Abstract
Ytterbium-doped cesium lead halides are quantum cutting materials with exceptionally high photoluminescence quantum yields, making them promising materials as scintillators. In this work, we report ytterbium-doped cesium lead chloride (Yb3+:CsPbCl3) with an X-ray scintillation light yield of 102,000 photons/MeV at room temperature, which is brighter than the current state-of-the-art commercial scintillators. The high light yield was achieved based on a novel method of synthesizing Yb3+:CsPbCl3 powders using water and low-temperature processing. The combination of high light yield and the simple and inexpensive manufacturing method reported in this work demonstrates the great potential of Yb3+:CsPbCl3 for scintillation applications.
1. Introduction
Detection of X-ray photons is critically important in various fields, such as security, medical radiography, industrial manufacturing, and astronomy.1−5 Scintillators play key roles in many of these applications by absorbing X-ray photons and emitting visible or near-infrared photons for detection with conventional photodetectors. However, the state-of-the-art commercial scintillators are manufactured using complex fabrication methods such as single-crystal growth at high temperature (∼1700 °C), which make their cost prohibitively high for applications that require large area detection.6,7 Alternatives such as plastic scintillators8−10 can be inexpensively manufactured into large dimensions at low temperature, but they are not commonly used in high-energy X-ray systems due to their low stopping power, low light yield, and poor energy resolution that do not meet the performance criteria.
Metal halide perovskites (MHPs) have recently been identified as promising scintillator materials.11,12 MHPs are low-band gap (1.6–2.2 eV) materials composed of high-Z atoms with chemical composition of ABX3, where A = Cs+, CH3NH3+, and so forth, B = Pb2+, Sn2+, and X = Cl–, Br–, and I–. With a combination of relatively high effective atomic number, high density, and low band gap, MHPs are efficient hard radiation absorbers with potential light yields as high as 250,000 photons/MeV.13−15 MHPs are composed of entirely earth abundant, low-cost elements, and can be synthesized into single crystals, polycrystalline powders, or thin films using simple solution processing methods at low temperatures (25–150 °C). Therefore, MHP-based scintillators are expected to be drastically cheaper compared to current commercial scintillators.16,17 Even in highly defective polycrystalline forms, MHPs exhibit low charge trapping due to defect tolerance.18,19 For this reason, charge carrier diffusion length can be as long as few microns in polycrystalline MHP powders synthesized at low temperature.20 These attributes can be exploited to obtain high performance in optoelectronic devices such as solar cells and photodetectors.
However, pure MHP compositions by themselves have so far demonstrated limited scintillation performance due to the low exciton binding energy in MHPs, suppressing the formation of excitons and radiative recombination.12,21 To overcome this limitation, liquid nitrogen temperatures were required to obtain sufficient light yield.12,21 Formation of nanostructured MHPs into quantum dots or quantum wells to increase the exciton binding energy has shown to increase the light yield22,23 but at the expense of increased band gap and drastic cost increase associated with nanoparticle synthesis. Moreover, pure MHP composition scintillators suffer from the large overlap between photoluminescence and absorbance spectra which causes reabsorption of emitted photons.
In this work, we sought to address all of the challenges mentioned above using ytterbium (Yb3+)-doped cesium lead chloride perovskites (Yb3+:CsPbCl3) as scintillators. Doping of cesium lead halide (CsPbX3) with Yb3+ can boost the photoluminescence quantum yield (PLQY) above 100% through the quantum cutting process,24−32 wherein one photon absorbed by the CsPbX3 matrix can be converted into two photons emitted from the Yb3+ dopants. The bright emission through the quantum cutting process, combined with high atomic number compositions, makes Yb3+:CsPbCl3 compelling for scintillator applications, and yet, there has been no report on scintillation from Yb3+-doped MHPs. In this paper, we report a novel method of synthesizing Yb3+:CsPbCl3 powders using only water as the solvent and mild thermal annealing (below 200 °C). Our manufacturing method can produce a large quantity of Yb3+:CsPbCl3 powder in a low-cost and scalable fashion. The Yb3+:CsPbCl3 powder can be sintered into any shape and size for various target applications. Radioluminescence activity was optimized as a function of Yb doping amount. The highest performance was achieved with 5 mol % Yb doping amount Yb3+:CsPbCl3 with light yield of 102,000 photons/MeV under room temperature operation, which is higher than light yields of state-of-the-art commercial scintillators. The Yb3+:CsPbCl3 powder was pressed into a pellet for radiographic imaging of various objects with a complementary metal-oxide-semiconductor photodetector. Our results demonstrate the promising potential of Yb3+:CsPbCl3 for scintillators that can simultaneously achieve high performance, large area detection, and low cost.
2. Results and Discussion
The Yb3+:CsPbCl3 powder samples were prepared using simple solution processing with water as the solvent, as illustrated in Figure 1a. Stoichiometric amounts of cesium chloride (CsCl, water solubility 1910 g/L at 25 °C), lead chloride (PbCl2, water solubility 0.99 g/100 mL at 20 °C), and ytterbium chloride (YbCl3, water solubility 17 g/100 mL at 25 °C) precursors were combined in de-ionized water, stirred at 40 °C for 1 h, after which the water was removed through evaporation at 65 °C. The resultant powder was then finely crushed in a mortar and pestle and annealed at 200 °C for 1 h, with mechanical stirring and mixing during the process. Seven powder samples were produced with varying Yb3+ ion contents from 0.5 mol % up to 60 mol %.
Figure 1.
Synthesis and physical characterization of Yb3+:CsPbCl3 powders. (a) Schematic of Yb3+:CsPbCl3 synthesis process. (b) XRD patterns of Yb3+:CsPbCl3 samples with different Yb contents and the orthorhombic phase of CsPbCl3. (c) Scanning electron microscopy (SEM) image of a 5% Yb3+:CsPbCl3 powder sample. (d–g) Energy-dispersive X-ray spectroscopy (EDXS) overlay of Cl–, Pb2+, Cs+, and Yb3+.
The X-ray diffraction (XRD) patterns of the Yb3+:CsPbCl3 samples are shown in Figure 1b, along with a reference pattern of the orthorhombic structural phase (Pnma) of CsPbCl3. The XRD patterns from Yb3+:CsPbCl3 with less than 25% Yb3+ content match well to the CsPbCl3 reference pattern, in agreement with previous reports which show that Yb3+ doping at low concentrations retain the bulk CsPbCl3 crystal structure.33 The orthorhombic (Pnma) structure of these powder samples is consistent with Yb3+:CsPbCl3 powders formed through the solid-state reaction33 and vapor deposition34 and is in contrast with the cubic (Pmm) structure of Yb3+:CsPbCl3 nanocrystals and quantum dots.29,35−37 The well-matched patterns between CsPbCl3 and Yb3+:CsPbCl3 at and below 5% Yb3+ content, without any presence of peaks from precursor materials and other possible phases, suggest that the Yb3+ ions are incorporated into the CsPbCl3 host lattice through placement into the Pb2+ sites. The (101) peak location at 2θ = 22.4° does not shift significantly as a function of Yb3+ amount, suggesting that the substitution of the smaller Yb3+ ion in the Pb2+ position does not result in any significant lattice compression. In the 25, 45, and 60% Yb3+:CsPbCl3 compositions, XRD peaks not matching to the CsPbCl3 structure are observed, indicating the presence of impurity species at these higher Yb3+ doping concentrations that reduce the orthorhombic crystallinity of the samples (Figure S1). The majority of the impurity phase in high (>25%) Yb amount samples is likely to be Cs4PbCl6, as shown in Figure S2. These results show that our water-based and low-temperature synthesis method results in high purity Yb:CsPbCl3 with the Yb3+ dopants substituted into the Pb2+ sites in the lattice in Yb3+ concentrations at and below 5%.
The morphology of the 5% Yb3+:CsPbCl3 powder is shown in the SEM image in Figure 1c. The powder was found to be composed of micrometer scale grains. EDXS images show that the Cl–, Pb2+, Cs+, and Yb3+ are distributed throughout the entirety of the grains (Figures 1d–g, S3). Few areas of higher concentration of Cs+ and Yb3+ were observed (Figure 1f,g), which suggests that there are some regions of the samples with higher Yb3+ concentration. The average surface elemental composition of the 5% Yb3+:CsPbCl3 sample obtained through EDS shows that the Yb3+ doping amount is close to the stoichiometric amounts of the precursor used in the synthesis based on the measured Yb3+ to Pb2+ ratios (Table S1).
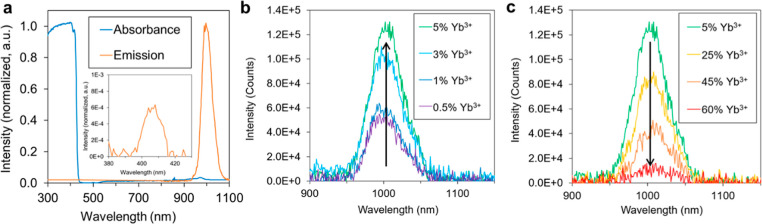
The absorbance and photoluminescence spectra of 5% Yb3+:CsPbCl3 sample are shown in Figure 2a. A spectrophotometer and a spectrofluorometer, both equipped with an integrating sphere, were employed for these measurements to account for any light scattering in the powder samples (see details in the Materials and Methods section). Our results show that there is a large Stokes shift between the absorption onset at 440 nm and the photoluminescence emission peak at 1000 nm. This is consistent with previous reports on Yb3+:CsPbCl3 wherein the light absorption occurs in the CsPbCl3 with the onset of 440 nm, and the emission peak at 1000 nm originates from the 2F5/2 → 2F7/2 transition in Yb3+ ions.27,29 The photoluminescence from the CsPbCl3 matrix at 406 nm is greatly suppressed as a result of efficient sensitization with the Yb3+-ion dopants (inset in Figure 2a). The emission wavelength of 1000 nm is well-matched with silicon photodiodes that are cost effective and widely available for coupling with scintillators in various applications. Moreover, the large Stokes shift is advantageous for the scintillator performance as it prevents the re-absorption of radioluminescence photons by the material, enabling increased scintillator thickness without attenuating the emitted photon flux.38
Figure 2.
Optical characterization and radioluminescence of Yb3+:CsPbCl3 powder. (a) Absorbance and photoluminescence of 5% Yb3+:CsPbCl3. (b,c) X-ray radioluminescence spectra of 0.5–5% Yb3+:CsPbCl3 and 5–60% Yb3+:CsPbCl3.
Radioluminescence spectra of the Yb3+:CsPbCl3 samples were measured using a spectrofluorometer equipped with a Moxtek MAGPRO X-ray source operating at 40 kV and 300 μA. To ensure consistent amounts of excited scintillator material and X-ray excitation conditions, all Yb3+:CsPbCl3 samples in the powder form were loaded into identical Kapton capillary tubes and placed into an integrating sphere with consistent sample placement (see the Materials and Methods section for the detailed experimental procedure). Our measurements show a trend of increasing radioluminescence intensity from Yb3+:CsPbCl3 samples with increasing Yb3+-ion contents up to 5% (Figure 2b). This is consistent with previous papers that showed larger PLQYs in Yb3+-doped CsPbX3 with more Yb3+ ions in the low Yb3+ concentration regime.27,29 Our results indicate that similar behavior occurs in radioluminescence emission as well. This is likely caused by more efficient sensitization of Yb3+ dopants due to larger number of Yb3+ defect complex sites and reduced average distances for charge carriers to travel. However, at above the 5% Yb3+ doping level, we observed the opposite trend of decreasing radioluminescence intensity with higher Yb3+ concentration (Figure 2c). We attribute this to presence of impurity species based on our XRD results (Figure 1b) that show an increased amount of impurity species with increasing Yb3+ ion content above 5%.
An important performance metric for scintillators is the light yield, which is defined to be the number of photons emitted per absorbed radiation energy. Given the same radiation source, scintillators with higher light yield produce brighter radioluminescence emission for more sensitive detection with photodetectors. In various applications, such as medical imaging and national security, sensitive detection of weaker intensity radiation is highly desired. The scintillation light yields of our Yb3+:CsPbCl3 samples, as well as commercially available reference scintillators CaF2(Eu) and CsI(TI), were quantified using excitation from the X-ray source operating at 40 kV and 300 μA (see the Materials and Methods section for details).
Consistent with the trend observed in the radioluminescence spectra intensity as a function of Yb3+ amount, we found that the 5% Yb3+:CsPbCl3 provided the highest performance with a light yield of 102,000 photons/MeV (Figure 3a). This is a significantly higher light yield than the state-of-the-art commercially available scintillators such as CsI(Tl) with a light yield of 65,000 photons/MeV (see Table S3 for comparison of light yields from various scintillators). The high light yield from Yb3+:CsPbCl3 can be attributed to the combination of relatively low band gap of CsPbCl3, resulting in greater charge carrier generation with high energy radiation absorption, long charge carrier lifetime, and diffusion length even in polycrystalline samples20 and bright emission from the quantum cutting process.24−32 Previous studies in the context of downconverters for solar cells have found that Yb3+-doped CsPbX3 exhibits luminescence saturation at increasing photoexcitation fluences, limiting the emission of the material.25,29 To check whether our 5% Yb3+:CsPbCl3 sample is limited by the saturation effect, we measured the radioluminescence intensity as a function of excitation X-ray flux. As shown in Figure 3b, the photons emitted from the sample varies linearly with the X-ray source current, indicating that saturation does not occur within the operating conditions of the X-ray source employed in this study.
Figure 3.
Radioluminescence performance and radiographic imaging of 5% Yb3+:CsPbCl3. (a) X-ray radioluminescence spectrum of 5% Yb3+:CsPbCl3 with light yield of 102,000 photons/MeV. (b) Linear radioluminescence response of 5% Yb3+:CsPbCl3 as a function of the X-ray source current. (c,d) X-ray imaging on the surface of 25 mm pressed 5% Yb3+:CsPbCl3 powder pellet of a thumbtack and a micro-SIM card.
To demonstrate the applicability of our Yb3+:CsPbCl3 for radiographic imaging, a cylindrical pellet of the 5% Yb3+:CsPbCl3 powder sample was manufactured by hydraulically pressing the powder under 6 tons of pressure at 70 °C for 3 h. Images of various objects under X-ray irradiation formed on the scintillator pellet were captured using a Basler NIR GigE camera (Figure 3c,d). In Figure 3c, a thumbtack is shown, in which the embedded metal pin inside the plastic head is clearly visible. Figure 3d shows a micro-SIM phone card, in which the metal contact is differentiated from the plastic casing.
3. Conclusions
We have shown the first detection and application of radioluminescence in a Yb3+-doped cesium lead halide perovskite. Our water-based synthesis method produces Yb3+:CsPbCl3 powder over a wide composition range that shows incorporation of the Yb3+ dopant with high purity up to a maximum of 5 mol %. The water evaporation and low-temperature annealing method presented here is among the simplest methods of producing Yb3+:CsPbX3 but does not sacrifice the crystallinity of the CsPbCl3 host lattice. The 5% Yb3+:CsPbCl3 sample has a large Stokes shift and the strong characteristic Yb3+ emission at 1000 nm. Suppressed CsPbCl3 excitonic emission at 406 nm proves efficient transfer of charge carriers from the bulk lattice to the defect Yb3+ emission sites. Radioluminescence is exhibited by all Yb3+:CsPbCl3 samples and is optimized at 5 mol % due to a balance of high Yb3+ ion content and high material purity. The champion 5% Yb3+:CsPbCl3 composition has a measured light yield of 102,000 photons/MeV, which is higher than those from the commercial scintillator options. Radiographic imaging was demonstrated using a pressed powder pellet.
4. Materials and Methods
4.1. Synthesis of Yb3+-Doped CsPbCl3 Powder
Cesium iodide (CsCl, >99.0%) was purchased from Tokyo Chemical Industry (TCI), lead(II) chloride (PbCl2, 99.999%) was purchased from Alfa Aesar, and ytterbium(III) chloride hexahydrate (YbCl3·6H2O, 99.9%-Yb) was purchased from STREM Chemicals. The precursors were combined according to the YbxCsPb1–1.5xCl3 stoichiometry, assuming 2Yb3+:1VPb substitution at the Pb2+ B-sites, in Yb3+ molar percents of 0, 0.5, 1, 3, 5, 25, 45, and 60%. The precursors were dissolved in de-ionized water at a concentration of 5 g/L and stirred for 1 h at 40 °C. The solution was then transferred to a crystallization dish, and the water was slowly evaporated off at a temperature of 65 °C while stirring. The resultant powder was finely crushed using a mortar and pestle, transferred back to the crystallization dish, and annealed for 1 h at 200 °C, stirring four times during the annealing process. The pressed powder pellet of 5% Yb3+:CsPbCl3 was fabricated using a 25 mm pellet die and an Atlas Manual Hydraulic Press under 6 tons of pressure for 2 h while under vacuum at 70 °C.
4.2. Physical Characterization
XRD patterns of the Yb3+:CsPbCl3 powders were measured using an Empyrean Multipurpose X-ray diffractometer with a Cu anode, at 45 kV and 40 mA with step size 0.02°. SEM and EDXS were measured on an FEI Quanta 650 Scanning Electron Microscope.
4.3. Optical Characterization
The steady-state photoluminescence spectra were measured using a PTI QuantaMaster 400 spectrofluorometer, using a Xe arc lamp excitation source monochromatized at a wavelength of 405 nm. The absorbance spectra were measured using a PerkinElmer UV/vis/NIR Lambda 950S spectrometer equipped with an integrating sphere.
4.4. Radioluminescence Characterization and Light Yield Calculation
4.4.1. Materials
A Moxtek 60 kV 12 W MagPro X-ray imaging source was used for X-ray generation. Commercial scintillators CaF2(Eu) and CsI(TI) were purchased from Epic-Crystal Co. with light yields of 19,000 and 60,000 ph/MeV, respectively. Both commercial scintillators were grown via the Bridgeman method and cut to be 25.4 × 25.4 × 25.4 mm3 cubes with all sides polished. An acrylic glass cube with dimensions 25.4 × 25.4 × 25.4 mm3 with all sides polished was purchased from U.S. Plastic Corporation. Power meters equipped with a silicon photodiode (PM16-120) and a germanium photodiode (PM16-122) were purchased from Thor Labs. A PTI QuantaMaster 400 spectrofluorometer equipped with a UV–vis photomultiplier tube (PMT) (PFR Technologies, LLC, R2658) and NIR PMT (Hamamatsu Photonics, H10330-75) was used to measure radioluminescence and reference light signals.
4.4.2. Approach
The light yield of the Yb3+:CsPbCl3, reported in photons per MeV of energy deposited, was determined in two parts. The energy deposited by the X-ray source was quantified using the known light yields of two commercial scintillators, CaF2(Eu) and CsI(TI). The energy-deposited values were corrected for non-proportionality of CaF2(Eu) and CsI(TI) at the specific keV X-ray energies produced by the X-ray source used in the measurement. To quantify the Yb3+:CsPbCl3 photon output, the power of a reference light source was correlated to the PMT detector response; correcting for spectral sensitivity of the detector at different wavelengths.
Radioluminescence signals were obtained for the commercial scintillators and the Yb3+:CsPbCl3 scintillators using the Moxtek 60 kV 12 W MagPro X-ray Imaging Source at 300 μA and 40 kV. All scintillators studied were placed inside an integrating sphere and confirmed to completely absorb the X-ray beam, which was collimated using the inlet of the integrating sphere. All luminescence signals were determined to be within the linear range of the PMT detector and within the documented operating range of the power meters. The reference light power measurements were performed and cross-checked using both a silicon photodiode power meter and a germanium photodiode power meter.
4.4.3. Powder Sample Preparation
Powder samples were packed into 12 Kapton capillaries purchased from Cole-Parmer (0.0710″ ID, 0.0750″ OD, cut to a length of 50 mm). Capillaries were sealed on both ends with Sigillum Wax sealant purchased from Thomas Scientific and arranged into two rows of six, which was confirmed to achieve total attenuation of X-rays.
4.4.4. Quantifying Photon Output of Scintillators
The photon output of the xenon lamp light source with a monochromator was used in conjunction with a power meter and PMT detector to determine the photon output of the commercial CaF2(Eu) and CsI(TI) scintillators and the Yb3+:CsPbCl3 scintillators. Wavelengths matching the emission of commercial and Yb3+:CsPbCl3 scintillators were selected for the reference light power meter measurements, as detailed in Table S4. The reference light was scattered in the same geometry as the scintillators. To match the commercial CaF2(Eu) and CsI(TI) scintillators, the reference light measurement was performed with a polished glass cube with the same dimensions and geometry as the commercial scintillators inside an integrating sphere (Figure S4). For the Yb3+:CsPbCl3 scintillators, the reference light was scattered using CsPbCl3 powder in the same capillary configuration and with the same geometry as the Yb3+:CsPbCl3 scintillator. The number of emitted photons from each scintillator was calculated with calibration using reference light power measurements and PMT detector counts.
4.4.5. Quantifying Energy Deposited Using Commercial Scintillators
Complete X-ray absorption by each of our sample was confirmed using the methodology illustrated in Figure S5a,b. The sample being tested for total X-ray absorption was positioned right outside the integrating sphere port in the path of the X-ray beam. A commercial scintillator [CsI(Na)] was positioned inside the integrating sphere to detect any X-rays transmitted through the sample. Complete X-ray absorption by the tested scintillator was confirmed by the absence of CsI(Na) scintillated photons. The cubic CaF2(Eu) and CsI(TI) scintillators completely absorbed the X-ray beam, as did 12 Yb3+:CsPbCl3 powder filled capillaries, stacked into two rows (Figure S5c–e).
Finally, the energy deposited by the X-ray source was calculated using the CaF2(Eu) and CsI(TI) commercial scintillators. The values were corrected using the non-proportionalities of CaF2(Eu) and CsI(TI), 85 and 115%, respectively. The deposited energy was determined to be 2.08 × 107 ± 0.28 × 107 MeV CaF2(Eu) and 2.19 × 107 ± 0.24 × 107 MeV with CsI(TI).
4.4.6. Light Yield of Yb3+:CsPbCl3
The light yield of the 5% Yb3+:CsPbCl3 scintillator was calculated using an energy deposited value of 2.4 × 107 MeV, which is the highest value in the range of the measurements described in Materials and MethodsSection 4.4.5. We chose the highest value for the deposited energy to be conservative and obtain lower limit value of the light yield. Resolution of the radioluminescence emission spectra of Yb3+:CsPbCl3 was not affected by equipment parameters within the linear range of the detector. The light yield of the 5% Yb3+:CsPbCl3 was determined to be 102,000 photons/MeV with a standard deviation of 877.
4.5. Radiographic Imaging
Radiographic images were captured using a Basler ace acA1300-60gm-NIR GigE camera with a 4.5 mm C Series vis–NIR fixed focal length lens. The images were captured on the surface of the 25 mm 5% Yb3+:CsPbCl3 pressed powder pellet using the benchtop X-ray source at 40 kV and 300 μA and a 5.5 s exposure time.
Acknowledgments
This material is based upon work supported by the U.S. Department of Homeland Security under grant award number, 20CWDARI0003. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied of the U.S. Department of Homeland Security.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c01712.
XRD results, EDXS images, elemental composition, and details of experimental methods (PDF)
Author Contributions
§ K.A.D. and A.M.C. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Bailes M.; Berger B. K.; Brady P. R.; Branchesi M.; Danzmann K.; Evans M.; Holley-Bockelmann K.; Iyer B. R.; Kajita T.; Katsanevas S.; Kramer M.; Lazzarini A.; Lehner L.; Losurdo G.; Lück H.; McClelland D. E.; McLaughlin M. A.; Punturo M.; Ransom S.; Raychaudhury S.; Reitze D. H.; Ricci F.; Rowan S.; Saito Y.; Sanders G. H.; Sathyaprakash B. S.; Schutz B. F.; Sesana A.; Shinkai H.; Siemens X.; Shoemaker D. H.; Thorpe J.; van den Brand J. F. J.; Vitale S. Gravitational-wave physics and astronomy in the 2020s and 2030s. Nat. Rev. Phys. 2021, 3, 344–366. 10.1038/s42254-021-00303-8. [DOI] [Google Scholar]
- Glodo J.; Wang Y.; Shawgo R.; Brecher C.; Hawrami R. H.; Tower J.; Shah K. S. New Developments in Scintillators for Security Applications. Phys. Procedia 2017, 90, 285–290. 10.1016/j.phpro.2017.09.012. [DOI] [Google Scholar]
- Günther S.; Reinke P. Y. A.; Fernández-García Y.; Lieske J.; Lane T. J.; Ginn H. M.; Koua F. H. M.; Ehrt C.; Ewert W.; Oberthuer D.; Yefanov O.; Meier S.; Lorenzen K.; Krichel B.; Kopicki J.-D.; Gelisio L.; Brehm W.; Dunkel I.; Seychell B.; Gieseler H.; Norton-Baker B.; Escudero-Pérez B.; Domaracky M.; Saouane S.; Tolstikova A.; White T. A.; Hänle A.; Groessler M.; Fleckenstein H.; Trost F.; Galchenkova M.; Gevorkov Y.; Li C.; Awel S.; Peck A.; Barthelmess M.; Schlünzen F.; Lourdu Xavier P.; Werner N.; Andaleeb H.; Ullah N.; Falke S.; Srinivasan V.; França B. A.; Schwinzer M.; Brognaro H.; Rogers C.; Melo D.; Zaitseva-Doyle J. J.; Knoska J.; Peña-Murillo G. E.; Mashhour A. R.; Hennicke V.; Fischer P.; Hakanpää J.; Meyer J.; Gribbon P.; Ellinger B.; Kuzikov M.; Wolf M.; Beccari A. R.; Bourenkov G.; von Stetten D.; Pompidor G.; Bento I.; Panneerselvam S.; Karpics I.; Schneider T. R.; Garcia-Alai M. M.; Niebling S.; Günther C.; Schmidt C.; Schubert R.; Han H.; Boger J.; Monteiro D. C. F.; Zhang L.; Sun X.; Pletzer-Zelgert J.; Wollenhaupt J.; Feiler C. G.; Weiss M. S.; Schulz E.-C.; Mehrabi P.; Karničar K.; Usenik A.; Loboda J.; Tidow H.; Chari A.; Hilgenfeld R.; Uetrecht C.; Cox R.; Zaliani A.; Beck T.; Rarey M.; Günther S.; Turk D.; Hinrichs W.; Chapman H. N.; Pearson A. R.; Betzel C.; Meents A. X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science 2021, 372, 642–646. 10.1126/science.abf7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold D. C.; Siciliano E. R.; Schweppe J. E.; Ely J. H.; Milbrath B. D.; Kouzes R. T.; Geelhood B. D.. 2003 IEEE Nuclear Science Symposium. Conference Record (IEEE Cat. No. 03CH37515), 2003. [Google Scholar]
- Withers P. J.; Bouman C.; Carmignato S.; Cnudde V.; Grimaldi D.; Hagen C. K.; Maire E.; Manley M.; Du Plessis A.; Stock S. R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 1, 18. 10.1038/s43586-021-00015-4. [DOI] [Google Scholar]
- Dujardin C.; Auffray E.; Bourret-Courchesne E.; Dorenbos P.; Lecoq P.; Nikl M.; Vasil’ev A. N.; Yoshikawa A.; Zhu R.-Y. Needs, Trends, and Advances in Inorganic Scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. 10.1109/tns.2018.2840160. [DOI] [Google Scholar]
- Nikl M.; Yoshikawa A. Recent R&D Trends in Inorganic Single-Crystal Scintillator Materials for Radiation Detection. Adv. Opt. Mater. 2015, 3, 463–481. 10.1002/adom.201400571. [DOI] [Google Scholar]
- Bertrand G. H. V.; Hamel M.; Sguerra F. Current Status on Plastic Scintillators Modifications. Chem.—Eur. J. 2014, 20, 15660–15685. 10.1002/chem.201404093. [DOI] [PubMed] [Google Scholar]
- Hajagos T. J.; Liu C.; Cherepy N. J.; Pei Q. High-Z Sensitized Plastic Scintillators: A Review. Adv. Mater. 2018, 30, 1706956. 10.1002/adma.201706956. [DOI] [PubMed] [Google Scholar]
- Lance M. J.; Zaitseva N. P.; Payne S. A.; Kouzes R. T.; Myllenbeck N. R.; Janos A. Nature of Moisture-Induced fogging defects in scintillator plastic. Nucl. Instrum. Methods Phys. Res., Sect. A 2020, 954, 161806. 10.1016/j.nima.2019.01.033. [DOI] [Google Scholar]
- Maddalena F.; Tjahjana L.; Xie A.; Arramel; Zeng S.; Wang H.; Coquet P.; Drozdowski W.; Dujardin C.; Dang C.; Birowosuto M. Inorganic, Organic, and Perovskite Halides with Nanotechnology for High-Light Yield X- and γ-ray Scintillators. Crystals 2019, 9, 88. 10.3390/cryst9020088. [DOI] [Google Scholar]
- Zhou Y.; Chen J.; Bakr O. M.; Mohammed O. F. Metal Halide Perovskites for X-ray Imaging Scintillators and Detectors. ACS Energy Lett. 2021, 6, 739–768. 10.1021/acsenergylett.0c02430. [DOI] [Google Scholar]
- Wei H.; Fang Y.; Mulligan P.; Chuirazzi W.; Fang H.-H.; Wang C.; Ecker B. R.; Gao Y.; Loi M. A.; Cao L.; Huang J. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photonics 2016, 10, 333–339. 10.1038/nphoton.2016.41. [DOI] [Google Scholar]
- Xie A.; Nguyen T. H.; Hettiarachchi C.; Witkowski M. E.; Drozdowski W.; Birowosuto M. D.; Wang H.; Dang C. Thermal Quenching and Dose Studies of X-ray Luminescence in Single Crystals of Halide Perovskites. J. Phys. Chem. C 2018, 122, 16265–16273. 10.1021/acs.jpcc.8b03622. [DOI] [Google Scholar]
- Yakunin S.; Sytnyk M.; Kriegner D.; Shrestha S.; Richter M.; Matt G. J.; Azimi H.; Brabec C. J.; Stangl J.; Kovalenko M. V.; Heiss W. Detection of X-ray photons by solution-processed lead halide perovskites. Nat. Photonics 2015, 9, 444–449. 10.1038/nphoton.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinckemalie L.; Valli D.; Roeffaers M. B. J.; Hofkens J.; Pradhan B.; Debroye E. Challenges and Opportunities for CsPbBr3 Perovskites in Low- and High-Energy Radiation Detection. ACS Energy Lett. 2021, 6, 1290–1314. 10.1021/acsenergylett.1c00007. [DOI] [Google Scholar]
- Moseley O. D. I.; Doherty T. A. S.; Parmee R.; Anaya M.; Stranks S. D. Halide perovskites scintillators: unique promise and current limitations. J. Mater. Chem. C 2021, 9, 11588–11604. 10.1039/d1tc01595h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W.; Zheng Q.; Prezhdo O. V.; Zhao J.; Saidi W. A. Low-frequency lattice phonons in halide perovskites explain high defect tolerance toward electron-hole recombination. Sci. Adv. 2020, 6, eaaw7453 10.1126/sciadv.aaw7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. W.; Petrozza A. Defect Tolerance and Intolerance in Metal-Halide Perovskites. Adv. Energy Mater. 2020, 10, 2001959. 10.1002/aenm.202001959. [DOI] [Google Scholar]
- Stranks S. D.; Eperon G. E.; Grancini G.; Menelaou C.; Alcocer M. J. P.; Leijtens T.; Herz L. M.; Petrozza A.; Snaith H. J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- Mykhaylyk V. B.; Kraus H.; Kapustianyk V.; Kim H. J.; Mercere P.; Rudko M.; Da Silva P.; Antonyak O.; Dendebera M. Bright and fast scintillations of an inorganic halide perovskite CsPbBr3 crystal at cryogenic temperatures. Sci. Rep. 2020, 10, 8601. 10.1038/s41598-020-65672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Wu J.; Ou X.; Huang B.; Almutlaq J.; Zhumekenov A. A.; Guan X.; Han S.; Liang L.; Yi Z.; Li J.; Xie X.; Wang Y.; Li Y.; Fan D.; Teh D. B. L.; All A. H.; Mohammed O. F.; Bakr O. M.; Wu T.; Bettinelli M.; Yang H.; Huang W.; Liu X. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88–93. 10.1038/s41586-018-0451-1. [DOI] [PubMed] [Google Scholar]
- Li X.; Meng C.; Huang B.; Yang D.; Xu X.; Zeng H. All-Perovskite Integrated X-Ray Detector with Ultrahigh Sensitivity. Adv. Opt. Mater. 2020, 8, 2000273. 10.1002/adom.202000273. [DOI] [Google Scholar]
- Cohen T. A.; Milstein T. J.; Kroupa D. M.; MacKenzie J. D.; Luscombe C. K.; Gamelin D. R. Quantum-cutting Yb3+-doped perovskite nanocrystals for monolithic bilayer luminescent solar concentrators. J. Mater. Chem. A 2019, 7, 9279–9288. 10.1039/c9ta01261c. [DOI] [Google Scholar]
- Erickson C. S.; Crane M. J.; Milstein T. J.; Gamelin D. R. Photoluminescence Saturation in Quantum-Cutting Yb3+-Doped CsPb(Cl1-xBrx)3 Perovskite Nanocrystals: Implications for Solar Downconversion. J. Phys. Chem. C 2019, 123, 12474–12484. 10.1021/acs.jpcc.9b01296. [DOI] [Google Scholar]
- Ferro S. M.; Wobben M.; Ehrler B. Rare-earth quantum cutting in metal halide perovskites—a review. Mater. Horiz. 2021, 8, 1072–1083. 10.1039/d0mh01470b. [DOI] [PubMed] [Google Scholar]
- Kroupa D. M.; Roh J. Y.; Milstein T. J.; Creutz S. E.; Gamelin D. R. Quantum-Cutting Ytterbium-Doped CsPb(Cl1–xBrx)3 Perovskite Thin Films with Photoluminescence Quantum Yields over 190%. ACS Energy Lett. 2018, 3, 2390–2395. 10.1021/acsenergylett.8b01528. [DOI] [Google Scholar]
- Li X.; Duan S.; Liu H.; Chen G.; Luo Y.; Ågren H. Mechanism for the Extremely Efficient Sensitization of Yb3+Luminescence in CsPbCl3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 487–492. 10.1021/acs.jpclett.8b03406. [DOI] [PubMed] [Google Scholar]
- Milstein T. J.; Kroupa D. M.; Gamelin D. R. Picosecond Quantum Cutting Generates Photoluminescence Quantum Yields Over 100% in Ytterbium-Doped CsPbCl3 Nanocrystals. Nano Lett. 2018, 18, 3792–3799. 10.1021/acs.nanolett.8b01066. [DOI] [PubMed] [Google Scholar]
- Pan G.; Bai X.; Yang D.; Chen X.; Jing P.; Qu S.; Zhang L.; Zhou D.; Zhu J.; Xu W.; Dong B.; Song H. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. 10.1021/acs.nanolett.7b04575. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhang Y.; Zhang X.; Yin W.; Wang Y.; Wang H.; Lu M.; Li Z.; Gu Z.; Yu W. W. Yb3+ and Yb3+/Er3+ doping for near-infrared emission and improved stability of CsPbCl3 nanocrystals. J. Mater. Chem. C 2018, 6, 10101–10105. 10.1039/c8tc03957g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D.; Liu D.; Pan G.; Chen X.; Li D.; Xu W.; Bai X.; Song H. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Downconverter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149. 10.1002/adma.201704149. [DOI] [PubMed] [Google Scholar]
- Stefanski M.; Ptak M.; Sieradzki A.; Strek W. Optical characterization of Yb3+:CsPbCl3 perovskite powder. Chem. Eng. J. 2021, 408, 127347. 10.1016/j.cej.2020.127347. [DOI] [Google Scholar]
- Crane M. J.; Kroupa D. M.; Roh J. Y.; Anderson R. T.; Smith M. D.; Gamelin D. R. Single-Source Vapor Deposition of Quantum-Cutting Yb3+:CsPb(Cl1-xBrx)3 and Other Complex Metal-Halide Perovskites. ACS Appl. Energy Mater. 2019, 2, 4560–4565. 10.1021/acsaem.9b00910. [DOI] [Google Scholar]
- Lesage A.; van der Laan M.; Gomez L.; Gregorkiewicz T. Substitutional Doping of Yb3+ in CsPbBrxCl3-x Nanocrystals. J. Phys. Chem. C 2020, 124, 6413–6417. 10.1021/acs.jpcc.9b11393. [DOI] [Google Scholar]
- Ma J.-P.; Chen Y.-M.; Zhang L.-M.; Guo S.-Q.; Liu J.-D.; Li H.; Ye B.-J.; Li Z.-Y.; Zhou Y.; Zhang B.-B.; Bakr O. M.; Zhang J.-Y.; Sun H.-T. Insights into the local structure of dopants, doping efficiency, and luminescence properties of lanthanide-doped CsPbCl3 perovskite nanocrystals. J. Mater. Chem. C 2019, 7, 3037–3048. 10.1039/c9tc00237e. [DOI] [Google Scholar]
- Zhou L.; Liu T.; Zheng J.; Yu K.; Yang F.; Wang N.; Zuo Y.; Liu Z.; Xue C.; Li C.; Cheng B.; Wang Q. Dual-Emission and Two Charge-Transfer States in Ytterbium-doped Cesium Lead Halide Perovskite Solid Nanocrystals. J. Phys. Chem. C 2018, 122, 26825–26834. 10.1021/acs.jpcc.8b07906. [DOI] [Google Scholar]
- Lian L.; Zheng M.; Zhang W.; Yin L.; Du X.; Zhang P.; Zhang X.; Gao J.; Zhang D.; Gao L.; Niu G.; Song H.; Chen R.; Lan X.; Tang J.; Zhang J. Efficient and Reabsorption-Free Radioluminescence in Cs 3 Cu 2 I 5 Nanocrystals with Self-Trapped Excitons. Adv. Sci. 2020, 7, 2000195. 10.1002/advs.202000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.