Abstract
Desulfotomaculum thermobenzoicum, but not Desulfotomaculum nigrificans, Desulfotomaculum ruminis, or Desulfosporosinus orientis, grew by disproportionation of thiosulfate, forming stoichiometric amounts of sulfate and sulfide; sulfite was not disproportionated. The addition of acetate enhanced growth and thiosulfate disproportionation by D. thermobenzoicum compared to those observed with thiosulfate alone.
Disproportionation is a general term used to describe a process by which an element or a compound in a state of intermediate oxidation is converted to substances in higher and lower oxidation states. Specifically, the biological disproportionation of thiosulfate occurs via an intramolecular redox change at each of the sulfur atoms (21) and produces 1 mol of sulfate and 1 mol of sulfide, with a Gibb's free energy change of approximately −22 kJ/mol (equation 1).
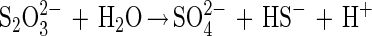 |
1 |
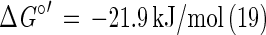 |
Although the disproportionation of thiosulfate yields only a small amount of free energy, microorganisms capable of this metabolism are abundant in both freshwater and marine environments (1, 6), and it is a dominant process for thiosulfate transformation in marine and freshwater sediments and in benthic cyanobacterial mats (6–9).
To date, the only microorganisms known to metabolize inorganic sulfur compounds by a disproportionation mechanism are gram-negative, sulfate-reducing bacteria that cluster within the delta subclass of the Proteobacteria (11). Desulfovibrio sulfodismutans, Desulfocapsa thiozymogenes, Desulfocapsa sulfoexigens, and strain NTA3 are the only known microorganisms capable of growth by thiosulfate disproportionation (1, 2, 4, 5), while other sulfate reducers have been reported to disproportionate but not grow with thiosulfate, sulfur, or sulfite (11). This communication reports on the growth of a gram-positive, thermophilic sulfate-reducing bacterium by thiosulfate disproportionation.
Organisms and methods of cultivation.
The type strains of Desulfotomaculum thermobenzoicum (49756), Desulfotomaculum nigrificans (19998), Desulfotomaculum ruminis (23193), and Desulfosporosinus orientis (19365) were obtained from the American Type Culture Collection (Manassas, Va.). The microorganisms were cultivated in a basal medium containing (per liter) 0.4 g of MgCl2 · 6H2O, 0.1 g of CaCl2 · 2H2O, 1.0 g of NaCl, 0.25 g of NH4Cl, 0.1 g of KH2PO4, 0.1 g of yeast extract (Difco), 2.0 g of NaHCO3, 2.0 g of TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid], 0.001 g of resazurin, and 0.5 g of dithiothreitol. A trace metal solution and vitamin solution (12) were added to the medium in amounts of 5.0 and 10.0 ml per liter, respectively. The trace metal solution contained the following (per liter): 2.0 g of nitrilotriacetic acid (adjusted to pH 6.0 with 1.0 M KOH), 1.0 g of MnCl2 · 4H2O, 1.0 g of FeCl2 · 4H2O, 0.2 g of CoCl2 · 6H2O, 0.2 g of ZnCl2, 0.5 g of H3BO3, 0.02 g of CuCl2 · 2H2O, 0.02 g of NiCl2 · 6H2O, 0.02 g of Na2MoO4 · 2H2O, 0.02 g of Na2SeO4, and 0.02 g of Na2WO4. The medium was adjusted to a pH of 7.4 and boiled under a gas stream of 80% N2–20% CO2.
All procedures for the anaerobic preparation and use of media and solutions were essentially those of Balch and Wolfe (3). The basal medium was boiled and dispensed in 10-ml aliquots into serum tubes (18 by 150 mm) under a stream of an oxygen-free, 80% N2–20% CO2 gas mixture. The tubes were sealed with blue rubber stoppers (Bellco Glass, Vineland, N.J.) and crimped with aluminum seals, and the pressure of each tube was brought to 135 kPa with the above-described gas mixture prior to autoclaving. When hydrogen was the electron donor, the headspace of each tube was aseptically exchanged three times by evacuation with vacuum and repressurization with an 80% H2–20% CO2 (170 kPa) gas mixture. All additions to anaerobic and sterile media were performed aseptically using syringes and needles flushed with oxygen-free nitrogen gas. Stock solutions of thiosulfate, sulfate, sulfite, and acetate were sterilized by filtration (0.2 μm pore size). The headspace of each solution then was exchanged with a 100% N2 gas phase, as described above. The initial concentration of thiosulfate ranged from 5 to 15 mM. For some experiments, acetate was added as a carbon source at an initial concentration of 3 mM. Medium volumes varied from 10.5 to 12.5 ml after additions; volume was constant among treatments within an experiment.
Inocula for experiments were obtained from cultures grown in basal medium with 5 mM thiosulfate and an 80% H2–20% CO2 gas phase (180 kPa) to late exponential phase. Each tube was inoculated with 2 ml of culture, about a 20% (vol/vol) inoculum size. Inoculations were performed aseptically using syringes and needles flushed with oxygen-free nitrogen gas. D. ruminis and D. orientis cultures were incubated at 37°C, and D. thermobenzoicum and D. nigrificans cultures were incubated at 62°C. All cultures were incubated without shaking unless hydrogen was the electron donor. Cultures with hydrogen as the electron donor were shaken at 200 rpm.
To minimize the transfer of sulfur compounds, a washed cell suspension was used as the inoculum for performing a detailed sulfur balance of D. thermobenzoicum. All manipulations were done aseptically and anaerobically using an anaerobic chamber and sterile materials. All plasticware was placed inside the anaerobic chamber 24 h prior to use. Cultures of D. thermobenzoicum were grown on hydrogen and thiosulfate to late exponential phase as described above. The cultures (20 ml) were transferred to a sterile centrifuge tube inside the anaerobic chamber. The centrifuge tube was sealed, brought outside the anaerobic chamber, and then centrifuged (11,500 × g; 15 min; 4°C). The cell pellet was resuspended in 2 ml of anaerobic, sterile basal medium. Two hundred microliters of the cell suspension was used to inoculate each experimental tube.
Analytical techniques.
Growth of cultures was monitored spectrophotometrically by measuring the increase in absorbance at 600 nm. Direct cell counts were performed at 400-fold magnification with a phase-contrast microscope and a Petroff-Hausser counting chamber (10). A total sample volume of 500 μl was removed to quantify all sulfur species. Samples were immediately analyzed for volatile sulfides and total reduced inorganic sulfur. Volatile sulfides were quantified spectrophotometrically by the methylene blue assay as described by Tanner (16). Total reduced inorganic sulfur was determined by using a modified single-extraction chromium reduction assay as previously reported (21). Elemental sulfur was determined as previously reported (20). Sulfoxyanions (thiosulfate, sulfate, and sulfite) were quantified by suppressed ion chromatography using a Dionex Ion Chromatograph DX-500 equipped with a CD-20 conductivity detector and an AS-11 column. Thiosulfate and sulfite were also quantified with the ion chromatography system by measuring the absorbance at 230 nm with an AD-20 absorbance detector. In either case, the chromatograph was run isocratically with a mobile phase of 7 mM NaOH at a flow rate of 2.0 ml min−1 for 5 min, and then the mobile phase concentration was linearly increased to 35 mM NaOH for an additional 7 min.
Growth by thiosulfate disproportionation.
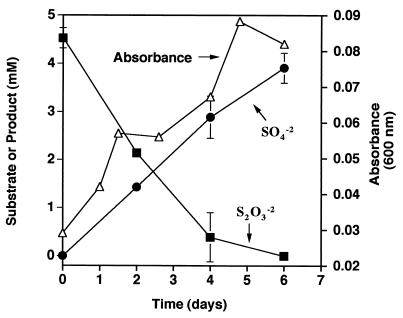
A small amount of growth (a change of 0.06 absorbance units) was observed when D. thermobenzoicum was incubated in basal medium with 4.5 mM thiosulfate (Fig. 1). Sulfate was produced concomitant with growth and thiosulfate disappearance. After 6 days, the concentration of sulfide produced was 4.7 mM. Thus, D. thermobenzoicum transformed approximately 4.5 mM thiosulfate to approximately 4.0 mM sulfate and 4.7 mM sulfide (96.7% sulfur recovery) after 6 days of incubation. The ratio of sulfate produced to thiosulfate consumed was about 0.9, which is close to that theoretically predicted for thiosulfate disproportionation (equation 1). In basal medium without thiosulfate, no increase in absorbance was observed and sulfate was not produced. Abiotic transformation of thiosulfate to sulfate or sulfide was not observed. Additionally, D. thermobenzoicum cultures did not show an increase in absorbance in basal medium with 5 mM sulfate, indicating that the small amount of yeast extract in the medium did not support growth.
FIG. 1.
Growth and thiosulfate disproportionation by D. thermobenzoicum in basal medium with 4.5 mM thiosulfate. Results are expressed as the means and standard deviations of triplicate determinations.
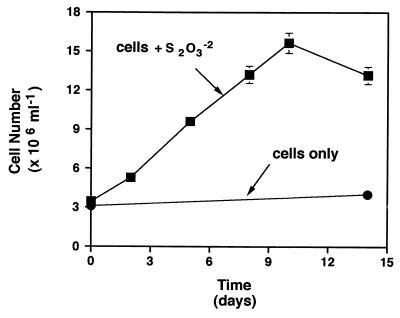
When growth was followed by total cell counts (Fig. 2), the number of D. thermobenzoicum cells increased linearly from approximately 3.5 × 106 to 1.6 × 107 cells/ml over a period of 10 days in sulfate-free basal medium with 8 mM thiosulfate. In basal medium without thiosulfate, cell numbers did not increase. Growth of D. thermobenzoicum in basal medium with thiosulfate was sustained through at least four successive transfers.
FIG. 2.
Increase in cell numbers of D. thermobenzoicum in basal medium with 8 mM thiosulfate. Data are the means and average deviations of duplicate determinations.
In basal medium with 10 mM thiosulfate, growth or thiosulfate transformation by D. nigrificans, D. ruminis, or D. orientis was not observed. In addition, thiosulfate metabolism by these organisms was not stimulated even after 3 mM acetate was added as a carbon source for biosynthesis. Sulfite (5 mM) was not transformed by any of the four species tested. However, all four species grew with hydrogen (170 kPa of an 80% H2–20% CO2 gas phase) and 10 mM thiosulfate or 5 mM sulfite as the electron acceptor, indicating that this medium does support growth of these organisms if a suitable electron donor is supplied (data not shown).
Stoichiometry and effect of acetate on thiosulfate disproportionation by D. thermobenzoicum.
D. thermobenzoicum disproportionated approximately 26 μmol of thiosulfate to 24 μmol of acid volatile sulfide and 25 μmol of sulfate when grown in basal medium with 15 mM thiosulfate (Table 1). The ratio of sulfate to sulfide was 1.0, a value that is nearly identical to that for the theoretical stoichiometry of thiosulfate disproportionation (equation 1). When the small amount of sulfur (approximately 4 μmol or 7% of the total sulfur) recovered in the total reduced inorganic sulfur pool is included, the sulfur recovery is 101%. The latter material may be in the form of iron sulfides, since elemental sulfur was not detected; however, this observation was not investigated further.
TABLE 1.
| Substance added | Amt (μmol) of:
|
Growth (ΔOD600)b | Ratio (sulfate/sulfide) | |||
|---|---|---|---|---|---|---|
| Thiosulfate used | Sulfite formed | Sulfate formed | Sulfide formed | |||
| S2O32− | 26.4 ± 1.7d | 0.5 ± 1.0 | 24.9 ± 3.1 | 23.8 ± 2.5 | 0.05 | 1 |
| Acetate + S2O32− | 145.5 ± 10.6e | 9.8 ± 9.8 | 135.4 ± 21.3 | 168.7 ± 5.3 | 0.11 | 0.8 |
| Acetate | BDLf | BDL | BDL | 0.4 ± 0.4 | 0.0 | NAg |
| S2O32− (no cells) | 2.7 | BDL | BDL | BDL | 0.0 | NA |
Incubated at 62°C for 7 days in basal medium with 15 mM thiosulfate.
OD600, optical density at 600 nm.
Values represent the mean and standard deviation of triplicate determination.
101% sulfur recovery when sulfur recovered as iron sulfides is included; elemental sulfur was not detected.
107% sulfur recovery.
BDL, below detection limit.
NA, not applicable.
When 3 mM acetate was added to the medium, the extent of both growth and thiosulfate disproportionation significantly increased (Table 1). D. thermobenzoicum is not known to utilize acetate as an electron donor for sulfate or thiosulfate reduction and presumably uses acetate only as a carbon source for biosynthesis (17). Consistent with this, we did not observe any growth of D. thermobenzoicum in the presence of acetate and sulfate (Table 1). D. thermobenzoicum converted approximately 146 μmol of thiosulfate to 135 μmol of sulfate, 169 μmol of sulfide, and 10 μmol of sulfite. The sulfur recovery was 107%, and the ratio of sulfate to sulfide was 0.8, again in agreement with the stoichiometry of thiosulfate disproportionation (equation 1). Growth was not observed in tubes that contained acetate alone (Table 1). Thiosulfate has been reported to chemically decompose at elevated temperatures (13). However, less than 2% of the initial thiosulfate was abiotically transformed in control tubes amended only with thiosulfate (without cells) under the experimental conditions used here, i.e., 62°C and 135 kPa of N2/CO2 (Table 1). In addition, thiosulfate was not transformed in the presence of heat-killed cells.
Conclusions.
D. thermobenzoicum is phylogenetically distinct from previously described thiosulfate-disproportionating bacteria, clustering with low-G+C-content gram-positive bacteria (clostridia) within a separate division of the domain Bacteria. To date, a disproportionation metabolism has been conclusively documented only within the gram-negative, mesophilic sulfate-reducing bacterial group, whose members phylogenetically cluster within the delta subclass of the Proteobacteria. Physiologically, our results are consistent with the fact that the ability to disproportionate sulfoxyanions seems to be limited to bacteria that reduce sulfate (11). However, a disproportionation metabolism is apparently not unique to gram-negative, sulfate-reducing bacteria but is found among gram-positive bacteria as well.
The addition of acetate significantly stimulated both growth and thiosulfate disproportionation. D. thermobenzoicum achieved twice the cell density and disproportionated sixfold more thiosulfate when grown in medium with acetate added as the carbon source than it did in medium without acetate. The final cell concentration for growth of D. thermobenzoicum by thiosulfate disproportionation was very similar to that observed by Bak and Pfennig (2) for D. sulfodismutans. Thiosulfate disproportionation did support the growth of D. thermobenzoicum through repeated transfers in medium without acetate added. This medium contained low levels of yeast extract that could have served as the carbon source (0.05 g/liter). Alternatively, D. thermobenzoicum may have used carbon dioxide as a carbon source, since D. thermobenzoicum possesses the enzymatic ability to assimilate carbon via the carbon monoxide dehydrogenase pathway (18). To date, only strain NTA 3 has been observed to grow autotrophically by thiosulfate disproportionation (1).
Disproportionating bacteria are numerically significant and have been detected in high numbers in freshwater mud and marine sediments (2, 9). The genus Desulfotomaculum has the ability, unique among sulfate-reducing bacteria, to form endospores, indicating that bacteria possessing a disproportionation metabolism can persist through extreme or unfavorable environmental conditions. The finding that D. thermobenzoicum effectively disproportionates thiosulfate suggests that this transformation may occur in thermophilic as well as freshwater and marine environments.
REFERENCES
- 1.Bak F, Cypionka H. Novel type of energy metabolism involving fermentation of inorganic sulphur compounds. Nature. 1987;326:891–892. doi: 10.1038/326891a0. [DOI] [PubMed] [Google Scholar]
- 2.Bak F, Pfennig N. Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov., by disproportionation of inorganic sulfur compounds. Arch Microbiol. 1987;147:184–189. [Google Scholar]
- 3.Balch W E, Wolfe R S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finster K, Liesack W, Thamdrup B. Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediment. Appl Environ Microbiol. 1998;64:119–125. doi: 10.1128/aem.64.1.119-125.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen P H, Schuhmann A, Bak F, Liesack W. Disproportionation of inorganic sulfur compounds by the sulfate-reducing bacterium Desulfocapsa thiozymogenes gen. nov., sp. nov. Arch Microbiol. 1996;166:184–192. [Google Scholar]
- 6.Jorgensen B B, Bak F. Pathways and microbiology of thiosulfate transformations and sulfate reduction in a marine sediment (Kattegat, Denmark) Appl Environ Microbiol. 1991;57:847–856. doi: 10.1128/aem.57.3.847-856.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen B B. Sulfate reduction and thiosulfate transformations in a cyanobacterial mat during a diel oxygen cycle. FEMS Microbiol Ecol. 1994;13:303–312. [Google Scholar]
- 8.Jorgensen B B. Sulfur cycle of freshwater sediments: role of thiosulfate. Limnol Oceanogr. 1990;35:1329–1342. [Google Scholar]
- 9.Jorgensen B B. Thiosulfate shunt in the sulfur cycle of marine sediments. Nature. 1990;249:152–154. doi: 10.1126/science.249.4965.152. [DOI] [PubMed] [Google Scholar]
- 10.Koch A L. Methods for general and molecular bacteriology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 11.Kramer M, Cypionka H. Sulfate formation via ATP sulfurylase in thiosulfate- and sulfite-disproportionating bacteria. Arch Microbiol. 1989;151:232–237. [Google Scholar]
- 12.McInerney M J, Bryant M P, Pfennig N. Anaerobic bacterium that degrades fatty acids in syntrophic association with methanogens. Arch Microbiol. 1979;122:129–135. doi: 10.1007/s002030050685. [DOI] [PubMed] [Google Scholar]
- 13.Mizoguchi T, Takei Y, Okabe T. Chemical behavior of low valence sulfur compounds. X. Disproportionation of thiosulfate, trithionate, tetrathionate and sulfite under acidic conditions. Bull Chem Soc Jpn. 1976;49:70–75. [Google Scholar]
- 14.Nilsen R K, Torsvik T, Lien T. Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot North Sea oil reservoir. Int J Syst Bacteriol. 1996;46:397–402. [Google Scholar]
- 15.Rosnes J T, Torsvik T, Lien T. Spore-forming thermophilic sulfate-reducing bacteria isolated from North Sea oil field waters. Appl Environ Microbiol. 1991;57:2302–2307. doi: 10.1128/aem.57.8.2302-2307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner R S. Monitoring sulfate-reducing bacteria: comparison of enumeration media. J Microbiol Methods. 1989;10:83–89. [Google Scholar]
- 17.Tasaki M, Kamagata Y, Nakamura K, Mikami E. Isolation and characterization of a thermophilic benzoate-degrading, sulfate-reducing bacterium, Desulfotomaculum thermobenzoicum sp. nov. Arch Microbiol. 1991;155:348–352. [Google Scholar]
- 18.Tasaki M, Kamagata Y, Nakamura K, Okamura K, Minami K. Acetogenesis from pyruvate by Desulfotomaculum thermobenzoicum and differences in pyruvate metabolism among three sulfate-reducing bacteria in the absence of sulfate. FEMS Microbiol Lett. 1993;106:259–264. [Google Scholar]
- 19.Thauer R K, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troelsen H, Jorgensen B B. Seasonal dynamics of elemental sulfur in two coastal sediments. Estuar Coast Shelf Sci. 1982;15:255–266. [Google Scholar]
- 21.Ulrich G A, Krumholz L R, Suflita J M. A rapid and simple method for estimating sulfate reduction activity and quantifying inorganic sulfides. Appl Environ Microbiol. 1997;63:1627–1630. doi: 10.1128/aem.63.4.1627-1630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vairavamurthy A, Manowitz B, Luther III G W, Jeon Y. Oxidation state of sulfur in thiosulfate and implications for anaerobic energy metabolism. Geochim Cosmochim Acta. 1993;57:1619–1623. [Google Scholar]