
Note: Error bars represent standard error values.
When the interaction term for the melatonin differences for T1 – T2 by T2 – T3 was entered into the correlation matrix, significant correlations were found with the number of awakenings (rs = 0.530, p = 0.028), duration of awakenings (rs = 0.576, p = 0.015) and sleep efficiency (rs = 0.576, p = 0.015).
Absolute changes in cortisol values from T1 to T2 were positively related to the duration of awakenings (rs = 0.478, p = 0.045), total sleep time rs = 0.491, p = 0.038) and sleep efficiency rs = 0.474, p = 0.047), indicating higher concentrations were related to longer durations.
The interaction of difference values (difference T1 to T2 by difference T2 to T3) for cortisol correlated negatively with sleep latency rs = −0.477, p = 0.045. As noted, sleep latency was significantly different between groups.
mTBI group analyses
The Acute Stress Checklist for Kids was administered only to the injured group as stress due to injury was not relevant for the control group. The median score was 40.5 symptom-items (range 29–69). The median distress score was 6 symptoms (range 4–9). All but one participant endorsed symptoms that met the exposure criteria, 7 of 11 participants met criteria for symptoms of intrusion, dissociation, avoidance and/or arousal, and all participants met criteria for distress or impairment due to the event. Thus, 63.6% of the subjects met the criteria for Acute Stress Disorder. A strong, positive correlation between PedsQL (fatigue) and ASC-Kids (stress) (rs = 0.789, p = 0.004) was found.
In addition to the relationships between the two rating scales, there were positive correlations between stress ratings and the time at which sleep onset was achieved (rs = 0.815, p = 0.004). Further, sleep onset also was related to total sleep time (rs = −.924, p= <0.001) and sleep efficiency (rs = −0.638, p = 0.047).
Note that the PedsQL ratings for both groups were correlated with sleep latency and with post-sleep cortisol changes; however, these relationships did not hold up in the injured group alone. Note that one injured subject had a very short latency (6-min) compared to the others, likely impacting that correlation.
Discussion
During the acute phase of concussed youth compared to matched non-concussed youth, sleep latency was significantly longer in the injured group, with high levels of subjective stress related to both sleep latency and high levels of fatigue that was not seen in the control group. The injured group was awakened more often and for longer periods than the control group. In the injured group, differences in the metabolism of both cortisol and melatonin also were observed, whereas relationships of these hormones to sleep metrics were not significantly different, but did approach significance. Although these sleep disturbances are consistent with previous studies in adults, this study is the first to document sleep-wake disturbances in the acute phase in youth. Finding a co-occurrence of stress and fatigue is supported by the results from Losoi et al. who noted that individuals having modifiable psychological risk factors such as stress commonly reported fatigue persisting up to one year after injury.26
Total sleep time and sleep efficiency were found to be less in the injured group. Wylie and Flashman noted that individuals with mTBI typically report significantly lower sleep quality than non-injured controls, along with longer sleep onset latency, poorer sleep efficiency, longer sleep duration, more frequent daytime napping, and greater total sleep duration.9
Additionally, modest support for the effects of melatonin and cortisol concentrations on sleep were observed. In general, melatonin did impact awakenings (number and duration) and sleep efficiency. In an adult group of mTBI participants, Ayalon et al. delayed melatonin production, impacting circadian rhythmicity associated with disrupted sleep-wake patterns.27
Changes in overnight cortisol concentrations impacted duration of awakenings, total sleep time, and sleep efficiency. Further, an interaction between cortisol concentration differences were reversed between the groups: overnight increases in cortisol (T1 to T2) were greater for the injured group than the controls. However, the post-sleep changes (from T2 to T3) were reversed with the control group having greater increases. Differences in the trajectory of cortisol concentrations between groups were notable, though not statistically significant. Both injured and control groups started and ended at virtually the same cortisol levels (see Fig. 2).
The findings reported in this study point to likely causal mechanisms in sleep disruption after concussion with both melatonin and cortisol changes influencing sleep characteristics. Importantly, these findings point towards two potential avenues for treatment. Poorer sleep was a hallmark of the injured group with delayed sleep onset standing out as a significant problem. Subjective stress was directly related to sleep latency in the injured group with added tentative support found for melatonin and cortisol changes being contributory. Lower melatonin concentrations at bedtime and at the time of awakening suggested that an exogenous melatonin supplement may provide some help. Although current research provides mixed results, Kemp et al. obtained encouraging results for the effectiveness of melatonin treatment in a pilot randomized control trial.28 Combining melatonin with psychological stress reduction may likewise be effective.
Present findings support that reducing subjective stress should be considered early in concussion recovery. While psychotherapy is effective later in the recovery process, targeted behavioral interventions trials addressing sleep latency due to anxiety is a possible approach earlier in the course of injury. Unfortunately, as of now, little empirical is available evidence for using this approach,29,30 but cognitive behavioral therapy for insomnia is a promising post-concussion treatment.31
One aim of this study was to determine the feasibility of in-home study of sleep after concussion. The uncontrolled nature of a home environment created challenges and impacted data quality and limited subject participation because of busy parents. In the end, the small group sizes could not be overcome. Another limitation was the short timeframe for data collection. Collecting over multiple days is desirable but was logistically not possible in this design. However, despite these challenges, use of actigraphy in conjunction with sleep logs and obtaining saliva samples was effective. The lack of a stress metric for the control group was another limitation. The stress questionnaire used was aimed directly at a particular stressor and could not be adapted for control subjects.
Conclusions
In this study of multi-modal assessments of factors related to sleep after concussion in youth, several relationships emerged that lend support to a theory of sleep disruption following pediatric concussion. This theory suggests acute injury alters cortisol metabolism in such a way as to disrupt sleep with melatonin playing a role altering sleep latency. Stress immediately following the concussive event can impact cortisol and melatonin metabolic processes which in turn impacts sleep quantity and quality.
Although these findings are broadly consistent with previous studies of brain-injured adults, the small group sizes precluded strong inferences when considering methodological approaches for further study. Use of such multimodal assessment with larger groups will likely provide clearer direction and understanding of the mechanisms underlying sleep disruption in concussions and lend support for treatment approaches. This study demonstrated the value of multiple approaches to complex biological and psychological processes, while providing direction for treatment studies.
Authors' contributions
Funding provided by the Nebraska Research Initiative grant was awarded to DM and AM;
AM, DM, and JC developed the study plan, conducted the analyses and contributed to the writing;
RM recruited participants and provided the clinical evaluation; he also contributed to the writing;
CM and TC provided data collection including discussions with parents, traveling to their homes two-times, and entering the data. They both contributed to the writing.
Submission statement
This manuscript has not been published and is not under consideration for publication elsewhere.
Ethical approval
The study was approved by the Ethics Committee or Institutional Review Board of the University of Nebraska – Lincoln (IRB #15462) in cooperation with the University of Nebraska Medical School at Omaha.
Written informed consent and assent were obtained from all parents and participants before baseline measurements were started, and a copy of the signed consent and assent forms were given to each participant.
Conflict of interest
There are no other disclosures or conflicts of interest to report. There are no competing interests to report.
Acknowledgements
This work was supported by the Nebraska Research Initiative (NRI), USA with a grant to the first and last authors (grant title: "Interdisciplinary-Intercampus Investigation into the Neurophysiological and Neruopsychological Effects of Concussion in School-aged Children"). There are no other disclosures to report.
Amanda Prohansky's assistance was greatly appreciated for the actigraph data tabulation. We also thank the parents and youth who allowed us to come into their homes to collect data.
References
- 1.Hollis S.J., Stevenson M.R., McIntosh A.S., Shores E.A., Finch C.F. Compliance with return-to-play regulations following concussion in Australian schoolboy and community rugby union players. Br J Sports Med. 2012;46(10):735–740. doi: 10.1136/bjsm.2011.085332. [DOI] [PubMed] [Google Scholar]
- 2.Limond Jenny, Dorris Liam, McMillan T. Quality of life in children with axquired brain injury: parent perspectives 105 years after injury. Brain Inj. 2009;23:7–8. doi: 10.1080/02699050902997870. [DOI] [PubMed] [Google Scholar]
- 3.Page Margaretta, Berger Ann M., Johnson L.B. Putting evidence into practice : evidence-based interventions for sleep-wake disturbances. Clin J Oncol Nurs. 2006;10(6):753–768. doi: 10.1188/06.CJON.753-767. [DOI] [PubMed] [Google Scholar]
- 4.Rao V., Spiro J., Vaishnavi S., Rastogi P., Schretlen D., Makley M. Prevalence and types of sleep disturbance acutely after traumatic brain injury. 2. Brain Inj. 2008;22(5):381–386. doi: 10.1080/02699050801935260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekleton J.A., Parcell D.L., Redman J.R., Phipps-Nelson J., Ponsford J.L., Rajaratnam S.M.W. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology. 2010;74(21):1732–1738. doi: 10.1212/WNL.0b013e3181e0438b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdaugh D.L., Ono K.E., Resiner A., Burns T.G. Assessment of sleep quantity and sleep disturbances during recovery from sports-related concussion in youth athletes. Arch Phys Med Rehabil. 2018;99:960–996. doi: 10.1016/j.apmr.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Ponsford J.L., Sinclair K. Sleep and fatigue following traumatic brain injury. Psychiatry Clin N Am. 2014;37:77–89. doi: 10.1016/j.psc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.International Classification of Functioning, Disability and Health. vol. 16. 2001. WHO. International classification of functioning, disability and health; pp. 1–3. 123-124, 168-170. [DOI] [Google Scholar]
- 9.Wylie G.R., Flashman L.A. Understanding the interplay between mild traumatic brain injury and cognitive fatigue: models and treatments. Concussion. 2017;2(4):CNC50. doi: 10.2217/cnc-2017-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernier A., Carlson S.M., Carrier J. Relations between physiological and cognitive regulatory systems : infant sleep regulation and subsequent executive functioning. Child Dev. 2010;81(6):1739–1752. doi: 10.1111/j.1467-8624.2010.01507.x. [DOI] [PubMed] [Google Scholar]
- 11.Jaffee M.S., Winter W.C., Jones C.C., Ling G. Sleep disturbance in athletic concussion. Brain Inj. 2015;29:221–227. doi: 10.3109/02699052.2014.983978. [DOI] [PubMed] [Google Scholar]
- 12.Mollayeva T., Colantonio A., Cassidy J.D., Vernich L., Moineddin R., Shapiro C.M. Sleep stage distribution in persons with mild traumatic brain injury: a polysomnographic study according to American Academy of Sleep Medicine standards. Sleep Med. 2017;34:179–192. doi: 10.1016/j.sleep.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Mesquita G., Reimão R. Stress and sleep quality in high school brazilian adolescents. An Acad Bras Cienc. 2010;82(2):545–551. doi: 10.1590/S0001-37652010000200029. [DOI] [PubMed] [Google Scholar]
- 14.El-Sheikh M., Buckhalt J.A., Keller P.S., Granger D.A. Children's objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol. 2008;27(1):26–33. doi: 10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- 15.Meltzer L.J., Logan D.E., Mindell J.A. Sleep and cortisol in response to stress. J Behav Sleep Med. 2005;3(4):177–192. doi: 10.1207/s15402010bsm0304. [DOI] [Google Scholar]
- 16.Meltzer L.J., Montgomery-Downs H.E., Insana S.P., Walsh C.M. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumpter R.E., Dorris L., Kelly T., McMillan T.M. Pediatric sleep difficulties after moderate-severe traumatic brain injury. J Int Neuropsychol Soc. 2013;19(7):829–834. doi: 10.1017/S1355617713000465. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman Y., Tzischinsky O., Epstein R., Etzioni A., Lavie P., Pillar G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2001;24(2):129–134. doi: 10.1016/S0887-8994(00)00254-X. [DOI] [PubMed] [Google Scholar]
- 20.Sadeh A., Raviv A., Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. doi: 10.1037//0012-I549.36.3.29I. [DOI] [PubMed] [Google Scholar]
- 21.Doane L.D., Thurston E.C. Associations among sleep, daily experiences, and loneliness in adolescence: evidence of moderating and bidirectional pathways. J Adolesc. 2014;37(2):145–154. doi: 10.1016/j.adolescence.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Williamson D.E., Dahl R.E., Birmaher B., Goetz R.R., Nelson B., Ryan N.D. Stressful life events and EEG sleep in depressed and normal control adolescents. Biol Psychiatr. 1995;37(12):859–865. doi: 10.1016/0006-3223(94)00240-4. [DOI] [PubMed] [Google Scholar]
- 23.Åkerstedt T., Kecklund G., Axelsson J. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76(3):170–173. doi: 10.1016/j.biopsycho.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Haynes S.N., Adams A., Franzen M. The effects of presleep stress on sleep-onset insomnia. J Abnorm Psychol. 1981;90(6):601–606. doi: 10.1037/0021-843X.90.6.601. [DOI] [PubMed] [Google Scholar]
- 25.Mollayeva T., Colantonio A., Cassidy J.D., Vernich L., Moineddin R., Shapiro C.M. Sleep stage distribution in persons with mild traumatic brain injury: a polysomnographic study according to American Academy of Sleep Medicine standards. Sleep Med Rev. 2017;34 doi: 10.1016/j.sleep.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Losoi H., Silverberg N.D., Wäljas M., et al. Recovery from mild traumatic brain injury in previously healthy adults. J Neurotrauma. 2016;33(8):766–776. doi: 10.1089/neu.2015.4070. [DOI] [PubMed] [Google Scholar]
- 27.Ayalon L., Borodkin K., Dishon L., Kanety H., Dagan Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology. 2007;68(14):1136–1140. doi: 10.1212/01.wnl.0000258672.52836.30. [DOI] [PubMed] [Google Scholar]
- 28.Kemp S., Biswas R., Neuman V., Caughlin A. The value of melatonin for sleep disorders occurring post-head injury: a pilot RTC. Brain Inj. 2004;18:911–919. doi: 10.1080/02699050410001671892. [DOI] [PubMed] [Google Scholar]
- 29.McCrory P., Meeuwisse W., Dvořák J., et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 30.Al Sayegh A., Sandford D., Carson A.J. Psychological approaches to treatment of postconcussion syndrome: a systematic review. J Neurol Neurosurg Psychiatry. 2010;81(10):1128–1134. doi: 10.1136/jnnp.2008.170092. [DOI] [PubMed] [Google Scholar]
- 31.Tomfohr-Madsen L., Madsen J.W., Bonneville D., et al. A pilot randomized controlled trial of cognitive-behavioral therapy for insomnia in adolescents with persistent postconcussion symptoms. J Head Trauma Rehabil. June. 2019;1 doi: 10.1097/HTR.0000000000000504. [DOI] [PubMed] [Google Scholar]