Abstract
The World Health Organization has identified antimicrobial resistance as a public health emergency and developed a global priority pathogens list of antibiotic-resistant bacteria that can be summarized in the acronym ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacterales species), reminding us of their ability to escape the effect of antibacterial drugs. We previously tested new heteroaryl-ethylene compounds in order to define their spectrum of activity and antibacterial capability. Now, we focus our attention on PB4, a compound with promising MIC and MBC values in all conditions tested. In the present study, we evaluate the activity of PB4 on selected samples of ESKAPE isolates from nosocomial infections: 14 S. aureus, 6 E. faecalis, 7 E. faecium, 12 E. coli and 14 A. baumannii. Furthermore, an ATCC control strain was selected for all species tested. The MIC tests were performed according to the standard method. The PB4 MIC values were within very low ranges regardless of bacterial species and resistance profiles: from 0.12 to 2 mg/L for S. aureus, E. faecalis, E. faecium and A. baumannii. For E. coli, the MIC values obtained were slightly higher (4–64 mg/L) but still promising. The PB4 heteroaryl-ethylenic compound was able to counteract the bacterial growth of both high-priority Gram-positive and Gram-negative clinical strains. Our study contributes to the search for new molecules that can fight bacterial infections, in particular those caused by MDR bacteria in hospitals. In the future, it would be interesting to evaluate the activity of PB4 in animal models to test for its toxicity.
Keywords: ESKAPE, heteroaryl-ethylene, clinical strains, antimicrobial activity
1. Introduction
An important development of modern medicine is the efficient treatment of bacterial infections with antibiotics but, over the years, bacteria have found ways to resist the action of antimicrobial drugs [1,2].
Antimicrobial resistance (AMR) is a crucial global concern due to the increased and irresponsible use of antibiotics promoting the selection and spread of antibiotic-resistant pathogens responsible for difficult-to-treat infections, especially in Intensive Care Units (ICUs) [3].
Infections caused by multi-drug resistant (MDR) pathogens can lead to additional complications such as prolonged hospital stays and protracted treatment with last-line antibiotics, which increases the selection of resistant microbiota and results in higher healthcare costs. Bacterial resistance can be both intrinsic and acquired, the latter possibly being greatly enhanced by antimicrobial drugs exposure [4].
In 2017, the World Health Organization (WHO) created an acronym, ESKAPE, to represent the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacterales species [5]; to date, we are still facing a worldwide prevalence of MDR pathogens.
Gram-positive bacteria (GPB) have developed particular resistance profiles, such as Methicillin-Resistant Staphylococcus aureus (MRSA) [6] or Vancomycin-Resistant enterococci (VRE) [7] and are often related to nosocomial infections. Both these pathogens are responsible for increased morbidity and mortality due to the ineffectiveness of “last resort” antibiotics [8,9].
MRSA strains are characterized by a mobile genetic element carrying the methicillin-resistance gene (mecA), called Staphylococcal Chromosomal Cassette (SCCmec), usually type I, II or III [10,11]. Clinically, the acquisition of resistance to multiple antibiotic classes by S. aureus complicates treatment [12]. E. faecalis and E. faecium are responsible for the majority of human infections and related to the presence of bladder catheter, and/or neutropenia [13,14]. Nosocomial infections with enterococci are often associated with peculiar antimicrobial resistance profiles exhibiting a high level of intrinsic resistance to penicillins, cephalosporins, aminoglycosides and carbapenems [15]. As shown above, the WHO list mainly consists of Gram-negative bacteria (GNB). Enterobacterales resistance to third generation cephalosporins and carbapenems is increasing worldwide and is now above 10% and 2–7%, respectively [4]. This is due to the spread of Extended Spectrum Beta-Lactamases (ESBLs) (CTX-M, TEM, SHV) and carbapenemases (VIM, IMP, NDM, KPC and OXA) [16].
E. coli and K. pneumoniae are ESBL producers; compared with non-ESBLs, they express more TEM1, TEM2 and SHV capable of hydrolyzing narrow spectrum cephalosporins, carbapenems and monobactams. The administration of piperacillin/tazobactam, a treatment that alternates a β-lactam and β-lactamase inhibitor, has been considered as a carbapenem-sparing regimen for ESBL infections, although the global trend of AmpC β-lactamase-producing bacteria should be carefully monitored [17]. A. baumannii is one of the most successful pathogens in causing nosocomial infections, and carbapenem-resistant A. baumannii (CRAB) is often isolated in hospital settings. Current antimicrobials for CRAB (i.e., polymyxins, tigecycline, and sometimes aminoglycosides) are far from perfect therapeutic options due to their pharmacokinetic properties and increasing resistance rates [18,19].
In such a critical situation, the introduction in the clinical practice of new molecules able to counteract the continuous increase of multidrug-resistant pathogens is unquestionably a current priority [20]. In fact, while the constant introduction of new drugs has long made it possible to bypass the issue of antibiotic resistance, the current lack of molecules effective against MDR pathogens is becoming of growing concern [21].
In a previous study by Bongiorno et al. [22], the spectrum of ability of eight heteroaryl-ethylene compounds, called PBn, as antimicrobial agents capable of inhibiting the proliferation of a selected sample of Gram-positive and Gram-negative ATCC strains was tested by MIC (minimal inhibition concentration) and MBC (minimal bactericidal concentration) assays. Furthermore, the presence of an inoculum effect was assessed at scalar inoculum concentrations, and the cytotoxicity of the new molecules on colorectal adenocarcinoma cancer cells (CaCo2) was analyzed [22]. Our preliminary results were highly encouraging and pave the way for further investigations of heteroaryl-ethylenes as antimicrobial agents in the treatment of several Gram-positive and Gram-negative bacterial infections, especially those caused by MRSA, VRE, ESBL and A. baumannii.
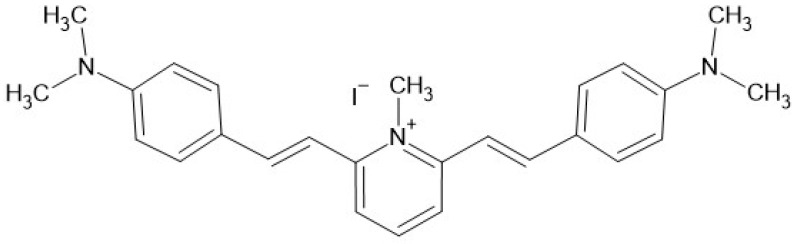
In the present study, we have focused our attention on PB4 (Figure 1) which emerged previously as the most promising PBn heteroaryl-ethylene compound with low MIC and MBC values in all conditions tested [22].
Figure 1.
Chemical structure of PB4, the figure was created using BioRender bioinformatic tool.
PB4 activity was evaluated on selected samples of MRSA, VRE, ESBL and CRAB freshly isolated from nosocomial infections. The workflow is shown in Figure 2.
Figure 2.
Experimental workflow, the figure was created using BioRender bioinformatic tool.
2. Results
In this study, we selected Gram-positive and Gram-negative clinical strains with different antibiotic resistance profiles, including 14 S. aureus, 6 E. faecalis, 7 E. faecium, 14 A. baumannii, and 12 E. coli, aiming to understand the potency and antimicrobial capacity of PB4. Furthermore, an ATCC control strain was selected for all species tested.
2.1. Gram-Positive Clinical Strains
PB4 was tested against both MRSA and MSSA isolates (11 and 3 strains, respectively). PB4 showed an antimicrobial activity of 0.25 mg/L MIC on S. aureus ATCC29213. The MIC values of PB4 were within a range of 0.12 mg/L to 0.5 mg/L for all S. aureus strains tested. PB4 was active on 3 MSSA and 8 MRSA at a concentration of 0.12 mg/L, proving effective at very low concentrations despite the greater aggressiveness of the control MRSA strains included in the study. Slightly higher concentrations of PB4 (0.25 mg/L and 0.5 mg/L) were sufficient to inhibit the growth of the three remaining clinical MRSA strains (see Table 1).
Table 1.
Activity of PB4 on S. aureus ATCC 29213 control strain and on 14 clinical S. aureus strains (3 MSSA and 11 MRSA).
| Antimicrobial Resistance Profile | PB4 MIC | ||
|---|---|---|---|
| Strain | mg/L | µmol/L | |
| S. aureus ATCC 29213 | 0.25 | 0.49 | |
| MSSA | 1-CT | 0.12 | 0.234 |
| 2-CT | 0.12 | 0.234 | |
| 3-CT | 0.12 | 0.234 | |
| MRSA | 4-CT | 0.12 | 0.234 |
| 5-CT | 0.12 | 0.234 | |
| 6-CT | 0.12 | 0.234 | |
| 7-CT | 0.12 | 0.234 | |
| 8-CT | 0.12 | 0.234 | |
| 9-CT | 0.12 | 0.234 | |
| 10-CT | 0.12 | 0.234 | |
| 11-CT | 0.12 | 0.234 | |
| 12-CT | 0.25 | 0.49 | |
| 13-CT | 0.5 | 0.977 | |
| 14-CT | 0.5 | 0.977 | |
Enterococcus spp. clinical strains were selected for different antibiotic resistance profiles, including 6 E. faecalis (VRE, MDR, Not MDR) and 7 E. faecium (VRE, Linezolid Resistant (LinR), MDR, Not MDR). E. faecalis ATCC 29212 and ATCC 51299, with a PB4 MIC value of 0.5 mg/L, were included. The MIC values for PB4 were in a range between 0.12 mg/L and 2 mg/L when tested on Enterococci.
In particular, PB4, independently of their antibiotic profile, showed a MIC range of 0.25 to 0.5 mg/L for E. faecalis strains, and of 0.12 to 2 mg/L for E. faecium. The MIC values for PB4 in the Enterococcus spp. sample are reported in Table 2.
Table 2.
Activity of PB4 on E. faecalis ATCC 29212 and E. faecalis ATCC 51299 control strains, on six E. faecalis and on seven E. faecium clinical strains (VRE, LinR, MDR, not MDR).
| Antimicrobial Resistance Profile |
PB4 MIC | |||
|---|---|---|---|---|
| Strain | mg/L | µmol/L | ||
| E. faecalis ATCC 29212 | 0.5 | 0.977 | ||
| E. faecalis ATCC 51299 | 0.5 | 0.977 | ||
| E. faecalis | VRE | 15-CT | 0.25 | 0.488 |
| 16-CT | 0.25 | 0.488 | ||
| 17-CT | 0.5 | 0.977 | ||
| MDR | 18-CT | 0.25 | 0.488 | |
| NOT MDR | 19-CT | 0.25 | 0.488 | |
| 20-CT | 0.5 | 0.977 | ||
| E. faecium | VRE | 21-CT | 0.25 | 0.488 |
| 22-CT | 1 | 1.95 | ||
| Lin R | 23-CT | 0.5 | 0.977 | |
| 24-CT | 1 | 1.95 | ||
| MDR | 25-CT | 0.12 | 0.234 | |
| 26-CT | 1 | 1.95 | ||
| NOT MDR | 27-CT | 2 | 3.91 | |
2.2. Gram-Negative Clinical Strains
Furthermore, 12 clinical strains were selected for their different antibiotic resistance profiles, in particular 3 ESBL and 9 non-ESBL E. coli.
E. coli ATCC 25922 was chosen as a control strain in order to test the activity of PB4 on this species, and the compound showed a MIC value of 1 mg/L. The MIC values for PB4 were in a range between 4 mg/L and 64 mg/L. The MIC values for PB4 were heterogeneous on E. coli, showing different behaviors for the three different ESBL clinical strains. Regarding non-ESBL E. coli, PB4 MIC values of 4 mg/L were obtained for five clinical strains, 8 mg/L for one clinical strain, and 32 mg/L for the two remaining clinical strains (see Table 3).
Table 3.
Activity of PB4 on E. coli ATCC 25922 control strain and on 12 E. coli clinical strains (ESBL and susceptible).
| Antimicrobial Resistance Profile |
PB4 MIC | ||
|---|---|---|---|
| Strain | mg/L | µmol/L | |
| E. coli ATCC 25922 | 1 | 1.95 | |
| ESBL | 28-CT | 4 | 7.82 |
| 29-CT | 16 | 31.28 | |
| 30-CT | 64 | 125.12 | |
| Susceptible | 31-CT | 4 | 7.82 |
| 32-CT | 4 | 7.82 | |
| 33-CT | 4 | 7.82 | |
| 34-CT | 4 | 7.82 | |
| 35-CT | 4 | 7.82 | |
| 36-CT | 8 | 15.64 | |
| 37-CT | 32 | 62.56 | |
| 38-CT | 32 | 62.56 | |
| 39-CT | 64 | 125.12 | |
Regarding clinical strains, we selected 14 clinical MDR strains based on their carbapenem-resistance profiles, particularly Carbapenem Resistant A. baumannii (CRAB). We obtained PB4 MIC values in a range between <0.12 mg/L and 1 mg/L (see Table 4).
Table 4.
Activity of PB4 on A. baumannii ATCC 17978 control strain and 14 CRAB clinical strains.
| Antimicrobial Resistance Profile |
PB4 MIC | ||
|---|---|---|---|
| Strain | mg/L | µmol/L | |
| A. baumannii ATCC 17978 | 0.5 | 0.97 | |
| CRAB | 40-CT | <0.12 | <0.23 |
| 41-CT | <0.12 | <0.23 | |
| 42-CT | <0.12 | <0.23 | |
| 43-CT | <0.12 | <0.23 | |
| 44-CT | 0.25 | 0.488 | |
| 45-CT | 0.25 | 0.488 | |
| 46-CT | 0.25 | 0.488 | |
| 47-CT | 0.25 | 0.488 | |
| 48-CT | 0.25 | 0.488 | |
| 49-CT | 0.25 | 0.488 | |
| 50-CT | 0.5 | 0.977 | |
| 51-CT | 0.5 | 0.977 | |
| 52-CT | 0.5 | 0.977 | |
| 53-CT | 1 | 1.955 | |
A previous observation showed that PB4 may be active on A. baumannii ATCC 17978 control strain, with an antimicrobial activity of 0.5 mg/L MIC.
A summary table (see Table 5) of the results obtained clarifies the activity of PB4. The PB4 MIC values obtained on the control strains are reported on the far left of the table as a reference.
Table 5.
The table reports the MIC values of PB4 on the control strains used as references. The number of clinical strains tested for each species, the PB4 MIC ranges obtained and the distribution of the results are shown alongside.
| PB4 MIC Value (mg/L) | |||||||||||||||
| Control Strain | MIC Value (mg/L) | Species | n. | Range | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | 0.5 | 0.25 | 0.12 |
| S. aureus ATCC 29213 | 0.25 | S. aureus | 14 | 0.12–0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 11 |
| E. faecalis ATCC 29212 | 0.5 | E. faecalis | 6 | 0.25–0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 |
| E. faecalis ATCC 51299 VRE | 0.5 | E. faecium | 7 | 0.12–2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 1 |
| E. coli ATCC 25922 | 1 | E. coli | 12 | 4–64 | 0 | 2 | 2 | 1 | 1 | 6 | 0 | 0 | 0 | 0 | 0 |
| A. baumannii ATCC 17978 | 0.5 | A. baumannii | 14 | ≤0.12–1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 6 | 4 |
| PB4 MIC Value (µmol/L) | |||||||||||||||
| Control Strain | MIC Value (mg/L) | Species | n. | Range | 250.2 | 125.1 | 62.5 | 31.2 | 15.6 | 7.8 | 3.9 | 1.9 | 0.977 | 0.488 | 0.234 |
| S. aureus ATCC 29213 | 0.488 | S. aureus | 14 | 0.234–0.977 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 11 |
| E. faecalis ATCC 29212 | 0.977 | E. faecalis | 6 | 0.488–0.977 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 0 |
| E. faecalis ATCC 51299 VRE | 0.977 | E. faecium | 7 | 0.234–3.91 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 1 | 1 |
| E. coli ATCC 25922 | 1.9 | E. coli | 12 | 7.82–125.12 | 0 | 2 | 2 | 1 | 1 | 6 | 0 | 0 | 0 | 0 | 0 |
| A. baumannii ATCC 17978 | 0.977 | A. baumannii | 14 | ≤0.234–1.955 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 6 | 4 |
In general, we have seen that PB4 is effective at very low ranges, and this is true for all Gram-positive and Gram-negative clinical strains tested regardless of the species and their particular antibiotic resistance profile.
On S. aureus clinical strains, PB4 showed an inhibitory efficacy in a range between 0.12 and 0.5 mg/L: 11/14 clinical strains exhibited the lowest recorded MIC values of 0.12 mg/L; 1/14 had values of 0.25 mg/L; and 2/14 had values of 0.5 mg/L. A PB4 MIC value of 0.25 mg/L was measured for S. aureus ATCC 29213.
On E. faecalis clinical strains, PB4 was effective in a range between 0.25 and 0.5 mg/L: 4/6 clinical strains had the lowest recorded MIC value of 0.25 mg/L; and 2/6 strains had values of 0.5 mg/L.
On E. faecium clinical strains, PB4 activity was detected in a range between 0.12 and 2 mg/L: 3/7 clinical strains had low MIC values in a range of 0.12 to 0.25–0.5 mg/L; 3/7 showed an intermediate MIC value of 1 mg/L, and the last clinical strain exhibited the highest MIC value of 2 mg/L. For E. faecalis ATCC 29212 and E. faecalis ATCC 51299, a PB4 MIC value of 0.5 mg/L was measured.
On E. coli clinical strains, PB4 efficacy was assessed in a range between 4 and 64 mg/L: 6/12 clinical strains exhibited the lowest MIC value of 4 mg/L; 1/12 had a value of 8 mg/L; 1/12 had a value of 16 mg/L; 2/12 had a value of 32 mg/L; and only 2/12 strains had the highest MIC value of 64 mg/L. For E. coli ATCC 25922, a PB4 MIC value of 1 mg/L was measured.
On A. baumannii clinical strains, PB4 inhibition activity ranged between ≤0.12 and 1 mg/L: 4/14 clinical strains had the lowest MIC value of less than or equal to 0.12 mg/L; 6/14 had values of 0.25 mg/L; 3/14 had values of 0.5 mg/L; and 1/14 had a value of 1 mg/L. For A. baumannii ATCC 17978, a PB4 MIC value of 0.5 mg/L was measured.
3. Discussion
In 2017, the World Health Organization (WHO) published a list of antibiotic-resistant priority pathogens that can be summarized in the acronym ESKAPE (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter spp.), reminding us of their ability to escape the effect of antibacterial drugs, which, in turn, accounts for their ability to cause serious, difficult-to-control Gram-positive and Gram-negative bacterial infections, especially in the hospital setting [23].
Thus, we focused our attention on S. aureus (MSSA and MRSA), VR and LinR E. faecalis and E. faecium, ESBL E. coli, and carbapenem resistant A. baumannii (CRAB). For this purpose, in a previous study we had tested newly synthesized molecules derived from the condensation of heterocyclic aromatic aldehydes: the PB compounds. These eight molecules were tested on Gram-positive and Gram-negative ATCC control strains to characterize their antimicrobial activity and determine their MIC and MCB values [22].
Based on previous results, PB4 was selected as the compound candidate to be tested on MSSA and MRSA, VRE and LinR Enterococci, ESBL E. coli, and CRAB.
Surprisingly, despite the antimicrobial resistance profile which characterizes every single clinical strain, PB4 showed a good level of activity. Indeed, the MIC values of PB4 were within very low ranges regardless of bacterial species and resistance profiles: from 0.12 to 0.5 mg/L for both MSSA and MRSA; from 0.25 to 0.5 mg/L for E. faecalis, and from 0.12 to 2 mg/L for E. faecium.
Only for E. coli, including the ATCC strain, which showed a MIC value of 1 mg/L, the MIC values obtained were from 4 mg/L to 64 mg/L, slightly higher than for other microorganisms tested but still promising. Remarkably, for A. baumannii clinical strains, the inhibitory activity of PB4 ranged from ≤0.12 mg/L to 1 mg/L, despite their MDR, especially, their resistance to carbapenems (CRAB).
As discussed above, PB4 acts on all clinical strains, both Gram-positive and Gram-negative, in very low ranges. Furthermore, values were comparable for organisms belonging to the same species but with different antibiotic resistance profiles. For E. coli, we observed a slightly higher PB4 MIC range but there was no difference between the ESBL and Susceptible strains that were analyzed. Thus, the different PB4 action is due to the unique characteristics of each strain.
It is very important to highlight that several ranges of action in microorganisms belonging to different species does not indicate a poor molecule functionality. The MIC value and its action range are usually species specific [24]. To date, the PB4 target is still unknown. Probably, the molecule does not act on a site characteristic to Gram-positive or Gram-negative but on their common structure. We intend to study the toxicity, pharmacokinetics and pharmacodynamics of PB4 to confirm this surprising antimicrobial activity acting on difficult-to-treat infections.
In this study, we observed that PB4, a heteroaryl-ethylenic compound, can counteract the bacterial growth of both high-priority Gram-positive and Gram-negative clinical strains. Our aim was to contribute to the search for new molecules that can fight bacterial infections, in particular those caused by MDR bacteria in hospitals, where serious infections, combined with impaired pathophysiology and immunity of patients, cannot but worsen their clinical picture and outcome [25].
In the future, in order to better investigate the good antimicrobial capability found in bacterial strains through in vitro studies and in prokaryotic cells, it would be interesting to test PB4 for its toxicity and antimicrobial activity using in vivo models.
This is a crucial aspect, because the molecule could fail in vivo depending on its toxicity or rate of elimination by the organism to which it is administered.
4. Materials and Methods
4.1. Characteristics of Molecule Tested in the Study
PB4 (Pyridinium, 2,6-bis[(1E)-2-[4-(dimethylamino)phenyl]ethenyl]-1-methyl-, iodide CAS: 793694-41-4) was synthesized as previously reported [26] and characterized by 1H NMR, 13C NMR, and MS spectrometry. The purity of PB4 was confirmed through NMR and TGA analysis; the batch employed for these experiments reached a purity degree of 96%.
4.2. Microorganisms and Growth Conditions
Control strains of Gram-positive and Gram-negative bacteria, E. faecalis ATCC 29212 VSE, E. faecalis ATCC 51299 VRE, S. aureus ATCC 29213, E. coli ATCC 25922, A. baumannii ATCC 17978, were obtained from the American Type Culture Collection (Manassas, VA, USA).
In this study, fourteen S. aureus, six E. faecalis, seven E. faecium and fourteen A. baumannii clinical strains were selected for their resistance profile among the bacterial culture collections of the Medical Molecular Microbiology and Antibiotic Resistance Laboratory (MMARL) of the University of Catania.
The identification of Gram-positive strains was previously conducted using API system (Biomérieux, Craponne , France) and confirmed by sequencing the 16S gene [27,28,29].
The identification of A. baumannii strains was confirmed by Matrix-Assisted Laser Desorption Ionization–Time of Flight (MALDI87 TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA) [30].
Twelve E. coli clinical strains were provided by the Laboratory of Microbiology and Clinical Virology, Gaspare Rodolico Hospital of Catania, Vittorio Emanuele University Hospital.
The identification of the strains was performed by Vitek2 GN (Biomérieux, France) systems.
S. aureus was grown on Mannitol Salt Agar (CM0085B, Thermo ScientificTM OxoidTM, Basingstoke, UK), E. faecalis on Bile Aesculin Agar (CM0888, Thermo ScientificTM OxoidTM, Basingstoke, UK), A. baumannii and E. coli on MacConkey Agar (CM0007, Thermo ScientificTM OxoidTM, Basingstoke, UK) at 37 °C for 24 h.
4.3. Minimum Inhibitory Concentration (MIC)
Microtiter plate assays were performed to determine the minimum inhibitory concentration of PB4 according to the standard method [31,32,33,34], with some modifications.
The PB4 compound was at a concentration of 8000 mg/L in 100% DMSO (85190, Thermo ScientificTM OxoidTM, Basingstoke, UK). Further dilutions of the substance were prepared using Mueller Hinton II Broth [Cation-Adjusted] (CA-MHB) (212322, BD BBLTM, Franklin Lakes, NJ, USA).
The concentration range tested was between 128 mg/L and 0.25 mg/L. A 100 μL aliquot of PB4 was inoculated in 96-well microplates containing sterile CA-MHB, and serial dilutions were performed.
Subsequently, starting from different 0.5 McFarland (108 CFU/mL) bacterial suspensions, scalar dilutions of the inoculum were carried out and a concentration of 105 CFU/mL was inoculated in 96-well microplates. The microplates were incubated for 18 ± 2 h at 37 °C. MIC values were determined at the lowest concentrations of the antimicrobial agent inhibiting bacterial growth. The MIC values were expressed in mg/L. The tests were repeated in duplicate by two different researchers.
Acknowledgments
We would like to thank the BRIT laboratory at the University of Catania (Italy) for the valuable technical assistance and the use of its facility. The authors wish to thank PharmaTranslated (http://www.pharmatranslated.com/, accessed data 10 May 2022) and, in particular, Silvia Montanari.
Author Contributions
Conceptualization, investigation, data curation, formal analysis and writing—original draft preparation D.A.B. and A.M. (Alessia Mirabile); formal analysis, investigation, resources and data curation C.B. (Carmelo Bonomo) and P.G.B.; validation and review and editing S.S. (Stefano Sracqudanio), A.M. (Andrea Marino). and F.C.; data curation, validation, visualization C.B. (Carmela Bonaccorso), resources, supervision, funding acquisition, writing—review and the original draft, C.G.F. and S.S. (Stefania Stefani); methodology, investigation, supervision and writing—review and editing, N.M. and D.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The manuscript was partially supported by a research project grant (number PRIN2020) from the Ministry of Research (MIUR) Italy.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mühlberg E., Umstätter F., Kleist C., Domhan C., Mier W., Uhl P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2020;66:11–16. doi: 10.1139/cjm-2019-0309. [DOI] [PubMed] [Google Scholar]
- 2.Aminov R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 4.Martinez J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014;11:33–39. doi: 10.1016/j.ddtec.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Karaman R., Jubeh B., Breijyeh Z. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules. 2020;25:2888. doi: 10.3390/molecules25122888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goll C., Balmer P., Schwab F., Rüden H., Eckmanns T. Different trends of MRSA and VRE in a German hospital, 1999–2005. Infection. 2007;35:245–249. doi: 10.1007/s15010-007-6234-5. [DOI] [PubMed] [Google Scholar]
- 7.Huycke M.M., Sahm D.F., Gilmore M.S. Multiple-drug resistant enterococci: The nature of the problem and an agenda for the future. Emerg. Infect. Dis. 1998;4:239–249. doi: 10.3201/eid0402.980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z., Cao B., Liu Y., Gu L., Wang C. Investigation of the prevalence of patients co-colonized or infected with methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in China: A hospital-based study. Chin. Med. J. 2009;122:1283–1288. [PubMed] [Google Scholar]
- 9.Rincón S., Panesso D., Díaz L., Carvajal L.P., Reyes J., Munita J.M., Arias C.A. Resistance to ‘last resort’ antibiotics in Gram-positive cocci: The post-vancomycin era. Biomed. Rev. Inst. Nac. Salud. 2014;34((Suppl. S1)):191–208. doi: 10.1590/S0120-41572014000500022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deurenberg R.H., Vink C., Kalenic S., Friedrich A.W., Bruggeman C.A., Stobberingh E.E. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2007;13:222–235. doi: 10.1111/j.1469-0691.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 11.Schulte R.H., Munson E. Staphylococcus aureus Resistance Patterns in Wisconsin: 2018 Surveillance of Wisconsin Organisms for Trends in Antimicrobial Resistance and Epidemiology (SWOTARE) Program Report. Clin. Med. Res. 2019;17:72–81. doi: 10.3121/cmr.2019.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby W.M. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 13.Werner G., Neumann B., Weber R.E., Kresken M., Wendt C., Bender J.K. Thirty years of VRE in Germany—‘Expect the unexpected’: The view from the National Reference Centre for Staphylococci and Enterococci. Drug Resist. Updat. 2020;53:100732. doi: 10.1016/j.drup.2020.100732. [DOI] [PubMed] [Google Scholar]
- 14.Arias C.A., Contreras G.A., Murray B.E. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 2010;16:555–562. doi: 10.1111/j.1469-0691.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller W.R., Murray B.E., Rice L.B., Arias C.A. Resistance in Vancomycin-Resistant Enterococci. Infect. Dis. Clin. N. Am. 2020;34:751–771. doi: 10.1016/j.idc.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moxon C.A., Paulus S. Beta-lactamases in Enterobacteriaceae infections in children. J. Infect. 2016;72:S41–S49. doi: 10.1016/j.jinf.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Chong Y., Shimoda S., Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018;61:185–188. doi: 10.1016/j.meegid.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Piperaki E.-T., Tzouvelekis L.S., Miriagou V., Daikos G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019;25:951–957. doi: 10.1016/j.cmi.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Isler B., Doi Y., Bonomo R.A., Paterson D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2019;63:e01110-18. doi: 10.1128/AAC.01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terreni M., Taccani M., Pregnolato M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules. 2021;26:2671. doi: 10.3390/molecules26092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafiq N., Pandey A.K., Malhotra S., Holmes A., Mendelson M., Malpani R., Balasegaram M., Charani E. Shortage of essential antimicrobials: A major challenge to global health security. BMJ Glob. Health. 2021 doi: 10.1136/bmjgh-2021-006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bongiorno D., Musso N., Bonacci P.G., Bivona D.A., Massimino M., Stracquadanio S., Bonaccorso C., Fortuna C.G., Stefani S. Heteroaryl-Ethylenes as New Lead Compounds in the Fight against High Priority Bacterial Strains. Antibiotics. 2021;10:1034. doi: 10.3390/antibiotics10091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendleton J.N., Gorman S.P., Gilmore B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti.-Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 24.EuropeanCommittee on Antimicrobial Suceptibility Testing. [(accessed on 30. May 2022)]. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/
- 25.Haney E.F., Mansour S.C., Hancock R.E.W. Antimicrobial Peptides: An Introduction. Methods Mol. Biol. 2017;1548:3–22. doi: 10.1007/978-1-4939-6737-7_1. [DOI] [PubMed] [Google Scholar]
- 26.Fortuna C.G., Barresi V., Bonaccorso C., Consiglio G., Failla S., Trovato-Salinaro A., Musumarra G. Design, synthesis and in vitro antitumour activity of new heteroaryl ethylenes. Eur. J. Med. Chem. 2012;47:221–227. doi: 10.1016/j.ejmech.2011.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Campanile F., Bongiorno D., Perez M., Mongelli G., Sessa L., Benvenuto S., Gona F., Varaldo P.E., Stefani S., Floriana Gona b AMCLI—S. aureus Survey Participants Epidemiology of Staphylococcus aureus in Italy: First nationwide survey, 2012. J. Glob. Antimicrob. Resist. 2015;3:247–254. doi: 10.1016/j.jgar.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Lazzaro L.M., Cassisi M., Stefani S., Campanile F. Impact of PBP4 Alterations on β-Lactam Resistance and Ceftobiprole Non-Susceptibility Among Enterococcus faecalis Clinical Isolates. Front. Cell Infect. Microbiol. 2021;11:816657. doi: 10.3389/fcimb.2021.816657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarti M., Campanile F., Sabia C., Santagati M., Gargiulo R., Stefani S. Polyclonal Diffusion of Beta-Lactamase-Producing Enterococcus faecium. J. Clin. Microbiol. 2012;50:169–172. doi: 10.1128/JCM.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruker Maldi-TOF/TOF Solution. [(accessed on 30 May 2022)]. Available online: https://www.bruker.com/it/products-and-solutions/mass-spectrometry/maldi-tof.html.
- 31.Weinstein M.P., Lewis J.S. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: Background, Organization, Functions and Processes. J. Clin. Microbiol. 2020;58:e01864-19. doi: 10.1128/JCM.01864-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CLSI . CLSI Supplement M100. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. [Google Scholar]
- 33.European Commettee on Antimicrobial Susceptibility Testing EUCAST Reading Guide for Broth Microdilution. 2022. [(accessed on 30 May 2022)]. Available online: https://www.eucast.org/
- 34.GOLDBIO: Broth Microdilution and Disk Diffusion. 2018. [(accessed on 30 May 2022)]. Available online: https://www.goldbio.com/documents/2531/Broth%20Microdilution%20and%20Disk%20Diffusion.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.