Abstract
The ability of physical activity to ameliorate cardiovascular disease and improve cardiovascular health is well accepted, but many aspects of the molecular mechanisms underlying these benefits are incompletely understood. Exercise increases the levels of reactive oxygen species (ROS) through various mechanisms. This triggers the activation of Nrf2, a redox-sensitive transcription factor activated by increases in oxidative stress. Activation of Nrf2 mitigates oxidative stress by increasing the nuclear transcription of many antioxidant genes while also mediating additional beneficial effects through the cytoprotective nature of Nrf2 signaling. Understanding the transcriptional patterns of Nrf2 caused by exercise can help in the design of pharmacological mimicry of the process in patients who are unable to exercise for various reasons.
Keywords: Exercise, Oxidative stress, Antioxidants, Nrf-2, Cardiovascular system
Abbreviations: Nrf2, nuclear factor erythroid 2-related factor 2; PKC, protein kinase C; MnSOD, manganese superoxide dismutase; tBHQ, tertiary butylhydroquinone
Introduction
Physical activity promotes metabolic and cardiovascular health benefits that derive in part from the transcriptional responses to exercise within the cardiovascular system and other organs. There is great interest in discovering pharmacologic exercise mimetics that could impart wellness and alleviate disease burden especially in individuals with metabolic diseases, such as type 2 diabetes mellitus, and also in others who are unable to undertake an exercise of sufficient duration or intensity. Pharmacological approaches that could recapitulate the beneficial effects of exercise could potentially benefit individuals with spinal cord injury, paraplegia, morbid obesity.1 However, the molecular physiology by which exercise signals the transcriptional response is complex, making it challenging to identify a single target for pharmacological mimicry.2 Exercise mimetics can be classified as either full or specific mimetics, where full mimetics attempt to simulate all the complex local and systemic changes induced by physical exercise while specific mimetics only target specific organs. Full exercise mimetics that were previously suggested included activators of signaling molecules (e.g. GW501516, AICAR) such as peroxisome proliferator-activated receptor γ coactivator 1a (PGC-1a), peroxisome proliferator-activated receptor delta (PPAR δ), and adenosine monophosphate-activated protein kinase (AMPK).3 We propose that Nrf2 activation, which is strongly associated with cyto-protection, can mimic the effects of exercise on the cardiovascular system by acting as a specific mimetic.
Exposure to stress or exercise can transiently expand or contract the homeostatic range as a result of exposure to sub-toxic, non-damaging, signaling molecules.4 Examples of such signaling molecules are free radicals including ubisemiquinone, superoxide, and other (non-radical) reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and nitric oxide (NO), which are all generated during acute and regular exercise. Increases in the concentrations of these free radicals lead to oxidative stress from a state of oxidative eustress; for example, concentrations of H2O2 exceeding 10 nM activates adaptive stress responses via regulation of master switches such as Nrf2/Keap1 (Kelch-like ECH-associated protein 1) or NF-κB.5 Furthermore, physical exercise, by acting as a moderate stressor, triggers ischemic preconditioning and prevents the cytotoxicity caused by reperfusion, largely through the activation of the Nrf2 pathway.6
Nrf2 is a basic region-leucine zipper (bZip) transcription factor that forms heterodimers with small musculoaponeurotic fibrosarcoma protein (MAF) K, G, and F in the nucleus. The heterodimer recognizes an enhancer sequence termed antioxidant response element (ARE) that is present in the regulatory regions of more than 250 ARE genes. These ARE genes encode a network of cooperating enzymes involved in antioxidant mechanisms that generate NADPH, glutathione (GSH), and thioredoxin.7 The Nrf2 interactome is functionally linked to cytoprotection in low-grade stress, chronic inflammation, metabolic alterations, mechanical stress, and ROS formation.8 Analysis of these molecular profiles suggests alterations of Nrf2 expression and activity as a common mechanism in a subnetwork of diseases referred to as the Nrf2 diseasome.8 In addition to the role of Nrf2 in various diseases, acute and regular exercise can also induce a state of “low stress” through the production of ROS and stimulation of other mechanoreceptors that activates Nrf2 to modulate endogenous antioxidant systems.9 Activation of Nrf2 reduces cell damage while also conferring other cytoprotective effects on cardiac cells.10,11 The cardiovascular system is highly susceptible to oxidative stress-induced damage, in part due to a high metabolic rate.12
Therefore, the function of Nrf2 activated by exercise is not only to reduce oxidative stress, but also to protect cardiomyocytes from cell injury and apoptosis. Several studies have detailed the importance of pharmacologic Nrf2 activation in cardiac protection and performance enhancement; for example, curcumin, an Nrf2 activator, improves exercise performance of mice with coronary artery ligation.13 Trimetazidine, another Nrf2 activator, attenuates exhaustive exercise-induced myocardial injury in rats via regulation of the Nrf2/NF-κB signaling pathway.14 In summary, Nrf2 can be activated both by pharmacological agents and also by exercise, which can be viewed as a “low stress” condition that can generate oxidative stress, as well as mechanical stress that is able to activate Nrf2. Therefore, compounds such as sulforaphane, bardoxolone, curcumin, and resveratrol that can activate Nrf2 can also mimic the cardiovascular benefits of exercise because of the cytoprotective nature of Nrf2 signaling. Such compounds are putative-specific mimetics of the beneficial effects of exercise. This review summarizes different mechanisms that can lead to Nrf2 activation either by exercise or Nrf2 activators to deliver cardiovascular benefits.
Regular exercise and Nrf2 levels
Activation of Nrf2 during exercise
Nrf2 normally resides in the cytosolic compartment by association with a cytosolic actin-binding protein, Keap1 (Kelch-like ECH-associated protein 1), which is also less commonly known as an inhibitor of Nrf2. Keap1 plays a central role in the regulation of Nrf2 activity, and exists as dimers inside the cells, where Keap1 functions as a substrate linker protein for the interaction of Cul3/Rbx1-based E3-ubiquitin ligase complex with Nrf2, leading to the continuous ubiquitination of Nrf2 and its subsequent proteasomal degradation. Hence, the continuous degradation of Nrf2 under basal conditions maintains Nrf2 at low levels, leading to low basal levels of Nrf2-regulated antioxidants. When cells encounter stress, such as exposure to mild oxidative or electrophilic stress, or chemical inducers, Nrf2 (via modification of critical cysteine thiols of Keap1 and Nrf2 and other mechanisms as discussed below) dissociates from Keap1, becomes stabilized, and translocates into the nuclei, where it forms heterodimers with small musculoaponeurotic fibrosarcoma protein (MAF) K, G, and F in the nucleus. The heterodimer recognizes an enhancer sequence termed antioxidant response element (ARE) that is present in the regulatory regions of over 250 genes (ARE genes).
Findings from both in vivo and in vitro experiments indicate that exercise activates Nrf2. Cell culture studies using skeletal muscle cells support the concept that Nrf2 is activated by ROS, and that this activation is suppressed by antioxidants such as N-acetylcysteine.15 Additionally, H2O2 treatment caused a rapid increase in endogenous Nrf2 protein levels in rat cardiomyocytes.16 Earlier work by Muthusamy et al.9 confirmed that acute exercise stress (AES) activates Nrf2/ARE (antioxidant response element) signaling in the mouse heart, with subsequent enhancement of antioxidant defense pathways while excessive oxidative stress, along with blunted defense mechanisms, was observed in Nrf2-null mice. There are a limited number of reports on the effects of physical activity on human Nrf2 levels. A recent study evaluated Nrf2 signaling in response to two 30-min cycling protocols in healthy young men, and reported increases in nuclear Nrf2 activation (measured by protein expression of Nrf2 in the whole-cell and nuclear fractions) during acute aerobic exercise regardless of exercise intensity.17 We next discuss some mechanisms of signaling activated by Nrf2 during exercise.
Mechanisms of Nrf2 activation during exercise
Increase in oxidative stress
An increase in ROS levels is one of the main activators of Nrf2 during exercise. The role of ROS in Nrf2 activation during exercise gained traction with the identification of ROS-generating NADPH oxidase-4 (Nox4) as an essential regulator of exercise performance in mice. Myocardial Nox4 levels increase during acute exercise and trigger activation of Nrf2, with the induction of multiple endogenous antioxidants. Cardiomyocyte-specific Nox4-deficient (csNox4KO) mice display a loss of exercise-induced Nrf2 activation, cardiac oxidative stress and have reduced exercise performance.18 Keap1 is a redox and electrophile sensor that upon modification of critical cysteine residues loses its ability to repress Nrf2. Keap1 is a cysteine-rich protein, with mouse Keap1 having a total of 25, and human with 27, cysteine residues, most of which can be modified in vitro by different oxidants and electrophiles.19 Three of these residues (C151, C273, and C288) alter the conformation of Keap1 leading to nuclear translocation of Nrf2 and subsequent target gene expression (Fig. 1, Pathway A). The Keap1-Nrf2 is a key regulator of cytoprotective responses to endogenous and exogenous stresses caused by ROS and electrophiles.20 The main mechanism regulating the transcriptional activity of Nrf2 occurs by the control of protein stabilization by Keap1. Keap1 is a homodimeric protein that bridges Nrf2 with the E3 ligase complex formed by Cullin 3 and RING-box protein 1 (CUL3/RBX1). Under homeostatic conditions, the N-terminal domain of the Keap1 homodimer binds one molecule of Nrf2 at two amino acid sequences with low (aspartate, leucine, and glycine; DLG) and high (glutamate, threonine, glycine, and glutamate; ETGE) affinity, and hence presents Nrf2 to ubiquitination by CUL3/RBX121 and subsequent degradation by the proteasome. Moderate increases in ROS levels during exercise activates Nrf2 by altering the conformation of Keap1, which allows Nrf2 to escape ubiquitination, with subsequent translocation into the nucleus.
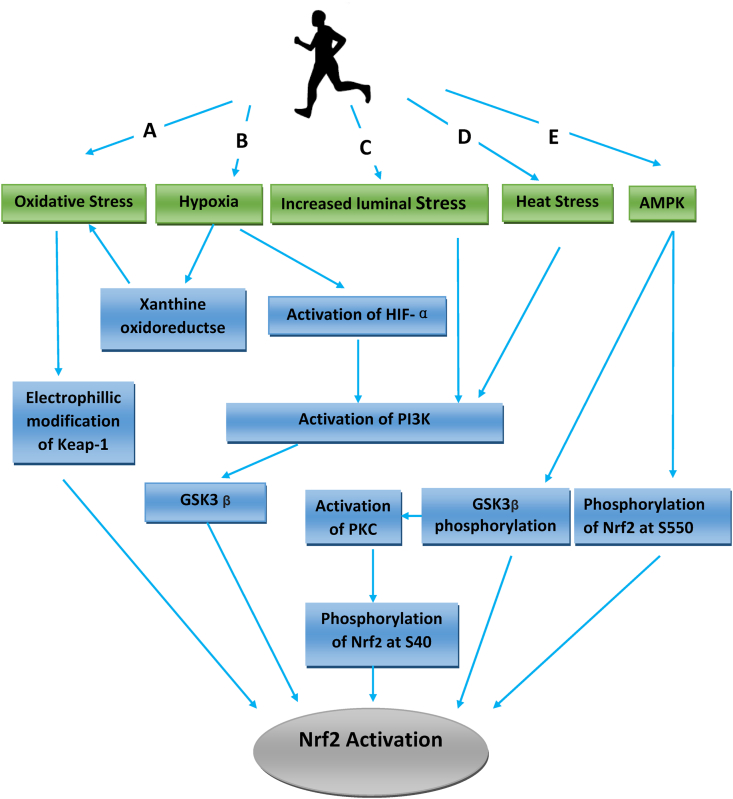
Fig. 1.
Pathways leading to activation of Nrf2 by exercise. A: Increases in ROS modifies Keap – Nrf2 interactions, B: Shear stress in blood vessels activates PI3K, C: Tissue hypoxia activates HIF-1α, PI3K and xanthine oxidoreductase, D: Heat stress leading to increase in ROS and Nrf2 activation, E: Activation of AMPK leading to GS3K and Nrf2 phosphorylation.
Hypoxia
Hypoxia is a component of exercise22 and has at least two consequences on Nrf2 activation: first is the increased expression of hypoxia-inducible factor-1α (HIF-1 α) leading to the stimulation of the PI3K/Akt GSK-3 β pathway,23 which then causes the phosphorylation of serine residues on glycogen synthase kinase-3 beta and prevention of Nrf2 ubiquitination.24 The second consequence of exercise-related hypoxia is the conversion of xanthine dehydrogenase to xanthine oxidase during exercise, perhaps due to transient hypoxia and the subsequent generation of reactive oxygen and nitrogen species (Nrf2 activators) from the xanthine oxidase produced25,26 (See Fig. 1, Pathway B). Other effects of hypoxia on angiogenesis are discussed later.
Increase in luminal stress
The vascular endothelium experiences constant hemodynamic stress resulting from frictional forces of blood flow across its surface, and also by pressure changes with each cardiac cycle; both effects are enhanced during exercise. The interaction between fluid shear stress and endothelial function is critical in maintaining vascular homeostasis via the integration of biomechanical forces with signal transduction mechanisms to maintain redox balance. Endothelial cells respond to changes in shear stress by modulating redox signaling27 and other non-redox mechanisms through the activation of the PI3K pathway (see Fig. 1, pathway C). When shear stress activates PI3K, it also phosphorylates PIP2 to form PIP3, allowing phosphoinositide-dependent kinase 1 (PDK1), which can activate PKC, a downstream substrate of PDK1) to be phosphorylated and activated. Activation of Nrf2 by PKC can then occur through the phosphorylation of S40 at the Neh2 domain of Nrf2, which interacts with Keap1 and increases the nuclear translocation of Nrf2.28,29 Thus, Nrf2 acts as an endothelial mechanoreceptor transcription factor in the coupling of intravascular physical forces with redox responses. Physical exercise also stimulates endothelium-dependent relaxation of collateral coronary arteries and arterioles through increased eNOS expression at the mRNA and protein levels,30 leading to increased nitric oxide (NO) production by the endothelium.31 It has been suggested that NO generated during exercise also signals the transcriptional up-regulation of NAD(P)H: quinone oxidoreductase 1 (NQ01) and other detoxifying enzymes and protective genes through Nrf2 activation.32
Heat stress
The body's heat production rate can exceed 1000 W during exercise largely due to the contraction of skeletal muscles. Some of the heat produced is stored, increasing body core temperature to as high as 104 °F (40 °C).33 The exposure of cells to this low dose of heat stress induces adaptive survival responses that protect cells against subsequent exposure to heat stress by a process known as thermotolerance, which involves the Nrf2 antioxidant pathway,34 leading to increases in protein expression of the Nrf2 transcription factor and resulting in increased levels of Nrf2 targets such as MnSOD, catalase, HO-1, glutamate-cysteine ligase, and Hsp70 (See Fig. 1, Pathway D).
Increase in AMPK
AMPK (AMP-activated protein kinase) is a phylogenetically conserved fuel-sensing enzyme present in all mammalian cells. It is activated in skeletal muscle and other tissues during exercise in response to an increase in the AMP/ATP ratio. Activation of AMPK has long been regarded as one of the crucial signaling nodes responsible for the transcriptional response to exercise, and the benefits of exercise have been attributed to AMPK activation in part through Nrf2 activation. AMPK phosphorylates Nrf2 at the Ser550 residue, which, in conjunction with AMPK-mediated GSK3β inhibition, promotes nuclear accumulation of Nrf2 for antioxidant (Fig. 1 Pathway E) response element (ARE)-driven gene transactivation. This is demonstrated by the ability of xanthohumol (an AMPK activator) to suppresses inflammation, leading to an increase in the transcription of Nrf2-targeted antioxidative genes (NADPH quinone oxidoreductase-1 [NQO-1], heme oxygenase-1 [HO-1]), as well as an increase in nuclear localization and phosphorylation of Nrf2 protein.35 Additionally, AMPK activators are also able to induce Nrf2 activation through a pathway that is AMPK independent.36 Other activators of AMPK include metformin and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR). Metformin can activate AMPK by phosphorylation of the key regulatory site (Thr-172) on the catalytic subunit of AMPK and confers benefits that are similar to that produced by exercise,37 while AICAR can stimulate Nrf2 through AMPK dependent and independent pathways.38 Genetic ablation and pharmacological inhibition of AMPK blunts Nrf2-dependent HO-1 expression by xanthohumol at the mRNA level.39 Therefore, AMPK dependent activation of Nrf2 is one of the molecular activation pathways of Nrf2 in exercise. (See Fig. 1, pathway E).
Summarily, two major mechanisms participate in the activation of Nrf2: a) increases in levels of ROS and other stressful conditions such as hypoxia, heat stress, and b) increased luminal stress through the PI3K/Akt pathway. Both mechanisms play key roles in Nrf2 activation and associated with the cardiovascular benefits of exercise, as summarized in Table 1.
Table 1.
Nrf2 activation in different tissues.
| Activation of the heart | Activation of blood vessels | Activation of skeletal muscles | |
|---|---|---|---|
| Increase in luminal stress (Pathway A) | √ | ||
| Increase in oxidative stress (Pathway B) | √ | √ | √ |
| Hypoxia (Pathway C) | √ | √ | √ |
| Heat stress (Pathway D) | √ | ||
| Increase in AMPK (Pathway E) | √ | √ | √ |
Role of Nrf2 in regular exercise mediated cardiovascular benefits
Regular exercise improves cardiovascular health by promoting adaptive responses through activation of Nrf2. Rhythmic activation of Nrf2 upregulates the transcription of several endogenous cardiac antioxidants, which can reduce oxidative stress in cardiomyocytes.40 Other benefits of Nrf2 activation include increased mitochondrial biogenesis and capacity, physiologic remodeling of the heart muscle, improved angiogenesis, and reduced arteriosclerosis.
Nrf2 maintains mitochondrial health
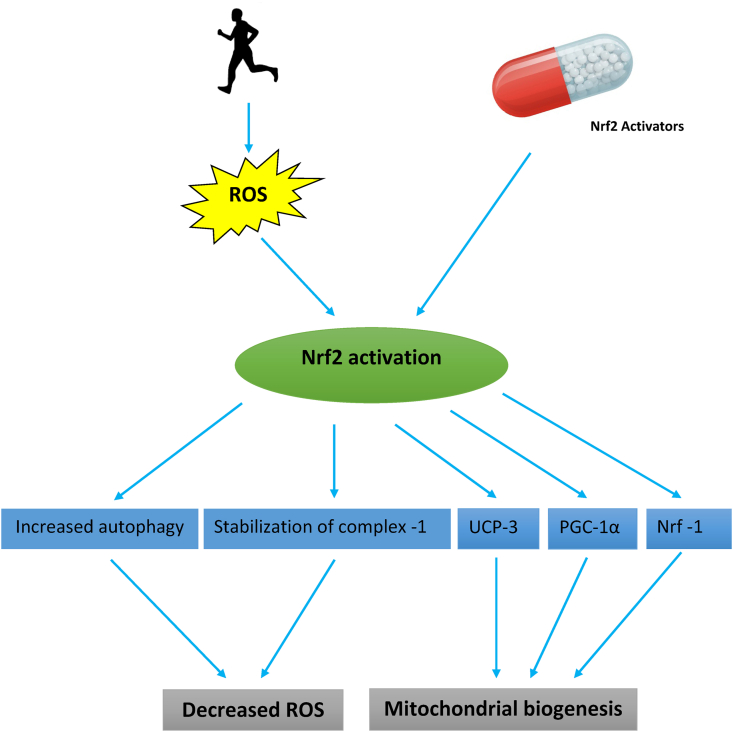
Mitochondria are the primary source of cellular ROS, and play a central role in energy metabolism, control of the stress responses, cell death as well as being the hub for several biosynthetic processes. Also, mitochondria modulate ROS production to regulate apoptosis. Regular physical activity increases muscle mitochondrial metabolic capacity by increasing muscle mitochondrial DNA and ATP production rates,41 while long-term aerobic exercises reduce age-related loss of mitochondrial DNA function in humans via the effects of transcription factor Nrf2.42 The Nrf2 gene allows for adaptation and survival under conditions of stress, such as during exercise, by regulating the gene expression of several cytoprotective proteins, including antioxidant, anti-inflammatory, and detoxification enzymes, and other proteins that assist in the repair or removal of damaged macromolecules by promoting autophagy as evidenced in a model of sepsis, where increasing levels of the autophagosome marker MAP1 light chain 3-II (LC3-II) and the cargo protein p62 at 24 h post-infection which are suppressed in Nrf2-KO mice43 (Fig. 2).
Fig. 2.
Effect of Nrf2 on mitochondrial ROS generation and biogenesis.
Abbreviations: Nrf-1 = Nuclear respiratory factor-1, PGC-1α = Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, ROS = Reactive oxygen species, UCP3= Uncoupling protein-3.
Excessive ROS levels from exhaustive exercise release cytochrome c from mitochondria, which triggers caspase activation leading to apoptosis, whereas moderate exercise activates Nrf2 to counteract increased mitochondrial ROS production by causing transcriptional upregulation of uncoupling protein 3 (UCP3) (Fig. 2). One of the functions of uncoupling proteins (UCP1, UCP2, and UCP3) and adenine nucleotide translocase is to induce proton leak in response to increases in oxidative stress. UCP3 therefore actively lowers the rate of ROS production in isolated energized mitochondria in the absence of exogenous activators.44 High succinate levels and a high membrane potential in the mitochondria, both of which occur in exercising muscles, induce reverse electron transfer from complex II into complex I and is associated with increased superoxide production.45,46 Embryonic fibroblasts from Nrf2 knockout mice are unable to regulate their membrane potential and therefore generate high levels of ROS, unlike cells from wild-type mice which have a decreased mitochondrial membrane potential that allows them to regulate excessive ROS production.47
Enhancing mitochondrial biogenesis is another mechanism by which Nrf2 promotes mitochondrial health; as discussed earlier, Nrf2 stimulates UCP3, which in addition to reducing ROS levels, also maintains levels of nuclear respiratory factor 1 (NRF-1). NRF-1 encodes a protein that homodimerizes and functions as a transcription factor and activates the expression of some key metabolic genes regulating cellular growth and nuclear genes required for respiration, heme biosynthesis, and mitochondrial DNA transcription and replication. UCP-3 also activates peroxisome proliferator-activated receptor γ coactivator 1α (making the two classes of nuclear transcriptional regulators important for mitochondrial biogenesis) and promotes purine nucleotide biosynthesis (Fig. 2). Transcription factors such as NRF-1 &2 control the expression of both the genes encoding subunits of the five respiratory complexes and mitochondrial translational components and also those for heme biosynthetic enzymes that are localized in the mitochondrial matrix.48 Nrf2-dependent transcriptional upregulation of NRF-1 promotes mitochondrial biogenesis (by increasing mitochondrial protein content) and protects against cardiac cytotoxicity produced by the anthracycline chemotherapeutic agent doxorubicin.49 Sulforaphane mimics these effects by reversing the doxorubicin-associated reduction in nuclear Nrf2 binding activity and restoring cardiac expression of Nrf2-regulated genes, at both the RNA and protein levels.
Summarily, Nrf2 activation during moderate exercise is critical to mitochondrial homeostasis and survival by reducing excessive ROS production, preventing cardiomyocyte apoptosis, and enhancing mitochondrial biogenesis.
Nrf2 inhibits cardiac pathological remodeling
Cardiac remodeling in humans and animals is often an adaptation to underlying pathologic or physiologic changes. Enlargement of the ventricular cavities, or increases in myocardial wall thickness, occurs in response to an enhanced volume load or increased wall stress, which can either be physiologic or pathologic. Physiologic changes result from the increased hemodynamic loading of the heart during exercise, which can be in the form of either an increased volume load or an enhanced “pressure” afterload, or a combination of both, depending on the specific exercise stimulus. Therefore, structured exercise over prolonged periods, as experienced by competitive athletes, stimulates cardiac adaptation and physiologic remodeling50 and is termed “the athlete's heart”. In pathologic remodeling, there is increased left ventricular (LV) volume and perturbations of the normal elliptical LV chamber configuration which is driven, on a histologic level, by myocyte hypertrophy and apoptosis and increased interstitial collagen. Moderate exercise is protective against pathologic remodeling induced by isoproterenol via the expression of Nrf2-dependent antioxidant genes; furthermore, Nrf2 attenuates oxidative stress-induced myocardial damage, while echocardiography analysis revealed impaired diastolic ventricular function in untrained mice receiving isoproterenol.51 A role for Nrf2 in cardiac remodeling was also demonstrated in aged WT and Nrf2 knockout animals undergoing chronic endurance exercise, where aged WT and Nrf2 knockout animals both exhibited hypertrophy. The older Nrf2 knockout animals showed ventricular remodeling coupled with profound cardiac functional abnormalities and diastolic dysfunction.52 This suggests that moderate exercise can prevent pathologic remodeling in cardiac tissue by activation of Nrf2.
Both physiologic and pathologic remodeling is characterized by initial stress and activation of Nrf2. While activation produced by regular exercise is rhythmic, pathologic activation is continuous and unrelenting, eventually leading to a fall in Nrf2 levels. The loss of Nrf2 activity in pathologic remodeling is therefore in part linked to a constant and unwavering stress as demonstrated in mice where transverse aortic constriction (as a source of hemodynamic stress or pressure overload) in mice initially induced adaptive hypertrophy (days 1–14) with preserved cardiac function; however, sustained pressure overload caused maladaptive cardiac remodeling and left ventricular dysfunction (days 14–28). These changes coincided with a transient enhancement of Nrf2 expression at both mRNA and protein levels in the heart that peaked on day 7. This then decreased to near basal levels on day 28, coinciding with ventricular dysfunction after transverse aortic constriction.53
Nrf2 activation is therefore associated with an ability to prevent apoptosis and pathologic remodeling in cardiac tissue by exerting an indirect control of nuclear factor kappa-B (NF-kB) activity. This is possible because the NRF2 gene is a master regulator of redox homeostasis. Lipopolysaccharide (LPS) simultaneously activates a fast, pro-inflammatory NF-kB response and slow Nrf2 response. The NF-kB response is subsequently inhibited when Nrf2 is maximally active.54 Moreover, exercise training inhibited NF-kB expression induced by water pipe smoke in mice while exercise increased Nrf2 activity.55
Another important effect of Nrf2 is in cardiac remodeling where it is related to the expression of metallothioneins Mt1 and Mt2 genes. The effect of exercise on cardiac fibroblasts is due to differentially expressed NRF2-dependent antioxidant genes - Mt1 and Mt2 which are induced in cardiac fibroblasts during exercise. Mice lacking Mt1/2 exhibit signs of cardiac dysfunction in exercise, including cardiac fibrosis, vascular rarefaction, and functional decline.56 These results indicate that cardiac tissue can benefit from exercise by prevention of pathologic cardiac remodeling through the activation of Nrf2 and its action on Mt1 and Mt2 genes.
Role of Nrf2 in arteriosclerosis
Regular exercise is a deterrent to cardiovascular diseases, and its anti-atherogenic effects have been described in several animal models.57,58 Oxidative stress is a leading contributor to the atherosclerotic process.59 Knock-out of several antioxidant enzymes and downstream products of Nrf2 activation such as glutathione peroxidase 1 (Gpx1), peroxiredoxin 1 (Prdx1) and heme oxygenase 1 (HO-1) worsen atherosclerotic plaque formation,60,61 suggesting a key role for Nrf2 in this process. Moderate levels of regular exercise are beneficial by activating Nrf2 which upregulates endogenous antioxidant enzymes such as manganese superoxide dismutase, heme oxygenase, and catalase,62,63 as well as promoting the synthesis of glutathione.64 These pathways are part of a plethora of protective resources against oxidation and inflammation in the development of arteriosclerosis.
Our current understanding is that the effects of Nrf2 in preventing atherosclerosis may be mediated through the inducible form of HO-1, an Nrf2 regulated gene. HO-1 is responsible for the oxidative cleavage of heme groups, leading to the generation of biliverdin, carbon monoxide, and the release of ferrous iron. HO-1 has important antioxidant, anti-inflammatory, anti-apoptotic, anti-proliferative, and immunomodulatory effects in vascular cells through the formation of bilirubin, which is the most potent endogenous low-molecular-weight peroxynitrite scavenger currently known. Bilirubin prevents lipid peroxidation, making the Nrf2/HO-1 axis a potent inhibitor of (endothelial nitric oxide synthase) eNOS uncoupling, prostacyclin synthase nitration, and soluble guanylate cyclase (sGC) oxidation. These actions prevent many pro-atherogenic vascular signaling pathways, with significant roles in the protection against atherogenesis.63,65,66 The only known human case of HO-1-deficiency exhibited marked endothelial cell injury and early atherosclerotic changes, as reflected by an irregular distribution of foamy macrophages with iron pigments, fatty streaks, and fibrous plaques in the aorta.67,68 Another important protective role of Nrf2 during atherosclerotic plaque formation is to reduce macrophage infiltration and foam cell formation following macrophage absorption of LDL cholesterol. Exposing macrophages to oxidized LDLs leads to increased Nrf2 expression, which indirectly protects macrophages from oxidized LDL-mediated injury via phase II antioxidant enzyme activity.69
Another Nrf2 downstream target that also inhibits monocyte migration is the activation of glutathione-cysteine ligase modifying subunit and NAD(P)H dehydrogenase [quinone] 1 (NQO1), both of which offer protection against atherosclerosis.70 Low serum glutathione levels are an independent risk factor for coronary heart disease in adolescents71; low levels of glutathione peroxidase (GPx) levels, for which glutathione is a cofactor,72 combined with low HDL levels, may be partly responsible for increased atherosclerosis-related mortality rates in humans.73 Nrf2 is an important regulator of glutathione peroxidase,74 making Nrf2 crucial in protection against atherosclerosis.
Nrf2 also protects endothelial cells from developing a pro-inflammatory state by suppressing p38–VCAM-1 signaling, which is another mechanism for Nrf2 protection against atherosclerosis. Early atherosclerotic lesions contain monocytes and T-lymphocytes, recruited from the circulation by adhesion to activated vascular endothelial cells in a process triggered by pro-inflammatory mediators (e.g., TNF-α). Pro-inflammatory mediators activate cellular adhesion molecules (e.g., VCAM-1) via signaling intermediaries including phosphorylated p38 mitogen-activated protein (MAP) kinases 3 and 6 (MKK 3/6).75 Suppression of p38-VCAM-1 signaling by Nrf2 prevents the development of these events.76 Sulforaphane (an Nrf2 activator) reduces TNF-α mediated secretion of endothelin-1, VCAM1, ICAM1, and E-selectin, and reduces endothelial permeability in placental explants and also the secretion of TNF-α induced soluble Flt-1, soluble endoglin, and activin A.77
Despite the intuitive role of Nrf2 and the persuasive evidence of its antioxidant, anti-inflammatory, and anti-atherogenic properties, the Nrf2 signaling pathway has demonstrated both pro-and anti-atherogenic effects in animal models of disease. Deletion of the Nrf2 gene in ApoE knockout mice decreases atherosclerotic lesions at a late stage, whereas it does not affect atherosclerotic lesions during earlier stages.78 These observations suggest that Nrf2 inhibition may be atheroprotective in advanced plaques.
Nrf2 improves angiogenesis and endothelial health
The endothelium is an interface between circulating blood and tissues that regulates vascular tone, thromboresistance, inflammation of the vascular wall, and cellular adhesion. The endothelium is exquisitely susceptible to injury as it can respond to a variety of physical and chemical stimuli.79,80 Aged or senescent endothelial cells become dysfunctional, lose re-endothelialization, angiogenesis capacity and become pro-atherogenic and pro-thrombotic.81 Regular exercise induces coronary vascular adaptations including increased coronary blood flow reserve,82,83 and this increase in coronary blood flow has been linked to increased angiogenesis.84
Angiogenesis is controlled by several factors including Nrf2 signaling. It is enhanced by treating endothelial cells with angiogenic cytokines promoting nuclear localization of Nrf2 and an increase in expression of HO-1. Nrf2 activation stimulates tube network formation, unlike its inhibition which decreases angiogenic responses of human endothelial cells. Moreover, a lack of Nrf2 attenuates the survival, proliferation, migration, and angiogenic potential of murine proangiogenic cells and negatively impacts the angiogenic transcriptome in vitro.85
Another important factor in angiogenesis and tissue repair is the level of circulating endothelial progenitor cells (EPCs), which are bone marrow-derived cells. These cells can target sites of ischemic injury and repair damaged vessels. Several studies show that physical activity significantly increases circulating EPCs, and in particular, there is evidence of the beneficial effects of exercise on EPCs activity in coronary artery disease, heart failure, and peripheral artery disease.86,87 The role of Nrf2 in enhancing the function of EPCs was demonstrated in streptozotocin-induced diabetic mice by silencing the NRF2 gene which decreased EPC biological functions leading to accelerated cell senescence and increased oxidative stress e.g., ROS and malondialdehyde upregulation, decreased SOD activity. Furthermore, activation of the Nrf2 gene protected EPCs from diabetic mice from oxidative stress and cell senescence.12
Hypoxia is a strong activator of angiogenesis, in part due to its ability to activate Nrf2 during exercise.88 Hypoxia activates the HIF-1α - PI-3K/AKT pathway,23 leading to decreased ubiquitinization and stabilization of Nrf2 through phosphorylation and inactivation of GSK-3beta. Hypoxic conditions increase mRNA and protein expression of Nrf2 and HO-1 in rat cardiac microvascular endothelial cells. Furthermore, knockdown of Nrf2 suppresses the migration and vascular tube formation of rat cardiac microvascular endothelial cells in response to hypoxia, while also decreasing HO-1 and VEGF expression. Finally, overexpression of Nrf2 in cardiac microvascular endothelial cells increases HO-1 expression, cell migration, and vascular tube formation induced by hypoxia; these effects were attenuated by an HO-1 inhibitor such as ZnPP.89 Thus, Nrf2 generated during exercise helps to maintain endothelial health by enhancing angiogenesis and preventing endothelial cell senescence.
Role of Nrf2 in reducing hypertension
Hypertension is a risk factor for several cardiovascular diseases including coronary disease, left ventricular hypertrophy, valvular heart diseases, cardiac arrhythmias, stroke, and renal failure. The effects of exercise interventions on systolic blood pressure remain understudied, especially in hypertensive populations. A recent meta-analysis of randomized controlled trials of patients treated with antihypertensives such as angiotensin-converting enzyme inhibitors, angiotensin-2 receptor blockers, β-blockers, calcium channel blockers, diuretics, and exercise interventions on systolic blood pressure concluded that exercise lowered blood pressure to a similar extent as that produced by commonly used antihypertensive medications.90
The ability of Nrf2 to mitigate blood pressure reduction stems from its role as a mechanosensitive transcription factor that is activated in several models of hypertension: DOCA-salt-induced hypertension,91 chronic pressure overload92 and aortic constriction.93 Similarly, treatment with verapamil, an FDA-approved drug for the treatment of hypertension, causes Nrf2 activation due to KEAP1 degradation,94 which might play a part in the reduction in blood pressure exhibited by verapamil.
Nrf2 influences redox signaling in small arteries and isolated vascular smooth muscle cells of control Wistar Kyoto (WKY) and stroke-prone spontaneously hypertensive rats (SHRSP). Increased vascular ROS production produced by angiotensin II was associated with reduced Nrf2 activity in arteries and vascular smooth muscle cells in SHRSP, leading to increased vascular contractility and decreased endothelial-dependent relaxation in SHRSP; these changes were corrected by l-sulforaphane, an activator of Nrf2.95
The short-term regulation of blood pressure has also been shown to be under the control of Nrf2. For example, tBHQ (Nrf2 activator) decreases mean arterial pressure and plasma norepinephrine levels in rats, while knockout of Nrf2 in the hypothalamic paraventricular nucleus (which influences sympathetic outflow in the central nervous system) blunted the blood pressure lowering effects of tBHQ,96 suggesting the blood pressure-lowering effect of tBHQ is due to Nrf2 activation.
Heme oxygenase 1 (HO-1) is the stress-induced isoform of heme oxygenase that is activated in part by Nrf2 activation and has a profound effect on endothelial function and hypertension. Cardinal pathophysiological features of hypertension (enhanced vascular inflammation, vascular remodeling, vascular contractility) are all alleviated by the heme oxygenase (HO) system. Induction of HO-1 prevents pulmonary hypertension in response to chronic hypoxia,97 and alterations in the activity and expression of HO correlate with the pathophysiology of hypertension and related complications such as hypertrophy, myocardial infarction, and heart failure.67 Additionally, a spectrum of drugs has been used to up-regulate HO-1 expression and HO activity to lower blood pressure. Stannous chloride (SnCl2) lowers blood pressure in spontaneously hypertensive rats.98 Metalloporphyrins (such as heme, heme arginate, and CoPP) induce HO-1 expression and HO activity and normalize blood pressure in animals and humans.99,100
Nrf2 activators mimicking exercise-induced cardiovascular benefits
Unlike ROS scavengers, Nrf2 activators do not interfere with stimulation of antioxidant systems, but they can directly activate Nrf2 through various mechanisms. This activation can result in excessive NRF2 levels and so lowering reductive stress.51 Most pharmacological Nrf2 activators are electrophilic molecules that covalently modify cysteine residues in the thiol-rich Keap1 protein by oxidation or alkylation. Examples of these Nrf2 activators include sulforaphane, bardoxolone, curcumin, and resveratrol (see Table 2).
Table 2.
Nrf2 activators that can mimic exercise-induced cardiovascular benefits.
| Compound | Mechanism of action | Cardiovascular benefit | Reference |
|---|---|---|---|
| Sulforaphane | Electrophilic modification of Keap1-Cys-151 | Increases mitochondrial mass and augments PGC1α and PGC1β activity | 103 |
| Curcumin | Electrophilic modification of Keap1-Cys-151 | Protects against pathological remodeling. Increases exercise capacity in heart failure | 13, 104 |
| Resveratrol | Electrophilic modification of Keap1-Cys-151 | Improves •NO availability and decreases endothelial dysfunction. Reduction in blood pressure. |
105, 106 |
| Trimetazidine | Not determined yet | Prevents apoptosis attenuates exhaustive exercise-induced myocardial injury | 14 |
Many cysteine residues of Keap1 are modified by electrophiles.101,102 As discussed earlier, Keap1 is a cysteine-rich protein, with 27 cysteine residues in humans, most of which can be modified in vitro by different oxidants and electrophiles.19 Three of these residues (C151, C273, and C288) can alter the conformation of KEAP1 leading to nuclear translocation of Nrf2 and subsequent target gene expression. Other sensitive cysteines are Cys-226, Cys-434, and Cys-613. This “cysteine-code” controls Keap1 activity during the protective response mediated by Nrf2. Examples of these Nrf2 activators include sulforaphane, bardoxolone, curcumin, and resveratrol.
Exercise vs Nrf2 activators
Physical exercise provokes whole-body homeostatic responses characterized by the increased metabolic activity of contracting skeletal muscle, such as increased skeletal muscle energy and oxygen demand. To meet this challenge, multiple integrated signaling pathways, including those in skeletal muscle, cardiovascular, and endocrine systems, are activated. Trying to mimic this process has led to the discovery of full and specific exercise mimetics. The full mimetics mimic all aspects of exercise, while specific mimetic only target particular organs. Activating Nrf2 signaling will be better described as a specific mimetic because it is mainly involved with cytoprotection and most Nrf2 activators only activate Nrf2 by electrophilic modification of Keap1, which is similar to the action of ROS in exercise, and not by other mechanism employed by exercise. Even though there is emerging evidence to support the concept that aspects of the cardiovascular benefits of exercise can be replicated by Nrf2 activators, the side effects of these activators should be also be taken into consideration. One such activator is bardoxolone methyl, which has been associated with endothelial toxicity along, as is the case for other agents such as rofecoxib (COX-2 inhibitor) and torcetrapib (CETP-inhibitor).107 This is counter-intuitive based on the ability of bardoxolone to activate Nrf2 and confer endothelial benefits. But high doses of most Nrf2 activators tend to inhibit Nrf2 stimulation. For example, sulforaphane (at a concentration as low as 0.2 μM) doubles the activity of NQO1 (index of Nrf2 activation).108,109 Sulforaphane has time and concentration-dependent inhibitory effects on hypoxia-induced mRNA expression of vascular endothelial growth factor and HIF-1A and c-Myc, two angiogenesis-associated transcription factors, at higher concentrations (0.8–25 mmol/L). Overall, doses of Nrf2 activators should be tailored to reduce endothelial toxicity.
Conclusion
Nrf2 is activated by regular exercise and plays an important role in exercise-induced cardiovascular benefits such as increased mitochondrial biogenesis and capacity, physiologic remodeling of the heart muscle, and reduced arteriosclerosis. Enhancing Nrf2 signaling with Nrf2 activators can provide cardiovascular benefits because of the cytoprotective nature of Nrf2 signaling. Caution should be taken in using these activators because they can antagonize Nrf2 signaling at higher doses.
Submission statement
The authors confirm that this material has not been submitted elsewhere for publication, either in part or in whole, and that this work is an original presentation.
Authors' contributions
The text was written by BF and SL, who also prepared the tables and figures. IL supervised and edited the text.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Li S., Laher I. Exercise pills: at the starting line. Trends Pharmacol Sci. 2015;36(12):906–917. doi: 10.1016/j.tips.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Li S., Laher I. Exercise mimetics: running without a road map. Clin Pharmacol Ther. 2017;101(2):188–190. doi: 10.1002/cpt.533. [DOI] [PubMed] [Google Scholar]
- 3.Narkar V.A., Downes M., Yu R.T., et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;8(3):405–415. doi: 10.1016/j.cell.2008.06.051. 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies K.J.A. Cardiovascular adaptive homeostasis in exercise. Front Physiol. 2018 doi: 10.3389/fphys.2018.00369. Accessed May 01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 6.Fratta Pasini A.M., Stranieri C., Rigoni A.M., et al. Physical exercise reduces cytotoxicity and up-regulates Nrf2 and UPR expression in circulating cells of peripheral artery disease patients: an hypoxic adaptation? J Atherosclerosis Thromb. 2018;25(9):808–820. doi: 10.5551/jat.42432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A., Kang M.I., Watai Y., et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2015;26(1):221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuadrado A., Manda G., Hassan A., et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 9.Muthusamy V.R., Kannan S., Sadhaasivam K., et al. Acute exercise stress activates Nf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52:366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q.M. Maltagliati AJ Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genom. 2018;50(2):77–97. doi: 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan E.L., McCord J.M., Reuland D.J., et al. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxid Med Cell Longev. 2012;2012:132931. doi: 10.1155/2012/132931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R.Y., Liu L.H., Liu H., et al. Nrf2 protects against diabetic dysfunction of endothelial progenitor cells via regulating cell senescence. Int J Mol Med. 2018;42:1327–1340. doi: 10.3892/ijmm.2018.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wafi A.M., Hong J., Rudebush T.L., et al. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J Appl Physiol. 2019;126:477–486. doi: 10.1152/japplphysiol.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Liu M., Zhang Y., Li X. Trimetazidine attenuates exhaustive exercise-induced myocardial injury in rats via regulation of the Nrf2/NF-κB signaling pathway. Front Pharmacol. 2019;10:175. doi: 10.3389/fphar.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie M., Warabi E., Komine S., Oh S., Shoda J. Cytoprotective role of Nrf2 in electrical pulse stimulated C2C12 myotube. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0144835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purdom-Dickinson S.E., Lin Y., Dedek M., et al. Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor. J Mol Cell Cardiol. 2007;42(1):159–176. doi: 10.1016/j.yjmcc.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Done A.J., Newell M.J., Traustadóttir T. Effect of exercise intensity on Nrf2 signalling in young men. Free Radic Res. 2017;51(6):646–655. doi: 10.1080/10715762.2017.1353689. [DOI] [PubMed] [Google Scholar]
- 18.Hancock M., Hafstad A.D., Nabeebaccus A.A. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. Elife. 2018;7 doi: 10.7554/eLife.41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kansanen E., Kivela A.M., Levonen A.L. Regulation of Nrf2-dependent gene expression by 15-deoxy-Δ12,14-prostaglandin J2. Free Radic Biol Med. 2009;47(9):1310–1317. doi: 10.1016/j.freeradbiomed.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Kansanen E., Jyrkkänen H.K., AL Levonen. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic Biol Med. 2012;52(6):973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 21.Tong K.I., Padmanabhan B., Kobayashi A., et al. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol Cell Biol. 2007;27(21):7511–7521. doi: 10.1128/MCB.00753-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dempsey J.A., McKenzie D.C., Haverkamp H.C., Eldridge M.W. Update in the understanding of respiratory limitations to exercise performance in fit, active adults. Chest. 2008;134(3):613–622. doi: 10.1378/chest.07-2730. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Tejado M., Naranjo-Suárez S., Jiménez C., et al. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 24.Cheng X.Y., Gu X.Y., Gao Q., Zong Q.F., Li X.H., Zhang Y. Effects of dexmedetomidine postconditioning on myocardial ischemia and the role of the PI3K/Akt-dependent signaling pathway in reperfusion injury. Mol Med Rep. 2016;14:797–803. doi: 10.3892/mmr.2016.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heunks L.M., Viña J., van Herwaarden C.L., et al. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277(6):R1697–R1704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- 26.Viña J., Gimeno A., Sastre J., et al. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. 2000;49(6):539–544. doi: 10.1080/15216540050167098. [DOI] [PubMed] [Google Scholar]
- 27.Takabe W., Alberts-Grill N., Jo H. Disturbed flow: p53 SUMOylation in the turnover of endothelial cells. J Cell Biol. 2011;193(5):805–807. doi: 10.1083/jcb.201104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom D.A., Jaiswal A.K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278(45):44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 29.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277(45):42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin M.H., Pollock J.S., Amann J.F., et al. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90(2):501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 31.Ramírez-Vélez R., Bustamante J., Czerniczyniec A., et al. Effect of exercise training on enos expression, NO production and oxygen metabolism in human placenta. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0080225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhakshinamoorthy S., Porter A.G. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J Biol Chem. 2004;279(19):20096–20107. doi: 10.1074/jbc.M312492200. [DOI] [PubMed] [Google Scholar]
- 33.Gleeson M. Temperature regulation during exercise. Int J Sports Med. 1998;19:S96–S99. doi: 10.1055/s-2007-971967. [DOI] [PubMed] [Google Scholar]
- 34.Glory A., Averill-Bates D.A. The antioxidant transcription factor Nrf2 contributes to the protective effect of mild thermotolerance (40°C) against heat shock-induced apoptosis. Free Radic Biol Med. 2016;99:485–497. doi: 10.1016/j.freeradbiomed.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Loboda A., Damulewicz M., Pyza E. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessen N., Pold R., Buhl E.S., et al. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol. 2003;94(4):1373–1379. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- 37.Hawley S.A., Gadalla A.E., Olsen G.S., Hardie D.G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51(8):2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 38.Sid B., Glorieux C., Valenzuela M., et al. AICAR induces Nrf2 activation by an AMPK-independent mechanism in hepatocarcinoma cells. Biochem Pharmacol. 2014;91(2):168–180. doi: 10.1016/j.bcp.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann K., Baldinger J., Mayerhofer B., et al. Activated AMPK boosts the Nrf2/HO-1 signaling axis--A role for the unfolded protein response. Free Radic Biol Med. 2015;88(Pt B):417–426. doi: 10.1016/j.freeradbiomed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajos-Draus A., Duda M., Beręsewicz A. Cardiac and renal upregulation of Nox2 and NF-κB and repression of Nox4 and Nrf2 in season- and diabetes-mediated models of vascular oxidative stress in Guinea-pig and rat. Physiol Rep. 2017;5(20) doi: 10.14814/phy2.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eluamai A., Brooks K. Effect of aerobic exercise on mitochondrial DNA and aging. J Exerc Sci Fit. 2013;11:1–5. doi: 10.1016/j.jesf.2013.03.003. [DOI] [Google Scholar]
- 42.Marcuello A., González-Alonso J., Calbet J.L., et al. Skeletal muscle mitochondrial DNA content in exercising humans. J Appl Physiol. 2005;99:1372–1377. doi: 10.1152/japplphysiol.00289.2005. [DOI] [PubMed] [Google Scholar]
- 43.Chang A.L., Ulrich A., Suliman H., Piantadosi C.A. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radic Biol Med. 2015;78:179–189. doi: 10.1016/j.freeradbiomed.2014.10.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toime L.J., Brand M.D. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med. 2010;49:606–611. doi: 10.1016/j.freeradbiomed.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller F.L., Liu Y., Abdul-Ghani M.A., et al. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J. 2008;409:491–499. doi: 10.1042/BJ20071162. [DOI] [PubMed] [Google Scholar]
- 46.Zoccarato F., Cavallini L., Bortolami S., Alexandre A. Succinate modulation of H2O2 release at NADH:ubiquinone oxidoreductase (Complex I) in brain mitochondria. Biochem J. 2007;406:125–129. doi: 10.1042/BJ20070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holmström K.M., Baird L., Zhang Y., et al. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2(8):761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scarpulla R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S., Wang W., Niu T., et al. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med Cell Longev. 2014;2014:748524. doi: 10.1155/2014/748524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mihl C., Dassen W.R.M., Kuipers H. Cardiac remodeling: concentric versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J. 2008;16:129–133. doi: 10.1007/BF03086131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shanmugam G., Challa A.K., Devarajan A., et al. Exercise mediated Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling. Front Cardiovasc Med. 2019;6:68. doi: 10.3389/fcvm.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ShanmugamG, NarasimhanM, Conley R.L., et al. Chronic endurance exercise impairs cardiac structure and function in middle-aged mice with impaired Nrf2 signaling. Front Physiol. 2017;8:268. doi: 10.3389/fphys.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Ichikawa T., Villacorta L., et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;11:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 54.Cuadrado A., Martín-Moldes Z., Ye J., Lastres-Becker I. Transcription factors NRF2 and NF-κB are coordinated effectors of the rho family, GTP-binding protein RAC1 during inflammation. J Biol Chem. 2014;289:15244–15258. doi: 10.1074/jbc.M113.540633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nemmar A., Al-Salam S., Yuvaraju P., Beegam S., Ali B.H. Exercise training mitigates water pipe smoke exposure-induced pulmonary impairment via inhibiting NF-κB and activating Nrf2 signalling pathways. Oxid Med Cell Longev. 2018:7459612. doi: 10.1155/2018/7459612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lighthouse J.K., Burke R.M., Velasquez L.S., et al. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4(1) doi: 10.1172/jci.insight.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okabe T.A., Kishimoto C., Murayama T., Yokode M., Kita T. Effects of exercise on the development of atherosclerosis in apolipoprotein E-deficient mice. Exp Clin Cardiol. 2006;11:276–279. [PMC free article] [PubMed] [Google Scholar]
- 58.Williams J.K., Kaplan J.R., Suparto I.H., Fox J.L., Manuck S.B. Effects of exercise on cardiovascular outcomes in monkeys with risk factors for coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:864–871. doi: 10.1161/01.ATV.0000067934.12783.6A. [DOI] [PubMed] [Google Scholar]
- 59.Nowak W.N., Deng J., Ruan X.Z., Xu Q. Reactive oxygen species generation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37(5):e41–e52. doi: 10.1161/ATVBAHA.117.309228. [DOI] [PubMed] [Google Scholar]
- 60.Torzewski M., Ochsenhirt V., Kleschyov A.L., et al. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 61.Yet S.F., Layne M.D., Liu X., et al. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. Faseb J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 62.Kinscherf R., Deigner H.P., UsingerC, et al. Induction of mitochondrial manganese superoxide dismutase in macrophages by oxidized LDL: its relevance in atherosclerosis of humans and heritable hyperlipidemic rabbits. Faseb J. 1997;11:1317–1328. doi: 10.1096/fasebj.11.14.9409551. [DOI] [PubMed] [Google Scholar]
- 63.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 64.Darley-Usmar V.M., Severn A., O'Leary V., Rogers M. Treatment of macrophages with oxidized low-density lipoprotein increases their intracellular glutathione content. Biochem J. 1991;278(Pt 2):429–434. doi: 10.1042/bj2780429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araujo J.A., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jansen T., Hortmann M., Oelze M., Opitz, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010;49:186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 67.Kawashima A., Oda Y., Yachie A., Koizumi S., Nakanishi I. Heme oxygenase–1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 68.Yachie A., Niida Y., Wada T., et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu H., Jia Z., Li Y.R. Nrf2 signaling in macrophages. React Oxyg Species (Apex) 2016;2(6):417–420. doi: 10.20455/ros.2016.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jyrkkänen H.K., Kansanen E., Inkala M., et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res. 2008;103:e1. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 71.Morris R.T., Laye M.J., Lees S.J., et al. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- 72.Bierl C., Voetsch B., Jin R.C., Handy D.E., Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi: 10.1074/jbc.M401907200. [DOI] [PubMed] [Google Scholar]
- 73.Buijsse B., Lee D.H., Steffen L., et al. Low serum glutathione peroxidase activity is associated with increased cardiovascular mortality in individuals with low HDLc's. PloS One. 2012;7 doi: 10.1371/journal.pone.0038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A., Rangasamy T., Thimmulappa R.K., et al. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietersma A., Tilly B.C., Gaestel M., et al. p38 mitogen activated protein kinase regulates endothelial VCAM-1 expression at the post-transcriptional level. Biochem Biophys Res Commun. 1997;230:44–48. doi: 10.1006/bbrc.1996.5886. [DOI] [PubMed] [Google Scholar]
- 76.Zakkar M., Van der Heiden K., Luong le A., et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 77.Cox A.G., Gurusinghe S., Abd Rahman R., et al. Sulforaphane improves endothelial function and reduces placental oxidative stress in vitro. Pregnancy Hypertens. 2019;16:1–10. doi: 10.1016/j.preghy.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 78.Harada N., Ito K., Hosoya T., et al. Nrf2 in bone marrow-derived cells positively contributes to the advanced stage of atherosclerotic plaque formation. Free Radic Biol Med. 2012;53(12):2256–2262. doi: 10.1016/j.freeradbiomed.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Chiu J.J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gimbrone M.A., Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22(1):9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J.X., Pan Y.Y., Wang X.X., Qiu Y.G., Mao W. Endothelial progenitor cells in age-related vascular remodeling. Cell Transplant. 2018;27:786–795. doi: 10.1177/0963689718779345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laughlin M.H., Overholser K.A., Bhatte M.J. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol. 1989;67:1140–1149. doi: 10.1152/jappl.1989.67.3.1140. [DOI] [PubMed] [Google Scholar]
- 83.Laughlin M.H., Tomanek R.J. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol. 1987;63:1481–1486. doi: 10.1152/jappl.1987.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 84.Nasu R., Kimura H., Akagi K., Murata T., Tanaka Y. Blood flow influences vascular growth during tumour angiogenesis. Br J Canc. 1999;79:780–786. doi: 10.1038/sj.bjc.6690125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Florczyk U., Jazwa A., Maleszewska M., et al. Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxidants Redox Signal. 2014;20:1693–1708. doi: 10.1089/ars.2013.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoetzer G.L., Van Guilder G.P., Irmiger H.M., Keith R.S., Stauffer B.L., DeSouza C.A. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102:847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 87.Sandri M., Adams V., Gielen S., et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 88.Kim T.H., Hur E., Kang S.J., et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α. Canc Res. 2011;71:2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 89.Kuang L., Feng J., He G., Jing T. Knockdown of Nrf2 inhibits the angiogenesis of raardiac micro-vascular endothelial cells under hypoxic conditions. Int J Biol Sci. 2013;9:656–665. doi: 10.7150/ijbs.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naci H., Salcher-Konrad M., Dias S., et al. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br J Sports Med. 2019;53(14):859–869. doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 91.Gómez-Guzmán M., Jiménez R., Sánchez M., et al. Epicatechin lowers blood pressure, restores endothelial function, and decreases oxidative stress and endothelin-1 and NADPH oxidase activity in DOCA-salt hypertension. Free Radic Biol Med. 2012;52:70–79. doi: 10.1016/j.freeradbiomed.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Smyrnias I., Zhang X., Zhang M., et al. Nicotinamide adenine dinucleotide phosphate oxidase-4-dependent upregulation of nuclear factor erythroid-derived 2-like 2 protects the heart during chronic pressure overload. Hypertension. 2015;65(3):547–553. doi: 10.1161/HYPERTENSIONAHA.114.04208. [DOI] [PubMed] [Google Scholar]
- 93.Li J., Ichikawa T., Villacorta L., et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29(11):1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 94.Lee D.H., Park J.S., Lee Y.S., Sung S.H., Lee Y.H., Bae S.H. The hypertension drug, verapamil, activates Nrf2 by promoting p62-dependent autophagic Keap1 degradation and prevents acetaminophen-induced cytotoxicity. BMB Rep. 2017;50(2):91–96. doi: 10.5483/bmbrep.2017.50.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopes R.A., Neves K.B., Tostes R.C., Montezano A.C., Touyz R.M. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension. 2015;66(6):1240–1250. doi: 10.1161/HYPERTENSIONAHA.115.06163. [DOI] [PubMed] [Google Scholar]
- 96.Bai J., Yu X.J., Liu K.L., et al. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol Appl Pharmacol. 2017;15(333):100–109. doi: 10.1016/j.taap.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Chan S.Y., Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44(1):14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Escalante B., Sacerdoti D., Davidian M.M., Laniado-Schwartzman M., McGiff J.C. Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension. 1991;17(6 Pt 1):776–779. doi: 10.1161/01.hyp.17.6.776. [DOI] [PubMed] [Google Scholar]
- 99.Abraham N.G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 100.Lever J.M., Boddu R., George J.F., Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxidants Redox Signal. 2016;25(3):165–183. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hur W., Gray N.S. Small molecule modulators of antioxidant response pathway. Curr Opin Chem Biol. 2011;15(1):162–173. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Satoh T., McKercher S.R., Lipton S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic Biol Med. 2013;65:645–657. doi: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88(Pt B):179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Correa F., Buelna-Chontal M., Hernández-Reséndiz S., et al. Curcumin maintains cardiac and mitochondrial function in chronic kidney disease. Free Radic Biol. 2013;61:119–129. doi: 10.1016/j.freeradbiomed.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 105.Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8(5):250. doi: 10.3390/nu8050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zordoky B.N., Robertson I.M., Dyck J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852(6):1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 107.Wojcik T., Szczesny E., Chlopicki S. Detrimental effects of chemotherapeutics and other drugs on the endothelium: a call for endothelial toxicity profiling. Pharmacol Rep. 2015;67(4):811–817. doi: 10.1016/j.pharep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 108.Fahey J.W., Stephenson K.K., Dinkova-Kostova A.T., Egner P.A., Kensler T.W., Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis. 2005;26(7):1247–1255. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- 109.Zhang Y., Talalay P., Cho C.G., Posner G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;15(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]