Abstract
The objectives of this study were to investigate the incidence of candidemia, as well as the factors associated with Candida species distribution and fluconazole resistance, among patients admitted to the intensive care unit (ICU) during the COVID-19 pandemic, as compared to two pre-pandemic periods. All patients admitted to the ICU due to COVID-19 from March 2020 to October 2021, as well as during two pre-pandemic periods (2005–2008 and 2012–2015), who developed candidemia, were included. During the COVID-19 study period, the incidence of candidemia was 10.2%, significantly higher compared with 3.2% and 4.2% in the two pre-pandemic periods, respectively. The proportion of non-albicans Candida species increased (from 60.6% to 62.3% and 75.8%, respectively), with a predominance of C. parapsilosis. A marked increase in fluconazole resistance (from 31% to 37.7% and 48.4%, respectively) was also observed. Regarding the total patient population with candidemia (n = 205), fluconazole resistance was independently associated with ICU length of stay (LOS) before candidemia (OR 1.03; CI: 1.01–1.06, p = 0.003), whereas the presence of shock at candidemia onset was associated with C. albicans (OR 6.89; CI: 2.2–25, p = 0.001), and with fluconazole-susceptible species (OR 0.23; CI: 0.07–0.64, p = 0.006). In conclusion, substantial increases in the incidence of candidemia, in non-albicans Candida species, and in fluconazole resistance were found in patients admitted to the ICU due to COVID-19, compared to pre-pandemic periods. At candidemia onset, prolonged ICU LOS was associated with fluconazole-resistant and the presence of shock with fluconazole-susceptible species.
Keywords: candidemia, ICU, incidence, epidemiology, Candida species, non-albicans Candida species, fluconazole resistance, COVID-19, critically ill
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), being declared a pandemic by the World Health Organization on 11 March 2020 [1], spread rapidly around the world, causing a global health emergency [2]. Severe forms are complicated by hypoxemic acute respiratory failure requiring intensive care unit (ICU) admission [3,4]. In these patients, secondary infections, both bacterial and fungal, have been increasingly reported [5,6,7,8,9], resulting in the widespread use of antibiotics for the empirical treatment of suspected as well as of microbiologically confirmed infections, hence contributing to an increase in multidrug-resistant bacteria and fungi and increased costs of care [10].
Regarding fungal infections, a growing number of studies have mainly focused on Aspergillus superinfections in mechanically ventilated patients admitted to the ICU due to COVID-19, whereas bloodstream infections due to Candida species have been less studied thus far [11,12,13,14,15,16]. On the other hand, candidemia’s incidence, often cited as the fourth most common bloodstream infection in the ICU [17], is increasing, particularly in ICU patients [18,19]. In addition, the epidemiology of candidemia may change over time and can vary significantly across different geographic regions and hospitals. Furthermore, emerging azole resistance displays major challenges for therapeutic strategies [20,21]. Information on the epidemiology of candidemia in the ICU remains limited in the context of the ongoing COVID-19 pandemic. The objectives of the present study were to investigate the incidence of candidemia, as well as the factors associated with Candida species distribution and fluconazole resistance, among patients admitted to the intensive care unit (ICU) during the COVID-19 pandemic, as compared to two pre-pandemic periods.
2. Methods
2.1. Study Setting and Design
All patients with SARS-CoV-2 infection confirmed by reverse transcription polymerase chain reaction on nasopharyngeal swabs, and acute respiratory failure, admitted to the COVID-19 ICUs of ‘Evangelismos’ Hospital, a tertiary-care medical center, from March 2020 to October 2021, who developed candidemia during their ICU stay, constituted the COVID-19 candidemia cohort. Candidemia cases were identified through the electronic system. Approval for the use of the de-identified data was obtained from the ethics committee of the hospital (approval number 116/03-2021). Demographics, dates of hospital and ICU admissions, date of candidemia, detected Candida species, admission diagnosis classified as medical or surgical, main co-morbidities including diabetes mellitus and current malignancy, illness severity, length of stay (LOS) in ICU and ICU clinical outcome were recorded. The severity of acute illness was evaluated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score [22] on ICU admission. The severity of organ dysfunction was assessed by the Sequential Organ Failure Assessment (SOFA) score [23], calculated on the first day of ICU admission and, additionally, on the day of candidemia. The difference (Delta) in the SOFA score, defined as the SOFA score on the ICU day that the positive blood culture for Candida species was collected minus the SOFA score on ICU admission, was also calculated. For the management and therapy of the ICU patients with COVID-19, international recommendations were followed [24]. For the treatment of candidemia, recommendations for application in non-immunocompromised critically ill patients were followed [25]. Accordingly, after candidemia diagnosis, antifungal treatment, mainly an echinocandin, was given, with the exception of three patients who died because of the severity of their acute illness before blood culture results were available. After the susceptibility results became available, the initial treatment could be modified by the attending intensivists.
Characteristics of COVID-19 patients who developed candidemia were compared with those of two historical candidemia cohorts from our ICU before the COVID-19 pandemic—in particular, an earlier cohort including all ICU patients who developed candidemia from 2005 to 2008 (n = 66) and a later one comprising all ICU patients who developed candidemia from 2012 to 2015 (n = 77).
2.2. Definitions
ICU-acquired candidemia was defined as the presence of at least one positive blood culture for any Candida species in the blood specimen collected more than 48 h after ICU admission. Blood cultures were performed in the presence of signs and symptoms of sepsis or when infection was suspected on clinical rounds. The onset of candidemia was defined as the specimen collection date for the positive Candida blood culture.
2.3. Species Identification and Antifungal Susceptibility Testing
The BD Bactec (Becton Dickinson, Sparks, MD, USA) automated blood culture system was used for monitoring blood culture bottles. Fungal isolates were identified at species level by using the VitekMS (BioMeriéux, Marcy l’Etoile, France) device and MALDI-TOF MS method. Antifungal susceptibility was evaluated with the Vitek2 (BioMeriéux, Marcy l’Etoile, France) automated system. The phenotypic susceptibility profile for each fungal isolate was interpreted according to the EUCAST standard (European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs for antifungal agents, Version 10.0, valid from 4 February 2020). In addition, for the period of March 2020 to October 2021, directly from the signal-positive blood culture vials with yeasts in Gram staining, a multiplex syndromic approach was applied, namely the FilmArray Blood Culture Identification 2 panel (BCID2 assay, BioMeriéux, Marcy l’Etoile, France), for the early detection of the emerging species Candida auris.
2.4. Statistical Analysis
Statistical data analysis was performed using the R software, Version 4.1.1 (R Foundation for Statistics, Vienna, Austria). Data are described as mean ± SD or median and interquartile range (IQR) in case of variables with non-normal distribution, and as number and percentage (%) in case of categorical variables. In order to compare the distributions of numerical variables between two groups of patients, we used the two-sample t-test, or, alternatively, the Mann–Whitney U test in case of variables with non-normal distribution, whereas associations between qualitative factors were appropriately investigated via the chi-squared (X2) statistic or Fisher’s exact test. Incidence between the various cohorts was also compared via the statistical test of proportions. Univariate and multivariate binary logistic regression models were built for the determination of risk factors for bloodstream infection with albicans versus non-albicans Candida species and for potentially fluconazole-resistant species, reporting odds ratios (OR) and corresponding 95% confidence intervals (CI) in relation to the model covariates. The level of statistical significance was set at 0.05.
3. Results
3.1. COVID-19 Candidemia Cohort
In the 18-month study period during the pandemic, among 600 patients who were admitted to the ICU due to COVID-19, 62 patients developed candidemia during the ICU stay, accounting for an incidence of 10.2%. The median [IQR] age of the patients with candidemia was 69 [15.8] years, and 72.4% were males. Admission APACHE II and SOFA scores were 15 [7] and 8 [3], respectively.
The median [IQR] time between hospital and ICU admission and positive Candida culture was 28.5 [19.5] days and 22 [18.2] days, respectively. Non-albicans Candida species predominated (in 47 out of 62 patients, 77%). Among the non-albicans species, the most frequently isolated was C. parapsilosis (31 patients, 50%), followed by C. auris (9 patients, 14%) and C. glabrata (6 patients, 9.7%).
3.2. Comparison between COVID-19 Candidemia Cohort and the Pre-COVID-19 Cohorts
Baseline characteristics of the ICU patients with candidemia development, stratified according to the time period of ICU admission, are presented in Table 1. Compared with patients without COVID-19, patients with COVID-19 were older and had lower illness severity as expressed by APACHE II and SOFA scores on ICU admission. However, on candidemia day, they were more likely to present circulatory shock and they had a higher SOFA score. As a result, the Delta SOFA score was significantly higher in COVID-19 patients than in the non-COVID-19 ones (3 (6) versus 0 (4) and −1 (3.5), respectively, p < 0.001). As expected, patients with COVID-19 were less likely to have a surgical diagnosis on ICU admission. Patients with and without COVID-19 had similar hospital and ICU LOS before candidemia development. While the incidence of candidemia did not change significantly between 2005–2008 and 2012–2015, a significant increase was observed in the COVID-19 cohort compared to the two pre-pandemic cohorts (10.2% (62/600) versus 3.8% (66/1737) and 4.2% (77/1833), respectively, p < 0.001). All-cause ICU mortality was 47.8% for C. albicans and 59% for non-albicans Candida. There were no differences in mortality rates among the three periods; see Table 1.
Table 1.
Characteristics of ICU patients with candidemia in the three candidemia cohorts.
| Variables | Pre-COVID-19 Cohorts | COVID-19 Cohort | p-Value | |
|---|---|---|---|---|
| 2005–2008 n = 66 |
2012–2015 n = 77 |
2019–2021 n = 62 |
||
| Age, median (IQR) | 67 (21) | 63 (31) | 69 (15.8) | 0.001 |
| males, n (%) | 45 (68.1) | 46 (59.7) | 45 (72.4) | 0.27 |
| APACHE II score on ICU admission, median (IQR) | 19 (8.8) | 20 (10) | 15 (7) | <0.001 |
| SOFA score on ICU admission, median (IQR) | 9 (4) | 10 (5) | 8 (3) | 0.001 |
| SOFA score on candidemia day, median (IQR) | 8.5 (6) | 7 (5) | 11 (6) | 0.001 |
| Delta SOFA score, median (IQR) | 0 (4) | −1 (3.5) | 3 (6) | <0.001 |
| ICU admission diagnosis Medical, n (%) Surgical, n (%) |
22 (33) 44 (66.7) |
40 (53.3) 35 (46.7) |
62 (100) 0 (0) |
<0.001 |
| Co-morbidities Diabetes mellitus, n (%) Current malignancy, n (%) |
5 (7.6) 6 (9.1) |
3 (3.9) 5 (6.5) |
16 (25.8) 5 (8.1) |
<0.001 0.99 |
| Hospital stay before candidemia onset, days, median (IQR) | 24 (18) | 30 (41.8) | 28.5 (19.5) | 0.15 |
| ICU stay before candidemia onset, days, median (IQR) | 15.5 (19.8) | 25 (34.5) | 22 (18.2) | 0.69 |
| ICU length of stay, days, median (IQR) | 35.5 (34.5) | 49 (51) | 34.5 (39.8) | 0.029 |
| Presence of shock on candidemia day, n (%) | 34 (52.3) | 30 (46.1) | 45 (75) | 0.001 |
| Incidence of candidemia, (%) | 3.8 | 4.2 | 10.3 | <0.001 |
| Mortality, n (%) | 42 (63.6) | 35 (46.7) | 35 (56.5) | 0.92 |
ICU, intensive care unit; IQR, interquartile range; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; Delta SOFA, SOFA score on candidemia day minus SOFA score on ICU admission.
3.3. Candida Species Distribution and Fluconazole Resistance
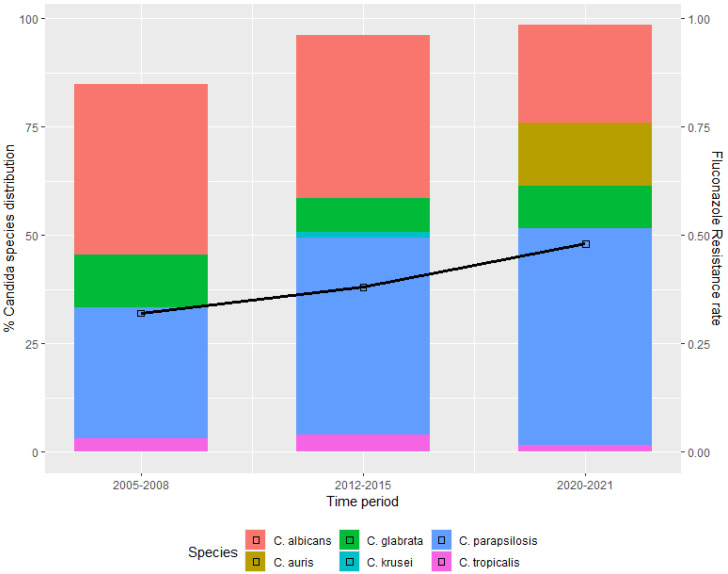
The distribution of Candida species and the antifungal susceptibility during the three study periods are shown in Table 2. Non-albicans Candida species predominated in all cohorts, with C. parapsilosis being the most commonly isolated. Considerable differences in Candida species distributions were observed over the years. In particular, a gradual decrease in the incidence of C. albicans was observed in the COVID-19 pandemic cohort (from 39.4% in 2005–2008 and 37.7% in 2012–2015 to 24.2% in COVID-19 cohort), accompanied by a corresponding increase in non-albicans Candida species, including the emergence of C. auris; see Figure 1.
Table 2.
Candida species and fluconazole resistance in the three candidemia cohorts.
| Pre-COVID-19 Cohorts | COVID-19 Cohort | p | ||
|---|---|---|---|---|
| 2005–2008 n = 66 |
2012–2015 n = 77 |
2020–2021 n = 62 |
||
| Candida species | ||||
| C. albicans, n (%) | 26 (39.4) | 29 (37.7) | 15 (24.2) | 0.069 |
| non-albicans Candida, n (%) | 40 (60.6) | 48 (62.3) | 47 (75.8) | 0.069 |
| C. parapsilosis | 28 (70) | 36 (75) | 31 (66) | 0.16 |
| C. glabrata | 8 (20) | 6 (12.5) | 5 (10.6) | 1 |
| C. tropicalis | 2 (5) | 3 (6.3) | 1 (2) | 0.77 |
| C. krusei | 0 (0) | 1 (2) | 0 (0) | 1 |
| C. auris | 0 (0) | 0 (0) | 9 (19) | <0.001 |
| other Candida species | 2 (5) | 2 | 1 | |
| Fluconazole resistance | ||||
| Fluconazole-resistant, n (%) | 21 (31.8) | 29 (37.7) | 30/62 (48.4) | 0.098 |
| C. albicans | 4/26 (15.4) | 1/29 (3.4) | 2/15 (13.3) | |
| C. parapsilosis | 10/28 (35.7) | 20/36 (55.6) | 17/31 (48.6) | |
| C. glabrata | 7/8 (87.5) | 2/6 (33.3) | 2/5 (40) | |
| C. tropicalis | 0/2 (0) | 0/3 (0) | 0/1 (0) | |
| C. krusei | NA | 1/1(100) | NA | |
| C. auris | NA | NA | 9/9 (100) | |
NA: non-applicable.
Figure 1.
Species distribution of Candida bloodstream isolates and fluconazole resistance before and during the COVID-19 pandemic era.
During the COVID-19 period, fluconazole resistance occurred in 30 (48.4%) candidemia cases: 2/15 in C. albicans, 17/31 in C. parapsilosis, 3/6 in C. glabrata, 9/9 in C. auris; see Table 2. Fluconazole resistance considerably increased over the three time periods, from 31.8% in 2005–2008, to 37.7% in 2012–2015, and to 48.4% in the COVID-19 period, p = 0.098; see Table 2 and Figure 1.
Regarding the susceptibility tests for other antifungal agents, we did not observe resistance of the aforementioned Candida species to amphοtericin B, echinocandins and voriconazole.
3.4. Factors Associated with Non-Albicans Candidemia
Regarding the entire cohort of patients who developed candidemia during the three time periods (n = 205), factors associated with non-albicans Candida species, according to univariate and multivariate models, are shown in Table 3.
Table 3.
Factors associated with candidemia development due to Candida albicans versus non-albicans Candida species in the overall study population: univariate and multivariate models. OR (95% CI) takes non-albicans Candida as the reference group.
| Patients with Candidemia, n = 205 | ||||
|---|---|---|---|---|
| Candida albicans Species, n = 70 | Non-albicans Candida Species, n = 135 | OR (95% CI) | p-Value | |
| Univariate analysis | ||||
| Age (years) ‡ | 63.0 (22.0) | 67.0 (21.0) | 0.98 (0.97–1.01) b | 0.19 |
| Gender (Female), n (%) | 24 (34.3%) | 45 (33.3%) | 1.04 (0.56–1.91) | 0.89 |
| ICU stay before candidaemia onset, days ‡ | 15.0 (19.2) | 23.0 (24.5) | 0.98 (0.9–1.00) c | 0.08 |
| Hospital stay before candidaemia onset, days ‡ | 23 (25) | 29.5 (24) | 0.99 (0.98–1.01) c | 0.17 |
| Delta SOFA | −0.32 ± 4.07 | 1.10 ± 4.15 | 0.91 (0.84–0.98) d | 0.03 |
| ICU length of stay, days ‡ | 39.0 (36.5) | 38.0 (37.0) | 0.99 (0.99–1.01) c | 0.90 |
| Diagnosis (surgical), n (%) | 30 (43.5%) | 49 (36.6%) | 1.33 (0.73–2.41) | 0.33 |
| Presence of shock on candidemia day, n (%) | 36 (54.5%) | 73 (58.9%) | 0.83 (0.45–1.53) | 0.56 |
| COVID-19 | 15 (24.2%) | 47 (75.8%) | 0.51 (0.25–0.98) | 0.049 |
| Multivariate analysis a | ||||
| ICU stay before candidemia onset, days |
0.97 (0.95–1.00) c | 0.08 | ||
| Delta SOFA | 0.74 (0.60–0.89) d | 0.002 | ||
| Presence of shock on candidemia day | 6.89 (2.2–25.0) | 0.001 | ||
‡: Median (IQR) for skewed parameters; OR: odds ratio; CI: confidence interval. a Significant results adjusted for other variables in the model; b per each year increase; c per each day increase; d per each unit increase; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; Delta SOFA, SOFA score on candidemia day minus SOFA score on ICU admission.
Multivariate logistic regression analysis revealed that an increased SOFA score on candidemia day (compared to that on ICU admission) was independently associated with candidemia due to Candida albicans, whereas the presence of shock on candidemia day was independently associated with candidemia due to non-albicans Candida species; see Table 3.
3.5. Factors Associated with Fluconazole Resistance
Resistance to fluconazole was significantly associated with non-albicans Candida species (54.8% versus 8.6%, in non-albicans Candida species and C. albicans, respectively, p < 0.001); see Figure 2. Factors associated with fluconazole resistance are shown in Table 4. Compared to patients who developed candidemia due to fluconazole-susceptible Candida species, patients with fluconazole-resistant strains had longer hospital and ICU LOS before the onset of candidemia (33 (27) versus 23 (22.8) days, p = 0.03, and 26 (22.5) versus 16 (21) days, p = 0.003, respectively). Multivariate analysis showed that prolonged ICU LOS before candidemia onset was significantly associated with the development of candidemia due to fluconazole-resistant Candida species (OR 1.03, CI: 1.01–1.06, p = 0.003), whereas the presence of shock at candidemia onset was independently associated with candidemia due to fluconazole-susceptible Candida species (OR 0.23, CI: 0.07–0.64, p = 0.006); see Table 4.
Figure 2.
Fluconazole resistance in all (n = 205) bloodstream-isolated Candida albicans and non-albicans Candida species.
Table 4.
Factors associated with candidemia development due to fluconazole-resistant Candida species in the overall patient population: univariate and multivariate models. OR (95% CI) takes fluconazole-susceptible as the reference group.
| Patients with Candidemia, n = 205 | ||||
|---|---|---|---|---|
| Fluconazole-Resistant Species, n = 80 | Fluconazole-Susceptible Species, n = 125 | OR (95% CI) | p-Value | |
| Univariate analysis | ||||
| Age (years) ‡ | 67.0 (20.8) | 65.5 (22.5) | 1.01 (0.98–1.03) b | 0.22 |
| Gender (Female), n (%) | 29 (36.2%) | 40 (32.0%) | 1.20 (0.66–2.17) | 0.53 |
| ICU stay before candidemia onset, days ‡ | 26.0 (22.5) | 16 (21.0) | 1.02 (1.01–1.04) c | 0.003 |
| Hospital stay before candidemia onset, days ‡ | 33.0 (27.0) | 23.0 (22.8) | 1.01 (1.00–1.03) c | 0.03 |
| Delta SOFA | 0.49 ± 4.64 | 0.68 ± 3.87 | 0.98 (0.91–1.06) d | 0.76 |
| ICU length of stay, days ‡ | 43.0 (48.0) | 36.0 (37.0) | 1.01 (0.99–1.02) c | 0.09 |
| Diagnosis (surgical), n (%) | 30 (38.0%) | 49 (39.5%) | 0.93 (0.52–1.66) | 0.82 |
| Presence of shock on candidemia day, n (%) | 37 (52.9%) | 72 (60.0%) | 0.74 (0.41–1.35) | 0.33 |
| COVID-19 | 30 (48.4%) | 32 (51.6%) | 1.74 (0.95–3.20) | 0.07 |
| Multivariate analysis a | ||||
| ICU stay before candidemia onset | 1.03 (1.01–1.06) | 0.003 | ||
| Presence of shock on candidemia day | 0.23 (0.07–0.64) | 0.006 | ||
‡: Median (IQR) for skewed parameters; OR: odds ratio; CI: confidence interval. a Significant results adjusted for other variables in the model; b per each year increase; c per each day increase; d compared to Day 0; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment; Delta SOFA, SOFA score on candidemia day minus SOFA score on ICU admission.
4. Discussion
This study investigated the incidence and epidemiology of candidemia in patients admitted to the ICU due to COVID-19, compared to two previous non-COVID-19 ICU candidemia cohorts. The main findings are the following: (i) candidemia incidence was 10%, more than two-fold higher compared to the pre-pandemic era; (ii) there was an epidemiological shift to non-albicans Candida species from 60.6% to 75.8% with a predominance of C. parapsilosis and (iii) there was a considerable increase in the rate of fluconazole resistance from 31.8% to 48.4%. In addition, for the whole cohort of patients with candidemia, fluconazole resistance was independently associated with ICU LOS before candidemia onset, whereas fluconazole susceptibility was independently associated with the presence of shock at candidemia onset.
The increase in the incidence of candidemia shown in our study during the ongoing pandemic is striking, though consistent with initial findings from our ICU in the first pandemic wave [8], as well as with findings of other institutions in different geographic regions [6,11,14,16,26,27,28]. In particular, in studies comparing patients with and without COVID-19, a two-fold increase in the incidence of candidemia in COVID-19 compared to non-COVID-19 patients was observed in two ICUs in India [29], whereas a nearly five-fold increase has been reported in Brazil [16], and a 10-fold rise in another report [27]. Similarly, in another case series from Italy, a higher incidence of candidemia in COVID-19 patients compared with a historical control has been reported [11], though, in the latter two studies, information about patients’ hospital location (i.e., ICU or ward) was not reported.
There was no difference in the incidence of candidemia in our ICU between the two pre-pandemic periods. This is in accordance with nationwide data from Germany showing that there was no increase in the ICU-acquired candidemia incidence during the period from 2006 to 2011 [30]. However, an increasing incidence of candidemia has been reported in internal medicine wards in our country, possibly associated with the financial crisis [20]; the present study shows that COVID-19 has further accelerated the phenomenon.
In fact, the above findings are not surprising since critically ill patients with COVID-19 have similar risk factors for candidemia development with the other, non-neutropenic ICU patients and they also received corticosteroid treatment, as recommended after the first pandemic wave [31], which might have been an additional risk factor, as already commented elsewhere [27]. Furthermore, over-occupancy of the ICU, along with the higher workload of healthcare workers and the subsequent relaxation in compliance with the infection control measures, might be additional causes [28].
The increased incidence of non-albicans Candida species detected in our study is consistent with comparable data previously reported from our ICU [32] and elsewhere [19], as well as with recent data demonstrating an increasing incidence of candidemia in a nationwide study from Greece, with a species shift towards C. parapsilosis [33]. The increased incidence of non-albicans Candida species is in accordance with an epidemiological shift across the globe, including the emergence of non-albicans Candida species. Indicatively, in a recent study [27], non-albicans Candida collectively constituted the majority of isolates in candidemic patients, considering non-COVID-19 and COVID-19 cases together. Similarly, in another study from India during the current pandemic [30], 64% of candidemia cases were due to non-albicans Candida species. On the contrary, C. albicans remains the predominant pathogen of candidemia in COVID-19 patients in Europe [11,13,34,35,36], as well as in the pre-pandemic era, according to German data for candidemia in the ICUs [31].
Introduced in the early 1990s, fluconazole is an often-preferred treatment for many systemic Candida infections as it is inexpensive and exhibits limited toxicity; it is implicated, however, in the subsequent resistance acquisition due to its extensive use over the years [19,37].
According to our results, concomitantly to the increase in non-albicans Candida species, a worrisome increase in the rate of fluconazole resistance was observed, from around 32% in the pre-COVID-19 era to 48% in the COVID-19 period. Although not surprising, since fluconazole resistance is closely associated with non-albicans Candida species, such an increase in fluconazole-resistant Candida species is of major concern. Notably, among the various isolated species, C. parapsilosis presented the highest proportion of resistance of around 50% across all three time periods, with the exception of C. auris, which has expected fluconazole resistance as a potential multidrug-resistant yeast. All-cause mortality did not differ significantly among the three study periods.
In our analysis, at candidemia onset, the SOFA score was significantly higher and the presence of shock was more frequent in patients with COVID-19 compared to those in the pre-pandemic periods, indicating an excess severity of the multi-organ dysfunction in those patients, though without a significant increase in mortality.
Interestingly, considering non-COVID-19 and COVID-19 cases together, the results of multivariate analyses revealed that the presence of shock at candidemia onset was independently associated with the isolation of Candida albicans and with fluconazole-susceptible species. This is a novel finding, possibly suggesting a more virulent capacity of the Candida albicans compared to non-albicans species. Although this finding is consistent with recent experimental data [38], it deserves further research.
Certain limitations of the present study should be pointed out. The first is the absence of a contemporaneous group to the COVID-19 pandemic cohort, i.e., critically ill patients admitted to the non-COVID-19 ICUs during the current pandemic; thus, the comparisons have been made with pre-pandemic cohorts. In fact, such a design was not feasible since the majority of our ICU beds were dedicated entirely to the admission of COVID-19 patients. However, thanks to the availability of data from the two historical non-COVID-19 cohorts, the trends in the incidence and epidemiology of candidemia in our ICU have been shown. Secondly, consumption of antifungal drugs, namely fluconazole, at the individual patient level before the onset of candidemia has not been recorded. However, in our ICU, there was no routine prophylactic use of antifungals, as has already been reported [31]; therefore, pre-exposure to fluconazole is less likely to have influenced these results.
Finally, comparisons between patients with and without candidemia during the study periods were not available, since only patients who developed candidemia have been included in the analysis. However, despite the above limitations, the present study highlights the importance for the critical care teams to be aware of the increased incidence of candidemia and of fluconazole resistance in COVID-19 ICU patients, in order to recognize cases early and treat them accordingly, as well as the urgent need to integrate antimicrobial stewardship activities in the pandemic response.
5. Conclusions
In summary, the present study provides temporal trends for candidemia in an ICU setting. The incidence of candidemia was significantly increased during the COVID-19 pandemic compared to previous non-COVID-19 periods. Additionally, a substantial increase in the incidence of non-albicans Candida and in fluconazole-resistant species was observed during the COVID-19 era. Prolonged ICU LOS was associated with fluconazole-resistant and the presence of shock with flunonazole-susceptible species. Further study is needed to clarify the reasons for the increased incidence of candidemia and of fluconazole resistance in the COVID-19 ICU patients. Meanwhile, the present findings underscore the urgent need for increased awareness, as well as for the implementation of antimicrobial and antifungal stewardship programs in order to diminish the incidence of candidemia and fluconazole resistance rates.
Author Contributions
Conceptualization and writing, C.R., J.M., E.P. (Elizabeth Paramythiotou) and E.P. (Efstathia Perivolioti); statistical analysis, E.C. and S.K. (Stelios Kokkoris); data collection, C.R., A.G., S.K. (Stavros Karageorgiou), C.G. and D.K.; microbiological data, E.P. (Efstathia Perivolioti), P.A. and A.A.; review and editing, S.K. (Stelios Kokkoris), I.V. and G.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The ethics committee of the hospital approved this study (Protocol 116/03-2021).
Informed Consent Statement
Informed consent was waived as the data were anonymized and retrospectively obtained.
Data Availability Statement
Data supporting the results can be provided from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Coronavirus Disease 2019 (COVID-19) Situation Report–51. [(accessed on 30 January 2022)]. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10.
- 2.World Health Organization Coronavirus Disease (COVID-19) Pandemic. 2021. [(accessed on 30 January 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQiAhs79BRD0ARIsAC6XpaXhxHeN64r7-j5rvv0ZDtNGxNkA0e2EWCAUr8QWWj-qi_PPrXOljroaAjXBEALw_wcB.
- 3.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., Bonanomi E., Cabrini L., Carlesso E., Castelli G., et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacobbe D.R., Battaglini D., Ball L., Brunetti I., Bruzzone B., Codda G., Crea F., De Maria A., Dentone C., Di Biagio A., et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020;50:e13319. doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla B.S., Warde P.R., Knott E., Arenas S., Pronty D., Ramirez D., Rego A., Levy M., Zak M., Parekh D.J., et al. Bloodstream infection risk, incidence, and deaths for hospitalized patients during coronavirus disease pandemic. Emerg. Infect. Dis. 2021;27:2588–2594. doi: 10.3201/eid2710.210538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel P.R., Weiner-Lastinger L.M., Dudeck M.A., Fike L.V., Kuhar D.T., Edwards J.R., Pollock D., Benin A. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control. Hosp. Epidemiol. 2021;15:1–4. doi: 10.1017/ice.2021.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kokkoris S., Papachatzakis I., Gavrielatou E., Ntaidou T., Ischaki E., Malachias S., Vrettou C., Nichlos C., Kanavou A., Zervakis D., et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J. Hosp. Infect. 2021;107:95–97. doi: 10.1016/j.jhin.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cona A., Tavelli A., Renzelli A., Varisco B., Bai F., Tesoro D., Za A., Biassoni C., Battaglioli L., Allegrini M., et al. Incidence, risk factors and impact on clinical outcomes of bloodstream infections in patients hospitalised with COVID-19: A prospective cohort study. Antibiotics. 2021;10:1031. doi: 10.3390/antibiotics10091031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segala F.V., Bavaro D.F., Di Gennaro F., Salvati F., Marotta C., Saracino A., Murri R., Fantoni M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses. 2021;13:2110. doi: 10.3390/v13112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastrangelo A., Germinario N., Ferrante M., Frangi C., Voti R.L., Muccini C., Ripa1 M., On behalf of COVID-BioB Study Group Candidemia in COVID-19 patients: Incidence and characteristics in a prospective cohort compared to historical non-COVID-19 controls. Clin. Infect. Dis. 2021;73:e2838–e2839. doi: 10.1093/cid/ciaa1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White P.L., Dhillon R., Cordey A. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin. Infect. Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seagle E.E., Jackson B.R., Lockhart S.R., Georgacopoulos O., Nunnally N.S., Roland J., Barter D.M., Johnston H.L., Czaja C.A., Kayalioglu H., et al. The landscape of candidemia during the COVID-19 pandemic. Clin. Infect. Dis. 2022;74:802–811. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 15.Bishburg E., Okoh A., Nagarakanti S.R., Lindner M., Migliore C., Patel P. Fungemia in COVID-19 ICU patients, a single medical center experience. J. Med. Virol. 2021;93:2810–2814. doi: 10.1002/jmv.26633. [DOI] [PubMed] [Google Scholar]
- 16.Nucci M., Barreiros G., Guimaraes L.F., Deriquehem V.A.S., Castineiras A.C., Nouer S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses. 2021;64:152–156. doi: 10.1111/myc.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 18.Lortholary O., Renaudat C., Sitbon K., Madec Y., Denoeud-Ndam L., Wolff M., Fontanet A., Bretagne S., Dromer F., Dromer F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010) Intensive Care Med. 2014;40:1303–1312. doi: 10.1007/s00134-014-3408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goemaere B., Becker P., Van Wijngaerden E., Maertens J., Spriet I., Hendrickx M., Lagrou K. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61:127–133. doi: 10.1111/myc.12714. [DOI] [PubMed] [Google Scholar]
- 20.Siopi M., Tarpatzi A., Kalogeropoulou E., Damianidou S., Vasilakopoulou A., Vourli S., Pournaras S., Meletiadis J. Epidemiological trends of fungemia in Greece with a focus on candidemia during the recent financial crisis: A 10-year survey in a tertiary care academic hospital and review of literature. Antimicrob. Agents Chemother. 2020;64:e01516–e01519. doi: 10.1128/AAC.01516-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014;20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 22.Knaus W., Draper E., Wagner D.P., Zimmerman J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J.L., de Mendonça A., Cantraine F., Moreno R., Takala J., Suter P., Sprung C., Colardyn F., Serge B. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit. Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Alhazzani W., Evans L., Alshamsi F., Møller M.H., Ostermann M., Prescott H.C., Arabi Y.M., Loeb M., Ng Gong M., Fan E., et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Loeches I., Antonelli M., Cuenca-Estrella M., Dimopoulos G., Einav S., De Waele J., Garnacho-Montero J., Kanj S.S., Machado F.R., Montravers P., et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 26.Macauley P., Epelbaum O. Epidemiology and mycology of Candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: Overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses. 2021;64:634–640. doi: 10.1111/myc.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riche C.V.W., Cassol R., Pasqualotto A.C. Is the frequency of candidemia increasing in COVID-19 patients receiving corticosteroids? J. Fungi. 2020;6:286. doi: 10.3390/jof6040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulet Bayona J.V., Tormo Palop N., Salvador García C., Fuster Escrivá B., Chanzá Aviñó M., Ortega García P., Gimeno Cardona C. Impact of the SARS-CoV-2 pandemic in candidaemia, invasive aspergillosis and antifungal consumption in a tertiary hospital. J. Fungi. 2021;7:440. doi: 10.3390/jof7060440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajni E., Singh A., Tarai B., Jain K., Shankar R., Pawar K., Mamoria V., Chowdhary A. A high frequency of Candida auris blood stream infections in Coronavirus disease 2019 patients admitted to intensive care units, Northwestern India: A Case Control Study. Open Forum Infect. Dis. 2021;8:ofab452. doi: 10.1093/ofid/ofab452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer E., Geffers C., Gastmeier P., Schwab F. No increase in primary nosocomial candidemia in 682 German intensive care units during 2006 to 2011. [(accessed on 30 January 2022)];Eurosurveillance. 2013 18:20505. doi: 10.2807/ese.18.24.20505-en. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20505. [DOI] [PubMed] [Google Scholar]
- 31.The Recovery Collaborative Group Dexamethazone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratikaki M., Platsouka E., Sotiropoulou C., Douka E., Paramythiotou E., Kaltsas P., Kotanidou A., Paniara O., Roussos C., Routsi C. Epidemiology, risk factors for and outcome of candidaemia among non-neutropenic patients in a Greek intensive care unit. Mycoses. 2011;54:154–161. doi: 10.1111/j.1439-0507.2009.01787.x. [DOI] [PubMed] [Google Scholar]
- 33.Mamali V., Siopi M., Charpantidis S., Samonis G., Tsakris A., Vrioni G. On Behalf of the Candi-Candi network.increasing incidence and shifting epidemiology of candidemia in Greece: Results from the first nationwide 10-year survey. J. Fungi. 2022;8:116. doi: 10.3390/jof8020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardi T., Pintado V., Gomez-Rojo M., Escudero-Sanchez R., Azzam Lopez A., Diez-Remesal Y., Castro N.M., Ruiz-Garbajosa P., Pestaña D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agrifoglio A., Cachafeiro L., Figueira J.C., Añón J.M., de Lorenzo A.G. Critically ill patients with COVID-19 and candidaemia: We must keep this in mind. J. Mycol. Med. 2020;30:10101. doi: 10.1016/j.mycmed.2020.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berrouane Y.F., Herwaldt L.A., Pfaller M.A. Trends in antifungal use and epidemiology of nosocomial yeast infections in a university hospital. J. Clin. Microbiol. 1999;37:531–537. doi: 10.1128/JCM.37.3.531-537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirayama T., Miyazaki T., Ito Y., Wakayama M., Shibuya K., Yamashita K., Takazono T., Saijo T., Shimamura S., Yamamoto K., et al. Virulence assessment of six major pathogenic Candida species in the mouse model of invasive candidiasis caused by fungal translocation. Sci. Rep. 2020;10:3814. doi: 10.1038/s41598-020-60792-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results can be provided from the corresponding author on request.