Abstract
Fermentation strategies for the production of poly(3-hydroxybutyrate) (PHB) from whey by recombinant Escherichia coli strain CGSC 4401 harboring the Alcaligenes latus polyhydroxyalkanoate (PHA) biosynthesis genes were developed. The pH-stat fed-batch cultures of E. coli CGSC 4401 harboring pJC4, a stable plasmid containing the A. latus PHA biosynthesis genes, were carried out with a concentrated whey solution containing 280 g of lactose equivalent per liter. Final cell and PHB concentrations of 119.5 and 96.2 g/liter, respectively, were obtained in 37.5 h, which resulted in PHB productivity of 2.57 g/liter/h.
Whey is a major by-product in the manufacturer of cheese or casein from bovine milk, representing 80 to 90% of the volume of milk transformed. It contains approximately 4.5% (wt/vol) lactose, 0.8% (wt/vol) protein, 1.0% (wt/vol) salts, and 0.1 to 0.8% (wt/vol) lactic acid (18). Only half of the whey produced annually in the United States is recycled into useful products such as food ingredients and animal feed, and the rest is regarded as a pollutant due to its high biological oxygen demand. Disposal of whey is being managed at considerable cost. Polyhydroxyalkanoates (PHAs) are polyesters, which are accumulated as energy and/or carbon storage materials by numerous microorganisms, usually when a nutritional component such as nitrogen, phosphorus, sulfur, oxygen, or magnesium is limited in the presence of an excess carbon source (1, 10, 11, 14, 16). PHAs have been considered to be good substitutes for petroleum-derived synthetic plastics because of their similar material properties to synthetic polymers and complete biodegradability after disposal (3). A major problem in commercializing PHAs is their high production cost. Much effort has been devoted to lower the production cost of PHA by developing better bacterial strains and efficient strategies for fermentation and recovery of PHAs (5, 10, 12). Among these bacterial strains, recombinant Escherichia coli strains harboring the Ralstonia eutropha and Alcaligenes latus PHA biosynthesis genes have been successfully employed for the production of poly (3-hydroxybutyrate) (PHB) to a high concentration with high productivity (6, 15, 17). Economic evaluation of the process for the production of PHB suggested that the major contributor to the overall PHB production cost was carbon substrate cost (up to 50%) (4). Therefore, it is desirable to produce PHB from cheap carbon source or even from a waste product such as whey by using recombinant E. coli. Several researchers reported that PHB could be produced from whey-based medium by recombinant E. coli in flask culture (8, 9, 13). Recently, we carried out high-cell-density culture of a recombinant E. coli strain harboring the R. eutropha PHA biosynthesis genes for the production of PHB from whey (18). Even though a relatively high concentration of PHB (69 g/liter) could be produced, cell broth had to be intermittently removed due to the volumetric limitation of the fermentor caused by the low solubility of lactose in the feeding solution (ca. 210 g of lactose per liter) (18).
In this study, we report the fermentation strategies for the production of PHB from whey in recombinant E. coli without removing culture broth during fermentation. A highly concentrated whey solution was successfully employed for the efficient production of PHB from whey to a high concentration by recombinant E. coli.
Experimental procedures.
E. coli CGSC 4401 (E. coli Genetic Stock Center, New Haven, Conn.), CGSC 3121, CGSC 2507, DSM 499 (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), and KCTC 2223 (Korean Collection for Type Cultures, Taejon, Korea) were used in this study. The plasmid pJC4 containing the A. latus PHA biosynthesis genes has been described previously (6). E. coli strains were transformed with pJC4 by electroporation (7). Cells were maintained as a 15% (vol/vol) glycerol stock at −75°C after growing in Luria-Bertani (LB) medium (pH 6.7) or chemically defined MR medium (described below) containing 20 g of lactose per liter.
Bovine whey powder was obtained from SamIk Co., Seoul, Korea. Crude whey solution was prepared by dissolving 700 g of whey powder in 1 liter of distilled water. To remove excessive proteins in whey solution, the pH of the whey solution was adjusted to 4.5 by the addition of 37% (wt/vol) HCl (19). The solution was autoclaved at 121°C for 15 min and centrifuged at 11,000 × g in a sterilized bottle for 15 min to remove aggregates. By adding diatomaceous earth (Sigma Co., St. Louis, Mo.) to 2% (wt/vol), small protein particles could be removed by filtration with Whatman no. 3 filter paper (Whatman Co., Maidstone, England). The pH of the filtered solution was adjusted to 6.5 with 12 N NaOH.
Flask cultures were carried out in a 250-ml flask containing 100 ml of MR medium in a shaking incubator at 30°C and 200 rpm. Whey powder (40 g/liter) was added as a carbon source in flask culture. The MR medium (pH 6.9) contains (per liter) 6.67 g of KH2PO4, 4 g of (NH4)2HPO4, 0.8 g of MgSO4 · 7H2O, 0.8 g of citric acid, and 5 ml of trace metal solution. The trace metal solution contains (per liter of 5 M HCl) 10 g of FeSO4 · 7H2O, 2 g of CaCl2, 2.2 g of ZnSO4 · 7H2O, 0.5 g of MnSO4 · 4H2O, 1 g of CuSO4 · 5H2O, 0.1 g of (NH4)6Mo7O24 · 4H2O, and 0.02 g of Na2B4O7 · 10H2O. Two different feeding solutions were used for the fed-batch cultures. In fermentation A, a concentrated whey solution (containing 210 g of lactose equivalent per liter) plus 4.5 g of MgSO4 · 7H2O per liter was used. In fermentations B and C, highly concentrated whey solution (containing 280 g of lactose equivalent per liter) plus 6 g of MgSO4 · 7H2O per liter was used. Feeding solutions were prepared as described above.
With the recombinant E. coli strain CGSC 4401(pJC4), fed-batch cultures were carried out at 30°C in a 6.6-liter jar fermentor (Bioflo 3000; New Brunswick Scientific Co., Edison, N.J.) containing 1.3 liter of MR medium plus pretreated whey solution equivalent to 20 g of lactose per liter. Seed cultures (130 ml) were prepared in the same medium. The culture pH was controlled at 6.95 by the automatic addition of 28% (vol/vol) NH4OH. The level of dissolved oxygen concentration (DOC) was controlled by automatically increasing the agitation speed up to 1,000 rpm and varying the pure oxygen percentage. Nutrient feeding solution was added by the pH-stat feeding strategy. When the pH rose to a value greater than its set point (6.95) by 0.1 due to the depletion of lactose, an appropriate volume of feeding solution was automatically added to increase the lactose concentration in the culture broth to 20 g/liter. The feeding volume was calculated online with the fermentation software AFS3.42 (New Brunswick Scientific Co.). Foam formation was suppressed by adding Antifoam 289 (Sigma Chemical Co., St. Louis, Mo.) during the initial stage of fed-batch cultures.
Cell growth was monitored by measuring the optical density at 600 nm (OD600; DU Series 600 Spectrophotometer; Beckman, Fullerton, Calif.). The PHB concentration was determined by measuring the concentration of 3-hydroxybutyric acid methyl ester, which was prepared by methanolysis of PHB, by gas chromatography (Donam Co., Seoul, Korea) with a fused silica capillary column (SPB-5, 30 m by 0.32 mm [inside diameter], 0.25-μm-thick film; Supelco, Bellefonte, Pa.) with benzoic acid as an internal standard (2). Cell concentration, defined as dry weight of cells per liter of culture broth, was determined as previously described (14). The residual cell concentration was defined as the cell concentration minus the PHB concentration. The PHB content (percentage of weight) was defined as the percentage of the ratio of PHB concentration to cell concentration. The concentrations of lactose, galactose, and glucose were measured by high-performance liquid chromatography (L-4200 UV-Vis detector, L-600 pump, D-2500 chromato-integrator; Hitachi, Tokyo, Japan) equipped with an ion-exchange column (Aminex HPX-87H, 300 mm by 7.8 mm; Hercules, Calif.) using 0.01 N H2SO4 as a mobile phase.
Flask cultures.
E. coli CGSC 4401, CGSC 3121, CGSC 2507, DSM 499, and KCTC 2223 harboring pJC4 were cultivated for 96 h in chemically defined MR medium supplemented with 40 g of whey powder per liter at 30°C to examine the efficiency of PHB production from whey. The results of flask cultures are summarized in Table 1. The highest cell and PHB concentrations of 6.6 and 5.0 g/liter, respectively, were obtained when E. coli CGSC 4401(pJC4) was cultivated in MR plus 40 g of whey per liter. From these flask culture results, recombinant E. coli CGSC 4401(pJC4) was chosen as the strain for PHB production from whey.
TABLE 1.
PHB production from whey in flask cultures of various recombinant E. coli strains (pJC4)
| E. coli strain | Cell concn (g/liter) | PHB concn (g/liter) | PHB content (wt%) |
|---|---|---|---|
| CGSC 4401 | 6.6 | 5.0 | 76 |
| CGSC 2507 | 6.8 | 3.4 | 50 |
| CGSC 3121 | 3.0 | 1.3 | 43 |
| DSM 499 | 6.1 | 2.7 | 45 |
| KCTC 2223 | 6.2 | 0.57 | 9.2 |
Fed-batch culture with whey solution containing 210 g of lactose per liter.
Fed-batch culture of E. coli CGSC 4401 harboring pJC4 was carried out by the pH-stat feeding strategy using the whey solution containing 210 g of lactose per liter (fermentation A). During the cultivation, the DOC was initially maintained at 30%. When the OD600 reached 180 (cell concentration of ca. 60 g/liter), the DOC was maintained at 20% until the end of cultivation. In 49 h, cell and PHB concentrations had reached 83.1 and 46.8 g/liter, respectively, resulting in a PHB content of 56.3 wt% and a productivity of 1.15 g/liter/h. Because the lactose concentration in the feeding solution was somewhat low (210 g/liter), a relatively large volume of nutrient feeding solution (7.4 liter) was added to the fermentor to achieve high cell density. Due to the volumetric limitation of the fermentor caused by the large volume of feeding solution, culture broth had to be removed to prevent flooding during the cultivation. When lactose was depleted, 2, 2.5, and 2 liters of culture broth were sequentially removed from the fermentor. During the entire cultivation, 7.4 liters of nutrient feeding solution was added and 6.5 liters of culture broth was removed.
Fed-batch culture with highly concentrated whey solution containing 280 g of lactose per liter.
In fermentation A, a large volume of culture broth had to be removed due to the volumetric limitation of the fermentor caused by the low concentration of the carbon source (210 g of lactose per liter), which resulted in a relatively low cell concentration and PHB productivity. To solve this problem, we examined the possibility of further concentrating the whey solution. In our previous study (18), the whey solution was concentrated based on the solubility data for lactose in water (200 to 210 g/liter). It was reasoned that the actual solubility of lactose in whey solution might be different from this value. It was found that whey solution could be highly concentrated to contain 280 g of lactose equivalent per liter by pretreatment of whey solution. The feeding solution containing 280 g of lactose per liter was sufficient to allow us to achieve a high density of cells and high concentration of PHB with a higher cell yield than that obtained with whey solution containing 210 g of lactose per liter (cell yield increased from 0.42 to 0.52 g/g of lactose) without removing culture broth during fermentation. Even though lactose was concentrated to a level greater than its water solubility (210 g/liter) at room temperature, whey solution containing 280 g of lactose per liter was stable at room temperature, which seemed to be mainly due to the presence of impurities, such as the remaining proteins and salts in whey solution. Lactose precipitates appeared in oversaturated whey solution containing 280 g of lactose per liter, only when it was deposited in a −4°C refrigerator for longer than 1 week. Concentrated whey solution can be easily prepared on a large scale by evaporation. For example, a falling film evaporator, which is widely employed in the milk industry, can be used. The pH-stat fed-batch culture of E. coli CGSC 4401 harboring pJC4 was carried out by using highly concentrated whey solution containing 280 g of lactose per liter (fermentation B). Culture broth removal during fed-batch cultivation could be avoided and a higher cell concentration could be achieved by using this highly concentrated whey feeding solution. The initial DOC was maintained at 30% of air saturation. When the OD600 reached 240 (cell concentration of ca. 80 g/liter), the DOC was lowered to 20%. In fermentation B, the final cell concentration, PHB concentration, and PHB content of 102.9 g/liter, 59.6 g/liter, and 57.9 wt%, respectively, were obtained in 42 h, which resulted in a productivity of 1.42 g of PHB/liter/h.
A new strategy to achieve high PHB content.
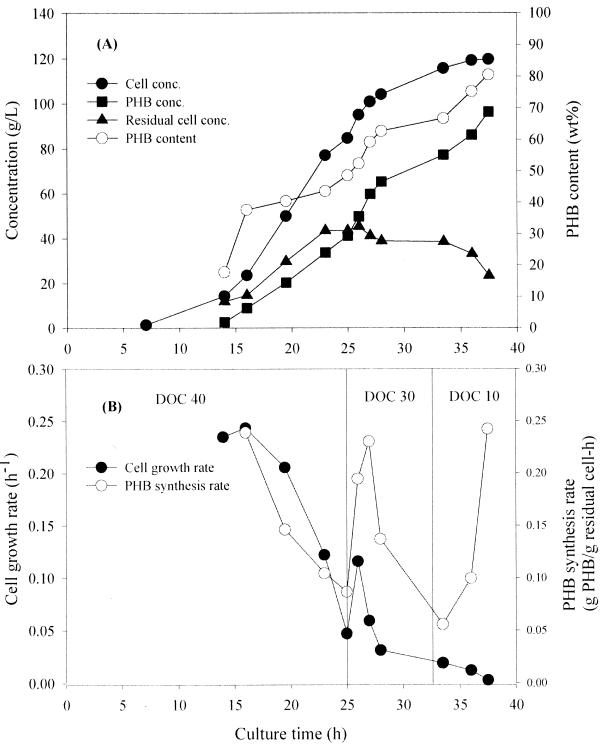
Even though a higher cell concentration of 102.9 g/liter was achieved without removing culture broth by employing highly concentrated whey solution, the final PHB content was still lower than 60%. The PHB content is a very important factor, which contributes significantly to the cost of production of PHB in large-scale fermentation (4). A new strategy had to be developed to obtain higher PHB content. Therefore, we examined the effect of the DOC during the fed-batch culture to achieve high PHB content and cell concentration at the same time. In fermentations A and B, the DOC was lowered from 30% to 20% when the cell concentration reached 70% of the final cell concentration. When the DOC was lowered to 20%, it was observed that the cell growth rate rapidly increased initially and then continuously decreased; however, the PHB synthesis rate reincreased (results not shown). From these results, we reasoned that appropriate control of the DOC could allow us to obtain higher PHB content and PHB concentration. This strategy was applied in the fed-batch culture by using highly concentrated whey solution containing 280 g of lactose per liter (fermentation C). Wang and Lee (17) suggested that fed-batch cultivation with recombinant E. coli could be divided into two phases: an active growth phase during which PHB content is kept constant at a low level and an active PHB synthesis phase during which PHB is actively accumulated with the concomitant increase in PHB content (17). It was reported that a sufficient DOC during the active growth phase was important to achieve high final cell and PHB concentrations. In fermentation C, the DOC was maintained at 40% during the active growth phase. By doing this, the cell growth rate and PHB synthesis rate increased much higher than those obtained in fermentations A and B after lowering the DOC in the later phase of fermentation. In another fed-batch cultivation similar to fermentation B, the DOC was rapidly lowered from 40% to 5% at the OD600 of 180 and was maintained at 5% until the end of fermentation. However, the final cell and PHB concentrations obtained were only 79 and 63.2 g/liter, respectively (results not shown). Even though the PHB content (80%) was high, the cell concentration was lower than that obtained in fermentation B. These results suggested that the timing of decreasing the DOC and how to decrease the DOC have considerable effects on the final cell concentration and PHB content. To achieve a higher PHB content, the DOC was decreased stepwise from 40% to 30% and then to 15% in fermentation C (Fig. 1B). Whenever the DOC was decreased during the active PHB synthesis phase, the PHB synthesis rate sharply increased. The final PHB synthesis rate was much higher than that obtained in fermentations A and B. The final cell concentration, PHB concentration, and PHB productivity obtained in fermentation C were 119.5 g/liter, 96.2 g/liter, and 2.57 g/liter/h, respectively, which are much higher than those reported previously (18). By optimally controlling the level of DOC, the higher PHB concentration and PHB content could be achieved at the same time.
FIG. 1.
Time profiles of cell concentration (●), PHB concentration (■), residual cell concentration (▴), and PHB content (○) (A) and cell growth rate (●) and PHB synthesis rate (○) (B) during fed-batch culture of E. coli CGSC 4401(pJC4) with feeding solution containing 280 g of lactose equivalent per liter (fermentation C).
In this study, we have demonstrated that environmentally friendly PHB polymer can be efficiently produced from environmentally polluting whey with high PHB content and productivity by the pH-stat fed-batch culture with a highly concentrated whey solution. It was also found that higher cell and PHB concentrations could be obtained by optimal timing of the DOC reduction. The strategies developed in this study should provide an industrially attractive solution to whey disposal and utilization problems by allowing economical production of higher-value biodegradable polymer.
Acknowledgments
We thank Mary Berlyn for kindly providing the CGSC strains.
This work was supported by the Korea-Australia International Cooperative Project from the Korean Ministry of Science and Technology (MOST) and by the Brain Korea 21 program.
REFERENCES
- 1.Anderson A J, Dawes E A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev. 1990;54:450–472. doi: 10.1128/mr.54.4.450-472.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunegg G, Sonnleitner B, Lafferty R M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol. 1978;6:29–37. [Google Scholar]
- 3.Byrom D. Polymer synthesis by micro-organisms: technology and economics. Trends Biotechnol. 1987;5:246–250. [Google Scholar]
- 4.Choi J, Lee S Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng. 1997;17:335–342. [Google Scholar]
- 5.Choi J, Lee S Y. Efficient and economical recovery of poly(3-hydroxybutyrate) from recombinant Escherichia coli by simple digestion with chemicals. Biotechnol Bioeng. 1999;62:546–553. doi: 10.1002/(sici)1097-0290(19990305)62:5<546::aid-bit6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Choi J, Lee S Y, Han K. Cloning of the Alcaligenes latus polyhydroxyalkanoate biosynthesis genes and use of these genes for enhanced production of poly(3-hydroxybutyrate) in Escherichia coli. Appl Environ Microbiol. 1998;64:4897–4903. doi: 10.1128/aem.64.12.4897-4903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidler S, Dennis D. Polyhydroxyalkanoate production in recombinant Escherichia coli. FEMS Microbiol Rev. 1992;103:231–236. doi: 10.1016/0378-1097(92)90314-e. [DOI] [PubMed] [Google Scholar]
- 9.Janes B, Hollar J, Dennis D. Molecular characterization of the poly-beta-hydroxybutyrate biosynthetic pathway of Alcaligenes eutrophus H16. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic; 1990. pp. 175–190. [Google Scholar]
- 10.Lee S Y. Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol. 1996;14:431–438. [Google Scholar]
- 11.Lee S Y. Bacterial polyhydroxyalkanoates. Biotechnol Bioeng. 1996;49:1–14. doi: 10.1002/(SICI)1097-0290(19960105)49:1<1::AID-BIT1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Lee S Y. Poly(3-hydroxybutyrate) extrusion by cells of recombinant Escherichia coli. J Microbiol Biotechnol. 1996;6:147–149. [Google Scholar]
- 13.Lee S Y, Middelberg A P J, Lee Y K. Poly(3-hydroxybutyrate) production from whey using recombinant Escherichia coli. Biotechnol Lett. 1997;19:1033–1035. [Google Scholar]
- 14.Lee S Y, Chang H N. Effect of complex nitrogen source on the synthesis and accumulation of poly(3-hydroxybutyric acid) by recombinant Escherichia coli in flask and fed-batch cultures. J Environ Polym Degrad. 1994;2:169–176. [Google Scholar]
- 15.Schubert P, Steinbüchel A, Schlegel H G. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-β-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988;170:5837–5847. doi: 10.1128/jb.170.12.5837-5847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinbüchel A. Biodegradable plastics. Curr Opin Biotechnol. 1992;3:291–297. [Google Scholar]
- 17.Wang F, Lee S Y. Production of poly(3-hydroxybutyrate) by fed-batch culture of filamentation-suppressed recombinant Escherichia coli. Appl Environ Microbiol. 1997;12:4765–4769. doi: 10.1128/aem.63.12.4765-4769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong H H, Lee S Y. Poly(3-hydroxybutyrate) production from whey by high-density cultivation of recombinant Escherichia coli. Appl Microbiol Biotechnol. 1998;50:30–33. doi: 10.1007/s002530051252. [DOI] [PubMed] [Google Scholar]
- 19.Yellore V, Desai A. Production of poly-3-hydroxybutyrate from lactose and whey by Methylobacterium sp. ZP24. Lett Appl Microbiol. 1998;26:391–394. doi: 10.1046/j.1472-765x.1998.00362.x. [DOI] [PubMed] [Google Scholar]