Abstract
Exercise mitigates obesity-associated pathologies; however, there is controversy regarding optimal exercise interventions. Autophagy, is known to decrease during obesity and is an important moderator for exercise adaptations.
Purpose
To investigate individual and combined effects of different exercise interventions and autophagy inhibition on exercise adaptations during obesity.
Methods
C57BL/6J mice initiated 45% high fat diet at 8 weeks of age. After 6 weeks of diet, animals were divided into moderate (MOD) or high intensity interval training interventions (HIIT), animals were further divided into autophagy inhibition or vehicle conditions (n = 10/group). Animals exercised and autophagy was inhibited 3X/week by NSC185058 injections, thereby blocking autophagosome formation. Interventions continued for 4 weeks.
Results
High fat diet impaired glucose handling ∼17%; exercise interventions normalized glucoregulation to pre-high fat diet levels, without differences between any interventions. High fat diet induced ∼25% decrease in aerobic capacity, which returned to baseline after exercise interventions, with no differences between any interventions. No effects of autophagy inhibition were noted.
Conclusions
HIIT and MOD training confer similar health-related adaptations.
Keywords: Insulin resistance, Exercise capacity, Glucose tolerance, Obesity
Introduction
Obesity, metabolic syndrome, and type 2 diabetes mellitus (T2DM) remain significant maladies in Western society.1 Insulin medication costs have more than doubled, causing significant economic burdens and a reduction in overall quality of life for millions of patients.2 Exercise is known to decrease T2DM-associated insulin resistance (IR) and mortality regardless of weight loss.3 Recently, controversy surrounding the relative effectiveness of high intensity interval training (HIIT) compared to moderate intensity continuous training (MOD) has emerged.4 HIIT has become exponentially more popular over the past decade, finishing in the top three fitness trends in each of the last five years.5 Clinically, HIIT has become increasingly widespread as an exercise intervention across a greater breadth of populations, including those with metabolic impairments.4,6, 7, 8, 9 More so, recent meta-analyses suggest HIIT to be more effective compared to traditional moderate intensity continuous training (MOD) to improve insulin sensitivity.10 However, mechanisms that may correspond to the purported augmented benefits have yet to be elucidated. More so, it is difficult to draw strong conclusions regarding any potential additional benefits associated with HIIT compared to MOD, as many “comparative” protocols in the past did not match total work completed or average intensity of exercise.7,11,12
One cellular mechanism recently implicated in the development of exercise adaptations is autophagy.13, 14, 15 Autophagy is a process that removes dysfunctional cellular components by sequestering organelles within an autophagosome and degrading them by lysosomal reaction.16 Previous research has demonstrated reductions in autophagy during IR.17,18 and transgenic models blocking autophagy initiation demonstrate diminished glucose handling capacity compared to healthy controls.19 Additionally, inhibition of Beclin-mediated autophagy impairs exercise training adaptations in otherwise healthy mice.20 Conversely, exercise interventions are known to increase autophagy.20, 21, 22 Yet, the precise stimuli needed to activate autophagy with exercise training and the subsequent effects on IR remain uninvestigated, specifically the exercise intensity or volume necessary to induce adaptations. For example, a significant proportion of research investigating autophagy activation in relation to exercise adaptations utilized wheel running20,22,23 or swimming24,25 models in rodents. While these studies provide excellent evidence for a general relationship between exercise and autophagy activation, these models inherently cannot control for intensity or volume of exercise across all animals. These limitations restrict our ability to understand how intensity or volume moderate autophagy activation and subsequently, exercise adaptations. Additionally, the role of autophagy to metabolic health has primarily been investigated utilizing genetic knock down models.19,20,25 These genetic models however, determine the influence of lifelong autophagy inhibition, and do not assess the effect of obesity mediated, acute autophagy inhibition, on exercise adaptions. Arguably, this approach therefore yields potentially confounding interpretations. We have recently found evidence of a correlational relationship between LC3II/I ratio (indicative of autophagosome formation) and glucose handling in exercised mice.22 potentially suggesting a mechanistic role for LC3 activation and glucose handling. However, to date, this precise mechanism has not been directly investigated. As autophagy activation is believed to moderate some exercise adaptations, and HIIT is postulated to provide superior benefits compared to MOD, it is plausible that HIIT induces greater increases in autophagy activation compared to MOD, allowing for augmented exercise adaptations such as improved protection from insulin resistance.
Therefore, the purpose of this study was two-fold: (1) to determine if HIIT exhibits additive effects compared to MOD on adaptions to glucose handling and exercise capacity when matched for volume and intensity during high fat diet-induced obesity. (2) Determine if LC3-mediated autophagy is necessary for these exercise induced adaptations. We hypothesized that HIIT would provide additional protections against metabolic pathologies in high fat fed mice compared to MOD, and that autophagy inhibition would hinder exercise-induced adaptations to glucose tolerance and exercise capacity in high fat fed mice. Herein, we demonstrate that MOD and HIIT training are equally efficacious to improve glucose handling and exercise capacity in obesity so long as volume and intensity of exercise are matched. These data suggest that autophagosome inhibition at the level of LC3 does not appear to directly moderate exercise induced adaptations.
Methods
Animal interventions
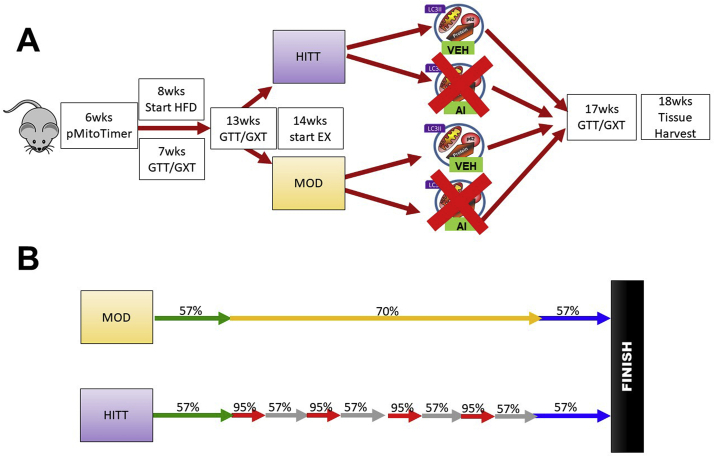
All methods and procedures were approved by the University of Arkansas Institutional Animal Care and Use Committee (AUP Approval #18053). The overall animal protocol is depicted in Fig. 1A. 40 male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed on a 12:12 light—dark cycle. At 6 weeks of age, C57BL/6J mice were transfected in the flexor digitorum brevis muscle (FDB) with pMitoTimer to assess mitochondrial quality as we have previously described (More thoroughly described below).26
Fig. 1.
Pictorial description of animal protocols for the present study. A: Animal experimental protocol from the present study. B: Animal exercise protocols for each exercise session from the present study. MOD = Moderate intensity continuous exercise, HIIT=High intensity interval training exercise, VEH = vehicle control (peanut oil), AI = autophagy inhibitor NSC185058 (dissolved in peanut oil).
After 1 week of recovery (7 weeks age) animals underwent baseline glucose tolerance tests (GTTs) and graded exercise tests (GXTs), which served as healthy control conditions to compare physiological phenotypes throughout the duration of the study. The following week (8 weeks age) all animals began high fat diet interventions. Animals were switched from 17% fat, 54% CHO, and 29% protein diet (3.0 kcal/g, Teklad, Indianapolis, IN, Product #8640) to 45% fat, 35% CHO, and 20% protein diet (4.7 kcal/g Research Diets, New Brunswick, NJ, Product #D12451) to induce obesity and glucose intolerance. Animals continued high fat diets for the remainder of the study. After 5 weeks of high fat diet (13 weeks age) animals underwent a second series of glucose tolerance and graded exercise tests to assess the effects of high fat diet. Following 6 weeks of high fat diet (14 weeks old) animals began treadmill exercise interventions using either moderate intensity exercise (MOD) or high intensity interval training exercise (HIIT) via treadmill (interventions described below). Additionally, animals were further divided into either peanut oil injections as vehicle control (VEH) or autophagy inhibition drug (NSC185058) injections (AI), described below. This experimental design created 4 conditions: MOD-VEH, MOD-AI, HIIT-VEH, HIIT-AI (n = 10/group) with all animals receiving high fat diet. Animals continued interventions for an additional 4 weeks. After 3 weeks of exercise interventions (17 weeks age) animals underwent a final series of glucose tolerance and graded exercise tests to investigate the effects of the exercise interventions and the autophagy inhibition. At 18 weeks age (6 weeks of high fat diet + 4 weeks of exercise and drug interventions), 48 h after the final exercise bout, animals were anaesthetized using 3% isoflurane. Hindlimb muscles, livers, and epididymal fat were excised, weighed using a scientific scale, and snap frozen in liquid nitrogen for analysis of autophagy activation and insulin cascade signaling. Animals were then euthanized while anaesthetized by thoracotomy. The selected tissues were collected to investigate potential contributions of each tissue to overall whole body glucose handling. 10 min prior to euthanasia, animals were intraperitoneally injected with insulin at a concentration of 5 units/kg of bodyweight to specifically investigate tissue response to insulin stimulation in experimental animals. Animals were in the fed state for tissue harvest to prevent potential insulin shock from the insulin stimulation. For the purpose of this study our “sedentary control” condition was animals’ pre-high fat diet glucose tolerance and exercise capacity as repeated measures variables. Prior works have established high fat feeding to result in aberrations to many of our outcome variables relative to chow-fed or sedentary control animals.21,27, 28, 29, 30, 31 Therefore, we opted for a longitudinal experimental design to determine effects of high fat feeding and experimental conditions of exercise modality and autophagy inhibition on physiological parameters of glucose and exercise tolerance. This allowed us to more precisely investigate the research questions, including: 1. Are there additional benefits with HIIT training compared to MOD during high fat feeding and 2. Does autophagy inhibition affect exercise adaptations during high fat feeding.
pMitoTimer transfection
To transfect pMitoTimer, animals were anaesthetized with 3% isoflourane. The plantar side of the right foot was then injected with 10 μL of 0.36 mg/ml hyaluronidase dissolved in sterile saline. 60 min after hyaluronidase injection, the right foot was then injected with 20 μg of pMitoTimer dissolved in 10 μL sterile saline. After 15 min of recovery, animals were anaesthetized a final time and the flexor digitorum brevis (FDB) underwent electroporation, with 10 pulses at 75 V/cm, 1 Hz and 20 ms/pulse. Post-transfection, pMitoTimer becomes incorporated into the (FDB) and changes from green to red fluorescence with mitochondrial damage.27 Conversely, pMitoTimer is also responsive to adaptions to mitochondrial and metabolic health with favorable stimuli such as exercise.27 The FDB muscle is a small thin muscle that can be fixed and mounted on a microscope slide immediately following tissue harvest. Additionally, prior works have confirmed the FDB's sensitivity to exercise training27 As such, the FDB muscle is ideal for this imaging method. It was recently demonstrated that autophagy protects and promotes mitochondrial function in muscle following exercise.32 Due to the closely tied mechanisms of autophagy, mitochondrial health and overall cellular health.27 this method was used to more thoroughly quantify cellular metabolic health after interventions.
Glucose tolerance tests
Glucose Tolerance Tests (GTTs) were performed as we previously described.22,31 Briefly, animals were fasted for 6 h, after which fasting baseline glucose was measured using a Relion® Ultima diabetic glucometer (Abbott Diabetes Care, Alameda, CA). Animals were then intraperitoneally injected with a bolus of glucose dissolved in saline (1.0 g glucose/kg of bodyweight). Glucose was measured at 0 min (baseline), 30 min, 60 min, and 120 min. Data were analyzed as area under the curve (AUC). All glucose tolerance tests were completed at least 48 h s after most recent session of exercise to wash out effects of the most recent exercise session.
Graded exercise test (GXTs)
Mice were first acclimated to the treadmill by placing mice on a customized treadmill with apparatus for mouse lanes (detailed in our prior works31) and allowing mice to walk at the slowest speed (13.4 m/min) for 5 min. This acclimation procedure was repeated on consecutive days at least 3 times before GXTs. For GXTs, mice began at a speed of 13.4 m/min and 5% incline, every 3 min the speed was increased 2.7 m/min with no change in incline. Mice ran until they reached volitional exhaustion, which was defined as refusal to continue exercise for at least 10 s. Exercise capacity was determined based on the speed and grade at exhaustion and expressed as workload/min∗g body weight (workload = speed (m/min)∗bodyweight(g)∗incline of the treadmill), similar to other measures of aerobic capacity measurements in humans (e.g. VO2max, mlO2/min/kg).
Exercise interventions
Exercise Interventions began after 6 weeks of high fat diet (14 week old animals). All animals exercised 3 days/week with 48–72 h between each exercise bout (Exercise performed Monday, Wednesday, Friday). This protocol was designed as a murine analog to ACSM Guidelines.33 specifically completing ∼30 min of aerobic exercise 3–5 days/week. This protocol allowed for the investigation of these modalities when following commonly utilized exercise protocols for humans. For MOD groups, animals exercised at 70.0% of their overall exercise capacity (corresponding to 16.1 m/min and 5% incline) for 20 min. For HIIT groups, animals competed 20 min of repeating intervals of 30 s at 95% of overall exercise capacity (21.5 m/min) and 60 s of 57% of overall exercise capacity (13.4 m/min). Both groups completed a 5 min warm up and cool down at 57% of exercise capacity (13.4 m/min) and 5% grade. Exercise capacities were based on 13 week GXTs. All groups were matched on exercise capacity and glucose handling before the initiation of exercise interventions. This protocol matched both MOD and HIIT animals on average exercise intensity throughout the bout (69.0% of work capacity during the workout and 57% of work capacity during the cool down) and on total work and distance throughout the bout (455.4 ± 1.6 m). A pictorial description of the exercise protocol can be found in Fig. 1B.
Autophagy inhibition
To inhibit autophagy at the level of LC3, autophagy inhibition was implemented concurrently with exercise interventions by intraperitoneal injections of N-(pyridin-2-yl)pyridine-2-carbothioamide (also referred to as NSC185058, purchased from Enamine Ltd., Kiev, Ukraine cat#EN300-214523) at a concentration of 100 mg/kg body weight as previously described.34 The drug was dissolved in sterile peanut oil at a concentration of 0.025 mg/μL and mixed overnight on a rocking plate. N-(pyridin-2-yl)pyridine-2-carbothioamide blocks the conversion of LC3I → LC3II, thereby blocking the formation of autophagosomes.34 This method was chosen over genetic models or other drugs, such as chloroquine, because N-(pyridin-2-yl)pyridine-2-carbothioamide allows for direct investigation of the research question; specifically, the relationship between LC3 activation and glucose regulation. Control animals (VEH) received sterile peanut oil intraperitoneal injections as vehicle control. All animals received injections 3 days/week, which prior works have demonstrated sufficient to inhibit autophagy in vivo.34 Injections were performed within 1 h of completing exercise to ensure the drug or the injection did not impede the animals’ ability to complete the exercise session.
Histology
pMitoTimer analysis was completed as we have previously described.26 At tissue harvest FDB muscles were excised, fixed in 4% paraformaldehyde solution for 20 min, washed in phosphate buffered saline (PBS) for 5 min and then mounted on a gelatin coated microscope slides with 10% glycerol/PBS. During fixing and mounting samples were kept under aluminum foil to minimize light exposure. Slides were imaged using a Nikon Ti–S inverted epiflourescent microscope (Melville, NY) and associated computer software. Red and Green fluorescence was measured using TRITC filters (excitation/emission 543/572 nm) and FITC filters (excitation/emission 488/518 nm). Data were collected using highly controlled acquisition parameters such as exposure time and intensity based on preliminary experiments. All images were collected on the day of tissue harvest. Once images were collected, Red and Green fluorescence were quantified using MATLAB programing (generous gift of Dr. Zhen Yan, University of Virginia) as we previously described.26 Red:Green ratio as well as number of red puncta (degenerated mitochondria tagged for autophagosomal degradation) were quantified.
Immunoblotting
Western blotting for autophagy and insulin signaling cascade were completed as previously described.31 Primary antibodies included: LC3 A/B (Cell Signaling, Danvers, MA 4108), Akt (Cell Signaling 9272), and p-Akt (Cell Signaling 9271, Ser473). Bands were imaged using infrared (IR) secondaries (Li-Cor, Lincoln, NE) as appropriate for the primary antibodies and Li-Cor Odyssey Fc Imaging System (Li-Cor, Lincoln, NE). Bands were quantified using Image Studio Lite software (Li-Cor) and normalized to Ponceau Stain which was used to ensure equal loading across the membrane.
Statistics
All physiological data (longitudinal data from GTTs and GXTs) were analyzed by repeated measures ANOVA with factors of time (7 weeks v. 13 weeks v. 17 weeks), drug intervention (CON v. AI) and exercise protocol (MOD v. HIIT). Cross sectional data (tissue weights, histology and cellular signaling data) were analyzed by 2X2 ANOVA with factors of drug intervention and exercise protocol. When significant F ratios were found, a Tukey post-hoc was used to determine differences between means. Significance was denoted a p < 0.05, all data were analyzed using the Statistical Analysis System (SAS, version 9.3, Cary, NC) and are presented as mean ± SEM.
Results
High fat diet induced weight gain across mice, however exercise interventions blunted weight gain
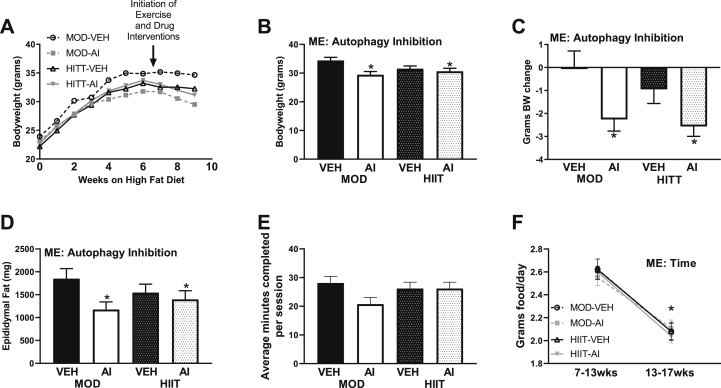
All groups experienced similar weight gain throughout the 6 weeks of 45% high fat diet (Fig. 2A). With the introduction of exercise interventions and autophagy inhibition interventions, weight gain appeared to plateau (Fig. 1A). Interestingly, AI animals appeared to experience weight loss (Fig. 2A–C). This pattern was corroborated by AI animals overall weighing ∼3 g less at the final timepoint compared to VEH animals (Main effect AI F(1,33) = 7.44, p = 0.010, Fig. 2B). More so, differences in weight were due to weight loss in AI animals, not additional weight gain in VEH animals, as AI animals lost ∼2.5 g of body weight between the start of autophagy inhibition interventions and the completion of the study (Main effect AI F(1,34) = 11.47, p = 0.002, Fig. 2C). Interestingly, this weight loss was not attributed to muscle loss, as most of hindlimb muscles did not demonstrate any differences between groups (Table 1). However, AI animals did have ∼20% less epididymal fat compared to VEH animals (∼1696 mg v. 1285 mg, Main effect AI F(1,33) = 4.43, p = 0.043, Fig. 2D), suggesting a majority of weight loss differences were attributed to fat mass loss. These differences did not appear to be moderated by differences in duration of exercise completed or food intake, as no differences in volume of exercise were noted (F(3,34) = 1.84. p = 0.158, Fig. 2E). Whereas all animals had a mean decrease in food consumption following onset of interventions (week 13 to week 17, Main effect Time F(7,71) = 166.14, p < 0.0001, Fig. 2F).
Fig. 2.
Phenotypic data from the present study. A: Body weight growth curves with the initiation of 45% high fat diet. B: Body weight at tissue harvest C: Body weight loss from initiation of exercise and drug interventions to tissue harvest. D: Epididymal fat mass at tissue harvest. E: Average minutes of exercise completed each session, with a maximum time of 30 min. F: Average food consumption from pre-exercise and drug interventions to post-exercise and drug interventions. ME denotes a Main Effect. ∗ denotes p < 0.05.
Table 1.
Tabulated tissue weights from each group from the current study.
| Tissue | MOD-VEH | MOD-AI | HIIT-VEH | HIIT-AI |
|---|---|---|---|---|
| Gastrocnemius (mg) | 141.3 ± 1.7 | 144.3 ± 3.5 | 139.5 ± 2.2 | 140.4 ± 1.1 |
| Plantaris (mg) | 22.3 ± 0.7 | 20.5 ± 1.0 | 20.6 ± 0.8 | 21.0 ± 0.5 |
| Soleus (mg) | 10.9 ± 0.3 | 10.8 ± 0.5 | 10.9 ± 0.3 | 11.0 ± 0.4 |
| EDL (mg) | 12.1 ± 0.5 | 12.6 ± 0.9 | 11.7 ± 0.5 | 11.9 ± 0.5 |
| TA∗ (mg) | 57.4 ± 1.1 | 58.6 ± 1.48 | 53.7 ± 0.9 | 54.6 ± 1.26 |
| Liver (mg) | 1141.6 ± 63.6 | 1169.4 ± 35.3 | 1065.5 ± 22.5 | 1171.7 ± 47.9 |
| Heart∗ (mg) | 140.7 ± 2.6 | 137.4 ± 3.5 | 130.9 ± 3.14 | 127.8 ± 3.8 |
MOD = Moderate intensity continuous exercise, HIIT=High Intensity Interval Training, VEH=Vehicle control, AI = Autophagy inhibition, through the drug NSC185058. ∗ = Main effect of AI.
Exercise interventions restored high fat diet-induced impaired glucose handling and aerobic capacity without differences between interventions
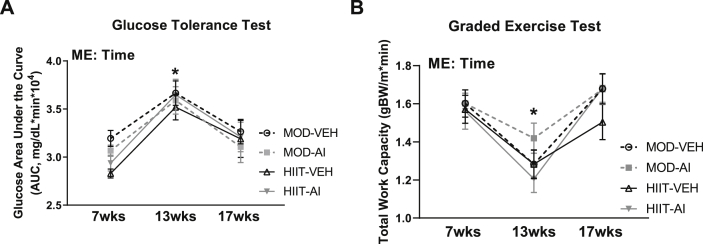
Animals were matched for body weight, glucose tolerance and aerobic capacity at baseline (7 weeks old) and after high fat diet interventions and then assigned to experimental conditions (13 weeks old) (Fig. 3A, B). High fat diet consumption resulted in ∼17% increase in Glucose Area Under the Curve (AUC), indicative of impaired glucose tolerance (Main effect Time F(2,70) = 24.21, p < 0.001). After exercise and autophagy inhibition interventions, glucose AUC values were restored to pre-high fat levels, with no significant differences noted between any groups or between 7 week and 17 week time points (p = 0.080, Fig. 3A). Similarly, high fat diet consumption resulted in an ∼25% decrease in exercise capacity (Main effect Time F(2, 58) = 31.24, p < 0.0001, Fig. 3B). Exercise interventions restored exercise capacity, with no significant effect of intervention or autophagy inhibition (p = 0.177 and p = 0.49 respectively, Fig. 3B). Additionally, there were no statistical differences between the 7 week and 17 week time points for exercise capacity (p = 0.512).
Fig. 3.
Longitudinal data for glucose handling and aerobic capacity. A: Glucose handling data measured by glucose tolerance tests. B: Aerobic capacity measured by graded exercise tests. ME denotes a Main Effect. ∗ denotes p < 0.05.
Neither exercise intervention nor autophagy inhibition demonstrated differences in muscle metabolic health as measured by pMitoTimer
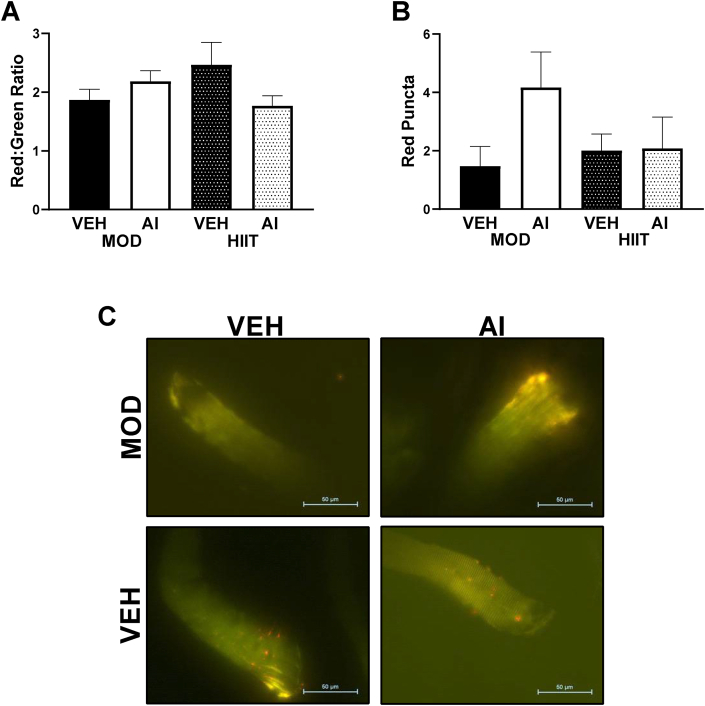
pMitoTimer was analyzed in the FDB muscle as a surrogate measure of muscular metabolic health. Mitochondrial quality as assessed by pMitoTimer has previously been demonstrated to improve with exercise training in high fat fed animals compared to sedentary controls in the FDB muscle.27 Here, we utilized pMitoTimer to determine if either mode of exercise or AI demonstrated distinct effects on mitochondrial and intramuscular health in exercise-trained high fat fed animals. Neither exercise interventions nor autophagy inhibition exhibited different Red:Green ratios (F(3, 27) = 1.68, p = 0.195, Fig. 4A, C), compared to any other groups. Additionally, number of red puncta, a measurement of completely degenerated mitochondria, was also not statistically different between groups (F(3,29) = 1.64, p = 0.202, Fig. 4B, C).
Fig. 4.
Intramuscular metabolism measured by pMitoTimer. A: Red/Green pMitoTimer ratio. B: Red Puncta a measure of completely degenerated mitochondria. C: Representative images. ME denotes a Main Effect. ∗ denotes p < 0.05.
AI interventions appeared to block autophagosome formation although these effects appear tissue and exercise dependent
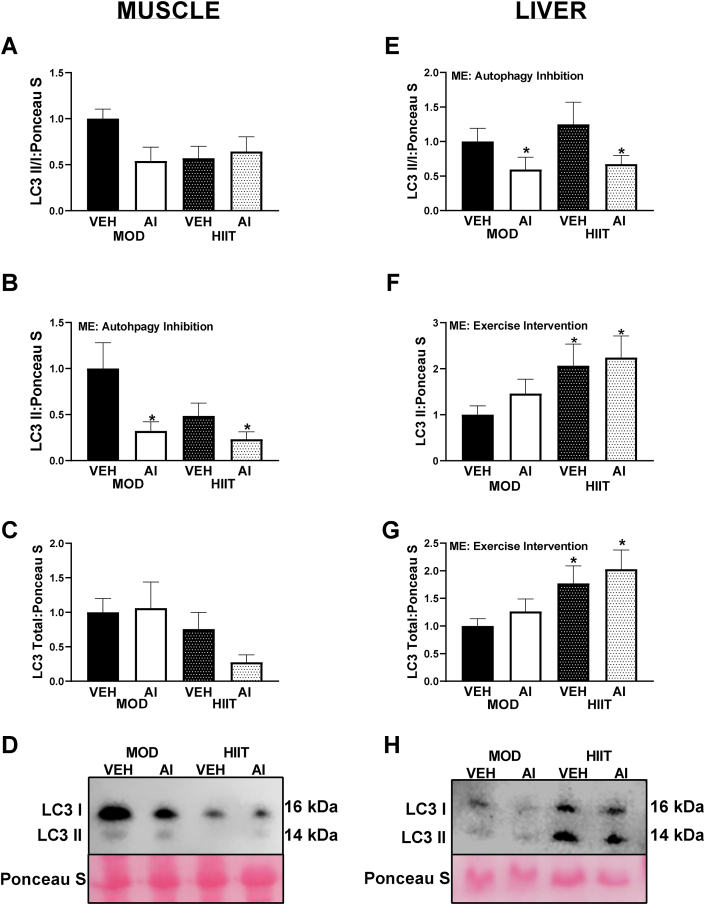
In order to thoroughly understand what tissues may be contributing to the alterations in whole body glucose handling, both muscle and liver were analyzed as both tissues can greatly contribute to glucose handling and insulin resistance. With regards to LC3 in the muscle, LC3II/I ratio differences did not reach statistical difference (F(3,30) = 2.30, p = 0.097, Fig. 5A, E). However, this appeared to be largely driven by a mean lower LC3 content in the HIIT-VEH animals, with the interaction effect approaching statistical significance (Interaction F(1,30) = 3.64, p = 0.066, Fig. 5A, E). Moreover, LC3II content did reach statistical significance (F(3,29) = 3.65, p = 0.024, Fig. 5B). Specifically, an effect of AI was noted (Main effect F(1,29) = 7.01, p = 0.013), with AI animals having ∼60% lower LC3II compared to VEH animals (Fig. 5B, E). No significant differences in total LC3 content were noted in muscle (F(3,29) = 1.60, p = 0.21, Fig. 5C and D). However, the effect of exercise type approached statistical significance (Main effect Exercise F(1,29) = 3.25, p = 0.068, Fig. 5C, D), with MOD animals having ∼50% greater mean total LC3 content compared to HIIT animals (Fig. 5C, D).
Fig. 5.
LC3 Western blot data from the present study in muscle and liver tissue. A: LC3II/I ratio in muscle. B: LC3II content in muscle. C: Total LC3 content in muscle. D: Representative images of LC3 Western blot data from muscle tissue. E: LC3II/I ratio in liver. F: LC3II content in liver. G: Total LC3 content in liver. H: Representative images of Western blot data from liver tissue. ME denotes a Main Effect. ∗ denotes p < 0.05. All immunoblot data are arbitrary units and represented as percent difference relative to MOD-VEH.
In the liver, LC3II/I ratio was approximately 50% lower in AI animals compared to VEH (Main effect AI F(1,30) = 4.36, p = 0.045), with AI animals having ∼50% less LC3II/I compared to VEH animals (Fig. 4E, H). However, no differences were noted in LC3II content between AI and VEH (F(3,29) = 2.10, p = 0.122), (Fig. 5F). Interestingly, LC3II content was ∼30% greater in HIIT compared to MOD animals (Main effect Exercise F(1,29) = 5.52, p = 0.026, (Fig. 5F, H). Total LC3 content followed a similar pattern to LC3II content (Global statistic F(3,30) = 3.12, p = 0.041), with HIIT animals having ∼45% more LC3 content compared to MOD animals (Main effect F(1,30) = 8.38, p = 0.007, (Fig. 5G, H).
Drug interventions differentially affected insulin signaling between muscle and liver
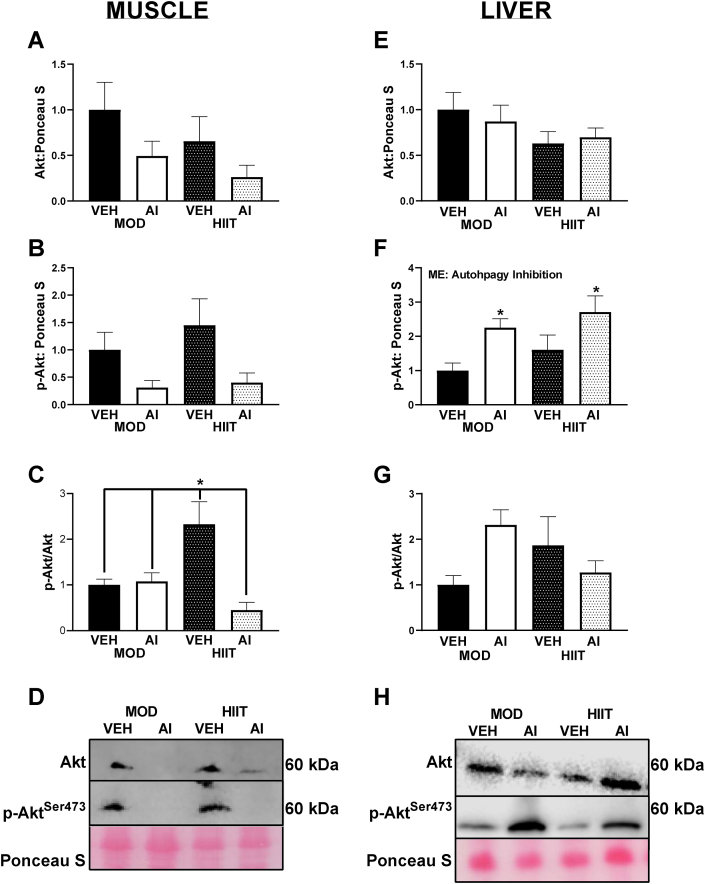
Using Akt phosphorylation as a marker for insulin signaling, we noted no significant differences in total Akt protein content. However the main effect of AI approached significance (F(1, 36) = 3.77, p = 0.062) with VEH animals having approximately 100% greater Akt content compared to AI animals (Fig. 6A, D). Total Akt phosphorylation (P-Aktser473) in the muscle was ∼1.5-fold lower in AI compared to VEH animals (F(1,29) = 6.37, p = 0.017, Fig. 6B, D). With regards to the p-Akt ser473/Akt ratio in the muscle an interaction between AI and exercise intervention was noted (F(1, 29) = 10.23, p = 0.003), with HIIT-VEH animals exhibiting 100% greater p-Akt ser473/Akt ratios compared to MOD-VEH and MOD-AI (p = 0.031 and 0.020 respectively) and 5-fold greater p-Aktser473/Akt ratio compared to HIIT-AI animals (p = 0.0007, Fig. 6C and D).
Fig. 6.
Akt Western blot data from the present study in muscle and liver tissue. A: Akt content in muscle. B: p-Aktser473 content in muscle. C: p-Akt ser473/Akt ratio in muscle. D: Representative images of Western blot data from muscle tissue. E: Akt content in liver. F: p-Aktser473content in liver. G: p-Akt ser473/Akt content in liver. H: Representative images of Western blot data from liver tissue. ME denotes a Main Effect. ∗ denotes p < 0.05. All immunoblot data are arbitrary units and represented as percent difference relative to MOD-VEH.
In the liver, no significant effects were observed for total Akt (F(3,32) = 1.11, p = 0.361, Fig. 6E, H). However, for total p-Aktser473 a main effect of AI was noted (F(1,30) = 10.57, p = 0.003) with AI animals having ∼2-fold greater total p-Aktser473 compared to VEH animals (Fig. 6F, H). However, despite a significant interaction between exercise and AI (F(1,31) = 5.06, p = 0.032), pairwise comparisons for the ratio of p-Aktser473/Akt did not reach statistical significance (p values range 0.15–0.96, Fig. 6G and H).
Discussion
To our knowledge, this is one of the first studies to directly investigate the mechanistic role of exercise-induced autophagy activation in mediating adaptations to variable exercise training regiments. We find when exercise volumes and average intensities are matched following hypercaloric-induced obesity, there does not appear to be any differences in training mediated improvements to exercise capacity or glucose handling between exercise MOD and HIIT based exercise regimens, suggesting there are not necessarily additive benefits of HIIT compared to MOD with regard to these outcomes. More so, we find pharmacological autophagy inhibition at the level of LC3 does not influence these physiologic exercise adaptations, suggesting the mechanistic role of autophagy in mediating these events likely occurs upstream of LC3 lipidation.
Similar to our prior investigations, we find a 45% high fat diet sufficient to stimulate lipid-induced pathologies, such as impaired glucose handling and reduced exercise capacity.22,31,35 Prior works have extended exercise interventions for 8–12 weeks19,23,25 or had much larger exercise volumes.20 Yet, despite these differences in running volume compared to prior studies, animals restored both exercise capacity and glucose handling to pre-high fat levels following 4 weeks of exercise training, strongly supporting our and others prior findings in murine and human models that small amounts of physical activity can be sufficient to improve glucose handling and exercise tolerance capabilities during obesity.22,31,36,37 Importantly, this reduction in insulin resistance occurs despite no weight loss in VEH animals, strongly demonstrating the sufficiency of exercise as a therapeutic modality for insulin resistance. However, our findings contradict prior works suggesting HIIT is superior to MOD exercise for maximizing favorable adaptations to insulin sensitivity.10,36,38 Importantly, many prior works examining these two modalities have not matched exercise volume or average intensity.7,11,12,36 Arguably this could have resulted in HIIT groups working at either a higher intensity or completing more total work; therefore yielding confounding insights into the relative benefits of each. With the current study, no differences for physiologic adaptations were noted when exercise workloads and average intensities are precisely matched. We should acknowledge this study was conducted in mice and not humans; therefore, the possibility of underlying physiological differences between murine and human models that could account for these discrepancies cannot be ruled out. Therefore, it is important for similar experiments to the current study be repeated in human models to ensure translational efficacy of the current results. However, while we did not see differences in systemic glucose handling (AUCs) it is interesting to note that in the muscle we did find that VEH-HIIT animals had substantially greater p-Akt/Akt ratios (indicative of enhanced insulin-stimulated signaling), whereas in the liver we observed no such effect. This may suggest that HIIT may have greater capacity to increase muscular insulin sensitivity, however this does not appear to correspond to global differences in glucoregulation.
We also found no differences between exercise interventions and autophagy inhibition on intramuscular metabolic health measured by pMitoTimer. Similar to other outcome variables, this largely seems to suggest no differences between HIIT and MOD when volumes are matched. It is important to note that prior works using pMitoTimer to assess mitochondrial quality have demonstrated improved Red:Green ratios, demonstrating improved muscle metabolic and mitochondrial health, with exercise training in high fat fed mice.27, 28, 29, 30 Based on the prior establishment by Laker et al. that exercise restores intramuscular health in high fat fed animals.27 we specifically focused on the comparison of MOD v HIIT exercise trained animals. Comparing MOD to HIIT allowed us to investigate the primary research question regarding MOD and HIIT exercise; however, we acknowledge this design did not allow for and did not include sedentary control animals. It is likely that all animals experienced a mean decrease in Red:Green ratios after exercise interventions compared to pre-exercise high fat diet interventions. However due to methodological considerations of pMitoTimer, longitudinal measurement of Red:Green ratio multiple times throughout the study is not feasible.
Contrary to our hypothesis, we found no influence of autophagy inhibition on exercise adaptations. While our insulin signaling data may indicate alterations to insulin sensitivity to specific tissues by AI, total body glucose tolerance and aerobic capacity were unaffected by AI. This finding of no effect of autophagy inhibition at the whole body level contradicts prior reports finding autophagy inhibition by other methods, such as genetic knockdowns or chloroquine, decreases exercise capacity and glucose tolerance in mice.19,20,25,32,39 Our data may suggest that the point where autophagy is inhibited may be important for such outcomes, specifically NSC185058 prevents the lipidation of LC3I to LC3II, thus inhibiting autophagosomal formation.16 Inhibition at this step of autophagy was specifically selected in the current study due to our prior findings of a correlational relationship between LC3 lipidation and glucose tolerance. In contrast, chloroquine, acts downstream of autophagosomal formation by inhibiting the binding of the autophagosome to the lysosome.16 Prior works have found chloroquine itself to induce reductions in exercise capacity.25 suggesting point of autophagy blockade may largely influence overall physiological implications. Conversely, recent works have found no alterations to autophagy in the soleus muscle of high-fat fed rat that completed 10 weeks of treadmill training, potentially suggesting autophagy activation is not necessary for exercise adaptions40; however this may vary between muscle fiber types. Other approaches have utilized heterozygous knockout of Beclin, which blocked training adaptations with exercise interventions in normal chow fed animals.20 Since these different models appear to produce different effects, manipulation of specific points in the autophagy process may exert varying impacts on overall health. Additionally, to our knowledge this is one of the first studies to initiate autophagy interventions concurrently with exercise interventions. As exercise is known to increase autophagy activation (including LC3 content), it is possible that the exercise interventions were sufficient to “protect” autophagy and subsequent LC3 levels despite the drug treatment. For the purpose of this study our “sedentary control” condition was animals' pre-high fat diet glucose tolerance and exercise capacity as repeated measures variables. We must acknowledge this lack of a “sedentary control” limits our ability to make strong inferences on the influence of our interventions on the outcome variables. However, the overall purpose of this study was to compare the effect of different exercise interventions, not necessarily exercise vs. no-exercise. As such, we opted for a longitudinal experimental design to have animals’ baseline values serve as a “sedentary control”. We believe this design maximized our statistical power to detect differences between “sedentary controls” (7wk time point), high fat diet (13wk time point) and interventions (17wk time point), while minimizing the animal number required. However, this longitudinal design cannot account for outcomes that inherently are cross-sectional data (e.g. tissue weights, pMitoTimer, and Western Blot data), and must be noted as significant limitation of our study. Future works investigating this drug or exercise interventions would benefit from stronger control groups to more fully elucidate possible exercise/drug interactions during high fat feeding.
Another interesting finding from our study was the reduction in body and fat mass in AI animals despite no differences in overall exercise time or food consumption compared to VEH animals. To our knowledge, this is the first report using this drug to report body weight alterations, which may support this drug as useful for weight loss as it did not appear to generate negative side effects when used in combination with exercise. However, we must note that the additional fat loss did not confer additional benefits such as improved glucose handling or exercise capacity, though it is quite possible that these animals reached a “ceiling effect” for these variables based on the specific exercise intervention. Conversely, the loss of body fat in the AI animals may have confounded potential detriments to glucose handling and exercise capacity. We did not anticipate loss of body fat within AI animals, and to our knowledge this result has not been reported in the literature.34,41 As such, more research may be warranted to understand this drug and subsequent LC3 activity in relation to exercise adaptations during high fat feeding.
Perhaps more intriguing, this compound is currently being tested as a therapeutic agent for cancer.34,41,42 Many chemotherapeutic agents are known to cause muscle loss, which can complicate treatment effectiveness and overall mortality.43,44 It is noticeable in our study, when mice were given NSC185058 concurrent to an exercise training regimen at doses similar to pharmacological studies34,41 we do not see muscle loss. Whether this retention of muscle mass is an inherent characteristic of the drug itself or an interaction of drug and exercise is currently unknown. However, that this compound does not dramatically induce muscle atrophy appears to be a potentially positive attribute to this pharmacological intervention. While not the initial purpose of this study, our finding of fat mass loss without concurrent muscle mass loss likely warrants further investigation.
Taken together, our data demonstrate when exercise intensities and total work are matched, high intensity interval training and moderate intensity training confer similar adaptations on exercise capacity and glucose tolerance in diet-induced obese mice. These effects of exercise training were imparted despite only moderate total volumes of exercise. Additionally, late stage autophagy inhibition does not influence physiologic exercise training adaptations toward exercise capacity and glucose tolerance, but does appear to influence body weight, which warrants further investigation. Overall, our study does not suggest any additional benefits for HIIT compared to MOD for general exercise adaptations during lipid overload when both volume and average exercise intensity are matched, furthering the notion that regardless of the specific intervention, exercise can be medicine.
Conflict of interest
The authors declare no financial or other conflicts of interest that could influence the interpretations of this work.
Submission statement
This manuscript has not been published and is not under consideration for publication elsewhere.
Authors' contributions
M.E.R. synthesized experimental design, wrote animal use protocol, implemented animal experiments, collected and analyzed data, wrote and edited manuscript. L.T.J. Implemented animal experiments, collected and analyzed data, edited and revised manuscript. S.L., K.R.D. and W.S.H. assisted with animal experiments, collected data, edited and revised manuscript. T. A. W. analyzed, interpreted data wrote and edited manuscript. N.P.G. Synthesized experimental design, wrote animal use protocol, analyzed and interpreted data, wrote and edited manuscript.
Acknowledgments
This study was funded by the Central States Chapter of the American College of Sports Medicine and The University of Arkansas Human Health, Performance and Recreation Departmental Graduate Student Grant. The authors would like to thank the hard working students, faculty and staff at the Exercise Science Research Center for their continual support of our research.
References
- 1.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. J Am Med Assoc. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hua X., Carvalho N., Tew M., Huang E.S., Herman W.H., Clarke P. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. J Am Med Assoc. 2016;315(13):1400–1402. doi: 10.1001/jama.2016.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slentz C.A., Bateman L.A., Willis L.H., et al. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial. Diabetologia. 2016;59(10):2088–2098. doi: 10.1007/s00125-016-4051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wewege M., van den Berg R., Ward R.E., Keech A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: a systematic review and meta-analysis. Obes Rev. 2017;18(6):635–646. doi: 10.1111/obr.12532. [DOI] [PubMed] [Google Scholar]
- 5.Thompson W.R. Worldwide survey OF fitness trends for 2019 : ACSM's health & fitness journal. ACSM's Health & Fit J. 2019;22(6):10–17. [Google Scholar]
- 6.Hwang C.L., Yoo J.K., Kim H.K., et al. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp Gerontol. 2016;82:112–119. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes-Neto M., Duraes A.R., Reis H., Neves V.R., Martinez B.P., Carvalho V.O. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(16):1696–1707. doi: 10.1177/2047487317728370. [DOI] [PubMed] [Google Scholar]
- 8.Hussain S.R., Macaluso A., Pearson S.J. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol Rev. 2016;24(6):273–281. doi: 10.1097/CRD.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 9.Tjonna A.E., Lee S.J., Rognmo O., et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelleyman C., Yates T., O'Donovan G., et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015;16(11):942–961. doi: 10.1111/obr.12317. [DOI] [PubMed] [Google Scholar]
- 11.Wang N., Liu Y., Ma Y., Wen D. High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice. Life Sci. 2017;191:122–131. doi: 10.1016/j.lfs.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 12.de Matos M.A., Vieira D.V., Pinhal K.C., et al. High-intensity interval training improves markers of oxidative metabolism in skeletal muscle of individuals with obesity and insulin resistance. Front Physiol. 2018;9:1451. doi: 10.3389/fphys.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene N.P., Brown J.L., Rosa-Caldwell M.E., Lee D.E., Blackwell T.A., Washington T.A. Skeletal muscle insulin resistance as a precursor to Diabetes: beyond glucoregulation. Curr Diabetes Rev. 2018;14(2):113–128. doi: 10.2174/1573399813666161122123636. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez A.M., Bernardi H., Py G., Candau R.B. Autophagy is essential to support skeletal muscle plasticity in response to endurance exercise. Am J Physiol Regul Integr Comp Physiol. 2014;307(8):R956–R969. doi: 10.1152/ajpregu.00187.2014. [DOI] [PubMed] [Google Scholar]
- 15.Laker R.C., Drake J.C., Wilson R.J., et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017;8(1):548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klionsky D.J., Abdelmohsen K., Abe A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12(1) doi: 10.1080/15548627.2015.1100356. 1-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Stefanis D., Mastrocola R., Nigro D., Costelli P., Aragno M. Effects of chronic sugar consumption on lipid accumulation and autophagy in the skeletal muscle. Eur J Nutr. 2017;56(1):363–373. doi: 10.1007/s00394-015-1086-8. [DOI] [PubMed] [Google Scholar]
- 18.Kruse R., Vind B.F., Petersson S.J., Kristensen J.M., Højlund K. Markers of autophagy are adapted to hyperglycaemia in skeletal muscle in type 2 diabetes. Diabetologia. 2015;58(9):2087–2095. doi: 10.1007/s00125-015-3654-0. [DOI] [PubMed] [Google Scholar]
- 19.He C., Bassik M.C., Moresi V., et al. Exercise–induced BCL2–regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lira V.A., Okutsu M., Zhang M., et al. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. Faseb J. 2013;27(10):4184–4193. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa-Caldwell M.E., Lee D.E., Brown J.L., et al. Moderate physical activity promotes basal hepatic autophagy in diet-induced obese mice. Appl Physiol Nutr Metabol. 2017;42(2):148–156. doi: 10.1139/apnm-2016-0280. [DOI] [PubMed] [Google Scholar]
- 22.Rosa-Caldwell M.E., Brown J.L., Lee D.E., et al. Autophagy activation, not peroxisome proliferator-activated receptor gamma coactivator 1alpha, may mediate exercise-induced improvements in glucose handling during diet-induced obesity. Exp Physiol. 2017;102(9):1194–1207. doi: 10.1113/EP086406. [DOI] [PubMed] [Google Scholar]
- 23.Santos-Alves E., Marques-Aleixo I., Rizo-Roca D., et al. Exercise modulates liver cellular and mitochondrial proteins related to quality control signaling. Life Sci. 2015;135:124–130. doi: 10.1016/j.lfs.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Lenhare L., Crisol B.M., Silva V.R.R., et al. Physical exercise increases Sestrin 2 protein levels and induces autophagy in the skeletal muscle of old mice. Exp Gerontol. 2017;97:17–21. doi: 10.1016/j.exger.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Ju J.S., Jeon S.I., Park J.Y., et al. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci. 2016;66(5):417–430. doi: 10.1007/s12576-016-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown J.L., Rosa-Caldwell M.E., Lee D.E., et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle. 2017;8(6):926–938. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laker R.C., Xu P., Ryall K.A., et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2014;289(17):12005–12015. doi: 10.1074/jbc.M113.530527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson R.J., Drake J.C., Cui D., et al. Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy. Mitochondrion. 2019;44:20–26. doi: 10.1016/j.mito.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottlieb R.A., Stotland A. MitoTimer: a novel protein for monitoring mitochondrial turnover in the heart. J Mol Med. 2015;93(3):271–278. doi: 10.1007/s00109-014-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Call J.A., Wilson R.J., Laker R.C., Zhang M., Kundu M., Yan Z. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am J Physiol Cell Physiol. 2017;312(6):C724–C732. doi: 10.1152/ajpcell.00348.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greene N.P., Lee D.E., Brown J.L., et al. Mitochondrial quality control, driven by PGC-1α, is dysregulated by Western Diet-induced obesity and partially restored by moderate physical activity in mice. Physiol Rep. 2015;3 doi: 10.14814/phy2.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Verso F., Carnio S., Vainshtein A., Sandri M. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. 2014;10(11):1883–1894. doi: 10.4161/auto.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riebe D., Ehrman J.K., Liguori G., Magal M. tenth ed. Wolters Kluwer; Philadelphia, PA: 2018. Medicine ACoS. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 34.Akin D., Wang S.K., Habibzadegah-Tari P., et al. A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors. Autophagy. 2014;10(11):2021–2035. doi: 10.4161/auto.32229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho J., Lee I., Kim D., et al. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nut Biochem. 2014;18(4):339–346. doi: 10.5717/jenb.2014.18.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connolly L.J., Nordsborg N.B., Nyberg M., Weihe P., Krustrup P., Mohr M. Low-volume high-intensity swim training is superior to high-volume low-intensity training in relation to insulin sensitivity and glucose control in inactive middle-aged women. Eur J Appl Physiol. 2016;116(10):1889–1897. doi: 10.1007/s00421-016-3441-8. [DOI] [PubMed] [Google Scholar]
- 37.Sun S., Zhang H., Kong Z., Shi Q., Tong T.K., Nie J. Twelve weeks of low volume sprint interval training improves cardio-metabolic health outcomes in overweight females. J Sports Sci. 2019;37(11):1257–1264. doi: 10.1080/02640414.2018.1554615. [DOI] [PubMed] [Google Scholar]
- 38.Winding K.M., Munch G.W., Iepsen U.W., Van Hall G., Pedersen B.K., Mortensen S.P. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes Metabol. 2018;20(5):1131–1139. doi: 10.1111/dom.13198. [DOI] [PubMed] [Google Scholar]
- 39.He C., Sumpter R., Jr., Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8(10):1548–1551. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho D.K., Choi D.H., Cho J.Y. Effect of treadmill exercise on skeletal muscle autophagy in rats with obesity induced by a high-fat diet. J Exerc Nut Biochem. 2017;21(3):26–34. doi: 10.20463/jenb.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang T., Kim C.K., Alvarez A.A., et al. MST4 phosphorylation of ATG4B regulates autophagic activity, tumorigenicity, and radioresistance in glioblastoma. Canc Cell. 2017;32(6):840–855. doi: 10.1016/j.ccell.2017.11.005. e848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maruyama T., Noda N.N. Autophagy-regulating protease Atg4: structure, function, regulation and inhibition. J Antibiot (Tokyo) 2017;71(1):72–78. doi: 10.1038/ja.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Vledder M.G., Levolger S., Ayez N., Verhoef C., Tran T.C., Ijzermans J.N. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99(4):550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 44.Prado C.M., Baracos V.E., McCargar L.J., et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Canc Res. 2007;13(11):3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]