Abstract
Polyphenols, which are probably the most important secondary metabolites produced by plants, have attracted tremendous attention due to their health-promoting effects, including their antioxidant, anti-inflammatory, antibacterial, anti-adipogenic, and neuro-protective activities, as well as health properties. However, due to their complicated structures and high molecular weights, a large proportion of dietary polyphenols remain unabsorbed along the gastrointestinal tract, while in the large intestine they are biotransformed into bioactive, low-molecular-weight phenolic metabolites through the residing gut microbiota. Dietary polyphenols can modulate the composition of intestinal microbes, and in turn, gut microbes catabolize polyphenols to release bioactive metabolites. To better investigate the health benefits of dietary polyphenols, this review provides a summary of their modulation through in vitro and in vivo evidence (animal models and humans), as well as their possible actions through intestinal barrier function and gut microbes. This review aims to provide a basis for better understanding the relationship between dietary polyphenols, gut microbiota, and host health.
Keywords: dietary polyphenols, host health, gut microbiota, biotransformation
1. Introduction
Polyphenols, described as plants’ secondary metabolites, are probably the most abundant antioxidants in our daily life. The main dietary sources of these compounds include fruits, vegetables, grains, green tea, coffee, etc. [1]. Total dietary polyphenol intake is as high as 1 g per day for each adult, which is about 10-times higher than the intake of Vitamin C, and even 100-times higher than that of Vitamin E and carotenoids [2]. During the last few decades, there has been tremendous research output related to the health-promoting effects of polyphenols, including their antioxidant, anti-inflammatory, antibacterial, anti-adipogenic, and neuro-protective activities [3,4].
It has been reported that most dietary polyphenol intake remains unabsorbed in the small intestine, while the unabsorbed parts may accumulate in the large intestine and are extensively metabolized by the gut microbiota [5]. Therefore, intestinal microbiota play an important role in the biotransformation and metabolism of the original polyphenolic structures into low-molecular-weight metabolites, which can be readily absorbed and contribute to host healthy benefits. However, little is currently known regarding the possible mechanism among dietary polyphenols, gut microbes, and host health.
Dietary polyphenols influence gut microbiota compositions in the host, which further affect the host’s metabolism. In turn, intestinal microbiota can metabolize polyphenols into bioactive, low-molecular-weight phenolic metabolites to modulate the regulatory metabolism network. In this regard, this review aims to provide an assessment of dietary polyphenols’ biological significances on host health, a summary of their modulation through in vitro and in vivo evidence (animal models and humans), as well as their possible action through intestinal barrier function and gut microbes.
2. Dietary Polyphenols and Their Sources
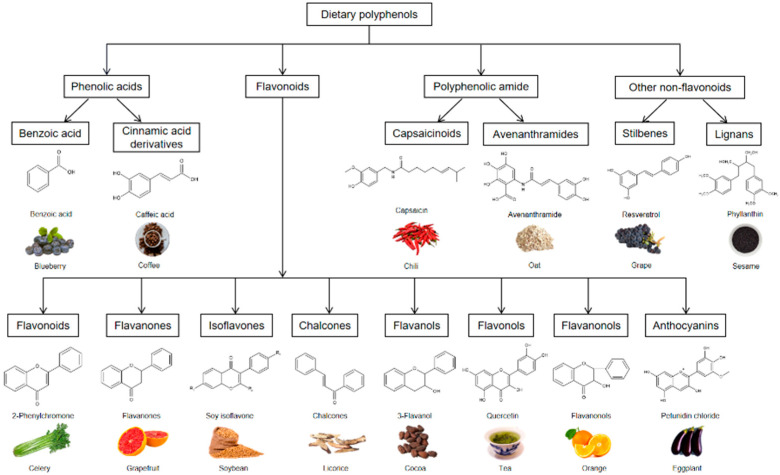
Dietary polyphenols are one of the most abundant and widely distributed natural products in plants. At present, according to the structure, dietary polyphenols are divided into four categories: phenolic acids, flavonoids (the largest subclass of polyphenols), polyphenolic amide, and other non-flavonoids (Figure 1). Phenolic acids can be further divided into two main types, benzoic acid and cinnamic acid derivatives based on C1–C6 and C3–C6 backbones [6]. Flavonoids include flavonoids, flavanones, isoflavones, chalcones, flavanols, flavonols, flavanonols, anthocyanins, and so on [7]. Polyphenolic amides have N-containing functional substituents, two such groups are capsaicinoids and avenanthramides. The non-flavonoids include mainly stilbenes and lignans. In addition to phenolic acids, flavonoids, and phenolic amides, there are several non-flavonoid polyphenols found in foods that are considered important to human health, such as resveratrol, ellagic acid and its derivatives, curcumin, etc. The remarkable feature of the chemical structure is that it has a different amount of phenolic hydroxyl groups, which can be divided into phenolic monomers and polymerized polyphenols. Phenolic monomers include flavonoids and non-flavonoids. The former generally involves a common carbon skeleton of diphenyl propane in which two benzene rings are connected by a linear three-carbon chain, while the latter is two benzene rings connected by the vinyl group [8]. Polymeric polyphenols are oligomers or polymers polymerized by monomers known as tannins.
Figure 1.
Classification of dietary polyphenols and their sources.
Polyphenols are widely distributed in nature, including in fruits, vegetables, cereals, beans, tea, coffee, honey, and red wine, which are the main sources of dietary polyphenols. Specifically, caffeic acid and ferulic acid are the most common phenolic acids in food. Caffeic acid is abundant in vegetables, fruits, and coffee, while ferulic acid is mainly distributed in rice bran, wheat bran, and other cereals. Among the flavonols, quercetin is the most common, which is commonly found in onions. Flavanols or flavan-3-ols are often commonly called catechins, which are abundant in red wine, chocolate, and lotus root. Isoflavones are mainly found in the leguminous family of plants. Anthocyanidins in plants mainly exist in glycosidic forms, which are commonly referred to as anthocyanins [9] and are largely distributed in strawberries, blueberries, and cherries. Some polyphenols have N-containing functional substituents, such as capsaicinoids in chili peppers and avenanthramides in oats, which belong to polyphenolic amides. The second major non-flavonoid group mainly consists of stilbenes, with resveratrol being the main representative, which is found in red and purple grape skins and grape wine. Another important nonflavonoid group is the lignans, which exist in bound forms in flax, sesame, and many grains.
3. Dietary Polyphenols and Their Biological Significance
As the most general plant-derived bioactive components in our diet, dietary polyphenols have received tremendous attention among nutritionists, food researchers, and consumers. Phenolic compounds are generally involved in defenses against plant pathogens and atmospheric agents, including bacteria, fungi, and viruses, and many abiotic stresses like drought, salinity, and UV. Polyphenols exhibit antimicrobial and antioxidant properties that can help plants to evade pathogenic infections and, at the same time, protect the major tissues from the toxic effects of reactive oxygen species [10]. Currently, they represent a topic of great scientific attention due to interest in their biological significance for humans. Both in vitro and in vivo studies have shown their health-promoting effects, including their antioxidant, anti-inflammatory, antibacterial, anti-adipogenic, and neuro-protective activities.
3.1. Antioxidant Properties
The effectiveness of phenolic compounds in the inhibition of oxidative processes is potentially related to their reactive species scavenging activity. Due to the structure of the hydroxyl group on the benzene ring, polyphenols scavenge free radicals by H-atom transfer from the active OH group of the polyphenol to the free radical [6]. This allows polyphenols to indirectly activate antioxidant responses and generate non-toxic levels of intermediates, specifically the electrophilic forms of hydroquinone and quinone [11]. On the other hand, polyphenols inhibit the formation of or deactivation of the active species and precursors of free radicals, thus reducing the rate of oxidation and ultimately suppressing the generation of free radicals. They donate an electron to the free radical, neutralizing the radicals, and causing themselves to become stable (less reactive) radicals, thus stopping the reactions [12]. Treatment of HepG2 cells with (−)-epigallocatechin-3-gallate from green tea stimulates the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2), which modulates the expression of antioxidant genes [13]. Resveratrol improves antioxidant defenses in pancreatic tissue because it enhances the activity of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione-S-transferase (GST) [14].
3.2. Anti-Inflammatory Properties
Oxidative-stress-induced inflammation is mediated by the activation of cellular signaling processes of nuclear factor-kappa B (NF-kB) activation and activator protein-1 (AP-1) DNA binding [15]. It affects the expression of pro-inflammatory genes such as interleukin-1beta (IL-1β), IL-6, tumor necrotic factor alpha (TNF-a), and inducible nitric oxide synthase (iNOS) [16]. Preclinical and clinical studies suggest that polyphenols are able to express anti-inflammatory properties [17]. Although the precise mechanisms deserve further clarification, dietary polyphenols have shown benefits in distinct disorders [18]. Dihydroxylated phenolic acids produced from dietary proanthocyanidins potentially lowered the secretion of cytokines, including TNF-α, IL-1β, and IL-6, from healthy individuals [19]. Supplementation with 0.8% quercetin decreased interferon-γ, IL-1α, and IL-4 in male C57Bl/6j mice [20]. The administration of 10 mg/kg of quercetin also reduced the plasma nitrate plus nitrite (NOx) concentration and TNF-α production in adipose tissue of obese Zucker rats, resulting in an important anti-inflammatory effect [21].
3.3. Antibacterial Properties
Dietary polyphenols and plants rich in polyphenols have been demonstrated to be natural antimicrobials against both Gram-positive and Gram-negative bacteria. Epigallocatechin gallate (EGCG) was able to bind directly to the peptidoglycan from Staphylococcus aureus, affecting its cell integrity and destroying the osmotic protection of the cell wall [22]. Other than bacterial cell walls, tea polyphenols also damaged the inner cytoplasmic membrane of Serratia marcescens, increasing its permeability and releasing small cellular molecules [23]. Moreover, polyphenols can exhibit antibacterial activity via anti-biofilm agents. Cranberry proanthocyanidins limited the motility—particularly swarming motility—and reduced the biofilm formation of Pseudomonas aeruginosa [24]. However, due to the structural diversity of polyphenol classes, the mechanisms of their antimicrobial activities have not yet been fully resolved.
3.4. Anti-Adipogenic Properties
Stimulating the development of beige adipocytes (so called ‘browning’) can reduce adverse obesity effects and help to improve metabolic health [25,26]. Dietary polyphenols have been demonstrated to effectively activate adipose tissue browning and relieve obesity and lipid accumulation through the induction of beige adipocytes. Daily ingestion of a catechin-rich beverage increases brown adipose tissue density in healthy young women, supporting the brown adipogenesis of polyphenols [27]. Also, in mice fed with a high energy diet, vanillic acid could accelerate thermogenesis and mitochondrial synthesis in both classical brown adipose tissue (BAT) and inguinal white adipose tissue (WAT) [28]. Resveratrol decreased triglycerides (TG) accumulation in the liver by suppressing the expression of adipogenesis-related genes, such as acetyl-CoA carboxylase (ACC), peroxisome proliferator-activated receptor (PPAR-γ), and sterol regulatory element binding protein (SREBP-1) [26,29]. Piceatannol treatment suppressed protein levels of the adipogenic transcription factors PPAR-γ, while it increased ACC protein expression [30]. Therefore, a positive relationship may exist between dietary polyphenol and anti-adipogenesis, and the underlying mechanisms are worthy of exploration.
3.5. Neuro-Protective Properties
The neuro-protective effects of dietary polyphenols have received considerable attention in recent years, suggesting that polyphenols may be effective in reversing neurodegenerative pathology and age-related declines in neurocognitive performance. Animal evidence demonstrates that blueberries are effective at reversing age-related deficits in rat spatial working memory, and (−)-epicatechin enhances the retention of mice spatial memory and may relate to their potential to influence the synthesis of neurotrophic factors [31,32]. In addition, curcumin could disrupt existing plaques and partially restore distorted neurites in an Alzheimer mouse model [33]. Resveratrol can activate the phosphorylation of protein kinase C and secretes transthyretin to prevent Aβ aggregation in cultured rat hippocampal cells [34]. However, a direct association between dietary polyphenol and an improvement in neurological health has not been made at present.
4. Impact of Dietary Polyphenols on Gut Microbiota
Emerging evidence demonstrates that gut microbiota plays an important role in maintaining the physiological function of host health and the pathogenesis of various diseases, including obesity, diabetes, inflammatory bowel disease, and even neurodegenerative disorders. Diet can alter the composition of gut microbiota, which in turn affects host metabolism. The alteration of gut microbiota by the administration of probiotics, prebiotics, or fecal microbiota transplantation is already well established. However, the gut microbiota-modulating effects of polyphenol are less clear. Nevertheless, there is growing evidence showing that dietary polyphenol may directly modulate the gut microbiome, i.e., increasing beneficial microbial or decreasing harmful microbial species in the gut microbiota. In this part, we summarize the in vitro and in vivo studies that studied the effects of polyphenol supplementation on the gut microbiota.
4.1. In Vitro Modulation of Dietary Polyphenols on Gut Microbiota
In vitro experiments on polyphenols and polyphenol-rich food sources have been studied through extraction, digestion, and fermentation to demonstrate that they could modulate the resident bacteria. A series of in vitro studies with polyphenol from different sources have been listed in Table 1, including grapes, berries, tea, pomegranate, and other plants, to demonstrate the regulatory effect of polyphenol supplementation on intestinal micro-organisms.
Table 1.
Study on the effect of polyphenols on gut microbiota in vitro.
| Polyphenol and Source | Model | Impact on Microbiota | Reference |
|---|---|---|---|
| Flavonoids, Red wine | In vitro feces fermentation | Inhibit Clostridium histolyticum group | [35] |
| Grape polyphenol, Grape seeds | In vitro feces fermentation | Increase Bifidobacterium spp. and Lactobacillus-Enterococcus group; Inhibit Clostridium histolyticum group and the Bacteroides-Prevotella group | [36] |
| Ellagic acid and anthocyanins, Raspberry | In vitro colonic fermentation | Increase the abundance of Escherichia coli, butyric acid-producing bacteria, Lactobacillus and Akkermansia; Decrease Bacteroides and Ruminococcus. | [37] |
| Anthocyanins, flavonoids, neochlorogenic acids, tart cherry | The Simulator of the Human Intestinal Microbial Ecosystem | Increase Bacteroidetes, Firmicutes, Proteobacteria Decrease Verrumicrobia |
[38] |
| Catechins and Flavonol, Black tea | The Simulator of the Human Intestinal Microbial Ecosystem | Increase Klebsiella, enterococci, Akkermansia. Reduce bifidobacteria, B. coccoides, Anaeroglobus, Victivallis |
[39] |
| Green tea, oolong tea and black tea | In vitro fermentation Intestinal absorption |
Increase Bifidobacterium spp., Lactobacillus/Enterococcus spp.; Decrease Firmicutes/Bacteroidetes ratio and Clostridium histolyticum | [40] |
| Ellagitannins, Pomegranate by-product | In vitro feces fermentation | Enhance Bifidobacterium spp. and Lactobacillus spp. | [41] |
| Mango peel | In vitro model of the colon | Enhance Bifidobacterium and Lactobacillus | [42] |
| Red fruit | In vitro fermentation | Decrease B. cereus, S. aureus, E. coli | [43] |
| Olive pomace | In vitro feces fermentation | Increase Firmicutes and Bacteroidetes groups | [44] |
| 6-gingerols, Ginger | Simulated digestion model in vitro | Increase Bifidobacterium and Enterococcus | [45] |
| Proanthocyanidins, Sorghum bran | In vitro model of the colon | Increase Bifidobacterium spp., Lactobacillus–Enterococcus group; Decrease Clostridium histolyticum group, Bacteroides–Prevotella group | [46] |
Polyphenols can selectively inhibit the growth of pathogenic bacteria. Flavonoids in red wine showed a slight inhibition of the Clostridium [35]. Ellagic acid and anthocyanins in raspberry juice may inhibit the growth of Ruminococcus [37]. Grape polyphenols can inhibit the growth of Clostridium histolyticum [36]. On the other hand, polyphenols can promote the growth of beneficial bacteria in the gut, such as Bifidobacterium. Tannin in pomegranate, gingerol in ginger, grape polyphenols, and sorghum polyphenols can promote the growth of Bifidobacterium [41,45,46]. Tannin can also promote the growth of Lactobacillus [45]. Gingerol and grape polyphenols can promote the growth of Enterococci [36,45]. Sorghum polyphenols can cooperate with fructooligosaccharides to enhance the abundance of lactic acid bacteria, Roseburia, and Prevotella [46]. However, Kemperman’s research shows that polyphenols in red wine and black tea can reduce the abundance of Bifidobacterium [39]. They conducted in vitro experiments using fluids from the colon and found that catechins and flavonoids in black tea could stimulate Klebsiella, Enterococci, and Akkermansia and reduce Bifidobacteria, B. coccoids, Anaeroglobus, and Victivallis. Anthocyanins and catechins in red wine can promote the growth of Klebsiella, Alistipes, Cloacibacillus, Victivallis, and Akkermansia, and reduce the growth of Bifidobacteria, B. coccoides, Anaeroglobus, Subdoligranulum, and Bacteroides [39]. Mango peel is another high-polyphenol food, with gallates, flavonoids, gallotannins, gallic acid, and so on, and in vitro fermentation of mango peel could increase the growth of Bifidobacterium and Lactobacillus.
4.2. In Vivo Modulation of Dietary Polyphenols on Gut Microbiota of Animal Models
Similarly, in vivo studies have shown that polyphenol supplementation can modulate gut microbiota in animal models, including the increase of beneficial microbes and the decrease of harmful microbes. Detailed information on the published in vivo studies, from invertebrate Drosophila and zebrafish to vertebrate rat, mouse, chick, pig, etc., have been listed in Table 2. Both vertebrate and invertebrate model organisms confirmed that polyphenol supplementation can increase the number of beneficial bacteria in the gut, such as Bifidobacterium and Lactobacillus. Mango supplementation in mice fed with a high-fat diet can prevent the loss of beneficial intestinal bacteria, especially Bifidobacteria, Akkermansia, and Aldercrutzia [47]. Orso applied a diet of chestnut shell extract rich in tannin to a zebrafish intestinal inflammation model and found that it promoted the growth of healthy and beneficial bacteria (Enterobacteriaceae and Pseudomonas) [48]. Supplementation with polyphenols can also change the ratio of Firmicutes to Bacteroides. Cranberry extract is rich in phenolic acids, flavonoids, anthocyanins, and other polyphenols, which can reduce the ratio of Firmicutes to Bacteroides in mice induced by a high-fat/high-sugar diet [49]. Moreover, a polyphenol diet intervention can selectively inhibit pathogenic bacteria. Polyphenols from Smilax china L. rhizome can reduce the relative abundance of Desulfovibrionaceae, Lachnospiraceae, and Streptococcaceae [50], and grape pomace reduces potentially pathogenic bacteria to humans, such as Salmonella, E. coli, Shigella, Yersinia, and Proteus [51]. The combination of quercetin and resveratrol can significantly inhibit the relative abundance of Desulfovibrionaceae, Acidaminococcaceae, Coriobacteriaceae, Bilophila, and Lachnospiraceae, which may be related to diet-induced obesity [52]. Blueberry polyphenols were used to interfere with ovariectomized rats, with an upregulation of Bacteroides dorei and Lachnoclostridium and a decrease of Rickenellaceae and Eubacterium [53].
Table 2.
Effect of polyphenols on animal gut microbiota.
| Polyphenol and Source | Model | Impact on Microbiota | Reference |
|---|---|---|---|
| Rat | |||
| Epicatechin and catechin, Commercial |
Wistar rats | Decrease Bacteroides, Clostridium and Staphylococcus | [54] |
| Quercetin and Resveratrol, Commercial | HFD (High-fat-diet) rats | Reduce Firmicutes and the proportion of Firmicutes to Bacteroidetes. | [52] |
| Sinapic acid and resveratrol, Commercial | HFD rats | Increase Lachaospiraceae; Decrease Bacteroides and Desulfovibrionaceaesp | [55] |
| Chlorogenic acid, Commercial |
Wistar male rats | Increase Burkholderiales, Bifidobacterium; Decrease Desulfovibrionales, Desulfovibrio, Klebsiella, | [56] |
| Hesperetin, Commercial |
Rats | Increase Bifidobacterium, Lactobacillales; Decrease Clostridium subcluster XIVa | [57] |
| Blueberry polyphenols, Blueberry |
Rats | Reduce the Firmicutes to Bacteroidetes ratio; Increase Proteobacteria Bacteroides dorei and Lachnoclostridium. | [53] |
| Epicatechin and procyanidin, Cocoa |
Male Zucker diabetic fatty rats | Increase acetate-producing bacteria such as Blautia; Prevent lactate-producing bacteria (Enterococcus and Lactobacillus genera) | [58] |
| Gallic acid | Rats | Increase Lactobacillus, Bifidobacterium, Enterobacteriaceae | [59] |
| Pomegranate peel | HFD rats | Decrease Firmicutes to Bacteroidetes ratio; Increase Bacteroidales, Lactobacillus | [60] |
| Persimmon tannin | Rats | Decrease Firmicutes/Bacteroidetes ratio; Increase Bifidobacterium spp., Lactobacillus spp | [61] |
| Seaweed polyphenols | HFD/streptozotocin rats | Increase Odoribacter, Muribaculum, Parabacteroides; Decrease Firmicutes/Bacteroidetes ratio | [62] |
| Phenolic acids, flavan-3-ols | A high salt diet fed rats | Increase Bacteroidetes, Ruminococcaceae; Decrease Proteobacteria, Erysipelotrichaceae | [63] |
| Ellagic acid, gallic acid, and quercetin-3-rutinoside | Colon cancer rats | Increase Bacteroidetes; Decrease Firmicutes | [64] |
| Mice | |||
| Resveratrol, Commercial | HFD mice | Increase Bacteroidetes; Decrease Firmicutes | [65] |
| Chlorogenic acid, Commercial |
HFD mice | Increase Bacteroidaceae, Lactobacillaceae; Decrease Desulfovibrionaceae, Ruminococcaceae, Lachnospiraceae | [66] |
| Tea polyphenols, Commercial |
HFD mice | Increase Actinobacteria; Decrease Proteobacteria | [67] |
| Anthocyanins, Commercial |
Mice | Increase Lachnospiraceae; Decrease Bacilli, Clostridia | [3] |
| Flavonoid apigenin, Commercial |
Mice | Increase Actinobacteria; Decrease Firmicutes | [68] |
| Phenolic acids, flavonoids, anthocyanins, Cranberry | High fat/sucrose mice | Reduce the Firmicutes to Bacteroidetes ratio; Stimulate the growth of Akkermansia spp. | [49] |
| Caffeoylquinic acid, Quercetin, Smilax china L. rhizome | High fat/high sucrose mice | Decrease ratios of Firmicutes to Bacteroidetes; Increase Desulfovibrionaceae, Streptococcaceae, Akkermansiaceae | [50] |
| Betacyanins, Red pitayas | HFD mice | Decrease the ratio of Firmicutes to Bacteroidetes; Increase the relative abundance of Akkermansia. | [69] |
| Flavonoids, Painong-San | Colitis mice | Increase Romboutsia, Lactobacillus, Bifidobacterium, Akkermansia; Decrease Oscillospiraceae, Helicobacter | [70] |
| Gallic acid, Canarium album | HFD mice | Increase Firmicutes, Verrucomicrobia, Akkermansia; Decrease of Bacteroidetes | [71] |
| Gallic acid, anthocyanins, epicatechin, Berry | High-fat/sucrose mice | Increase Akkermansiaceae; Decrease Firmicutes, Lachnospiraceae, Ruminococcaceae, Peptostreptococcaceae | [72] |
| Flavonoid, Penthorum chinense pursh | Mice | Increase Bacteroidetes, Proteobacteria, Verrucomicrobia; Decrease Firmicutes, Actinobacteria, Deferribacteres | [73] |
| Grape polyphenols, Grape | Mice | Increase Akkermansia, Lactobacillus | [74] |
| Anthocyanins, Lycium ruthenicum Murray | Mice | Increase Barnesiella, Alistipes, Eisenbergiella, Coprobacter, Odoribacter | [75] |
| Camellia japonica bee pollen kaempferol | Oxonate-induced mice | Increase Firmicutes; Decrease Bacteroidetes, Actinobacteria, Proteobacteria | [76] |
| Ellagitannins, ellagic acid, anthocyanins, Raspberry | Mice | Increase Lactobacillus; Decrease Blautia, Ruminiclostridium | [37] |
| Anthocyanidins, Lycium ruthenicum |
Mice | Increase Verrucomicrobia, Bacteroidetes, Akkermansia, Odoribacter, Bifidobacterium; Decrease Firmicutes | [77] |
| Tea polyphenol, Kombucha |
HFD/streptozotocin mice | Increase Lactobacillus, Butyricicoccus; Decrease Proteobacteria, Desulfovibrio, Escherichia-Shigella, Bacteroidetes | [78] |
| 3-hydroxybenzylhydrazine, isophorone, Millet shells | HFD mice | Increase Bacteroidetes; Decrease Verrucomicrobia, Actinobacteria | [79] |
| Tea polyphenol, Tea extract |
Colitis Mice | Increase Faecalibaculum, Bifidobacterium; Decrease Bacteroids, Mucispirillum | [80] |
| Mango Polyphenols, Mango pulp |
HFD mice | Prevent the loss of beneficial gut bacteria, specifically Bifidobacteria, Akkermansia, and Aldercrutzia. | [47] |
| Chlorogenic acid, Chicory root |
Mice | Increase Prevotellaceae, Lachnospiraceae bacterium A2, Clostridium ASF356, Decrease Oscillospirales, Ruminococcus, the ratio Firmicutes/Bacteroidetes | [81] |
| Pig | |||
| Gallic acid, ethyl gallate, Red-osier dogwood |
Pig | Increase class Bacilli, Lactobacillales and family lactobacillaceae | [82] |
| Proanthocyanidin, Grape seed |
Pig | Increase Lachnospiraceae, Clostridales, Lactobacillus and Ruminococcacceae. | [83] |
| Chlorogenic acid, Commercial |
Pig | Increase Lactobacillus spp., Prevotella spp., Anaerovibrio spp., and Alloprevotella spp.; Decrease Proteobacteria | [84] |
| Chick | |||
| Procyanidins and anthocyanidins, Grape | Broiler chicks | Increase the populations of Enterococcus, Escherichia coli, Lactobacillus; Decrease the counts of Clostridium. | [85] |
| Pentagalloyl glucose, Eucalyptus |
Broiler chicks | Increase the Firmicutes to Bacteroidetes ratio, Verrucomicrobia; Decrease Actinobacteria, Proteobacteria | [86] |
| Epicatechin and quercetin 3-glucoside, Carioca Bean | Broiler chicks | Increase Coriobacteriaceae, Dehalobacteriaceae, Lachnospiraceae | [87] |
| Lamb | |||
| Resveratrol, catechin, epicatechin, procyanidins, Grape pomace | Lambs | Enhance the growth of facultative probiotic bacteria and inhibit the growth of pathogen populations such as Enterobacteriaceae and E. coli. | [51] |
| Zebrafish | |||
| Tannins, Chestnut shells | Inflammation zebrafish | Increase the Enterobacteriaceae, Pseudomonas spp. and anaerobic bacteria (e.g., Lactobacilli and Bifidobacteria) | [48] |
| Dendrobium candidum | Inflammation zebrafish | Increase Lactobacillus, Faecalibacterium, Rummeliibacillus; Decrease Shewanella, Geodermatophilus | [88] |
| Drosophila | |||
| Eigallocatechin-3-gallate (EGCG), commercial | Rotenone-treated flies | Decrease Proteobacteria, Acetobacter, Lactobacillus; Increase the relative abundance of Firmicutes and Bacteroidetes | [89] |
4.3. In Vivo Modulation of Dietary Polyphenols on Gut Microbiota of Humans
Clinical studies further confirmed the regulatory effect of polyphenols on human intestinal micro-organisms (Table 3). Consistent with in vitro and in vivo animal studies, supplementation with polyphenols such as anthocyanins and flavonoids increase the abundance of Bifidobacterium and Lactobacillus, which are two intestinal protective agents in the human gut [90,91]. Blueberries are rich in anthocyanins, which can increase the number of Bifidobacteria and lactic acid bacteria in healthy volunteers [92]. Almonds and almond skins are heavily rich in a range of flavonoids, including catechin, flavonol, and flavanone glycosides, and adding almonds or almond skins to the diet can increase the number of Bifidobacteria and Lactobacillus in feces [93]; Moreno-Indias found that polyphenols in red wine can increase the number of Bifidobacteria and Lactobacillus [94]. Besides, a diet rich in polyphenols can regulate the ratio of Firmicutes to Bacteroides in the human body. Daily consumption of cranberries rich in proanthocyanidins can reduce the number of Firmicutes in the body and increase the number of Bacteroides [95]; however, Yuan used tea polyphenols in tea to intervene in healthy volunteers and found different results. The diet that intervened with tea polyphenols resulted in an increase in the number of Firmicutes in feces, a decrease in the number of Bacteroides, and an increase in the ratio of Firmicutes to Bacteroides [96]. Queipo-Ortu found that the combined action of alcohol and polyphenols could increase the number of Enterococcus, Prevotella, Bacteroides, Bifidobacterium, Bacteroides uniformis, Eggerthella lenta, and Blautia coccoides–Eubacterium, but had no significant effect on the changes of Lactobacillus [97].
Table 3.
Effect of polyphenols on human gut microbiota.
| Polyphenol and Source | Impact on Microbiota | Reference |
|---|---|---|
| Anthocyanins, Blackcurrant |
Increase Lactobacilli, Bifidobacteria; Decrease Bacteroides spp., Clostridium spp. | [90] |
| Flavanols, Cocoa |
Increase Bifidobacterial, Lactobacilli, E. rectale-C. coccoides; Decrease Clostridia; While low–cocoa group: Increase Clostridia, E. rectale-C. coccoides | [91] |
| Proanthocyanins, Blueberry |
Increase Bifidobacterium, Prevotella spp., Bacteroides spp., Clostridium coccoides; Decrease Enterococcus spp. | [92] |
| Flavonoid, Almond |
Increase Bifidobacterium spp. and Lactobacillus spp.; Repress pathogen Clostridum perfringens | [93] |
| Red wine polyphenols | Increase Bifidobacteria, Lactobacillus and butyrate-producing (Faecalibacterium prausnitzii and Roseburia); Decrease Lipopolysaccharide (LPS)-producing (Escherichia coli and Enterobacter cloacae) | [94] |
| Proanthocyanidins, Cranberry |
Increase abundance of Bacteroidetes, Lachnospira and Anaerostipes.; Decrease abundance of Firmicutes, Clostridia, Oribacterium | [95] |
| Catechins, Green tea |
Increase Firmicutes and Actinobacteria, Lachnospiraceae.; Reduce Bacteroidetes and increase the FIR:BAC (Firmicutes: Bacteroidetes) | [96] |
| Red wine polyphenols | Increase the relative abundance of Enterococcus, Prevotella, Bacteroides, Bifidobacterium, Bacteroides uniformis groups | [97] |
| Anthocyanins, Tart cherry |
High-Bacteroide: Increase Lachnospiraceae, Ruminococcus, Collinsella; Decrease Bacteroides, Bifidobacterium. Low-Bacteroides: Increase Bacteroides or Prevotella and Bifidobacterium; Decrease Lachnospiraceae, Ruminococcus and Collinsella. | [38] |
| Polyphenolic, Schisandra chinensis |
Increase Akkermansia, Roseburia, Bacteroides, Prevotella, and Bifidobacterium | [98] |
| Increase | Increase Clostridium, Lactobacillus, Faecalibacterium, Bifidobacterium | [99] |
| Cocoa flavanols, Dark chocolate |
Increase Lactobacillus; Decrease Bacteroidetes | [100] |
| Phenolic acids, Dietary raisin |
Increase Faecalibacterium prausnitzii, Bacteroidetes spp., Ruminococcus spp.; Decrease Klebsiella spp., Prevotella spp., Bifidobacterium spp. | [101] |
| Apple polyphenol | Increase Lactobacillus, Streptococcus; Decrease lecithinase-positive clostridia, Enterobacteriaceae, Pseudomonas | [102] |
| Flavanones, Orange |
Increase Lactobacillus; Decrease Blautia coccoides, Clostridium leptum | [103] |
The effect of polyphenols on gut microbiota is related to the number of initial microbiota in the intestinal tract. Mayta-Apaza classified them according to the initial number of Bacteroides in the body, and different microbial compositions led to different performances after receiving a dietary intervention. After receiving sour cherry juice, the volunteers with high initial Bacteroides reduced Bacteroides and Bifidobacterium and increased the Lachnospiraceae, Ruminococcus, and potential polyphenol metabolite Collinsella. The volunteers with low Bacteroides responded to the increase of Bacteroides and Bifidobacterium and the decrease in the relative abundance of Lachnospiraceae, Ruminococcus, and Collinsella [38]. The effect of polyphenols on gut microbiota is related to the intake of polyphenols. Tzounis found that high-dose cocoa flavanone beverages increase the number of Bifidobacterium, lactic acid bacteria, and Enterococci; increase the number of E.rectale–C.coccoides; and reduce the number of Histolytic Chlamydia. A low dose of cocoa flavanone beverage will not cause a significant change in the number of Bifidobacteria, but will increase Clostridia [91].
5. Mechanism of Dietary Polyphenol and Gut Microbiota Affecting Host Health
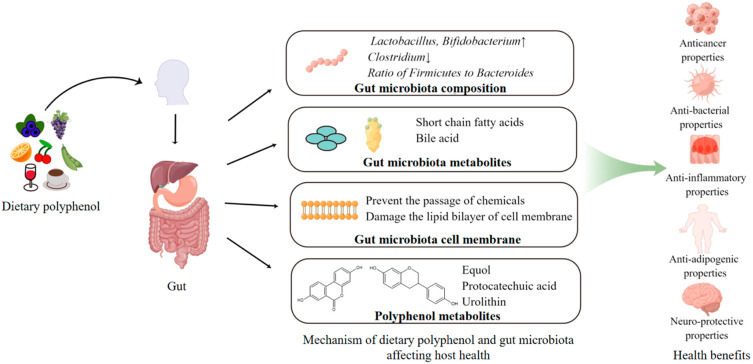
The gut microbiota and the host maintain normal physiological function and morphology of the intestine by forming a mutually beneficial relationship. Gut microbiota not only play a bridge role between the diet and host in digesting dietary food complexes, but also yields short-chain fatty acids and other metabolites to regulate human health. Studies have shown that only a small portion of polyphenols (5–10% of the total polyphenol intake) are absorbed in the small intestine, while most (90–95% of the total polyphenol intake) are transported to the human large intestine [104]. Diet polyphenol can modulate the gut microbial composition, and, at the same time, gut microbiota also improve the bioavailability of polyphenols by converting them to bioavailable metabolites (Figure 2).
Figure 2.
Possible mechanisms among dietary polyphenols, gut microbiota, and host health.
5.1. Dietary Polyphenols Affect the Composition of Gut Microbiota
Dietary polyphenol has a definite role in the composition and functional profile of the gut microbiota. Polyphenols promote the growth of beneficial microbes, such as Lactobacillus and Bifidobacterium, which are two major health beneficial probiotics and bring benefits to human health, such as improving gastrointestinal disorders, suppressing diarrhea and constipation [105], alleviating lactose intolerance [106], relieving irritable bowel symptoms [107], and preventing inflammatory bowel disease [108]. A systematic review by Ma et al. with a meta-analysis revealed that polyphenol supplementation profoundly increased the abundance of Lactobacillus by 220% and Bifidobacterium by 56%. On the other hand, polyphenols can inhibit the growth of harmful microbiota, and Clostridium histolyticum and Clostridium perfringens in Clostridium are common pathogenic bacteria. Clostridium histolyticum causes inflammatory bowel disease [5] and Clostridium perfringens produces many toxins and hydrolytic enzymes, which are related to gastrointestinal disease and necrotizing enteritis [109]. Ma’s review system by meta-analysis showed that polyphenols derived from different foods all suppress the abundance of Clostridium pathogen species in the human gut microbiota, with tea being the most effective polyphenol food source for reducing Clostridium [110]. Dietary polyphenols can also regulate the ratio of Firmicutes to Bacteroides, which is related to body weight, and the ratio of Firmicutes to Bacteroides in obese patients is higher [111]. Xue’s studies have shown that four dietary polyphenols, rutin, quercetin, chlorogenic acid, and caffeic acid, can reduce the ratio of Firmicutes to Bacteroides in in vitro gut microbiota experiments [112]. However, due to the different types of polyphenols, polyphenol dosage, and research methods, the results of different studies are different to some extent, resulting in the changes between microbes not being completely consistent.
5.2. Dietary Polyphenols Affect the Metabolites of Gut Microbiota
Short-chain fatty acids (SCFAs) are the most well-studied microbial metabolites so far. SCFAs are a saturated aliphatic organic acid [113] that are produced by the incomplete metabolism of plant-derived carbohydrates by intestinal flora present in an anaerobic environment [114]. Acetate, propionate, and butyrate are the main SCFAs in the gut (accounting for 90% of the total SCFAs) [115]. Wu’s studies have shown that EGCG can significantly increase the number of SCFAs-producing bacteria, especially Akkermansia, and then promote the production of SCFAs, thereby enhancing anti-inflammatory effects and colon barrier integrity, which reduces enteritis [116]. Previous studies have shown that Akkermansia muciniphila can promote the production of acetate and propionate, and the nutritional interaction between Akkermansia muciniphila and butyrate-producing bacteria promotes butyrate production [117]. Liu’s experiment showed that after a week-long intervention with an Aronia-berry-rich diet, the polyphenol diet extracted by Aronia berry was 57% higher than that in the control group [3]. In the human model intestinal system, the in vitro fermentation of wild cherry juice increased the microbial production of propionate and butyrate [118]. McDougall found that after ingesting anthocyanin-rich raspberry, the concentration of bile acid in an ideal fluid of ileostomy subjects changed significantly, wherein the glycine and taurine derivatives of cholate and deoxycholate increased [119]. Fotschki further described the beneficial effects of raspberry dregs on the bile acid profile of the cecum in a hyperlipidemic mouse model [120]. Studies by Huang have shown that EGCG can significantly reduce the content of intestinal bile acid; increase the excretion of bile acid, cholesterol, and total lipids in feces; and alleviate metabolic abnormalities and fatty liver induced by a high-fat diet in mice [121]. Therefore, after dietary polyphenols reach the gut, microbiota can then further produce metabolites, and, once absorbed and transported to target tissues and organs, contribute to metabolite health.
5.3. Dietary Polyphenols Affect the Bacterial Cell Membrane
Dietary polyphenol can interfere with the bacterial cell function of the cell membrane. For example, flavonols and flavones in the Staphylococcus genus can increase membrane cytoplasm permeability. Studies have shown that the antibacterial effect of polyphenols is more effective against Gram-positive bacteria. Inouye pointed out that because of the hydrophilic outer membrane outside the cell wall of Gram-negative bacteria, the passage of chemicals is prevented. Gram-negative bacteria are more resistant to plant secondary metabolites, including phenols [122]. When polyphenols were ingested, the growth of Gram-negative Salmonella and Escherichia strains was inhibited, but the growth of Gram-positive lactic acid bacteria was not affected [123]. The effect of polyphenols on bacteria depends on the interaction between compounds and the bacterial cell surface, which can inhibit bacterial growth by disturbing the function of the cell membrane [124]. Tea polyphenols, such as tea catechins, have a strong affinity to the lipid bilayers of the cell membrane through hydrogen bonds with the bilayer surface, thus penetrating underneath the surface and giving play to antibacterial, anticancer, and other beneficial effects [125]. EGCG has antibacterial activity against Staphylococcus; possible mechanisms include damaging the lipid bilayer of the cell membrane, reducing mucus production and affecting the formation of biofilm, and binding and neutralizing with enterotoxin B [126]. Therefore, the effect of polyphenols on the bacterial cell membranes is considered to be one of the mechanisms for regulating metabolic health.
5.4. Biotransformation of Polyphenols by Gut Microbiota
With respect to the complicated structures and high molecular weights, dietary polyphenols have low bioavailability and are difficult to be absorbed in the small intestine. About 90% of dietary polyphenols arrive at the colon in an intact form where they are biotransformed and metabolized into bioactive, low-molecular-weight phenolic metabolites through the residing microbiota [127]. Chen discovered that gut bacteria can deconjugate mulberry anthocyanin (cyanidin-3-glucoside, cyanidin-3-rutin, and delphinidin-3-rutinoside) to lower molecular-weight metabolites, and metabonomic data showed that the first two compounds were decomposed into protocatechuic, vanillic acid, and p-coumaric acids, while the latter was converted to syringic acid and gallic acid [128,129]. The core bacteria that can metabolize anthocyanins are Bifidobacterium spp. and Lactobacillus spp. [130,131] with probiotic effects to produce antibacterial substances, to compete with pathogens for adhering to the epithelium and for nutrients, to regulate the host immune system, and to inhibit the production of bacterial toxins [132]. The flavonoids (flavonols, flavones, and flavanones) can be biotransformed into p-hydroxyphenylacetic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid, hydrocaffeic acid, coumaric acid, 3-(4-hydroxyphenyl) propionic acid, and other aromatic metabolites [133]. Soybean isoflavones can be converted to dihydrodaidzein, dihydrogenistein,6′-OH-O-desmethylangolensin, and cis-4-OH-equol by anaerobic bacteria in the distal region of the small intestine and colon [134,135,136]. The bioavailability of ellagic tannin, which was found in pomegranate and grape, is low, but they can be metabolized by intestinal micro-organisms into urolithins with antioxidant activity and preventive effects for chronic diseases such as cancer, diabetes, and cardiovascular and neurodegenerative diseases [137,138]. Therefore, polyphenol metabolites produced by gut microbiota have potentially beneficial effects on the host.
6. Conclusions
There is increasing evidence in the literature to emphasize that dietary polyphenols have potentially beneficial effects on host health through interactions with gut microbiota. Numerous studies listed in this review, both in vitro and in vivo, demonstrated the relationship between dietary polyphenols and gut microbiota, while the possible mechanism may be through the alteration of gut microbiota composition, the production of gut microbiota metabolites, the modulation of intestinal barrier function, and the biotransformation and metabolism of dietary polyphenols. However, a clear and deep understanding of these mechanisms between polyphenols and gut microbiota is necessitated, especially considering the metabolic pathways, which will allow for new therapeutic targets in the future.
Acknowledgments
Figure is drawn by Figdraw (www.figdraw.com accessed on 30 May 2020).
Author Contributions
Writing—original draft preparation, Y.Q. and X.W., writing—review and editing, X.W. and H.Z.; supervision, H.Z.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Chinese Universities Scientific Fund, grant number 2022TC072.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aravind S.M., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021;142:110189. doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- 2.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Martin D.A., Valdez J.C., Sudakaran S., Rey F., Bolling B.W. Aronia berry polyphenols have matrix-dependent effects on the gut microbiota. Food Chem. 2021;359:129831. doi: 10.1016/j.foodchem.2021.129831. [DOI] [PubMed] [Google Scholar]
- 4.Aso T. Equol Improves Menopausal Symptoms in Japanese Women. J. Nutr. 2010;140:1386S–1389S. doi: 10.3945/jn.109.118307. [DOI] [PubMed] [Google Scholar]
- 5.Ma G., Chen Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods. 2020;66:103829. doi: 10.1016/j.jff.2020.103829. [DOI] [Google Scholar]
- 6.Papuc C., Goran G.V., Predescu C.N., Nicorescu V., Stefan G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017;16:1243–1268. doi: 10.1111/1541-4337.12298. [DOI] [PubMed] [Google Scholar]
- 7.Kamiloglu S., Tomas M., Ozdal T., Capanoglu E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. Technol. 2021;117:15–33. doi: 10.1016/j.tifs.2020.10.030. [DOI] [Google Scholar]
- 8.Singla R.K., Dubey A.K., Garg A., Sharma R.K., Fiorino M., Ameen S.M., Haddad M.A., Al-Hiary M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019;102:1397–1400. doi: 10.5740/jaoacint.19-0133. [DOI] [PubMed] [Google Scholar]
- 9.Sigurdson G.T., Robbins R.J., Collins T.M., Giusti M.M. Impact of location, type, and number of glycosidic substitutions on the color expression of o-dihydroxylated anthocyanidins. Food Chem. 2018;268:416–423. doi: 10.1016/j.foodchem.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 10.Šamec D., Karalija E., Šola I., Vujčić Bok V., Salopek-Sondi B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants. 2021;10:118. doi: 10.3390/plants10010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X., Li M., Xiao Z., Daglia M., Dragan S., Delmas D., Vong C.T., Wang Y., Zhao Y., Shen J., et al. Dietary polyphenols for managing cancers: What have we ignored? Trends Food Sci. Technol. 2020;101:150–164. doi: 10.1016/j.tifs.2020.05.017. [DOI] [Google Scholar]
- 12.Tsao R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi Y., Zhang W., Tian H., Li R., Huang S., Li X., Qi G., Liu X. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018;9:1510–1523. doi: 10.1039/C7FO01554B. [DOI] [PubMed] [Google Scholar]
- 14.Palsamy P., Subramanian S. Modulatory effects of resveratrol on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–nicotinamide-induced diabetic rats. Chem. Biol. Interact. 2009;179:356–362. doi: 10.1016/j.cbi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu T., Zhang L., Joo D., Sun S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opal S.M., DePalo V.A. Anti-Inflammatory Cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 18.Li H., Christman L.M., Li R., Gu L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020;11:4878–4891. doi: 10.1039/D0FO00713G. [DOI] [PubMed] [Google Scholar]
- 19.Monagas M., Khan N., Andrés-Lacueva C., Urpí-Sardá M., Vázquez-Agell M., Lamuela-Raventós R.M., Estruch R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009;102:201–206. doi: 10.1017/S0007114508162110. [DOI] [PubMed] [Google Scholar]
- 20.Stewart L.K., Soileau J.L., Ribnicky D., Wang Z.Q., Raskin I., Poulev A., Majewski M., Cefalu W.T., Gettys T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–S46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera L., Morón R., Sánchez M., Zarzuelo A., Galisteo M. Quercetin Ameliorates Metabolic Syndrome and Improves the Inflammatory Status in Obese Zucker Rats. Obesity. 2008;16:2081–2087. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 22.Zhao W.-H., Hu Z.-Q., Hara Y., Shimamura T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002;46:2266–2268. doi: 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi S., Wang W., Bai F., Zhu J., Li J., Li X., Xu Y., Sun T., He Y. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2014;30:451–460. doi: 10.1007/s11274-013-1464-4. [DOI] [PubMed] [Google Scholar]
- 24.Ulrey R.K., Barksdale S.M., Zhou W., Van Hoek M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2014;14:499. doi: 10.1186/1472-6882-14-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartelt A., Heeren J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 26.Hu J., Wang Z., Tan B.K., Christian M. Dietary polyphenols turn fat “brown”: A narrative review of the possible mechanisms. Trends Food Sci. Technol. 2020;97:221–232. doi: 10.1016/j.tifs.2020.01.013. [DOI] [Google Scholar]
- 27.Nirengi S., Amagasa S., Homma T., Yoneshiro T., Matsumiya S., Kurosawa Y., Sakane N., Ebi K., Saito M., Hamaoka T. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. SpringerPlus. 2016;5:1363. doi: 10.1186/s40064-016-3029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X., Guo J., You Y., Yin M., Liang J., Ren C., Zhan J., Huang W. Vanillic acid activates thermogenesis in brown and white adipose tissue. Food Funct. 2018;9:4366–4375. doi: 10.1039/C8FO00978C. [DOI] [PubMed] [Google Scholar]
- 29.Andrade J.M.O., Paraíso A.F., de Oliveira M.V.M., Martins A.M., Neto J.F., Guimaraes A., de Paula A.M., Qureshi M., Santos S.H.S. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Tung Y.-C., Lin Y.-H., Chen H.-J., Chou S.-C., Cheng A.-C., Kalyanam N., Ho C.-T., Pan M.-H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules. 2016;21:1419. doi: 10.3390/molecules21111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casadesus G., Shukitt-Hale B., Stellwagen H.M., Zhu X., Lee H.-G., Smith M.A., Joseph J.A. Modulation of Hippocampal Plasticity and Cognitive Behavior by Short-term Blueberry Supplementation in Aged Rats. Nutr. Neurosci. 2004;7:309–316. doi: 10.1080/10284150400020482. [DOI] [PubMed] [Google Scholar]
- 32.Van Praag H., Lucero M.J., Yeo G.W., Stecker K., Heivand N., Zhao C., Yip E., Afanador M., Schroeter H., Hammerstone J., et al. Plant-Derived Flavanol (-)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta B., Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Patán F., Cueva C., Monagas M., Walton G.E., Gibson G.R., Quintanilla-López J.E., Lebrón-Aguilar R., Martín-Álvarez P.J., Moreno-Arribas M., Bartolomé B. In Vitro Fermentation of a Red Wine Extract by Human Gut Microbiota: Changes in Microbial Groups and Formation of Phenolic Metabolites. J. Agric. Food Chem. 2012;60:2136–2147. doi: 10.1021/jf2040115. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L., Wang W., Huang J., Ding Y., Pan Z., Zhao Y., Zhang R., Hu B., Zeng X. In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota. Food Funct. 2016;7:1959–1967. doi: 10.1039/C6FO00032K. [DOI] [PubMed] [Google Scholar]
- 37.Wu T., Chu X., Cheng Y., Tang S., Zogona D., Pan S., Xu X. Modulation of Gut Microbiota by Lactobacillus casei Fermented Raspberry Juice In Vitro and In Vivo. Foods. 2021;10:3055. doi: 10.3390/foods10123055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayta-Apaza A.C., Pottgen E., De Bodt J., Papp N., Marasini D., Howard L., Abranko L., Van de Wiele T., Lee S.-O., Carbonero F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018;59:160–172. doi: 10.1016/j.jnutbio.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Kemperman R.A., Gross G., Mondot S., Possemiers S., Marzorati M., Van de Wiele T., Doré J., Vaughan E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013;53:659–669. doi: 10.1016/j.foodres.2013.01.034. [DOI] [Google Scholar]
- 40.Xu M., Yang K., Zhu J. Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules. 2020;25:5101. doi: 10.3390/molecules25215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bialonska D., Ramnani P., Kasimsetty S.G., Muntha K.R., Gibson G.R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 42.Sáyago-Ayerdi S.G., Venema K., Tabernero M., Sarriá B., Bravo L.L., Mateos R. Bioconversion by gut microbiota of predigested mango (Mangifera indica L) ‘Ataulfo’ peel polyphenols assessed in a dynamic (TIM-2) in vitro model of the human colon. Food Res. Int. 2021;139:109963. doi: 10.1016/j.foodres.2020.109963. [DOI] [PubMed] [Google Scholar]
- 43.Jiao X., Wang Y., Lin Y., Lang Y., Li E., Zhang X., Zhang Q., Feng Y., Meng X., Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019;64:88–100. doi: 10.1016/j.jnutbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Coman M.M., Oancea A.M., Verdenelli M.C., Cecchini C., Bahrim G.E., Orpianesi C., Cresci A., Silvi S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2017;244:735–745. doi: 10.1007/s00217-017-2997-9. [DOI] [Google Scholar]
- 45.Wang J., Chen Y., Hu X., Feng F., Cai L., Chen F. Assessing the Effects of Ginger Extract on Polyphenol Profiles and the Subsequent Impact on the Fecal Microbiota by Simulating Digestion and Fermentation In Vitro. Nutrients. 2020;12:3194. doi: 10.3390/nu12103194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sost M.M., Ahles S., Verhoeven J., Verbruggen S., Stevens Y., Venema K. A Citrus Fruit Extract High in Polyphenols Beneficially Modulates the Gut Microbiota of Healthy Human Volunteers in a Validated In Vitro Model of the Colon. Nutrients. 2021;13:3915. doi: 10.3390/nu13113915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojo B., El-Rassi G.D., Payton M.E., Perkins-Veazie P., Clarke S., Smith B.J., Lucas E.A. Mango Supplementation Modulates Gut Microbial Dysbiosis and Short-Chain Fatty Acid Production Independent of Body Weight Reduction in C57BL/6 Mice Fed a High-Fat Diet. J. Nutr. 2016;146:1483–1491. doi: 10.3945/jn.115.226688. [DOI] [PubMed] [Google Scholar]
- 48.Orso G., Solovyev M.M., Facchiano S., Tyrikova E., Sateriale D., Kashinskaya E., Pagliarulo C., Hoseinifar H.S., Simonov E., Varricchio E., et al. Chestnut Shell Tannins: Effects on Intestinal Inflammation and Dysbiosis in Zebrafish. Animals. 2021;11:1538. doi: 10.3390/ani11061538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anhê F.F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T.V., Garofalo C., Moine Q., Desjardins Y., Levy E., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2014;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Yang L., Xu M., Qiao G., Li C., Lin L., Zheng G. Smilax china L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. J. Funct. Foods. 2021;77:104332. doi: 10.1016/j.jff.2020.104332. [DOI] [Google Scholar]
- 51.Kafantaris I., Kotsampasi B., Christodoulou V., Kokka E., Kouka P., Terzopoulou Z., Gerasopoulos K., Stagos D., Mitsagga C., Giavasis I., et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017;101:e108–e121. doi: 10.1111/jpn.12569. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L., Zhang Q., Ma W., Tian F., Shen H., Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8:4644–4656. doi: 10.1039/C7FO01383C. [DOI] [PubMed] [Google Scholar]
- 53.Cladis D.P., Simpson A.M.R., Cooper K.J., Nakatsu C.H., Ferruzzi M.G., Weaver C.M. Blueberry polyphenols alter gut microbiota & phenolic metabolism in rats. Food Funct. 2021;12:2442–2456. doi: 10.1039/d0fo03457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massot-Cladera M., Pérez-Berezo T., Franch A., Castell M., Pérez-Cano F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012;527:105–112. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Yang C., Deng Q., Xu J., Wang X., Hu C., Tang H., Huang F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019;116:1202–1211. doi: 10.1016/j.foodres.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Song J., Zhou N., Ma W., Gu X., Chen B., Zeng Y., Yang L., Zhou M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019;10:2947–2957. doi: 10.1039/C8FO02599A. [DOI] [PubMed] [Google Scholar]
- 57.Unno T., Hisada T., Takahashi S. Hesperetin Modifies the Composition of Fecal Microbiota and Increases Cecal Levels of Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2015;63:7952–7957. doi: 10.1021/acs.jafc.5b02649. [DOI] [PubMed] [Google Scholar]
- 58.Álvarez-Cilleros D., Ramos S., López-Oliva M.E., Escrivá F., Álvarez C., Fernández-Millán E., Martín M.Á. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020;132:109058. doi: 10.1016/j.foodres.2020.109058. [DOI] [PubMed] [Google Scholar]
- 59.da Silva-Maia J.K., Batista A.G., Correa L.C., Lima G.C., Bogusz S.B., Jr., Marostica M.R., Jr. Aqueous extract of berry (Plinia jaboticaba) byproduct modulates gut microbiota and maintains the balance on antioxidant defense system in rats. J. Food Biochem. 2019;43:e12705. doi: 10.1111/jfbc.12705. [DOI] [PubMed] [Google Scholar]
- 60.Zhao R., Long X., Yang J., Du L., Zhang X., Li J., Hou C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019;10:8273–8285. doi: 10.1039/C9FO02077B. [DOI] [PubMed] [Google Scholar]
- 61.Zhu W., Lin K., Li K., Deng X., Li C. Reshaped fecal gut microbiota composition by the intake of high molecular weight persimmon tannin in normal and high-cholesterol diet-fed rats. Food Funct. 2018;9:541–551. doi: 10.1039/C7FO00995J. [DOI] [PubMed] [Google Scholar]
- 62.Yuan Y., Zheng Y., Zhou J., Geng Y., Zou P., Li Y., Zhang C. Polyphenol-Rich Extracts from Brown Macroalgae Lessonia trabeculate Attenuate Hyperglycemia and Modulate Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2019;67:12472–12480. doi: 10.1021/acs.jafc.9b05118. [DOI] [PubMed] [Google Scholar]
- 63.Gomes A., Oudot C., Macià A., Foito A., Carregosa D., Stewart D., Van De Wiele T., Berry D., Motilva M.-J., Brenner C., et al. Berry-Enriched Diet in Salt-Sensitive Hypertensive Rats: Metabolic Fate of (Poly)Phenols and the Role of Gut Microbiota. Nutrients. 2019;11:2634. doi: 10.3390/nu11112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fidelis M., Santos J.S., Escher G.B., Rocha R.S., Cruz A.G., Cruz T.M., Marques M.B., Nunes J.B., do Carmo M.A.V.D., de Almeida L.A., et al. Polyphenols of jabuticaba [Myrciaria jaboticaba (Vell.) O.Berg] seeds incorporated in a yogurt model exert antioxidant activity and modulate gut microbiota of 1,2-dimethylhydrazine-induced colon cancer in rats. Food Chem. 2021;334:127565. doi: 10.1016/j.foodchem.2020.127565. [DOI] [PubMed] [Google Scholar]
- 65.Wang P., Li D., Ke W., Liang D., Hu X., Chen F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020;44:213–225. doi: 10.1038/s41366-019-0332-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Lam K.-L., Hu J., Ge S., Zhou A., Zhou A., Zheng B., Zeng S., Lin S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019;7:579–588. doi: 10.1002/fsn3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma H., Zhang B., Hu Y., Wang J., Liu J.-M., Qin R., Lv S., Wang S. Correlation Analysis of Intestinal Redox State with the Gut Microbiota Reveals the Positive Intervention of Tea Polyphenols on Hyperlipidemia in High Fat Diet Fed Mice. J. Agric. Food Chem. 2019;67:7325–7335. doi: 10.1021/acs.jafc.9b02211. [DOI] [PubMed] [Google Scholar]
- 68.Bian S., Wan H., Liao X., Wang W. Inhibitory Effects of Apigenin on Tumor Carcinogenesis by Altering the Gut Microbiota. Mediat. Inflamm. 2020;2020:7141970. doi: 10.1155/2020/7141970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H., Chu Q., Yan F., Yang Y., Han W., Zheng X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016;31:1462–1469. doi: 10.1111/jgh.13278. [DOI] [PubMed] [Google Scholar]
- 70.Wang K., Guo J., Chang X., Gui S. Painong-San extract alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota, restoring intestinal barrier function and attenuating TLR4/NF-κB signaling cascades. J. Pharm. Biomed. Anal. 2022;209:114529. doi: 10.1016/j.jpba.2021.114529. [DOI] [PubMed] [Google Scholar]
- 71.Zhang N.-N., Guo W.-H., Hu H., Zhou A.-R., Liu Q.-P., Zheng B.-D., Zeng S.-X. Effect of A Polyphenol-Rich Canarium album Extract on the Composition of the Gut Microbiota of Mice Fed a High-Fat Diet. Molecules. 2018;23:2188. doi: 10.3390/molecules23092188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodríguez-Daza M.-C., Roquim M., Dudonné S., Pilon G., Levy E., Marette A., Roy D., Desjardins Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020;11:2032. doi: 10.3389/fmicb.2020.02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin J., Ren W., Wei B., Huang H., Li M., Wu X., Wang A., Xiao Z., Shen J., Zhao Y., et al. Characterization of chemical composition and prebiotic effect of a dietary medicinal plant Penthorum chinense Pursh. Food Chem. 2020;319:126568. doi: 10.1016/j.foodchem.2020.126568. [DOI] [PubMed] [Google Scholar]
- 74.Lu F., Li Y., Wang X., Hu X., Liao X., Zhang Y. Early-life polyphenol intake promotes Akkermansia growth and increase of host goblet cells in association with the potential synergistic effect of Lactobacillus. Food Res. Int. 2021;149:110648. doi: 10.1016/j.foodres.2021.110648. [DOI] [PubMed] [Google Scholar]
- 75.Peng Y., Yan Y., Wan P., Dong W., Huang K., Ran L., Mi J., Lu L., Zeng X., Cao Y. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res. Int. 2020;130:108952. doi: 10.1016/j.foodres.2019.108952. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y., Cao X., Zhao H., Yang E., Wang Y., Cheng N., Cao W. Impact of Camellia japonica Bee Pollen Polyphenols on Hyperuricemia and Gut Microbiota in Potassium Oxonate-Induced Mice. Nutrients. 2021;13:2665. doi: 10.3390/nu13082665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian B., Zhao J., An W., Zhang J., Cao X., Mi J., Zhao J., Zhang Y., Li J. Lycium ruthenicum diet alters the gut microbiota and partially enhances gut barrier function in male C57BL/6 mice. J. Funct. Foods. 2019;52:516–528. doi: 10.1016/j.jff.2018.11.034. [DOI] [Google Scholar]
- 78.Xu S., Wang Y., Wang J., Geng W. Kombucha Reduces Hyperglycemia in Type 2 Diabetes of Mice by Regulating Gut Microbiota and Its Metabolites. Foods. 2022;11:754. doi: 10.3390/foods11050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu F., Shan S., Li H., Shi J., Hao R., Yang R., Li Z. Millet shell polyphenols prevent atherosclerosis by protecting the gut barrier and remodeling the gut microbiota in ApoE−/− mice. Food Funct. 2021;12:7298–7309. doi: 10.1039/D1FO00991E. [DOI] [PubMed] [Google Scholar]
- 80.Liu Y., Wang X., Chen Q., Luo L., Ma M., Xiao B., Zeng L. Camellia sinensis and Litsea coreana Ameliorate Intestinal Inflammation and Modulate Gut Microbiota in Dextran Sulfate Sodium-Induced Colitis Mice. Mol. Nutr. Food Res. 2020;64:e1900943. doi: 10.1002/mnfr.201900943. [DOI] [PubMed] [Google Scholar]
- 81.Pouille C.L., Ouaza S., Roels E., Behra J., Tourret M., Molinié R., Fontaine J.-X., Mathiron D., Gagneul D., Taminiau B., et al. Chicory: Understanding the Effects and Effectors of This Functional Food. Nutrients. 2022;14:957. doi: 10.3390/nu14050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng S., Song J., Qin X., Yang K., Liu M., Yang C., Nyachoti C.M. Dietary supplementation of red-osier dogwood polyphenol extract changes the ileal microbiota structure and increases Lactobacillus in a pig model. AMB Express. 2021;11:145. doi: 10.1186/s13568-021-01303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choy Y.Y., Quifer-Rada P., Holstege D.M., Frese S.A., Calvert C.C., Mills D.A., Lamuela-Raventos R.M., Waterhouse A.L. Phenolic metabolites and substantial microbiome changes in pig feces by ingesting grape seed proanthocyanidins. Food Funct. 2014;5:2298–2308. doi: 10.1039/C4FO00325J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J., Yu B., Chen D., Zheng P., Luo Y., Huang Z., Luo J., Mao X., Yu J., He J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019;103:8157–8168. doi: 10.1007/s00253-019-10025-8. [DOI] [PubMed] [Google Scholar]
- 85.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- 86.Li W., Zhang X., He Z., Chen Y., Li Z., Meng T., Li Y., Cao Y. In vitro and in vivo antioxidant activity of eucalyptus leaf polyphenols extract and its effect on chicken meat quality and cecum microbiota. Food Res. Int. 2020;136:109302. doi: 10.1016/j.foodres.2020.109302. [DOI] [PubMed] [Google Scholar]
- 87.Dias D.M., Kolba N., Binyamin D., Ziv O., Regini Nutti M., Martino H.S.D., Glahn R.P., Koren O., Tako E. Iron Biofortified Carioca Bean (Phaseolus vulgaris L.)—Based Brazilian Diet Delivers More Absorbable Iron and Affects the Gut Microbiota In Vivo (Gallus gallus) Nutrients. 2018;10:1970. doi: 10.3390/nu10121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gong X., Jiang S., Tian H., Xiang D., Zhang J. Polyphenols in the Fermentation Liquid of Dendrobium candidum Relieve Intestinal Inflammation in Zebrafish Through the Intestinal Microbiome-Mediated Immune Response. Front. Immunol. 2020;11:1542. doi: 10.3389/fimmu.2020.01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Y., Xie M., Xue J., Xiang L., Li Y., Xiao J., Xiao G., Wang H. EGCG ameliorates neuronal and behavioral defects by remodeling gut microbiota and TotM expression in Drosophila models of Parkinson’s disease. FASEB J. 2020;34:5931–5950. doi: 10.1096/fj.201903125RR. [DOI] [PubMed] [Google Scholar]
- 90.Molan A.-L., Liu Z., Plimmer G. Evaluation of the Effect of Blackcurrant Products on Gut Microbiota and on Markers of Risk for Colon Cancer in Humans. Phytotherapy Res. 2014;28:416–422. doi: 10.1002/ptr.5009. [DOI] [PubMed] [Google Scholar]
- 91.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 92.Vendrame S., Guglielmetti S., Riso P., Arioli S., Klimis-Zacas D., Porrini M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011;59:12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 93.Liu Z., Lin X., Huang G., Zhang W., Rao P., Ni L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe. 2014;26:1–6. doi: 10.1016/j.anaerobe.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 94.Moreno-Indias I., Sánchez-Alcoholado L., Pérez-Martínez P., Andrés-Lacueva C., Cardona F., Tinahones F.J., Queipo-Ortuño M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016;7:1775–1787. doi: 10.1039/C5FO00886G. [DOI] [PubMed] [Google Scholar]
- 95.Rodríguez-Morató J., Matthan N.R., Liu J., de la Torre R., Chen C.-Y.O. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: A randomized crossover controlled feeding trial. J. Nutr. Biochem. 2018;62:76–86. doi: 10.1016/j.jnutbio.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 96.Yuan X., Long Y., Ji Z., Gao J., Fu T., Yan M., Zhang L., Su H., Zhang W., Wen X., et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018;62:e1800178. doi: 10.1002/mnfr.201800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Queipo-Ortuño M.I., Boto-Ordóñez M., Murri M., Gomez-Zumaquero J.M., Clemente-Postigo M., Estruch R., Cardona Diaz F., Andrés-Lacueva C., Tinahones F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 98.Song M.-Y., Wang J.-H., Eom T., Kim H. Schisandra chinensis fruit modulates the gut microbiota composition in association with metabolic markers in obese women: A randomized, double-blind placebo-controlled study. Nutr. Res. 2015;35:655–663. doi: 10.1016/j.nutres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Clavel T., Fallani M., Lepage P., Levenez F., Mathey J., Rochet V., Sérézat M., Sutren M., Henderson G., Bennetau-Pelissero C., et al. Isoflavones and Functional Foods Alter the Dominant Intestinal Microbiota in Postmenopausal Women. J. Nutr. 2005;135:2786–2792. doi: 10.1093/jn/135.12.2786. [DOI] [PubMed] [Google Scholar]
- 100.Wiese M., Bashmakov Y., Chalyk N., Nielsen D.S., Krych L., Kot W., Klochkov V., Pristensky D., Bandaletova T., Chernyshova M., et al. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. BioMed Res. Int. 2019;2019:4625279. doi: 10.1155/2019/4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wijayabahu A.T., Waugh S.G., Ukhanova M., Mai V. Dietary raisin intake has limited effect on gut microbiota composition in adult volunteers. Nutr. J. 2019;18:14. doi: 10.1186/s12937-019-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shinohara K., Ohashi Y., Kawasumi K., Terada A., Fujisawa T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe. 2010;16:510–515. doi: 10.1016/j.anaerobe.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 103.Cuervo A., Valdés L., Salazar N., de los Reyes-Gavilán C.G., Ruas-Madiedo P., Gueimonde M., González S. Pilot Study of Diet and Microbiota: Interactive Associations of Fibers and Polyphenols with Human Intestinal Bacteria. J. Agric. Food Chem. 2014;62:5330–5336. doi: 10.1021/jf501546a. [DOI] [PubMed] [Google Scholar]
- 104.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 105.Lye H.-S., Kuan C.-Y., Ewe J.-A., Fung W.-Y., Liong M.-T. The Improvement of Hypertension by Probiotics: Effects on Cholesterol, Diabetes, Renin, and Phytoestrogens. Int. J. Mol. Sci. 2009;10:3755–3775. doi: 10.3390/ijms10093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pelletier X., Laure-Boussuge S., Donazzolo Y. Hydrogen excretion upon ingestion of dairy products in lactose-intolerant male subjects: Importance of the live flora. Eur. J. Clin. Nutr. 2001;55:509–512. doi: 10.1038/sj.ejcn.1601169. [DOI] [PubMed] [Google Scholar]
- 107.Hoveyda N., Heneghan C., Mahtani K.R., Perera R., Roberts N.W., Glasziou P. A systematic review and meta-analysis: Probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Golowczyc M.A., Mobili P., Garrote G.L., Abraham A., De Antoni G.L. Protective action of Lactobacillus kefir carrying S-layer protein against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2007;118:264–273. doi: 10.1016/j.ijfoodmicro.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 109.Kleessen B., Kroesen A.J., Buhr H.J., Blaut M. Mucosal and Invading Bacteria in Patients with Inflammatory Bowel Disease Compared with Controls. Scand. J. Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 110.Petit L., Gibert M., Popoff M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 111.Guo X., Xia X., Tang R., Zhou J., Zhao H., Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008;47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 112.Parkar S.G., Trower T.M., Stevenson D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12–19. doi: 10.1016/j.anaerobe.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 113.Cait A., Hughes M.R., Antignano F., Cait J., Dimitriu P.A., Maas K.R., Reynolds L.A., Hacker L., Mohr J., Finlay B.B., et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018;11:785–795. doi: 10.1038/mi.2017.75. [DOI] [PubMed] [Google Scholar]
- 114.Rauf A., Khalil A.A., Rahman U.-U., Khalid A., Naz S., Shariati M.A., Rebezov M., Urtecho E.Z., de Albuquerque R.D.D.G., Anwar S., et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2021:1–21. doi: 10.1080/10408398.2021.1895064. [DOI] [PubMed] [Google Scholar]
- 115.Den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.-J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu Z., Huang S., Li T., Li N., Han D., Zhang B., Xu Z.Z., Zhang S., Pang J., Wang S., et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. 2021;9:184. doi: 10.1186/s40168-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhai Q., Feng S., Arjan N., Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 118.Wu T., Grootaert C., Pitart J., Vidovic N.K., Kamiloglu S., Possemiers S., Glibetic M., Smagghe G., Raes K., Van de Wiele T., et al. Aronia (Aronia melanocarpa) Polyphenols Modulate the Microbial Community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and Decrease Secretion of Proinflammatory Markers in a Caco-2/endothelial Cell Coculture Model. Mol. Nutr. Food Res. 2018;62:e1800607. doi: 10.1002/mnfr.201800607. [DOI] [PubMed] [Google Scholar]
- 119.McDougall G.J., Allwood J.W., Pereira-Caro G., Brown E.M., Ternan N., Verrall S., Stewart D., Lawther R., O’Connor G., Rowland I., et al. Nontargeted LC-MSn Profiling of Compounds in Ileal Fluids That Decrease after Raspberry Intake Identifies Consistent Alterations in Bile Acid Composition. J. Nat. Prod. 2016;79:2606–2615. doi: 10.1021/acs.jnatprod.6b00532. [DOI] [PubMed] [Google Scholar]
- 120.Fotschki B., Juskiewicz J., Jurgoński A., Rigby N., Sójka M., Kołodziejczyk K., Mackie A., Zdunczyk Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet. J. Nutr. Biochem. 2017;46:13–20. doi: 10.1016/j.jnutbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 121.Huang J., Feng S., Liu A., Dai Z., Wang H., Reuhl K., Lu W., Yang C.S. Green Tea Polyphenol EGCG Alleviates Metabolic Abnormality and Fatty Liver by Decreasing Bile Acid and Lipid Absorption in Mice. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Inouye S., Yamaguchi H., Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001;7:251–254. doi: 10.1007/s101560170022. [DOI] [PubMed] [Google Scholar]
- 123.Puupponen-Pimia R., Nohynek L., Hartmann-Schmidlin S., Kahkonen M., Heinonen M., Maatta-Riihinen K., Oksman-Caldentey K.-M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005;98:991–1000. doi: 10.1111/j.1365-2672.2005.02547.x. [DOI] [PubMed] [Google Scholar]
- 124.Bouarab-Chibane L., Forquet V., Lantéri P., Clément Y., Léonard-Akkari L., Oulahal N., Degraeve P., Bordes C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019;10:829. doi: 10.3389/fmicb.2019.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sirk T.W., Friedman M., Brown E.F. Molecular Binding of Black Tea Theaflavins to Biological Membranes: Relationship to Bioactivities. J. Agric. Food Chem. 2011;59:3780–3787. doi: 10.1021/jf2006547. [DOI] [PubMed] [Google Scholar]
- 126.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015;2015:905215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gowd V., Karim N., Shishir M.R.I., Xie L., Chen W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019;93:81–93. doi: 10.1016/j.tifs.2019.09.005. [DOI] [Google Scholar]
- 128.Chen Y., Li Q., Zhao T., Zhang Z., Mao G., Feng W., Wu X., Yang L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017;237:887–894. doi: 10.1016/j.foodchem.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 129.Hanske L., Engst W., Loh G., Sczesny S., Blaut M., Braune A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013;109:1433–1441. doi: 10.1017/S0007114512003376. [DOI] [PubMed] [Google Scholar]
- 130.Verediano T.A., Stampini Duarte Martino H., Dias Paes M.C., Tako E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients. 2021;13:1331. doi: 10.3390/nu13041331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Faria A., Fernandes I., Norberto S., Mateus N., Calhau C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 132.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin W., Wang W., Yang H., Wang D., Ling W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J. Food Sci. 2016;81:H3026–H3034. doi: 10.1111/1750-3841.13544. [DOI] [PubMed] [Google Scholar]
- 134.Decroos K., Eeckhaut E., Possemiers S., Verstraete W. Administration of Equol-Producing Bacteria Alters the Equol Production Status in the Simulator of the Gastrointestinal Microbial Ecosystem (SHIME) J. Nutr. 2006;136:946–952. doi: 10.1093/jn/136.4.946. [DOI] [PubMed] [Google Scholar]
- 135.Heinonenab S., Wähälä K., Adlercreutz H. Identification of Isoflavone Metabolites Dihydrodaidzein, Dihydrogenistein, 6′-OH-O-dma, and cis-4-OH-equol in Human Urine by Gas Chromatography–Mass Spectroscopy Using Authentic Reference Compounds. Anal. Biochem. 1999;274:211–219. doi: 10.1006/abio.1999.4279. [DOI] [PubMed] [Google Scholar]
- 136.Mayo B., Vázquez L., Flórez A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients. 2019;11:2231. doi: 10.3390/nu11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Espin J.C., Larrosa M., Garcia-Conesa M.T., Tomas-Barberan F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid. Based Complement. Alternat. Med. 2013;2013:2704. doi: 10.1155/2013/270418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cerdá B., Periago P., Espín A.J.C., Tomás-Barberán F.A. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005;53:5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]