Abstract
Simple Summary
The genus Gaidropsarus is a poorly known group of marine fishes found from the intertidal zone to the deep sea in all three major oceans. The present taxonomic study describes a new deep-sea species of this genus originating from Galicia and Porcupine Banks, two seamount-like structures in the Northeast Atlantic. The results suggest that deep-water coral reefs could be an essential habitat for this species. The existence of this new species was previously flagged by the analysis of mitochondrial DNA sequences of the species of the genus described in the North Atlantic, and has been corroborated by morphological examination of the specimens.
Abstract
A new species of rockling fish genus Gaidropsarus is described based on six specimens collected in Galicia and Porcupine Banks, in Atlantic European waters. An analysis of morphological characters has confirmed the specific status of specimens of a previously described clade by comparison of DNA sequences. Gaidropsarus gallaeciae sp. nov. it is distinguished from congeners by the following combination of characters: 43–44 vertebrae; 54–60 third dorsal fin rays; 44–52 anal fin rays; 21–23 pectoral fin rays; head length 21.1–25.2% of standard length (SL); length of the pelvic fin 16.2–19% SL; length of the first dorsal fin ray 15.8–27% of head length (%HL); eye diameter 15.8–20.5% HL; and interorbital space 21.7–28% HL. Using the nucleotide sequence of the 5’ end of the mitochondrial COI gene as a molecular marker, the genetic p-distance between the new species and its congeners far exceeds the usual 2%, granting the former the status of an independent taxon, which is in accordance with the morphological identification. A comparison with the other 12 valid species of the genus is presented. The study also highlights the morphological diversity resulting from the meristic and biometric variability of Gaidropsarus species and lays the groundwork for future taxonomic studies on this genus.
Keywords: Teleostei, taxonomy, rocklings, deep-sea
1. Introduction
The genus Gaidropsarus Rafinesque, 1810 shows a remarkable ecological diversity ranging from intertidal and near-shore to deeper areas up to 2000 m depth, and from arctic to temperate and subtropical waters. Species in this genus show an antitropical distribution, with most of them known only from the northern hemisphere, in the Atlantic Ocean, while species from the southern hemisphere produce a circumglobal ring of forms comprising the sub-Antarctic waters of the Atlantic, Indian and Pacific Oceans [1].
Following recent revisions [2,3] and the report of a new species [4], there are currently 12 valid species worldwide: Gaidropsarus argentatus (Reinhardt, 1837), Gaidropsarus ensis (Reinhardt, 1837), Gaidropsarus granti (Regan, 1903), Gaidropsarus macrophthalmus (Günther, 1867), Gaidropsarus mediterraneus (Linnaeus, 1758), Gaidropsarus vulgaris (Cloquet, 1824), Gaidropsarus mauli Biscoito & Saldanha 2018, Gaidropsarus capensis (Kaup, 1858) Gaidropsarus insularum Sivertsen, 1945, Gaidropsarus novaezealandiae (Hector, 1874) Gaidropsarus pakhorukovi Shcherbachev, 1995, and Gaidropsarus parini Svetovidov, 1986.
The classification of this genus is controversial, having been placed alternatively within the family Gaidropsaridae, Gadidae or Lotidae, although recent research that appears to be definitive includes it in the first group, forming its own family [5]. Fishes from this genus, commonly known as rocklings, are characterized by an elongated and relatively slender body, barbels present on the chin and at each anterior nostril on the snout, a first dorsal fin ray followed by a row of small fleshy filaments, a not indented anal fin and an uninterrupted lateral line is along its entire length [6].
The taxonomy of the genus Gaidropsarus is incomplete and inadequately known, due to the absence in the museums of any representative collections of the numerous species widely distributed in the World Ocean [1]. Further studies are required to evaluate the small differences between many of the described species, which at one time or another have been included in several nominal genera. Hence, a key and a complete list of species are not feasible at present [6]. Some recent publications based on molecular data of North Atlantic species highlighted several inconsistencies with existing morphology-based taxonomic concepts [2,7]. Moreover, the presence of probably undescribed species [2], new distribution records and extension ranges [8,9] and the finding of new species [4] confirm the limited knowledge of the genus.
Seamounts are hotspots of marine biodiversity with high species richness [10], but the composition and diversity of fish fauna on these habitats is not well documented. In addition to traditional fisheries sampling techniques, the use of non-invasive technologies such as remotely operated vehicles (ROVs), autonomous underwater vehicles (AUVs), and baited cameras (BCs) have improved knowledge of the seamounts fish fauna, including the discovery of potentially new species. However, specimen-based examination is needed to clarify their detailed taxonomy [11,12]. In fact, the report of new fish species found on seamounts is not uncommon [13,14].
The use of these “in vivo” sampling techniques has revealed the presence of unidentified rocklings at different deep-sea habitats of the NE Atlantic and the Mediterranean in association with the coral Desmophyllum pertusum (Linnaeus, 1758) or in cracks and crevices of chimneys [15,16,17].
The Galicia and the Porcupine Banks constitute two of the 557 seamount-like structures included in the limits of the OSPAR Convention (North-East Atlantic) [18]. The ichthyofauna of the Galicia Bank was studied from the 1990s onwards, resulting in a total of 139 catalogued species [8]. The Porcupine Bank has also been the subject of numerous ichthyological studies [19,20,21], but an updated species composition list has not yet been produced.
Molecular taxonomy is especially valuable for groups in which distinctive morphological features are difficult to observe or compare. During DNA analysis of a large sample of individuals of the genus Gaidropsarus, employing both mitochondrial (cytochrome oxidase subunit I, COI; cytochrome b, CytB; NADH dehydrogenase 2, ND2) and nuclear (Rhodopsine, Rho, Zic Family Member 1, ZIC1) markers, a set of sequences was revealed that differed significantly from those ascribed to recognized species [2,3]. This study provides a formal description of this set of individuals as a new species and explores the current state of knowledge of the genus.
2. Materials and Methods
Specimens come from two Atlantic seamount-like structures (Figure 1). The Galicia Bank is a non-volcanic seamount located off the northwest of the Iberian Peninsula, between 42°15′ N–43° N and 11°30′ W–12°15′ W, at water depths from 625 to 1800 m and at about 125 nautical miles from the coast, and is 50 km long in the E–W direction and 90 km on the N–S axis [8]. The presence of vulnerable species and habitats in this bank, such as Lophelia and Madrepora communities and black and bamboo coral aggregations were the basis for its inclusion in the Natura 2000 network as a Site of Community Importance [22]. The Porcupine Bank is located in the Northeast Atlantic, from 13° W to 15° W longitude and from 51° N to 54° N latitude, 200 km off the west coast of Ireland. It extends from a depth of 150 m to 4000 m of the abyssal plain, forming a seamount-like structure, which is connected by a narrow strip to the continental shelf. The closed water recirculation system in this area favours the retention of nutrients and plankton, creating an area of high productivity.
Figure 1.
Map showing the location of catches of Gaidropsarus gallaeciae sp. nov. specimens in the Galicia and Porcupine Banks, in the northeast Atlantic.
Sampling on the Galicia Bank was conducted aboard the R/V “Thalassa”, as part of the INDEMARES project (BanGal0810), an exploratory multi-gear survey, whereas sampling on the Porcupine Bank was conducted aboard the R/V “Vizconde de Eza” during the annual bottom-trawl surveys (Porcupine 2019), using a Baca-GAV 39/52 with a cod-end mesh size of 20 mm. Specimens were taken from the catch and frozen on board. In the laboratory, specimens were thawed, examined and photographed. With the exception of total length (TL) and standard length (SL), measurements are distances perpendicular to the length of the fish measured with a digital calliper to the nearest 0.1 mm on the type specimens and to nearest mm on the comparative material. Counts and measurements were recorded following Svetovidov [23,24]. All measurements are expressed as the percentage of standard length (%SL) or head length (%HL). Voucher specimens were deposited in the ichthyological collection of the Museo de Historia Natural, Universidade de Santiago de Compostela (MHNUSC).
To improve the knowledge of the natural variation of the species of the genus, a comprehensive review of the morphological characters, distribution and coloration of valid Gaidropsarus species reported in the ichthyological literature, mainly compiled in Svetovidov [23,24], Barros-García et al. [2] and Biscoito and Saldanha [4], was complemented with measurements and counts of own comparative material, when available.
A single COI sequence representative for each valid Gaidropsarus species available (n = 7) was retrieved from Genbank specimens belonging to the author’s project ‘Molecular identification of Gaidropsarus fishes’ (Code GSRUS) in BOLD systems (https://www.boldsystems.org/, accessed on 27 April 2022), including the sequence of the holotype of Gaidropsarus gallaeciae sp. nov. (KY250297). A sequence of Notacanthus bonaparte Risso, 1840 (KP845234) was used as outgroup.
These sequences were employed to construct a molecular cladogram using the Neighbour-Joining (NJ) method [25] in MEGA 11 [26] with confidence limits tested through a bootstrap procedure [27], after 2000 replicates.
3. Results
Gaidropsarus gallaeciae sp. nov.
http://zoobank.org/FA7C4849-4D40-45F1-9285-81B289ADE402 (accessed on 27 April 2022)
Figure 2, Figure 3 and Figure 4; Table 1
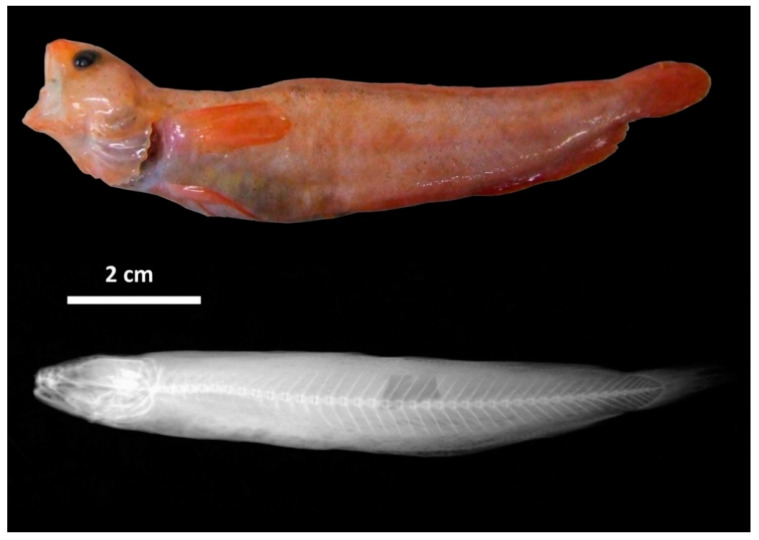
Figure 2.
Gaidropsarus gallaeciae sp. nov., holotype (MHNUSC 10126-1) 98.5 mm SL (top), X-ray of entire body (down).
Figure 3.
Gaidropsarus gallaeciae sp. nov., paratype (MHNUSC 10126-2) 76.4 mm SL (top), X-ray of entire body (down).
Figure 4.
Details of the head structures of the holotype of Gaidropsarus gallaeciae sp. nov.: (A) mouth; (B) upper jaw teeth; (C) vomer teeth; (D) lower jaw teeth; (E) first gill arch; (F) gill rakers.
Table 1.
Morphological data of the type material of Gaidropsarus gallaeciae sp. nov.
| Holotype | Paratypes | Mean ± SE | |||||
|---|---|---|---|---|---|---|---|
| TL | 111.4 | 88.2 | 101.6 | 105.5 | 74.3 | 65.8 | 91.1 ± 7.4 |
| SL | 98.5 | 76.4 | 89.2 | 95.3 | 65.1 | 57.4 | 80.3 ± 6.8 |
| As % SL | |||||||
| Head length | 22.4 | 21.1 | 25.2 | 22.9 | 24 | 23.9 | 23.3 ± 0.6 |
| 1st Predorsal length | 21.8 | 27.7 | 22.6 | 22.4 | 23 | 24.7 | 23.7 ± 0.9 |
| 3rd Predorsal length | 37.9 | 34.4 | 36.4 | 33.7 | 40.4 | 38 | 36.8 ± 1 |
| 2nd dorsal base fin length | 11.7 | 9.8 | 11.3 | 10.3 | 11.4 | 11.7 | 11 ± 0.3 |
| 3rd dorsal base fin length | 56.4 | 59.7 | 64.4 | 59 | 60.1 | 55.9 | 59.3 ± 1.2 |
| Anal base fin length | 46.4 | 48 | 47.2 | 46.6 | 39.6 | 45.6 | 45.6 ± 1.2 |
| Pectoral fin length | 15.6 | 16.1 | 17.5 | 16.1 | 16.9 | 15.3 | 16.3 ± 0.3 |
| Pelvic fin length | 16.2 | 18.8 | 17.8 | 16.4 | 18 | 19 | 17.7 ± 0.5 |
| Preanal length | 48.9 | 45.9 | 48.4 | 43.4 | 47.2 | 49.1 | 47.2 ± 0.9 |
| Body depth | 19.9 | 15.7 | 19.4 | 21.6 | 19.7 | 19.5 | 19.3 ± 0.8 |
| Prepectoral length | 22.2 | 22 | 22.8 | 22 | 24.1 | 27.5 | 23.4 ± 0.9 |
| Prepelvic length | 17.9 | 18.6 | 19.3 | 16.8 | 20.4 | 21.3 | 19.1 ± 0.7 |
| Caudal peduncle height | 6.3 | 6.2 | 7.4 | 6.9 | 7.2 | 7.7 | 7 ± 0.2 |
| As % HL | |||||||
| Snout length | 24 | 21.1 | 20.9 | 24.3 | 25 | 22.6 | 23 ± 0.7 |
| Eye diameter | 15.8 | 20.5 | 19.6 | 19.7 | 18.6 | 17.5 | 18.6 ± 0.7 |
| Postorbital length | 64.3 | 58.4 | 54.7 | 56 | 56.4 | 60.6 | 58.4 ± 1.5 |
| Interorbital space | 24.4 | 21.7 | 23.1 | 28 | 25 | 26.3 | 24.8 ± 0.9 |
| Upper jaw length | 37.9 | 44.7 | 39.6 | 47.2 | 41 | 43.1 | 42.3 ± 1.4 |
| Lower jaw length | 34.4 | 37.3 | 32 | 36.7 | 36.5 | 40.1 | 36.2 ± 1.1 |
| Chin barbel length | 16.7 | 16.8 | 18.6 | 21.1 | 21.8 | 22.6 | 19.6 ± 1.1 |
| 1st dorsal fin ray length | 15.8 | 19.3 | 24 | 22 | 26.3 | 27 | 22.4 ± 1.8 |
| Meristic | |||||||
| 3rd dorsal fin rays | 60 | 57 | 57 | 58 | 54 | 58 | 57.3 ± 0.8 |
| Anal fin rays | 44 | 52 | 46 | 49 | 50 | 50 | 48.5 ± 1.2 |
| Pectoral fin rays | 22 | 23 | 21 | 22 | 23 | 22 | 22.2 ± 0.3 |
| Ventral fin rays | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Gill rakers (inner) | 1 + 8 | 1 + 5 | 1 + 6 | 1 + 6 | 1 + 8 | 1 + 6 | 7.5 ± 0.5 |
| Gill rakers (outer) | 1 + 8 | 1 + 7 | 1 + 7 | 1 + 8 | 1 + 6 | 1 + 7 | 8.2 ± 0.3 |
| Vertebrae | 44 | 43 | – | – | – | – | 43.5 ± 0.3 |
Gaidropsarus sp. 1: [2] (p. 26); Gaidropsarus sp.: [3].
3.1. Holotype
MHNUSC 10126-1 (Figure 2 and Figure 4), 111.4 mm TL, 98.5 mm SL, Galicia Bank, bottom trawl, 18 August 2010, 42.751 °N, −11.7713° W, 788.5 m; sample ID: ROL002; GenBank registration: KY250297.
3.2. Paratypes
MHNUSC 10126-2 (Figure 3), 88.2 mm TL, 76.4 mm SL, Galicia Bank, beam trawl; 19 August 2010, 42.4535° N, −11.4549° W, 788 m; sample ID: ROL001, GenBank registration: KY250298; MHNUSC-10126-3, 101.6 mm TL, 89.2 mm SL, Porcupine Bank, bottom trawl, 11 September 2019, 51.0316° N, −14.4061° W, 751 m; sample ID: ROL003, GenBank registration: MZ198255; MHNUSC-10126-4, 105.5 mm TL, 95.3 mm SL, Porcupine Bank, bottom trawl, 11 September 2019, 51.0316° N, −14.4061° W, 751 m; sample ID: ROL004, GenBank registration: MZ198256; MHNUSC-10126-5, 74.3 mm TL, 65.1 mm SL, Porcupine Bank, bottom trawl, 11 September 2019, 51.0316° N, −14.4061° W, 751 m; sample ID: ROL005, GenBank registration: MZ198257; MHNUSC-10126-6, 65.8 mm TL, 57.4 mm SL, Porcupine Bank, bottom trawl, 11 September 2019, 51.0316° N, −14.4061° W, 751 m; sample ID: ROL006, GenBank registration: MZ198258.
3.3. Comparative Material Examined
Morphological data of the comparative material examined are shown in Table S1.
Gaidropsarus vulgaris MHNUSC 25196-1, 320 mm TL, 8 February 2022, 42.3442, −8.9799, 62 m depth; MHNUSC 25196-2, 239 mm TL, 20 March 2014, 42.7937, −9.0212, 7 m depth; MHNUSC 25196-3, 295 mm TL, 16 January 2014, 42.3045, −8.8464, 7 m depth; MHNUSC 25196-4, 262 mm TL, 21 January 2014, 42.5003, −9.0499, 25 m depth; MHNUSC 25196-5, 258 mm TL 26 February 2014, 43.7598, −7.6387, 12 m depth.
Gaidropsarus macrophthalmus MHNUSC 25199-1, 201 mm TL, 10 September 2015, 52.1237, −13.8644, 405 m depth; MHNUSC 25199-2, 171 mm TL, 10 September 2015, 52.1237, −13.8644, 405 m depth; MHN USC 25199-3, 157 mm TL, 14 September 2017, 53.5014, −11.9862, 290 m depth; MHNUSC 25199-4, 185 mm TL, 24 September 2015, 52.0796, −13.0109, 729 m depth; MHNUSC 25199-5, 187 mm TL, 24 September 2015, 52.0796, −13.0109, 729 m depth.
Gaidropsarus mediterraneus MHNUSC 25195-1, 277 mm TL, 16 October 2015, 42.7346, −9.0906, 7 m depth; MHNUSC 25195-2, Madeira.
Gaidropsarus ensis MHNUSC 25197-1, 230 mm TL, MHNUSC 25197-2, 222 mm TL, 30 July 2015, 48.1953, −46.9417, 1004 m depth; MHNUSC 25197-3, 355 mm TL, 6 June 2015, 42.8497, −50.7967, 981 m depth; MHNUSC 25197-4, 245 mm TL, 30 July 2015, 48.1953, −46.9417, 1004 m depth.
Gaidropsarus argentatus MHNUSC-25198-1, 330 mm TL, 30 June 2015, 77.7206, −10.1256, 884 m depth; MHNUSC-25198-2, 282 mm TL, 30 June 2015, 77.1842, −11.4722, 657 m depth; MHNUSC-25198-3, 305 mm TL, 30 June 2015, 77.7206, −10.1256, 884 m depth.
3.4. Diagnosis
The new species belongs to the genus Gaidropsarus as defined by Iwamoto and Cohen [28] as having three dorsal fins barely separated from each other; the first with a single thickened unsegmented ray, the second with small, unsegmented rays in a fleshy ridge that rises within a groove and the third with segmented rays in an elongate fin, and five prominent individual barbels, four on the snout and one at the tip of the lower jaw. Gaidropsarus gallaeciae sp. nov. is morphologically distinct from all congeners by the following combination of characters: third dorsal-fin rays 54–60, anal-fin rays 44–52, pectoral fin rays 21–23, total vertebrae 43–44; anal fin base short, its length 39.6–48% SL; first dorsal fin ray moderately elongated, its length 15.8–27% HL and a wider interorbital space, 21.7–28% HL.
3.5. Differential Diagnosis
A detailed comparison between the Gaidropsarus gallaeciae sp. nov. and the other 12 valid congeners is provided in Table 2 and Table 3. According to our current knowledge of the genus, only three species, G. macrophthalmus, G. capensis and G. granti, share the low number of vertebrae found in the new species, while all other species have more than 45 vertebrae. It differs from G. capensis by having more third dorsal fin rays (54–60 vs. 43–52), anal fin rays (44–52 vs. 37–43) and pectoral fin rays (21–23 vs. 18–21), a wider interorbital space (21.7–28% HL vs. 13.5–19.5% HL), a different distribution area (NE Atlantic vs. SE Atlantic and SW Indian Oceans); from G. macrophthalmus by having more pectoral fin rays (21–23 vs. 17–22), a shorter anal fin base (39.6–48% SL vs. 48.5–50% SL), a longer pelvic fin (16.2–19% SL vs. 9.6–16.1% SL) and by the coloration pattern (uniform vs. mottled); and from G. granti by a wider interorbital space (21.7–28% HL vs. 10.5–17.6% HL), a longer first dorsal fin ray (15.8–27% HL vs. 12.7–14.9% HL) and by the coloration pattern (uniform vs. mottled).
Table 2.
Comparison of morphological data between Gaidropsarus gallaeciae sp. nov and 12 valid species of the genus. Abbreviations are as follow: Gaidropsarus gallaecia sp. nov. (GGAL), G. mauli (GMAU), G. argentatus (GARG), G. ensis (GENS), G. mediterraneus (GMED), G. vulgaris (GVUL), G. granti (GGRA), G. macrophthalmus (GMAC), G. insularum (GINS), G. novaezealandiae (GNOV), G. capensis (GCAP), G. pakhorukovi (GPAK), and G. parini (GPAR).
| Species | GGAL | GMAU | GARG | GENS | GMED |
|---|---|---|---|---|---|
| As % SL | |||||
| Head length | 21.1–25.2 | 23.4–25.4 | 19.7–25.1 | 19–22 | 18.8–24 |
| 1st Predorsal length | 21.8–27.7 | 22.8–23.9 | 20.7–22.6 | 18.7–20.2 | 18.6–18.9 |
| 3rd Predorsal length | 33.7–40.4 | 36–36.3 | 31.7–36.8 | 29.1–32.3 | 20.1–38.5 |
| 2nd dorsal base fin length | 9.8–11.7 | — | 8.6–11.4 | 8–11.3 | 13.2–18.4 |
| 3rd dorsal base fin length | 55.9–64.4 | — | 57.1–62.4 | 59.3–64.4 | 54.1–60.9 |
| Anal base fin length | 39.6–48 | — | 38.7–39.8 | 39.9–46.3 | 45–52.2 |
| Pectoral fin length | 15.3–17.5 | 17.8–19.4 | 16.1–18.9 | 17–20 | 12.3–14.6 |
| Pelvic fin length | 16.2–19 | 27.5–33.3 | 18.1–21.5 | 17–26.3 | 13–15.5 |
| Preanal length | 43.4–49.1 | 50.8–53.1 | 51.4–53.4 | 48–50 | 44.1–51.1 |
| Body depth | 15.7–21.6 | 15.2–21.9 | 15.6–23.5 | 16.7–25.2 | 14–19.3 |
| Prepectoral length | 22–27.5 | — | 20.7–28 | 17.8–21.3 | 20.5–22.7 |
| Prepelvic length | 16.8–21.3 | — | 16.9–19.9 | 12.7–16 | 15–17.2 |
| Caudal peduncle height | 6.2–7.7 | 5.6–6.8 | 5.5–7.4 | 5.1–7.2 | 4.5–6.3 |
| As% HL | |||||
| Snout length | 20.9–25 | 25.4–26.7 | 25.2–27 | 23.6–27.9 | 18.8–30.4 |
| Eye diameter | 15.8–20.5 | 10.4–12 | 14.8–21.8 | 17.3–24.5 | 13.3–22.5 |
| Postorbital length | 54.7–64.3 | — | 57.3–58.4 | 54.1–59.1 | 60.63.5 |
| Interorbital space | 21.7–28 | 20.9–21.3 | 13.1–23.1 | 14.4–25.1 | 9.1–25.7 |
| Upper jaw length | 37.9–47.2 | — | 44.7–47.7 | 45.3–64.8 | 42.9–45.6 |
| Lower jaw length | 32–40.1 | — | 36.6–41.1 | 36.1–60.3 | 38.9–40.2 |
| Chin barbel length | 16.7–22.6 | 26.9 | 19.8–23.8 | 15.1–20.8 | 15.3–18.5 |
| 1st dorsal fin ray length | 15.8–27 | 21.3–25.4 | 24.1–43 | 82.1–145.5 | 14.9–42 |
| Meristic | |||||
| 3rd dorsal fin rays | 54–60 | 57–58 | 52–65 | 52–64 | 48–63 |
| Anal fin rays | 44–52 | 46–47 | 43–51 | 40–48 | 41–53 |
| Pectoral fin rays | 21–23 | 25–26 | 22–25 | 20–27 | 15–19 |
| Ventral fin rays | 7 | 9 | 7–8 | 6–7 | 5–8 |
| Gill rakers (inner) | 6–9 | 9 | 10–11 | 12–14 | 9–11 |
| Gill rakers (outer) | 7–9 | 7–8 | 8–11 | 11–13 | 7–10 |
| Vertebrae | 43–44 | 47–48 | 49–53 | 50–54 | 46–50 |
| Species | GVUL | GGRA | GMAC | GINS | GNOV |
| As % SL | |||||
| Head length | 23.6–25.9 | 20.9–25.5 | 19.3–23.2 | 18.7–21.5 | 17.9–20.7 |
| 1st Predorsal length | 22.1–24 | — | 20.5–22.9 | — | — |
| 3rd Predorsal length | 36.4–38.1 | 21.1–37.9 | 33.3–38.3 | — | — |
| 2nd dorsal base fin length | 11.3–13.9 | 10.7 | 8.6–11.7 | 8.5–9.8 | — |
| 3rd dorsal base fin length | 54.9–61.1 | 54.4–59.7 | 55.6–66.3 | 65.1–67.5 | 58.5–65.3 |
| Anal base fin length | 40.5–45.3 | 43.6–45.6 | 48.5–50 | 46.4–49.6 | 48.2–51.5 |
| Pectoral fin length | 14.1–15.4 | 13.8–15.4 | 14.7–15.5 | — | — |
| Pelvic fin length | 17.4–20.3 | 15.5–23.1 | 9.6–16.1 | — | — |
| Preanal length | 48.9–54.8 | 48.7–54.8 | 44–47.6 | — | — |
| Body depth | 14.8–20.4 | 13.1–14 | 14.2–19.5 | — | — |
| Prepectoral length | 23.5–25.4 | — | 19.3–25 | — | — |
| Prepelvic length | 18.6–20.7 | — | 16.3–20.2 | — | — |
| Caudal peduncle height | 7–8.5 | 5.6–6.9 | 4.8–7.1 | 6.8–8.5 | 6.3–8.1 |
| As % HL | |||||
| Snout length | 21.2–26.6 | 19.3–29.2 | 21–26 | 27.6 | — |
| Eye diameter | 10.5–16.7 | 13.7–18.8 | 16–23.7 | — | 15.2–19 |
| postorbital length | 55.3–65.2 | 59.6 | 54.3–59.1 | — | |
| Interorbital space | 14.4–19.5 | 10.5–17.6 | 12.5–26.5 | 16.7–19.4 | 15.2–18.7 |
| Upper jaw length | 42.3–49.3 | 42.9 | 46.2–52.9 | 59.2–61.3 | — |
| Lower jaw length | 36.8–40 | 41.7 | 36.6–44 | 48.4–55.1 | — |
| Barbel length | 19.6–24.2 | — | 14–22.2 | — | — |
| 1st dorsal ray length | 9.5–16.9 | 12.7–14.9 | 10.1–25.1 | 11.2–25 | 20–27.9 |
| Meristic | |||||
| 3rd dorsal fin rays | 56–64 | 55–60 | 48–59 | 66–70 | 56–69 |
| Anal fin rays | 46–54 | 45–52 | 40–50 | 50–57 | 50–59 |
| Pectoral fin rays | 20–24 | 20–22 | 17–22 | 19–22 | 20–21 |
| Ventral fin rays | 6–7 | 7–8 | 6–7 | — | 7–8 (5) |
| Gillraker (inner) | 10–11 | — | 8–11 | 9 | 9–10 |
| Gillraker (outer) | 7–9 | 10 | 6–9 | 7 | 6–8 |
| Vertebrae | 46–49 | 44–47 | 43–47 | 47–49 | 46–49 |
| Species | GCAP | GPAK | GPAR | ||
| As % SL | |||||
| Head length | 19.4–22.5 | 23.7–24.7 | 22.1–22.8 | ||
| 1st Predorsal length | — | 24.4–25.3 | 17.8–18.5 | ||
| 3rd Predorsal length | — | — | — | ||
| 2nd dorsal base fin length | 12.2–13.2 | 12.8–15.5 | 10.4–11.6 | ||
| 3rd dorsal base fin length | — | 55.3 | 56–58.1 | ||
| Anal base fin length | 48.4–49 | 41.7 | 43.8–48.6 | ||
| Pectoral fin length | — | 17.7–19.2 | 17.3–17.8 | ||
| Pelvic fin length | — | — | 19.9–20.7 | ||
| Preanal length | — | — | 45.2–48.5 | ||
| Body depth | 16.5–17.3 | — | — | ||
| Prepectoral length | — | — | — | ||
| Prepelvic length | — | 22.4 | — | ||
| Caudal peduncle height | 7–8.1 | 6.5 | 6.7–7.1 | ||
| As % HL | |||||
| Snout length | 28.2–33.2 | — | — | ||
| Eye diameter | 16.1–20.9 | 17.2–19.8 | 13.9–16.4 | ||
| postorbital length | — | — | |||
| Interorbital space | 13.5–19.5 | 16 | — | ||
| Upper jaw length | 48.8–52.1 | — | — | ||
| Lower jaw length | — | — | — | ||
| Barbel length | — | — | — | ||
| 1st dorsal ray length | 19.5–32.5 | 12–15.1 | 26.7–28 | ||
| Meristic | |||||
| 3rd dorsal fin rays | 43–52 | 60–62 | 60–64 | ||
| Anal fin rays | 37–43 | 50–51 | 52–53 | ||
| Pectoral fin rays | 18–21 | 22–26 | 23–25 | ||
| Ventral fin rays | 6–7 | 7–8 | 7–8 | ||
| Gillraker (inner) | 8–9 | 9 | 10 | ||
| Gillraker (outer) | 4–9 | 9 | 7 | ||
| Vertebrae | 41–43 | 46–47 | 47–48 | ||
Table 3.
Comparison of coloration, geographical and depth distributions between Gaidropsarus gallaeciae sp. nov and 12 valid species of the genus.
| Species | Coloration | Distribution |
|---|---|---|
| Gaidropsarus gallaeciae sp. nov. | Pinkish overall, greyish on the abdominal region | NE Atlantic: Galicia Bank and Porcupine Bank, 751–788 m depth |
|
Gaidropsarus
mauli |
Pinkish overall, less intense on the abdominal region, varying from more or less uniform to a mottled pattern | Atlantic Ocean: Azores and Bay of Biscay; 850–1700 m depth |
| Gaidropsarus argentatus | Uniform reddish-brown to light brick red; pink ventrally | Arctic and Atlantic Oceans, from west Spitzbergen, Norwegian Sea, Iceland, south Greenland and off Labrador; 150–2260 m depth |
| Gaidropsarus ensis | Light brick red, belly tinted red with blue-grey tinge | N Atlantic: Off Newfoundland and Labrador and west of British Isles; 600–1500 m depth |
| Gaidropsarus mediterraneus | Varied, back and upper flank brown, sometimes reddish brown, grading to pale brown-white on the ventral, with pale spots along the sides; blackish with white blotches mainly in the Macaronesian specimens | NE Atlantic, from Norway and British Isles south to Morocco, including Canaries, Azores and Madeira, and Mediterranean; 0–40 m depth |
|
Gaidropsarus
vulgaris |
Pale cream to pink or reddish with chocolate brown spots on head and body | NE Atlantic, from Norway and Iceland south to Gibraltar, including Madeira and Mediterranean; 10–120 m depth |
|
Gaidropsarus
granti |
Back brown, with irregular brown creamy blotches and spots and a whitish longitudinal sinuous band along upper flank | NE Atlantic, in Porcupine Bank (southwest of Ireland); Galicia Bank off Spain; Azores, Madeira and Canary Islands and Mediterranean; 20–823 m depth |
| Gaidropsarus macrophthalmus | Back mottled deep brown, flanks reddish, belly pink |
NE Atlantic, from Faroe Islands and British Isles to south of the Azores and Mediterranean; 150–600 m depth |
| Gaidropsarus insularum | Chocolate-brown | SE Atlantic: southern tip of Africa, Tristan da Cunha and Gough islands; SW Indic: St. Paul and Amsterdam Islands; littoral |
| Gaidropsarus novaezealandiae | Head, body and fins dark reddish brown, purplish grey ventrally | SW Pacific: New Zealand and south of Tasmania; 0–50 m, but two specimens collected at 300–500 m |
|
Gaidropsarus
capensis |
Unknown in live fish | SE Atlantic and SW Indian Oceans: southern Africa; to 45 m depth |
| Gaidropsarus pakhorukovi | Brownish-grey, darker dorsally | SW Atlantic: Rio Grande Seamount; 670–1190 m depth |
| Gaidropsarus parini | Chocolate-brown to grey | SE Pacific: Nazca Ridge; 310–680 m depth |
Gaidropsarus gallaeciae sp. nov. differs from G. novaezealandiae by having fewer vertebrae (43–44 vs. 46–49), more pectoral fin rays (21–23 vs. 20–21), a wider Interorbital space (21.7–28% HL vs. 15.2–18.7% HL), a shorter anal fin base (39.6–48% SL vs. 48.2–51.5% SL), a deeper distribution range (751–788 m vs. 300–500 m) and a different geographical area (Northeast Atlantic vs. Southwest Pacific).
It differs from G. insularum by having fewer vertebrae (43–44 vs. 47–49), fewer third dorsal fin rays (54–60 vs. 66–70), fewer anal fin rays (44–52 vs. 50–57), a wider interorbital space (21.7–28% HL vs. 16.7–19.4% HL), a deeper distribution range (751–788 m vs. littoral) and a different geographical area (Northeast Atlantic vs. Southeast Atlantic).
It differs from G. pakhorukovi by having fewer vertebrae (43–44 vs. 46–47), fewer pectoral fin rays (21–23 vs. 22–26), a deeper distribution range (751–788 m vs. 670–1190 m) and a different geographical area (Northeast Atlantic vs. Southeast Atlantic).
It differs from G. parini by having fewer vertebrae (43–44 vs. 47–48), fewer third dorsal fin rays (54–60 vs. 60–64), fewer anal fin rays (44–52 vs. 52–53), fewer pectoral fin rays (21–23 vs. 23–25), a deeper distribution range (751–788 m vs. 310–680 m) and a different geographical area (Northeast Atlantic vs. Pacific Ocean: Nasca)
It differs from G. mauli by having fewer vertebrae (43–44 vs. 46–47), fewer pectoral fin rays (21–23 vs. 25–26), a shorter snout (20.9–25% HL vs. 25.4–26.7%HL), a shorter chin barbel (16.7–22.6% HL vs. 26.9% HL), a shorter pelvic fin (16.2–19% SL vs. 27.5–33.3% SL), a wider interorbital space (21.7–28% HL vs. 20.9–21.3% HL) and larger eyes (eye diameter 15.8–20.5% HL vs. 10.4–12% HL).
It differs from G. vulgaris by having fewer vertebrae (43–44 vs. 46–49), a wider interorbital space (21.7–28% HL vs. 14.4–19.5% HL), a deeper distribution range (751–788 vs. 10–120) and by the coloration pattern (uniform vs. spotted).
It differs from G. mediterraneus by having fewer vertebrae (43–44 vs. 46–50), more pectoral fin rays (21–23 vs. 15–19), a longer pelvic fin (16.2–19%SL vs. 13–15.5%SL), a deeper distribution range (751–788 vs. 0–40) and different coloration (pinkish vs. brown).
It differs from G. argentatus by having fewer vertebrae (43–44 vs. 49–53), a larger anal fin base (39.6–48 vs. 38.7–39.8), a shorter snout (20.9–25% HL vs. 25.2–27%HL) and a shorter first dorsal fin ray (15.8–27 % HL vs. 24.1–43% HL).
It differs from G. ensis by having fewer vertebrae (43–44 vs. 50–54), fewer gill rakers (6–9 vs. 12–14 in the inner row) and a shorter first dorsal fin ray (15.8–27% HL vs. 82.1–145.5% HL).
3.6. Etymology
The name gallaeciae derives from the latin Gallaecia, an ancient Roman Iberian province, now called Galicia, the westernmost region of Spain, in reference to the name of the Galicia Bank where the holotype was collected.
3.7. Description
Counts and measurements of type specimens are shown in Table 1. Body elongate and relatively slender, maximum body depth is contained from 5 to 6.4 times in SL; moderate and round eyes, horizontal eye diameter 1 to 1.5 times in snout length; snout short and rounded, its length 4.1 to 4.8 times in head length; mouth large (Figure 4A), slightly oblique, reaching a vertical through the posterior margin of orbit; upper jaw slightly protruding beyond lower jaw; first dorsal fin short, contained 3.7 to 6.3 times in HL; a small anterior nostril placed near de base of the barbel; posterior nostril oval, large, close to orbit; barbel present on chin, its length approximately equal to eye diameter and 1 to 1.4 times in snout lenght, and one barbel at each anterior nostril on the snout; first dorsal fin ray elongated, followed by a second dorsal fin of short fleshy filaments. The dentition consists of densely packed bands of small conical elements in both jaws (Figure 4B,D); the outermost row in the upper jaw and the innermost row in the lower jaw are larger; fang-like teeth absent in both jaws; conical teeth on the vomer boomerang-formed (Figure 4C); palatine teeth absent; gillrakers in the form of dentated tubercles (Figure 4F), 1 + 6 – 8 on the outer side of the first arch and 1+5–8 on the inner side. The colouration of fresh specimens is pinkish-reddish on the head, body and fins, and greyish on the ventral visceral part (Figure 2 and Figure 3).
3.8. Molecular Taxonomic Remarks
Figure 5 shows a molecular cladogram of valid COI Gaidropsarus species sequences publicly available. In this figure, Gaidropsarus gallaeciae sp. nov. is located at an independent and well differentiated branch. The genetic distance of the sequence of the new species from those of its congeners far exceeds 2%, which is the cut-off value for species delimitation in teleost marine fishes [29]. This figure partially illustrates previously obtained results, in which this species is reported as Gaidropsarus sp. 1 [2] or as Gaidropsarus sp. [3].
Figure 5.
Neighbour-Joining tree, based on p-distances, for COI sequences of Gaidropsarus analyzed in this study. Numbers at the main nodes are bootstrap percentages after 2000 replicates. Only values higher than 70% are shown. The scale refers to the number of substitutions per nucleotide.
3.9. Habitat and Distribution
Known specimens were collected from two seamount-like structures in the Northeast Atlantic, the Galicia and Porcupine Banks, at 788 and 751 m depth, respectively (Figure 1). All specimens were caught together with a large amount of live and dead cold-water coral of D. pertusum, Desmophyllum dianthus (Esper, 1794) and Madeprora oculata Linnaeus, 1758, a fact that support this being the preferred habitat of the species. In the Galicia Bank, this habitat was named as “Summit Sands with CW coral reef patches“, corresponding with A6.611 Deep-sea D. pertusum reefs in the EUNIS classification [22]. Considering the presence of cold-water coral communities around the world, the new species it is likely to be widely distributed, but most probably throughout the eastern Atlantic and Mediterranean areas.
3.10. Accompanying Fauna
The two specimens of the Galicia Bank were collected along with 21 other fish species, including several gadiform such as Guttigadus latifrons (Holt & Byrne, 1908), Halargyreus johnsonii Günther, 1862, Lepidion lepidion (Risso, 1810), Mora moro (Risso, 1810) and Nezumia aequalis (Günther, 1878). The list of invertebrates caught included around 70 different species of crustaceans, molluscs, echinoderms and cnidarians (dead coral, D. pertusum, M. oculata).
The four specimens of the Porcupine Bank were collected along with one another rockling species, G. granti [9], and 33 other fish species, including also several gadiform fishes such as Trachyrincus scabrus (Rafinesque, 1810), L. lepidion, Phycis blennoides (Brünnich, 1768) or H. johnsonii among others. The list of bottom living invertebrates collected from the same site included 38 species of crustaceans, molluscs, echinoderms and cnidarians (dead coral, D. pertusum, D. dianthus).
4. Discussion
The morphology of the genus Gaidropsarus is conservative, making it difficult to find diagnostic characters and to establish an identification key for all known species. Gaidropsarus gallaeciae sp. nov. shares many morphological characters with the other congeneric species. A combination of meristic, biometric, colouration, geographical distribution and depth characters is therefore needed to differentiate the new species from all congeneric species.
The number of vertebrae is an important diagnostic character in distinguishing species of Gaidropsarus. On this basis, the species of this genus can be divided into two groups, either those that may have 45 vertebrae or less or those with more than 45 vertebrae. Our newly described species, Gaidropsarus gallaeciae sp. nov. is included in the first group together with G. macrophthalmus, G. capensis and G. granti.
Traditional taxonomy is descriptive, but the diagnostic characters of many hitherto unrevised fishes come from early manuscripts, which often refer to the examination of only a few specimens, and these results have come down to the present day with minimal changes. However, the magnitude of the variation of morphological characters in fishes, mainly biometrics and meristics, is not properly known and they are often underestimated, being the cause of erroneous denominations and the emergence of synonymies [30]. This seems to be the case for Gaidropsarus species. For example, G. gutattus, now considered a synonym of G. mediterraneus [2,3], was originally described by Collett [31] based on two specimens and only three more were subsequently analysed [23]. Therefore, the morphology of this species has only been based on the examination of five specimens, which is clearly insufficient to know the natural morphological variation of a species. The number of specimens examined was also low for the rest of the species. The specific distinction of G. insularum, G. novaezealandiae and G. parini carried out by Svetovidov [23] is based on only three, ten and two specimens, respectively, so it is not surprising that the valid status of these species has been questioned by Andrew et al. [32].
The main distinctive characters found in Gaidropsarus gallaeciae sp. nov. were the aforementioned number of vertebrae, the interorbital space, the length of the anal fin base, the length of the first dorsal fin ray and the length of the chin barbel. However, given the small number of specimens of Gaidropsaurus species examined, the number of distinctive characters may be reduced in the future.
Among the descriptive characters, the meristic ones have traditionally been used in the identification keys of Gaidropsarus. In fact, the number of fin rays is an important feature for taxonomic discrimination between species of this genus [23,24]. Short, non-overlapping ranges of morphological characters will favour the detection of distinctive characters, while wide, overlapping ranges make it difficult. The main diagnostic characters of the genus were compiled by Svetovidov [23,24] and successive updates [2,4]. In their revision, Barros-García et al. [2] show a large interspecific overlap of the meristic features, resulting in a set of conservative morphological traits. On the other hand, only a few other characters were used as diagnostics. The length of the first dorsal fin ray is longer in the boreal G. ensis and G. argentatus with respect to other species, whereas G. macrophthalmus is distinguished by the presence of enlarged canine teeth on the upper jaw [23,24,28]. Small eyes, contained five or more times in the head length separate G. vulgaris, G. granti and G. mediterraneus from G. macrophthalmus, with large eyes, contained less than five times in the head length [28]. However, measurements of comparative material of G. macrophthalmus show the eye diameter is contained 4.9 to 6.3 times in the head length, which refutes this character as diagnostic. Further sampling effort would be needed to gain knowledge of the true morphological variation of Gaidropsarus species in order to create more reliable identification keys.
The coloration pattern is also a diagnostic character in the genus Gaidropsarus [4,23,24,28]. The coloration of G. granti, with a whitish sinuous longitudinal band is a quick and useful diagnostic character for the species [9], and the presence of dark spots on the dorsal parts of head and body, and on the second dorsal and caudal fins is also a diagnostic character for G. vulgaris [28]. The colour pattern of this genus varies with depth, from a polymorphic colour in shallower species to a uniform coloration in the deeper ones, most likely tied to how light penetrate the ocean water and camouflaging is needed. Thus, G. mediterraneus, the shallower species of the genus, shows a cryptic and variable coloration, which has probably led to the consideration of two different species, G. mediterraneus from the continental area and G. gutattus from the insular one, when, in fact, they are one and the same [2,3]. Whereas G. mediterraneus has a more or less uniform brown colouration, G. gutattus exhibits a whitish mottled one. This synonymy was already pointed out by Orsi Relini & Relini [33], who reported that G. mediterraneus sometimes showed the typical coloration of G. gutattus, with irregular light spots on its dorsal and lateral dark brown surfaces. This is also the most probable cause of the erroneous record of G. guttatus in continental area [34].
In contrast, the North Atlantic deeper species such as G. granti, G. argentatus, G. mauli and Gairopsarus gallaeciae sp. nov. show a similar homogeneous pink-reddish coloration which could difficult their correct identification, particularly in juvenile stages.
The analyses of Barros-García et al. [2,3] seems to point to the real composition of species of this genus, with a reduction of valid shallower species, due to synonymy recognition, suggesting, furthermore, the existence of a greater diversity hidden in the deep. The recent record of G. mauli [4] and Gaidropsarus gallaeciae sp. nov. themselves would confirm this hypothesis. Moreover, according to the molecular results, other deep-sea species remain yet undescribed [2]. Considering that most of the deep-sea areas are unexplored and that the occurrence of Gaidropsarus species reported by in vivo sampling techniques (ROVs, AUVs, BCs) is not unusual, an increased number of undiscovered deep-sea species of this genus is predictable.
Eggs, larvae and juveniles of Gaidropsarus species are pelagic [6]. Pelagic early stages and the absence of physical barriers in the ocean should prevent rapid speciation events, but this statement contrasts with the fact of finding greater diversity at depth. However, depth-related ecological niche axis along which divergence occurs is due to local adaptation to diverse feeding habitats, light conditions, spawning sites, or other ecological factors [35]. For instance, speciation in the Pacific rockfish genus Sebastes is associated with divergence in habitat depth and a depth-associated morphology, in the absence of geographic barriers [36].
Therefore, diversification processes according to deep-sea environments is proposed for the genus Gaidropsarus. Ecological speciation occurs when adaptation to different environments or resources causes reproductive isolation [37]. Changing habitats with depth can create ecological opportunities for colonisation processes, promoting species diversification. The recently discovered species G. mauli was first found in a hydrothermal vent site, in crevices along rocky walls in the vicinity of the venting fluids [4]. Given the abundant presence of live and dead coral in the catches of all of the specimens, the occurrence of Gaidropsarus gallaeciae sp. nov. could be associated with the presence of cold-water coral reefs, as also occurs with G. granti [9]. In fact, both species were caught in the same haul in the Porcupine Bank, and both were also found in samples with coral in the Galicia Bank [38] which reinforces this likely coexistence and niche overlap. Several other fish species caught alongside the new species such as M. moro, L. lepidion and G. latifrons are also associated with the occurrence of cold-water corals [39], reinforcing the above. Association between Gaidropsarus species and cold-water corals has been often observed in live specimens on and between live and dead coral thickets [12,17,39,40]. Underwater observations have also shown much imagery evidence of a strict territorial behaviour of Gaidropsarus sp. in D. pertusum or M. oculata colonies [41]. The size of Gaidropsarus gallaeciae sp. nov. appears to be small, up to 11 cm TL, which could also be a morphological adaptation to shelter among the branches of corals as protection against predators. Hydrothermal vent fields and cold-water corals are two of the varied habitats responsible for the high biodiversity found in the deep ocean [42].
DNA barcoding greatly facilitates the grouping of individuals into putative species, which must then be validated through morphological scrutiny by taxonomic experts. Though morphology is the traditional technique used in alpha taxonomy, genetic tools are becoming increasingly common in studies describing new species, especially when morphological data are ambiguous [43]. In this aspect, Renner et al. [44] recommended DNA-based diagnoses of new species in all taxonomic groups, not just bacterial. A DNA barcoding analysis including the calculation of COI genetic distances [2] clearly differentiated Gaidropsarus gallaeciae sp. nov. (then reported as Gaidropsarus sp. 1) from six valid and two unidentified species from the North Atlantic. Considering that this previous study was based only on mitochondrial DNA sequences, a multilocus species delimitation analysis was carried out, including both mitochondrial (COI, CytB, ND2) and nuclear (Rho, ZIC1) genetic markers, that finally confirmed this previous finding [3].
Although no southern hemisphere species sequences are available, a BOLD search of Gaidropsarus gallaeciae sp. nov. returns a difference of 4.11%, a typical species differentiation distance, with a private COI sequence assigned to southern species G. novaezealandiae. Interestingly, this would be the smallest genetic distance found between the new species and all other congeneric species, as this species is more distantly related to all of the North Atlantic species examined, ranging from 13.21 to 17.36% [2].
A revision of this genus based on extensive collections of specimens and DNA sequencing is needed [4]. Therefore, it is necessary to complete the sequence database with specimens from southern hemisphere to better understand the interspecific relationships of this genus. Without the slightest doubt, the integrative study of traditional and molecular taxonomy can highlight identification mistakes and incongruities between the two disciplines, helping to reveal cryptic species, to identify immature specimens, and to clarify synonymies [45].
5. Conclusions
The occurrence of a new fish species Gaidropsarus gallaeciae sp. nov. is well supported by morphological and molecular analyses that clearly differentiate it from other known species. The taxonomy of the genus Gaidropsarus remains poorly understood. The relatively large number of species in this genus, the scattered distribution of many of them, and the small number of specimens examined and/or found are probably the main reasons for this insufficient knowledge. Although morphological characters are conservative, overlapping to a large extent between species, some diagnostic characters can be identified. These, together with colouration, geographical distribution and depth range can currently be applied for species identification. Molecular taxonomy of North Atlantic species has helped to resolve some taxonomic inaccuracies, but also flags the presence of undescribed species. Examination of more specimens and obtaining DNA sequences of southern hemisphere species should be the next objective to clarify the taxonomy of this difficult group.
Acknowledgments
The authors would like to thank the staff involved in the research surveys INDEMARES 2010 and PORCUPINE 2019 of the Spanish Institute of Oceanography (IEO) on board the R/V “Thalassa” and “Vizconde de Eza” (Ministry of Agriculture, Fisheries and Food, Spain). We also thank Francisco Sánchez, (ECOMARG-IEO Santander) for the donation of the underwater image of Gaidropsarus sp. used in the Graphical Abstract and to Lucía Ruiloba (IIM-CSIC) for her assistance with Figure 4.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11060860/s1, Table S1. Morphological data of the material comparative examined.
Author Contributions
Conceptualization, R.B.; resources, A.d.C., F.B. and A.S.; funding acquisition A.d.C., F.B. and A.S; data curation, R.B., F.B., A.S., D.B.-G. and A.d.C.; analysis, R.B., A.d.C. and D.B.-G.; writing—original draft preparation, R.B., F.B. and D.B.-G.; writing—review and editing, R.B., F.B., A.S., D.B.-G. and A.d.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval was waived for this study because all specimens examined were from fishery catches and were already dead.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequences employed in the current study are available in the BOLD systems (https://www.boldsystems.org/, accessed on 27 April 2022) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 27 April 2022) repositories. All specimens used in this study for taxonomical purposes are deposited in the fish collection of the Museo de Historia Natural, Universidade de Santiago de Compostela (MHNUSC) in Santiago de Compostela, Spain (see methods). All other data are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Spanish Bottom Trawl Survey on the Porcupine Bank (SP-PORC-Q3) was funded in part by the EU through the European Maritime and Fisheries Fund (EMFF) within the Spanish National Program of collection, management and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy. Galicia Bank data used in this work were obtained from the EC contract INDEMARES-LIFE (07/NAT /E/000732). D.B.-G. was supported by national funds by FCT -Foundation for Science and Technology through the projects UIDB/04423/2020; UIDP/04423/2020 and 2020.04364.CEECIND. A.d.C. was funded by a grant from the Xunta de Galicia’s program for the consolidation and structuring of competitive research units (code ED431C 2019/28).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balushkin A.V. On the first occurrence of the rockling Gaidropsarus pakhorukovi Shcherbachev (Gairdropsarini, Lotidae, Gadidae) and on species diagnostics of G. pakhorukovi and G. parini Svetovidov. J. Ichthyol. 2009;49:723–729. doi: 10.1134/S0032945209090033. [DOI] [Google Scholar]
- 2.Barros-García D., Bañón R., Arronte J.C., Fernández-Peralta L., García R., Iglésias S.P., Sellos D.Y., Pedro-Barreiros J., Sebastián-Comesaña A., de Carlos A. New insights into the systematics of North Atlantic Gaidropsarus (Gadiformes, Gadidae): Flagging synonymies and hidden diversity. Mar. Biol. Res. 2018;14:17–29. doi: 10.1080/17451000.2017.1367403. [DOI] [Google Scholar]
- 3.Barros-García D., Sebastián-Comesaña A., Bañón R., Baldó F., Arronte J.C., Froufe E., de Carlos A. Multilocus species delimitation analyses show junior synonyms and deep-sea unknown species of genus Gaidropsarus (Teleostei: Gadiformes) in the North Atlantic/Mediterranean Sea area. Mar. Biol. :2022. doi: 10.21203/rs.3.rs-1497892/v1. submitted . [DOI] [Google Scholar]
- 4.Biscoito M., Saldanha L. Gaidropsarus mauli a new species of three-bearded rockling (Gadiformes, Gadidae) from the Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge) and the Biscay Slope (Northeastern Atlantic) Zootaxa. 2018;4459:301–314. doi: 10.11646/zootaxa.4459.2.5. [DOI] [PubMed] [Google Scholar]
- 5.Roa-Varón A., Dikow R.B., Carnevale G., Tornabene L., Baldwin C.C., Li C., Hilton E.J. Confronting sources of systematic error to resolve historically contentious relationships: A case study using Gadiform fishes (Teleostei, Paracanthopterygii, Gadiformes) Syst. Biol. 2021;70:739–755. doi: 10.1093/sysbio/syaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen D.M., Inada T., Iwamoto T., Scialabba N. FAO species catalogue. Gadiform fishes of the world (Order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fish. Synop. 1990;10:1–442. [Google Scholar]
- 7.Francisco S.M., Robalo J.I., Stefanni S., Levy A., Almada V.C. Gaidropsarus (Gadidae, Teleostei) of the North Atlantic Ocean: A brief phylogenetic review. J. Fish Biol. 2014;85:473–487. doi: 10.1111/jfb.12437. [DOI] [PubMed] [Google Scholar]
- 8.Bañón R., Arronte J.C., Rodríguez-Cabello C., Piñeiro C.G., Punzón A., Serrano A. Commented checklist of marine fishes from the Galicia Bank seamount (NW Spain) Zootaxa. 2016;4067:293–333. doi: 10.11646/zootaxa.4067.3.2. [DOI] [PubMed] [Google Scholar]
- 9.Bañón R., Ruiz-Pico S., Baldó F., de Carlos A. Unexpected deep-sea fish species on the Porcupine Bank (NE Atlantic): Biogeographical implications. J. Fish Biol. 2020;97:908–913. doi: 10.1111/jfb.14418. [DOI] [PubMed] [Google Scholar]
- 10.Morato T., Hoyle S.D., Allain V., Nicol S.J. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. USA. 2010;107:9707–9711. doi: 10.1073/pnas.0910290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koeda K., Takashima S., Yamakita T., Tsuchida S., Fujiwara Y. Deep-sea fish fauna on the seamounts of southern Japan with taxonomic notes on the observed species. J. Mar. Sci. Eng. 2021;9:1294. doi: 10.3390/jmse9111294. [DOI] [Google Scholar]
- 12.Linley T.D., Lavaleye M., Maiorano P., Bergman M., Capezzuto F., Cousins N.J., Priede I.G. Effects of cold-water corals on fish diversity and density (European continental margin: Arctic, NE Atlantic and Mediterranean Sea): Data from three baited lander systems. Deep-Sea Res. II Top. Stud. Oceanogr. 2017;145:8–21. doi: 10.1016/j.dsr2.2015.12.003. [DOI] [Google Scholar]
- 13.Iwamoto T., Okamoto M. A new grenadier fish of the genus Lucigadus (Macrouridae, Gadiformes, Teleostei) from the Emperor Seamounts, Northwestern Pacific. Proc. Calif. Acad. Sci. 2015;62:369–380. [Google Scholar]
- 14.Møller P.R., Schwarzhans W.W., Lauridsen H., Nielsen J.G. Bidenichthys okamotoi, a new species of the Bythitidae (Ophidiiformes, Teleostei) from the Koko Seamount, Central North Pacific. J. Mar. Sci. Eng. 2021;9:1399. doi: 10.3390/jmse9121399. [DOI] [Google Scholar]
- 15.Cuvelier D., Sarrazin J., Colaco A., Copley J., Desbruyeres D., Glover A.G., Tyler P., Santos R. Distribution and spatial variation of hydrothermal faunal assemblages at Lucky Strike (Mid-Atlantic Ridge) revealed by high-resolution video image analysis. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2009;56:2026–2040. doi: 10.1016/j.dsr.2009.06.006. [DOI] [Google Scholar]
- 16.Söffker M., Sloman K.A., Hall-Spencer J.M. In situ observations of fish associated with coral reefs off Ireland. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2011;58:818–825. doi: 10.1016/j.dsr.2011.06.002. [DOI] [Google Scholar]
- 17.Wienberg C., Beuck L., Heidkamp S., Hebbeln D., Freiwald A., Pfannkuche O., Monteys X. Franken Mound: Facies and biocoenoses on a newly-discovered “carbonate mound” on the western Rockall Bank, NE Atlantic. Facies. 2008;54:1–24. doi: 10.1007/s10347-007-0118-0. [DOI] [Google Scholar]
- 18.Morato T., Kvile K.Ø., Taranto G.H., Tempera F., Narayanaswamy B.E., Hebbeln D., Menezes G., Wienberg C., Santos R.S., Pitcher T.J. Seamount physiography and biology in North-East Atlantic and Mediterranean Sea. Biogeosciences. 2013;10:3039–3054. doi: 10.5194/bg-10-3039-2013. [DOI] [Google Scholar]
- 19.Bañón R., de Carlos A., Farias C., Vilas-Arrondo N., Baldó F. Exploring deep-sea biodiversity in the Porcupine Bank (NE Atlantic) through fish integrative taxonomy. J. Mar. Sci. Eng. 2021;9:1075. doi: 10.3390/jmse9101075. [DOI] [Google Scholar]
- 20.Johnston G., O’Hea B., Dransfeld L. Fish species recorded during deepwater trawl surveys on the continental shelf and the Porcupine Bank, 2006–2008. Ir. Nat. J. 2010;31:130–134. [Google Scholar]
- 21.O’Riordan C.E. Some interesting fishes and other marine fauna from the Porcupine Bank. Ir. Nat. J. 1984;21:321–323. [Google Scholar]
- 22.Serrano A., González-Irusta J.M., Punzón A., García-Alegre A., Lourido A., Ríos P., Blanco M., Gómez-Ballesteros M., Druet M., Cristobo J., et al. Deep-sea benthic habitats modeling and mapping in a NE Atlantic seamount (Galicia Bank) Deep-Sea Res. Part I Oceanogr. Res. Pap. 2017;126:115–127. doi: 10.1016/j.dsr.2017.06.003. [DOI] [Google Scholar]
- 23.Svetovidov A.N. Review of three-bearded rocklings of the genus Gaidropsarus Rafinesque, 1810 (Gadidae) with description of a new species. J. Ichthyol. 1986;62:115–135. [Google Scholar]
- 24.Svetovidov A.N. Gadidae. In: Whitehead P.J.P., Bauchot M.L., Hureau J.C., Nielsen J., Tortonese E., editors. Fishes of the North-Eastern Atlantic and the Mediterranean. Volume 2. UNESCO; Paris, France: 1986. pp. 680–710. [Google Scholar]
- 25.Saitou N., Nei M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto T., Cohen D.M. Gaidropsaridae. In: Carpenter K.E., De Angelis N., editors. The Living Marine Resources of the Eastern Central Atlantic. Bony fishes. Part 1. Elopiformes to Scorpaeniformes. Volume 3. FAO; Rome, Italy: 2016. pp. 2015–2022. [Google Scholar]
- 29.Ward R.D., Hanner R., Hebert P.D.N. The campaign to DNA barcode all fishes, FISH-BOL. J. Fish Biol. 2009;74:329–356. doi: 10.1111/j.1095-8649.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 30.Bañón R., Arronte J.C., Vázquez-Dorado S., del Río J.L., de Carlos A. DNA barcoding of the genus Lepidion (Gadiformes: Moridae) with recognition of Lepidion eques as a junior synonym of Lepidion lepidion. Mol. Ecol. Resour. 2013;13:189–199. doi: 10.1111/1755-0998.12045. [DOI] [PubMed] [Google Scholar]
- 31.Collett R. Diagnoses de poissons nouveaux provenant des campagnes de “L’Hirondelle.” V. Descriptions de deux espèces nouvelles de genre Onus Risso. Bull. Soc. Zool. Fr. 1890;15:105–109. [Google Scholar]
- 32.Andrew T.G., Hecht T., Heemstra P.C., Lutjeharms J.R.E. Fishes of the Tristan da Cunha Group and Gough Island, South Atlantic Ocean. Ichthyol. Bull. 1995;63:1–43. [Google Scholar]
- 33.Orsi Relini L., Relini G. Gaidropsarus granti from a Ligurian seamont: A Mediterranean native species? Mar. Ecol. 2014;35:35–40. doi: 10.1111/maec.12122. [DOI] [Google Scholar]
- 34.Fernández A., Pereiro F.X., Iglesias S., Porteiro C., Pallarés P. La Pesquería demersal gallega, estrategias de pesca para su regulación racional en base a la Merluza. Bol. Inst. Esp. Oceanogr. 1978;4:69–109. [Google Scholar]
- 35.Roy D., Hurlbut T.R., Ruzzante D.E. Biocomplexity in a demersal exploited fish, white hake (Urophycis tenuis): Depth-related structure and inadequacy of current management approaches. Can. J. Fish. Aquat. Sci. 2012;69:415–429. doi: 10.1139/f2011-178. [DOI] [Google Scholar]
- 36.Ingram T. Speciation along a depth gradient in a marine adaptive radiation. Proc. R. Soc. B Biol. Sci. 2011;278:613–618. doi: 10.1098/rspb.2010.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendry A.P. Ecological speciation! Or the lack thereof? Can. J. Fish. Aquat. Sci. 2009;66:1383–1398. doi: 10.1139/F09-074. [DOI] [Google Scholar]
- 38.González-Irusta J.M., De la Torriente A., Punzón A., Blanco M., Arronte J.C., Bañón R., Cartes J., Serrano A. Living at the top. Connectivity limitations and summit depth drive fish diversity patterns in an isolated seamount. Mar. Ecol. Prog. Ser. 2021;670:121–137. doi: 10.3354/meps13766. [DOI] [Google Scholar]
- 39.Milligan R.J., Spence G., Murray Roberts J., Bailey D.M. Fish communities associated with cold-water corals vary with depth and substratum type. Deep-Sea Res. Part I Oceanogr. Res. Pap. 2016;114:43–54. doi: 10.1016/j.dsr.2016.04.011. [DOI] [Google Scholar]
- 40.Quattrini A.M., Nizinski M.S., Chaytor J.D., Demopoulos A.W., Roark E.B., France S.C., Moore J.A., Heyl T., Auster P.J., Kinlan B., et al. Exploration of the canyon-incised continental margin of the Northeastern United States reveals dynamic habitats and diverse communities. PLoS ONE. 2015;10:e0139904. doi: 10.1371/journal.pone.0139904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freiwald A. ((Executive Director Senckenberg am Meer, Wilhelmshaven, Hamburg, Germany)). Personal communication. 2022.
- 42.Paulus E. Shedding light on deep-sea biodiversity—A highly vulnerable habitat in the face of anthropogenic change. Front. Mar. Sci. 2021;8:667048. doi: 10.3389/fmars.2021.667048. [DOI] [Google Scholar]
- 43.Daly-Engel T.S., Baremore I.E., Grubbs R.D., Gulak S.J., Graham R.T., Enzenauer M.P. Resurrection of the sixgill shark Hexanchus vitulus Springer & Waller, 1969 (Hexanchiformes, Hexanchidae), with comments on its distribution in the northwest Atlantic Ocean. Mar. Biodivers. 2019;49:759–768. doi: 10.1007/s12526-018-0849-x. [DOI] [Google Scholar]
- 44.Renner S.S. A Return to Linnaeus’s Focus on Diagnosis, Not Description: The Use of DNA Characters in the Formal Naming of Species. Syst. Biol. 2016;65:1085–1095. doi: 10.1093/sysbio/syw032. [DOI] [PubMed] [Google Scholar]
- 45.Pires A.C., Marinoni L. DNA barcoding and traditional taxonomy unified through integrative taxonomy: A view that challenges the debate questioning both methodologies. Biota Neotrop. 2010;10:339–346. doi: 10.1590/S1676-06032010000200035. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences employed in the current study are available in the BOLD systems (https://www.boldsystems.org/, accessed on 27 April 2022) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/, accessed on 27 April 2022) repositories. All specimens used in this study for taxonomical purposes are deposited in the fish collection of the Museo de Historia Natural, Universidade de Santiago de Compostela (MHNUSC) in Santiago de Compostela, Spain (see methods). All other data are included in this article.