Abstract
Crocus sativus L. has various pharmacological properties, known for over 3600 years. These properties are attributed mainly to biologically active substances, which belong to the terpenoid group and include crocins, picrocrocin and safranal. The aim of the current work was to examine the effects of crocins (CRCs) and their methyl ester derivate dimethylcrocetin (DMCRT) on glioblastoma and rhabdomyosarcoma cell lines, in terms of cytotoxicity and gene expression, implicated in proapoptotic and cell survival pathways. Cell cytotoxicity was assessed with Alamar Blue fluorescence assay after treatment with saffron carotenoids for 24, 48 and 72 h and concentrations ranging from 22.85 to 0.18 mg/mL for CRCs and 11.43 to 0.09 mg/mL for DMCRT. In addition, BAX, BID, BCL2, MYCN, SOD1, and GSTM1 gene expression was studied by qRT-PCR analysis. Both compounds demonstrated cytotoxic effects against glioblastoma and rhabdomyosarcoma cell lines, in a dose- and time-dependent manner. They induced apoptosis, via BAX and BID upregulation, MYCN and BCL-2, SOD1, GSTM1 downregulation. The current research denotes the possible anticancer properties of saffron carotenoids, which are considered safe phytochemicals, already tested in clinical trials for their health promoting properties.
Keywords: crocins (CRCs), dimethylocrocetin (DMCRT), saffron, glioblastoma (GBM), rhabdomyosarcoma, cytotoxicity
1. Introduction
The World Health Organization has declared that cancer currently constitutes a major public health challenge worldwide, accounting for one in six deaths, that is nearly 10 million deaths globally, in 2020. The most worrying fact is that the number of new cases and deaths are estimated to rise significantly due to the global trend for longevity, epidemiological and demographic transitions and the adoption of cancer-associated choices, including smoking, sedentary lifestyle and ‘‘westernized’’ diets; hence, the global burden of all cancers is predicted to reach 27.5–37 million new cases/year by 2040 [1,2,3,4]. Surgery, radiotherapy and chemotherapy represent the mainstay of medical oncology therapeutics, however the disadvantage with the last two treatment modalities is the lack of selectivity and the insult of vital cellular targets shared by both cancer and normal cells, e.g., cell cycle and metabolism, DNA synthesis and repair and protein synthesis [5]. Thus, cancer survivors usually suffer from serious and sometimes, life-threatening short- or long-term complications, e.g., myelosuppression, secondary tumors, gastrointestinal toxicity, alopecia, cardiotoxicity, lung fibrosis and cognitive impairment. It is nowadays evident that conventional chemotherapy and radiation therapy have reached a plateau on efficacy as sole and/or primary treatment choices, in terms of cancer remission, survival rates and tolerance in side effects.
Medicinal plants, their secondary metabolites and synthesized chemical compounds still provide some of the most original and promising approaches for discovering new anticancer therapeutic agents. It is nevertheless noteworthy that 60% of commercially available antitumor therapies derive from natural sources; e.g., Vinca alkaloids (vinorelbine, vindesine, vincristine and vinblastine) were isolated from Catharanthus roseus (Madagascar Periwinkle), the taxane paclitaxel from the bark and leaf of Taxus baccata and Taxus canadensis and its semi-synthetic derivative docetaxel from T. baccata, Campothecin (topoisomerase I inhibitor) from Camptotheca acuminata, etoposide and teniposide from Podophyllum peltatum (May Apple) [6,7,8]. The growing burden of cancer worldwide, along with several limitations in conventional therapies, including high cost and severe toxicities, has led the research towards natural compounds, as they provide a huge variety, whereas some of them are endowed with strong biological activities.
There is a growing body of compelling evidence that Crocus sativus L. could be considered a medicinal plant, probably possessing anticancer, antioxidant, anti-inflammatory, hepatoprotective, antiatherosclerotic, hypolipidemic, hypotensive, antidiabetic, anticonvulsant, antidepressant, anxiolytic and hypnotic effects, improver of cognitive performance and memory impairment [9,10,11,12]. The red stigmas of Crocus sativus L., commonly named as saffron have three major characteristic components: (i) crocins (CRCs) (8′-diapocarotene-8,8′-dioic acid), which are mainly responsible for the red pigmentation of stigmas; (ii) picrocrocin (C16H26O7), a monoterpene glycoside of the aglycone 4-hydroxy-2,6,6-trimethyl-1-carboxaldehyde-1-cyclohexene (HTCC), responsible for its distinctive bitter flavor; and (iii) safranal (2,6,6-trimethylcyclohexane-1,3-dien-1-carboxaldehyde) (C10H14O), an aromatic monoterpene aldehyde and the main component of the essential oil, providing the characteristic odor to saffron. Other constituents include carotenoids (alpha-, beta-, gamma-carotene, lycopene, zeaxanthin and xanthone-carotenoid glycosidic conjugate), phenolic and flavonoid compounds [13,14,15,16,17,18,19,20].
CRCs are water soluble carotenoids and the prevailing constituent of Crocus sativus L. stigmas, primarily responsible for the pharmacologic activity of saffron [21]. They are either mono- or di-glycosyl esters of a dicarboxylic acid crocetin (CRT; 2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioic acid; C20H24O4), in which d-glucose and/or d-gentiobiose occur as carbohydrate residues. The methyl ester derivate dimethylcrocetin (DMCRT) is similar to CRT. Their difference is attributed to the presence of a methoxy group at positions C-1 and C-16 of the carbon chain instead of the carboxyl group found in CRT. DMCRT is soluble in many organic solvents acceptable in cell experiments [13,14,18,22].
Constituents and metabolites of saffron are relatively safe as demonstrated in several studies on animal models, in terms of acute, subacute, sub chronic and chronic exposure. Saffron and its metabolites did not cause any significant hematological or biochemical toxic effects, nor were associated with histotoxicity in heart, liver, spleen and kidney, or genotoxic and mutagenic effects [23,24,25,26,27,28,29,30,31,32]. CRCs in preclinical mice models were well tolerated in doses up to 3 g/kg, in intraperitoneal or oral administration for acute exposure and 400 mg/kg in intraperitoneal administration for subacute exposure (3–4 weeks) [26,33,34,35,36,37,38,39,40,41,42].
Saffron and its constituents have been studied for their potential anticancer and antioxidant properties and their cytotoxic effect on several cancer cell lines [43,44,45,46,47,48,49]. However, there is not any evidence on the effects of CRCs and DMCRT on glioblastoma (GBM) and rhabdomyosarcoma cell lines. Therefore, the aim of the current work is to examine, using biological assays, the effects of CRCs and DMCRT on GBM and rhabdomyosarcoma tumor cell lines, in terms of cytotoxicity and alterations on the expression of key signaling molecules, implicated in proapoptotic pathways and of cellular antioxidants.
2. Materials and Methods
2.1. Extraction of Crocins and Dimethylcrocetin
CRCs were extracted from the stigmas of Crocus sativus L. (5 g of dried stigmas) as previously described [50]. The chemical profile of CRCs has been elucidated in previous studies [16,50,51,52,53,54]. At this study, the analysis of the extract was re-evaluated using the same technique and under the same operation parameters as reported in the study of Kakouri et al. (2020) [50]. In particular, samples were analyzed using an LC/Q-TOF/HRMS technique (Agilent Series 1260-Agilent Technologies, Boeblingen, Germany). Identification of the compounds detected was based on literature and UV data [16,50,51,52,53,54]. Identification was further ascertained by the fact that a Q-TOF analyzer provides the chemical formula of each detected compound based on accurate mass measurement, which in our study, was adjusted to less than 5 ppm. Nomenclature of the identified compounds was based on the proposal of Carmona et al., i.e., the first part describes the cis/trans form of the aglycon part, then follows the total number of sugar moieties (glycose monomers) and finally is indicated the type of sugar in each part of crocin structure [51].
In the case of DMCRT, five grams of powdered dried stigmas were first extracted with 50 mL of petroleum ether and after that with diethyl ether. Extraction took place 5 times for each solvent used in an ultrasound water bath, at 25 °C and at 35 kHz ultrasound frequency for 10 min each (total time 50 min, i.e., [5 times for each solvent] × 10 min). The received powder was dried under nitrogen steam and extracted with 200 mL of methanol (HPLC grade). The extract was left on a magnetic stirrer for 120 min, protected from light. Extraction with methanol was repeated under the same conditions twice. The extract (400 mL) was kept at 4 °C and a new extraction followed, where the extract remained under stirring for 24 h. Extracts were then combined (600 mL final volume) and alkaline hydrolysis followed. An aqueous KOH solution (2N) was gradually added to the final extract under continuous stirring, until solid particles were formed, indicating the presence of DMCRT. The received extract was centrifuged (SIGMA 3K) at 8000 rpm (7155× g) for 15 min and the solid residue was collected. Chloroform was then added in order to receive a more purified extract and evaporation of the organic solvent took place. The purity of the final material was tested with the diffuse reflectance infrared Fourier transform (DRIFT), using a Nicolet 6700 FTIR spectrometer operating at the region of 4000–400 cm–1 and equipped with a deuterated triglycine sulphate detector (DTGs). Spectra were recorded with a total of 100 scans at a resolution of 4 cm−1, using the Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTs) technique. Baseline correction and smoothing of the generated spectra took place before analysis of the data, which was performed using the OMNIC 3.1 software. Characteristic peaks at 1696 cm−1, 1225 and 1106 cm−1 confirmed the presence of DMCRT [55]. The final powdered material was kept at 4 °C until further use.
2.2. Cell Culture and Reagents
A172 glioblastoma (ECACC 88062428) and TE-671 (ECACC 89071904) rhabdomyosarcoma cell lines were purchased from the European Collection of Cell Cultures (ECACC, London, UK). Cell lines were maintained as exponentially growing monolayer cultures in DMEM, supplemented with 1% l-Glutamine-penicillin/streptomycin (Invitrogen-Gibco Inc., Carlsbad, CA, USA) and 15% fetal bovine serum (FBS) (Invitrogen-Gibco Inc., Carlsbad, CA, USA) in the Steri-Cycle CO2 incubator (Thermo Scientific, Waltham, MA, USA) at 37 °C, 95% humidity and 5% CO2. Experiments were conducted in the laminar flow hood (ESI-FLUFRANCE, Wissous, France) to reduce the risk of cell contamination. The culture medium was renewed, depending on the needs of cell growth, usually every three days to remove toxic metabolic derivatives, which could interfere with normal cell growth and proliferation.
Cells were allowed to grow at ~80% confluence and harvested using 0.1% trypsin and centrifugation at 2400 rpm (708× g) for 10 min. Supernatant was discarded and pellet was kept for further processing. Cell population was determined with the use of a NIHON KOHDEN CellTaq-a (Nihon Kohden EUROPE GmbH, Rosbach, Germany) hematology analyzer.
2.3. Experimental Setup
Experiments were performed in 96-well plates (CellStar®, Sigma-Aldrich Chemie GmbH, Taufkirchen, DE, USA). Experimental setup included a column of cell culture medium only, a column of cell culture medium and the respective staining chemical, a column of cultured cells only (no drug, nor staining agent) and a column of cultured cells and the respective staining chemical, whereas the remaining wells were used for the testing of several concentrations of the testing agents (CRCs, DMCRT). Wells containing cell culture medium only and cells with no staining agent or drug (untreated cells) were used as blank, whereas wells with cultured cells with staining agent were used as positive controls.
2.4. Assessment of Cell Viability
CRCs were diluted to nuclease and protease free water and DMCRT to 5% DMSO. In order to prepare different concentrations of each testing agent, serial dilutions were performed. Specifically, for DMCRT the successive dilutions led to a final concentration of DMSO in the testing wells lower than 1%. Cell viability was assessed after incubation with the testing agents via resazurin reduction experiments, using an Alamar Blue viability assay (Invitrogen-Gibco Inc., Carlsbad, CA, USA), as previously described [56,57]. In brief, cells were cultured in 96-well plates (Cellstar, Greiner GmbH, Frickenhausen, Germany) at a density of 2 × 104 cells (C = 105 cells/lt) per well in their respective medium, following 24-h incubation at 37 °C, until reaching confluency. Then, saffron compounds were added separately to the wells by serial dilution to final concentrations of 22.85, 11.429, 5.714, 2.857, 1.429, 0.714, 0.357 and 0.179 mg/mL for CRCs and 11.429, 5.714, 2.857, 1.429, 0.714, 0.357, 0.179 and 0.090 mg/mL for DMCRT. Higher doses than 22.85 mg/mL and 11.429 mg/mL for CRCs and DMCRT respectively were not applied, as at higher doses the compounds would not dilute and precipitated. Cells were incubated with the aforementioned phytochemicals for 24, 48 and 72 h.
To quantify the number of viable cells at each time point (24, 48 and 72 h) of exposure, Alamar Blue was added to wells, to a final concentration of 10% and cells were incubated for 6 h. The reduced form of Alamar Blue was determined by fluorescence measurements at excitation wavelength 550 nm and emission of 590 nm. Measurements were carried out using an automated-microtiter plate reader Victor3 (Perkin Elmer Inc., Waltham, MA, USA). Signal intensities were normalized by subtracting the blank signal intensity from the signal intensity of the experimental wells. Each drug testing was performed in triplicates and performed at least two independent times.
2.5. RNA Extraction and cDNA Synthesis
A172 glioblastoma and TE671 rhabdomyosarcoma cells were cultured in 25 cm2 flasks and incubated for 24, 48 and 72 h with CRCs and DMCRT. CRCs were diluted in cell culture media, while DMCRT was dissolved in DMSO, with its final concentration in flasks not exceeding 1%. Each reagent was added to the flasks in a final concentration of 1 mg/mL and left for incubation at 37 °C for 48 and 72 h. RNA isolation/purification was carried out at each time point. Total RNA isolation/ purification was performed using QIAGEN RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. cDNA synthesis was performed using 500 ng of total RNA, using a QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany) protocol in a total volume of 20 μL. The reaction mixture was incubated at 42 °C for 50 min, followed by heat inactivation at 70 °C for 15 min. The complementary DNA (cDNA) was stored at −20 °C until further use.
2.6. Reverse Transcription Real-Time PCR (qRT-PCR)
The StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was used for conducting the RT-qPCR analysis while SYBR™ Select Master Mix (Applied Biosystems, Foster City, USA) was utilized for target genes expression. Primers pairs were designed using PrimerExpress 2.0 software (Thermo Fisher Scientific, Waltham, MA, USA) and their sequences are shown in Table 1. In order to confirm the reaction specificity, dissociation curve analysis was demonstrated. The expression levels of Beta-Actin (ACTB) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as reference genes. The reaction started at 95 °C for a 15 min time period, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The calculation of the relative transcript levels of the gene of interest was made by the comparative threshold cycle (CT) method [(1 + E)−ΔΔCT] [58]. LinRegPCR software was used for the calculation of PCR efficiency for each amplicon [59]. All the results were expressed as mean ± SEM. One-way analysis of variance (ANOVA) following by Tukey’s multiple comparisons test was used to detect statistical differences. The level of significant difference was set at p-value < 0.05. The SigmaPlot software 12.0 (Systat Software Inc., San Jose, CA, USA) was used to carry out the statistical analysis.
Table 1.
Gene name, Accession No., Primers.
| Gene Symbol | Gene Name | Accession No | Primer F (5′-3′) | Primer R (5′-3′) |
|---|---|---|---|---|
| ACTB | actin, beta | NM_001101.3 | CTGTCCACCTTCCAGCAGATGT | AGCATTTGCGGTGGACGAT |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | NM_001256799.2 | TTGCCCTCAACGACCACTTT | CACCCTGTTGCTGTAGCCAAA |
| BAX | BCL2 Associated X, Apoptosis Regulator | NM_001291428.1 | GGTTGTCGCCCTTTTCTA | CGGAGGAAGTCCAATGTC |
| BID | BH3 interacting domain death agonist | NM_001196.3 | TCCTTGCTCCGTGATGTCTTTC | AAGCTCCTCACGTAGGTGCGTA |
| BCL2 | BCL2 apoptosis regulator | NM_000633.2 | GATGTGATGCCTCTGCGAAG | CATGCTGATGTCTCTGGAATCT |
| MYCN | MYCN proto-oncogene, bHLH transcription factor | NM_001293228.1 | CCCCTGGGTCTGCCCCGTTT | GCCGAAGTAGAAGTCATCTT |
| SOD1 | superoxide dismutase 1 | NM_000454.4 | GGATGAAGAGAGGCATGTTGGA | TAGACACATCGGCCACACCAT |
| GSTM1 | glutathione S-transferase mu 1 | NM_000561.3 | ACTTGATTGATGGGGCTCAC | TCTCCAAAATGTCCACACGA |
2.7. Data Analysis and Statistics
Multiparameter analyses were performed with GraphPad Prism 8 version 8.0.0 (GraphPad Software for Windows, San Diego, CA, USA, www.graphpad.com; accessed on 21 April 2022). Results in cytotoxicity are expressed as the mean ± SD. of triplicate plates. Each experiment was repeated at least twice. Multiple comparisons two-way ANOVA was used to calculate the significance of the mean differences between groups. p-values < 0.05 were considered statistically significant and confidence intervals were at ±95% confidence intervals (±95% CI). Continuous variables are expressed as the mean ± standard deviation (SD), unless otherwise indicated. Nonlinear regressions were performed using the equation:
| (1) |
A four parameter logistic model was used to evaluate IC50, using the equation:
| (2) |
The percentage of cell viability was calculated as follows:
| (3) |
where FL is Fluorescence in nm.
Gene Ontology (GO) enrichment was performed using the gprofiler [60] and WebGestalt web tools [61].
Finally, we calculated the “velocity” of the cell viability (i.e., the rate by which cell viability changes) by calculating the first derivative of cell viability with respect to time as follows:
| (4) |
3. Results
3.1. LC/Q-TOF/HRMS Analysis
The compounds detected at the extract were the following: cis/trans crocin-5, cis/trans crocin-4, cis/trans crocin 2, cis/trans crocin-3 and trans crocin-1. Our results are in accordance with previously reported literature data [16]. Trans crocin-4 and trans crocin-3 were detected in abundance, according to the area under the peak, as automatically generated by the Q-TOF analyzer.
3.2. The Biological Effects on Glioblastoma Cells (A172)
3.2.1. The Dose-Dependent Effect of CRCs and DMCRT on Glioblastoma Cells (A172)
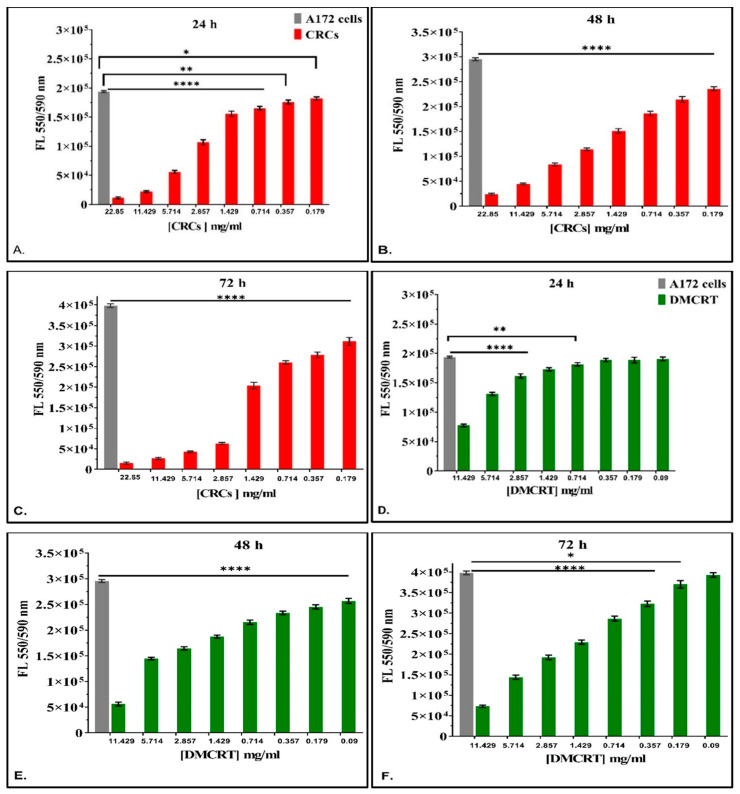
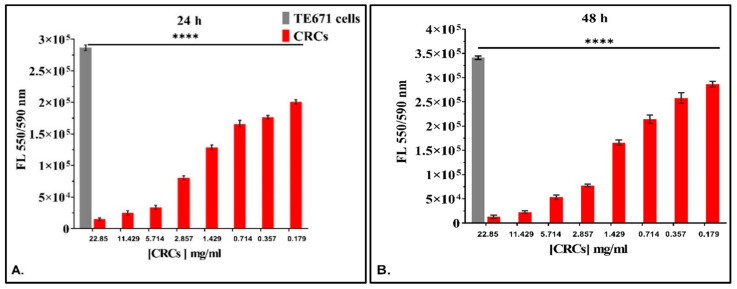
A172 cells were incubated with CRCs and DMCRT in various concentrations as described in the Section 2. In particular, when analyzing drug effects with respect to concentration for each time interval studied (24, 48 and 72 h) CRCs and DMCRT were indifferent at 0 h of exposure (data not shown), for any concentration, as expected. CRCs (Figure 1A–C) exhibited at all-time points and for concentrations ≥ 0.179 mg/mL a significant dose-dependent cytotoxic effect; the cytotoxicity increased, as the compound’s concentration augmented. DMCRT did not manifest any effect at concentrations ≤ 0.71 mg/mL and 0.09 mg/mL, at 24 and 72 h, respectively, whereas for the rest of concentrations, especially at 48 h there was a clear dose-dependent effect, when compared with the untreated cells (Figure 1D–F).
Figure 1.
Dose-dependent effect of CRCs and DMCRT on A172 cells. CRCs exhibited a significant dose-dependent effect at all time points and for concentrations ≥0.179 mg/mL tested (p < 0.05 for cells exposed to any concentration of CRCs vs untreated A172) (A–C). Similarly, DMCRT was also effective from concentrations ≥0.714 mg/mL and ≥0.179 mg/mL, at 24 and 72 h respectively and for all concentrations, at 48 h, manifesting significant increasing cell fatal effects with increasing concentrations (D–F). Legend: CRCs: Crocins, DMCRT: dimethylocrocetin, FL: Fluorescence Intensity. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (* p < 0.05; ** p < 0.01; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
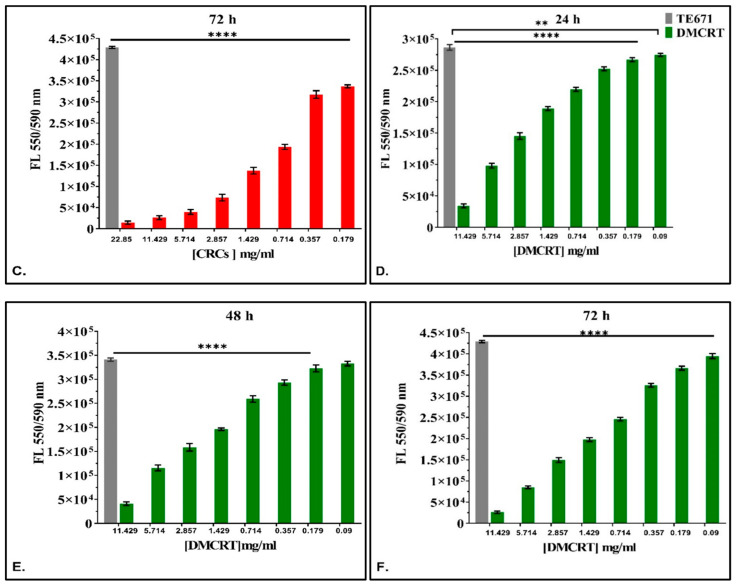
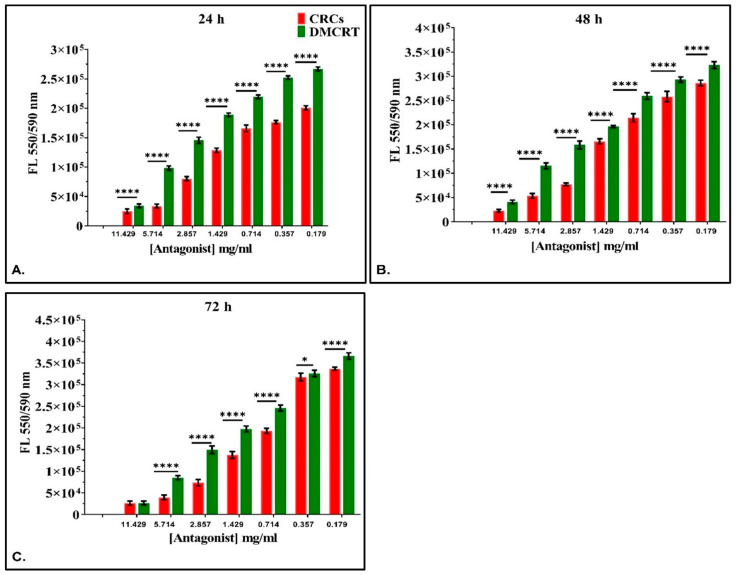
3.2.2. The Comparative Dose-Dependent Effect of CRCs and DMCRT on Glioblastoma Cells (A172)
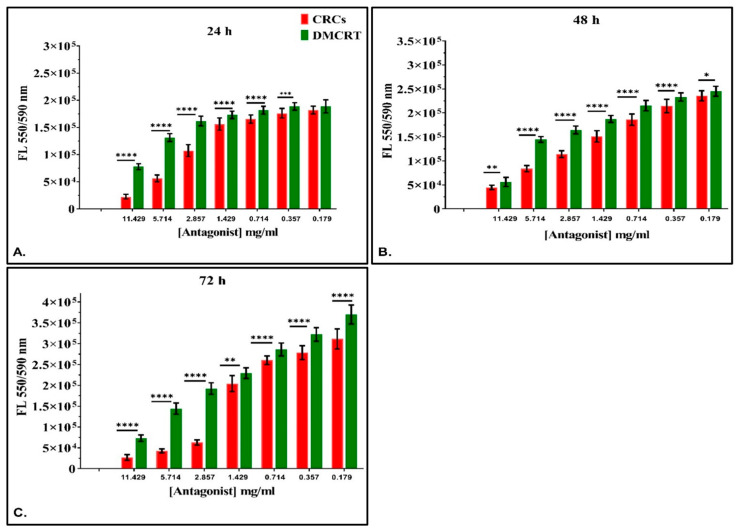
Significant differences among CRCs and DMCRT were observed at all time points (24, 48 and 72 h) and for all concentrations; CRCs generally demonstrated better cytotoxic effects as compared to DMCRT. More specifically, CRCs were more potent than DMCRT on A172 cells for concentrations ≥0.36 mg/mL at 24 h (Figure 2A) and for all concentrations at 48-(Figure 2B) and 72-h’ time points (Figure 2C).
Figure 2.
Comparative dose-dependent effect of CRCs and DMCRT on A172 cells. CRCs and DMCRT followed a similar pattern at all time points. CRCs exhibited higher efficacy than DMCRT. This effect was found to be true at all time points that is 24 (A), 48 (B) and 72 h (C) Legend: CRCs: Crocins, DMCRT: dimethylocrocetin, FL: Fluorescence Intensity. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
3.2.3. The Time-Dependent Effect of CRCs and DMCRT on Glioblastoma Cells (A172)
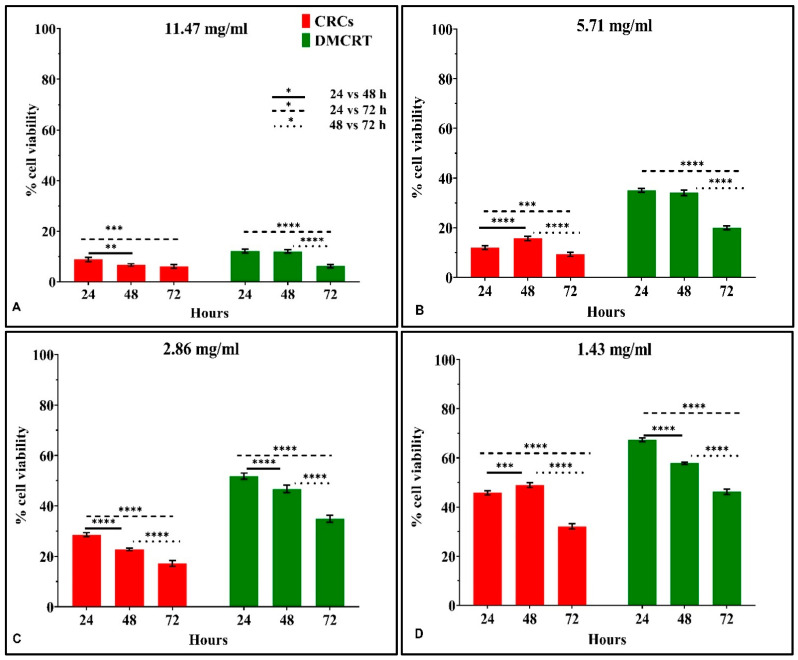
Further on, the time-dependent efficacy of CRCs and DMCRT was studied, for all concentrations tested. Measurements were performed at 6 h, meaning at the time just after the addition of the drug and the staining agent and every 24 h thereafter i.e., at 24, 48 and 72 h. When examining the drug effect with respect to time for each concentration we observed that at 6 h, as expected, no significant differences were present with respect to any tested bioactive compound (not depicted).
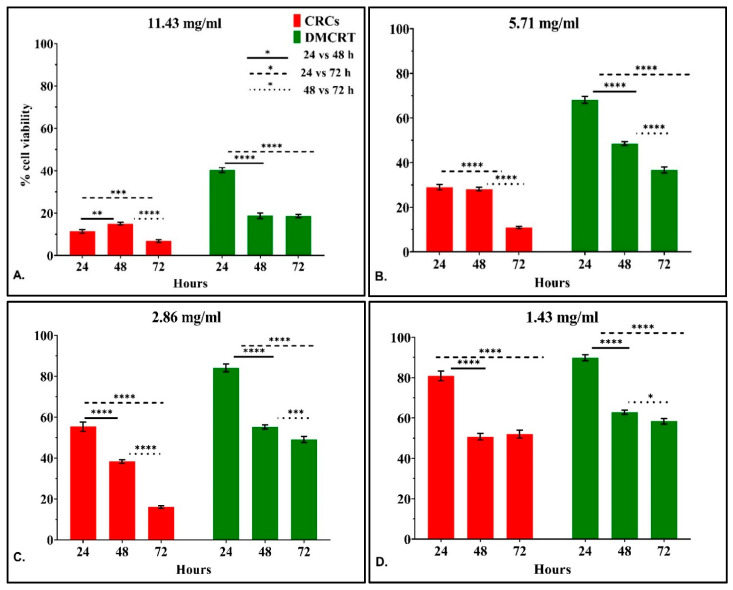
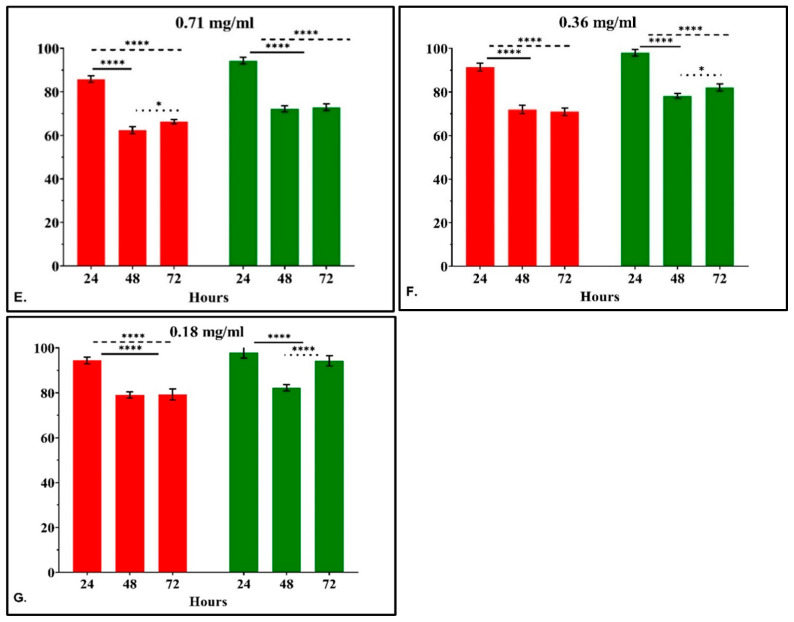
CRCs at the high concentrations 2.86–11.43 were more cytotoxic at 72 h, verifying a time-dependent effect (p < 0.0001 for 48 vs. 72 h and p < 0.0001 for 24 vs. 72 h). For the lowest concentrations 0.18–1.43 mg/mL we observed a significant cytotoxic effect, when comparing 72 vs. 24 h (p < 0.0001 for 24 vs. 72 h), whereas for 48 vs. 72 h we did not observe significant differences, indicating that the cytotoxic effect might reach a plateau. When comparing 48 vs. 24 h we observed time-dependent cytotoxicity at concentrations lower than 2.86 mg/mL (Figure 3A–G).
Figure 3.
Time-dependent effect of CRCs and DMCRT on A172 cells. Time-dependent effect of CRCs and DMCRT at concentrations 11.429 mg/mL (A), 5.174 mg/mL (B), 2.857 mg/mL (C), 1.429 mg/mL (D), 0. 714 mg/mL, (E) 0.357 mg/mL (F), 0,18 mg/mL (G) Legend: CRCs: Crocins, DMCRT: dimethylocrocetin. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
DMCRT manifested a significant time-dependent cytotoxic effect for all concentrations, when comparing 48 vs. 24 h (p < 0.0001 for 24 vs. 48 h) and for concentrations ≥ 0.36 mg/mL, when considering 72 vs. 24 h (p < 0.0001 for 24 vs. 72 h). When comparing 48 vs. 72 h, DMCRT was more potent at 72 h for concentrations 1.43–5.71 mg/mL, whereas when considering the highest concentration of 11.43 mg/mL the effect was already maximized at 48 h of exposure (Figure 3A–G).
An interesting effect, concerning a “rescue” point of A172 cells, was observed, when cells were exposed at the lowest concentrations of DMCRT 0.18–0.36 mg/mL, as they gradually recovered at 72 h from the effects of DMCRT at 48 h (Figure 3F,G). The same effect was observed for cells exposed to CRCs at 0.71 mg/mL, as at 72 h, they slightly recovered from the compound’s cytotoxic effect (Figure 3E). However, although cells partially recovered at 72 h, attempting to overcome the cytotoxic effect of the tested compounds at 48 h, they do not completely succeed when compared with the effect at 24 h.
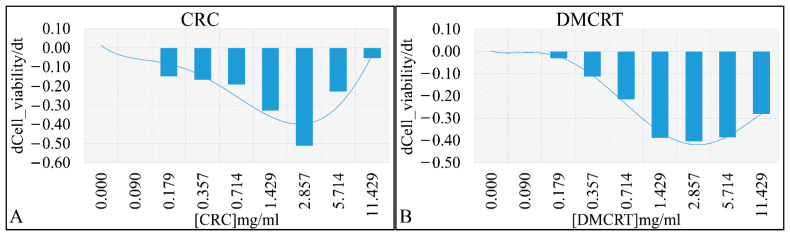
Based on the previous observations, we calculated the more efficient concentration of each phytochemical with respect to cell viability reduction velocity. Of note, the “speed” of alterations in cell viability is not identical to the effectiveness of a specific concentration, but demonstrates how fast a specific concentration could alter cellular viability observed at 24 h to the viability at 72 h. The concentration of 2.857 mg/mL for both compounds, manifested such effectiveness. In particular, CRCs demonstrated the local maximum at the 2.857 mg/mL concentration (Figure 4A). DMCRT manifested also a local maximum at the 2.857 mg/mL concentration, yet with comparable “speeds” for the 1.429 and 5.174 mg/mL concentrations (Figure 4B). Of note, negative values indicate that viability is lower at 72 h as compared to 24 h. A low first derivative, is compatible with higher effectiveness of the compound for a specific concentration. In addition, as the derivative approaches to zero, the more the compound exerted its effects from the first hours of treatment, whereas a derivative farther from zero, indicates that the compound requires more time in order to exert its effects. Thus, the effects of CRCs were changing dose-dependently for concentrations 0.09–2.86 mg/mL and thereafter CRCs’ action was already evident at 24 h of treatment (Figure 4A). On the contrary, DMCRT manifested a dose-dependent effect for concentrations 0.09–5.71 mg/mL, achieving a plateau thereafter, indicating that its effects remained time-dependent (Figure 4B). Interestingly, CRCs manifested one concentration with almost pure time-dependency (2.86 mg/mL), while DMCRT manifested three concentrations (1.43, 2.86, 5.71 mg/mL).
Figure 4.
The “speed” by which CRCs and DMCRT reduce cell viability. CRCs manifested a dose-dependent effect from 0–2.857 mg/mL, indicating that the induced “damage” was time dependent. The 5.174 and 11.429 mg/mL concentrations showed that probably all effects were already induced at 24 h and time did not play a role thereafter (A). Similarly, DMCRT manifested a dose-dependent increase in “velocity” yet it reached a plateau after 2.857 mg/mL, indicating that its effects were mostly time-dependent in all cases (B) (Legend: CRCs: Crocins, DMCRT: dimethylocrocetin).
3.2.4. The IC50 Curves of CRCs and DMCRT on Glioblastoma Cells (A172)
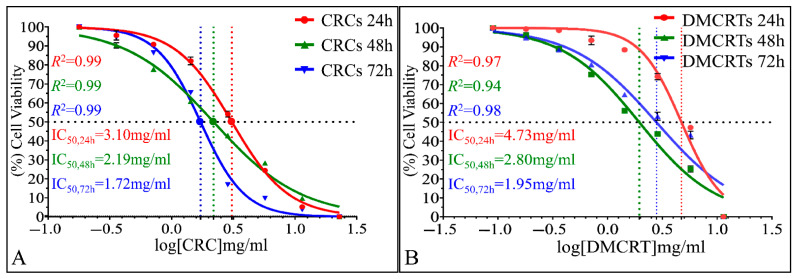
The time dependent effect of CRCs and DMCRT, as well as the superior in vitro sensitivity of A172 cells on CRCs were also depicted via IC50 calculations. IC50 were calculated 3.10 mg/mL (R2 = 0.99), 2.19 mg/mL (R2 = 0.99) and 1.72 mg/mL (R2 = 0.99), for CRCs, at 24, 48 and 72 h, respectively (Figure 5A). Further on, DMCRT manifested IC50 values of 4.73 mg/mL (R2 = 0.97), 2.80 mg/mL (R2 = 0.94) and 1.95 mg/mL (R2 = 0.98) at 24, 48 and 72 h, respectively (Figure 5B).
Figure 5.
Calculation of IC50 of CRCs and DMCRT on A172 cells. IC50 were found 3.10 mg/mL (R2 = 0.99), 2.19 mg/mL (R2 = 0.99) and 1.72 mg/mL (R2 = 0.99), for CRCs, at 24, 48 and 72 h, respectively (A). IC50 for DMCRT were estimated 4.73 mg/mL (R2 = 0.97), 2.80 mg/mL (R2 = 0.94) and 1.95 mg/mL (R2 = 0.98) at 24, 48 and 72 h, respectively (B).
The IC50 calculations confirmed our previous results and in particular, the fact that CRCs were more efficient, as compared to DMCRT. In addition, IC50 regressions produced better simulation results for CRCs, indicating that they exhibit superior time- and dose- dependent cytotoxicity, as compared to DMCRT.
3.2.5. Gene Expression under CRCs and DMCRT on Glioblastoma Cells (A172)
Gene expression concerning the effects of CRCs and DMCRT on glioblastoma cells, was investigated and compared between the two compounds (Figure 6). Hence, gene expression was examined for BAX (Figure 6A), BID (Figure 6B), BCL2 (Figure 6C), MYCN (Figure 6D), SOD1 (Figure 6E) and GSTM1 (Figure 6F) when cells were treated with 1 mg/mL CRCs and DMCRT. Although it is well established that exposure of cancer cells to concentrations of saffron or its compounds up to 16 mg/mL are related to apoptotic cell death and not necrosis, via alterations in the expression of relevant apoptotic genes, the dose treatment of 1 mg/mL was selected, based on the significant cytotoxic effects observed on both cell lines, without however complete cellular abolishment or exceeding the IC50 concentration for 48 and 72 h [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76].
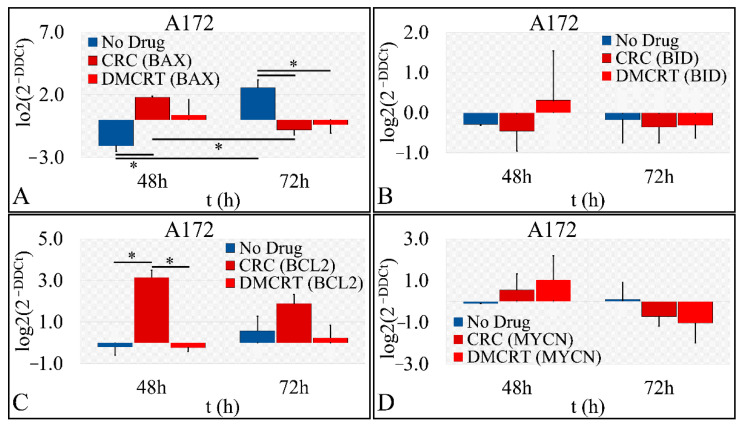
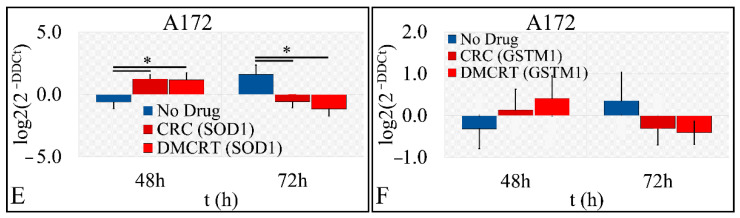
Figure 6.
Gene expression under CRCs and DMCRT treatment. We performed indicative gene expression experiments for cells treated with CRCs and DMCRT. In particular, we investigated and compared to CRCs’ gene expression, gene expression for BAX (A), BID (B), BCL2 (C), MYCN (D), SOD1 (E) and GSTM1 (F) (the asterisks depict a significance at p < 0.05 level).
Initially, the putative apoptosis initiation mechanisms after treatment with saffron carotenoids were investigated, by measuring the expression of the BAX, BID and BCL2 genes, which are involved in mitochondrial apoptotic pathways. BAX expression manifested significant differences between cells without treatment and cells exposed to CRCs or DMCRT at 48 and 72 h (Figure 6A). BAX was up-regulated at 48 h, whereas was down-regulated at 72 h, when cells were exposed to any of the compounds (Figure 6A). This effect signifies that saffron carotenoids exert a pro-apoptotic effect, from which cells attempt to recover later on. Similarly, the expression of the pro-apoptotic BID, which in response to apoptotic signaling interacts with BAX protein, facilitating its insertion into the mitochondrial membrane, was upregulated at 48 h of DMCRT treatment, whereas was down-regulated at 72 h for cells treated with CRCs or DMCRT, in accordance with BAX expression profile (Figure 6B). Further on, BCL2 oncoprotein expression demonstrated significant differences between untreated cells and cells exposed to saffron phytochemicals. Although untreated glioblastoma cells do not strongly express the antiapoptotic BCL2 protein, BCL2 was significantly up-regulated at 48 h when cells were treated with CRCs and at 72 h when cells were exposed to any of the carotenoids (Figure 6C). MYCN proto-oncogene was down-regulated at 72 h of treatment with any of saffron phytochemicals, although at 48 h it was upregulated (Figure 6D). Finally, another possible trigger of cellular apoptosis could be alterations in the expression of genes implicated in the antioxidant cellular machinery. Thus, SOD1 (Figure 6E) and GSTM1 (Figure 6F), both implicated in free radical scavenging, were down-regulated, at 72 h of treatment with CRCs or DMCRT, although initially upregulated at 48 h.
3.3. The Biological Effects on Rhabdomyosarcoma Cells (TE671)
3.3.1. The Dose-Dependent Effect of CRCs and DMCRT on Rhabdomyosarcoma Cells (TE671)
TE671 cells were incubated with various concentrations of CRCs and DMCRT. When analyzing drug effects with respect to concentration for each time interval studied (24, 48 and 72 h), CRCs and DMCRT were indifferent at 0 h of exposure, as expected (data not shown). Overall, both CRCs (Figure 7A–C) and DMCRT (Figure 7D–F) exhibited significant dose-dependent cytotoxicity at all-time points, when compared with untreated TE671 cells; as their concentration increased, cell viability was reduced with the exception of the lowest concentration of DMCRT (0.09 mg/mL) at 48 h.
Figure 7.
Dose-dependent effect of CRCs and DMCRT on TE671 cells. CRCs exerted a significant dose-dependent effect at all time intervals (p < 0.005 for cells exposed to any concentration of CRCs vs untreated TE671) (A–C). Similarly, DMCRT was also effective, manifesting significant increasing cytotoxicity, with increasing concentrations (D–F). Legend: CRCs: Crocins, DMCRT: dimethylocrocetin, FL: Fluorescence Intensity. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (** p < 0.01; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments and CRCs (** p < 0.01; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
3.3.2. The Comparative Dose-Dependent Effect of CRCs and DMCRT on Rhabdomyosarcoma Cells (TE671)
Similar to A172 cells, we examined the effect of saffron phytochemicals comparatively. Significant differences between CRCs and DMCRT were observed at all time points (24, 48 and 72 h) and for all concentrations, as CRCs manifested better cytotoxic effects, when compared to DMCRT (Figure 8). The exemption was the concentration of 11.43 mg/mL, at 72 h, in which their effect on TE671 did not differ.
Figure 8.
Comparative dose-dependent effect of CRCs and DMCRT on TE671 cells. The comparative effectiveness of CRCs and DMCRT, was investigated and presented together. CRCs and DMCRT followed a similar pattern at all time points and in particular CRCs exhibited higher efficacy than DMCRT. This effect was found to be true at all time points that is 24 h (A), 48 h (B) and 72 h (C) (Legend: CRCs: Crocins, DMCRT: dimethylocrocetin, FL: Fluorescence Intensity. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (* p < 0.05; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
3.3.3. The Time-Dependent Effect of CRCs and DMCRT on Rhabdomyosarcoma Cells (TE671)
In addition to the previous analyses, the time-dependent efficacy of CRCs and DMCRT at each concentration tested was investigated. Measurements were initially performed at 6 h, meaning at the time just after the addition of the drug and the staining agent and every 24 h thereafter i.e., at 24, 48 and 72 h. At 6 h, as expected, no significant differences were present for any tested bioactive compound (data not shown).
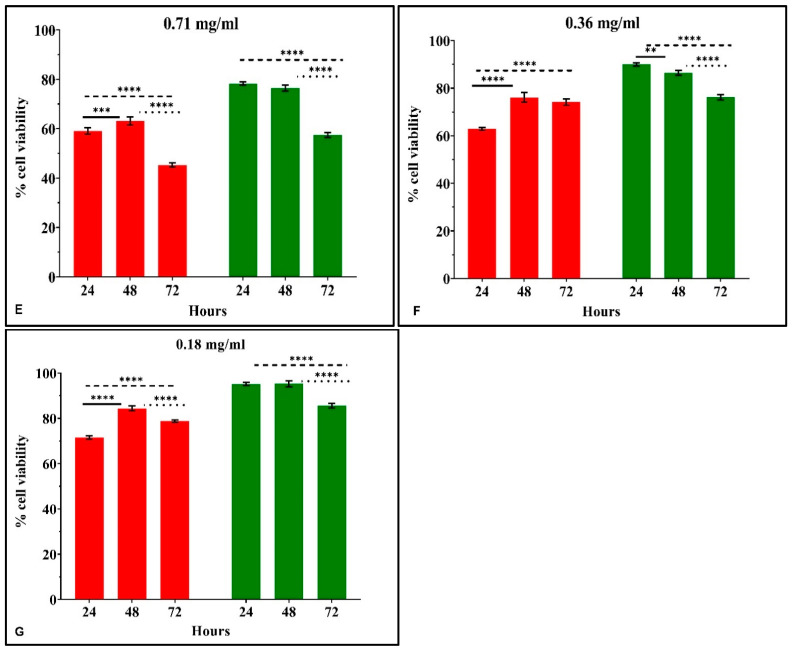
The time-dependent effect of CRCs on TE671 was more complicated as compared to A172 cells. CRCs at concentrations 0.71–11.43 mg/mL were more cytotoxic at 72 h, as compared to 24 h (p < 0.01 for 24 vs. 72 h). On the contrary, for the lowest concentrations of 0.18–0.36 mg/mL the effect of CRCs was more prominent at 24 h, when comparing 72 vs. 24 h, as cells partially recovered. When comparing 48 vs. 72 h, CRCs exhibited significant time-dependent cytotoxicity at 72 h for concentrations ≤ 5.71 mg/mL. The time dependent effect was not observed, when comparing 48 vs. 24 h, with the exception of concentrations 2.86 and 11.43 mg/mL as the cytotoxic effect of CRCs was more prominent at 24 h (Figure 9A–G).
Figure 9.
Time-dependent effect of CRCs and DMCRT on TE671 cells. Time-dependent effect of CRCs and DMCRT at concentrations 11.429 mg/mL (A), 5.174 mg/mL (B), 2.857 mg/mL (C), 1.429 mg/mL (D), 0.714 mg/mL (E), 0.357 mg/mL (F), 0.18 mg/mL (G) (Legend: CRCs: Crocins, DMCRT: dimethylocrocetin. Asterisks (*) depict at least p < 0.05 significance level between control experiments and CRCs (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). Each value represents the mean ± S.D. of triplicate experiments.
DMCRT exhibited significant time-dependent cytotoxicity for all concentrations at 72 h, as compared to both 24 and 48 h (p < 0.01 for 72 vs. 24 or 48 h), manifesting a more stable cytotoxic effect with a gradual decline in cell viability. The same time-dependent effect was observed when comparing 24 and 48 h, for concentrations 1.43–2.86 mg/mL (p < 0.05 for 48 vs. 24 h), whereas for high concentrations ≥ 5.71 mg/mL the effect was already significant at 24 h (Figure 9A–G).
TE671 cells manifested a “clearer” “rescue” behavior at 48 h, when treated with CRCs at concentrations ≤ 1.43 mg/mL. Cell recovery at 48 h, indicated that cells attempted to overcome the cytotoxic effect of CRCs at 24 h, yet they do not succeed and cells continue to die, as demonstrated by the significant cytotoxicity at 72 h.
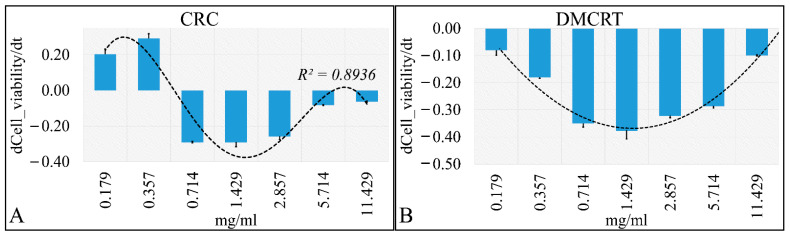
Similar to A172 cells, the first derivative of cell viability with respect to individual concentrations was calculated. Cells exposed to CRCs manifested a higher cytotoxicity rate for concentrations between 0.714 mg/mL and 2.857 mg/mL (Figure 10A), whereas DMCRT manifested a quasi-normal distribution, with higher cell cytotoxicity rates at 1.429 mg/mL (Figure 10B). TE671 cells exposed to CRCs manifested three concentrations with similar effectiveness (0.71, 1.43 and 2.86 mg/mL), indicating three concentrations which are more time-dependent (Figure 9A). In addition, the cells manifested two concentrations (0.18, 0.36 mg/mL), with positive derivative, indicating that cells recovered during treatment (Figure 9A). At the same time, cells exposed to the higher concentrations (5.71, 11.43 mg/mL) manifested a derivative close to zero, indicating a dose-dependent effect, as cell viability at 72 h was close to cell viability observed at 24 h (Figure 9A).
Figure 10.
The “speed” by which CRCs and DMCRT reduces cell viability. CRCs manifested a threshold effect from 0.71–2.86 mg/mL, indicating that the induced cytotoxicity was time—independent (A). DMCRT manifested a dose-dependent “bell”-shaped behavior, with local maxima at 1.43 mg/mL, indicating that its effects were mostly time-dependent in all cases (B) (Legend: CRCs: Crocins, DMCRT: dimethylocrocetin).
On the other hand, DMCRT manifested a normal-like distribution with respect to the first derivative (Figure 9B) and the most effective concentration identified was 1.43 mg/mL. Additionally, concentrations 0.71 and 2.86 mg/mL manifested similar behavior, indicating a time-dependent pattern of activity, whereas concentrations 0.18 and 11.43 mg/mL demonstrated a dose-dependent pattern (Figure 9B). DMCRT’s effect on TE671 cells, resembled the effect of DMCRT on A172 cells. The difference is that A172 cells manifested a slight shift of the normal-like curve towards the 2.86 mg/mL.
3.3.4. The IC50 Curves of CRCs and DMCRT on Rhabdomyosarcoma Cells (TE671)
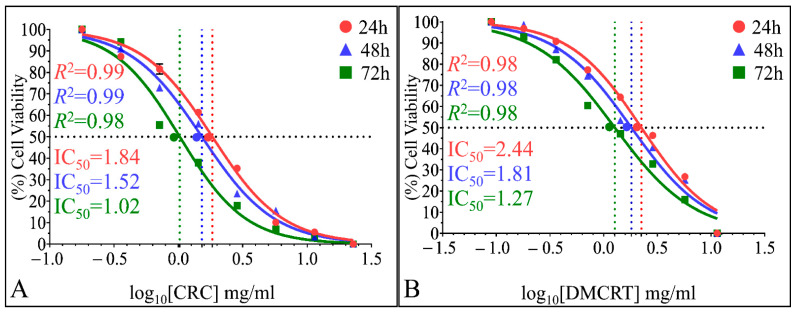
The time dependent effect of CRCs and DMCRT, as well the in vitro sensitivity of TE671 cells on CRCs were verified via IC50 calculations. IC50 were calculated 1.84 mg/mL (R2 = 0.99), 1.52 mg/mL (R2 = 0.99) and 1.02 mg/mL (R2 = 0.98), for CRCs, at 24, 48 and 72 h, respectively (Figure 11A). DMCRT manifested IC50 values of 2.44 mg/mL (R2 = 0.98), 1.81 mg/mL (R2 = 0.98) and 1.27 mg/mL (R2 = 0.98) at 24, 48 and 72 h, respectively (Figure 11B). In addition, IC50 regressions produced similar simulation results for CRCs and DMCRT.
Figure 11.
Calculation of IC50 of CRCs and DMCRT on TE671 cells. IC50 were found 1.84 mg/mL (R2 = 0.99), 1.52 mg/mL (R2 = 0.99) and 1.02 mg/mL (R2 = 0.98), for CRCs, at 24, 48 and 72 h, respectively (A). In addition, the IC50 for DMCRT was estimated to be 2.44 mg/mL (R2 = 0.98), 1.81 mg/mL (R2 = 0.98) and 1.27 mg/mL (R2 = 0.98) at 24, 48 and 72 h, respectively (B).
3.3.5. Gene Expression under CRCs and DMCRT on Rhabdomyosarcoma Cells (TE671)
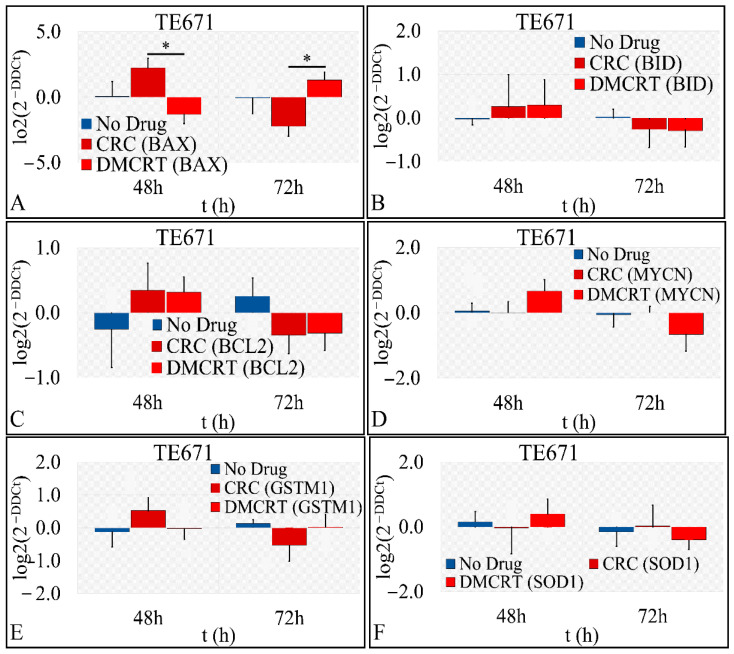
The expression of several genes, implicated in cellular apoptosis and survival and antioxidant defense mechanisms of TE671 cells, and treated with 1 mg/mL CRCs and DMCRT, was studied at a concentration which was close to the IC50 for both 48 and 72-h time intervals. Significant differences were observed in the case of BAX expression at 48 and 72 h of treatment. The apoptotic BAX was upregulated at 48 and 72 h for cells exposed to CRCs and DMCRT, respectively, whereas it was downregulated at 48 and 72 h for cells exposed to DMCRT and CRCs respectively (Figure 12A). On the other hand, BID was up-regulated at 48 h for cells treated with any of saffron phytochemicals but was down-regulated at 72 h (Figure 12B). The same expression pattern was observed when considering the pro-survival BCL2 gene (Figure 12C). MYCN proto-oncogene was down-regulated at 72 h, only under DMCRT treatment (Figure 12D). When considering the antioxidant cellular mechanisms, CRCs downregulated GSTM1 expression (Figure 12E), whereas DMCRT down-regulated SOD1 (Figure 12F); both alterations were observed at 72 h. DMCRT and CRCs did not have any effect on GSTM1 and SOD1 expression, respectively, at any time point studied.
Figure 12.
Gene expression under CRCs and DMCRT treatment for BAX (A), BID (B), BCL2 (C), MYCN (D), SOD1 (E) and GSTM1 (F) (* depicts a significance at the p < 0.05 level).
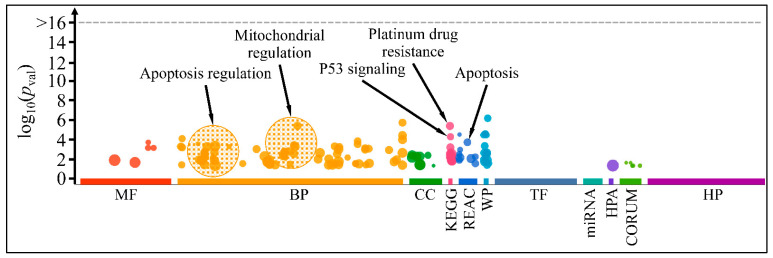
3.4. Functional Annotations of Selected Genes
Functional annotation included gene ontology (GO) annotation of selected genes (Figure 13). This analysis preceded the gene expression analyses, as we have selected the investigated genes based on their interest and probable functions. Selected genes manifested major functions, such as apoptosis and mitochondrial regulation, whereas additional performances included cellular nitrogen utilization and response to toxic substances (Table S1). Interestingly, some of these genes, i.e., BAX, SOD1 and GSTM1, manifested a dose- and time-dependent effect, in agreement with the observed cell viability and treatment.
Figure 13.
Gene ontology annotation of investigated genes (BAX, BID, BCL2, MYCN, SOD1, GSTM1). Major functions included apoptosis regulation, mitochondrial regulation, p53 signaling, platinum drug resistance and apoptosis (Legend: MF: Molecular function; BP: Biological process; CC: Cellular component; KEGG: KEGG pathway database; REAC: Reactome pathway database; WP: WikiPathways; TF: Transcription factor binding motifs; MIRNA: miRNA targets; HPA: The human protein atlas; CORUM: The comprehensive resource of mammalian protein complexes; HP: Human phenotype ontology).
4. Discussion
Medicinal plants, their secondary metabolites and synthesized chemical compounds have provided effective drug products for several diseases. The US National Cancer Institute has screened approximately 35,000 plant species for their potential anticancer action, founding that almost 3000 plant species have demonstrated significant and reproducible anticancer properties [77]. Among natural compounds, CRCs are endowed with antioxidant, anticancer and genoprotective properties, confirmed in in vitro and in vivo studies, such as on HL-60 human promyelocytic leukemia cells, K-562 human myelogenous leukemia cells, MCF-7 and MDA-MB-231 breast cancer cells, HO-8910 ovarian cancer cells, HeLa cervical carcinoma cells, A549 and SPC-A1 lung adenocarcinoma cells, BPH-1, LnCaP, 22rv1, CWR22, PC3, LapC-4 and PC3 prostate cells, MIAPaCa-2, BxPC3, Capan-1 and ASPC-1 pancreatic cancer cells, HepG2 hepatocellular carcinoma cells, AGS gastric cancer cells, HCT-116 SW-480 and HT-29 colorectal cancer cells, oral squamous cell carcinoma HN5, Ehrich sarcoma S-180, Erlich ascites carcinoma EAC and Dalton’s lymphoma DLA cells [74,75,78,79,80,81,82,83,84].
An important parameter that differentiates saffron carotenoids from current anticancer drugs is their selective cytotoxicity, as they inhibit cell proliferation of cancer cells, without affecting healthy cells, which is the cornerstone of effective cancer treatment [75,78,81,82,83,85,86,87,88]. Specifically, CRCs at doses of 9.77–48.85 μg/mL protected rat neural stem cells against hypoglycemia, and/or ischemia/reperfusion apoptosis via activation of the Notch signaling pathway, which induces neural stem cell proliferation and differentiation [89]. In dendritic cells CRCs at doses 1.25–5 mg/mL stimulated cell proliferation and promoted cell maturation [90]. Exposure of BV2 mouse microglial cells to 4.88–39.07 μg/mL of CRCs or 1.642–13.13 μg/mL of CRT protected cells from LPS (lipopolysaccharide)-induced cytotoxicity via downregulating the productions of intracellular reactive oxygen species (ROS), tumor necrosis factor-α and interleukin-1β [91]. Assessment of cell viability of neuro-2a mouse neuroblasts when exposed to CRCs for 24, 48 and 72 h at doses 24.424–195.392 μg/mL demonstrated significant positive effects on cell viability and attenuation of cellular apoptosis [92]. CRCs at doses of 0.078–0.156 mg/mL protected primary retinal cell cultures, containing a mixture of retinal cells enriched in photoreceptors, Müller and bipolar cells, against blue or white light–mediated apoptosis [93]. In normal human liver cell lines (L02 cells) CRT doses up to 13.13 μg/mL did not affect cell viability after 48 h of treatment and restored mitochondrial morphology and function in cells with FFA (free fatty acid)-induced lipid accumulation [94]. CRCs in a dose up to 0.977 mg/mL on human keratinocytes NHEKs (CC-2507) and dermal fibroblasts (C-013-5C) exhibited strong antioxidant capacity, comparable to vitamin E and higher than vitamin C, protecting against UVA-induced peroxidation and demonstrated significant anti-inflammatory properties [95]. Treatment of human normal fibroblastic cells (HFSF-PI3) with 1.9539–3.9078 mg/mL of CRCs or fibroblast mouse (L929) cells with 1–2 mg/mL of saffron ethanolic extract did not elicit any apoptogenic effect [11,96]. Similarly, CRCs in doses up to 0.088 mg/mL did not demonstrate any toxicity to the normal thyroid (PCCL3) cells [97]. CRCs in a dose of 1–4 mg/mL on breast epithelial MCF-10A cells did not affect the NF-κB-mediated inflammation and proliferation [98]. The cytoprotective properties of CRCs at doses up to 9.77 μg/mL were also demonstrated in bovine aortic endothelial cells (BAECs), exposed to significant oxidative stress [99,100]. CRCs at doses 9.77–39.079 μg/mL had no cytotoxic effect on primary bone marrow-derived macrophages (BMMs), even after 72 h of treatment [101]. T lymphocytes isolated from the peripheral blood of children with acute lymphoblastic leukemia (ALL) when treated with CRCs for 72 hours at a final concentration of 0.625–2.5 mg/mL exhibited a dose-dependent increase in their proliferation index and cytotoxic function [102]. Exposure of human umbilical vein endothelial cells (HUVEC) to saffron ethanolic or aqueous extracts and CRT at doses 0.1–1 mg/mL at various time intervals (24, 48 and 72 h) had no cytotoxic effects [103]. Saffron extracts at doses ranging from 50 to 400 μg/mL and 1 to 3 mg/mL had no effect on the viability of the human normal lung cells (CCD-18Lu) and non-cancer young adult mouse colon (YAMC) cells, respectively [84,104]. In normal fetal lung fibroblasts the IC50 of saffron extracts was calculated at 19.9 mg/mL, which was more than 10 times higher for tested human malignant cells, i.e., SKNSH neuroblastoma cells, HeLa cervical adenocarcinoma cells and MCF-7 breast cancer cells [105].
Retinoids, such as isotretinoin (13-cis-retinoic acid), ATRA (all-trans retinoic acid), etretinate and its metabolite acitretin, fenretinide (4-HPR), bexarotene and tazarotene, are used in the treatment of a broad spectrum of cancers, such as acute promyelocytic leukemia, ovarian carcinoma, head and neck squamous cell carcinoma, bladder cancer and neuroblastoma, due to their growth inhibitory, differentiating, antioxidant, anti-angiogenic, anti-inflammatory and immunomodulatory properties [11]. Nevertheless, significant toxicity and long-term storage in liver and adipose tissue may limit their usage at high therapeutic doses, outweighing their benefits in cancer treatment [106,107,108,109,110]. CRCs are fully water-soluble carotenoids, thus spreading easily throughout the body, reaching every tissue, whereas excess would be excreted via urine and not stored in tissues, such as adipose tissue or liver [20]. Thus, saffron’s carotenoids could represent an alternative to the toxicity of retinoids, harboring adequate anticancer, antioxidant and genoprotective properties [5,111,112].
4.1. In Vitro Cytotoxicity of Saffron’s Metabolites
In our study, in vitro results on glioblastoma A172 cells demonstrated that CRCs manifested a significant dose-dependent effect, at all-time points (24, 48 and 72 h); as their concentration increased, the reduction of resazurin of the staining agent to resorufin decreased, indicating reduction in cell viability. DMCRT’s cytotoxicity was dose dependent at 48 h and for concentrations ≥1.43 and ≥0.18 mg/mL, at 24 and 72 h, respectively. In the case of rhabdomyosarcoma TE671 cells CRCs exhibited significant dose-dependent cytotoxicity for all tested time points. DMCRT manifested a dose-dependent effect at 24- and 72 h and for concentrations ≥ 0.179 mg/mL at 48 h. For both cell lines, CRCs exhibited higher cytotoxicity, when compared with DMCRT.
CRCs on A172 cells manifested a time-dependent effect for all concentrations (11.43, 5.71, 2.86, 1.43, 0.71, 0.36 and 0.18 mg/mL) at 72 h, when compared with 24 h, whereas when compared with 48 h the time-dependent cytotoxicity was observed for concentrations ≥ 2.86 mg/mL. The absence of a time dependent effect between 72 vs. 48 h at the lowest concentrations 0.18–1.43 mg/mL could be attributed to the cytotoxicity effect reaching a plateau after 48 h of exposure. The time-dependent effect was also observed when comparing 48 and 24 h at concentrations ≤ 2.86 mg/mL, whereas for higher concentrations significant cytotoxicity was already present at 24 h. The time-dependent effect was clearer in the case of DMCRT; when comparing 48 vs. 24 h for all concentrations (11.43, 5.71, 2.86, 1.43, 0.71, 0.36 and 0.18 mg/mL) a significant reduction in cell viability was observed, as the time of exposure increased. The time-dependent effect was evident for concentrations ≥ 0.36 mg/mL, when considering 72 vs. 24 h, since the lowest concentration of 0.18 mg/mL did not produce any further cytotoxic effect, as it was demonstrated from dose-dependent analysis of DMCRT on A172 cells (Figure 1F). When comparing 72 vs. 48 h, the time-dependent result was evident at concentrations 1.43–5.71 mg/mL, whereas at lowest concentrations 0.18–0.36 mg/mL cells recovered at 72 h from the effects of DMCRT at 48 h, probably because the low concentrations could not sustain a prolonged cytotoxic effect.
For the TE671 cells the time-dependent efficiency was also evident for saffron’s carotenoids at most concentrations. CRCs demonstrated time-dependent cytotoxicity at 72 h, when compared with 24 h at concentrations 0.71–11.43 mg/mL, whereas the lowest concentrations 0.18–0.36 mg/mL could not sustain a prolonged cytotoxic effect. When comparing the activity of CRCs on TE671 cells at 72 vs. 48 h, the time-dependent effect was evident for concentrations ≤ 5.71, as the highest concentration of 11.43 mg/mL produced significant cellular alterations and cytotoxicity at 24 h, reaching a plateau at 48 h. In the case of DMCRT, a clear time -dependent cytotoxic action for all concentrations at 72 h was observed, when comparing both with 24 and 48 h, as it was evident with A172 cells. When comparing 48 vs. 24 h, a time-dependent cytotoxicity was observed, however was not significant, with the exclusion of concentrations near the calculated IC50 for 48 h (1.43–2.86 mg/mL), as the cytotoxic effect was maximized at 24 h, whereas sustained exposure for 72 h was needed to produce further significant cytotoxicity.
The observed partial cellular recovery, i.e., for concentrations ≤1.43 mg/mL and ≤0.18 mg/mL of CRCs and DMCRT respectively at 72 vs. 48 h for A172 cells, ≤1.43 mg/mL and ≤0.36 mg/mL of CRCs, at 48 vs. 24 h and 72 vs. 48 h respectively for TE cells could be associated with a possible cytostatic effect of CRCs, causing cell cycle arrest at the G0/G1 phase, an effect already studied in several cancer cell lines [74,75,82,113,114,115]. Cells which are blocked in the G0/G1 phase successfully repair DNA lesions and are able to re-enter the cell cycle in a process called checkpoint recovery, allowing cell survival and proliferation. This phenomenon suggests a possible acquired resistance of cancer cells to saffron metabolites, as observed during usual chemotherapy and radiation therapy, attenuating their effect and leading to chemoresistance and tumor recurrence [116,117,118].
The calculation of IC50s demonstrated the time dependent effect of CRCs and DMCRT, as well the superior in vitro activity of CRCs on both cell lines and the sensitivity of TE671 cells, when compared with A172 cells for both carotenoids. More specifically, the IC50 values for both compounds for A172 cells were almost 2× and 1.5× higher at 24 and 48/72 h respectively, in comparison with the respective values for TE671 cells. The calculated IC50 are in accordance with other studies on several cancer lines and within the reference range of safety for normal cells (Table 2) [11,90,93,95,96,103,105]. To our knowledge, it is the first time that the aforementioned cell lines, are exposed to CRCs and DMCRT and relevant IC50 are calculated.
Table 2.
Antiproliferative effect of CRCs and DMCRT on several cell lines.
| Cancer | Cell Line | IC50 | Range of Concentrations | Ref(s) |
|---|---|---|---|---|
| Bladder cancer | 5637 | 0.2 mg/mL | 0.05–4 mg/mL of SAE | [23,119] |
| Breast cancer | MDA-MB-231 | 0.5 mg/mL of SAE | 0.1–1 mg/mL of SAE | [25,62,66,80,81,98,105,120,121,122] |
| >0.195 mg/mL of trans-CRC-4 | 9.7696 μg/mL–0.976 mg/mL of trans-CRC-4 | |||
| BT-474 HER2+ | 3.5 mg/mL at 24 h of CRCs | 1–5 mg/mL of CRCs | ||
| MCF-7 | 0.35–0.78 mg/mL of SAE | 0.1–1 mg/mL of SAE | ||
| >0.195 mg/mL of trans-CRC-4 | 9.7696 μg/mL–0.976 mg/mL of trans-CRC-4 | |||
| 3.5 mg/mL at 48 h of treatment with CRCs | 1.5–6 mg/mL of CRCs | |||
| 0.05 mg/mL of CRCs | 0.01–0.2 mg/mL of CRCs | |||
| 3.5 mg/mL of CRCs | 2–5 mg/mL of CRCs | |||
| >4 mg/mL of CRCs | 1–4 mg/mL of CRCs | |||
| BT-549 | ~4 mg/mL of CRCs | 1–4 mg/mL of CRCs | ||
| MDA-MB-468 | 3–4 mg/mL of CRCs | 2–5 mg/mL of CRCs | ||
| 3 mg/mL of CRCs at 24 h 2.5 mg/mL of CRCs at 48 h 1.5 mg/mL of CRCs at 72 h |
1–5 mg/mL of CRCs | |||
| Cervical cancer | HeLa | 2.3 mg/mL of saffron ethanolic extract | 1–5 mg/mL of saffron ethanolic extract | [63,76,105,123,124,125,126] |
| 1.92 mg/mL of saffron extracts | 0.25–4 mg/mL of saffron extracts | |||
| 3–3.5 mg/mL of CRCs | 0.976–9.7696 mg/mL of CRCs | |||
| 0.072248 mg/mL of CRT | 0.328–1.31 mg/mL of CRT | |||
| SiHa | 3.9078 mg/mL of CRCs | 0.0587–15.613 mg/mL of CRCs | ||
| Sensitive human cervical cancer cell line OV2008 cells | 3 mg/mL of CRCs at 24 h 1.5–2.7 mg/mL of CRCs at 48 h 1–1.5 mg/mL of CRCs at 72 h |
1–5 mg/mL of CRCs | ||
| Resistant human cervical cancer cell line C13 | 7 mg/mL of CRCs at 24 h 5 mg/mL of CRCs at 48 h 2.5 mg/mL of CRCs at 72 h |
1–4 mg/mL of CRCs | ||
| Colorectal cancer | SW480 | >1.0 mg/mL of Crocus sativus extract | 0.25–3 mg/mL of Crocus sativus extract | [67,104,127,128] |
| 0.977 mg/mL of CRCs | 0.029–0.977 mg/mL of CRCs | |||
| HCT116 | 1.0 mg/mL of Crocus sativus extract | 0.25–3 mg/mL of Crocus sativus extract | ||
| 1.94 mg/mL of CRCs | 0.977–4 mg/mL of CRCs | |||
| >0.293 mg/mL of CRCs | 0.029–0.977 mg/mL of CRCs | |||
| 4.89–7.82 mg/mL of CRCs | 0.488–14.65 mg/mL of CRCs | |||
| 0.052 mg/mL of CRT | 0.328–1.31 mg/mL CRT | |||
| HT-29 human colon adenocarcinoma cells | >1.0 mg/mL of Crocus sativus extract | 0.25–3 mg/mL of Crocus sativus extract | ||
| >0.293 mg/mL of CRCs | 0.029–0.977 mg/mL of CRCs | |||
| 0.39 mg/mL of CRCs | 4.88 μg/mL–1.9539 mg/mL of CRCs | |||
| >40 μg/mL of CRCs | 10–40 μg/mL of CRCs | |||
| Caco-2 | ~40 μg/mL of CRCs | 10–40 μg/mL of CRCs | ||
| DHD/K12-PROb rat colon adenocarcinoma cells | 0.97696 mg/mL of CRCs | 4.88 μg/mL–1.9539 mg/mL of CRCs | ||
| Cutaneous squamous cell carcinoma | A431 | 3.9078 mg/mL of CRCs | 0.977–3.9078 mg/mL of CRCs | [129] |
| SCL-1 | 3.9078 mg/mL of CRCs | 0.977–3.9078 mg/mL of CRCs | ||
| Esophageal squamous carcinoma | KYSE-150 | 65.68 μg/mL of CRT | 4.105–65.68 μg/mL of CRT | [130,131] |
| Gastric cancer | AGS | 2.026–4 mg/mL of CRCs | 0.003–19.68 mg/mL of CRCs | [48,69,70,132,133] |
| 2 mg/mL of CRCs | 2–6 mg/mL of CRCs | |||
| 2.405 mg/mL of CRCs | 0.003–19.68 mg/mL of CRCs | |||
| SGC-7901 | 2.527 mg/mL of CRCs | 0.003–19.68 mg/mL of CRCs | ||
| HGC-27 | 2–4 mg/mL of CRCs | 2–6 mg/mL of CRCs | ||
| BGC-823 | 2.321 mg/mL of CRCs | 8–16 mg/mL of CRCs | ||
| EPG85-257 | ~78.15 μg/mL | 9.77–97.7 μg/mL | ||
| Head and neck | HN5 | 0.58 mg/mL of CRCs at 48 h 0.48 mg/mL of CRCs at 72 h 12.5–50 µg/mL of CRCs for 24, 48 and 72 h had no inhibitory effects on cell proliferation |
12.5–1000 μg/mL of CRCs | [45,132] |
| Hepatocellular carcinoma | HepG2 | 3 mg/mL of CRCs | 0.977–5 mg/mL of CRCs | [64,71,125] |
| 2.75–3.25 mg/mL of CRCs at 48 h | 0–10 mg/mL of CRCs | |||
| 0.2 mg/mL CRT | 0.328–1.31 mg/mL of CRT | |||
| HCCLM3 | 3 mg/mL of CRCs | 3–5 mg/mL | ||
| Leukemia | HL60 | 5 mg/mL of CRCs at 24 h | 0.625–10 mg/mL of CRCs | [25,72,73,134,135,136,137] |
| 3 mg/mL of CRCs at 48 h | 0.625–10 mg/mL of CRCs | |||
| >0.0328 mg/mL of CRT | 0.0016–0.0328 mg/mL of CRT | |||
| 11–39 mg/mL of CRCs | Non applicable | |||
| 7–30 mg/mL of DMCRT | Non applicable | |||
| MOLT-4 human T-cell leukemia cell line | >0.488 mg/mL of CRCs | 0.0488–0.488 mg/mL of CRCs | ||
| Jurkat cells | 2.5 mg/mL of CRCs at 24 h 1.25 mg/mL at 48 h of CRCs | 0.625–10 mg/mL of CRCs | ||
| CO 88BV59-1 EBV-transformed B-lymphocyte | 0.17 mg/mL of CRCs at 24 h 0.109 mg/mL of CRCs at 48 h 0.0774 mg/mL of CRCs at 72 h |
0.195 μg/mL–0.195 mg/mL of CRCs | [42] | |
| Lung adenocarcinoma | A549 | 170–380 μg/mL of SAE | 100–800 μg/mL of SAE | [11,74,125,138] |
| 1.5 mg/mL of saffron ethanolic extract at 24 h 0.565 mg/mL of saffron ethanolic extract at 48 h |
0.5–2 mg/mL of saffron ethanolic extract | |||
| 5.3537 mg/mL of CRCs | 0.977–4 mg/mL of CRCs | |||
| 4–5 mg/mL | 1–16 mg/mL of CRCs | |||
| 0.134 mg/mL CRT | 0.328–1.31 mg/mL of CRT | |||
| 4–5 mg/mL | 1–16 mg/mL | |||
| SPC-A1 | 4–5 mg/mL | 1–16 mg/mL | ||
| Neuroblastoma | SKNSH | 1.66 mg/mL of saffron extracts | 0.25–4 mg/mL of saffron extracts | [105] |
| Ovarian cancer | SK-OV-3 | 3.3 mg/mL of CRCs | 0.977–4 mg/mL of CRCs | [125,139] |
| 0.0623 mg/mL CRT | 0.328–1.31 mg/mL of CRT | |||
| A2780 | >0.0781 mg/mL of CRCs | 0.00977–0.0977 mg/mL of CRCs | ||
| Oral squamous cell carcinoma | KB | 1.9246 mg/mL of CRCs | 0.0488–3.9078 mg/mL of CRCs | [68] |
| Osteosarcoma | MG63 | 1.95–3.9 mg/mL of CRCs | 0.488–3.9 mg/mL of CRCs | [65] |
| OS732 | ||||
| Pancreatic cancer | BxPC-3 | >10 mg/mL of CRCs | 1–10 mg/mL of CRCs | [140] |
| Prostate cancer | Hormone-sensitive LAPC-4, CWR22 and LnCaP cells |
4.2-> 8 mg/mL of aqueous saffron extracts | 0.1–8 mg/mL of aqueous and alcoholic saffron extracts | |
| Hormone-insensitive 22rv1, C4–2B, DU145, PC3 cells | 0.8–7.9 mg/mL of alcoholic saffron extracts | 0.1–8 mg/mL of aqueous and alcoholic saffron extracts | [75] | |
| 0.254–0.928 mg/mL of CRCs | 0.0977–3.91 mg/mL of CRCs | |||
| Retinoblastoma | Y-79 | 0.0195–0.07815 mg/mL of CRCs | 0.00488–0.07815 mg/mL of CRCs | [141] |
| WERI-Rb-1 | 0.0195–0.07815 mg/mL of CRCs | 0.00488–0.07815 mg/mL of CRCs | ||
| Tongue Squamous Cell Carcinoma | Tca8113 | 0.218 mg/mL of CRCs | 0.098–0.781 mg/mL of CRCs | [142] |
A challenge arising from cytotoxicity experiments of drugs and phytochemicals, is the projection of the calculated IC50s to optimized dosing and the prediction of in vivo efficacy and safety, so as to facilitate drug development for future clinical trials. However, in order to predict in vivo efficacy and project optimized dosing for clinical trials it requires physiologically-based pharmacokinetic/pharmacodynamic (PK/PD) mathematical models. Of note, drug efficacy is a highly complex process which depends on several factors, including the xenobiotic’s physicochemical properties, the route of administration, the characteristics of the formulation and characteristics of the recipient, affecting the absorption, distribution, metabolism and excretion, e.g., cytochrome P450 enzymes (CYP) activity, liver or kidney diseases and underlying physiological properties of the gastrointestinal tract.
The safety of saffron and its metabolites have been evaluated in clinical trials of both adult and pediatric patients, administered mainly p.o., in different dosages or formulations (14–450 mg of saffron, 5–30 mg of CRCs, or 7.5–37.5 mg of CRT) [143,144,145]. Administration of Crocus sativus L. stigma tablets (200 and 400 mg/day, for 7 days) in healthy adult volunteers, was safe, without significantly affecting any hematological, biochemical and coagulation parameters [146,147,148]. Adult patients suffering from mild-to-moderate depression and anxiety, when receiving 30–100 mg of dried saffron stigma in 4, 6, 8 or 12-week trials, demonstrated mild significant side effects, which resolved spontaneously, without any intervention [149,150,151,152,153,154,155]. Similarly, capsules of SAE, and/or CRCs were well tolerated in doses of 15 mg twice daily for 12 weeks in patients suffering from COPD (chronic obstructive pulmonary disease), PCOS (polycystic ovary syndrome), depression or schizophrenia [154,156,157,158]. Patients with Alzheimer’s disease (AD) who received saffron extract (30 mg/day) capsules for 12 months benefited significantly, in terms of cognitive improvement and management of associated neuropsychiatric disturbances, without adverse events [159,160]. A similar safety profile was demonstrated in other clinical trials on patients with AD who received a capsule saffron 30 mg/day for 12, 16, or 22 weeks [161,162]. Administration of 14 mg of a standardized saffron extract in an 8-week trial to children aged 12–16 years old, exhibited beneficiary antidepressant and anxiolytic effects and was adequately tolerated, without any adverse effects [163]. Additionally, the administration of CRCs tablets (20 mg) in healthy volunteers for one month did not elicit any major adverse events or affect hematological, biochemical and hormonal parameters [148]. CRCs at a dose of 30 mg/day for a period of 8 weeks in patients with metabolic syndrome was associated with a favorable antioxidant effect [26,164]. CRCs at doses 5–15 mg for 3 months in diabetic patients, suffering from diabetic maculopathy, had a significant hypoglycemic effect, decreased HbA1c and ameliorated oxidative stress and inflammation [165].
Interestingly, the pharmacokinetic profile in humans of saffron and its metabolites have not been adequately studied. In a recent report, p.o. administration of saffron extract (56 and 84 mg) resulted in high bioaccessibility of CRCs isomers and CRT; 40.77 and 66.67, respectively, even after degradation during salivary, gastric and duodenal digestion. However, only CRT was detected in the plasma instead of CRCs, confirming that CRCs isomers are subjected to intestinal hydrolysis in the epithelial cells, forming deglycosylase trans-CRT, which is subsequently absorbed by passive diffusion from the intestinal epithelium, before reaching the bloodstream [166,167,168]. The maximum concentrations (Cmax) achieved were 0.26 μg/mL at 60 min and 0.39 μg/mL and 90 min, following 56 and 84 mg administration of the saffron extract, respectively, indicating a dose-dependent response kinetics [169,170]. The results were similar to other studies, as the Cmax of CRT was calculated 0.28 μg/mL and 0.35 μg/mL following a single p.o. dose of 22.5 mg of G. jasminoides CRT and 16 mg of saffron CRT, respectively. Oral and intravenous administration in rodents of SFE (60 mg/kg, equivalent with 16.7 mg/kg of CRCs), rich in all-trans-crocin (27.8% w/w) was associated with Cmax of CRT 2.77 μg/mL in serum [167]. CRT in most clinical and in vivo studies was not detected after 3–4 h of administration, suggesting a relatively short half-life for CRT (~0.85 ± 0.04 h), although longer time of detection (~24 h) has been documented [169,171,172]. CRT does not accumulate in the plasma, even after repeated oral doses of CRCs for six days [173].
Collectively, doses of saffron up to 1.5 gr/day are considered safe, whereas mild adverse effects are reported with doses of more than 5 gr/day. Adverse effects include nausea, vomiting, diarrhea, vertigo, hematuria, hematemesis or melena, uterus bleeding [12]. Doses more than 10 gr/day are associated with the induction of abortion, whereas there is a study in which frequent exposure to saffron particles, even without ingestion, could cause miscarriage [174]. Overdose (12–20 g/day) may be fatal [24]. The oral LD50 of saffron was estimated 20.7 g/kg, when administered as decoction, whereas the LD50 value of oral administration of SAE or ethanolic saffron extract was 3.6–5 g/kg [155]. Intraperitoneal treatment with saffron stigma extract or petal extract was associated with an LD50 value of 1.6 g/kg and 6 g/kg, respectively [26,160,175,176,177]. The LD50 of CRCs is estimated at >3 g/kg [26,33,34,35,36,37,38,39,40,41,42].
With reference to the previous data, saffron and its major constituents present a rather safe pharmacokinetic profile, even in healthy volunteers, as according to the toxicity classification, substances with a LD50 value within the range of 0.5–5 g/kg are considered practically low-toxic and substances with LD50 > 5 g/kg may be considered practically non-toxic [23,25,147,178,179,180,181]. In the current study, although a wide range of concentrations was used, i.e., 0.179–22.85 mg/mL for CRCs and 0.09–11.429 for DMCRT, such high concentrations were also employed in studies of saffron metabolites on hormone-insensitive prostate cancer cells (22rv1, C4–2B, DU145, PC3 cells), lung adenocarcinoma cells (A549, SPC-A1), HL-60 leukemia cells, HCT116 colorectal carcinoma and gastric adenocarcinoma (BGC-823, SGC-7901) cells. Thus, it can be safely suggested that the lower to middle, in vitro administered doses in the present preclinical study, could also be applied in an in vivo experimental setup and clinical studies and the calculated IC50s are in agreement with several other studies on different cancerous cell lines (Table 2).
4.2. Gene Expression Alterations in Cancer Cell Lines in the Presence of Saffron’s Metabolites
Despite accumulating evidence demonstrating that saffron and its carotenoids may be promising anti-cancer and chemopreventive agents, mechanisms of their actions are still largely ambiguous. Our team suggested that CRCs and DMCRT at the dose of 1 mg/mL significantly enhanced apoptotic phenomena on both A172 and TE671 cell lines, by altering the expression of pro-apoptotic, pro-survival and detoxifying genes.
In A172 cells CRCs mediated apoptosis at 48 h via BAX upregulation. At 72 h however, CRCs triggered apoptosis, probably mainly via MYCN downregulation, as BAX and BID were downregulated. DMCRT induced apoptosis at 48 h via BID and BAX overexpression and BCL-2 downregulation. At 72 h MYCN downregulation was the main determinant of apoptosis of cells exposed to DMCRT. The antiapoptotic BCL-2 was significantly up-regulated at 48 h when cells were treated with CRCs and at 72 h when cells were exposed to CRCs or DMCRT, an effect which could also indicate a cellular attempt to counteract the BAX/BID apoptotic stimuli. The downregulation of the pro-apoptotic proteins BAX/BID at 72 h could explain the recovery effect from the compounds’ cytotoxic action.
In TE671 cells CRCs exerted an apoptotic effect at 48 h via BAX and BID upregulation, which was at some level counteracted by BCL-2, overexpression, leading to the observed cell recovery in cytotoxicity experiments. At 72 h however, CRCs triggered apoptosis, probably mainly via BCL-2 downregulation, as BAX and BID expression was also suppressed. DMCRT induced apoptosis at 48 h mainly via BID overexpression, whereas at 72 h BAX upregulation, with concomitant BCL-2 and MYCN, downregulation could justify the stronger cytotoxic effect of DMCRT at this time point. Although some gene expressions did not manifest significant differences, they still presented with an ascending or a descending pattern with respect to treatment, which should be taken into consideration.
Several phytochemicals exert their anti-cancer effects via inducing apoptosis in cancer cells. Apoptotic pathways are initiated via different entry sites, e.g., at the mitochondria (mitochondrial or intrinsic pathway) and the plasma membrane by death receptor signaling molecules (receptor or extrinsic pathway). Saffron and its active constituents stimulate apoptosis by activating and/or upregulating the expression of caspases, important in the initiation and execution of apoptosis, e.g., caspases-1, 3, 4, 5, 6, 7, 8 and-9, in a time-dependent manner. CRCs in several cancer cell lines up-regulate the expression of the proapoptotic proteins Bax, p53 and FasR receptor (FAS receptor)/APO-1 (apoptosis antigen 1). Bax and BID integrate apoptotic signals by disrupting the mitochondrial membrane potential, thus triggering the mitochondrial apoptotic pathway, as they facilitate permeabilization of the mitochondrial outer membrane and the release of apoptogenic factors to the cytosol, such as cytochrome c (CytC), Smac/Diablo, apoptosis-inducing factor (AIF) and endonuclease G (Endo-G). The tumor suppressor protein p53 induces both mitochondria-mediated apoptosis by activating other apoptotic proteins of BCL-2 family (Bax, Puma, Noxa) and the death receptor/extrinsic apoptosis pathway by promoting Fas/APO-1 death receptor expression [182,183]. Bcl-2 mediates antiapoptotic signals, preserving mitochondrial integrity, thus serving as a cellular mechanism to evade apoptosis and is associated with resistance to chemotherapeutic drugs. Conversely, CRCs down-regulate the expression of the anti-apoptotic Bcl-2, thus modulating the BAX/Bcl-2 ratio [74,81,82,83,96,135,138,184,185]. The balance between Bcl-2 and BAX expression dictates cellular behavior towards pro-survival or pro-apoptotic conditions [72].
Most studies agree on the fact that Bcl-2 and Bax manifest opposite expression patterns under treatment with saffron or its metabolites, i.e., Bcl-2 is down-regulated and Bax up-regulated (Table 3). To the best of our knowledge, this is the first report concerning the effects of CRCs and DMCRT on glioblastoma and rhabdomyosarcoma tumor cells. Yet in two known studies concerning neuronal cells (PC12 cells rat pheochromocytoma, retinal ganglion cells), exposure to saffron compounds elicited similar patterns of BCL2 and Bax expression, as to our study, i.e., Bcl-2 upregulation and Bax downregulation, protecting cells against oxidative stress-induced cell apoptosis [186,187]. Thus, as indicated in Table 3, saffron metabolites’ action on Bax and Bcl-2 expression is tissue and cellular specific or associated with cellular exposure to intense oxidative stress. In our study, cellular apoptosis at the dose of 1 mg/mL, could be initiated and executed by additional pathways, as indicated in other studies, i.e., suppression of STAT3 activation and p73-dependent FAS-FADD (Fas-Associated Death Domain) induction, leading to BID activation, disruption of DNA–protein interactions (e.g., topoisomerase II), thus inhibiting nucleic acid synthesis, inactivation of the pro-survival cascade of PI3K/AKT, decreased expression of several MAPK (ERK1/2, p38), downregulation of JAK/STAT pathway, up-regulation of p21/CIP1 and p27/KIP1 which bind to CDK2, preventing its kinase activity causing cell cycle arrest [88,113,187,188,189,190,191].
Table 3.
Summary of the effects of CRCs and CRT on BCL2 and BAX gene expression.
| Cell Line | BCL2 | Effect | Ref. |
|---|---|---|---|
| Normal human liver cell L02 | ↑ | Anti-apoptotic | [94] |
| Zebrafish NAFLD (Non-Alcoholic Fatty Liver Disease) model | ↑ | Anti-apoptotic | [94] |
| MC3T3-E1 osteoblasts | ↑ | Anti-apoptotic (after treatment with dexamethasone) | [209] |
| PC12 cells rat pheochromocytoma | ↑ | Anti-apoptotic (after treatment with acrylamide) | [186] |
| Retinal ganglion cells (RGCs) | ↑ | Anti-apoptotic (after retinal ischemia/reperfusion-IR injury) | [39] |
| Rat neural stem cells | ↑ | Anti-apoptotic (after glucose deprivation or IR injury) | [89] |
| Bovine aortic endothelial cells | ↑ | Anti-apoptotic (after cells are exposed to H2O2) | [100] |
| T24 cell of transitional cell carcinoma of bladder (TCCB) | ↓ | Pro-apoptotic | [114] |
| EBV-Transformed B-Lymphocytes | ↓ | Pro-apoptotic | [42] |
| BXPC3 and Capan-2 pancreatic adenocarcinoma | ↓ | Pro-apoptotic | [191] |
| KYSE-150 cells esophageal squamous cell carcinoma | ↓ | Pro-apoptotic | [190] |
| Acute promyelocytic leukemia cells HL60, NB4 and primary APL cells | ↓ | Pro-apoptotic | [210] |
| HL60 acute promyelocytic leukemia cells | ↓ | Pro-apoptotic | [72] |
| Hep3B and HepG2 hepatoblastoma | ↓ | Pro-apoptotic | [47] |
| Gastric adenocarcinoma (AGS) cells | ↓ | Pro-apoptotic | [96] |
| Human multiple myeloma cells | ↓ | Pro-apoptotic | [188] |
| MCF-7 breast cancer cells | ↓ | Pro-apoptotic | [184] |
| Human prostate cancer cells (LAPC-4, PC3) | ↓ | Pro-apoptotic | [75] |
| Lung adenocarcinoma A549 and SPC-A1 | ↓ | Pro-apoptotic | [74] |
| Normal human liver cell L02 | ↓ | Anti-apoptotic | [94] |
| Zebrafish | ↓ | Anti-apoptotic | [94] |
| MC3T3-E1 osteoblasts | ↓ | Anti-apoptotic | [209] |
| PC12 cells rat pheochromocytoma | ↓ | Anti-apoptotic (after treatment with acrylamide) | [186] |
| Retinal ganglion cells (RGCs) | ↓ | Anti-apoptotic (after retinal ischemia/reperfusion-IR injury) | [39] |
| Rat neural stem cells | ↓ | Anti-apoptotic (after glucose deprivation or IR injury) | [89] |
| Bovine aortic endothelial cells | ↓ | Anti-apoptotic (after cells are exposed to H2O2) | [100] |
| EBV-Transformed B-Lymphocytes | ↑ | Pro-apoptotic | [42] |
| KYSE-150 cells esophageal squamous cell carcinoma | ↑ | Pro-apoptotic | [190] |
| Acute promyelocytic leukemia cells HL60, NB4 and primary APL cells | ↑ | Pro-apoptotic | [210] |
| HL60 acute promyelocytic leukemia cells | ↑ | Pro-apoptotic | [72,210] |
| Hep3B and HepG2 hepatoblastoma | ↑ | Pro-apoptotic | [47] |
| Gastric adenocarcinoma (AGS) cells | ↑ | Pro-apoptotic | [96] |
| MCF-7 breast cancer cells | ↑ | Pro-apoptotic | [184] |
| Human prostate cancer cells (LAPC-4, PC3) | ↑ | Pro-apoptotic | [75] |
| T24 cell of transitional cell carcinoma of bladder (TCCB) | ↑ | Pro-apoptotic | [114] |
| HL-60 Human Leukemia Cells | ↑ | Pro-apoptotic | [72] |
| Lung adenocarcinoma A549 and SPC-A1 | ↑ | Pro-apoptotic | [74] |
| Human multiple myeloma cells | ↑ | Pro-apoptotic | [188] |
| BXPC3 and Capan-2 pancreatic adenocarcinoma | ↑ | Pro-apoptotic | [191] |
MYCN belongs to the MYC family of proto-oncogenes and transcription factors, encoding a helix-loop-helix (bHLH) domain-based protein, implicated in the progression of the cell cycle through the G1/S transition. MYCN represents a critical oncogene, maintaining the tumorigenic state in several cancer types, implicated in cancer initiation, progression and invasion [141,191,192]. C-Myc downregulation at 72 h in the presence of CRCs and DMCRT for A172 cells and in the presence of DMCRT for TE671 cells could represent another mode of saffron cytotoxicity and induction of apoptosis in a MYCN-dependent manner. Similarly, CRCs were found to abrogate c-Myc expression in BXPC3 and capan-2 pancreatic adenocarcinoma cells and Y-79 and WERI-Rb-1 human retinoblastoma cells [141,191]. Thus, saffron metabolites could convey apoptotic signals via several pathways. Further investigation is necessary to elucidate the molecular mechanism by which CRCs and DMCRT are implicated in apoptosis of neuronal tumor cells.
A commonly accepted mechanism for the antitumor action of saffron is the inhibitory effect on free radical chain reactions, acting as high-efficiency reactive oxygen species (ROS) scavengers. Saffron and its metabolites in non-cancerous cells reduce oxidative stress overload by protecting against mitochondrial dysfunction and increasing the activity of antioxidant enzymes [superoxide dismutase (SOD), catalase, the enzymes of glutathione thioredoxin system, i.e., glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST)], quenching free radicals [e.g., hydroxyl (•OH), superoxide (O2•−), nitric oxide (NO•), nitrogen dioxide (•NO2), peroxyl (RO2•), alkoxyl (RO-) and lipid peroxyl (LOO•)] and decreasing lipid peroxidation of cell membranes [MDA, 4-hydroxynonenal (HNE), 4-oxo-2-nonenal (ONE) and 2-propenal acrolein] [16,27,40,67,74,83,87,193,194,195,196,197,198,199,200,201,202,203,204]. In A172 cells both SOD1 and GSTM1 were down-regulated, at 72 h of treatment with CRCs or DMCRT, although initially upregulated at 48 h, probably as a cellular adaptation to avoid CRCs and DMCRT cytotoxicity. In TE671 cells GSTM1 and SOD1 expression was abrogated in the presence of CRCs and DMCRT, respectively, at 72 h of exposure. Saffron’s phytochemicals at 72 h of exposure mitigated cell’s ability to maintain oxidative equilibrium and defend against oxidative stress, thus initiating cascades of apoptosis [205,206,207,208].
Overall, our results agree with three possible pathways via which CRCs and DMCRT exert their cytotoxicity on glioblastoma and rhabdomyosarcoma cell lines: (a) enhancement of BAX and BID -mediated cellular apoptosis; (b) suppression of MYCN and BCL-2 cellular survival; and (c) suppression of the expression of GSTM1 and SOD1 oxidative species scavengers.
5. Conclusions
Our research endeavors for the first time to study the possible cytotoxic effects of CRCs and their semisynthetic derivative DMCRT on a glioblastoma and rhabdomyosarcoma cell lines and alterations in pathways implicated in cellular apoptosis and survival. Both CRCs and DMCRT manifested significant cytotoxic effects on A172 and TE671 cells, probably by altering the expression of genes implicated in apoptosis, i.e., BAX and BID, or cellular survival, i.e., MYCN and BCL-2 and detoxifying mechanisms, i.e., SOD1 and GSTM1. Saffron carotenoids and their semisynthetic derivates, as they are not the precursors of vitamin A, could be employed to minimize retinoid exposure and toxicity, even in high-dose and long-term application, especially in sensitive patient groups, such as children and pregnant women. A reasonable further approach would be further deciphering the mechanisms underlying saffron’s phytochemicals effects on cancer and normal cell lines and applying in vivo experiments to models of high-risk rhabdomyosarcoma and GBM, before employing relevant clinical trials, as CRCs are considered safe phytochemicals, already tested in humans mainly for psychiatric disorders and metabolic syndrome with tolerable side effects.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/antiox11061074/s1, Table S1. The detailed description of GO enrichment as derived from the gprofiler web-tool.
Author Contributions
Conceptualization, K.H., C.K.-G. and P.A.T.; Data curation, K.H., O.N., E.K. (Eleni Kakouri), and G.I.L.; Formal analysis, K.H., O.N., S.M., S.S.M. and G.I.L.; Funding acquisition, K.H., O.N. and P.A.T.; Investigation, K.H., O.N. and M.-E.Z.; Methodology, K.H., O.N., S.M., E.K. (Eleni Koniari), E.K. (Eleni Kakouri), M.-E.Z., S.S.M., C.K., E.F. and G.I.L.; Project administration, K.H., O.N. and S.M.; Resources, K.H.; Software, K.H., S.M. and S.S.M.; Supervision, K.H., E.F., G.P.C., S.K., C.K.-G. and P.A.T.; Validation, K.H.; Visualization, K.H.; Writing—original draft, K.H., O.N., E.K. (Eleni Koniari), and S.S.M.; Writing—review & editing, K.H., E.K. (Eleni Koniari), G.I.L., C.K.-G. and P.A.T. All authors have read and agreed to the published version of the manuscript.

Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Research Project is co-financed by Greece and the European Union (European Social Fund) through the Operational Program “Human Resources Development, Education and Lifelong Learning 2014–2020” and the Program encoded EDBM103, titled “Support for researchers with an emphasis on young researchers-cycle B” (MIS 5048464).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Namayandeh S.M., Khazaei Z., Lari Najafi M., Goodarzi E., Moslem A. GLOBAL Leukemia in Children 0–14 Statistics 2018, Incidence and Mortality and Human Development Index (HDI): GLOBOCAN Sources and Methods. Asian Pac. J. Cancer Prev. 2020;21:1487–1494. doi: 10.31557/APJCP.2020.21.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci V., Boffa I., De Masi G., Zollo M. Natural compounds for pediatric cancer treatment. Naunyn-Schmiedeberg's Arch. Pharmacol. 2016;389:131–149. doi: 10.1007/s00210-015-1191-5. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7:1129–1150. doi: 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- 7.Shoeb M. Anticancer agents from medicinal plants. Bangladesh J. Pharmacol. 2008;1:35–41. doi: 10.3329/bjp.v1i2.486. [DOI] [Google Scholar]
- 8.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou E., Kadoglou N.P., Kostomitsopoulos N., Valsami G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015;67:1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinzadeh H. Saffron: A herbal medicine of third millennium. Jundishapur J. Nat. Pharm. Prod. 2014;9:1–2. doi: 10.17795/jjnpp-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samarghandian S., Boskabady M.H., Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharm. Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M., Betti G., Hensel A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien. Med. Wochenschr. 2007;157:315. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 13.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Liakopoulou-Kyriakides M., Kyriakidis D.A. Croscus sativus-biological active constitutents. Stud. Nat. Prod. Chem. 2002;26:293–312. [Google Scholar]
- 15.Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother. Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 16.Tarantilis P.A., Tsoupras G., Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. 1995;699:107–118. doi: 10.1016/0021-9673(95)00044-N. [DOI] [PubMed] [Google Scholar]
- 17.Caballero-Ortega H., Pereda-Miranda R., Abdullaev F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.) sources. Food Chem. 2007;100:1126–1131. doi: 10.1016/j.foodchem.2005.11.020. [DOI] [Google Scholar]
- 18.Bolhassani A., Khavari A., Bathaie S.Z. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim. Biophys. Acta. 2014;1845:20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Rahaiee S., Hashemi M., Shojaosadati S.A., Moini S., Razavi S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017;99:401–408. doi: 10.1016/j.ijbiomac.2017.02.095. [DOI] [PubMed] [Google Scholar]
- 20.Rahaiee S., Moini S., Hashemi M., Shojaosadati S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015;52:1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bathaie S.Z., Mousavi S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010;50:761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 22.Winterhalter P., Straubinger M. Saffron-renewed interest in an ancient spice. Food Rev. Int. 2000;16:39–59. doi: 10.1081/FRI-100100281. [DOI] [Google Scholar]
- 23.Feizzadeh B., Afshari J.T., Rakhshandeh H., Rahimi A., Brook A., Doosti H. Cytotoxic effect of saffron stigma aqueous extract on human transitional cell carcinoma and mouse fibroblast. Urol. J. 2008;5:161–167. [PubMed] [Google Scholar]
- 24.Bostan H.B., Mehri S., Hosseinzadeh H. Toxicology effects of saffron and its constituents: A review. Iran. J. Basic Med. Sci. 2017;20:110–121. doi: 10.22038/ijbms.2017.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butnariu M., Quispe C., Herrera-Bravo J., Sharifi-Rad J., Singh L., Aborehab N.M., Bouyahya A., Venditti A., Sen S., Acharya K., et al. The Pharmacological Activities of Crocus sativus L.: A Review Based on the Mechanisms and Therapeutic Opportunities of its Phytoconstituents. Oxid. Med. Cell Longev. 2022;2022:8214821. doi: 10.1155/2022/8214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehri S., Razavi B.-M., Hosseinzadeh H. Chapter 34—Safety and toxicity of saffron. In: Koocheki A., Khajeh-Hosseini M., editors. Saffron. Woodhead Publishing; Cambridge, UK: 2020. pp. 517–530. [Google Scholar]
- 27.Premkumar K., Abraham S.K., Santhiya S.T., Ramesh A. Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother. Res. 2003;17:614–617. doi: 10.1002/ptr.1209. [DOI] [PubMed] [Google Scholar]
- 28.Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Linardaki Z.I., Orkoula M.G., Kokkosis A.G., Lamari F.N., Margarity M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol. 2013;52:163–170. doi: 10.1016/j.fct.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Shoyama Y., Sugiura M., Saito H. Effects of Crocus sativus L. on the Ethanol-Induced Impairment of Passive Avoidance Performances in Mice. Biol. Pharm. Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 31.Pitsikas N., Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav. Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura M., Shoyama Y., Saito H., Nishiyama N. Crocin Improves the Ethanol-induced Impairment of Learning Behaviors of Mice in Passive Avoidance Tasks. Proc. Jpn. Acad. Ser. B. 1995;71:319–324. doi: 10.2183/pjab.71.319. [DOI] [Google Scholar]
- 33.Hosseinzadeh H., Shariaty V.M., Sameni A.K., Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L. (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–951. [Google Scholar]
- 34.Taheri F., Bathaie S.Z., Ashrafi M., Ghasemi E. Assessment of crocin toxicity on the rat liver. Modares J. Med. Sci. Pathobiol. 2014;17:67–79. [Google Scholar]
- 35.Razavi B.M., Hosseinzadeh H., Movassaghi A.R., Imenshahidi M., Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in rats in subchronic exposure. Chem. Biol. Interact. 2013;203:547–555. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y.Q., Liu J.X., Wang J.N., Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 37.Salem M., Shaheen M., Tabbara A., Borjac J. Saffron extract and crocin exert anti-inflammatory and anti-oxidative effects in a repetitive mild traumatic brain injury mouse model. Sci. Rep. 2022;12:5004. doi: 10.1038/s41598-022-09109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghadrdoost B., Vafaei A.A., Rashidy-Pour A., Hajisoltani R., Bandegi A.R., Motamedi F., Haghighi S., Sameni H.R., Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Qi Y., Chen L., Zhang L., Liu W.-B., Chen X.-Y., Yang X.-G. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp. Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinzadeh H., Sadeghnia H.R., Ziaee T., Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J. Pharm. Pharm. Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 41.Mashmoul M., Azlan A., Yusof B.N.M., Khaza’ai H., Mohtarrudin N., Boroushaki M.T. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J. Funct. Foods. 2014;8:180–187. doi: 10.1016/j.jff.2014.03.017. [DOI] [Google Scholar]
- 42.Jahromi A.S., Kargar M., Kafilzadeh F., Jamalidoust M., Moradzadeh M. Crocin Promotes Apoptosis in Human EBV-Transformed B-Lymphocyte via Intrinsic Pathway. Mediterr J. Hematol. Infect Dis. 2021;13:e2021049. doi: 10.4084/MJHID.2021.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadnia H., Tavakkol Afshari J., Tabeshpour J., Younesi Rostami M., Mansourian E., Akhavan Rezayat A., Brook A. Cytotoxic Effect of Saffron Stigma Aqueous Extract on Human Prostate Cancer and Mouse Fibroblast Cell Lines. Urol. J. 2020;18:633–638. doi: 10.22037/uj.v16i7.6331. [DOI] [PubMed] [Google Scholar]
- 44.Amin A., Farrukh A., Murali C., Soleimani A., Praz F., Graziani G., Brim H., Ashktorab H. Saffron and Its Major Ingredients’ Effect on Colon Cancer Cells with Mismatch Repair Deficiency and Microsatellite Instability. Molecules. 2021;26:3855. doi: 10.3390/molecules26133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bijani F., Zabihi E., Bijani A., Nouri H.R., Nafarzadeh S., Seyedmajidi M. Evaluation of apoptotic effect of crocin, cisplatin, and their combination in human oral squamous cell carcinoma cell line HN5. Dent. Res. J. 2021;18:70. [PMC free article] [PubMed] [Google Scholar]
- 46.Colapietro A., Mancini A., Vitale F., Martellucci S., Angelucci A., Llorens S., Mattei V., Gravina G.L., Alonso G.L., Festuccia C. Crocetin Extracted from Saffron Shows Antitumor Effects in Models of Human Glioblastoma. Int. J. Mol. Sci. 2020;21:423. doi: 10.3390/ijms21020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim B., Park B. Saffron carotenoids inhibit STAT3 activation and promote apoptotic progression in IL-6-stimulated liver cancer cells. Oncol. Rep. 2018;39:1883–1891. doi: 10.3892/or.2018.6232. [DOI] [PubMed] [Google Scholar]
- 48.Shariat Razavi S.M., Mahmoudzadeh Vaziri R., Karimi G., Arabzadeh S., Keyvani V., Behravan J., Kalalinia F. Crocin Increases Gastric Cancer Cells’ Sensitivity to Doxorubicin. Asian Pac. J. Cancer Prev. 2020;21:1959–1967. doi: 10.31557/APJCP.2020.21.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmadabadi F., Saghebjoo M., Hedayati M., Hoshyar R., Huang C.J. Treatment-induced tumor cell apoptosis following high-intensity interval training and saffron aqueous extract in mice with breast cancer. Physiol. Int. 2021;108:19–26. doi: 10.1556/2060.2021.00009. [DOI] [PubMed] [Google Scholar]
- 50.Kakouri E., Agalou A., Kanakis C., Beis D., Tarantilis P.A. Crocins from Crocus sativus L. in the Management of Hyperglycemia. In Vivo Evidence from Zebrafish. Molecules. 2020;25:5223. doi: 10.3390/molecules25225223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmona M., Zalacain A., Sanchez A.M., Novella J.L., Alonso G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006;54:973–979. doi: 10.1021/jf052297w. [DOI] [PubMed] [Google Scholar]
- 52.Mohajeri S.A., Hosseinzadeh H., Keyhanfar F., Aghamohammadian J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J. Sep. Sci. 2010;33:2302–2309. doi: 10.1002/jssc.201000183. [DOI] [PubMed] [Google Scholar]
- 53.Karkoula E., Angelis A., Koulakiotis N.S., Gikas E., Halabalaki M., Tsarbopoulos A., Skaltsounis A.L. Rapid isolation and characterization of crocins, picrocrocin, and crocetin from saffron using centrifugal partition chromatography and LC-MS. J. Sep. Sci. 2018;41:4105–4114. doi: 10.1002/jssc.201800516. [DOI] [PubMed] [Google Scholar]
- 54.Assimiadis M.K., Tarantilis P.A., Polissiou M.G. UV-Vis, FT-Raman, and 1H NMR Spectroscopies of cis-trans Carotenoids from Saffron (Crocus sativus L.) Appl. Spectrosc. 1998;52:519–522. doi: 10.1366/0003702981944058. [DOI] [Google Scholar]
- 55.Tarantilis P.A., Beljebbar A., Manfait M., Polissiou M. FT-IR, FT-Raman spectroscopic study of carotenoids from saffron (Crocus sativus L.) and some derivatives. Spectrochim Acta A. 1998;54:651–657. doi: 10.1016/S1386-1425(98)00024-9. [DOI] [Google Scholar]
- 56.Hamid R., Rotshteyn Y., Rabadi L., Parikh R., Bullock P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. In Vitro. 2004;18:703–710. doi: 10.1016/j.tiv.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Petrenko Y.A., Gorokhova N.A., Tkachova E.N., Petrenko A.Y. The reduction of Alamar Blue by peripheral blood lymphocytes and isolated mitochondria. Ukr Biokhim Zh (1999) 2005;77:100–105. [PubMed] [Google Scholar]
- 58.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Ramakers C., Ruijter J.M., Deprez R.H., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 60.Raudvere U., Kolberg L., Kuzmin I., Arak T., Adler P., Peterson H., Vilo J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update) Nucleic Acids Res. 2019;47:W191–W198. doi: 10.1093/nar/gkz369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B., Schmoyer D., Kirov S., Snoddy J. GOTree Machine (GOTM): A web-based platform for interpreting sets of interesting genes using Gene Ontology hierarchies. BMC Bioinform. 2004;5:16. doi: 10.1186/1471-2105-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vali F., Changizi V., Safa M. Synergistic Apoptotic Effect of Crocin and Paclitaxel or Crocin and Radiation on MCF-7 Cells, a Type of Breast Cancer Cell Line. Int. J. Breast Cancer. 2015;2015:139349. doi: 10.1155/2015/139349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollaei H., Safaralizadeh R., Babaei E., Abedini M.R., Hoshyar R. The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed. Pharmacother. 2017;94:307–316. doi: 10.1016/j.biopha.2017.07.052. [DOI] [PubMed] [Google Scholar]
- 64.Yao C., Liu B.B., Qian X.D., Li L.Q., Cao H.B., Guo Q.S., Zhou G.F. Crocin induces autophagic apoptosis in hepatocellular carcinoma by inhibiting Akt/mTOR activity. OncoTargets Ther. 2018;11:2017–2028. doi: 10.2147/OTT.S154586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Huang T., Jiang G., Gong W., Qian H., Zou C. Synergistic apoptotic effect of crocin and cisplatin on osteosarcoma cells via caspase induced apoptosis. Toxicol. Lett. 2013;221:197–204. doi: 10.1016/j.toxlet.2013.06.233. [DOI] [PubMed] [Google Scholar]
- 66.Faridi N., Heidarzadeh H.R., Mohagheghi M.A., Bathaie S.Z.J.B., Research C.C. BT-474 Breast Cancer Cell Apoptosis Induced by Crocin, a Saffron Carotenoid. Basic Clin. Cancer Res. 2019;11:5–15. doi: 10.18502/bccr.v11i1.1646. [DOI] [Google Scholar]
- 67.Amin A., Bajbouj K., Koch A., Gandesiri M., Schneider-Stock R. Defective autophagosome formation in p53-null colorectal cancer reinforces crocin-induced apoptosis. Int. J. Mol. Sci. 2015;16:1544–1561. doi: 10.3390/ijms16011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jabini R., Ehtesham-Gharaee M., Dalirsani Z., Mosaffa F., Delavarian Z., Behravan J. Evaluation of the Cytotoxic Activity of Crocin and Safranal, Constituents of Saffron, in Oral Squamous Cell Carcinoma (KB Cell Line) Nutr. Cancer. 2017;69:911–919. doi: 10.1080/01635581.2017.1339816. [DOI] [PubMed] [Google Scholar]
- 69.Luo Y., Cui S., Tang F., Shen C., Qi Y., Lu D., Ma L., Yang Y., Li Y., Chen R., et al. The combination of crocin with cisplatin suppresses growth of gastric carcinoma cell line BGC-823 and promotes cell apoptosis. Pak. J. Pharm. Sci. 2017;30:1629–1634. [PubMed] [Google Scholar]
- 70.Luo Y., Yu P., Zhao J., Guo Q., Fan B., Diao Y., Jin Y., Zhang C. Pathogenesis and anti-proliferation mechanisms of Crocin in human gastric carcinoma cells. Int. J. Clin. Exp. Pathol. 2020;13:912–922. [PMC free article] [PubMed] [Google Scholar]
- 71.Noureini S.K., Wink M. Antiproliferative effects of crocin in HepG2 cells by telomerase inhibition and hTERT down-regulation. Asian Pac. J. Cancer Prev. 2012;13:2305–2309. doi: 10.7314/APJCP.2012.13.5.2305. [DOI] [PubMed] [Google Scholar]
- 72.Sun Y., Xu H.J., Zhao Y.X., Wang L.Z., Sun L.R., Wang Z., Sun X.F. Crocin Exhibits Antitumor Effects on Human Leukemia HL-60 Cells In Vitro and In Vivo. Evid-Based Complement. Altern. Med. 2013;2013:690164. doi: 10.1155/2013/690164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y., Wang Z., Wang L., Wang L.Z., Zang C., Sun L.R. The Effect and Mechanisms of Proliferative Inhibition of Crocin on Human Leukaemia Jurkat Cells. West Indian Med. J. 2015;64:473–479. doi: 10.7727/wimj.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen S., Zhao S., Wang X., Zhang L., Jiang E., Gu Y., Shangguan A.J., Zhao H., Lv T., Yu Z. Crocin inhibits cell proliferation and enhances cisplatin and pemetrexed chemosensitivity in lung cancer cells. Transl. Lung Cancer Res. 2015;4:775–783. doi: 10.3978/j.issn.2218-6751.2015.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’Alessandro A.M., Mancini A., Lizzi A.R., De Simone A., Marroccella C.E., Gravina G.L., Tatone C., Festuccia C. Crocus sativus stigma extract and its major constituent crocin possess significant antiproliferative properties against human prostate cancer. Nutr. Cancer. 2013;65:930–942. doi: 10.1080/01635581.2013.767368. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J., Yang S., Wang K., Huang Y., Yang N., Yang Z., Zheng Z., Wang Y. Crocin induces autophagic cell death and inhibits cell invasion of cervical cancer SiHa cells through activation of PI3K/AKT. Ann. Transl. Med. 2020;8:1180. doi: 10.21037/atm-20-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cragg G.M., Boyd M.R., Cardellina J.H., 2nd, Newman D.J., Snader K.M., McCloud T.G. Ethnobotany and drug discovery: The experience of the US National Cancer Institute. Ciba Found. Symp. 1994;185:178–190. doi: 10.1002/9780470514634.ch13. discussion 190–176. [DOI] [PubMed] [Google Scholar]
- 78.Abdullaev F.I., Frenkel G.D. The effect of saffron on intracellular DNA, RNA and protein synthesis in malignant and non-malignant human cells. BioFactors. 1992;4:43–45. [PubMed] [Google Scholar]
- 79.Chryssanthi D.G., Dedes P.G., Karamanos N.K., Cordopatis P., Lamari F.N. Crocetin inhibits invasiveness of MDA-MB-231 breast cancer cells via downregulation of matrix metalloproteinases. Planta Med. 2011;77:146–151. doi: 10.1055/s-0030-1250178. [DOI] [PubMed] [Google Scholar]
- 80.Chryssanthi D.G., Lamari F.N., Iatrou G., Pylara A., Karamanos N.K., Cordopatis P. Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res. 2007;27:357–362. [PubMed] [Google Scholar]
- 81.Lu P., Lin H., Gu Y., Li L., Guo H., Wang F., Qiu X. Antitumor effects of crocin on human breast cancer cells. Int. J. Clin. Exp. Med. 2015;8:20316–20322. [PMC free article] [PubMed] [Google Scholar]
- 82.Xia D. Ovarian cancer HO-8910 cell apoptosis induced by crocin in vitro. Nat. Prod. Commun. 2015;10:249–252. doi: 10.1177/1934578X1501000208. [DOI] [PubMed] [Google Scholar]
- 83.Tavakkol-Afshari J., Brook A., Mousavi S.H. Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem. Toxicol. 2008;46:3443–3447. doi: 10.1016/j.fct.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 84.Abdullaev F.I., Riverón-Negrete L., Caballero-Ortega H., Manuel Hernández J., Pérez-López I., Pereda-Miranda R., Espinosa-Aguirre J.J. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA. 2003;17:731–736. doi: 10.1016/S0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 85.Abdullaev F.I. Inhibitory effect of crocetin on intracellular nucleic acid and protein synthesis in malignant cells. Toxicol. Lett. 1994;70:243–251. doi: 10.1016/0378-4274(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 86.Dhar A., Mehta S., Dhar G., Dhar K., Banerjee S., Van Veldhuizen P., Campbell D.R., Banerjee S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009;8:315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- 87.Gutheil W.G., Reed G., Ray A., Anant S., Dhar A. Crocetin: An agent derived from saffron for prevention and therapy for cancer. Curr. Pharm. Biotechnol. 2012;13:173–179. doi: 10.2174/138920112798868566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ray P., Guha D., Chakraborty J., Banerjee S., Adhikary A., Chakraborty S., Das T., Sa G. Crocetin exploits p53-induced death domain (PIDD) and FAS-associated death domain (FADD) proteins to induce apoptosis in colorectal cancer. Sci. Rep. 2016;6:32979. doi: 10.1038/srep32979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An B., Ma Y., Xu Y., Liu X., Zhang X., Zhang J., Yang C. Crocin regulates the proliferation and migration of neural stem cells after cerebral ischemia by activating the Notch1 pathway. Folia Neuropathol. 2020;58:201–212. doi: 10.5114/fn.2020.100063. [DOI] [PubMed] [Google Scholar]
- 90.Xu H.J., Zhang K.P., Zhong R., Zhao Y.X., Li X.R., Lu Y., Song A.Q., Pang X.Y., Sun L.R. Influence of crocin on proliferation in vitro and function of dendritic cells derived from bone marrow of children with acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:57–61. [PubMed] [Google Scholar]
- 91.Nam K.N., Park Y.-M., Jung H.-J., Lee J.Y., Min B.D., Park S.-U., Jung W.-S., Cho K.-H., Park J.-H., Kang I., et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Du J., Li Y., Song D., Liu J., Huang Q., Li J., Li B., Li L. Protective effects of crocin against endogenous Aβ-induced neurotoxicity in N2a/APP695swe cells. Psychopharmacology. 2021;238:2839–2847. doi: 10.1007/s00213-021-05899-4. [DOI] [PubMed] [Google Scholar]
- 93.Laabich A., Vissvesvaran G.P., Lieu K.L., Murata K., McGinn T.E., Manmoto C.C., Sinclair J.R., Karliga I., Leung D.W., Fawzi A., et al. Protective Effect of Crocin against Blue Light– and White Light–Mediated Photoreceptor Cell Death in Bovine and Primate Retinal Primary Cell Culture. Investig. Ophthalmol. Vis. Sci. 2006;47:3156–3163. doi: 10.1167/iovs.05-1621. [DOI] [PubMed] [Google Scholar]
- 94.Xu Z., Lin S., Tong Z., Chen S., Cao Y., Li Q., Jiang Y., Cai W., Tong Y., Zahra B.S., et al. Crocetin ameliorates non-alcoholic fatty liver disease by modulating mitochondrial dysfunction in L02 cells and zebrafish model. J. Ethnopharmacol. 2022;285:114873. doi: 10.1016/j.jep.2021.114873. [DOI] [PubMed] [Google Scholar]
- 95.Fagot D., Pham D.M., Laboureau J., Planel E., Guerin L., Nègre C., Donovan M., Bernard B.A. Crocin, a natural molecule with potentially beneficial effects against skin ageing. Int. J. Cosmet. Sci. 2018;40:388–400. doi: 10.1111/ics.12472. [DOI] [PubMed] [Google Scholar]
- 96.Hoshyar R., Bathaie S.Z., Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013;32:50–57. doi: 10.1089/dna.2012.1866. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y., Zhu M., Krishna Mohan S., Hao Z. Crocin treatment promotes the oxidative stress and apoptosis in human thyroid cancer cells FTC-133 through the inhibition of STAT/JAK signaling pathway. J. Biochem. Mol. Toxicol. 2021;35:e22608. doi: 10.1002/jbt.22608. [DOI] [PubMed] [Google Scholar]
- 98.Xu Q., Yu J., Jia G., Li Z., Xiong H. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ. Cytokine. 2022;154:155888. doi: 10.1016/j.cyto.2022.155888. [DOI] [PubMed] [Google Scholar]
- 99.Xu G.-L., Qian Z.-Y., Yu S.-Q., Gong Z.-N., Shen X.-C. Evidence of crocin against endothelial injury induced by hydrogen peroxide in vitro. J. Asian Nat. Prod. Res. 2006;8:79–85. doi: 10.1080/10286020500044732. [DOI] [PubMed] [Google Scholar]
- 100.Xu G., Gong Z., Yu W., Gao L., He S., Qian Z. Increased Expression Ratio of Bcl-2/Bax Is Associated with Crocin-Mediated Apoptosis in Bovine Aortic Endothelial Cells. Basic Clin. Pharmacol. Toxicol. 2007;100:31–35. doi: 10.1111/j.1742-7843.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 101.Shi L., Zhao S., Chen Q., Wu Y., Zhang J., Li N. Crocin inhibits RANKL-induced osteoclastogenesis by regulating JNK and NF-κB signaling pathways. Mol. Med. Rep. 2018;17:7947–7951. doi: 10.3892/mmr.2018.8835. [DOI] [PubMed] [Google Scholar]
- 102.Zhang K., Wang L., Si S., Sun Y., Pei W., Ming Y., Sun L. Crocin improves the proliferation and cytotoxic function of T cells in children with acute lymphoblastic leukemia. Biomed. Pharmacother. 2018;99:96–100. doi: 10.1016/j.biopha.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 103.Gezici S. Comparative anticancer activity analysis of saffron extracts and a principle component, crocetin for prevention and treatment of human malignancies. J. Food Sci. Technol. 2019;56:5435–5443. doi: 10.1007/s13197-019-04014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aung H.H., Wang C.Z., Ni M., Fishbein A., Mehendale S.R., Xie J.T., Shoyama C.Y., Yuan C.S. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- 105.Trujillo-Jiménez F., García-López P., Garcí-Carrancá A., Abdullaev F.I. Effect of saffron on the viability of normal and malignant human cells in vitro. Acta Hortic. 2004;650:463–469. doi: 10.17660/ActaHortic.2004.650.56. [DOI] [Google Scholar]
- 106.See S.J., Levin V.A., Yung W.K., Hess K.R., Groves M.D. 13-cis-retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro-Oncology. 2004;6:253–258. doi: 10.1215/S1152851703000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mawson A.R. Retinoids in the treatment of glioma: A new perspective. Cancer Manag. Res. 2012;4:233–241. doi: 10.2147/CMAR.S32449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reynolds C.P., Matthay K.K., Villablanca J.G., Maurer B.J. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197:185–192. doi: 10.1016/S0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds C.P., Lemons R.S. Retinoid therapy of childhood cancer. Hematol. Oncol. Clin. N. Am. 2001;15:867–910. doi: 10.1016/S0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- 110.Masetti R., Biagi C., Zama D., Vendemini F., Martoni A., Morello W., Gasperini P., Pession A. Retinoids in pediatric onco-hematology: The model of acute promyelocytic leukemia and neuroblastoma. Adv. Ther. 2012;29:747–762. doi: 10.1007/s12325-012-0047-3. [DOI] [PubMed] [Google Scholar]
- 111.Zheng J., Zhou Y., Li Y., Xu D.P., Li S., Li H.B. Spices for Prevention and Treatment of Cancers. Nutrients. 2016;8:495. doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H., Khor T.O., Shu L., Su Z.Y., Fuentes F., Lee J.H., Kong A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. 2012;12:1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang G., Zhang B., Wang Y., Han S., Wang C. Crocin promotes apoptosis of human skin cancer cells by inhibiting the JAK/STAT pathway. Exp. Ther. Med. 2018;16:5079–5084. doi: 10.3892/etm.2018.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao P., Luo C.L., Wu X.H., Hu H.B., Lv C.F., Ji H.Y. Proliferation apoptotic influence of crocin on human bladder cancer T24 cell line. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Med. 2008;33:1869–1873. [PubMed] [Google Scholar]
- 115.Veisi A., Akbari G., Mard S.A., Badfar G., Zarezade V., Mirshekar M.A. Role of crocin in several cancer cell lines: An updated review. Iran. J. Basic Med. Sci. 2020;23:3–12. doi: 10.22038/IJBMS.2019.37821.8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Medema R.H., Macurek L. Checkpoint recovery in cells: How a molecular understanding can help in the fight against cancer. F1000 Biol. Rep. 2011;3:10. doi: 10.3410/B3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peng A. Working hard for recovery: Mitotic kinases in the DNA damage checkpoint. Cell Biosci. 2013;3:20. doi: 10.1186/2045-3701-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Vugt M.A., Medema R.H. Checkpoint adaptation and recovery: Back with Polo after the break. Cell Cycle. 2004;3:1383–1386. doi: 10.4161/cc.3.11.1248. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Z., Wang C.Z., Wen X.D., Shoyama Y., Yuan C.S. Role of saffron and its constituents on cancer chemoprevention. Pharm. Biol. 2013;51:920–924. doi: 10.3109/13880209.2013.771190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heidarzadeh H.R., Bathaie S.Z., Abroun S., Mohagheghi M.A. Evaluating the cytotoxic effect of crocin on MDA-MB-468 cell line based on apoptosis induction, ER stress, and autophagy markers. MJMS. 2018;20:37–51. [Google Scholar]
- 121.Hashemi S.A., Bathaie S.Z., Mohagheghi M.A.J.A.J.o.P. Crocetin and crocin decreased cholesterol and triglyceride content of both breast cancer tumors and cell lines. Avicenna J. Phytomed. 2020;10:384–397. [PMC free article] [PubMed] [Google Scholar]
- 122.Mostafavinia S.E., Khorashadizadeh M., Hoshyar R. Antiproliferative and Proapoptotic Effects of Crocin Combined with Hyperthermia on Human Breast Cancer Cells. DNA Cell Biol. 2016;35:340–347. doi: 10.1089/dna.2015.3208. [DOI] [PubMed] [Google Scholar]
- 123.Escribano J., Alonso G.L., Coca-Prados M., Fernandez J.A. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett. 1996;100:23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 124.Jiang Z., Gu M., Liu J., Li H., Peng J., Zhang Y. Anticancer activity of crocin against cervical carcinoma (HeLa cells): Bioassessment and toxicity evaluation of crocin in male albino rats. J. Photochem. Photobiology. B Biol. 2018;180:118–124. doi: 10.1016/j.jphotobiol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 125.Kim S.H., Lee J.M., Kim S.C., Park C.B., Lee P.C. Proposed cytotoxic mechanisms of the saffron carotenoids crocin and crocetin on cancer cell lines. Biochem. Cell Biol. 2014;92:105–111. doi: 10.1139/bcb-2013-0091. [DOI] [PubMed] [Google Scholar]
- 126.Mollaei H., Abedini M.R., Hoshyar R. Suppressive Effect of Crocin and Cisplatin on Pluripotency Genes Expression in Human Cervical Cancer Cells. Int. J. Cancer Manag. 2017;10:e11152. doi: 10.5812/ijcm.11152. [DOI] [Google Scholar]
- 127.García-Olmo D.C., Riese H.H., Escribano J., Ontañón J., Fernandez J.A., Atiénzar M., García-Olmo D. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): An experimental study in the rat. Nutr. Cancer. 1999;35:120–126. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- 128.Bakshi H.A., Quinn G.A., Nasef M.M., Mishra V., Aljabali A.A.A., El-Tanani M., Serrano-Aroca Á., Webba Da Silva M., McCarron P.A., Tambuwala M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells. 2022;11:1502. doi: 10.3390/cells11091502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bi X., Jiang Z., Luan Z., Qiu D. Crocin exerts anti-proliferative and apoptotic effects on cutaneous squamous cell carcinoma via miR-320a/ATG2B. Bioengineered. 2021;12:4569–4580. doi: 10.1080/21655979.2021.1955175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li S., Shen X.-Y., Ouyang T., Qu Y., Luo T., Wang H.-Q. Synergistic anticancer effect of combined crocetin and cisplatin on KYSE-150 cells via p53/p21 pathway. Cancer Cell Int. 2017;17:98. doi: 10.1186/s12935-017-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li S., Jiang S., Jiang W., Zhou Y., Shen X.Y., Luo T., Kong L.P., Wang H.Q. Anticancer effects of crocetin in human esophageal squamous cell carcinoma KYSE-150 cells. Oncol. Lett. 2015;9:1254–1260. doi: 10.3892/ol.2015.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vazifedan V., Mousavi S.H., Sargolzaei J., Soleymanifard S., Fani Pakdel A. Study of Crocin & Radiotherapy-induced Cytotoxicity and Apoptosis in the Head and Neck Cancer (HN-5) Cell Line. Iran. J. Pharm. Res. 2017;16:230–237. [PMC free article] [PubMed] [Google Scholar]
- 133.Luo Y., Yu P., Zhao J., Guo Q., Fan B., Diao Y., Jin Y., Wu J., Zhang C. Inhibitory Effect of Crocin Against Gastric Carcinoma via Regulating TPM4 Gene. OncoTargets Ther. 2021;14:111–122. doi: 10.2147/OTT.S254167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tarantilis P.A., Morjani H., Polissiou M., Manfait M. Inhibition of growth and induction of differentiation of promyelocytic leukemia (HL-60) by carotenoids from Crocus sativus L. Anticancer. Res. 1994;14:1913–1918. [PubMed] [Google Scholar]
- 135.Hoshyar R., Mollaei H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron, crocin. J. Pharm. Pharmacol. 2017;69:1419–1427. doi: 10.1111/jphp.12776. [DOI] [PubMed] [Google Scholar]
- 136.Moradzadeh M., Kalani M.R., Avan A. The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: A mini review. J. Cell. Biochem. 2019;120:4732–4738. doi: 10.1002/jcb.27525. [DOI] [PubMed] [Google Scholar]
- 137.Geromichalos G.D., Papadopoulos T., Sahpazidou D., Sinakos Z. Safranal, a Crocus sativus L constituent suppresses the growth of K-562 cells of chronic myelogenous leukemia. In silico and in vitro study. Food Chem. Toxicol. 2014;74:45–50. doi: 10.1016/j.fct.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 138.Samarghandian S., Borji A., Farahmand S.K., Afshari R., Davoodi S. Crocus sativus L. (saffron) stigma aqueous extract induces apoptosis in alveolar human lung cancer cells through caspase-dependent pathways activation. BioMed Res. Int. 2013;2013:417928. doi: 10.1155/2013/417928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Mahdizadeh S., Karimi G., Behravan J., Arabzadeh S., Lage H., Kalalinia F. Crocin suppresses multidrug resistance in MRP overexpressing ovarian cancer cell line. Daru. 2016;24:17. doi: 10.1186/s40199-016-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bakshi H., Sam S., Rozati R., Sultan P., Islam T., Rathore B., Lone Z., Sharma M., Triphati J., Saxena R.C. DNA fragmentation and cell cycle arrest: A hallmark of apoptosis induced by crocin from kashmiri saffron in a human pancreatic cancer cell line. Asian Pac. J. Cancer Prev. 2010;11:675–679. [PubMed] [Google Scholar]
- 141.Deng L., Li J., Lu S., Su Y. Crocin inhibits proliferation and induces apoptosis through suppressing MYCN expression in retinoblastoma. J. Biochem. Mol. Toxicol. 2019;33:e22292. doi: 10.1002/jbt.22292. [DOI] [PubMed] [Google Scholar]
- 142.Sun J., Xu X.M., Ni C.Z., Zhang H., Li X.Y., Zhang C.L., Liu Y.R., Li S.F., Zhou Q.Z., Zhou H.M. Crocin Inhibits Proliferation and Nucleic Acid Synthesis and Induces Apoptosis in the Human Tongue Squamous Cell Carcinoma Cell Line Tca8113. Asian Pac. J. Cancer Prev. 2011;12:2679–2683. [PubMed] [Google Scholar]
- 143.Jafarnia N., Ghorbani Z., Nokhostin M., Manayi A., Nourimajd S., Razeghi Jahromi S. Effect of Saffron (Crocus sativus L.) as an Add-On Therapy to Sertraline in Mild to Moderate Generalized Anxiety Disorder: A Double Blind Randomized Controlled Trial. Arch. Neurosci. 2017;4:e14332. doi: 10.5812/archneurosci.14332. [DOI] [Google Scholar]
- 144.Siddiqui S.A., Ali Redha A., Snoeck E.R., Singh S., Simal-Gandara J., Ibrahim S.A., Jafari S.M. Anti-Depressant Properties of Crocin Molecules in Saffron. Molecules. 2022;27:2076. doi: 10.3390/molecules27072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo Z.L., Li M.X., Li X.L., Wang P., Wang W.G., Du W.Z., Yang Z.Q., Chen S.F., Wu D., Tian X.Y. Crocetin: A Systematic Review. Front. Pharmacol. 2021;12:745683. doi: 10.3389/fphar.2021.745683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ayatollahi H., Javan A.O., Khajedaluee M., Shahroodian M., Hosseinzadeh H. Effect of Crocus sativus L. (Saffron) on Coagulation and Anticoagulation Systems in Healthy Volunteers. Phytother. Res. 2014;28:539–543. doi: 10.1002/ptr.5021. [DOI] [PubMed] [Google Scholar]
- 147.Modaghegh M.H., Shahabian M., Esmaeili H.A., Rajbai O., Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 148.Mohamadpour A.H., Ayati Z., Parizadeh M.R., Rajbai O., Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran. J. Basic Med. Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 149.Mazidi M., Shemshian M., Mousavi S.H., Norouzy A., Kermani T., Moghiman T., Sadeghi A., Mokhber N., Ghayour-Mobarhan M., Ferns G.A. A double-blind, randomized and placebo-controlled trial of Saffron (Crocus sativus L.) in the treatment of anxiety and depression. J. Complement. Integr. Med. 2016;13:195–199. doi: 10.1515/jcim-2015-0043. [DOI] [PubMed] [Google Scholar]
- 150.Akhondzadeh Basti A., Moshiri E., Noorbala A.A., Jamshidi A.H., Abbasi S.H., Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: A pilot double-blind randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31:439–442. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 151.Akhondzadeh S., Tahmacebi-Pour N., Noorbala A.-A., Amini H., Fallah-Pour H., Jamshidi A.-H., Khani M. Crocus sativus L. in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother. Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 152.Ghajar A., Neishabouri S.M., Velayati N., Jahangard L., Matinnia N., Haghighi M., Ghaleiha A., Afarideh M., Salimi S., Meysamie A., et al. Crocus sativus L. versus Citalopram in the Treatment of Major Depressive Disorder with Anxious Distress: A Double-Blind, Controlled Clinical Trial. Pharmacopsychiatry. 2017;50:152–160. doi: 10.1055/s-0042-116159. [DOI] [PubMed] [Google Scholar]
- 153.Moshiri E., Basti A.A., Noorbala A.-A., Jamshidi A.-H., Hesameddin Abbasi S., Akhondzadeh S. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607–611. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 154.Noorbala A.A., Akhondzadeh S., Tahmacebi-Pour N., Jamshidi A.H. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J. Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 155.Shahmansouri N., Farokhnia M., Abbasi S.-H., Kassaian S.E., Noorbala Tafti A.-A., Gougol A., Yekehtaz H., Forghani S., Mahmoodian M., Saroukhani S., et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J. Affect. Disord. 2014;155:216–222. doi: 10.1016/j.jad.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Mousavi B., Bathaie S.Z., Fadai F., Ashtari Z., Ali beigi N., Farhang S., Hashempour S., Shahhamzei N., Heidarzadeh H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- 157.Ghobadi H., Abdollahi N., Madani H., Aslani M.R. Effect of Crocin From Saffron (Crocus sativus L.) Supplementation on Oxidant/Antioxidant Markers, Exercise Capacity, and Pulmonary Function Tests in COPD Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2022;13:884710. doi: 10.3389/fphar.2022.884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rahimi G., Shams S., Aslani M.R. Effects of crocin supplementation on inflammatory markers, lipid profiles, insulin and cardioprotective indices in women with PCOS: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2022 doi: 10.1002/ptr.7474. [DOI] [PubMed] [Google Scholar]
- 159.Farokhnia M., Shafiee Sabet M., Iranpour N., Gougol A., Yekehtaz H., Alimardani R., Farsad F., Kamalipour M., Akhondzadeh S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014;29:351–359. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- 160.Pitsikas N. The Effect of Crocus sativus L. and Its Constituents on Memory: Basic Studies and Clinical Applications. Evid. Based Com. Altern. Med. 2015;2015:926284. doi: 10.1155/2015/926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akhondzadeh S., Sabet M.S., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 162.Akhondzadeh S., Shafiee Sabet M., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology. 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 163.Lopresti A.L., Drummond P.D., Inarejos-García A.M., Prodanov M. affron(®), a standardised extract from saffron (Crocus sativus L.) for the treatment of youth anxiety and depressive symptoms: A randomised, double-blind, placebo-controlled study. J. Affect Disord. 2018;232:349–357. doi: 10.1016/j.jad.2018.02.070. [DOI] [PubMed] [Google Scholar]
- 164.Nikbakht-Jam I., Khademi M., Nosrati M., Eslami S., Foroutan-Tanha M., Sahebkar A., Tavalaie S., Ghayour-Mobarhan M., Ferns G.A.A., Hadizadeh F., et al. Effect of crocin extracted from saffron on pro-oxidant–anti-oxidant balance in subjects with metabolic syndrome: A randomized, placebo-controlled clinical trial. Eur. J. Integr. Med. 2016;8:307–312. doi: 10.1016/j.eujim.2015.12.008. [DOI] [Google Scholar]
- 165.Sepahi S., Mohajeri S.A., Hosseini S.M., Khodaverdi E., Shoeibi N., Namdari M., Tabassi S.A.S. Effects of Crocin on Diabetic Maculopathy: A Placebo-Controlled Randomized Clinical Trial. Am. J. Ophthalmol. 2018;190:89–98. doi: 10.1016/j.ajo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 166.Hosseini A., Razavi B.M., Hosseinzadeh H. Pharmacokinetic Properties of Saffron and its Active Components. Eur. J. Drug Metab. Pharmacokinet. 2018;43:383–390. doi: 10.1007/s13318-017-0449-3. [DOI] [PubMed] [Google Scholar]
- 167.Christodoulou E., Grafakou M.E., Skaltsa E., Kadoglou N., Kostomitsopoulos N., Valsami G. Preparation, chemical characterization and determination of crocetin’s pharmacokinetics after oral and intravenous administration of saffron (Crocus sativus L.) aqueous extract to C57/BL6J mice. J. Pharm. Pharmacol. 2019;71:753–764. doi: 10.1111/jphp.13055. [DOI] [PubMed] [Google Scholar]
- 168.Lautenschläger M., Sendker J., Hüwel S., Galla H.J., Brandt S., Düfer M., Riehemann K., Hensel A. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine. 2015;22:36–44. doi: 10.1016/j.phymed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 169.Almodóvar P., Briskey D., Rao A., Prodanov M., Inarejos-García A.M. Bioaccessibility and Pharmacokinetics of a Commercial Saffron (Crocus sativus L.) Extract. Evid.-Based Complement. Altern. Med. eCAM. 2020;2020:1575730. doi: 10.1155/2020/1575730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jafarisani M., Bathaie S.Z., Mousavi M.F. Saffron carotenoids (crocin and crocetin) binding to human serum albumin as investigated by different spectroscopic methods and molecular docking. J. Biomol. Struct. Dyn. 2018;36:1681–1690. doi: 10.1080/07391102.2017.1331865. [DOI] [PubMed] [Google Scholar]
- 171.Chryssanthi D.G., Lamari F.N., Georgakopoulos C.D., Cordopatis P. A new validated SPE-HPLC method for monitoring crocetin in human plasma--application after saffron tea consumption. J. Pharm. Biomed. Anal. 2011;55:563–568. doi: 10.1016/j.jpba.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 172.Song Y.-N., Wang Y., Zheng Y.-H., Liu T.-L., Zhang C. Crocins: A comprehensive review of structural characteristics, pharmacokinetics and therapeutic effects. Fitoterapia. 2021;153:104969. doi: 10.1016/j.fitote.2021.104969. [DOI] [PubMed] [Google Scholar]
- 173.Xi L., Qian Z., Du P., Fu J. Pharmacokinetic properties of crocin (crocetin digentiobiose ester) following oral administration in rats. Phytomedicine. 2007;14:633–636. doi: 10.1016/j.phymed.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 174.Ajam M., Reyhani T., Roshanravan V., Zare Z. Increased Miscarriage Rate in Female Farmers Working in Saffron Fields: A Possible Effect of Saffron Toxicity. Asia Pac. J. Med. Toxicol. 2014;3:73–75. doi: 10.22038/apjmt.2014.3047. [DOI] [Google Scholar]
- 175.Abdullaev F.I. Cancer Chemopreventive and Tumoricidal Properties of Saffron (Crocus sativus L.) Exp. Biol. Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 176.Karimi G., Taiebi N., Hosseinzadeh H., Shirzad F. Evaluation of Subacute Toxicity of Aqueous Extract of Crocus sativus L. Stigma and Petal in Rats. Jmpir. 2004;3:29–35. [Google Scholar]
- 177.Ramadan A., Soliman G., Mahmoud S.S., Nofal S.M., Abdel-Rahman R.F. Evaluation of the safety and antioxidant activities of Crocus sativus and Propolis ethanolic extracts. J. Saudi Chem. Soc. 2012;16:13–21. doi: 10.1016/j.jscs.2010.10.012. [DOI] [Google Scholar]
- 178.Babaei A., Arshami J., Haghparast A., Danesh Mesgaran M. Effects of saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avicenna J. Phytomed. 2014;4:103–109. [PMC free article] [PubMed] [Google Scholar]
- 179.Hosseinzadeh H., Abootorabi A., Sadeghnia H.R. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 180.Skowroń J., Zapór L., Miranowicz-Dzierżawska K. Classification of the Substances on the Basis of the Acute-Toxic-Class Method (ATC) Int. J. Occup. Saf. Ergon. 1998;4:107–116. doi: 10.1080/10803548.1998.11076384. [DOI] [PubMed] [Google Scholar]
- 181.El Midaoui A., Ghzaiel I., Vervandier-Fasseur D., Ksila M., Zarrouk A., Nury T., Khallouki F., El Hessni A., Ibrahimi S.O., Latruffe N., et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients. 2022;14:597. doi: 10.3390/nu14030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chan S.-L., Yu V.C. Proteins of the Bcl-2 family in apoptosis signalling: From mechanistic insights to therapeutic opportunities. Clin. Exp. Pharmacol. Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 183.Azmi A.S., Wang Z., Philip P.A., Mohammad R.M., Sarkar F.H. Emerging Bcl-2 inhibitors for the treatment of cancer. Expert Opin. Emerg. Drugs. 2011;16:59–70. doi: 10.1517/14728214.2010.515210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bakshi H.A., Hakkim F.L., Sam S. Molecular Mechanism of Crocin Induced Caspase Mediated MCF-7 Cell Death: In Vivo Toxicity Profiling and Ex Vivo Macrophage Activation. Asian Pac. J. Cancer Prev. 2016;17:1499–1506. doi: 10.7314/APJCP.2016.17.3.1499. [DOI] [PubMed] [Google Scholar]
- 185.Wang K., Yin X.M., Chao D.T., Milliman C.L., Korsmeyer S.J. BID: A novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 186.Mehri S., Abnous K., Mousavi S.H., Shariaty V.M., Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Soeda S., Ochiai T., Paopong L., Tanaka H., Shoyama Y., Shimeno H. Crocin suppresses tumor necrosis factor-alpha-induced cell death of neuronally differentiated PC-12 cells. Life Sci. 2001;69:2887–2898. doi: 10.1016/S0024-3205(01)01357-1. [DOI] [PubMed] [Google Scholar]
- 188.Kim B., Lee K.Y., Park B. Crocin Suppresses Constitutively Active STAT3 Through Induction of Protein Tyrosine Phosphatase SHP-1. J. Cell Biochem. 2017;118:3290–3298. doi: 10.1002/jcb.25980. [DOI] [PubMed] [Google Scholar]
- 189.Beljebbar A., Sockalingum G.D., Morjani H., Angiboust J.F., Polissiou M., Manfait M. Differential Interaction Modes of Dimethylcrocetin in K562 and HL60 Tumor Cells As Probed by Near Infrared FT-Raman Microspectroscopy. In: Merlin J.C., Turrell S., Huvenne J.P., editors. Spectroscopy of Biological Molecules: 6th European Conference on the Spectroscopy of Biological Molecules, Villeneuve d’Ascq, France, 3–8 September 1995. Springer; Dordrecht, The Netherlands: 1995. pp. 475–476. [Google Scholar]
- 190.Li S., Qu Y., Shen X.Y., Ouyang T., Fu W.B., Luo T., Wang H.Q. Multiple Signal Pathways Involved in Crocetin-Induced Apoptosis in KYSE-150 Cells. Pharmacology. 2019;103:263–272. doi: 10.1159/000487956. [DOI] [PubMed] [Google Scholar]
- 191.Bakshi H.A., Zoubi M.S.A., Hakkim F.L., Aljabali A.A.A., Rabi F.A., Hafiz A.A., Al-Batanyeh K.M., Al-Trad B., Ansari P., Nasef M.M., et al. Dietary Crocin is Protective in Pancreatic Cancer while Reducing Radiation-Induced Hepatic Oxidative Damage. Nutrients. 2020;12:1901. doi: 10.3390/nu12061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schramm A., Lode H. MYCN-targeting vaccines and immunotherapeutics. Hum. Vaccines Immunother. 2016;12:2257–2258. doi: 10.1080/21645515.2016.1171430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Amin B., Abnous K., Motamedshariaty V., Hosseinzadeh H. Attenuation of oxidative stress, inflammation and apoptosis by ethanolic and aqueous extracts of Crocus sativus L. stigma after chronic constriction injury of rats. An. Da Acad. Bras. Cienc. 2014;86:1821–1832. doi: 10.1590/0001-3765201420140067. [DOI] [PubMed] [Google Scholar]
- 194.Bandegi A.R., Rashidy-Pour A., Vafaei A.A., Ghadrdoost B. Protective Effects of Crocus sativus L. Extract and Crocin against Chronic-Stress Induced Oxidative Damage of Brain, Liver and Kidneys in Rats. Adv. Pharm. Bull. 2014;4:493–499. doi: 10.5681/apb.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Broadhead G.K., Chang A., Grigg J., McCluskey P. Efficacy and Safety of Saffron Supplementation: Current Clinical Findings. Crit. Rev. Food Sci. Nutr. 2016;56:2767–2776. doi: 10.1080/10408398.2013.879467. [DOI] [PubMed] [Google Scholar]
- 196.Das I., Das S., Saha T. Saffron suppresses oxidative stress in DMBA-induced skin carcinoma: A histopathological study. Acta Histochem. 2010;112:317–327. doi: 10.1016/j.acthis.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 197.Giaccio M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004;44:155–172. doi: 10.1080/10408690490441433. [DOI] [PubMed] [Google Scholar]
- 198.Hosseinzadeh H., Sadeghnia H.R. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 199.Kanakis C.D., Tarantilis P.A., Pappas C., Bariyanga J., Tajmir-Riahi H.A., Polissiou M.G. An overview of structural features of DNA and RNA complexes with saffron compounds: Models and antioxidant activity. J. Photochem. Photobiol. B. 2009;95:204–212. doi: 10.1016/j.jphotobiol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 200.Kanakis C.D., Tarantilis P.A., Tajmir-Riahi H.A., Polissiou M.G. Crocetin, dimethylcrocetin, and safranal bind human serum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007;55:970–977. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 201.Mehri S., Abnous K., Khooei A., Mousavi S.H., Shariaty V.M., Hosseinzadeh H. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran. J. Basic Med. Sci. 2015;18:902–908. [PMC free article] [PubMed] [Google Scholar]
- 202.Naghizadeh B., Boroushaki M.T., Vahdati Mashhadian N., Mansouri S.M.T. Protective Effects of Crocin against Cisplatin-Induced Acute Renal Failure and Oxidative Stress in Rats. Iran. Biomed. J. 2008;12:93–100. [PubMed] [Google Scholar]
- 203.Serrano-Diaz J., Sanchez A.M., Maggi L., Martinez-Tome M., Garcia-Diz L., Murcia M.A., Alonso G.L. Increasing the applications of Crocus sativus flowers as natural antioxidants. J. Food Sci. 2012;77:C1162–C1168. doi: 10.1111/j.1750-3841.2012.02926.x. [DOI] [PubMed] [Google Scholar]
- 204.Ashrafi M., Bathaie S.Z., Abroun S., Azizian M. Effect of Crocin on Cell Cycle Regulators in N-Nitroso-N-Methylurea-Induced Breast Cancer in Rats. DNA Cell Biol. 2015;34:684–691. doi: 10.1089/dna.2015.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chu X., Zhang Y., Xue Y., Li Z., Shi J., Wang H., Chu L. Crocin protects against cardiotoxicity induced by doxorubicin through TLR-2/NF-κB signal pathway in vivo and vitro. Int. Immunopharmacol. 2020;84:106548. doi: 10.1016/j.intimp.2020.106548. [DOI] [PubMed] [Google Scholar]
- 206.Li X., Liu Y., Cao A., Li C., Wang L., Wu Q., Li X., Lv X., Zhu J., Chun H., et al. Crocin Improves Endothelial Mitochondrial Dysfunction via GPx1/ROS/KCa3.1 Signal Axis in Diabetes. Front. Cell Dev. Biol. 2021;9:651434. doi: 10.3389/fcell.2021.651434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nasimian A., Farzaneh P., Tamanoi F., Bathaie S.Z. Cytosolic and mitochondrial ROS production resulted in apoptosis induction in breast cancer cells treated with Crocin: The role of FOXO3a, PTEN and AKT signaling. Biochem. Pharmacol. 2020;177:113999. doi: 10.1016/j.bcp.2020.113999. [DOI] [PubMed] [Google Scholar]
- 208.Tang Y., Yang H., Yu J., Li Z., Xu Q., Ding B., Jia G. Crocin induces ROS-mediated papillary thyroid cancer cell apoptosis by modulating the miR-34a-5p/PTPN4 axis in vitro. Toxicol. Appl. Pharmacol. 2022;437:115892. doi: 10.1016/j.taap.2022.115892. [DOI] [PubMed] [Google Scholar]
- 209.Nie Z., Deng S., Zhang L., Chen S., Lu Q., Peng H. Crocin protects against dexamethasone-induced osteoblast apoptosis by inhibiting the ROS/Ca2+-mediated mitochondrial pathway. Mol. Med. Rep. 2019;20:401–408. doi: 10.3892/mmr.2019.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moradzadeh M., Ghorbani A., Erfanian S., Mohaddes S.T., Rahimi H., Karimiani E.G., Mashkani B., Chiang S.C., El-Khamisy S.F., Tabarraei A., et al. Study of the mechanisms of crocetin-induced differentiation and apoptosis in human acute promyelocytic leukemia cells. J. Cell Biochem. 2018;120:1943–1957. doi: 10.1002/jcb.27489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data is contained within the article and Supplementary Materials.