Abstract
Background
Declines in cardiorespiratory fitness (CRF) and muscle mass are both associated with advancing age and each of these declines is associated with worse health outcomes. Resistance exercise training (RET) has previously been shown to improve muscle mass and function in the older population. If RET is also able to improve CRF, as it has been shown to do in younger populations, it has the potential to improve multiple health outcomes in the expanding older population.
Methods
This systematic review aimed to identify the role of RET for improving CRF in healthy older adults. A search across CINAHL, MEDLINE, EMBASE and EMCARE databases was conducted with meta-analysis performed on eligible papers to identify improvements in established CRF parameters (VO2 peak, aerobic threshold (AT), 6-minute walking distance test (6MWT) following RET intervention. Main eligibility criteria included older adults (aged over 60), healthy cohorts (disease-specific cohorts were excluded) and RET intervention.
Results
Thirty-seven eligible studies were identified. Meta-analysis revealed a significant improvement in VO2 peak (MD 1.89 ml/kg/min; 95% confidence interval (CI) 1.21–2.57 ml/kg/min), AT (MD 1.27 ml/kg/min; 95% CI 0.44–2.09 ml/kg/min) and 6MWT (MD 30.89; 95% CI 26.7–35.08) in RET interventions less than 24 weeks. There was no difference in VO2 peak or 6MWT in interventions longer than 24 weeks.
Discussion
This systematic review adds to a growing body of evidence supporting the implementation of RET in the older population for improving whole-body health, particularly in time-limited timeframes.
Keywords: ageing, resistance exercise, cardiorespiratory fitness, health, older people, systematic review
Key Points
Resistance exercise training improves both cardiorespiratory fitness and muscle strength in older adults.
Resistance exercise training improves multiple measures of cardiorespiratory fitness within a 24-week intervention in older adult.
Resistance exercise training could provide improvement to whole body health within time-limited clinical pathways.
Introduction
Decades of increasing life expectancy have resulted in those over the age of 65 years becoming an increasing proportion of the UK population [1], with this trend replicated across much of the world. However, despite this increase in life span, there has not been an equal match in the rise of healthy life years lived. This disparity means that in the UK an estimated 20% of life is spent in poor health [2]. With predictions that 7% of the UK population will be over the age of 85 years by 2066 [3], it is crucial to develop evidence-based interventions, which can improve the proportion of life spent independent and healthy.
Cardiorespiratory fitness (CRF) is an important aspect of health and is known to decline in a non-linear manner as part of the ageing process [4–6]. Lower levels of CRF are associated with reduced life expectancy, increased healthcare costs and worse clinical outcomes [7–9]. The decline in CRF with advancing age is paralleled by a decrease in skeletal muscle mass and function, particularly muscle strength. This age-associated decline in muscle mass and function is termed sarcopenia [10, 11]. As with reduced CRF, sarcopenia has been linked with shorter life expectancy and is an independent predictor of poor clinical (i.e. surgical) outcomes [12–15]. Both low CRF and sarcopenia are viewed to be key components of frailty [16].
Exercise training-induced changes in CRF are thought to be due to the release and action of myokines [17–19]. Myokines induce changes locally in the muscle by regulating muscle development and enhancing muscle function. Myokines have distal effects, largely on adipose stores, enhancing metabolic pathways and inhibiting inflammatory responses [20].
Traditionally, aerobic exercise training (AET) has been used to improve CRF (including in older adults) [21, 22]; however, this form of training fails to improve muscle mass [23]. Conversely, resistance exercise training (RET) is the most employed modality to improve both muscle mass and function [24]. In young adults, RET has also been shown to improve CRF [25]; however, the impact of RET on the CRF of older adults is not well established. Building on observations in younger adults, this systematic review aims to explore the impact of RET on the CRF of healthy older adults.
Methods
The systematic review was registered on PROSPERO (ID CRD42020223356). A literature search was performed across CINAHL, MEDLINE, EMBASE, EMCARE, PubMed and Cochrane databases using a PICO protocol (Appendix 1). Databases were searched from database creation to 21 January 22. Only randomised control studies available in the English language were deemed eligible for inclusion.
Population
The population was determined to be healthy older adults (male or female, aged over 60 years), Given the high prevalence of chronic diseases within the older population, studies were included if they included a participant with chronic illnesses; however, studies were excluded if participants were recruited according to a disease profile (e.g. a group of participants all suffering from chronic obstructive pulmonary disease or heart failure would be excluded).
Intervention
The intervention was defined as strength or resistance-based exercise training involving multiple training sessions. Studies were excluded if the intervention provided combined exercise training (involving both aerobic and RET), or if assessment was conducted after a single training session (i.e. no training program was delivered). Studies with exercise programs longer than 24 weeks were included in separate sub-analysis to improve homogeneity between short-term and long-term studies. A control group was defined as a group performing no exercise or a sham exercise intervention.
Paper selection process
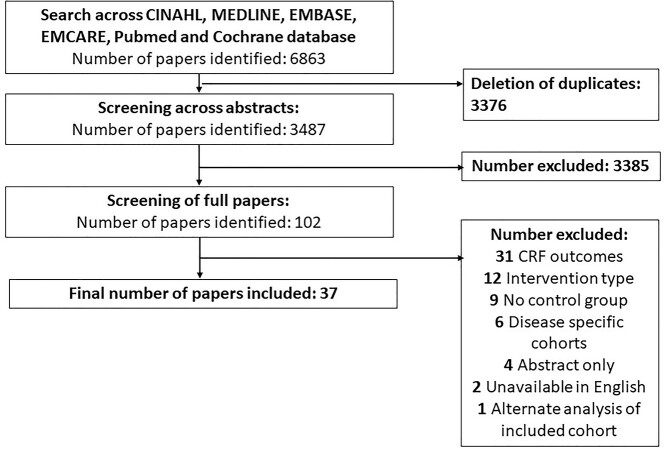
Two authors (M.P. and T.S.) independently screened abstracts using RAYYAN [26], full papers were then screened and any disagreements were resolved by consensus. A flow diagram of study identification can be seen in Figure 1.
Figure 1.
Paper identification via PRISMA Guidelines.
Outcome measures
The primary outcome measures of CRF included were maximal oxygen consumption (VO2 Peak), 6-minute walking test (6MWT) distance and anaerobic threshold (AT).
Statistical analysis
Effect estimates are reported as mean differences (MD) with 95% confidence intervals (CIs). Due to the clinical heterogeneity between shorter (<24 weeks) and longer term (>24 weeks) interventions, these were analysed separately. Due to inconsistent reporting of mean changes and change standard deviations (SDs), we calculated these using formulae from the Cochrane Handbook. We assumed a correlation coefficient of 0.7 between baseline and final values based on analysis of our previous, similar data [27]. When data were reported using l/min, we transformed data to ml/kg/min using average weight values from the study. We performed meta-analysis using a restricted maximum likelihood random effects model [28]. When more than 10 studies were included in a meta-analysis, we produced contour-enhanced funnel plots (P = 0.05 contour) and tested for possible publication bias using Egger’s linear regression test (P < 0.1). We used the I2 statistic to assess statistical heterogeneity. We also investigated heterogeneity by number of weeks of the intervention using a restricted maximum likelihood random effects meta-regression with Knapp–Hartung modification (>10 included studies with P < 0.1). This is reported with the R2 analogue. We used GRADE to assess the certainty of evidence for each outcome. We conducted sensitivity analysis by assuming different correlation coefficients when calculating change SDs (0.5 and 0.9). All data were extracted into Stata Version 16 for analysis.
Results
Included studies
Thirty-seven studies were identified as eligible. Study basic information and intervention design can be seen in Appendix 2A. Study demographics can be seen in Appendix 2B.
Risk of bias
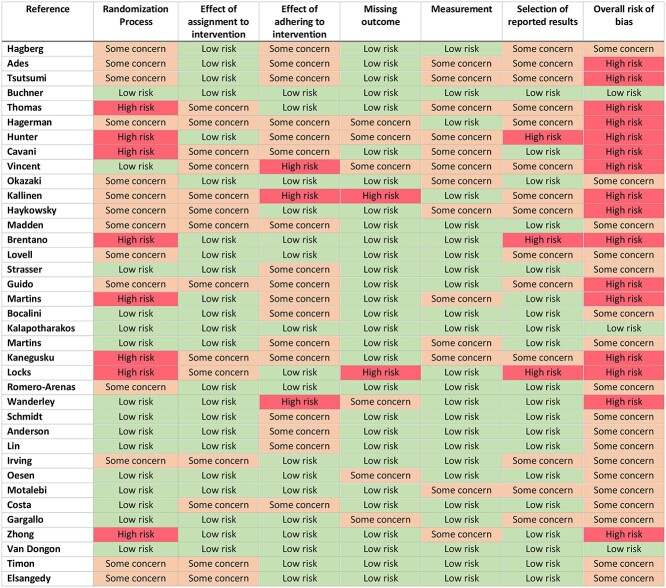
All papers were analysed for risk of bias using the Cochrane risk-of-bias tool for randomised control trials (RoB2) [29]. Details of this analysis are presented in Table 1.
Table 1.
Risk of bias analysis using RoB2
|
VO2 peak
We included 24 studies for VO2 peak; of these, 22 studies were eligible for meta-analysis as shown in Figure 2 [30–51]. Three of these studies had intervention periods over 24 weeks [32, 39, 43]. Overall, there was a significant increase in VO2 peak with RET if the intervention was 24 weeks or less (MD 1.89 ml/kg/min; 95% CI 1.21–2.57 ml/kg/min). There was no difference in VO2 peak with RET intervention periods longer than 24 weeks (MD −0.01 ml/kg/min; 95% CI −1.19 to 1.17 ml/kg/min). There was no funnel plot asymmetry on visual inspection and there was no evidence of possible publication bias on statistical testing (P = 0.54) (Appendix 3A). There was considerable statistical heterogeneity when the intervention was 24 weeks or less (I2 = 77%), but not when the intervention was longer than 24 weeks (I2 = 0%). On meta-regression analysis, the improvement in VO2 peak was not predicted by number of weeks of the intervention (R2 = 2%; P = 0.42). Similar estimates were obtained on sensitivity analysis. The certainty of evidence was moderate to high due to no evidence of publication bias and narrow confidence intervals with a large sample size, however given the risk of bias across the papers some concerns remained.
Figure 2.
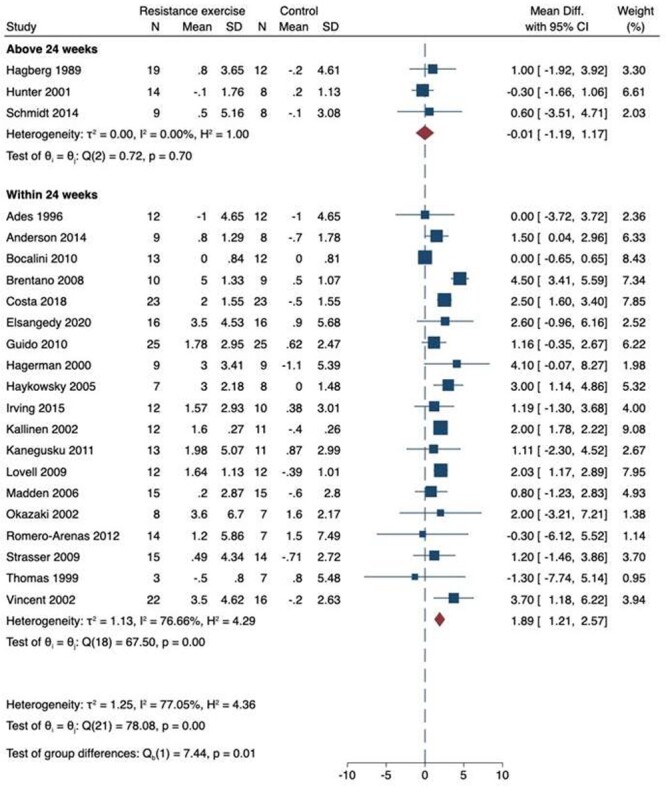
Meta-analysis of 22 studies exploring the effect of resistance exercise training on VO2 peak in healthy older adults.
Two papers reported VO2 outcomes as percentage change and were therefore not included in analysis. One paper reported no significant change when compared to control with a 24- to 26-week intervention (0.3% versus 0.3%) [41]. The other paper reported that there was a change between 0.2 and 2.9 ml/kg/min across all groups when comparing 12 weeks of high-intensity RET, low-intensity RET or control [52].
Six-minute walk test
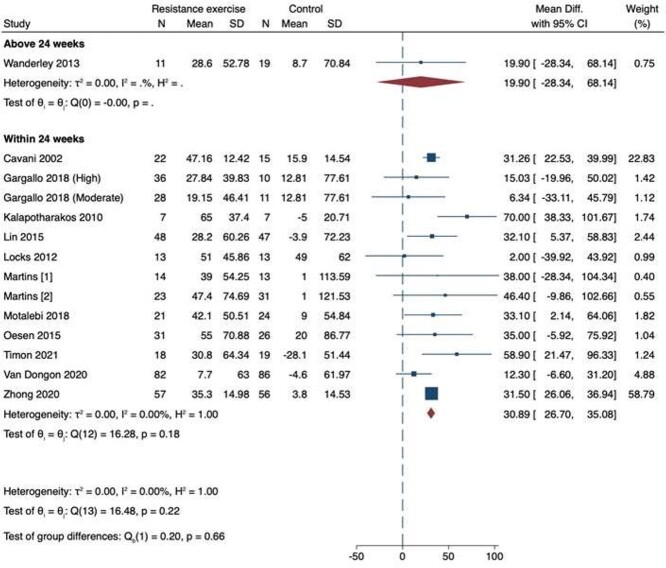
We included 13 studies for 6MWT as shown in Figure 3 [53–65]. One study had an intervention period above 24 weeks and 12 studies 24 weeks and below. There was an increase in 6MWT in the shorter (24 weeks and less) (MD 30.89; 95% CI 26.7–35.08) but not the longer (more than 24 weeks) (MD 19.9; 95% CI −28.34 to 68.14) studies. There was no evidence of statistical heterogeneity for either duration of intervention (I2 = 0%). There was no funnel plot asymmetry on visual inspection and there was no evidence of possible publication bias on statistical testing (P = 0.91) (Appendix 3B). On meta-regression, length of intervention did not predict increase in 6MWT (R2 = 27%; P = 0.81). Similar estimates were obtained on sensitivity analysis. The certainty of evidence was moderate for interventions less than 24 weeks given the number of studies included with no evidence of publication bias, however with high weighting on a single study with a high risk of bias concerns remain.
Figure 3.
Meta-analysis of 13 papers exploring the effect of resistance exercise training on 6MWT in healthy older adults.
Aerobic threshold
We included four studies for AT as shown in Figure 4 [37, 38, 48, 49]. All interventions were 24 weeks or less. Overall, there was a significant increase in AT with RET (MD 1.27 ml/kg/min; 95% CI 0.44–2.09 ml/kg/min). There was no evidence of statistical heterogeneity (I2 = 0%). There were too few studies to assess publication bias or conduct meta-regression. Similar estimates were obtained on sensitivity analysis. The certainty of evidence was very low, given the low number of papers included and high weighting to a single study.
Figure 4.
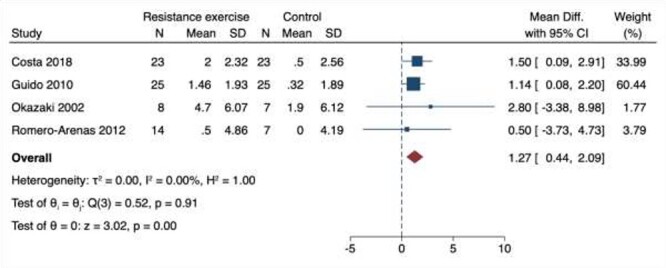
Meta-analysis of four papers exploring the effect of resistance exercise training on AT in healthy older adults.
Discussion
Given the known benefits of RET on muscle mass and strength, but limited knowledge of its impact upon CRF in older adults, we sought to systematically review and quantify the impacts of RET on CRF specifically in this age group. The main finding is that the CRF of older adults can be improved by a short-term RET program. Curiously, this improvement is not apparent with longer term RET.
This review includes a total of 1,641 older adults undergoing RET (832) or a no/sham-intervention control (809). The average age of those completing RET was 70.21, with the control participants aged 70.54. The dropout rate of participants across all studies was low at 10.2%, suggesting that RET is well tolerated by older adults. This dropout rate is comparable to the 12% previously reported for AET in older adults [66]. In this review, the most cited reasons for non-completion were non-clinical, including (i) geographical relocation, (ii) time commitment and (iii) failure to attend for reassessment. There was a low reported incidence of clinical reasons for dropout. Those that were reported included undergoing surgery unrelated to the study, or minor musculoskeletal injury. Kallinen et al. reported one death due to a myocardial infarction and another participant developing unstable angina [51]. Both cardiovascular events were reported to have started >2 days after a RET session and as such were not felt to be directly caused by the training. It should however be noted that in retrospect, an area of ischaemic change was identified on exercise ECG in the participant who developed unstable angina. Given that all modalities of exercise training carry cardiovascular risk in previously untrained individuals, and in accordance with guidelines published by the American College of Sports Medicine, all individuals commencing exercise training for the first time or after a prolonged hiatus are recommended to have a medical screening [67].
Despite the potential positive impact of RET for improving CRF in older adults highlighted by this review, there is difficulty in performing direct comparisons across studies given the wide variety of RET provided. When considering the FITT (frequency, intensity, time and type) principles of exercise training [67], length of training is only one important variable for exercise adaptation and training progression. Another core concept in exercise training is that of ‘overload’; the concept of a new challenge (often by altering FITT components) being required for progression [68]. The studies included in this review involved a variety of techniques to both set and progress the RET. These techniques included using a progressive rise in resistance according to the Borg Rating of Perceived Exertion scale [69], and performing one repetition maximum reassessments throughout the RET program [70]; with not all studies stipulating how the resistance was set or adjusted. There was also variety in the type of RET used with machine weights, free weights and alternatives (such as resistance bands) used. It is therefore perhaps even more promising that, despite this significant variability across studies, RET was still deemed able to improve the CRF of older adults within a short-term exercise program.
Another difficulty allowing for direct comparison is the different methods used to ascertain the measures. For example, in the most reported outcome, VO2 peak, both treadmills and cycle ergometers were used. This is with known differences in the O2 cost of these exercise modalities [71], and reported differences in maximum O2 uptake between them [72].
The improvement in CRF following a short-term RET programme is arguably of great importance when promoting exercise for older adults within clinical pathways. Improvement in CRF, combined with the established benefits of muscle mass and strength gains with RET [24], means that RET has the potential to impact on multiple facets of systems physiology known to decline with advancing age. RET could disrupt multiple processes of the frailty model and thus promote whole-body health. This proposition is supported by work demonstrating the impact of RET on functional outcomes such as reduced likelihood of falls [73, 74].
That all three of our validated measures of CRF (VO2, 6MWT and AT) showed improvements following short-term RET, also suggests that in a clinical or resource-limited setting, ‘bedside’ assessments of CRF such as 6MWT may be able to determine the benefits of RET, without the need for labour (personnel and equipment) intensive cardiopulmonary exercise testing. However, that longer RET did not seem to elicit improvements in CRF is curious. Beyond the small (n = 4) number of studies which included longer duration interventions [32, 43, 58, 75], one possible reason for this finding is that 50% of these studies employed an intervention, which was only two sessions per week, compared to three sessions each week being the most reported frequency in the shorter interventions. In addition, it may be speculated that individuals engaged in a regular RET intervention over a longer period may reduce their other activity outside of these sessions, although habitual physical activity was seldom reported in the studies included in this review.
In conclusion, this systematic review demonstrates that short-term RET improves CRF in healthy older adults, based on evidence using multiple measures of this health-related parameter. This finding, combined with the already established evidence base that RET improves muscle mass and function [24], suggests that RET should be an integral aspect of exercise promotion for an ageing population. Further, that ‘lack of time’ is a commonly cited barrier to exercise in older adults [76] and that older adults commonly face time-limited clinical pathways where improvements in physiological resilience have been shown to be beneficial (i.e. surgical prehabilitation [77]), RET may be able to elicit benefit in two key components of whole body health (i.e. muscle and CRF) in older adults.
Supplementary Material
Contributor Information
Thomas F F Smart, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK; Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Brett Doleman, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK; Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Jacob Hatt, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK; Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Melanie Paul, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK; Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Suzanne Toft, Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Jonathan N Lund, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK; Department of Surgery and Anaesthetics, Royal Derby Hospital, Derby DE22 3NE, UK.
Bethan E Phillips, Centre of Metabolism, Ageing and Physiology (COMAP), School of Medicine, Royal Derby Hospital Centre, University of Nottingham, Derby DE22 3DT, UK; MRC-Versus Arthritis Centre for Musculoskeletal Ageing Research and Nottingham National Institute for Health Research (NIHR) Biomedical Research Centre (BRC), Derby, UK.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This research was supported by the MRC/Versus Arthritis Centre for Musculoskeletal Ageing Research (grant number MR/R502364/1) and the National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre (BRC).
References
- 1. Living Longer. Office for National Statistics. [Google Scholar]
- 2. Health State Life Expectancies. UK-Office for National Statistics. [Google Scholar]
- 3. Estimates of the Very Old, Including Centenarians. QMI-Office for National Statistics. [Google Scholar]
- 4. Lee DC, Artero EG, Sui X, Artero EG, Xuemei Sui, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol 2010; 24: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fleg JL, Morrell CH, Bos AGet al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005; 112: 674–82. [DOI] [PubMed] [Google Scholar]
- 6. Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med 2009; 169: 1781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Snowden CP, Prentis J, Jacques Bet al. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg 2013; 257: 999–1004. [DOI] [PubMed] [Google Scholar]
- 8. Mandsager K, Harb S, Cremer P, Phelan D, Nissen SE, Jaber W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open 2018; 1: e183605. 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bachmann JM, DeFina LF, Franzini Let al. Cardiorespiratory fitness in middle age and health care costs in later life. J Am Coll Cardiol 2015; 66: 1876–85. [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997; 127: 990S–1S. [DOI] [PubMed] [Google Scholar]
- 11. Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab 2010; 35: 707–12. [DOI] [PubMed] [Google Scholar]
- 12. Pérez-Zepeda MU, Sgaravatti A, Dent E. Sarcopenia and post-hospital outcomes in older adults: a longitudinal study. Arch Gerontol Geriatr 2017; 69: 105–9. [DOI] [PubMed] [Google Scholar]
- 13. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010; 21: 543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wagner D, DeMarco M, Amini Net al. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg 2016; 8: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr 2013; 32: 772–6. [DOI] [PubMed] [Google Scholar]
- 16. Fried LP, Tangen CM, Walston Jet al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–57. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients 2020; 12: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb Perspect Med 2017; 7: a029793. 10.1101/CSHPERSPECT.A029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8: 457–65. [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Wang L, You W, Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. J Cell Physiol 2021; 236: 2393–412. [DOI] [PubMed] [Google Scholar]
- 21. Boileau RA, McAuley E, Demetriou Det al. Aerobic exercise training and cardiorespiratory fitness in older adults: a randomized control trial. J Aging Phys Act 1999; 7: 374–83. [Google Scholar]
- 22. Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 2016; 11: 216–22. [DOI] [PubMed] [Google Scholar]
- 23. Keating CJ, Párraga Montilla J, Latorre Román P, Moreno del Castillo R. Comparison of high-intensity interval training to moderate-intensity continuous training in older adults: a systematic review. J Aging Phys Act 2020; 28: 798–807. [DOI] [PubMed] [Google Scholar]
- 24. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010; 9: 226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashton RE, Tew GA, Aning JJ, Gilbert SE, Lewis L, Saxton JM. Effects of short-term, medium-term and long-term resistance exercise training on cardiometabolic health outcomes in adults: systematic review with meta-analysis. Br J Sports Med 2020; 54: 341–8. [DOI] [PubMed] [Google Scholar]
- 26. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blackwell JEM, Doleman B, Herrod PJJet al. Short-term (<8 wk) high-intensity interval training in diseased cohorts. Med Sci Sports Exerc 2018; 50: 1740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doleman B, Mathiesen O, Jakobsen JCet al. Methodologies for systematic reviews with meta-analysis of randomised clinical trials in pain, anaesthesia, and perioperative medicine. Br J Anaesth 2021; 126: 903–11. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JAC, Savović J, Page MJet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 30. Irving B, Lanza I, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab 2015; 100: 1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersen TR, Schmidt JF, Nielsen JJet al. Effect of football or strength training on functional ability and physical performance in untrained old men. Scand J Med Sci Sports 2014; 24: 76–85. [DOI] [PubMed] [Google Scholar]
- 32. Schmidt JF, Hansen PR, Andersen TRet al. Cardiovascular adaptations to 4 and 12 months of football or strength training in 65- to 75-year-old untrained men. Scand J Med Sci Sports 2014; 24: 86–97. [DOI] [PubMed] [Google Scholar]
- 33. Bocalini DS, Serra AJ, Santos L. Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J Aging Res 2010; 2010: 1–6. 10.4061/2010/760818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strasser B, Keinrad M, Haber P, Schobersberger W. Efficacy of systematic endurance and resistance training on muscle strength and endurance performance in elderly adults – a randomized controlled trial. Wien Klin Wochenschr 2009; 121: 757–64. [DOI] [PubMed] [Google Scholar]
- 35. Lovell DI, Cuneo R, Gass GC. Strength training improves submaximum cardiovascular performance in older men. J Geriatr Phys Ther 2009; 32: 117–24. [DOI] [PubMed] [Google Scholar]
- 36. Madden KM, Levy WC, Stratton JR. Exercise training and heart rate variability in older adult female subjects. Clin Invest Med 2006; 29: 20–8. [PubMed] [Google Scholar]
- 37. Okazaki K, Kamijo YI, Takeno Y, Okumoto T, Masuki S, Nose H. Effects of exercise training on thermoregulatory responses and blood volume in older men. J Appl Physiol (1985) 2002; 93: 1630–7. [DOI] [PubMed] [Google Scholar]
- 38. Guido M, Lima RM, Kaley Tet al. Effects of 24 Weeks of Resistance Training on Aerobic Fitness Indexes of Older Women, 2014.
- 39. Hunter GR, Wetzstein CJ, Mclafferty CLet al. High-Resistance versus Variable-Resistance Training in Older Adults, 2001. [DOI] [PubMed]
- 40. Hagerman FC, Walsh SJ, Staron RSet al. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci 2000; 55: B336–46. [DOI] [PubMed] [Google Scholar]
- 41. Buchner DM, Cress ME, Lateur BJet al. The effect of strength and endurance training on gait, balance, fall risk, and health services use in community-living older adults. J Gerontol A Biol Sci Med Sci 1997; 52A: M218–24. [DOI] [PubMed] [Google Scholar]
- 42. Ades PA, Ballor DL, Ashikaga T, Utton JL, Nair KS. Weight training improves walking endurance in healthy elderly persons. Ann Intern Med 1996; 124: 568–72. [DOI] [PubMed] [Google Scholar]
- 43. Hagberg JM, Graves JE, Limacher Met al. Cardiovascular responses of 70- to 79-yr-old men and women to exercise training. J Appl Physiol 1989; 66: 2589–94. [DOI] [PubMed] [Google Scholar]
- 44. Kanegusuku H, Queiroz ACC, Chehuen MRet al. Strength and power training did not modify cardiovascular responses to aerobic exercise in elderly subjects. Braz J Med Biol Res 2011; 44: 864–70. [DOI] [PubMed] [Google Scholar]
- 45. Elsangedy HM, Oliveira GTA, Machado DGSet al. Effects of self-selected resistance training on physical fitness and psychophysiological responses in physically inactive older women: a randomized controlled study. Percept Mot Skills 2021; 128: 467–91. [DOI] [PubMed] [Google Scholar]
- 46. Vincent KR, Braith RW, Feldman RA, Kallas HE, Lowenthal DT. Improved cardiorespiratory endurance following 6 months of resistance exercise in elderly men and women. Arch Intern Med 2002; 162: 673–8. [DOI] [PubMed] [Google Scholar]
- 47. Thomas CM, Pierzga JM, Kenney WL. Aerobic training and cutaneous vasodilation in young and older men. J Appl Physiol 1999; 86: 1676–86. [DOI] [PubMed] [Google Scholar]
- 48. Romero-Arenas S, Blazevich AJ, Martínez-Pascual Met al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol 2013; 48: 334–40. [DOI] [PubMed] [Google Scholar]
- 49. Costa R, Kanitz A, Reichert Tet al. Water-based aerobic training improves strength parameters and cardiorespiratory outcomes in elderly women. Exp Gerontol 2018; 108: 231–9. [DOI] [PubMed] [Google Scholar]
- 50. Brentano MA, Cadore EL, Silva EM. Et al . Physiological Adaptations to Strength and Circuit Training in Postmenopausal Women with Bone Loss [DOI] [PubMed]
- 51. Kallinen M, Sipilä S, Alen Met al. Improving cardiovascular fitness by strength or endurance training in women aged 76-78 years. A population-based, randomized controlled trial. Age Ageing 2002; 31: 247–54. [DOI] [PubMed] [Google Scholar]
- 52. Tsutsumi T, Don BM, Zaichkowsky LD, Delizonna LL. Physical fitness and psychological benefits of strength training in community dwelling older adults. Appl Human Sci 1997; 16: 257–66. [DOI] [PubMed] [Google Scholar]
- 53. Cavani V, Mier CM, Musto AA, Tummers N. Effects of a 6-week resistance-training program on functional fitness of older adults. J Aging Phys Act 2002; 10: 443–52. [Google Scholar]
- 54. Teixeira AM, Martins RA, Neves APet al. The effect of aerobic versus strength-based training on high-sensitivity C-reactive protein in older adults. Eur J Appl Physiol 2010; 110: 161–9. [DOI] [PubMed] [Google Scholar]
- 55. Martins RA, Coelho-e-Silva MJ, Pindus DMet al. Effects of strength and aerobic-based training on functional fitness, mood and the relationship between fatness and mood in older adults. J Sports Med Phys Fitness 2011; 51: 489–96. [PubMed] [Google Scholar]
- 56. Kalapotharakos VI, Diamantopoulos K, Tokmakidis SP. Effects of resistance training and detraining on muscle strength and functional performance of older adults aged 80 to 88 years. Aging Clin Exp Res 2010; 22: 134–40. [DOI] [PubMed] [Google Scholar]
- 57. Locks RR, Costa TC, Koppe S, Yamaguti AM, Garcia MC, Gomes ARS. Effects of strength and flexibility training on functional performance of healthy older people. Braz J Phys Ther 2012; 16: 184–90. [DOI] [PubMed] [Google Scholar]
- 58. Wanderley FAC, Moreira A, Sokhatska Oet al. Differential responses of adiposity, inflammation and autonomic function to aerobic versus resistance training in older adults. Exp Gerontol 2013; 48: 326–33. [DOI] [PubMed] [Google Scholar]
- 59. Lin SF, Sung HC, Li TLet al. The effects of tai-chi in conjunction with thera-band resistance exercise on functional fitness and muscle strength among community-based older people. J Clin Nurs 2015; 24: 1357–66. [DOI] [PubMed] [Google Scholar]
- 60. Oesen S, Halper B, Hofmann Met al. Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly--a randomized controlled trial. Exp Gerontol 2015; 72: 99–108. [DOI] [PubMed] [Google Scholar]
- 61. Motalebi SA, Iranagh JA, Mohammadi Fet al. Efficacy of elastic resistance training program for the institutionalized elderly. Topics in Geriatric Rehabilitation 2018; 34: 105–11. [Google Scholar]
- 62. Gargallo P, Colado JC, Juesas Aet al. The effect of moderate- versus high-intensity resistance training on systemic redox state and DNA damage in healthy older women. Biol Res Nurs 2018; 20: 205–17. [DOI] [PubMed] [Google Scholar]
- 63. Zhong Z. The effect of resistance training on the body composition and muscle functions of healthy elderly men. Int J Clin Exp Med 2020; 13: 7136–45. [Google Scholar]
- 64. Dongen EJI, Haveman-Nies A, Doets EL, Dorhout BG, de Groot LCPGM. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in practice. J Am Med Dir Assoc 2020; 21: 1065–1072.e3. [DOI] [PubMed] [Google Scholar]
- 65. Timon R, Camacho-Cardeñosa M, González-Custodio A, Olcina G, Gusi N, Camacho-Cardeñosa A. Effect of hypoxic conditioning on functional fitness, balance and fear of falling in healthy older adults: a randomized controlled trial. Eur Rev Aging Phys Act 2021; 18: 25. 10.1186/S11556-021-00279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang G, Gibson CA, Tran ZVet al. Controlled endurance exercise training and VO2max changes in older adults: a meta-analysis. Prev Cardiol 2005; 8: 217–25. [DOI] [PubMed] [Google Scholar]
- 67. ACSM’s Guidelines for Exercise Testing and Prescription - American College of Sports Medicine. Google Books. [DOI] [PubMed] [Google Scholar]
- 68. Hellebrandt FA, Houtz SJ. Mechanisms of muscle training in man: experimental demonstration of the overload principle. Phys Ther 1956; 36: 371–83. [DOI] [PubMed] [Google Scholar]
- 69. Williams N. The Borg rating of perceived exertion (RPE) scale. Occup Med 2017; 67: 404–5. [Google Scholar]
- 70. Bavaresco Gambassi B, David Lopes dos Santos M, Jesus Furtado Almeida F. Basic guide for the application of the main variables of resistance training in elderly. Aging Clin Exp Res 2019; 31: 1019–20. [DOI] [PubMed] [Google Scholar]
- 71. Shephard RJ. Tests of maximum oxygen intake a critical review. Sports Med 1984; 1: 99–124. [DOI] [PubMed] [Google Scholar]
- 72. Carter H, Jones AM, Barstow TJ, Burnley M, Williams CA, Doust JH. Oxygen uptake kinetics in treadmill running and cycle ergometry: a comparison. J Appl Physiol (1985) 2000; 89: 899–907. [DOI] [PubMed] [Google Scholar]
- 73. Cadore EL, Rodríguez-Mañas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res 2013; 16: 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherrington C, Fairhall NJ, Wallbank GKet al. New Cochrane review assesses the benefits and harms of exercise for preventing fall s in older people living in the community. Saudi Med J 2019; 40: 204–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 2004; 34: 329–48. [DOI] [PubMed] [Google Scholar]
- 76. Lees FD, Clark PG, Nigg CR, Newman P. Barriers to exercise behavior among older adults: a focus-group study. J Aging Phys Act 2005; 13: 23–33. [DOI] [PubMed] [Google Scholar]
- 77. Trépanier M, Minnella EM, Paradis Tet al. Improved disease-free survival after prehabilitation for colorectal cancer surgery. Ann Surg 2019; 270: 493–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.