The origin of the bacterial sacculus probably caused the origin of the domain Bacteria. Manufacture of this exoskeleton is complex because it involves the formation of polymerized peptidoglycan external to the cell membrane as a strong, stress-resistant sacculus. This process involves the formation of the disaccharide penta-muropeptide, its transport through the membrane, and its polymerization in the glycan and peptide directions to form an intact fabric covering the cell. Beyond that, the precursor must be inserted into the wall without risk of lysis and the wall must in some cases be safely turned over. These complex processes imply that in the time before the First Bacterium and in the time of the Last Universal Ancestor a set of diverse mechanisms arose and gave rise to variant mechanisms that functioned together to allow the formation of a strong covering for the bacterial cell.
Our understanding of the main aspects of the development of life on this planet are becoming established by the findings of biochemistry, biophysics, and cell biology, including those of the study of DNA sequences of different organisms (72). One important conclusion is that life leading to the organisms that are alive today started only once (2, 3, 55, 56). The other is that only after extensive evolution to gain the major attributes of cellular processes did organisms differentiate and develop stable diversity (31). We can be quite certain that the domain of bacteria arose as one line branching off from the remainder of organisms, which in turn later branched into the archaea and the eucarya. While many mutations occurred, only the most successful would have persisted, and thus stable diversity was probably delayed until after the general aspects of cell biology had been developed; this was simply because a successful mutant continued to eliminate the weaker competitors (4), according to the competitive-exclusion principle of Gause. The hypothesis that diversity finally arose because cell metabolism became more effective and caused the internal concentrations of solutes to increase has been suggested. These increased concentrations caused osmotic stress to become so great that the rupture of the cell membrane occurred (for a more detailed exposition of this argument, see references 31, 33, and 36). As a critical advance to compensate for this situation, bacteria that had developed an external wall, called the sacculus or the exoskeleton, arose. To deal with this osmotic stress, shortly thereafter the successful members of the remainder of organisms developed other ways to cope with osmotic problems, primarily by developing an endoskeleton. Thus, according to this hypothesis stable diversity probably arose because multiple noncompeting solutions to the same problem were independently developed (36).
The purpose of this review is to outline the technology and architecture of the cell wall that even the first successful bacterium must have developed. It is based on what we know about bacterial wall biology, i.e., the biochemistry, biophysics, and microbial physiology of the exoskeleton. So far, information for just a few bacteria is available. These are Enterococcus hirae, a gram-positive coccus, Bacillus subtilis, a gram-positive rod, and Escherichia coli, a gram-negative rod. Over the last 20 years, my research, speculation, and thinking have been aimed at trying to resolve how bacteria originated and to determine the physical and chemical bases of the bacterial wall, which is outside the cytoplasm and gives bacteria strength and shape. In this short review it is not possible to give a full explanation of the experiments and logic that gave rise to the concepts presented here. Moreover, much of the early work and ideas of workers in the cell wall field have not been given adequate coverage but are covered in references 5, 7, 10, 11, 15, 16, 17, 20, 22, 25, 30, 32, 36, 39, 48, 52, 54, 63, 68, and 70.
THE ROLE OF OSMOTIC PRESSURE
Some osmotic pressure in excess inside the cell relative to that on the outside was probably needed for wall enlargement and cell division even at the time of the first cell living on this planet (21). On the other hand, probably mechano-enzymes, like actin, developed or were perfected later, i.e., with the development of the eucarya (27). Before that time, forces resulting from biochemical syntheses were the only ones available to power cell growth and cell division. The internal osmotic pressure would have increased during the eons when cellular processes, including transport mechanisms, developed and became more efficient. At some point, the ability of the cell to accumulate materials led to the greater osmotic pressures inside than outside the cell, with the pressure increasing to the point where the cells with only phospholipid bilayer membranes had created the seeds of their own destruction and needed methods to cope with the tendency of water to enter the cell. Then countermeasures became essential and were evolved (31).
MANY MECHANISMS ARE BROUGHT TOGETHER FOR BACTERIAL WALL BIOSYNTHESIS
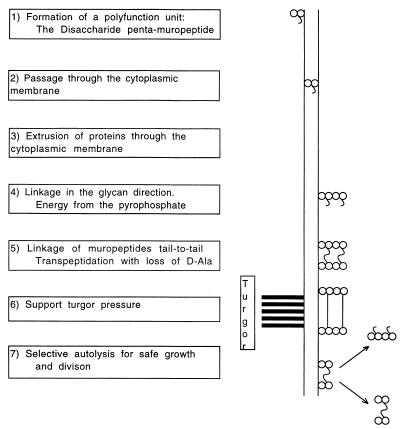
Wall biosynthesis is described in every microbiology text, but I particularly suggest references 7, 15, and 68. For the successful production of a bacterial sacculus, it can be hypothesized that at least seven quite different processes must have arisen independently and that they initially served essential functions other than that of wall synthesis. These processes are listed successively in Fig. 1. The formation of a sacculus involves many quite different processes and is quite complex, and dedicated versions of a number of existing processes must have been used for the production of the bacterial sacculus. Although these classes of processes must have originally evolved for other biological purposes, their variation for saccular formation and especially their ability to function together were essential for the generation of the then novel (∼3 billion years ago) process of sacculus formation.
FIG. 1.
Functions needed for saccular formation. The seven stages of the formation of the bacterial exoskeleton are listed. The stages of the production of a stress-bearing wall and its dissolution are indicated on the right.
Biochemistry to make disaccharide penta-muropeptides.
Intermediary metabolism evolved presumably because of the development of a progressive deficiency of abiotically produced low-molecular-weight substances needed for cytoplasm formation (18, 71). After the evolution of a network of a large number of enzymes, the construction of disaccharide penta-muropeptide from monosaccharides and amino acids was a relatively trivial variation of some processes of intermediary metabolism known today. The formation of a disaccharide penta-muropeptide is exceptional in only three ways (10, 11, 17, 68). The first is that its synthesis involves a special linkage to form N-acetyl muramic acid from N-acetylglucosamine and phosphoenolpyruvate. The second is that uncommon forms of amino acids are used. These include d-amino acids and diamino acids, such as diaminopimelic acid. The third is the linkage of glutamic acid into the structure by virtue of the second (gamma) carboxyl group instead of the usual alpha carboxyl group. There are other variations (68), but here I mention only the disaccharide penta-muropeptide of E. coli and B. subtilis, which is GlcNac-MurNAc-l-Ala-d-γ-Glu-m-A2pm-d-Ala-d-Ala, where GlcNAc is N-acetylglucosamine, MurNAc is N-acetyl muramic acid, and m-A2pm is meso-diaminopimelic acid.
Biophysics of extrusion through the lipid membrane with the help of bactoprenol.
Once the disaccharide penta-muropeptide is made, its export though the cytoplasmic membrane involves a different type of mechanism than that considered above for intermediary metabolism (54). Such a mechanism is quite common in modern organisms for the export of oligosaccharides. Moreover, this transport mechanism functions in all three domains and involves attaching oligosaccharides with a range of compositions to a long-chain molecule with 55 carbon atoms that is nonpolar except at one end. In animal systems, this molecule is called dolichol (67), and the slightly chemically modified form used in bacteria is called bactoprenol or undecaprenol. The long nonpolar portion can stretch back and forth several times across the bilayer. The polar end has a hydroxyl group that becomes linked to a phosphate residue. When the phosphate becomes coupled to a small polar molecule, the latter, too, is able to pass through the membrane. The necessary step for the export of the disaccharide penta-muropeptide precursor, after the completion of its synthesis, is that it is attached to the bactoprenol via a pyrophosphate linkage, with one of the phosphates being contributed by the bactoprenol phosphate and the other being contributed by the disaccharide penta-muropeptide UDP precursor molecule.
It is to be emphasized that this type of mechanism is a process that must have developed for export of polysaccharides for a variety of purposes in cells before the development of diversity with the Last Universal Ancestor. One purpose may have been to coat the outer surface of the primitive cell with polysaccharides in order to adjust the chemical nature of the surface to support the cell's adhesion to surfaces. Later in evolution such processes contributed to the binding of cells to other cells to form multicellular organisms.
Extrusion of proteins.
The first organisms could not even have been saprophytes and could absorb and consume only very small abiotically produced molecules (21, 29, 55, 56). The organisms that died represented a sink of nonutilizable biomass and not a resource for living organisms. Eventually, ways to harvest such resources developed, and for this purpose hydrolytic enzymes had to be synthesized and exported to the outside of living cells to degrade dead organisms (and larger resource molecules).
The export mechanism acting in modern bacteria is sophisticated (69, 72) requiring docking proteins, signal sequences, peptidases, and chaperones, all of which must have required extensive evolution. The seminal process of protein export is that the signal peptide, i.e., the initial portion of the protein to be exported, is hydrophobic and can insinuate itself into the phospholipid membrane. Once through, the remainder of the protein can be forced or threaded through the membrane since the thermodynamic barrier is thus lowered. This is because if a polar amino acid enters as another leaves the membrane, less biochemical work needs be expended. Subsequently, the signal peptide is removed after extrusion from the cell, requiring a previously secreted peptidase.
Some of the classes of proteins specifically needed to be secreted during bacterial wall formation beside the signal peptidase are penicillin binding proteins (PBPs) and autolysins that cleave the wall fabric in conjunction with enlargement. The development of such types of proteins and such transport methods, consequently, had to precede the development of a strong external wall. As mentioned, the impetus to secrete hydrolytic enzymes was, no doubt, in the first place for the generation of nutrients.
Energy for the synthesis of the wall fabric.
An essential and well-established feature of peptidoglycan formation is that two forms of energy transduction are provided by the cell through details of the structure of the exported disaccharide penta-muropeptide (10, 11, 68). This exported murein unit stores the needed energy in its chemical structure that has been trapped during its synthesis within the cytoplasm. It is expended externally both to couple the linkage of the disaccharides to each other (using the pyrophosphate linkage) and to couple the peptide portions of neighboring glycan chains to each other in a tail-to-tail linkage. The latter is by a transpeptidation that uses the energy in the terminal d-Ala-d-Ala bond of the muropeptides to create the cross-linkage of two muropeptides tail-to-tail with the loss of one d-Ala.
Coupling of autolysis and synthesis.
The strategy of forming an exoskeleton depends on being able to open the stress-bearing structure and insert new material. This must be done safely or else the cell structure ruptures as solutes and water enter (20, 29). Consequently, the original cell of the domain Bacteria must have arranged matters so that the cell added and linked new material before it cleaved the old wall. Somehow this had to be accomplished outside the cell proper. I have suggested that physical forces aided in this (20, 39, 45, 63). This may also have depended on autolysins with very special properties (25, 39, 63) or part of a holoenzyme (14, 15, 25).
The wall was an innovation at the time of the split of early life into domains.
When all seven of the classes of processes considered in Fig. 1 became workable and dedicated versions meshed together for wall enlargement, the synthesis to form a saccular structure would have become possible (29, 33). This would have allowed bacteria to grow (and grow faster) under conditions that would cause other metabolically successful organisms without strong walls to be impeded or succumb due to the self-created osmotic problem.
THE ROLE OF PHYSICS AND PHYSICAL CHEMISTRY IN WALL FORMATION
There are simple chemical mechanisms that may account for important parts of wall formation without invoking sophisticated biological mechanisms that would have required a great deal of evolutionary development (20, 32).
The first concept is that enzymes that attack macromolecules have an increased rate of reaction if their substrates are under tension. While this is not an original concept for chemists and, in fact, is a thermodynamic and kinetic truism, it has not been self-evident to many microbiologists. In fact, the response to stress is the only mechanism known which couples an increase in turgor pressure due to cytoplasmic growth to the activity of an enzyme acting on murein residing outside the cell membrane (20, 42).
The second possible chemical factor may be the geometry of the peptidoglycan layers. Evidently, murein addition can take place only adjacent to the cytoplasmic membrane, where enzymes are bound and where the precursor arrives from the cytoplasm. As shown in Fig. 2 and 3, for a gram-positive coccus and rod, the layers that are added are in the plane of the cytoplasmic membrane. There are two implications of this which are discussed below. One is the requirement that the sidewall murein must be progressively stretched after being laid down and eventually ruptured and discarded. The other is that when a septum is split to form new poles, the orientation of the layer becomes different and may become more resistant to autolytic attack. When the splitting occurs, the angle of the layers changes so that any further autolysis would not be in the plane of formation but at right angles to it. Consequently, it would not be surprising if hydrolysis “into the grain” would be slower than that “with the grain.” This is a hypothesis, but it may be a reason why existing poles do not provide a suitable substrate for cleavage and turnover. It has been a considerable puzzle for a number of years why the poles of B. subtilis (19), streptococci (1), and E. coli (47, 75) are quite inert and almost do not turn over at all whereas sidewalls of many rod-shaped cells where the geometry of the layer of murein is parallel to the surface of the cylinder have a half-time on the order of an hour (data for B. subtilis) (40).
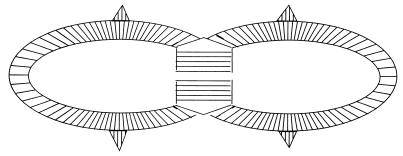
FIG. 2.
Growth pattern of E. hirae. The wall of a partially divided cell is shown diagrammatically. The layers of murein are added to the sidewall as shown on the sides of the figure. These move outward, are stretched, and eventually are autolyzed and discarded. The ingrowing septum is also added in an unstretched conformation as indicated by thick lines. When bisection occurs, the wall stretches and yields two daughter cells. The site for the ingrowth is located under a ridge that passes all around the equator of the cell.
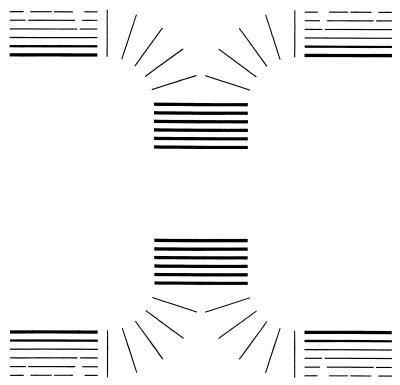
FIG. 3.
Growth pattern of B. subtilis. For the sidewall, new layers of wall are added repetitively on the inside of the existing wall but just outside the cytoplasmic membrane. The wall is stretched as it is forced outward and as the cell grows longer. Near the surface it becomes partially autolyzed and finally discarded. At certain locations a septum forms, growing inward by the same process of addition of new wall in successive layers as that shown in the central part of the figure. This wall is compact and unstressed until a central split exposes the septal wall to stress because the pressure inside the cell is higher than that outside. Then the wall covers more area, and when the septum splitting is finally complete, the same amount of murein that would constitute a planar septum becomes expanded in area by 50%.
Another physical factor may control and regulate the splitting of the septum: only the exposed septum is stressed but it is still in the right configuration and orientation (Fig. 1 and references 20 and 31). Once autolysis starts to split the septal wall and as autolysis continues, the physical stress forces lead to maximum tension in the middle of the thick septum. Because of this distribution of stresses, continued enzyme activity splits the septum evenly down the middle until cell separation occurs. Therefore, there may be no need for a special system to direct the autolysin activity (44).
The next part of the growth process is the transformation of the planar septum into an almost hemispherical pole. We have found that the septum of B. subtilis stretches 50% after the splitting of the septum and pole formation (37, 38). This occurs under conditions where no new murein is formed. Therefore, the generation of the pole shape depends on the elastic properties of the murein. This splitting process, moreover, can be catalyzed by hen egg white lysozyme, whose presumed function is to destroy gram-positive bacteria. This fact gives strong experimental support to the suggestion that the occurrence of the splitting in the middle of the septum is not a biological property requiring a special cellular process.
Random addition of murein to a layer simplifies the formation, and thus the enzymes involved do not need be moved systematically and progressively around the developing septum. The wall enlargement of the glycan chains requires a ternary interaction of donor, acceptor, and the linking enzyme. If there was a regimented mechanism that arranged and specified the location of the murein additions, it would have needed to control the movements of the bactoprenol molecules and the appropriate PBPs, as well as controlling both the existing recipient (acceptor) oligopeptidoglycan chain to be extended and the cross-linking of the peptide chains for the formation of a septal fabric. Ternary complexes are rare, and therefore such processes usually occur in two stages of binary complexes. The rules for solution chemistry, however, do not apply in quite the same way to membrane-bound molecules. Instead, they require the two-dimensional diffusion of the components associated with the surface. Some PBPs have their catalytic site on a flexible domain of the protein (66). That, no doubt, greatly increases the reaction rate but probably was not an essential part of the process of the formation of the wall of the earliest bacterium.
THE WALL GROWTH AND DIVISION OF THE FIRST BACTERIUM
The idea for consideration is that, in order to grow most simply, the first member of the domain Bacteria had to be the equivalent of a gram-positive coccus. This is consistent with considerations of the mechanisms of modern bacteria. As the strategy of saccular growth originated, a whole new set of mechanisms must have come into play in addition to the mechanisms listed in Fig. 1. While these strategic and control mechanisms could not be anywhere as sophisticated at the beginning of the existence of the domain as those used in modern bacteria, at the start they had to be sufficient to enlarge the sacculus safely.
A crucial point is that the assembly of an external strong wall required, at least at first, that the cell make use of physical forces and processes to a large extent. A corollary of this is that polymerization of the glycan and peptide chains should have occurred at random. It should be mentioned that I think that polymerization takes place at random during wall construction in today's organisms. This is an unpopular position (20, 22), but I still maintain it because there is no clear evidence from any organism that there is any order in the orientations of the glycan chains and no cogent reason has been presented why order is needed (see reference 32). Most fundamentally, an orderly sophisticated mechanism, such as that proposed in the current literature (14, 61a), would have had to be very precise and therefore not likely to have been developed at the dawn of the domain Bacteria (35). Systematic localization and movement of the region of new murein insertion would require a complex mechanism for which there is no precedent in other microbiological processes. Moreover, such a mechanism would have had no alternate or previous purpose and there is no known or easily envisioned function that could give rise to precise alignment. Consequently, if such an orderly process occurred, it would have had to have been evolved especially for the purpose of murein enlargement.
The possibility of a systematic type of growth mechanism is very appealing, and several have been proposed (14, 61A). However, the onus is then on the proposer to suggest how it could be regimented. The “three-for-one” model of Höltje (14, 15, 16), its precursor (25), and a modification thereof (34) have much in their favor, but the key point is that the wall is enlarged by a holoenzyme apparatus of several proteins that must remain as an aggregate during function and must act in an organized way that can scarcely have occurred in the first organism with an intact sacculus.
Before speculating on the original mechanism for growth and cell division, I will start by describing the cell division process as we know it in modern E. hirae and B. subtilis.
The growth of E. hirae.
The paradigm for this first bacterium may be the growth process of E. hirae as revealed by the numerous studies of the workers at Temple Medical School. In particular, they noticed that there is a ridge (12, 13) around this American-football-shaped coccus and that this ridge splits concomitantly with the ingrowth and development of a septum internal to the ridge. When the cell divides, each new daughter has a complete ridge that is capable of splitting in the next generation. The analogy to DNA replication jumps to mind. Seemingly, this is a process of semiconservative replication in which each asymmetric half generates the next whole ridge.
So it can be suggested that this type of ridge marks the site below which the murein is initially added and marks the site where further internal layers are added repetitively until the septum is completed (Fig. 2). The ridge marks the site of septal splitting. A more detailed analysis has been presented to account for the football shape of the cell (28). The mechanism for other types of gram-positive coccal cells, like Staphylococcus spp., must be much more complicated.
Figure 2 shows diagrammatically the layers of murein initially being added to the forming septum, but as it splits the layers come to be oriented closer to being approximately normal to the wall surface and become stretched. This proximity and stretching subsequently become important, as discussed below.
The growth of B. subtilis.
The rod-shaped organism diagrammed in Fig. 3, B. subtilis, actually has a very simple pattern of growth in which layer after layer of murein is added just outside the cytoplasmic membrane (20, 42). Layers as they are laid down are in a compact relaxed conformation and are unextended. However, as layers are successively added underneath and the cell grows longer, the older layers, even though no new material can be added, become elongated by being stretched. In the sidewall part shown in Fig. 3, I have tried to indicate the degree of stretching of a layer by how heavily the line has been drawn. The farther they get from the cytoplasmic membrane, the more stretched they become (and the thinner they are drawn). Eventually, the layer must rupture, causing crevasses to form. In the modern organism, rupture is aided by autolysins. This turnover process (40) leads to roughness of the exposed surface. Hydrolytic enzymes act in conjunction with the stress and, because of this, produce a roughened outside surface that has 50-fold more area than does the inner face of the cell wall (64). This process also causes the rotation of the two ends of the growing rod (26).
The Bacillus pole is made by formation of a septum, again made by addition of layers inside older layers, as shown in the central part of Fig. 3. Necessarily, there is no stress on a layer until the septum splits (shown by the heavy black lines). Experimental results showed that the surface area then increases by 50% as the planar septum is stretched into a nearly hemispherical pole (37, 38). This is shown with the thin lines as the abrupt extension of murein as soon as the splitting takes place, which will also become important below.
The control of autolysin action, of course, is necessary for any type of walled microorganism. However, the mechanism to achieve control can be quite different. For the sidewall of B. subtilis (42), it appears that the two major autolysins, glycosaminidase and amidase, are secreted across the cytoplasmic membrane, transported as the layers of murein move outward, and function only when they arrive on the outer surface.
A list of factors that might prevent the autolysins from functioning once they have been secreted through the cytoplasmic membrane but before they reach the surface of the turning over cell wall has been presented (32). Of these, possibly the most important factor is that the nascent inner portion of wall is not under tension and therefore is a poorer substrate for the autolysin. In the same place, a list of factors that might allow them to act effectively once they reach the surface has been presented. One factor is that autolysins have affinity for the cell wall (23) and might accumulate there. They do not diffuse away from the cell surface and may therefore hydrolyze its outer surface, causing inside-to-outside growth involving turnover of the outer layers.
Creation of an early murein septum.
The cell division strategies originally had to be simple, and I believe that modern gram-positive cocci offer the simplest paradigm. A coccus would have needed to be capable only of forming a septum, probably with a mechanism similar to that of the pole formation of E. hirae (13) or the cross-wall formation of B. subtilis (37, 38) as shown in Fig. 2 and 3. These cross-walls are much thicker than a monolayer of murein or slightly thicker than twice that of the organism's murein sidewall. As they are formed, the cytoplasmic membrane invaginates and new coatings of murein must be added to its inner margin. As growth takes place, the annulus becomes narrowed and, at the end, closes and completes the disk of the septum.
This septum-forming process requires that somehow the cell is able to (i) designate the septum location (this may have been a very imprecise process initially), (ii) permit the new murein precursors to be secreted only in a restricted zone (the width of the septum), and (iii) autolyze the septum in a special restricted way. That is, splitting must occur only in the precise middle of the septal thickness.
Except for the bisection of the septum, how splitting is done is still not known. If we assume that within this restricted septal region glycan chain elongation and cross-linking of peptide chains occurred at random, then the forces due to the turgor pressure inside the cell as the septum starts to split would give rise to the highest tension in the center of the septum (44) and center the splitting action.
For the simplest process imaginable, almost all that appears to be biologically needed is the accumulation of a number of protein factors and the disaccharide penta-muropeptide precursors at the ingrowing margin of the septum, i.e., just outside the cytoplasmic membrane. This accumulation and linkage would cause a layer of peptidoglycan to be formed on the inside of the wall, forming a covering sheet of limited size. It probably would be chemically attached to the earlier deposited layer of peptidoglycan for the septum.
Bisection of a septum.
For accurate bisection by physical forces, the polymerized chains would not need to be arranged in any precise order and their presence may be counterproductive. The hypothesized mechanism described in the last paragraph generates a circular planar septal disk, hopefully at the maximum diameter of the nearly round cell. The second necessary part of the process is the bisection of the septum to create two new poles. This need not mean that an autolysin with special selectivity is needed. The autolysin must not attack the pole wall but should attack the external central part of the septum. In addition, the catalytic activities of the autolysins should respond to the synthesis of cytoplasm and the consequent need of the cell to enlarge (20).
Location of the septum in cocci.
How does the septum become properly located? What type of machinery could find the middle of the cell? There may, or may not, be a fundamental difference between cocci and bacilli for this process. Many modern cocci, with no cylinder region, may depend on the semiconservative replication of the ridge as in E. hirea. I and my coworkers (41, 45) have presented a model for how cells can divide to form tetrads and octets. The model (explained in more detail in the next section) involves the attachment of the chromosome to the cell wall and specific binding sites located at the tips of poles. It also involves the replication of the binding sites at these locations. This type of process may be the paradigm for the location of the formation of septa in cocci.
Location of the septum in rod-shaped organisms.
The problem of a septal location is more difficult for rod-shaped bacteria. There are several possibilities. Consideration of the requirements for the mechanisms for division into two equal daughters led to the hypothesis that attachment of the chromosome to the ends of the cell envelope had to be the key (42). Of the various cell components, chromosomal DNA is the only molecule in the replicating cell that has a long enough dimension to be a measuring stick to enable the cell to bisect itself. A speculative model was proposed (41, 45) that may in the end prove valid. The suggestion was that the origin of replication, oriC, of the circular DNA is attached to the tip of the pole. Then on the initiation of chromosome replication, one of the two sister chromosomal origins is separated from the pole of origin and diffuses through the cell, possibly attached loosely to the membrane. At some point the oriC DNA finds and binds the terminus DNA and becomes attached to the binding site on the wall at the other pole. Then in our proposed mechanism, a special (but not unique) kind of action is needed. The receptor localized at the tip of the other pole is at that instant bound to the terminus DNA. The nascent oriC exchanges with the terminus DNA. This would happen if the binding site had a higher affinity for the origin DNA than it did for the terminus DNA. The ejected terminus DNA would not find a binding site and instead would be jockeyed through the cell, again probably loosely associated with the cytoplasmic membrane, and become associated with the replicating part of the DNA (the replisome). This jockeying would end up by positioning the terminus close to the center of the cell because the replicating structure of DNA is of a theta shape and the two symmetrical halves would force the terminus region to become located in the center. The eventual central location of the terminus in the cell would occur because its two oriC regions are attached at both poles. At some point the terminus complex would generate the formation of two new binding sites for the two termini that result from the completion of chromosome replication. Initially, this model was invented to explain the known fact that E. coli and B. subtilis divide almost precisely in half. Seemingly, there is no biological reason for such a degree of precision. We argued (33) that the linkage of DNA replication and cell division provided the incidentally observed high, but unnecessary, precision for the location of the division plane, almost precisely bisecting the cell. So this would be a default mechanism that would have required only the development of a mechanism so that the terminus DNA as the last stage of chromosome replication would code and locate the paired receptors for origin-terminus DNA at that site. For a primitive bacterium, the only addition is that this process must trigger the formation of the septum as well. Another possible mechanism has also been suggested (43).
CONCLUSIONS
The underlying basis for the considerations presented here is that formation of the strong peptidoglycan walls of bacteria was essential for the evolution of the domain Bacteria. At the time of the Last Universal Ancestor, much evolution had already occurred and created the general aspects of what we today call cell biology. Thus, biological mechanisms that could be varied and mobilized for saccular construction were in place and functioning. Moreover, physical processes could be harnessed in the process. The exoskeleton strategy of bacteria in surmounting osmotic challenges required the development of variations of at least seven kinds of ongoing processes in order to produce a strong polymer outside the cell proper. Thereafter, strategies to deal efficiently with growth and accurate cell division were soon required. This means that the very special processes needed for production of the sacculus and the division of the cell are key. Very special and critical issues in the development of bacteria are the roles and control of autolytic activities of bacteria that may in part be explained on the basis of physical forces.
ACKNOWLEDGMENTS
Workers at Temple University, Mike Higgins, Lolita Daneo-Moore, and Gerry Shockman, each with different approaches, studied the growth biology of Streptococcus faecalis, now known as E. hirae. Their work provided a basis for study of the mechanism behind the function of the bacterial wall in this gram-positive coccus. From this basis the strategy of the gram-positive rod B. subtilis could be examined. This examination was done with the help of Ron Doyle and Ian Burdett. An understanding of the growth biology of the gram-negative rod E. coli depended on the extensive work of Joachim-Volker Höltje and the members of his laboratory in Tübingen, Germany, and I was glad to be a small part of it.
Footnotes
Many others scientists have made important contributions to this field of study, but this paper is dedicated to Gerry Shockman, whose early and seminal contributions were critical. He has recently died.
REFERENCES
- 1.Cole R M, Hahn J J. Cell wall replication in Streptococcus pyrogenes. Immunofluorescent methods applied during growth show that new wall is formed equatorially. Science. 1962;125:722–724. doi: 10.1126/science.135.3505.722. [DOI] [PubMed] [Google Scholar]
- 2.Deamer D W. The first living system: a bioenergetic perspective. Microbiol Mol Biol Rev. 1997;61:239–261. doi: 10.1128/mmbr.61.2.239-261.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deamer D W, Fleischaker G R, editors. The origins of life: the central concepts. Boston, Mass: Jones and Bartlett Publishers; 1994. pp. 73–81. [Google Scholar]
- 4.De Pedro M A, Quintela J C, Höltje J-V, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle R J, Koch A L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15:169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- 6.Gause G F. The struggle for existence. Baltimore, Md: Williams & Wilkins; 1934. [Google Scholar]
- 7.Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. [Google Scholar]
- 8.Giesbrecht P, Kersten T, Wecke J. Fan-shaped ejections of regularly arranged murosomes involved in penicillin-induced death of staphylococci. J Bacteriol. 1992;17:2241–2252. doi: 10.1128/jb.174.7.2241-2252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldane, J. B. S. 1929. The origin of life. In Rational Annu. [Reprint, D. W. Deamer and G. R. Fleischaker (ed.), The origins of life: the central concepts, p. 73–81. Jones and Bartlett Publishers, Boston, Mass., 1994.]
- 10.Heijenoort J V. Biosynthesis of bacterial peptidoglycan unit. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 39–54. [Google Scholar]
- 11.Heijenoort J V. Murein synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. I. Washington, D.C.: American Society for Microbiology; 1996. pp. 48–57. [Google Scholar]
- 12.Higgins M L. Three-dimensional reconstruction of whole cells of Streptococcus faecalis from thin sections of cells. J Bacteriol. 1976;127:1337–1345. doi: 10.1128/jb.127.3.1337-1345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins M L, Shockman G D. Study of a cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstruction of thin sections of cells. J Bacteriol. 1976;127:1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höltje J-V. “Three-for-one”—a simple growth mechanism that guarantees a precise copy of the thin, rod-shaped murein sacculus of Escherichia coli. In: de Pedro M A, Höltje J-V, Loffelhardt W, editors. Bacterial growth and lysis. New York, N.Y: Plenum Press; 1993. pp. 419–426. [Google Scholar]
- 15.Höltje J-V. A hypothetical holoenzyme involved in the replication of the murein sacculus of Escherichia coli. Microbiology. 1996;142:1911–1919. doi: 10.1099/13500872-142-8-1911. [DOI] [PubMed] [Google Scholar]
- 16.Höltje J-V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höltje J-V, Schwarz U. Biosynthesis and growth of the murein sacculus. In: Nanninga N, editor. Molecular cytology of Escherichia coli. London, United Kingdom: Academic Press, Inc.; 1985. pp. 77–119. [Google Scholar]
- 18.Horowitz N H. On the evolution of biochemical syntheses. Proc Natl Acad Sci USA. 1945;31:153–157. doi: 10.1073/pnas.31.6.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchner G, Kemper M A, Koch A L, Doyle R J. Zonal turnover of cell poles of Bacillus subtilis. Ann Inst Pasteur/Microbiol (Paris) 1988;139:645–654. doi: 10.1016/0769-2609(88)90069-5. [DOI] [PubMed] [Google Scholar]
- 20.Koch A L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- 21.Koch A L. Primeval cells: possible energy-generating and cell-division mechanisms. J Mol Evol. 1985;21:270–277. doi: 10.1007/BF02102359. [DOI] [PubMed] [Google Scholar]
- 22.Koch A L. Biophysics of bacterial wall viewed as a stress-bearing fabric. Microbiol Rev. 1988;52:337–353. doi: 10.1128/mr.52.3.337-353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch A L. Partition of autolysins between the medium, the internal part of the wall, and the surface of the wall of Gram-positive rods. J Theor Biol. 1988;134:463–472. doi: 10.1016/s0022-5193(88)80052-3. [DOI] [PubMed] [Google Scholar]
- 24.Koch A L. The surface stress theory for the case of E. coli: the paradoxes of gram-negative growth. Res Microbiol. 1990;141:119–130. doi: 10.1016/0923-2508(90)90103-w. [DOI] [PubMed] [Google Scholar]
- 25.Koch A L. Additional argument for the key role of “smart” autolysins in the enlargement of the wall of gram-negative bacteria. Res Microbiol. 1990;141:529–541. doi: 10.1016/0923-2508(90)90017-k. [DOI] [PubMed] [Google Scholar]
- 26.Koch A L. The relative rotation of the ends of Bacillus subtilis. Arch Microbiol. 1990;153:569–573. doi: 10.1007/BF00245266. [DOI] [PubMed] [Google Scholar]
- 27.Koch A L. The wall of bacteria serves the role that mechano-proteins do in eukaryotes. FEMS Microbiol Rev. 1991;88:15–26. doi: 10.1111/j.1574-6968.1991.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 28.Koch A L. Differences in the formation of poles of Enterococcus and Bacillus. J Theor Biol. 1992;154:205–217. doi: 10.1016/s0022-5193(05)80403-5. [DOI] [PubMed] [Google Scholar]
- 29.Koch A L. Microbial genetic responses to extreme challenges. J Theor Biol. 1993;160:1–21. doi: 10.1006/jtbi.1993.1001. [DOI] [PubMed] [Google Scholar]
- 30.Koch A L. Current status of the surface stress theory. In: de Pedro M A, Höltje J-V, Löffëlhardt W, editors. Bacterial growth and lysis: metabolism and structure of the bacterial sacculus. New York, N.Y: Plenum Press; 1993. pp. 427–443. [Google Scholar]
- 31.Koch A L. Development and diversification of the Last Universal Ancestor. J Theor Biol. 1994;168:269–280. doi: 10.1006/jtbi.1994.1108. [DOI] [PubMed] [Google Scholar]
- 32.Koch A L. Bacterial growth and form. New York, N.Y: Chapman and Hall; 1995. [Google Scholar]
- 33.Koch A L. Origin of intracellular and intercellular pathogens. Q Rev Biol. 1995;70:423–437. [Google Scholar]
- 34.Koch A L. The ‘three-for-one’ model for the Gram-negative wall growth: a problem and a possible solution. FEMS Microbiol Lett. 1998;162:127–134. doi: 10.1111/j.1574-6968.1998.tb12989.x. [DOI] [PubMed] [Google Scholar]
- 35.Koch A L. Orientation of the peptidoglycan chains in the sacculus of Escherichia coli. Res Microbiol. 1998;149:689–701. doi: 10.1016/s0923-2508(99)80016-3. [DOI] [PubMed] [Google Scholar]
- 36.Koch A L. How did bacteria come to be? Adv Microb Physiol. 1998;40:354–399. doi: 10.1016/s0065-2911(08)60135-6. [DOI] [PubMed] [Google Scholar]
- 37.Koch A L, Burdett I D J. Normal pole formation during total inhibition of wall synthesis. J Gen Microbiol. 1986;132:3441–3449. doi: 10.1099/00221287-132-12-3441. [DOI] [PubMed] [Google Scholar]
- 38.Koch A L, Burdett I D J. Biophysics of pole formation of Gram-positive rods. J Gen Microbiol. 1986;132:3451–3457. doi: 10.1099/00221287-132-12-3451. [DOI] [PubMed] [Google Scholar]
- 39.Koch A L, Doyle R J. The growth strategy of the Gram-positive rod. FEMS Microbiol Rev. 1986;32:247–254. [Google Scholar]
- 40.Koch A L, Doyle R J. Inside-to-outside growth and the turnover of the Gram-positive rod. J Theor Biol. 1985;117:137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]
- 41.Koch A L, Doyle R J. Attachment of the chromosome to the cell poles: the strategy for the growth of bacteria in 2 and 3 dimensions. J Theor Biol. 1999;199:213–226. doi: 10.1006/jtbi.1999.0950. [DOI] [PubMed] [Google Scholar]
- 42.Koch A L, Higgins M L, Doyle R J. Surface tension-like forces determine bacterial shapes: Streptococcus faecium. J Gen Microbiol. 1981;123:151–161. doi: 10.1099/00221287-123-1-151. [DOI] [PubMed] [Google Scholar]
- 43.Koch A L, Höltje J-V. A physical model for the precise location of the division site during growth of rod-shaped bacterial cells. Microbiology. 1995;141:3171–3180. [Google Scholar]
- 44.Koch A L, Kirchner G, Doyle R J, Burdett I D J. How does a Bacillus split its septum right down the middle? Ann Inst Pasteur/Microbiol (Paris) 1985;136A:91–98. doi: 10.1016/s0769-2609(85)80028-4. [DOI] [PubMed] [Google Scholar]
- 45.Koch A L, Mobley H L T, Doyle R J, Streips U N. The coupling of wall growth and chromosome replication in Gram-positive rods. FEMS Microbiol Lett. 1981;12:201–208. [Google Scholar]
- 46.Koch A L, Woeste S W. The elasticity of the sacculus of Escherichia coli. J Bacteriol. 1992;174:4811–4819. doi: 10.1128/jb.174.14.4811-4819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch A L, Woldringh C L. The metabolic inertness of the poles of a Gram-negative rod. J Theor Biol. 1995;171:415–425. [Google Scholar]
- 48.Labischinski H, Barnickel G, Bradaczek H, Giesbricht P. On the secondary and tertiary structure of murein. Low and medium-angle X-ray evidence against chitin-based conformations of bacterial peptidoglycan. Eur J Biochem. 1979;95:147–155. doi: 10.1111/j.1432-1033.1979.tb12949.x. [DOI] [PubMed] [Google Scholar]
- 49.Labischinski H, Barnickel G, Leps B, Bradaczek H. Initial data for comparison of murein and pseudo murein conformation. Arch Microbiol. 1980;127:195–201. [Google Scholar]
- 50.Labischinski H, Barnickel G, Naumann D, Keller P. Conformational and topological aspects of the three-dimensional architecture of bacterial peptidoglycan. Ann Inst Pasteur/Microbiol (Paris) 1985;136A:45–50. doi: 10.1016/s0769-2609(85)80020-x. [DOI] [PubMed] [Google Scholar]
- 51.Labischinski H, Goodell E W, Goodell A, Hochberg M L. Direct proof of a “more-than-single-layered” peptidoglycan architecture of Escherichia coli W7, a neutron small-angle scattering study. J Bacteriol. 1991;173:751–756. doi: 10.1128/jb.173.2.751-756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Labischinski H, Maidhof H. Bacterial peptidoglycan: overview and evolving concepts. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 23–38. [Google Scholar]
- 53.Leps B, Labischinski H, Barnickel G, Bradaczek H, Giesbrecht P. A new proposal for the primary and secondary structure of the glycan moiety of pseudomurein. Conformational energy calculations on the glycan strands with talosaminuronic acid in 1C conformation and comparison with murein. Eur J Biochem. 1984;144:279–286. doi: 10.1111/j.1432-1033.1984.tb08461.x. [DOI] [PubMed] [Google Scholar]
- 54.Matsuhashi M. Utilization of lipid-linked precursors and the formation of peptidoglycan in the process of cell growth and division: membrane enzymes involved in the final steps of peptidoglycan synthesis and the mechanism of their regulation. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 55–71. [Google Scholar]
- 55.Miller S L, Orgel L E. The origins of life on earth. New York, N.Y: Prentice-Hall; 1973. [Google Scholar]
- 56.Morowitz H. Beginnings of cellular life: metabolism recapitulates biogenesis. New Haven, Conn: Yale University Press; 1992. [Google Scholar]
- 57.Norris V, et al. Cell cycle control: prokaryotic solutions to eukaryotic problems? J Theor Biol. 1994;168:227–230. doi: 10.1006/jtbi.1994.1102. [DOI] [PubMed] [Google Scholar]
- 58.Obermann W, Höltje J-V. Alterations of murein structure and of penicillin-binding proteins in minicells from Escherichia coli. Microbiology. 1994;140:79–87. doi: 10.1099/13500872-140-1-79. [DOI] [PubMed] [Google Scholar]
- 59.Oparin A I. The origin of life. New York, N.Y: Dover; 1936. [Google Scholar]
- 60.Orgel L E. Evolution of the genetic apparatus: a review. Cold Spring Harbor Symp Quant Biol. 1987;52:9–16. doi: 10.1101/sqb.1987.052.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 61a.Park J T. The murein sacculus. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. I. Washington, D.C.: American Society for Microbiology; 1996. pp. 48–57. [Google Scholar]
- 62.Schwarz U, Ryter A, Rambech A, Hellio R, Hirota Y. Process of cellular division in Escherichia coli: differentiation of growth zones in the sacculus. J Mol Biol. 1975;98:749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- 63.Shockman G D, Höltje J-V. Microbial peptidoglycan (murein) hydrolases. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 131–166. [Google Scholar]
- 64.Sonnenfeld E M, Beveridge T J, Koch A L, Doyle R J. Asymmetric distribution of charges on the cell wall of Bacillus subtilis. J Bacteriol. 1985;163:1167–1171. doi: 10.1128/jb.163.3.1167-1171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spratt B G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spratt B G, Bowler L D, Edelman A, Broome-Smith J K. Membrane topology of penicillin-binding proteins 1b and 3 of Escherichia coli and the production of water-soluble forms of high-molecular weight penicillin-binding proteins. In: Actor P, Daneo-Moore L, Higgins M L, Salton M R J, Shockman G D, editors. Antibiotic inhibition of bacterial cell surface assembly and function. Washington, D.C.: American Society for Microbiology; 1988. pp. 292–300. [Google Scholar]
- 67.Stryer L. Biochemistry. 4th ed. New York, N.Y: Freeman; 1995. [Google Scholar]
- 68.Tipper D J, Wright A. The structure and biosynthesis of bacterial cell walls. In: Sokatch J R, Ornston L N, editors. The Bacteria. Vol. 7. London, United Kingdom: Academic Press; 1979. pp. 291–426. [Google Scholar]
- 69.Wandersman C. Secretion across the bacterial outer membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. I. Washington, D.C.: American Society for Microbiology; 1996. pp. 955–966. [Google Scholar]
- 70.Weidel W, Pelzer H. Bag-shaped macromolecules—a new outlook on bacterial cell walls. Adv Enzymol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- 71.White D. The physiology and biochemistry of prokaryotes. New York, N.Y: Oxford University Press; 2000. pp. 295–304. [Google Scholar]
- 72.Wickner W, Driessen A J M, Hartl F-U. The enzymology of protein translocation across the plasma membrane of Escherichia coli. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- 73.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woese C R, Kandler O, Wheelis M L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woldringh C L, Huls P, Pas E, Brakenhoff G H, Nanninga N. Topography of peptidoglycan synthesis during elongation and polar cap formation in a cell division mutant of Escherichia coli MC43100. J Gen Microbiol. 1987;133:575–586. [Google Scholar]