Abstract
Despite intense investigation, the pathogenesis of COVID-19 and the newly defined long COVID-19 syndrome are not fully understood. Increasing evidence has been provided of metabolic alterations characterizing this group of disorders, with particular relevance of an activated tryptophan/kynurenine pathway as described in this review. Recent histological studies have documented that, in COVID-19 patients, indoleamine 2,3-dioxygenase (IDO) enzymes are differentially expressed in the pulmonary blood vessels, i.e., IDO1 prevails in early/mild pneumonia and in lung tissues from patients suffering from long COVID-19, whereas IDO2 is predominant in severe/fatal cases. We hypothesize that IDO1 is necessary for a correct control of the vascular tone of pulmonary vessels, and its deficiency in COVID-19 might be related to the syndrome’s evolution toward vascular dysfunction. The complexity of this scenario is discussed in light of possible therapeutic manipulations of the tryptophan/kynurenine pathway in COVID-19 and post-acute COVID-19 syndromes.
Keywords: COVID-19, IDO, post-acute COVID syndrome, PACS, SARS-CoV-2, tryptophan/kynurenine
1. Introduction
SARS-CoV-2 infection responsible for Coronavirus disease 2019 (COVID-19) is associated with a variability in clinical presentation and pathologic features, such as a minority of patients rapidly progressing to severe life-threatening respiratory failure requiring mechanical ventilation [1]. A proportion of COVID-19 patients suffer from post-acute sequelae, experiencing complications affecting different organs (a condition defined as “long COVID-19”, “long-haul” syndrome, or “post-acute COVID syndrome” (PACS) [2,3,4,5,6,7,8,9]. Most common symptoms often persisting for several weeks or months include systemic manifestations (fatigue, asthenia, poor concentration, wandering fever), symptoms and signs of pulmonary functional impairment (dyspnea, cough, reduced DLCO—diffusing capacity of the lungs for carbon monoxide), neuropsychiatric manifestations (sleep disturbances, cognitive dysfunction, depression, mood changes, anxiety, headache, taste, and/or smell loss), cardiac manifestations (chest pain, palpitations, tachycardia, dysrhytmias), as well as a variety of muscle-skeletal, renal, dermatological, and gastrointestinal manifestations [7,8]. The incidence of severe PACS is relatively low and most cases can resolve in less than six months, but due to the extremely large number of infected people the weight of long-COVID is a growing health concern [10,11,12,13]. Although general consensus has been reached regarding the major pathogenic mechanisms involved in the different phases and/or endotypes of acute COVID-19, the mechanisms accounting for clinical variability and the persistence of PACS symptoms are not fully understood, and different hypotheses have been formulated including autoimmune or inflammatory sequelae, persistent viral antigens, and others. The relevance of the tryptophan/kynurenine pathway has been fully recognized in different infectious diseases, but the precise role of these alterations in different clinical COVID-19 presentations and PACS is not fully understood. In this review, we describe the main data regarding these issues, and discuss the possible pathogenic role of abnormal vascular expression of enzymes that regulate this pathway (indoleamine 2,3-dioxygenases) in different COVID-19 endotypes and PACS [14,15,16,17,18].
1.1. Lung Involvement in Lung and PACS
The respiratory system is a major target of SARS-CoV-2 acute infection, with variable presentations from mild pneumonia to fatal Acute Respiratory Distress Syndrome (ARDS). Post-acute lung sequelae have been described in survivors of severe COVID-19 pneumonia, as well as in people recovering from either hospitalized or non-hospitalized mild COVID-19, with a risk that increases across the clinical severity [19,20,21,22]. Data from analysis of laboratory tests, High Resolution CT scans, and lung tissue obtained from patients with lung sequelae might provide useful information regarding both acute and post-acute COVID-19 pneumonias, revealing pathogenic mechanisms that occur and develop independently of the viral presence by nature. A variety of pulmonary clinical manifestations and radiological features of interstitial disease have been observed in PACS, characterized by frequent (up to 50%) clinico-radiological features of organizing pneumonia [19,23,24,25]. The most frequent functional abnormality in PACS is lung related decreases in diffusion capacity, followed by restrictive defects [20,26,27,28,29]. A possible explanation has been hypothesized, based on abnormalities of vascular volumes occurring in PACS lung [30]. There is general agreement on considering lung vascular abnormalities and vascular dysfunction as central factors in the pathogenesis of severe COVID-19 pneumonia, although consensus has not been reached on the mechanisms of their development. In particular, the role of direct endothelial infection by SARS-CoV2 is controversial, and uncertainty remains regarding the mechanisms of ventilation/perfusion (V/Q) mismatch leading to abnormal perfusion and hypoxemia in different COVID-19 endotypes [31,32,33,34]. The pathogenic links relating vascular abnormalities occurring in COVID-19 and PACS are currently unknown. To address these issues, we recently used lung histology and immunohistochemistry to investigate a series of transbronchial lung cryobiopsies from patients with persistent symptoms and computed tomography suggestive of residual lung disease after recovery from Sars-CoV-2 infection [35]. A variety of relevant changes were observed, ranging from minimal abnormalities to fibrosing interstitial disease. An intriguing finding in PACS cases of this series was the occurrence of morphological and immunophenotypical changes in the pulmonary vascular bed, similar to those observed in acute early/mild cCOVID-19 pneumonias (vascular enlargement and abnormal endothelial expression of IDO1, PD-L1 and STAT3) [35,36]. The persistence of these peculiar findings after virus clearance (as demonstrated by molecular analysis in lung tissue, BAL, and nasal swab in all PACS cases) strongly suggests that this phenotype occurs independently from active infection, and it is likely involved in the pathogenesis of both COVID-19 and post-COVID-19 sequelae. In our opinion, the abnormal expression of the enzyme indoleamine 2,3-dioxygenase (IDO1) by endothelial cells in acute and post-acute COVID-19 pneumonias deserves particular attention; this review addresses the topic.
1.2. IDO and the Tryptophan/Kynurenine Pathway
L-tryptophan (Trp) is a semi-essential amino acid utilized in protein synthesis and as a precursor of metabolites involved in a variety of important physiological mechanisms including pregnancy, neuronal function, and immune tolerance [37]. Trp consumption for protein synthesis is minimal, since >90% Trp is degraded through different pathways including the “serotonin” pathway (representing less than 10%) and the Tryptophan/Kynurenine Pathway (TKP) that largely predominates [38,39]. The TKP, regulated by a variety of enzymes expressed in different organs and conditions, determes the balanced concentration of Trp metabolites that can vary at the local and systemic levels [40]. The liver, where tryptophan-2,3-dioxygenase (TDO) is constitutive, is the predominant site for Trp degradation under physiological conditions, whereas extra-hepatic Trp degradation gains priority in inflammatory and immune activation, utilizing the catalytic activity of indoleamine-2,3-dioxigenases (IDO) [41]. The alternative serotonin pathway of Trp metabolic degradation is active in specialized sites (e.g., the brain and the pineal gland) where tryptophan hydroxylase can catalyze Trp hydroxylation, producing serotonin and melatonin [39]. The variety of Trp metabolites produced by TKP activation include kynurenine, anthranilic acid, kynurenic acid, 3-hydroxykynurenine, xanthurenic acid, 3-hydroxyanthranilic acid, quinolinic acid, picolinic acid, and finally NAD+ (a fundamental coenzyme for physiological processes such as DNA repair, cell growth, and energy metabolism). Trp metabolites have diverse biological properties, and their concentration may exert relevant roles in physiological and pathological mechanisms. The level of Trp metabolites in different body compartments is rigorously regulated, and the evaluation of blood concentration of kynurenine and the kynurenine/tryptophan ratio are considered as reliable markers of overall TKP activity at the systemic and local levels [42]. When the correct enzyme balance is altered (as in infection or cancer), the production of metabolites is modified, which affects several physiological functions [39,43].
The TKP-regulated availability of amino acids can limit the proliferation of some pathogenic microorganisms, and this competition has a protective role against infections in different species [44,45,46,47]. In mammals, this simple competitive strategy has evolved, providing novel functions in the regulation of immunity and other relevant physiological mechanisms [47,48].
1.3. Indoleamine 2,3-Dioxygenase (IDO1 and IDO2)
Indoleamine 2,3-dioxygenase enzyme activity, firstly identified as a Trp degrading enzyme in rabbit intestine [49], is the rate-limiting step of Trp degradation in extra-hepatic sites, where IDO is the main regulator of TKP activity, Trp consumption, and the production of Trp metabolites. Two closely related tryptophan catabolizing enzymes have been discovered, IDO1 and IDO2 [50,51,52]. IDO1 is highly induced by various inflammatory stimuli in different cell types and tissues, and its complex immunomodulatory functions are involved in physiologic and pathologic situations including maternal tolerance, inflammatory restraint in infection, tumor immune escape, neurodegenerative disorders, and autoimmune disorders [39]. IDO1 expression in normal tissues is negligible, but inflammatory stimuli can trigger its expression, mainly mediated by IFN [39,53,54]. Further positive and negative signals for IDO1 expression are also provided by Trp, nitric oxide (NO), H2O2, IL-6, and other cytokines [40,46,55,56,57,58].
IDO1 has relevant role in immune regulation, suppressing effector T-cell functions and favoring the development of regulatory T cells by different mechanisms including Trp depletion at the local site of inflammation and the production of immunosuppressive Trp metabolites (kynurenine, kynurenic acid, xanthurenic acid). Further immune-regulatory signals are provided by the increase in uncharged Trp-tRNAs (determined by local Trp depletion), a molecular mechanism that is able to activate the amino-acid sensitive GCN2 stress-kinase signaling, with eventual cell cycle arrest and/or anergy in T cells [59,60]. Essential amino acid deficiency can also interfere with the mTOR functional activity in dendritic cells, inducing the conversion of naïve T cells into Tregs [61,62].
Trp metabolites kynurenine and kynurenic acid are agonist ligands of the aryl hydrocarbon receptor (AHR), inducing T-cell apoptosis and favoring Treg development [63,64]. AHR is a multifunctional helix-loop-helix “biosensor” activated by a variety of naturally occurring and synthetic molecules, such as exogenous toxic compounds (e.g., polycyclic aromatic hydrocarbons), kynurenine, and other endogenous molecules [65]. The AHR response to exogenous or endogenous ligands can be divergent, either acting as a sensor to “danger signals” (favoring a proinflammatory Th17 response) or containing the inflammation by favoring suppressive Treg responses [65,66,67,68,69]. A further level of complexity is provided by the presence in the non-catalytic small domain of IDO1 proteins of immunoreceptor tyrosine-based inhibitory motifs (ITIMs), whose phosphorylation is dependent on the interaction with regulatory molecules; these interactions can lead to the suppression of cytokine signaling 3 (SOCS3) and the activation of the Src kinase [70,71]. In the presence of proinflammatory IL-6, proteosomal degradation of the enzyme occurs, thus interrupting tolerance [72,73]. IDO1 can in fact mediate its own expression and activity on the basis of microenvironmental molecular milieu (IL-6 and TGF-b) [65,72,74,75,76].
IDO2. IDO1 first appeared in placental animals through the duplication of the more ancestral paralog IDO2 gene (both located adjacent on chromosome 8) [50,77]; it is necessary to maintaining tolerance and providing protection to the fetus from T-lymphocytes [51,78,79].
Although IDO1 is genetically homologous to IDO2, the two enzymes have distinct expression patterns and roles: IDO2 exerting robust pro-inflammatory activity in autoimmune functions acting on B cells, whereas IDO1 is able to mediate the suppression of T cells, in opposition to the functions associated with autoimmunity [80,81,82,83,84]. Opposite functions are also exerted by IDO2 enzymes in experimental liver injury mediated by kynurenine and AHR signaling [85]. This intriguing functional difference may be related to the scarce catalytic activity of IDO2 in metabolizing TrpA compared to IDO1, and IDO2 may in fact represent a pseudoenzyme [52,86,87]. Pseudoenzymes (proteins that despite their evolutionarily similitude to active enzymes do not exert significant catalytic activity) have peculiar functions as regulators of relevant biological mechanisms; these functions include the control of substrates’ availability for their analog enzymes [88,89,90,91,92,93,94,95]. Another relevant difference is the lack of complete/functional ITIM sequences and signaling functions in IDO2, thus restraining microenvironmental regulation for its expression that mainly depends on aryl hydrocarbon receptor (AHR) rather than on interferons [73,96].
1.4. Kynurenine and Trp Metabolites as Biomarkers
The quantitative evaluation of kynurenine and kynurenine/tryptophan ratio is widely considered a reliable marker of TKP activation, and these values are perturbed in a variety of pathologies including COVID-19. Both kynurenine and K/T ratio are promising as diagnostic and prognostic biomarkers. Nevertheless, due to the large variety of cell types involved in enzyme regulation of Trp metabolism in different organs, the pathogenic significance of these biomarkers in different human diseases is not easy to decipher. This is particularly true when therapeutic manipulation of TKP abnormalities are hypothesized in different clinical contexts [92,93,94,95,96]. In general, TKP-related circulating biomarkers in the population can vary according to several parameters (age, gender, body mass index, physical activity, smoking, diabetes), and their cumulative effects likely determine the final physiologic or pathologic significance. In fact, TKP alterations of TKP-related biomarkers are associated with the risk of cancer and cardiovascular disease mortality independently of the cause [97,98,99,100].
Abnormal variations of kynurenine levels and K/T ratio in blood have been described in different pulmonary pathologies including infectious diseases (seasonal influenza, community-acquired pneumonia, pneumocystis infection, tuberculosis), lung transplantation and organ rejection, chronic inflammatory lung diseases, pulmonary hypertension, autoimmune diseases, and lung cancer [101,102,103,104,105,106,107,108,109,110,111].
1.5. Kynurenine and Trp Metabolites in COVID-19 and PACS
Over the past few months, a large number of studies has focused on the metabolic abnormalities occurring in COVID-19 patients, and significant changes in amino acid, lipid, and energy metabolism have been described. The methodological approaches in these studies were different, including sophisticated metabolomic analyses, but all studies documented the abnormalities in the tryptophan metabolism, as further supported by the increase in kynurenine and K/T ratio in the peripheral blood of SARS-CoV-2 positive patients [112,113,114,115,116,117,118,119,120]. These metabolic alterations had significant value as prognostic biomarkers to predict an increased risk of mortality, corresponding to different COVID-19 endotypes with increasing clinical severity [121,122,123,124,125]. Interestingly, derangement from normal values matched the occurrence of other inflammatory biomarkers (IL-6, CRP) [118,126,127].
Kynurenine signaling through the AHR may induce cell senescence andcontribute to aging-related pathologies of the musculoskeletal system, which can also complicate COVID-19 and PACS [128,129,130]. Modulation of the KTP with eventual systemic release of neuroprotective Trp metabolites occurs in skeletal muscles by physical exercise, and can partly explain the beneficial effects of physical exercise in different conditions, such as PACS [131,132,133].
In COVID-19 and PACS, several neurological complications can be observed, from isolated anosmia and/or dysgeusia to severe neuropsychiatric conditions, and the activation of TKP has been proposed as a mechanistic explanation and a promising therapeutic target [134,135,136,137,138,139,140]. In fact, the persistence of an abnormal K/T ratio and tryptophan decrease as well as vascular abnormalities occur in a number of patients and might be considered a feature of PACS [141,142,143]. The activation of TKP has been generally considered a possible cause of the neurological complications occurring in SARS-CoV-2 infection [118,134]. An abnormal KTP activation can significantly interfere with neurological physiology by decreasing the availability of the essential amino acid Trp for conversion to 5-HT and melatonin (molecules necessary for regulation of sleep, mood, and appetite), as well as by producing unbalanced proportions of neurotoxic (quinolinic acid, 3-hydroxykynurenine) versus neuroprotective and anti-depressive (kynurenic acid, picolinic acid, and the essential cofactor NAD+) Trp metabolites [94,144,145,146,147]. Within the brain, quinolinic acid concentrations are normally lower compared to blood, but IDO1-expressing dendritic cells, microglia, and macrophages raise the levels of the neurotoxic quinolinic acid during inflammation [148,149,150]. Accordingly, abnormal increases in the concentration of kynurenine and kynurenin/tryptophan ratio are observed in neurodegenerative and neuropsychiatric disorders [151,152,153,154].
1.6. IDO Expression in Cells and Tissues
The expression pattern and tissue distribution of the different enzymes involved in the TKP (TDO, IDO1, IDO2) has been evaluated in different species utilizing a variety of methodological strategies, and a detailed picture in human tissues is still partial [155]. Human IDO1, as in other mammals, has a restricted distribution, likely related to its distinctive functions [79]. IDO1 is mainly expressed in placenta (where the enzyme is considered to exert a relevant role in the maternal–fetal tolerance process) [78,156], in lymphoid tissues, both intestine and lung [157,158]. In lymph nodes and the thymus, dendritic cells with antigen presenting functions are the main cell type expressing IDO1 in immunohistochemical investigations [159].
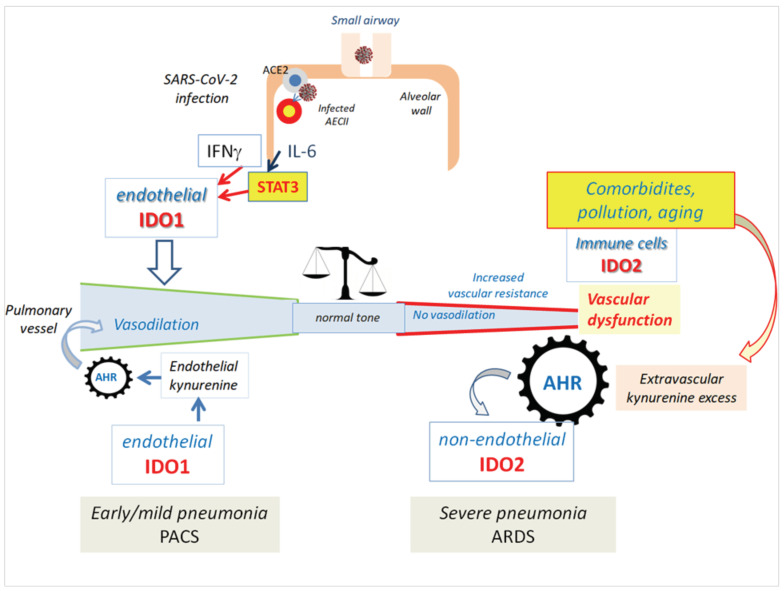
Consistent IDO1 immunohistochemical expression has been described in the lung, mainly confined to blood vessels [159]. The endothelial expression of IDO1 in other tissues is absent in normal conditions, but when up-regulated by IFNg the resulting vascular deprivation of Trp may provide antibacterial activity [160]. In our experience, the endothelial IDO1 expression in a normal lung is weak and/or restricted to scattered blood vessels [35,36]. A significantly different IDO1 expression pattern is observed in COVID-19 early/mild pneumonia and PACS patients, where most parenchymal blood vessels, both capillaries and venules, consistently show endothelial immunostaining (Figure 1) [35,36]. According to available data, IDO1 and IDO2 have overlapping but distinct functions and expression patterns. IDO1 expression in immune cells is variable and dependent on cytokines’ availability in the microenvironment, whereas IDO2 is constitutive in circulating myeloid DCs and plasmacytoid dendritic cells [161]. According to the few available studies, IDO2 expression in a normal lung is negligible [162].
Figure 1.
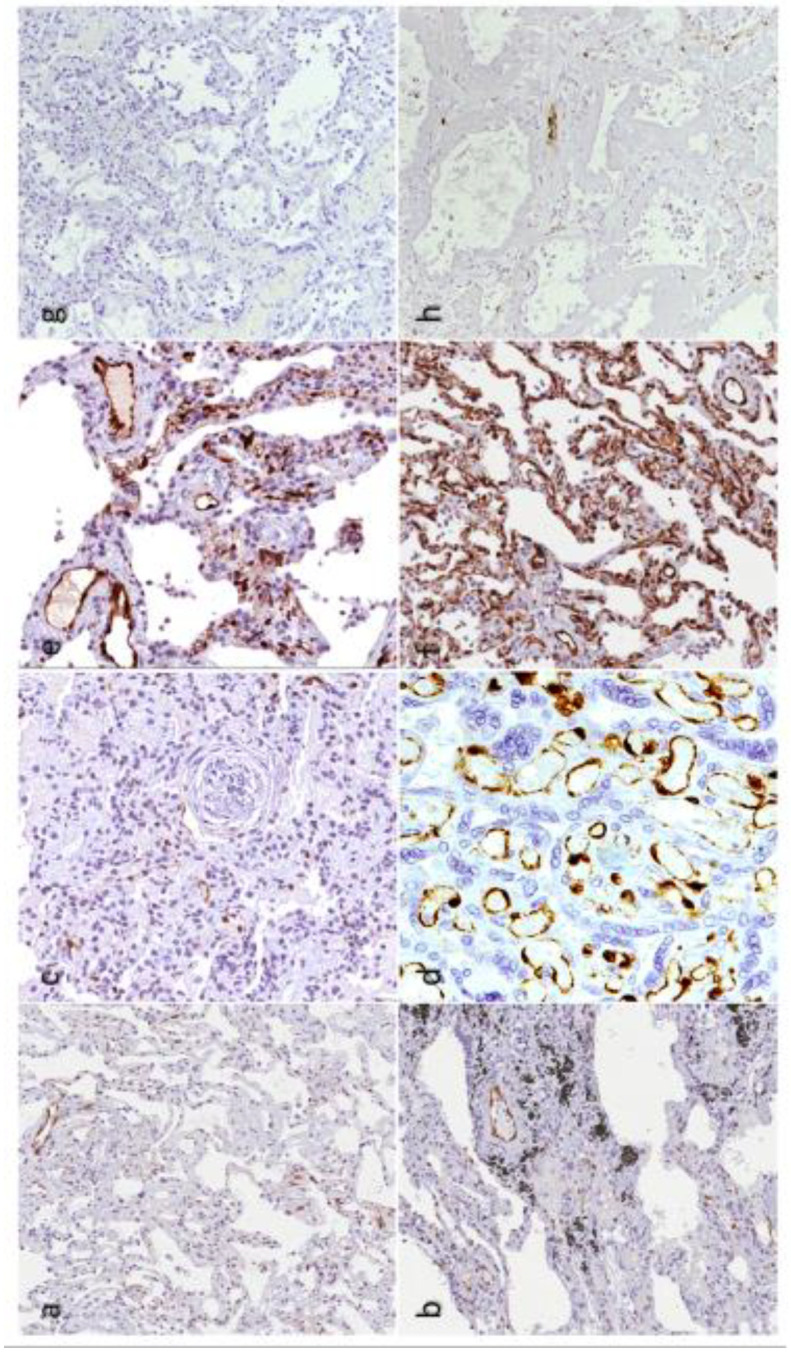
IDO1 endothelial expression is minimal in lung controls, where only scattered vessels are positive (a,b): normal lung; (c): organizing pneumonia. In human placenta, diffuse and strong endothelial IDO1 expression is observed in all vessels (d). Diffuse and strong endothelial IDO1 expression in a case of COVID-19 early/mild pneumonia (e). Diffuse and strong endothelial IDO1 expression in a case of post-COVID-19 pneumonia (f). Minimal/absent IDO1 endothelial expres-sion in two autoptic cases of severe COVID-19 (g,h). All cases were retrieved from the archive of Pathology Dept. of the San Raffaele Hospital, Milan, Italy and were immunostained with an-ti-IDO1 rabbit monoclonal antibody (dil.1:100, clone D5J4E, cod 86630, CellSignal, Danvers, MA, USA) with the Benchmark Ultra Instrument (Ventana-Roche). Original magnification in all images: 200×. Matched rabbit isotype control (cod. 3900 CellSignal, Danvers, MA, USA) on the same sections was always negative.
1.7. IDO1 Regulation of the Vascular Tone: Lessons from Placental Pathology and Pulmonary Hypertension
In the human placenta, IDO1 is constitutively expressed in chorionic vascular endothelium, with the highest levels found in the microvasculature. The endothelial expression increases in distribution from first trimester to term, paralleling the high increase in the kynurenine-to-tryptophan ratio occurring in chorionic villous tissue [163,164]. The IFNg secreted by Natural killer cells at the maternal–fetal interface can likely significantly contribute to local constitutive induction of IDO1 [165]. In addition to the well-established role in maintaining feto–maternal immune-tolerance and antimicrobial functions, IDO1 has been demonstrated to exert a relevant role in the regulation of vascular tone and placental perfusion, thus providing a regular blood flux to the growing fetus [166]. Accordingly, in experimental IDO1 deficiency, a number of pregnancy disorders can develop such as impairments in intrauterine growth restriction (IUGR) and pre-eclampsia [166,167,168,169,170]. Kynurenine is an endogenous relaxing factor for blood vessels [92,171], and the TKP has been proposed as a therapeutic target in pre-eclampsia and other hypertensive disorders [172,173]. Further complexity has been recently evidenced, since IDO1 can regulate vascular tone in inflammation by producing singlet molecular oxygen and the Trp metabolite cis-WOOH (cis-hydroperoxide (2S,3aR,8aR)-3a-hydroperoxy-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole-2-carboxylic acid) [174,175]. Endothelial IDO1 is likely necessary for exerting vascular relaxation, and critical levels of endothelial and/or perivascular concentration of vasoactive Trp metabolites may be necessary for effective control of the vascular tone [176]. Some Trp metabolites can easily diffuse and enter into cell cytoplasm, but some specific functions (i.e. the vascular tone control) likely depend on the actual local availability, and the “average” concentration can have different significances in different microenvironments. The activity of endothelial IDO1/kynurenine axis on vascular tone is likely more effective in organs characterized by peculiar circulatory systems such as the placenta and lung, both characterized by reduced blood pressure and both constitutively expressing IDO1 [159,177].
Increased kynurenine concentration in the vessel microenvironment can selectively operate in pulmonary hypertension by contrasting apoptosis in endothelial cells, but favoring apoptosis in smooth muscle cell [178]. In pulmonary hypertension, the observed increase of kynurenine serum levels, concomitant with Trp decrease, may serve as a negative feedback mechanism contrasting vascular pressure increase, and its negative prognostic significance as a biomarker can be treated as evidence of an insufficient protective effect on vascular dysfunction [179,180]. On the other hand, several studies have demonstrated a central role of IDO1 activity and kynurenine in inducing systemic vascular relaxation and hypotension in experimental and human septic shock [181,182,183,184,185]. An endothelial protective role of IDO1 has also been demonstrated in experimental ischemia-reperfusion, atherosclerosis, and acute lung allograft injury, thus suggesting a role for the TKP activation in mitigating vascular dysfunction and conditions exhibiting both excessive inflammation and a compromised balance between vasoconstrictor and vasodilator tone [186,187,188,189,190].
1.8. Vascular Dysfunction, COVID-19 and the TKP
Vascular dysfunction is associated with a variety of pathological conditions (cardiovascular disease, diabetes, obesity, older age, chronic lung disease, infections, etc.), and is a distinct feature of severe COVID-19 [191,192,193,194]. In fact, SARS-CoV-2 infection can induce vascular inflammation, disruption of the endothelial homeostasis, edema, and life-threatening coagulation abnormalities in severe cases, all features defining endothelial/vascular dysfunction [195,196,197,198,199]. The pathophysiology of endothelial dysfunction in COVID-19 is generally considered a consequence of the body’s uncontrolled inflammatory response, but the precise mechanisms accounting for its development have not been completely defined [31,193,200,201,202]. Direct infection has been considered a possible cause, but evidence of SARS-CoV-2 endothelial infection has only been rarely reported in pulmonary and extra-pulmonary sites [203,204,205], and experimental endotheliopathy can be triggered by plasma from severely ill patients [206]. Independent of viral infection, the interaction of SARS-CoV-2 spike proteins with different cell types may represent a plausible cause of endothelial damage, senescence, and impairment of endothelium-dependent vasodilation, thus representing a pathogenic trigger in COVID-19 and PACS [18,102,207,208,209,210,211,212,213].
1.9. IDO1 and IDO2 in COVID-19 Pneumonia: From Vasoplegia to Vascular Dysfunction?
In situ analyses have allowed a precise evaluation of IDO1 expression at the cellular level in acute early/mild COVID-19 pneumonias, revealing an intense and diffuse immunoreactivity in blood vessels, both capillaries and venules, at variance with what has been observed in control normal-lung samples and in a variety of other pulmonary pathologies; yet these results are similar to what has been observed in human placenta (Figure 1) [36]. In the same samples, SARS-CoV2 viral RNA was demonstrated in alveolar epithelial cells, concomitantly with IL-6 signals and STAT3 over-expression. Furthermore, a diffuse enlargement of interstitial vessels was noted, suggesting a pathogenic role of IDO1 in inducing COVID-19 V/Q mismatch and silent/happy-hypoxia [31,32]. These findings were associated with mild pneumonia, since all cases of that series did not need invasive ventilation [36]. Comparable endothelial IDO1 expression is not observed in pulmonary autoptic cases of severe COVID-19 pneumonia (Figure 1g,h; unpublished data). Interestingly, in fatal cases that enabled investigations through necropsy, strong and diffuse pulmonary expression of IDO2 was demonstrated, with no significant evidence of IDO1 [162]. Two harmful Trp-metabolites (3-hydroxy-anthranilic acid and quinolinic acid) were co-localized with IDO2 in the same samples, suggesting that most of the enzymatic activity was due to IDO2 [162]. A possible explanation of this unprecedented hyper-expression of IDO2 was proposed, centered on a peculiar positive feedback loop generated by the interaction of kynurenine and AHR, which favored the expression of IDO2 [91]. Different endogenous and exogenous AHR ligands can induce opposite effects in immunity: dendritic cells accumulation of kynurenine results in tolerogenic signals, whereas in pollution enhanced Th17 differentiation is observed via the AHR [214]. Due to this double-face behavior, AHR may be considered a determining force in lung pathology [215,216,217]. In COVID-19, AHR-binding environmental pollutants may amplify inflammation and contribute to disease severity [218,219]. The observed imbalance of IDO1 and IDO2 expression in COVID-19 may be ascribed to different mechanisms. The two enzymes differently respond to metabolic inhibitors and cytokines (in particular, IFNg, as suggested by their differential expression in malaria and influenza) [51,220,221,222,223]. Degradation of IDO1 may be ascribed to IDO2-mediated hyper-activation of the STAT3/IL-6 pathway that trigger the enzyme proteolysis [224,225]. Extensive evidence has been provided on the pathogenic role of STAT3/IL-6 signaling in COVID-19, and therapies blocking this pathway are utilized to avoid disease evolution [226,227]. A further possibility is provided by viral activation of AHR in an IDO-independent manner [228]. Finally, the progressive decrease in IDO1 may further up-regulate IDO2 expression [229]. These different mechanisms might be responsible for a vicious circle in which the abnormal accumulation of extra-vascular kynurenine may cause the AHR and IDO2 expression to switch off endothelial IDO1. In this scenario, the harmful products of TKP activity might exert their pathogenic effects in a microenvironment deprived of the protective role of IDO1 in endothelial cells. These findings open relevant issues regarding the molecular mechanisms occurring in COVID-19 pneumonia, and the role of predisposing conditions in development of vascular dysfunction in severe cases.
We propose a pathogenic model in which the differential expression of IDO1 and IDO2 in COVID-19 pneumonia has pathogenic and prognostic relevance. In this model two different conditions are hypothesized: (1) Early/mild COVID-19 pneumonia where the activation of inflammatory signals (i.e IFNg) induce vascular IDO1, TKP activation, and production of potential harmful Trp metabolites. The protective role of IDO1 is preserved, thereby avoiding further vascular damage. (2) Severe COVID-19 pneumonia where predisposing conditions (age, diabetes, obesity, etc.) provide a background activation of AHR, thereby leading to IDO1/IDO2 imbalance and the switch from protective vasodilatation to vascular dysfunction, as previously described. Interestingly, acute increase of kynurenine is able to induce loss of vascular-tone control and endothelial dysfunction [230]. Impairment of vascular-tone regulation and bioavailability of vasodilators (including IDO1 metabolites and NO) is considered central in the development of endothelial dysfunction and diffuse alveolar damage in different conditions, such as COVID-19 [231,232,233,234,235]. The early administration of inhaled NO has been considered a possible therapeutic approach for reducing pulmonary vascular resistance and enhancing the ventilation/perfusion matching [236]. IDO1 and NO have interconnected functions and reciprocal regulation in endothelial cells. They are both induced by IFNγ and participate in a complex feedback mechanism, where interaction with NO triggers IDO1 degradation through the proteasome pathway [237]. In addition, some Trp metabolites can regulate NO production, and IDO1 has a nitrite reductase activity likely involved in observed local production of NO under anaerobic conditions [238,239]. On the other hand, evidence of IDO2 acting as a vasodilator is lacking, and it is not expressed in placental and pulmonary vessels as IDO1. A further support to the pathogenic relevance of IDO1/IDO2 imbalance may be provided by their opposite roles in shaping the immune tolerance and susceptibility to autoimmune conditions [240]. In several experimental conditions IDO1 is protective, whereas IDO2 is pro-inflammatory and is able to mediate autoreactive responses [80,82,83,241,242,243,244]. Autoimmune complications are common in COVID-19, and the possible role of IDO1/IDO2 imbalance in their development warrants further investigation [245,246].
In summary, different morphological and immunophenotypical vascular patterns can be defined in COVID-19, where the IDO1+ and IDO1-/IDO2+ phenotypes may correspond, in our view, to the previously defined biphasic presentation of COVID-19 pneumonia, with an early type-L pattern, characterized by vascular enlargement, preserved compliance, hypoxemia, and an out-of-proportion hypocapnia; and a more severe and potentially fatal type-H pattern, characterized by reduced vascular relaxation and vascular dysfunction [31,247,248,249,250].
1.10. IDO1 Endothelial Expression in Post-COVID-19
Diffuse endothelial IDO1 expression and vascular enlargement were also observed in post-COVID pneumonia cases, independent from the severity of pulmonary pathologic pattern [35]. This finding opens possible scenarios for this still poorly defined condition, scenarios such as the neurological complications and the persistent respiratory impairment. In our view, persistently inflammatory stimuli in these patients may maintain endothelial IDO1 expression and TKP activity, despite the complete viral clearing. The persistent TKP activation may be able to maintain elevated kynurenine blood levels, thus explaining the neurological and immunological dysfunction, as well as the “encephalomyelitis/chronic fatigue syndrome” -like symptoms observed in PACS [251,252,253]. The endothelial IDO1-enzyme activity in PACS pneumonia could partly explain respiratory symptoms due to persistent Q/V mismatch as observed in mild COVID-19 pneumonia [32].
A schematic description of this hypothetical pathogenic scenario is described in Figure 2.
Figure 2.
Hypothetical mechanisms involved in COVID-19 pneumonia, as discussed in this review. After SARS-CoV-2 infection leading to early/mild pneumonia, inflammatory stimuli trigger endothelial IDO1 expression, kynurenine accumulation, and vascular relaxation. This mechanism may persist in post-COVID-19. In severe cases, a loss of vascular IDO1 expression is observed, likely resulting in impairement of vascular-tone control and induction of vascular dysfunction. The occurrence of antecedent abnormal AHR activation (related to old age, comorbidities, and/or pollution) may concur in altering the kynurenine levels and the switch from IDO1 to IDO2.
The pathogenic role of IDO1/IDO2 imbalance in COVID-19 and PACS should of course be considered within a wider and more complex scenario where different mechanisms occur, including other metabolic abnormalities involved in immune regulation [254,255,256]. Further studies regarding the expression pattern and functional activity of IDOs and TKP in different clinical presentations of COVID-19 and PACS are warranted.
1.11. Therapeutic Considerations
The complex involvement of the TKP activation in the diverse presentations of COVID-19 and PACS poses relevant issues when the possible manipulation of this pathway is considered as a therapeutic option [117]. The complex roles (protective versus harmful) of endothelial TKP activation in different physiological systems and tissues need to be carefully considered in therapeutic planning in different clinical contexts. In COVID-19, the TKP activation may be considered harmful in increasing the systemic concentration of kynurenines and toxic Trp-metabolites, potentially interfering with immune and neurological functions, as previously described. Nevertheless, the endothelial IDO1 expression likely exerts significant protection against vascular dysfunction in the lung, and under this context the inhibition of IDO1 does not appear to be safe, which means that alternative interventions are needed to correct metabolic abnormalities. The correct balance of Trp-metabolites is relevant for maintaining healthful functions, and specific approaches should be selected for treating different COVID-19 clinical presentations. This can be particularly true in PACS, where mild “supportive” therapeutic interventions may ameliorate symptoms and shorten the disease course.
Trp supplementation, either dietary or non-nutritional, has been experimentally investigated and proposed to ameliorate neurological disturbances and social behavior in humans by increasing 5-HT production, but its clinical effect is still controversial and prone to genetic variations [257,258,259,260,261,262,263,264,265]. In addition, potential side effects of excessive Trp intake should be evaluated in different clinical settings [266]. Deranged activation of the TKP is common in COVID-19, and Trp supplementation may potentially increase the systemic concentration of harmful Trp metabolites [267].
Melatonin supplementation. Divergence from the serotonin pathway of Trp metabolism induces a defective production of melatonin in COVID-19 patients, and this decrease has prognostic significance [268]. This deficiency may be either indirectly related to the over-activation of the TKP (eventually leading to lower available 5-HT), to the well-documented anti-oxidant and anti-inflammatory roles of melatonin, or both [134,269,270,271,272]. A possible role in protecting pulmonary endothelial cells can be also hypothesized, as observed in pre-eclampsia [273]. Several authors have proposed melatonin as a useful therapeutic tool in contrasting neurological complications and super-infections for both COVID-19 and PACS patients [274,275,276,277,278], and a significant vantage in mortality and recovery rate has been observed in severe cases [279,280,281]. Validation of the therapeutic role of melatonin and its metabolites in COVID-19 and PACS is needed, and clinical trials and the use of reliable animal models are warranted [282,283,284,285,286,287].
IDO inhibitors. The relevant pathogenic and prognostic roles of the different Trp-metabolites in COVID-19 suggest the possible use of specific inhibitors to modulate the enzymes that regulate the TKP [117,288,289]. IDOs and TDO are involved in tumor immunosurveillance and the potential use of inhibitors of these enzymes to restore antitumor immunity is a matter of intense clinical investigation [96,290,291]. Different specific inhibitors are in fact available, and clinical trials are ongoing on a variety of human neoplastic and non-neoplastic diseases [292,293,294,295]. If the pathogenic shift from early/mild to severe COVID-19 pneumonias is in part determined by the modulation of IDO1 and IDO2 expression in the pulmonary microenvironment as here hypothesized, the availability and selective use of specific IDO1 or IDO2 inhibitors might be crucial. A range of selective and potent TDO, IDO1, and IDO2 inhibitors are currently under investigation in cancer research and information from this field may be translationally utilized for new personalized therapies for patients suffering from COVID-19 and PACS [52,86,296,297,298,299].
The inhibition of AHR is feasible, and this approach might be safer in severe cases [300].
2. Conclusions
Although the pathogenesis of COVID-19 and PACS is complex and only partially understood, several lines of evidence have been provided on the relevant role of immune mechanisms in triggering the abnormal cascade of cytokine production in severe cases. The regulation of these mechanisms is mediated by a variety of factors, such as genetic background and occurrence of predisposing metabolic abnormalities. The Tryptophan/kynurenine pathway is central in the regulation of immunre responses and vascular tone and may represent a key factor in the development of vascular dysfunction in severe COVID-19 pneumonia. The possible pharmacological manipulation of this pathway in SARS-CoV-2 infectious diseases should be based on the precise understanding of the different pathogenetic and clinical contests, avoiding potentially harmful consequences in the vascular compartment.
Acknowledgments
We thank Fondazione Cariverona (ENACT Project) for its financial support.
Author Contributions
M.C., C.D. and V.P. contributed to the design of the work; M.C., C.D., V.P. and C.R. have drafted the work; M.C., C.D., C.R., G.M., G.L.S., G.P., V.B. and V.P. have revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., Chu H.Y. Sequelae in Adults at 6 Months after COVID-19 Infection. JAMA Netw. Open. 2021;4:e210830. doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darcis G., Bouquegneau A., Maes N., Thys M., Henket M., Labye F., Rousseau A.-F., Canivet P., Desir C., Calmes D., et al. Long-term clinical follow up of patients suffering from moderate to severe COVID-19 infection: A monocentric prospective observational cohort study. Int. J. Infect. Dis. 2021;109:209–216. doi: 10.1016/j.ijid.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.C., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Di Cristanziano V., Osebold L., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V., WHO Clinical Case Definition Working Group on Post-COVID-19 Condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Z., Yang M., Lai C.L. Long COVID-19 Syndrome: A Comprehensive Review of Its Effect on Various Organ Systems and Recommendation on Rehabilitation Plans. Biomedicines. 2021;9:966. doi: 10.3390/biomedicines9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou K.M., Vasarmidi E., Russell A.M., Andrejak C., Crestani B., Delcroix M., Dinh-Xuan A.T., Poletti V., Sverzellati N., Vitacca M., et al. European Respiratory Society Statement on Long COVID-19 Follow-Up. Eur. Respir. J. 2022 doi: 10.1183/13993003.02174-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carson G., Long Covid Forum Group Research priorities for Long Covid: Refined through an international multi-stakeholder forum. BMC Med. 2021;19:84. doi: 10.1186/s12916-021-01947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhawan R.T., Gopalan D., Howard L., Vicente A., Park M., Manalan K., Wallner I., Marsden P., Dave S., Branley H., et al. Beyond the clot: Perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir. Med. 2021;9:107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munblit D., Nicholson T.R., Needham D.M., Seylanova N., Parr C., Chen J., Kokorina A., Sigfrid L., Buonsenso D., Bhatnagar S., et al. Studying the post-COVID-19 condition: Research challenges, strategies and importance of core outcome set development. BMC Med. 2022;20:50. doi: 10.1186/s12916-021-02222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips S., Williams M.A. Confronting Our Next National Health Disaster—Long-Haul Covid. N. Engl. J. Med. 2021;385:577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- 14.Peluso M.J., Deitchman A.N., Torres L., Iyer N.S., Munter S.E., Nixon C.C., Donatelli J., Thanh C., Takahashi S., Hakim J., et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36:109518. doi: 10.1016/j.celrep.2021.109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Files J.K., Sarkar S., Fram T.R., Boppana S., Sterrett S., Qin K., Bansal A., Long D.M., Sabbaj S., Kobie J.J., et al. Duration of post-COVID-19 symptoms is associated with sustained SARS-CoV-2-specific immune responses. JCI Insight. 2021;6:e151544. doi: 10.1172/jci.insight.151544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siemińska I., Węglarczyk K., Surmiak M., Kurowska-Baran D., Sanak M., Siedlar M., Baran J. Mild and Asymptomatic COVID-19 Convalescents Present Long-Term Endotype of Immunosuppression Associated with Neutrophil Subsets Possessing Regulatory Functions. Front. Immunol. 2021;12:748097. doi: 10.3389/fimmu.2021.748097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan F.J., Hope C.M., Masavuli M.G., Lynn M.A., Mekonnen Z.A., Yeow A.E.L., Garcia-Valtanen P., Al-Delfi Z., Gummow J., Ferguson C., et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theoharides T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022;59:1850–1861. doi: 10.1007/s12035-021-02696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambardar S.R., Hightower S.L., Huprikar N.A., Chung K.K., Singhal A., Collen J.F. Post-COVID-19 Pulmonary Fibrosis: Novel Sequelae of the Current Pandemic. J. Clin. Med. 2021;10:2452. doi: 10.3390/jcm10112452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., Jia J.L., Li L.M., Mao H.L., Zhou X.M., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty S.E., Guo Y., Heath K., Dasmariñas M.C., Jubilo K.G., Samranvedhya J., Lipsitch M., Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel N., Goyal N., Kumar R. Long COVID Mimicking Interstitial Lung Disease: A Case Series. Curr. Health Sci. J. 2021;47:469–473. doi: 10.12865/CHSJ.47.03.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myall K.J., Mukherjee B., Castanheira A.M., Lam J.L., Benedetti G., Mak S.M., Preston R., Thillai M., Dewar A., Molyneaux P.L., et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021;18:799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronson K.I., Podolanczuk A.J. Lungs after COVID-19: Evolving Knowledge of Post-COVID-19 Interstitial Lung Disease. Ann. Am. Thorac. Soc. 2021;18:773–774. doi: 10.1513/AnnalsATS.202102-223ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., Lei C., Chen R., Zhong N., Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., Müller-Wieland D., Hartmann B., Dreher M., Müller T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Sar-Van der Brugge S., Talman S., Boonman-de Winter L.J.M., de Mol M., Hoefman E., van Etten R.W., De Backer I.C. Pulmonary function and health-related quality of life after COVID-19 pneumonia. Respir. Med. 2021;176:106272. doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salerno D., Oriaku I., Darnell M., Lanclus M., De Backer J., Lavon B., Gupta R., Jaffe F., Vega Sanchez M.E., Kim V. Temple University Covid-19 Research Group. Association of abnormal pulmonary vasculature on CT scan for COVID-19 infection with decreased diffusion capacity in follow up: A retrospective cohort study. PLoS ONE. 2021;16:e0257892. doi: 10.1371/journal.pone.0257892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chilosi M., Poletti V., Ravaglia C., Rossi G., Dubini A., Piciucchi S., Pedica F., Bronte V., Pizzolo G., Martignoni G., et al. The pathogenic role of epithelial and endothelial cells in early-phase COVID-19 pneumonia: Victims and partners in crime. Mod. Pathol. 2021;34:1444–1455. doi: 10.1038/s41379-021-00808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldani S., Ravaglia C., Bensai S., Bertolovic L., Ghirotti C., Puglisi S., Martinello S., Sultani F., Colinelli C., Piciucchi S., et al. Pathophysiology of light phenotype SARS-CoV-2 interstitial pneumonia: From histopathological features to clinical presentations. Pulmonology. 2021 doi: 10.1016/j.pulmoe.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santamarina M.G., Beddings I., Lomakin F.M., Boisier Riscal D., Gutiérrez Claveria M., Vidal Marambio J., Retamal Báez N., Pavez Novoa C., Reyes Allende C., Ferreira Perey P., et al. Sildenafil for treating patients with COVID-19 and perfusion mismatch: A pilot randomized trial. Crit. Care. 2022;26:1. doi: 10.1186/s13054-021-03885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piciucchi S., Ravaglia C., Vizzuso A., Giampalma E., Poletti V. Awake prone positioning for COVID-19 acute respiratory failure: Imaging and histological background. Lancet Respir. Med. 2022;10:e14. doi: 10.1016/S2213-2600(21)00554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravaglia C., Doglioni C., Chilosi M., Piciucchi S., Dubini A., Rossi G., Pedica F., Puglisi S., Donati L., Tomassetti S., et al. Clinical, radiological, and pathological findings in patients with persistent lung disease following SARS-CoV-2 infection. Eur. Respir. J. 2022 doi: 10.1183/13993003.02411-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doglioni C., Ravaglia C., Chilosi M., Rossi G., Dubini A., Pedica F., Piciucchi S., Vizzuso A., Stella F., Maitan S., et al. COVID-19 Interstitial Pneumonia: Histological and Immunohistochemical Features on Cryobiopsies. Respiration. 2021;100:488–498. doi: 10.1159/000514822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grifka-Walk H.M., Jenkins B.R., Kominsky D.J. Amino Acid Trp: The Far Out Impacts of Host and Commensal Tryptophan Metabolism. Front. Immunol. 2021;12:653208. doi: 10.3389/fimmu.2021.653208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leklem J.E. Quantitative aspects of tryptophan metabolism in humans and other species: A review. Am. J. Clin. Nutr. 1971;24:659–672. doi: 10.1093/ajcn/24.6.659. [DOI] [PubMed] [Google Scholar]
- 39.Yeung A.W., Terentis A.C., King N.J., Thomas S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015;129:601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 40.Badawy A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int J. Tryptophan Res. 2017;10:1178646917691938. doi: 10.1177/1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H., Gong J., Liu Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review) Mol. Med. Rep. 2018;17:4867–4873. doi: 10.3892/mmr.2018.8537. [DOI] [PubMed] [Google Scholar]
- 42.Marszalek-Grabska M., Walczak K., Gawel K., Wicha-Komsta K., Wnorowska S., Wnorowski A., Turski W.A. Kynurenine emerges from the shadows—Current knowledge on its fate and function. Pharmacol. Ther. 2021;225:107845. doi: 10.1016/j.pharmthera.2021.107845. [DOI] [PubMed] [Google Scholar]
- 43.Ala M. The footprint of kynurenine pathway in every cancer: A new target for chemotherapy. Eur. J. Pharmacol. 2021;896:173921. doi: 10.1016/j.ejphar.2021.173921. [DOI] [PubMed] [Google Scholar]
- 44.Murray H.W., Szuro-Sudol A., Wellner D., Oca M.J., Granger A.M., Libby D.M., Rothermel C.D., Rubin B.Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect. Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carlin J.M., Borden E.C., Byrne G.I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J. Interferon Res. 1989;9:329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 46.Adams O., Besken K., Oberdörfer C., MacKenzie C.R., Takikawa O., Däubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 2004;78:2632–2636. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grohmann U., Bronnte V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010;236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 48.Murray P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 2016;17:132–139. doi: 10.1038/ni.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J. Biol. Chem. 1967;242:5260–5266. doi: 10.1016/S0021-9258(18)99420-2. [DOI] [PubMed] [Google Scholar]
- 50.Yuasa H.J., Takubo M., Takahashi A., Hasegawa T., Noma H., Suzuki T. Evolution of vertebrate indoleamine 2,3-dioxygenases. J. Mol. Evol. 2007;65:705–714. doi: 10.1007/s00239-007-9049-1. [DOI] [PubMed] [Google Scholar]
- 51.Ball H.J., Sanchez-Perez A., Weiser S., Austin C.J., Astelbauer F., Miu J., McQuillan J.A., Stocker R., Jermiin L.S., Hunt N.H. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Meininger D., Zalameda L., Liu Y., Stepan L.P., Borges L., McCarter J.D., Sutherland C.L. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim. Biophys. Acta. 2011;1814:1947–1954. doi: 10.1016/j.bbapap.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Pfefferkorn E.R., Rebhun S., Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J. Interferon Res. 1986;6:267–279. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- 54.Taylor M.W., Feng G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. doi: 10.1096/fasebj.5.11.1907934. [DOI] [PubMed] [Google Scholar]
- 55.Liebau C., Baltzer A.W., Schmidt S., Roesel C., Karreman C., Prisack J.B., Bojar H., Merk H. Interleukin-12 and interleukin-18 induce indoleamine 2,3-dioxygenase (IDO) activity in human osteosarcoma cell lines independently from interferon-gamma. Anticancer Res. 2002;22:931–936. [PubMed] [Google Scholar]
- 56.Poljak A., Grant R., Austin C.J., Jamie J.F., Willows R.D., Takikawa O., Littlejohn T.K., Truscott R.J., Walker M.J., Sachdev P., et al. Inhibition of indoleamine 2,3 dioxygenase activity by H2O2. Arch. Biochem. Biophys. 2006;450:9–19. doi: 10.1016/j.abb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Thomas S.R., Terentis A.C., Cai H., Takikawa O., Levina A., Lay P.A., Freewan M., Stocker R. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J. Biol. Chem. 2007;282:23778–23787. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]
- 58.Orabona C., Pallotta M.T., Volpi C., Fallarino F., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Grohmann U., Puccetti P. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:20828–20833. doi: 10.1073/pnas.0810278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munn D.H., Sharma M.D., Baban B., Harding H.P., Zhang Y., Ron D., Mellor A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Sundrud M.S., Koralov S.B., Feuerer M., Calado D.P., Kozhaya A.E., Rhule-Smith A., Lefebvre R.E., Unutmaz D., Mazitschek R., Waldner H., et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biswas M., Sarkar D., Kumar S.R., Nayak S., Rogers G.L., Markusic D.M., Liao G., Terhorst C., Herzog R.W. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+CD25+FoxP3+Treg. Blood. 2015;125:2937–2947. doi: 10.1182/blood-2014-09-599266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turnquist H.R., Raimondi G., Zahorchak A.F., Fischer R.T., Wang Z., Thomson A.W. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J. Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 63.Mezrich J.D., Fechner J.H., Zhang X., Johnson B.P., Burlingham W.J., Bradfield C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torti M.F., Giovannoni F., Quintana F.J., García C.C. The Aryl Hydrocarbon Receptor as a Modulator of Anti-viral Immunity. Front. Immunol. 2021;12:624293. doi: 10.3389/fimmu.2021.624293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guarnieri T., Abruzzo P.M., Bolotta A. More than a cell biosensor: Aryl hydrocarbon receptor at the intersection of physiology and inflammation. Am. J. Physiol.-Cell Physiol. 2020;318:C1078–C1082. doi: 10.1152/ajpcell.00493.2019. [DOI] [PubMed] [Google Scholar]
- 66.Bessede A., Gargaro M., Pallotta M.T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E.M., Macchiarulo A., Vacca C., et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denison M.S., Pandini A., Nagy S.R., Baldwin E.P., Bonati L. Ligand binding and activation of the Ah receptor. Chem. Biol. Interact. 2002;141:3–24. doi: 10.1016/S0009-2797(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 68.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 69.Grohmann U., Puccetti P. The Coevolution of IDO1 and AhR in the Emergence of Regulatory T-Cells in Mammals. Front. Immunol. 2015;6:58. doi: 10.3389/fimmu.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondanelli G., Bianchi R., Pallotta M.T., Orabona C., Albini E., Iacono A., Belladonna M.L., Vacca C., Fallarino F., Macchiarulo A., et al. A Relay Pathway between Arginine and Tryptophan Metabolism Confers Immunosuppressive Properties on Dendritic Cells. Immunity. 2017;46:233–244. doi: 10.1016/j.immuni.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orabona C., Pallotta M.T., Grohmann U. Different partners, opposite outcomes: A new perspective of the immunobiology of indoleamine 2,3-dioxygenase. Mol. Med. 2012;18:834–842. doi: 10.2119/molmed.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grohmann U., Fallarino F., Bianchi R., Belladonna M.L., Vacca C., Orabona C., Uyttenhove C., Fioretti M.C., Puccetti P. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J. Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 73.Fallarino F., Grohmann U., Puccetti P. Indoleamine 2,3-dioxygenase: From catalyst to signaling function. Eur. J. Immunol. 2012;42:1932–1937. doi: 10.1002/eji.201242572. [DOI] [PubMed] [Google Scholar]
- 74.Belladonna M.L., Volpi C., Bianchi R., Vacca C., Orabona C., Pallotta M.T., Boon L., Gizzi S., Fioretti M.C., Grohmann U., et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 75.Albini E., Rosini V., Gargaro M., Mondanelli G., Belladonna M.L., Pallotta M.T., Volpi C., Fallarino F., Macchiarulo A., Antognelli C., et al. Distinct roles of immunoreceptor tyrosine-based motifs in immunosuppressive indoleamine 2,3-dioxygenase 1. J. Cell. Mol. Med. 2017;21:165–176. doi: 10.1111/jcmm.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Litzenburger U.M., Opitz C.A., Sahm F., Rauschenbach K.J., Trump S., Winter M., Ott M., Ochs K., Lutz C., Liu X., et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuasa H.J., Mizuno K., Ball H.J. Low efficiency IDO2 enzymes are conserved in lower vertebrates, whereas higher efficiency IDO1 enzymes are dispensable. FEBS J. 2015;282:2735–2745. doi: 10.1111/febs.13316. [DOI] [PubMed] [Google Scholar]
- 78.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 79.Ball H.J., Jusof F.F., Bakmiwewa S.M., Hunt N.H., Yuasa H.J. Tryptophan-catabolizing enzymes—Party of three. Front. Immunol. 2014;5:485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merlo L.M.F., Pigott E., DuHadaway J.B., Grabler S., Metz R., Prendergast G.C., Mandik-Nayak L. IDO2 is a critical mediator of autoantibody production and inflammatory pathogenesis in a mouse model of autoimmune arthritis. J. Immunol. 2014;192:2082–2090. doi: 10.4049/jimmunol.1303012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merlo L.M., Mandik-Nayak L. IDO2: A Pathogenic Mediator of Inflammatory Autoimmunity. Clin. Med. Insights Pathol. 2016;9:21–28. doi: 10.4137/CPath.S39930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merlo L.M.F., DuHadaway J.B., Montgomery J.D., Peng W.D., Murray P.J., Prendergast G.C., Caton A.J., Muller A.J., Mandik-Nayak L. Differential Roles of IDO1 and IDO2 in T and B Cell Inflammatory Immune Responses. Front. Immunol. 2020;11:1861. doi: 10.3389/fimmu.2020.01861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merlo L.M.F., Peng W., DuHadaway J.B., Montgomery J.D., Prendergast G.C., Muller A.J., Mandik-Nayak L. The Immunomodulatory Enzyme IDO2 Mediates Autoimmune Arthritis through a Nonenzymatic Mechanism. J. Immunol. 2022;208:571–581. doi: 10.4049/jimmunol.2100705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fatokun A.A., Hunt N.H., Ball H.J. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: Characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319–1329. doi: 10.1007/s00726-013-1602-1. [DOI] [PubMed] [Google Scholar]
- 85.Hoshi M., Osawa Y., Nakamoto K., Morita N., Yamamoto Y., Ando T., Tashita C., Nabeshima T., Saito K. Kynurenine produced by indoleamine 2,3-dioxygenase 2 exacerbates acute liver injury by carbon tetrachloride in mice. Toxicology. 2020;438:152458. doi: 10.1016/j.tox.2020.152458. [DOI] [PubMed] [Google Scholar]
- 86.Pantouris G., Serys M., Yuasa H.J., Ball H.J., Mowat C.G. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46:2155–2163. doi: 10.1007/s00726-014-1766-3. [DOI] [PubMed] [Google Scholar]
- 87.Mondanelli G., Mandarano M., Belladonna M.L., Suvieri C., Pelliccia C., Bellezza G., Sidoni A., Carvalho A., Grohmann U., Volpi C. Current Challenges for IDO2 as Target in Cancer Immunotherapy. Front. Immunol. 2021;12:679953. doi: 10.3389/fimmu.2021.679953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murphy J.M., Mace P.D., Eyers P.A. Live and let die: Insights into pseudoenzyme mechanisms from structure. Curr. Opin. Struct. Biol. 2017;47:95–104. doi: 10.1016/j.sbi.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Murphy J.M., Farhan H., Eyers P.A. Bio-Zombie: The rise of pseudoenzymes in biology. Biochem. Soc. Trans. 2017;45:537–544. doi: 10.1042/BST20160400. [DOI] [PubMed] [Google Scholar]
- 90.Adrain C. Pseudoenzymes: Dead enzymes with a lively role in biology. FEBS J. 2020;287:4102–4105. doi: 10.1111/febs.15535. [DOI] [PubMed] [Google Scholar]
- 91.Li Q., Harden J.L., Anderson C.D., Egilmez N.K. Tolerogenic Phenotype of IFN-γ-Induced IDO+ Dendritic Cells Is Maintained via an Autocrine IDO-Kynurenine/AhR-IDO Loop. J. Immunol. 2016;197:962–970. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 92.Hofmann F. Ido brings down the pressure in systemic inflammation. Nat. Med. 2010;16:265–267. doi: 10.1038/nm0310-265. [DOI] [PubMed] [Google Scholar]
- 93.Stone T.W., Forrest C.M., Darlington L.G. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- 94.Lovelace M.D., Varney B., Sundaram G., Lennon M.J., Lim C.K., Jacobs K., Guillemin G.J., Brew B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373–388. doi: 10.1016/j.neuropharm.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 95.Schwarcz R., Stone T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology. 2017;112:237–247. doi: 10.1016/j.neuropharm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Opitz C.A., Somarribas Patterson L.F., Mohapatra S.R., Dewi D.L., Sadik A., Platten M., Trump S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer. 2020;122:30–44. doi: 10.1038/s41416-019-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Theofylaktopoulou D., Midttun Ø., Ulvik A., Ueland P.M., Tell G.S., Vollset S.E., Nygård O., Eussen S.J. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013;173:121–130. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geisler S., Mayersbach P., Becker K., Schennach H., Fuchs D., Gostner J.M. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines. 2015;26:31–36. doi: 10.1515/pterid-2014-0015. [DOI] [Google Scholar]
- 99.Abedi S., Vessal M., Asadian F., Takhshid M.A. Association of serum kynurenine/tryptophan ratio with poor glycemic control in patients with type2 diabetes. J. Diabetes Metab. Disord. 2021;20:1521–1527. doi: 10.1007/s40200-021-00895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuo H., Ueland P.M., Ulvik A., Eussen S.J., Vollset S.E., Nygård O., Midttun Ø., Theofylaktopoulou D., Meyer K., Tell G.S. Plasma Biomarkers of Inflammation, the Kynurenine Pathway and Risks of All-Cause, Cancer and Cardiovascular Disease Mortality: The Hordaland Health Study. Am. J. Epidemiol. 2016;183:249–258. doi: 10.1093/aje/kwv242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pizzini A., Kurz K., Santifaller J., Tschurtschenthaler C., Theurl I., Fuchs D., Weiss G., Bellmann-Weiler R. Assessment of neopterin and indoleamine 2,3-dioxygenase activity in patients with seasonal influenza: A pilot study. Influenza Other Respir. Viruses. 2019;13:603–609. doi: 10.1111/irv.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer K., Patra T., Vijayamahantesh R.R. SARS-CoV-2 Spike Protein Induces Paracrine Senescence and Leukocyte Adhesion in Endothelial Cells. J. Virol. 2021;95:e0079421. doi: 10.1128/JVI.00794-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang M., Dong X., Huang Y., Su J., Dai X., Guo Y., Hu C., Zhou Q., Zhu B. Activation of the kynurenine pathway is associated with poor outcome in Pneumocystis pneumonia patients infected with HIV: Results of 2 months cohort study. BMC Infect. Dis. 2019;19:223. doi: 10.1186/s12879-019-3851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adu-Gyamfi C.G., Snyman T., Hoffmann C.J., Martinson N.A., Chaisson R.E., George J.A., Suchard M.S. Plasma Indoleamine 2, 3-Dioxygenase, a Biomarker for Tuberculosis in Human Immunodeficiency Virus-Infected Patients. Clin. Infect. Dis. 2017;65:1356–1358. doi: 10.1093/cid/cix550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suzuki Y., Suda T., Asada K., Miwa S., Suzuki M., Fujie M., Furuhashi K., Nakamura Y., Inui N., Shirai T., et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin. Vaccine Immunol. 2012;19:436–442. doi: 10.1128/CVI.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meloni F., Giuliano S., Solari N., Draghi P., Miserere S., Bardoni A.M., Salvini R., Bini F., Fietta A.M. Indoleamine 2,3-dioxygenase in lung allograft tolerance. J. Heart Lung Transplant. 2009;28:1185–1192. doi: 10.1016/j.healun.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 107.Meyer K.C., Arend R.A., Kalayoglu M.V., Rosenthal N.S., Byrne G.I., Brown R.R. Tryptophan metabolism in chronic inflammatory lung disease. J. Lab. Clin. Med. 1995;126:530–540. [PubMed] [Google Scholar]
- 108.Odler B., Foris V., Gungl A., Müller V., Hassoun P.M., Kwapiszewska G., Olschewski H., Kovacs G. Biomarkers for Pulmonary Vascular Remodeling in Systemic Sclerosis: A Pathophysiological Approach. Front. Physiol. 2018;9:587. doi: 10.3389/fphys.2018.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki Y., Suda T., Furuhashi K., Suzuki M., Fujie M., Hahimoto D., Nakamura Y., Inui N., Nakamura H., Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer. 2010;67:361–365. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Murakami Y., Hoshi M., Imamura Y., Arioka Y., Yamamoto Y., Saito K. Remarkable role of indoleamine 2,3-dioxygenase and tryptophan metabolites in infectious diseases: Potential role in macrophage-mediated inflammatory diseases. Mediat. Inflamm. 2013;2013:391984. doi: 10.1155/2013/391984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sawada L., Vallinoto A.C.R., Brasil-Costa I. Regulation of the Immune Checkpoint Indoleamine 2,3-Dioxygenase Expression by Epstein-Barr Virus. Biomolecules. 2021;11:1792. doi: 10.3390/biom11121792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu P., Chen D., Ding W., Wu P., Hou H., Bai Y., Zhou Y., Li K., Xiang S., Liu P., et al. The trans-omics landscape of COVID-19. Nat. Commun. 2021;12:4543. doi: 10.1038/s41467-021-24482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ansone L., Briviba M., Silamikelis I., Terentjeva A., Perkons I., Birzniece L., Rovite V., Rozentale B., Viksna L., Kolesova O., et al. Amino Acid Metabolism is Significantly Altered at the Time of Admission in Hospital for Severe COVID-19 Patients: Findings from Longitudinal Targeted Metabolomics Analysis. Microbiol. Spectr. 2021;9:e0033821. doi: 10.1128/spectrum.00338-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cai Y., Kim D.J., Takahashi T., Broadhurst D.I., Yan H., Ma S., Rattray N.J.W., Casanovas-Massana A., Israelow B., Klein J., et al. Kynurenic acid may underlie sex-specific immune responses to COVID-19. Sci. Signal. 2021;14:eabf8483. doi: 10.1126/scisignal.abf8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.D’Alessandro A., Thomas T., Akpan I.J., Reisz J.A., Cendali F.I., Gamboni F., Nemkov T., Thangaraju K., Katneni U., Tanaka K., et al. Biological and Clinical Factors Contributing to the Metabolic Heterogeneity of Hospitalized Patients with and without COVID-19. Cells. 2021;10:2293. doi: 10.3390/cells10092293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D’Amora P., Silva I.D.C.G., Budib M.A., Ayache R., Silva R.M.S., Silva F.C., Appel R.M., Júnior S.S., Pontes H.B.D., Alvarenga A.C., et al. Towards risk stratification and prediction of disease severity and mortality in COVID-19: Next generation metabolomics for the measurement of host response to COVID-19 infection. PLoS ONE. 2021;16:e0259909. doi: 10.1371/journal.pone.0259909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Danlos F.X., Grajeda-Iglesias C., Durand S., Sauvat A., Roumier M., Cantin D., Colomba E., Rohmer J., Pommeret F., Baciarello G., et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021;12:258. doi: 10.1038/s41419-021-03540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawler N.G., Gray N., Kimhofer T., Boughton B., Gay M., Yang R., Morillon A.C., Chin S.T., Ryan M., Begum S., et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARS-CoV-2 Infection and Inflammatory Cytokine Responses. J. Proteome Res. 2021;20:2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 119.Hasan M.R., Suleiman M., Pérez-López A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front. Genet. 2021;12:721556. doi: 10.3389/fgene.2021.721556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., Li K., Ran X., Long Q., Deng H., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blasco H., Bessy C., Plantier L., Lefevre A., Piver E., Bernard L., Marlet J., Stefic K., Benz-de Bretagne I., Cannet P., et al. The specific metabolome profiling of patients infected by SARS-COV-2 supports the key role of tryptophan-nicotinamide pathway and cytosine metabolism. Sci. Rep. 2020;10:16824. doi: 10.1038/s41598-020-73966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mangge H., Herrmann M., Meinitzer A., Pailer S., Curcic P., Sloup Z., Holter M., Prüller F. Increased Kynurenine Indicates a Fatal Course of COVID-19. Antioxidants. 2021;10:1960. doi: 10.3390/antiox10121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herrera-Van Oostdam A.S., Castañeda-Delgado J.E., Oropeza-Valdez J.J., Borrego J.C., Monárrez-Espino J., Zheng J., Mandal R., Zhang L., Soto-Guzmán E., Fernández-Ruiz J.C., et al. Immunometabolic signatures predict risk of progression to sepsis in COVID-19. PLoS ONE. 2021;16:e0256784. doi: 10.1371/journal.pone.0256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.López-Hernández Y., Monárrez-Espino J., Oostdam A.H., Delgado J.E.C., Zhang L., Zheng J., Valdez J.J.O., Mandal R., González F.L.O., Moreno J.C.B., et al. Targeted metabolomics identifies high performing diagnostic and prognostic biomarkers for COVID-19. Sci. Rep. 2021;11:14732. doi: 10.1038/s41598-021-94171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lionetto L., Ulivieri M., Capi M., De Bernardini D., Fazio F., Petrucca A., Pomes L.M., De Luca O., Gentile G., Casolla B., et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:166042. doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., Hudson K.E., Zimring J.C., Hansen K.C., Hod E.A., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5:e140327. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michaelis S., Zelzer S., Schnedl W.J., Baranyi A., Meinitzer A., Enko D. Assessment of tryptophan and kynurenine as prognostic markers in patients with SARS-CoV-2. Clin. Chim. Acta. 2021;525:29–33. doi: 10.1016/j.cca.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaiser H., Yu K., Pandya C., Mendhe B., Isales C.M., McGee-Lawrence M.E., Johnson M., Fulzele S., Hamrick M.W. Kynurenine, a Tryptophan Metabolite That Increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxid. Med. Cell. Longev. 2019;2019:9894238. doi: 10.1155/2019/9894238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel D., Potter M., Anaya J.M., McGee-Lawrence M.E., Hamrick M.W., Hill W.D., Isales C.M., Fulzele S. Kynurenine induces an age-related phenotype in bone marrow stromal cells. Mech. Ageing Dev. 2021;195:111464. doi: 10.1016/j.mad.2021.111464. [DOI] [PubMed] [Google Scholar]
- 130.Vyavahare S., Kumar S., Cantu N., Kolhe R., Bollag W.B., McGee-Lawrence M.E., Hill W.D., Hamrick M.W., Isales C.M., Fulzele S. Tryptophan-Kynurenine Pathway in COVID-19-Dependent Musculoskeletal Pathology: A Minireview. Mediat. Inflamm. 2021;2021:2911578. doi: 10.1155/2021/2911578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin K.S., Azzolini M., Lira Ruas J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020;318:C818–C830. doi: 10.1152/ajpcell.00580.2019. [DOI] [PubMed] [Google Scholar]
- 132.Gallardo-Gómez D., Del Pozo-Cruz J., Noetel M., Álvarez-Barbosa F., Alfonso-Rosa R.M., Del Pozo Cruz B. Optimal Dose and Type of Exercise to Improve Cognitive Function in Older Adults: A Systematic Review and Bayesian Model-Based Network Meta-Analysis of RCTs. Ageing Res. Rev. 2022;76:101591. doi: 10.1016/j.arr.2022.101591. [DOI] [PubMed] [Google Scholar]
- 133.Dotan A., David P., Arnheim D., Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun. Rev. 2022;21:103071. doi: 10.1016/j.autrev.2022.103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sen A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med. Hypotheses. 2021;153:110627. doi: 10.1016/j.mehy.2021.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iqbal Y., Al Abdulla M.A., Albrahim S., Latoo J., Kumar R., Haddad P.M. Psychiatric presentation of patients with acute SARS-CoV-2 infection: A retrospective review of 50 consecutive patients seen by a consultation-liaison psychiatry team. BJPsych Open. 2020;6:e109. doi: 10.1192/bjo.2020.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jain A. Deregulated kynurenine metabolism—An alternate hypothesis for COVID-19 associated anosmia. Med. Hypotheses. 2021;157:110721. doi: 10.1016/j.mehy.2021.110721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bouças A.P., Rheinheimer J., Lagopoulos J. Why Severe COVID-19 Patients Are at Greater Risk of Developing Depression: A Molecular Perspective. Neuroscientist. 2020;28:11–19. doi: 10.1177/1073858420967892. [DOI] [PubMed] [Google Scholar]
- 138.Shader R.I. COVID-19, interferons, and depression: A commentary. Psychiatry Res. 2020;291:113198. doi: 10.1016/j.psychres.2020.113198. [DOI] [PubMed] [Google Scholar]
- 139.Collier M.E., Zhang S., Scrutton N.S., Giorgini F. Inflammation control and improvement of cognitive function in COVID-19 infections: Is there a role for kynurenine 3-monooxygenase inhibition? Drug Discov. Today. 2021;26:1473–1481. doi: 10.1016/j.drudis.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tabacof L., Tosto-Mancuso J., Wood J., Cortes M., Kontorovich A., McCarthy D., Rizk D., Rozanski G., Breyman E., Nasr L., et al. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life and Participation. Am. J. Phys. Med. Rehabil. 2022;101:48–52. doi: 10.1097/PHM.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holmes E., Wist J., Masuda R., Lodge S., Nitschke P., Kimhofer T., Loo R.L., Begum S., Boughton B., Yang R., et al. Incomplete Systemic Recovery and Metabolic Phenoreversion in Post-Acute-Phase Nonhospitalized COVID-19 Patients: Implications for Assessment of Post-Acute COVID-19 Syndrome. J. Proteome Res. 2021;20:3315–3329. doi: 10.1021/acs.jproteome.1c00224. [DOI] [PubMed] [Google Scholar]
- 142.Jud P., Gressenberger P., Muster V., Avian A., Meinitzer A., Strohmaier H., Sourij H., Raggam R.B., Stradner M.H., Demel U., et al. Evaluation of Endothelial Dysfunction and Inflammatory Vasculopathy after SARS-CoV-2 Infection—A Cross-Sectional Study. Front. Cardiovasc. Med. 2021;8:750887. doi: 10.3389/fcvm.2021.750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lambadiari V., Mitrakou A., Kountouri A., Thymis J., Katogiannis K., Korakas E., Varlamos C., Andreadou I., Tsoumani M., Triantafyllidi H., et al. Association of COVID-19 with impaired endothelial glycocalyx, vascular function and myocardial deformation 4 months after infection. Eur. J. Heart Fail. 2021;23:1916–1926. doi: 10.1002/ejhf.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Braidy N., Grant R., Adams S., Brew B.J., Guillemin G.J. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox. Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 145.Lugo-Huitrón R., Ugalde Muñiz P., Pineda B., Pedraza-Chaverrí J., Ríos C., Pérez-de la Cruz V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013;2013:104024. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wirthgen E., Hoeflich A., Rebl A., Günther J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018;8:1957. doi: 10.3389/fimmu.2017.01957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lim C.K., Essa M.M., de Paula Martins R., Lovejoy D.B., Bilgin A.A., Waly M.I., Al-Farsi Y.M., Al-Sharbati M., Al-Shaffae M.A., Guillemin G.J. Altered kynurenine pathway metabolism in autism: Implication for immune-induced glutamatergic activity. Autism Res. 2016;9:621–631. doi: 10.1002/aur.1565. [DOI] [PubMed] [Google Scholar]
- 148.Moffett J.R., Espey M.G., Gaudet S.J., Namboodiri M.A. Antibodies to quinolinic acid reveal localization in select immune cells rather than neurons or astroglia. Brain Res. 1993;623:337–340. doi: 10.1016/0006-8993(93)91450-7. [DOI] [PubMed] [Google Scholar]
- 149.Heyes M.P., Achim C.L., Wiley C.A., Major E.O., Saito K., Markey S.P. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996;320:595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guillemin G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–1365. doi: 10.1111/j.1742-4658.2012.08485.x. [DOI] [PubMed] [Google Scholar]
- 151.Liang Y., Xie S., He Y., Xu M., Qiao X., Zhu Y., Wu W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Markers. 2022;2022:9484217. doi: 10.1155/2022/9484217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myint A.M., Kim Y.K. Network beyond IDO in psychiatric disorders: Revisiting neurodegeneration hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 153.Venkatesan D., Iyer M., Narayanasamy A., Siva K., Vellingiri B. Kynurenine pathway in Parkinson’s disease—An update. Eneurologicalsci. 2020;21:100270. doi: 10.1016/j.ensci.2020.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hunt C., Macedo E., Cordeiro T., Suchting R., de Dios C., Cuellar Leal V.A., Soares J.C., Dantzer R., Teixeira A.L., Selvaraj S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020;118:514–523. doi: 10.1016/j.neubiorev.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhai L., Ladomersky E., Bell A., Dussold C., Cardoza K., Qian J., Lauing K.L., Wainwright D.A. Quantification of IDO1 enzyme activity in normal and malignant tissues. Methods Enzymol. 2019;629:235–256. doi: 10.1016/bs.mie.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silvano A., Seravallii V., Strambi N., Cecchi M., Tartarotti E., Parenti A., Di Tommaso M. Tryptophan metabolism and immune regulation in the human placenta. J. Reprod. Immunol. 2021;147:103361. doi: 10.1016/j.jri.2021.103361. [DOI] [PubMed] [Google Scholar]
- 157.Yamazaki F., Kuroiwa T., Takikawa O., Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution and characterization of the placental enzyme. Biochem. J. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Munn D.H., Sharma M.D., Hou D., Baban B., Lee J.R., Antonia S.J., Messina J.L., Chandler P., Koni P.A., Mellor A.L. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Investig. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Théate I., van Baren N., Pilotte L., Moulin P., Larrieu P., Renauld J.C., Hervé C., Gutierrez-Roelens I., Marbaix E., Sempoux C., et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol. Res. 2015;3:161–172. doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 160.Däubener W., Schmidt S.K., Heseler K., Spekker K.H., MacKenzie C.R. Antimicrobial and immunoregulatory effector mechanisms in human endothelial cells. Thromb. Haemost. 2009;102:1110–1116. doi: 10.1160/TH09-04-0250. [DOI] [PubMed] [Google Scholar]
- 161.Trabanelli S., Očadlíková D., Ciciarello M., Salvestrini V., Lecciso M., Jandus C., Metz R., Evangelisti C., Laury-Kleintop L., Romero P., et al. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J. Immunol. 2014;192:1231–1240. doi: 10.4049/jimmunol.1300720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo L., Schurink B., Roos E., Nossent E.J., Duitman J.W., Vlaar A.P.J., van der Valk P., Vaz F.M., Yeh S.-R., Geeraerts Z., et al. Indoleamine 2,3-dioxygenase (IDO)-1 and IDO-2 activity and severe course of COVID-19. J. Pathol. 2021;256:256–261. doi: 10.1002/path.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blaschitz A., Gauster M., Fuchs D., Lang I., Maschke P., Ulrich D., Karpf E., Takikawa O., Schimek M.G., Dohr G., et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS ONE. 2011;6:e21774. doi: 10.1371/journal.pone.0021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kudo Y., Koh I., Sugimoto J. Localization of Indoleamine 2,3-Dioxygenase-1 and Indoleamine 2,3-Dioxygenase-2 at the Human Maternal-Fetal Interface. Int. J. Tryptophan Res. 2020;13:1178646920984163. doi: 10.1177/1178646920984163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang X., Wei H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021;12:728291. doi: 10.3389/fimmu.2021.728291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zardoya-Laguardia P., Blaschitz A., Hirschmugl B., Lang I., Herzog S.A., Nikitina L., Gauster M., Häusler M., Cervar-Zivkovic M., Karpf E., et al. Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia. Sci. Rep. 2018;8:5488. doi: 10.1038/s41598-018-23896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishizawa H., Hasegawa K., Suzuki M., Kamoshida S., Kato T., Saito K., Tsutsumi Y., Kurahashi H., Udagawa Y. The etiological role of allogeneic fetal rejection in pre-eclampsia. Am. J. Reprod. Immunol. 2007;58:11–20. doi: 10.1111/j.1600-0897.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 168.Nishizawa H., Suzuki M., Pryor-Koishi K., Sekiya T., Tada S., Kurahashi H., Udagawa Y. Impact of indoleamine 2,3-dioxygenase on the antioxidant system in the placentas of severely pre-eclamptic patients. Syst. Biol. Reprod. Med. 2011;57:174–178. doi: 10.3109/19396368.2011.587590. [DOI] [PubMed] [Google Scholar]
- 169.Santillan M.K., Pelham C.J., Ketsawatsomkron P., Santillan D.A., Davis D.R., Devor E.J., Gibson-Corley K.N., Scroggins S.M., Grobe J.L., Yang B., et al. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol. Rep. 2015;3:e12257. doi: 10.14814/phy2.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Broekhuizen M., Danser A.H.J., Reiss I.K.M., Merkus D. The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target? Int. J. Environ. Res. Public Health. 2021;18:11545. doi: 10.3390/ijerph182111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Y., Liu H., McKenzie G., Witting P.K., Stasch J.P., Hahn M., Changsirivathanathamrong D., Wu B.J., Ball H.J., Thomas S.R., et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Worton S.A., Greenwood S.L., Wareing M., Heazell A.E., Myers J. The kynurenine pathway; A new target for treating maternal features of preeclampsia? Placenta. 2019;84:44–49. doi: 10.1016/j.placenta.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 173.Worton S.A., Pritchard H.A.T., Greenwood S.L., Alakrawi M., Heazell A.E.P., Wareing M., Greenstein A., Myers J.E. Kynurenine Relaxes Arteries of Normotensive Women and Those with Preeclampsia. Circ. Res. 2021;128:1679–1693. doi: 10.1161/CIRCRESAHA.120.317612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stanley C.P., Maghzal G.J., Ayer A., Talib J., Giltrap A.M., Shengule S., Wolhuter K., Wang Y., Chadha P., Suarna C., et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature. 2019;566:548–552. doi: 10.1038/s41586-019-0947-3. [DOI] [PubMed] [Google Scholar]
- 175.Kass D.A. Fresh evidence overturns the identification of a factor involved in blood-vessel dilation. Nature. 2019;566:462–464. doi: 10.1038/d41586-019-00508-z. [DOI] [PubMed] [Google Scholar]
- 176.Watts S.W., Shaw S., Burnett R., Dorrance A.M. Indoleamine 2,3-diooxygenase in periaortic fat: Mechanisms of inhibition of contraction. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1236–H1247. doi: 10.1152/ajpheart.00384.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Struijk P.C., Mathews V.J., Loupas T., Stewart P.A., Clark E.B., Steegers E.A., Wladimiroff J.W. Blood pressure estimation in the human fetal descending aorta. Ultrasound Obstet. Gynecol. 2008;32:673–681. doi: 10.1002/uog.6137. [DOI] [PubMed] [Google Scholar]
- 178.Xiao Y., Christou H., Liu L., Visner G., Mitsialis S.A., Kourembanas S., Liu H. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2013;188:482–491. doi: 10.1164/rccm.201304-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jasiewicz M., Moniuszko M., Pawlak D., Knapp M., Rusak M., Kazimierczyk R., Musial W.J., Dabrowska M., Kaminski K.A. Activity of the kynurenine pathway and its interplay with immunity in patients with pulmonary arterial hypertension. Heart. 2016;102:230–237. doi: 10.1136/heartjnl-2015-308581. [DOI] [PubMed] [Google Scholar]
- 180.Nagy B.M., Nagaraj C., Meinitzer A., Sharma N., Papp R., Foris V., Ghanim B., Kwapiszewska G., Kovacs G., Klepetko W., et al. Importance of kynurenine in pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313:L741–L751. doi: 10.1152/ajplung.00517.2016. [DOI] [PubMed] [Google Scholar]
- 181.Pellegrin K., Neurauter G., Wirleitner B., Fleming A.W., Peterson V.M., Fuchs D. Enhanced enzymatic degradation of tryptophan by indoleamine 2,3-dioxygenase contributes to the tryptophan-deficient state seen after major trauma. Shock. 2005;23:209–215. [PubMed] [Google Scholar]
- 182.Jung I.D., Lee M.G., Chang J.H., Lee J.S., Jeong Y.I., Lee C.M., Park W.S., Han J., Seo S.K., Lee S.Y., et al. Blockade of indoleamine 2,3-dioxygenase protects mice against lipopolysaccharide-induced endotoxin shock. J. Immunol. 2009;182:3146–3154. doi: 10.4049/jimmunol.0803104. [DOI] [PubMed] [Google Scholar]
- 183.Changsirivathanathamrong D., Wang Y., Rajbhandari D., Maghzal G.J., Mak W.M., Woolfe C., Duflou J., Gebski V., dos Remedios C.G., Celermajer D.S., et al. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit. Care Med. 2011;39:2678–2683. doi: 10.1097/CCM.0b013e31822827f2. [DOI] [PubMed] [Google Scholar]
- 184.Darcy C.J., Davis J.S., Woodberry T., McNeil Y.R., Stephens D.P., Yeo T.W., Anstey N.M. An observational cohort study of the kynurenine to tryptophan ratio in sepsis: Association with impaired immune and microvascular function. PLoS ONE. 2011;6:e21185. doi: 10.1371/journal.pone.0021185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pedberg J.S., Van Meurs M., Kielstein J.T., Martens-Lobenhoffer J., Bode-Böger S.M., Zijlstra J.G., Kovesdy C.P., Kümpers P. Indoleamine-2,3-dioxygenase activity in experimental human endotoxemia. Exp. Transl. Stroke Med. 2012;4:24. doi: 10.1186/2040-7378-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu H., Liu L., Visner G.A. Nonviral gene delivery with indoleamine 2,3-dioxygenase targeting pulmonary endothelium protects against ischemia-reperfusion injury. Am. J. Transplant. 2007;7:2291–2300. doi: 10.1111/j.1600-6143.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- 187.Cole J.E., Astola N., Cribbs A.P., Goddard M.E., Park I., Green P., Davies A.H., Williams R.O., Feldmann M., Monaco C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc. Natl. Acad. Sci. USA. 2015;112:13033–13038. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Forteza M.J., Polyzos K.A., Baumgartner R., Suur B.E., Mussbacher M., Johansson D.K., Hermansson A., Hansson G.K., Ketelhuth D.F.J. Activation of the Regulatory T-Cell/Indoleamine 2,3-Dioxygenase Axis Reduces Vascular Inflammation and Atherosclerosis in Hyperlipidemic Mice. Front. Immunol. 2018;9:950. doi: 10.3389/fimmu.2018.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wolowczuk I., Hennart B., Leloire A., Bessede A., Soichot M., Taront S., Caiazzo R., Raverdy V., Pigeyre M., ABOS Consortium et al. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: An attempt to maintain immune homeostasis and vascular tone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R135–R143. doi: 10.1152/ajpregu.00373.2011. [DOI] [PubMed] [Google Scholar]
- 190.Lee S.M., Park H.Y., Suh Y.S., Yoon E.H., Kim J., Jang W.H., Lee W.S., Park S.G., Choi I.W., Choi I., et al. Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc. Natl. Acad. Sci. USA. 2017;114:E5881–E5890. doi: 10.1073/pnas.1615280114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z., Neil D., Hoefer I.E., Fragiadaki M., Waltenberger J., et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Potus F., Mai V., Lebret M., Malenfant S., Breton-Gagnon E., Lajoie A.C., Boucherat O., Bonnet S., Provencher S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2020;319:L277–L288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mejia-Renteria H., Travieso A., Sagir A., Martínez-Gómez E., Carrascosa-Granada A., Toya T., Núñez-Gil I.J., Estrada V., Lerman A., Escaned J. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int. J. Cardiol. 2021;345:153–155. doi: 10.1016/j.ijcard.2021.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nägele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mangalmurti N.S., Reilly J.P., Cines D.B., Meyer N.J., Hunter C.A., Vaughan A.E. COVID-19-associated Acute Respiratory Distress Syndrome Clarified: A Vascular Endotype? Am. J. Respir. Crit. Care Med. 2020;202:750–753. doi: 10.1164/rccm.202006-2598LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maruhashi T., Higashi Y. Pathophysiological Association of Endothelial Dysfunction with Fatal Outcome in COVID-19. Int. J. Mol. Sci. 2021;22:5131. doi: 10.3390/ijms22105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cimino G., Vizzardi E., Calvi E., Pancaldi E., Pascariello G., Bernardi N., Cersosimo A., Amore L., Inciardi R.M., Raddino R., et al. Endothelial dysfunction in COVID-19 patients assessed with Endo-PAT2000. Monaldi Arch. Chest Dis. 2022 doi: 10.4081/monaldi.2022.2213. [DOI] [PubMed] [Google Scholar]
- 199.Chen A.T., Wang C.Y., Zhu W.L., Chen W. Coagulation Disorders and Thrombosis in COVID-19 Patients and a Possible Mechanism Involving Endothelial Cells: A Review. Aging Dis. 2022;13:144–156. doi: 10.14336/AD.2021.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bernard I., Limonta D., Mahal L.K., Hobman T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses. 2020;13:29. doi: 10.3390/v13010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., et al. Pulmonary Vascular Endothelialitis, Thrombosis and Angiogenesis in COVID-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McCracken I.R., Saginc G., He L., Huseynov A., Daniels A., Fletcher S., Peghaire C., Kalna V., Andaloussi-Mäe M., Muhl L., et al. Lack of Evidence of Angiotensin-Converting Enzyme 2 Expression and Replicative Infection by SARS-CoV-2 in Human Endothelial Cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rauch A., Dupont A., Goutay J., Caplan M., Staessens S., Moussa M., Jeanpierre E., Corseaux D., Lefevre G., Lassalle F., et al. Endotheliopathy Is Induced by Plasma from Critically Ill Patients and Associated with Organ Failure in Severe COVID-19. Circulation. 2020;142:1881–1884. doi: 10.1161/CIRCULATIONAHA.120.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Suzuki Y.J., Nikolaienko S.I., Dibrova V.A., Dibrova Y.V., Vasylyk V.M., Novikov M.Y., Shults N.V., Gychka S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul. Pharmacol. 2021;137:106823. doi: 10.1016/j.vph.2020.106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Khan S., Shafiei M.S., Longoria C., Schoggins J.W., Savani R.C., Zaki H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife. 2021;10:e68563. doi: 10.7554/eLife.68563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Raghavan S., Kenchappa D.B., Leo M.D. SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins that Maintain Endothelial Barrier Integrity. Front. Cardiovasc. Med. 2021;8:687783. doi: 10.3389/fcvm.2021.687783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olajide O.A., Iwuanyanwu V.U., Lepiarz-Raba I., Al-Hindawi A.A. Induction of Exaggerated Cytokine Production in Human Peripheral Blood Mononuclear Cells by a Recombinant SARS-CoV-2 Spike Glycoprotein S1 and Its Inhibition by Dexamethasone. Inflammation. 2021;44:1865–1877. doi: 10.1007/s10753-021-01464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Robles J.P., Zamora M., Adan-Castro E., Siqueiros-Marquez L., Martinez de la Escalera G., Clapp C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin α5β1 and NF-κB signaling. J. Biol. Chem. 2022;298:101695. doi: 10.1016/j.jbc.2022.101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lei Y., Zhang J., Schiavon C.R., He M., Chen L., Shen H., Zhang Y., Yin Q., Cho Y., Andrade L., et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021;128:1323–1326. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim E.S., Jeon M.T., Kim K.S., Lee S., Kim S., Kim D.G. Spike Proteins of SARS-CoV-2 Induce Pathological Changes in Molecular Delivery and Metabolic Function in the Brain Endothelial Cells. Viruses. 2021;13:2021. doi: 10.3390/v13102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Julliard W., Fechner J.H., Mezrich J.D. The aryl hydrocarbon receptor meets immunology: Friend or foe? A little of both. Front. Immunol. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Poulain-Godefroy O., Bouté M., Carrard J., Alvarez-Simon D., Tsicopoulos A., de Nadai P. The Aryl Hydrocarbon Receptor in Asthma: Friend or Foe? Int. J. Mol. Sci. 2020;21:8797. doi: 10.3390/ijms21228797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Takei H., Yasuoka H., Yoshimoto K., Takeuchi T. Aryl hydrocarbon receptor signals attenuate lung fibrosis in the bleomycin-induced mouse model for pulmonary fibrosis through increase of regulatory T cells. Arthritis Res. Ther. 2020;22:20. doi: 10.1186/s13075-020-2112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wu S.M., Tsai J.J., Pan H.C., Arbiser J.L., Elia L., Sheu M.L. Aggravation of pulmonary fibrosis after knocking down the aryl hydrocarbon receptor in the insulin-like growth factor 1 receptor pathway. Br. J. Pharmacol. 2022 doi: 10.1111/bph.15806. [DOI] [PubMed] [Google Scholar]
- 218.Engin A.B., Engin E.D., Engin A. The effect of environmental pollution on immune evasion checkpoints of SARS-CoV-2. Environ. Toxicol. Pharmacol. 2021;81:103520. doi: 10.1016/j.etap.2020.103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Anderson G., Carbone A., Mazzoccoli G. Tryptophan Metabolites and Aryl Hydrocarbon Receptor in Severe Acute Respiratory Syndrome, Coronavirus-2 (SARS-CoV-2) Pathophysiology. Int. J. Mol. Sci. 2021;22:1597. doi: 10.3390/ijms22041597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ball H.J., Yuasa H.J., Austin C.J., Weiser S., Hunt N.H. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int. J. Biochem. Cell Biol. 2009;41:467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 221.Hansen A.M., Ball H.J., Mitchell A.J., Miu J., Takikawa O., Hunt N.H. Increased expression of indoleamine 2,3-dioxygenase in murine malaria infection is predominantly localised to the vascular endothelium. Int. J. Parasitol. 2004;34:1309–1319. doi: 10.1016/j.ijpara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 222.Fox J.M., Crabtree J.M., Sage L.K., Tompkins S.M., Tripp R.A. Interferon Lambda Upregulates IDO1 Expression in Respiratory Epithelial Cells after Influenza Virus Infection. J. Interferon Cytokine Res. 2015;35:554–562. doi: 10.1089/jir.2014.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Metz R., Duhadaway J.B., Kamasani U., Laury-Kleintop L., Muller A.J., Prendergast G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 224.Yamamoto Y., Yamasuge W., Imai S., Kunisawa K., Hoshi M., Fujigaki H., Mouri A., Nabeshima T., Saito K. Lipopolysaccharide shock reveals the immune function of indoleamine 2,3-dioxygenase 2 through the regulation of IL-6/stat3 signalling. Sci. Rep. 2018;8:15917. doi: 10.1038/s41598-018-34166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Belladonna M.L., Orabona C. Potential Benefits of Tryptophan Metabolism to the Efficacy of Tocilizumab in COVID-19. Front. Pharmacol. 2020;11:959. doi: 10.3389/fphar.2020.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S., Batani V., Trovato R., Fiore A., Petrova V., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grunewald M.E., Shaban M.G., Mackin S.R., Fehr A.R., Perlman S. Murine Coronavirus Infection Activates the Aryl Hydrocarbon Receptor in an Indoleamine 2,3-Dioxygenase-Independent Manner, Contributing to Cytokine Modulation and Proviral TCDD-Inducible-PARP Expression. J. Virol. 2020;94:e01743–19. doi: 10.1128/JVI.01743-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fukunaga M., Yamamoto Y., Kawasoe M., Arioka Y., Murakami Y., Hoshi M., Saito K. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: The absence of IDO1 upregulates IDO2 expression in the epididymis. J. Histochem. Cytochem. 2012;60:854–860. doi: 10.1369/0022155412458926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nakagawa K., Kobayashi F., Kamei Y., Tawa M., Ohkita M. Acute Kynurenine Exposure of Rat Thoracic Aorta Induces Vascular Dysfunction via Superoxide Anion Production. Biol. Pharm. Bull. 2022;45:522–527. doi: 10.1248/bpb.b21-01079. [DOI] [PubMed] [Google Scholar]
- 231.Hadi H.A., Carr C.S., Al Suwaidi J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005;1:183–198. [PMC free article] [PubMed] [Google Scholar]
- 232.Wang Q., Zhang M., Ding Y., Wang Q., Zhang W., Song P., Zou M.H. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ. Res. 2014;114:480–492. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Karmouty-Quintana H., Thandavarayan R.A., Keller S.P., Sahay S., Pandit L.M., Akkanti B. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020;21:8081. doi: 10.3390/ijms21218081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nikolaidis A., Kramer R., Ostojic S. Nitric Oxide: The Missing Factor in COVID-19 Severity? Med. Sci. 2021;10:3. doi: 10.3390/medsci10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Montiel V., Lobysheva I., Gérard L., Vermeersch M., Perez-Morga D., Castelein T., Mesland J.B., Hantson P., Collienne C., Gruson D., et al. Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients. EBioMedicine. 2022;77:103893. doi: 10.1016/j.ebiom.2022.103893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kamenshchikov N.O., Berra L., Carroll R.W. Therapeutic Effects of Inhaled Nitric Oxide Therapy in COVID-19 Patients. Biomedicines. 2022;10:369. doi: 10.3390/biomedicines10020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Samelson-Jones B.J., Yeh S.R. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry. 2006;45:8527–8538. doi: 10.1021/bi060143j. [DOI] [PubMed] [Google Scholar]
- 238.Melillo G., Cox G.W., Biragyn A., Sheffler L.A., Varesio L. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J. Biol. Chem. 1994;269:8128–8133. doi: 10.1016/S0021-9258(17)37169-7. [DOI] [PubMed] [Google Scholar]
- 239.Lim Y.J., Foo T.C., Yeung A.W.S., Tu X., Ma Y., Hawkins C.L., Witting P.K., Jameson G.N.L., Terentis A.C., Thomas S.R. Human Indoleamine 2,3-Dioxygenase 1 Is an Efficient Mammalian Nitrite Reductase. Biochemistry. 2019;58:974–986. doi: 10.1021/acs.biochem.8b01231. [DOI] [PubMed] [Google Scholar]
- 240.Prendergast G.C., Metz R., Muller A.J., Merlo L.M., Mandik-Nayak L. IDO2 in Immunomodulation and Autoimmune Disease. Front. Immunol. 2014;5:585. doi: 10.3389/fimmu.2014.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yan Y., Zhang G.X., Gran B., Fallarino F., Yu S., Li H., Cullimore M.L., Rostami A., Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wetzel L.A., Hurtado M., MacDowell Kaswan Z.A., McCusker R.H., Steelman A.J. Deletion of indoleamine 2,3 dioxygenase (Ido)1 but not Ido2 exacerbates disease symptoms of MOG35–55-induced experimental autoimmune encephalomyelitis. Brain Behav. Immun. Health. 2020;7:100116. doi: 10.1016/j.bbih.2020.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Duan R.N., Yang C.L., Du T., Liu A., Wang A.R., Sun W.J., Li X., Li J.X., Yan C.Z., Liu Q.J. Smek1 deficiency exacerbates experimental autoimmune encephalomyelitis by activating proinflammatory microglia and suppressing the IDO1-AhR pathway. J. Neuroinflammation. 2021;18:145. doi: 10.1186/s12974-021-02193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Correale J. Immunosuppressive Amino-Acid Catabolizing Enzymes in Multiple Sclerosis. Front. Immunol. 2021;11:600428. doi: 10.3389/fimmu.2020.600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhou Y., Han T., Chen J., Hou C., Hua L., He S., Guo Y., Zhang S., Wang Y., Yuan J., et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin. Transl. Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021;33:155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lv H., Chen T., Pan Y., Wang H., Chen L., Lu Y. Pulmonary vascular enlargement on thoracic CT for diagnosis and differential diagnosis of COVID-19: A systematic review and meta-analysis. Ann. Transl. Med. 2020;8:878. doi: 10.21037/atm-20-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Piciucchi S., Ravaglia C., Vizzuso A., Bertocco M., Poletti V. Reversibility of venous dilatation and parenchymal changes density in Sars-Cov-2 pneumonia: Toward the definition of a peculiar pattern. Pulmonology. 2021;27:353–357. doi: 10.1016/j.pulmoe.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., Camporota L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Simonato M., Dall’Acqua S., Zilli C., Sut S., Tenconi R., Gallo N., Sfriso P., Sartori L., Cavallin F., Fiocco U., et al. Tryptophan Metabolites, Cytokines, and Fatty Acid Binding Protein 2 in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomedicines. 2021;9:1724. doi: 10.3390/biomedicines9111724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Komaroff A.L., Lipkin W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021;27:895–906. doi: 10.1016/j.molmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Phetsouphanh C., Darley D.R., Wilson D.B., Howe A., Munier C.M.L., Patel S.K., Juno J.A., Burrell L.M., Kent S.J., Dore G.J., et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022;23:210–216. doi: 10.1038/s41590-021-01113-x. [DOI] [PubMed] [Google Scholar]
- 254.Shen T., Wang T. Metabolic Reprogramming in COVID-19. Int. J. Mol. Sci. 2021;22:11475. doi: 10.3390/ijms222111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Adebayo A., Varzideh F., Wilson S., Gambardella J., Eacobacci M., Jankauskas S.S., Donkor K., Kansakar U., Trimarco V., Mone P., et al. l-Arginine and COVID-19: An Update. Nutrients. 2021;13:3951. doi: 10.3390/nu13113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rees C.A., Rostad C.A., Mantus G., Anderson E.J., Chahroudi A., Jaggi P., Wrammert J., Ochoa J.B., Ochoa A., Basu R.K., et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA. 2021;118:e2101708118. doi: 10.1073/pnas.2101708118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Young S.N., Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol. Biochem. Behav. 2002;71:857–865. doi: 10.1016/S0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 258.Fernstrom J.D. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J. Nutr. 2012;142:2236S–2244S. doi: 10.3945/jn.111.157065. [DOI] [PubMed] [Google Scholar]
- 259.Mohajeri M.H., Wittwer J., Vargas K., Hogan E., Holmes A., Rogers P.J., Goralczyk R., Gibson E.L. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br. J. Nutr. 2015;113:350–365. doi: 10.1017/S0007114514003754. [DOI] [PubMed] [Google Scholar]
- 260.Steenbergen L., Jongkees B.J., Sellaro R., Colzato L.S. Tryptophan supplementation modulates social behavior: A review. Neurosci. Biobehav. Rev. 2016;64:346–358. doi: 10.1016/j.neubiorev.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 261.Gibson E.L., Vargas K., Hogan E., Holmes A., Rogers P.J., Wittwer J., Kloek J., Goralczyk R., Mohajeri M.H. Effects of acute treatment with a tryptophan-rich protein hydrolysate on plasma amino acids, mood and emotional functioning in older women. Psychopharmacology. 2014;231:4595–4610. doi: 10.1007/s00213-014-3609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gibson E.L. Tryptophan supplementation and serotonin function: Genetic variations in behavioural effects. Proc. Nutr. Soc. 2018;77:174–188. doi: 10.1017/S0029665117004451. [DOI] [PubMed] [Google Scholar]
- 263.Chojnacki C., Popławski T., Chojnacki J., Fila M., Konrad P., Blasiak J. Tryptophan Intake and Metabolism in Older Adults with Mood Disorders. Nutrients. 2020;12:3183. doi: 10.3390/nu12103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kikuchi A.M., Tanabe A., Iwahori Y. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet Suppl. 2021;18:316–333. doi: 10.1080/19390211.2020.1746725. [DOI] [PubMed] [Google Scholar]
- 265.Zamoscik V., Schmidt S.N.L., Bravo R., Ugartemendia L., Plieger T., Rodríguez A.B., Reuter M., Kirsch P. Tryptophan-enriched diet or 5-hydroxytryptophan supplementation given in a randomized controlled trial impacts social cognition on a neural and behavioral level. Sci. Rep. 2021;11:21637. doi: 10.1038/s41598-021-01164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Holeček M. Side effects of amino acid supplements. Physiol. Res. 2022;71:29. doi: 10.33549/physiolres.934790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Valente-Silva P., Cervenka I., Ferreira D.M.S., Correia J.C., Edman S., Horwath O., Heng B., Chow S., Jacobs K.R., Guillemin G.J., et al. Effects of Tryptophan Supplementation and Exercise on the Fate of Kynurenine Metabolites in Mice and Humans. Metabolites. 2021;11:508. doi: 10.3390/metabo11080508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sun C., Sun Y., Wu P., Ding W., Wang S., Li J., Liang L., Chai C., Fu Y., Li Z., et al. Longitudinal multi-omics transition associated with fatality in critically ill COVID-19 patients. Intensive Care Med. Exp. 2021;9:13. doi: 10.1186/s40635-021-00373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shukla M., Chinchalongporn V., Govitrapong P., Reiter R.J. The role of melatonin in targeting cell signaling pathways in neurodegeneration. Ann. N.Y. Acad. Sci. 2019;1443:75–96. doi: 10.1111/nyas.14005. [DOI] [PubMed] [Google Scholar]
- 270.Jauhari A., Baranov S.V., Suofu Y., Kim J., Singh T., Yablonska S., Li F., Wang X., Oberly P., Minnigh M.B., et al. Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Investig. 2020;130:3124–3136. doi: 10.1172/JCI135026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Luo F., Sandhu A.F., Rungratanawanich W., Williams G.E., Akbar M., Zhou S., Song B.J., Wang X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020;21:7174. doi: 10.3390/ijms21197174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Maher A.M., Saleh S.R., Elguindy N.M., Hashem H.M., Yacout G.A. Exogenous melatonin restrains neuroinflammation in high fat diet induced diabetic rats through attenuating indoleamine 2,3-dioxygenase 1 expression. Life Sci. 2020;247:117427. doi: 10.1016/j.lfs.2020.117427. [DOI] [PubMed] [Google Scholar]
- 273.Tang Y., Groom K., Chamley L., Chen Q. Melatonin, a Potential Therapeutic Agent for Preeclampsia, Reduces the Extrusion of Toxic Extracellular Vesicles from Preeclamptic Placentae. Cells. 2021;10:1904. doi: 10.3390/cells10081904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bologna C., Madonna P., Pone E. Efficacy of Prolonged-Release Melatonin 2 mg (PRM 2 mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study. J. Clin. Med. 2021;10:5857. doi: 10.3390/jcm10245857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jarrott B., Head R., Pringle K.G., Lumbers E.R., Martin J.H. “LONG COVID”-A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022;10:e00911. doi: 10.1002/prp2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sen A. Deficient synthesis of melatonin in COVID-19 can impair the resistance of coronavirus patients to mucormycosis. Med. Hypotheses. 2021;158:110722. doi: 10.1016/j.mehy.2021.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Brown G.M., Pandi-Perumal S.R., Pupko H., Kennedy J.L., Cardinali D.P. Melatonin as an Add-On Treatment of COVID-19 Infection: Current Status. Diseases. 2021;9:64. doi: 10.3390/diseases9030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shchetinin E., Baturin V., Arushanyan E., Bolatchiev A., Bobryshev D. Potential and Possible Therapeutic Effects of Melatonin on SARS-CoV-2 Infection. Antioxidants. 2022;11:140. doi: 10.3390/antiox11010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tan D.X., Reiter R.J. Mechanisms and clinical evidence to support melatonin’s use in severe COVID-19 patients to lower mortality. Life Sci. 2022;294:120368. doi: 10.1016/j.lfs.2022.120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lan S.H., Lee H.Z., Chao C.M., Chang S.P., Lu L.C., Lai C.C. Efficacy of melatonin in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. J. Med. Virol. 2022;94:2102–2107. doi: 10.1002/jmv.27595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hasan Z.T., Mal Allah Al Atrakji M.Q.Y., Mehuaiden D.A.K. The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients. Int. J. Infect. Dis. 2022;114:79–84. doi: 10.1016/j.ijid.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Essa M.M., Hamdan H., Chidambaram S.B., Al-Balushi B., Guillemin G.J., Ojcius D.M., Qoronfleh M.W. Possible role of tryptophan and melatonin in COVID-19. Int. J. Tryptophan Res. 2020;13:1178646920951832. doi: 10.1177/1178646920951832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kleszczyński K., Slominski A.T., Steinbrink K., Reiter R.J. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients. 2020;12:2561. doi: 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rodríguez-Rubio M., Figueira J.C., Acuña-Castroviejo D., Borobia A.M., Escames G., de la Oliva P. A phase II, single-center, double-blind, randomized placebo-controlled trial to explore the efficacy and safety of intravenous melatonin in patients with COVID-19 admitted to the intensive care unit (MelCOVID study): A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:699. doi: 10.1186/s13063-020-04632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ziaei A., Davoodian P., Dadvand H., Safa O., Hassanipour S., Omidi M., Masjedi M., Mahmoudikia F., Rafiee B., Fathalipour M. Evaluation of the efficacy and safety of Melatonin in moderately ill patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21:882. doi: 10.1186/s13063-020-04737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Iwashita H., Matsumoto Y., Maruyama Y., Watanabe K., Chiba A., Hattori A. The melatonin metabolite N1-acetyl-5-methoxykynuramine facilitates long-term object memory in young and aging mice. J. Pineal Res. 2021;70:e12703. doi: 10.1111/jpi.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Song Z., Bao L., Deng W., Liu J., Ren E., Lv Q., Liu M., Qi F., Chen T., Deng R., et al. Integrated histopathological, lipidomic, and metabolomic profiles reveal mink is a useful animal model to mimic the pathogenicity of severe COVID-19 patients. Signal Transduct. Target. Ther. 2022;7:29. doi: 10.1038/s41392-022-00891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schefold J.C., Zeden J.P., Pschowski R., Hammoud B., Fotopoulou C., Hasper D., Fusch G., Von Haehling S., Volk H.D., Meisel C., et al. Treatment with granulocyte-macrophage colony-stimulating factor is associated with reduced indoleamine 2,3-dioxygenase activity and kynurenine pathway catabolites in patients with severe sepsis and septic shock. Scand. J. Infect. Dis. 2010;42:164–171. doi: 10.3109/00365540903405768. [DOI] [PubMed] [Google Scholar]
- 289.Singh R., Salunke D.B. Diverse chemical space of indoleamine-2,3-dioxygenase 1 (Ido1) inhibitors. Eur. J. Med. Chem. 2021;211:113071. doi: 10.1016/j.ejmech.2020.113071. [DOI] [PubMed] [Google Scholar]
- 290.Zhai L., Spranger S., Binder D.C., Gritsina G., Lauing K.L., Giles F.J., Wainwright D.A. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin. Cancer Res. 2015;21:5427–5433. doi: 10.1158/1078-0432.CCR-15-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kim M., Tomek P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front. Immunol. 2021;12:636081. doi: 10.3389/fimmu.2021.636081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Breda C., Sathyasaikumar K.V., Sograte Idrissi S., Notarangelo F.M., Estranero J.G., Moore G.G., Green E.W., Kyriacou C.P., Schwarcz R., Giorgini F. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc. Natl. Acad. Sci. USA. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Brochez L., Chevolet I., Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer. 2017;76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 294.Cheong J.E., Ekkati A., Sun L. A patent review of IDO1 inhibitors for cancer. Expert Opin. Ther. Pat. 2018;28:317–330. doi: 10.1080/13543776.2018.1441290. [DOI] [PubMed] [Google Scholar]
- 295.Cheong J.E., Sun L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy—Challenges and Opportunities. Trends Pharmacol. Sci. 2018;39:307–325. doi: 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 296.Bakmiwewa S.M., Fatokun A.A., Tran A., Payne R.J., Hunt N.H., Ball H.J. Identification of selective inhibitors of indoleamine 2,3-dioxygenase 2. Bioorg. Med. Chem. Lett. 2012;22:7641–7646. doi: 10.1016/j.bmcl.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 297.Feng X., Shen P., Wang Y., Li Z., Bian J. Synthesis and in vivo antitumor evaluation of an orally active potent phosphonamidate derivative targeting IDO1/IDO2/TDO. Biochem. Pharmacol. 2019;168:214–223. doi: 10.1016/j.bcp.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 298.Capochiani de Iudicibus R., Tomek P., Palmer B.D., Tijono S.M., Flanagan J.U., Ching L.M. Parallel discovery of selective and dual inhibitors of tryptophan dioxygenases IDO1 and TDO2 with a newly-modified enzymatic assay. Bioorg. Med. Chem. 2021;39:116160. doi: 10.1016/j.bmc.2021.116160. [DOI] [PubMed] [Google Scholar]
- 299.He X., He G., Chu Z., Wu H., Wang J., Ge Y., Shen H., Zhang S., Shan J., Peng K., et al. Discovery of the First Potent IDO1/IDO2 Dual Inhibitors: A Promising Strategy for Cancer Immunotherapy. J. Med. Chem. 2021;64:17950–17968. doi: 10.1021/acs.jmedchem.1c01305. [DOI] [PubMed] [Google Scholar]
- 300.Nguyen C., Edgley A.J., Kelly D.J., Kompa A.R. Aryl Hydrocarbon Receptor Inhibition Restores Indoxyl Sulfate-Mediated Endothelial Dysfunction in Rat Aortic Rings. Toxins. 2022;14:100. doi: 10.3390/toxins14020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.